Abstract
Background
Current drug therapy for acute heart failure syndromes (AHFS) consists mainly of diuretics supplemented by vasodilators or inotropes. Nitrates have been used as vasodilators in AHFS for many years and have been shown to improve some aspects of AHFS in some small studies. The aim of this review was to determine the clinical efficacy and safety of nitrate vasodilators in AHFS.
Objectives
To quantify the effect of different nitrate preparations (isosorbide dinitrate and nitroglycerin) and the effect of route of administration of nitrates on clinical outcome, and to evaluate the safety and tolerability of nitrates in the management of AHFS.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3), MEDLINE (1950 to July week 2 2011) and EMBASE (1980 to week 28 2011). We searched the Current Controlled Trials MetaRegister of Clinical Trials (compiled by Current Science) (July 2011). We checked the reference lists of trials and contacted trial authors. We imposed no language restriction.
Selection criteria
Randomised controlled trials comparing nitrates (isosorbide dinitrate and nitroglycerin) with alternative interventions (frusemide and morphine, frusemide alone, hydralazine, prenalterol, intravenous nesiritide and placebo) in the management of AHFS in adults aged 18 and over.
Data collection and analysis
Two authors independently performed data extraction. Two authors performed trial quality assessment. We used mean difference (MD), odds ratio (OR) and 95% confidence intervals (CI) to measure effect sizes. Two authors independently assessed and rated the methodological quality of each trial using the Cochrane Collaboration tool for assessing risk of bias.
Main results
Four studies (634 participants) met the inclusion criteria. Two of the included studies included only patients with AHFS following acute myocardial infarction (AMI); one study excluded patients with overt AMI; and one study included participants with AHFS with and without acute coronary syndromes.
Based on a single study, there was no significant difference in the rapidity of symptom relief between intravenous nitroglycerin/N‐acetylcysteine and intravenous frusemide/morphine after 30 minutes (fixed‐effect MD ‐0.30, 95% CI ‐0.65 to 0.05), 60 minutes (fixed‐effect MD ‐0.20, 95% CI ‐0.65 to 0.25), three hours (fixed‐effect MD 0.20, 95% CI ‐0.27 to 0.67) and 24 hours (fixed‐effect MD 0.00, 95% CI ‐0.31 to 0.31). There is no evidence to support a difference in AHFS patients receiving intravenous nitrate vasodilator therapy or alternative interventions with regard to the following outcome measures: requirement for mechanical ventilation, systolic blood pressure (SBP) change after three hours and 24 hours, diastolic blood pressure (DBP) change after 30, 60 and 90 minutes, heart rate change at 30 minutes, 60 minutes, three hours and 24 hours, pulmonary artery occlusion pressure (PAOP) change after three hours and 18 hours, cardiac output (CO) change at 90 minutes and three hours and progression to myocardial infarction. There is a significantly higher incidence of adverse events after three hours with nitroglycerin compared with placebo (odds ratio 2.29, 95% CI 1.26 to 4.16) based on a single study. There was no consistent evidence to support a difference in AHFS patients receiving intravenous nitrate vasodilator therapy or alternative interventions with regard to the following secondary outcome measures: SBP change after 30 and 60 minutes, heart rate change after 90 minutes, and PAOP change after 90 minutes. None of the included studies reported healthcare costs as an outcome measure. There were no data reported by any of the studies relating to the acceptability of the treatment to the patients (patient satisfaction scores).
Overall there was a paucity of relevant quality data in the included studies. Assessment of overall risk of bias in these studies was limited as three of the studies did not give sufficient detail to allow assessment of potential risk of bias.
Authors' conclusions
There appears to be no significant difference between nitrate vasodilator therapy and alternative interventions in the treatment of AHFS, with regard to symptom relief and haemodynamic variables. Nitrates may be associated with a lower incidence of adverse effects after three hours compared with placebo. However, there is a lack of data to draw any firm conclusions concerning the use of nitrates in AHFS because current evidence is based on few low‐quality studies.
Plain language summary
Nitrates for acute heart failure syndromes
Heart failure occurs when the lower muscular heart chamber is unable to fill or eject blood normally due to heart disease of any origin. Acute heart failure syndromes (AHFS) are defined as gradual or rapid (over a period of less than 48 hours) deterioration in heart failure signs and symptoms resulting in a need for urgent therapy. There are many types of drugs and non‐drug based interventions used for the treatment of AHFS. The aim of this review has been to determine the effectiveness and safety of nitrates (one drug group used for the treatment of AHFS) compared with alternative interventions in the treatment of patients with AHFS.
The four studies in this review included 634 patients with AHFS and employed two types of nitrates (isosorbide dinitrate and nitroglycerin). The studies compared nitrates with frusemide and morphine, frusemide alone, hydralazine, prenalterol, intravenous nesiritide and placebo. The study population in the trials was predominantly male (469/634 or 74% of all the patients included in the studies were male). The findings of this review indicate that there is no significant difference between nitrates and alternative treatment interventions for patients with AHFS in terms of healthcare outcomes. Nitrates appeared to be well tolerated in all four studies. Headaches is the most common side effect reported by patients. Headaches occurred more frequently when compared with nesiritide. There appeared to be no significant difference in the occurrence of symptomatic hypotension, pain, nausea and angina between patients administered nitroglycerin and nesiritide. The included studies did not report healthcare costs as an outcome measure.
The limitations of the review include the following: there were few studies eligible for inclusion (only four); the quality of the studies were relatively poor; the study participants were predominantly male; and all the eligible studies were conducted in developed Western countries. Consequently, the findings may not be generalisable to other healthcare settings and to females. The review also found no consistent evidence to support a difference in AHFS patients receiving nitrates or alternative interventions with regard to many of the healthcare outcome measures studied. Due to these limitations, the results of the review preclude definitive conclusions regarding the effectiveness and safety of nitrates compared with alternative interventions in the treatment of patients with AHFS.
Background
Description of the condition
Heart failure is a syndrome in which the patients should have the following features: symptoms of heart failure, typically shortness of breath at rest or during exertion, or fatigue (or both); signs of fluid retention such as pulmonary congestion or ankle swelling; and objective evidence of an abnormality of the structure or function of the heart at rest (McMurray 2012). Acute heart failure syndromes (AHFS) are defined as gradual or rapid deterioration in heart failure signs and symptoms resulting in a need for urgent therapy (Gheorghiade 2005; McMurray 2012). AHFS are complex and can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood (Hunt 2001). For patients with pre‐existing chronic heart failure the following may be responsible for the acute phase of decompensation (episodes of AHFS) of the underlying condition: myocardial ischaemia, lack of compliance to diet or medication regimen, uncontrolled hypertension, cardiac arrhythmia, systemic infection or anaemia (DiGuili 2001; Fonarow 2008; McMurray 2012; Weintraub 2010; Whellan 2013).
Despite the fact that AHFS are one of the most common syndromes in emergency medicine, the exact pathogenesis has remained largely unknown. This is because the availability of haemodynamic data on which to base rational therapy in AHFS is limited by the difficulty in performing ethical clinical trials. However, the use of implantable device diagnostics is leading to a better understanding of the pathogenesis of AHFS (Adamson 2011; Whellan 2013; Zile 2011). For example, using an implantable haemodynamic monitor (HIM), it has recently been demonstrated that the product of the level to which estimated pulmonary artery diastolic (ePAD) pressure (P) is increased and the length of time (T) over which that pressure is increased (PxT) is the pressure‐based haemodynamic factor most closely associated with transition to AHFS in heart failure patients (Zile 2011). In the meantime, the fulminant presentation of AHFS, and rapid resolution with some traditional pharmacological agents, creates ethical barriers to justification of the need for clinical trials with new treatment modalities or 'non‐treatment'. Nevertheless, several studies have aimed to better characterise the pathogenesis of AHFS. These studies demonstrate that AHFS results from a rapidly deteriorating cycle of events in which patients with reduced baseline systolic and diastolic reserve are faced with an acute increase in systemic vascular resistance and, hence, afterload (Cotter 2003; Sharon 2000). This causes an acute decompensated state, leading to decreased peripheral perfusion, neurohormonal activation, decreasing left ventricular function and increasing vascular resistance (Kaluski 2003). The result of this vicious cycle is an increase in left ventricular end‐diastolic pressure that is transmitted backwards to the pulmonary vasculature, causing an acute increase in pulmonary capillary pressure and the transudation of fluid from the intravascular compartment to the lung interstitium and alveoli; ultimately the syndrome of acute cardiogenic pulmonary oedema may result (Kaluski 2003).
The International Working Group on Acute Heart Failure Syndromes determined that AHFS encompassed at least three clinical distinct entities (Gheorghiade 2005):
worsening chronic heart failure associated with reduced or preserved left ventricular ejection fraction (LVEF) (70% of all admissions);
de novo heart failure (e.g. after a large myocardial infarction; sudden increase in blood pressure superimposed on a noncompliant left ventricle) (25% of all admissions); and
advanced heart failure (i.e. refractory to therapy) with severe left ventricle systolic dysfunction, associated with a continually worsening low output state (5% of all admissions).
The two fundamental haemodynamic parameters relate to the presence or absence of elevated filling pressures (wet or dry) and perfusion that is adequate or critically limited (warm or cold). Over 90% of patients presenting with acute decompensated heart failure have clinical congestion (classified as being wet) and, if right heart catheterisation were performed, would show elevated pulmonary capillary wedge pressure (Nohria 2002). These patients may have adequate or reduced perfusion, with the majority showing elevation in systemic vascular resistance. Identification of congestion (elevated filling pressures) in acute decompensation of chronic heart failure relies heavily on the symptoms of dyspnoea and orthopnoea. Meanwhile, the most assessable indicator of perfusion is blood pressure and pulse pressure (Nohria 2002). The use of this haemodynamic classification system allows for more appropriate targeting therapy in patients presenting with acute heart failure. A minority (less than 1%) of patients present with cardiogenic shock (Cleland 2003). This is caused by a severe reduction in cardiac output which is not met by an adequate increase in peripheral vascular resistance leading to a significant decrease in blood pressure and end organ perfusion. Consequently, the management of cardiogenic shock is directed at improving cardiac performance (by optimising filling pressure, intra‐aortic balloon pump and emergent revascularisation) (Cotter 2000; Hochman 1999). The other AHFS (pulmonary oedema, hypertensive crisis and exacerbated heart failure) are caused by a combination of progressive excessive vasoconstriction superimposed on reduced left ventricular functional reserve (Cotter 2003). The impaired cardiac power and extreme vasoconstriction induces a vicious cycle of afterload mismatch resulting in a dramatic reduction of cardiac output and elevated left ventricular end diastolic pressure, which is transferred backwards to the pulmonary capillaries yielding pulmonary oedema (Cotter 2003). Therefore, the emergency management of these AHFS should be based on the administration of potent, rapidly acting intravenous vasodilators.
Heart failure complicating myocardial infarction is associated with a three to four‐fold increase in hospital mortality (Hellermann 2002; Spencer 1999; Spencer 2002; Steg 2004; Wu 2002). Depending on study design, the incidence of heart failure after myocardial infarction varies greatly from 3% to 53% (Ambrosioni 1995; Bueno 1995; Hellermann 2002). Meanwhile, because of their haemodynamically beneficial effects, nitrates are recommended in heart failure (Hunt 2001) and acute coronary syndromes (Peacock 2004), respectively. Intravenous nitrate vasodilators are well tolerated in acute myocardial infarction with clinically significant hypotension occurring in less than 4% of patients (GISSI‐3 1994), but this responds to dose reduction and fluid replacement. However, two mega‐trials have failed to demonstrate any mortality reduction attributable to nitrate use in acute coronary syndromes (GISSI‐3 1994; ISIS‐4 1995). The primary reason for the use of nitrates in acute coronary syndromes is for a beneficial haemodynamic effect and to decrease the pain of myocardial ischaemia (Peacock 2004).
Description of the intervention
Nitrates (for example, nitroglycerin, isosorbide dinitrate and sodium nitroprusside) are established intravenous vasodilators in clinical practice and are recommended for moderate to severe volume overload in acute decompensated heart failure (DiDomenico 2004). At low doses they induce only venodilatation, but as the dose is gradually increased they cause the arteries, including the coronary arteries, to dilate (Imhof 1980). They therefore cause balanced vasodilatation of the venous and arterial sides of the circulation, thereby reducing elevated left ventricular filling pressures and elevated systemic vascular resistance, without impairing tissue perfusion.
Nitrates can be administered sublingually, orally, transcutaneously and intravenously.
How the intervention might work
The main principles in the management of acute heart failure are symptom relief, reversal of acute haemodynamic abnormalities and a search for underlying precipitants of heart failure decompensation. Acute reduction in high left ventricular filling pressure with intravenous vasodilators corresponds most closely with symptomatic relief of dyspnoea at rest (Fonarow 2001; Stevenson 1999) and is the only significant haemodynamic predictor of subsequent mortality (Campana 1993; Lucas 2000). Thus using intravenous vasodilators to reverse acute heart failure decompensation is more physiologically rational in that it primarily targets elevated ventricular filling pressures and elevated systemic vascular resistance (Fonarow 2001). In addition, intravenous vasodilators reduce myocardial oxygen consumption and are not associated with worsening of myocardial ischaemia or precipitation of ventricular arrhythmias (Fonarow 2002; Nohria 2002).
Why it is important to do this review
The use of nitrates for AHFS is a clinically valid topic for a Cochrane systematic review for many reasons.
Firstly, AHFS are very common. With a prevalence of 5,700,000 (2.4% of the entire populace) in 2008, and an estimated yearly incidence of 670,000, the burden of heart failure in the United States is tremendous (Roger 2011).
Secondly, AHFS are associated with significant morbidity, healthcare costs and societal economic burden. In the United States, the mortality rate stood at 56,565 in 2007 and 990,000 hospital discharges pertained to heart failure admissions (Roger 2011). The EuroHeart Failure survey programme, one of the largest surveys of how patients hospitalised for heart failure are managed, reported 13% mortality during and after first hospitalisation for heart failure (Cleland 2003).
AHFS are presently the leading cause of hospitalisation in persons over 60 years of age in developed countries (Cleland 2001; Cowie 1997; Hunt 2001; Khand 1999; Stewart 2001). Patients with acute heart failure face a median six‐day duration of hospitalisation and a rehospitalisation rate over the next six months as high as 50% (AHA 2001; Fonarow 2003; Hunt 2001). It is estimated that AHFS account for almost 2% of National Health Service (NHS) expenditure in the United Kingdom, with the predominant cost component being hospitalisation (Berry 2001; Stewart 2002). These findings are consistent with those of several other countries (Doughty 1995; Eriksson 1991; Ghali 1990; Reitsma 1996; Rodriguez 1997).
Emergency department visits and subsequent hospitalisations for acute heart failure constitute a major public health problem. In 2006, there were nearly 658,000 annual emergency department encounters primarily for acute heart failure in the United States (Schappert 2008).
Ageing populations and improved post‐infarction survival lead to a high number of older patients with heart failure in more developed countries (Cleland 2001). A British study predicts that the annual number of male and female hospital admissions associated with a principal diagnosis of heart failure will increase by 34% (from 5500 to 7500) and by 12% (from 7800 to 8500), respectively, by 2020 (Stewart 2003)
Thirdly, despite the demonstrable beneficial effects of intravenous nitrate vasodilators as first‐line agents in the management of patients with acute heart failure, the most common therapy administered to these patients is intravenous loop diuretics, particularly frusemide (Nelson 1983; Verma 1987). In a United Kingdom emergency department study, the majority of patients with acute heart failure received frusemide, with less than 70% receiving nitrates (Crane 2002). Among patients in the Acute Decompensated Heart Failure National Registry (ADHERE 2003) in the United States, 64% of acute heart failure patients received intravenous diuretic therapy alone and only 27% of all admitted patients received intravenous vasoactive therapy (Fonarow 2003). A properly conducted quantitative systematic review may provide an objective evidence base for the role of nitrates in the management of patients with acute heart failure.
Fourthly, concerning the use of nitrates for AHFS, it is important to document any incidence of adverse effects, cost of treatment and acceptability to the patient in a systematic and scientifically valid way.
Furthermore, a Cochrane systematic review that is continuously updated as new evidence is published may lead to clinical practice guidelines which may improve the efficiency and quality of patient care. To date there has been no systematic review on this topic. This Cochrane review aims to synthesise current best evidence and will be continuously updated as relevant new trials regarding the role of nitrates in the management of AHFS are published.
Objectives
To quantify the effect of different nitrate preparations on clinical outcome in AHFS in adults.
To determine the effect of route of administration of nitrates on clinical outcome in AHFS in adults.
To evaluate the safety and tolerability of nitrates in AHFS in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) in all languages were eligible for inclusion in the review, with a randomised controlled trial defined as a study in which patients were allocated to treatment groups on the basis of a random or quasi‐random method (for example, using random number tables, hospital number, date of birth).
Types of participants
Adult patients aged 18 and older with AHFS (sudden impairment of the ability of the ventricle to fill with or eject blood resulting from any structural or functional cardiac disorder) of any severity.
Types of interventions
The target intervention was administration of a nitrate (for example nitroglycerin or isosorbide dinitrate) compared with an alternative intervention (pharmacological agent like frusemide, hydralazine, prenalterol and nesiritide or non‐pharmacological intervention such as non‐invasive positive pressure ventilation) for acute heart failure.
Types of outcome measures
Primary outcomes
Rapidity with which symptoms (for example dyspnoea, fatigue, self reported patient satisfaction score, global clinical status) are relieved.
Secondary outcomes
Requirement for mechanical ventilation.
Changes in haemodynamic variables (systolic blood pressure, diastolic blood pressure, heart rate, pulmonary artery occlusion pressure, cardiac output).
Progression to myocardial infarction.
Immediate adverse events.
Adverse events during hospitalisation.
Duration of hospitalisation.
Cost.
All‐cause mortality.
Search methods for identification of studies
Electronic searches
We made a computer‐assisted search for RCTs of nitrate therapy in patients with AHFS without language restriction in the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (2011, Issue 3), MEDLINE (1950 to July week 2 2011), EMBASE (1980 to week 28 2011) and the HERDIN database (Appendix 1). We used the Cochrane sensitivity‐maximising RCT filter (Lefebvre 2011). We searched the Current Controlled Trials MetaRegister of Clinical Trials (compiled by Current Science) (July 2011).
We applied no language restrictions.
Searching other resources
We also searched reference lists of review articles, relevant trials, relevant textbooks and abstracts of scientific meetings without language restriction to identify further RCTs. We reviewed the titles and abstracts to identify all potential RCTs. We obtained full‐text versions of these articles. We also made additional efforts to locate potential RCTs relevant to the topic from the following data sources:
'grey literature' (theses, internal reports, non‐peer reviewed journals) databases (Open Grey ‐ System for Information on Grey Literature in Europe);
references (and references of references, etc.) cited in primary sources;
other unpublished sources known to experts in the specialty (sought by personal communication); and
raw data from published trials (sought by personal communication).
Data collection and analysis
Selection of studies
Two authors (RK and SB) independently decided on inclusion of studies, having read the methods section of each review and applied the stated criteria. We assessed trial outcomes for comparability. Two authors (RS and DBD) independently entered data into the Review Manager software (RevMan 5.2) for statistical analysis. We resolved any disagreements by discussion. There was no occasion where uncertainty remained after this discussion.
Data extraction and management
We used a revised data extraction form to incorporate the new additions on quality assessment from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We extracted relevant data regarding inclusion criteria (study design, participants, interventions and outcomes), risk of bias (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias) and results. In cases where insufficient data were reported (for example, completeness of outcome data) we contacted the study authors for further information. Two review authors carried out data extraction. Excluded studies and reasons for exclusion are detailed in the table Characteristics of excluded studies. Where necessary, we contacted the authors of included studies for missing information.
We minimised publication bias by comprehensive literature searching (Glasziou 2001). In addition, we planned to use a graphical display (funnel plot) of the size of the treatment effect against the precision of the trial (one/standard error) to investigate publication bias.
Assessment of risk of bias in included studies
We assessed risk of bias in terms of sequence generation, allocation concealment, blinding (of participants, personnel and outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias (Higgins 2011). Two review authors assessed the risk of bias in eligible trials.
We evaluated each study and assessed them separately for these domains. We judged each explicitly as follows:
low risk of bias;
high risk of bias; and
unclear risk (lack of information or uncertainty over the potential for bias).
We resolved any disagreement by discussion.
Dealing with missing data
No simple solution exists for the problem of missing data. We handled this problem by contacting the investigators, whenever possible, to ensure that no data were missing for the studies. In addition, we planned to be explicit about the assumptions of whatever method we used to cope with missing data.
Assessment of heterogeneity
We evaluated clinical heterogeneity (differences between studies in key characteristics of the participants, interventions, or outcome measures). In the absence of clinical heterogeneity, we used the I2 statistic to describe the percentage of total variation across studies that was due to heterogeneity rather than chance (Higgins 2003). An I2 > 50% may represent substantial or considerable statistical heterogeneity (Higgins 2011). The importance we placed on the observed value of I2 depended on (i) the magnitude and direction of effects and (ii) the strength of evidence for heterogeneity (P value from the Chi2 test and confidence interval for I2).
We used visual inspection of the graphic representation of studies with their 95% CIs to assess heterogeneity. We generated tables and graphs using the analysis module included in RevMan 5.2. We represented pooled odds ratios pictorially as a 'forest plot' to permit visual examination of the degree of heterogeneity between studies.
Statistical and data analysis
The results concentrated on the objectives and comparisons specified in the protocol for the review. We identified post hoc analyses as such.
We considered the appropriateness of meta‐analysis in the presence of significant clinical or statistical heterogeneity. We performed meta‐analyses using RevMan software (RevMan 5.2). We calculated summary estimates of treatment effect with 95% confidence intervals (CIs) for each comparison.
For dichotomous (or binary) data, we described the results both as a relative measure (odds ratio (OR), risk ratio (RR) and relative risk reduction) and an absolute measure (risk difference (RD)). Relative measures (odds ratios and risk ratios) can be used to combine studies but absolute measures can be more informative than relative measures because they reflect the baseline risk as well as the change in risk with the intervention. Relative measures are used to combine studies but absolute measures (such as the risk difference) are particularly useful when considering trade‐offs between likely benefits and likely harms of an intervention (Deeks 2011).
For continuous data, we used the mean difference (MD) whenever outcomes were measured in a standard way across studies. This has the advantage of summarising results in natural units that are easily understood. When it was desirable to summarise results across studies with outcomes that are conceptually the same but measured in different ways (for example, different pain scores), we planned to use the standardised mean difference (SMD).
Subgroup analysis and investigation of heterogeneity
The effect of nitrates on heart failure complicating acute myocardial infarction is poorly defined. We performed subgroup analysis for the effects of nitrates on the subset of patients with AHFS complicating acute myocardial infarction.
Sensitivity analysis
We planned to perform sensitivity analyses to test how sensitive the results were to reasonable changes in the assumptions that we made in the protocol for combining the data (Lau 1998). We planned to perform sensitivity analysis for randomised versus quasi‐randomised studies and eventually good‐quality studies versus poor‐quality studies. We defined a good‐quality study as one which fulfils all of the following criteria: adequate allocation concealment, blinding of outcome assessment and data analysis performed according to the intention‐to‐treat principle. We defined a poor‐quality study as one which lacked one or more of these key domains.
Glossary
Please see http://www.cochrane.org/glossary for a glossary of terms.
Results
Description of studies
Results of the search
The electronic database search yielded a total of 622 publications. After reviewing titles and abstracts we retrieved six full‐text versions of the trials for possible inclusion. After examining the full‐text versions, we excluded two papers and included four studies. The study selection process is summarised in the PRISMA flow diagram shown in Figure 1.
1.
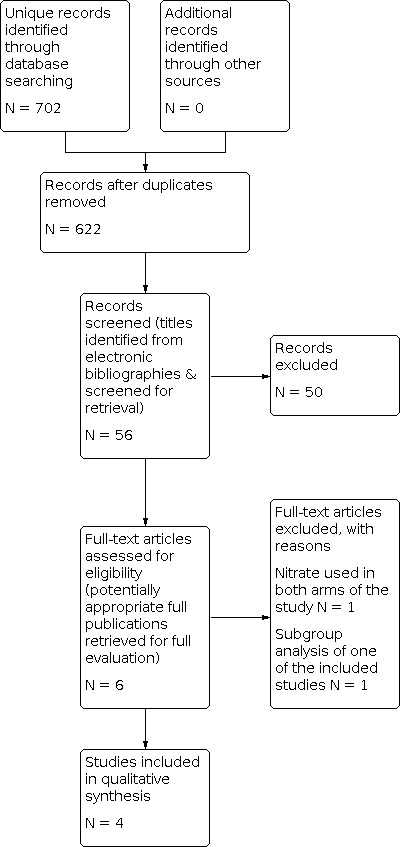
Study flow diagram.
Included studies
We included four trials (634 participants) comparing nitrates with alternative interventions in AHFS. Two of the studies had two comparison arms (Beltrame 1998; Nelson 1983), one of the studies had three comparison arms (VMAC 2002) and one of the studies had four comparison arms (Verma 1987).
Two studies each enrolled trial participants in a single coronary care unit (Nelson 1983; Verma 1987). One study enrolled and randomised trial participants in one emergency department (Beltrame 1998). One multi‐centre trial included patients with AHFS severe enough to require hospitalisation and intravenous therapy, but the authors did not specify the specific hospital setting where the trial participants were enrolled (VMAC 2002).
The specific intravenous nitrate drug employed was isosorbide dinitrate in two studies (Nelson 1983; Verma 1987) and nitroglycerin in two studies (Beltrame 1998; VMAC 2002). One of the studies which employed nitroglycerin also employed N‐acetylcysteine to potentiate the pharmacological effects of nitroglycerin (Beltrame 1998).
Two studies were UK‐based (Nelson 1983; Verma 1987), one was based in Australia (Beltrame 1998) and one was based in the US (VMAC 2002). The studies reported outcomes ranging from 24 hours to six months (Nelson 1983 48 hours; Verma 1987 five days; Beltrame 1998 24 hours; VMAC 2002 six months).
Two studies included only patients with AHFS following acute myocardial infarction (Nelson 1983; Verma 1987). One trial excluded patients with overt acute myocardial infarction as evidenced by ST segment elevation or severe anginal pain necessitating treatment with intravenous nitrates, morphine or both (Beltrame 1998). One trial included participants with AHFS with and without acute coronary syndromes (VMAC 2002).
Two studies recruited only male participants (Nelson 1983; Verma 1987), while two studies recruited both male and female participants (Beltrame 1998; VMAC 2002). Only one study detailed the ethnicity of the participants (VMAC 2002).
Only one study (VMAC 2002) reported the primary outcome measure of the review: rapidity with which symptoms are relieved. One of the primary outcome measures of VMAC 2002 was the patient's self evaluation of dyspnoea (all patients) from baseline to three hours after the start of the study drug.
Only two of the included studies (Nelson 1983; Verma 1987) were used for pooling data because they were the only included studies employing the same comparator arms (intravenous frusemide 1 mg/kg versus intravenous isosorbide dinitrate 50 to 200 μg/kg/h) in the same trial setting (coronary care unit).
Details of the included studies are shown in Characteristics of included studies and Figure 1.
Excluded studies
Two trials were ultimately excluded (Cotter 1998; Elkayam 2004). One of the trials (Elkayam 2004) was excluded because it was a subgroup analysis of an included study (VMAC 2002). One of the trials was excluded because both intervention arms of the trial included a nitrate (Cotter 1998). Details of the excluded studies are shown in Characteristics of excluded studies and Figure 1.
Risk of bias in included studies
The methodological quality graph (Figure 2) presents the review authors' judgements about each methodological quality item presented as percentages across all included studies. Given the small number of included studies we were unable to assess publication bias using a funnel plot approach (Higgins 2011).
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
In terms of random sequence generation and allocation concealment, three of the studies did not give sufficient detail to assess their potential risk of bias (Figure 3). Only one study reported sufficient detail concerning random sequence generation and allocation concealment to determine the potential risk of bias was low for these domains (VMAC 2002). Blinding of participants and personnel had a low potential risk of bias in all studies except one which had a high potential risk of bias for that domain (Beltrame 1998). Only one study had a low potential risk of bias in all domains (VMAC 2002).
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Primary outcome
Rapidity of symptom relief
Two studies reported the rapidity of symptom relief, the primary outcome measure of this review. It was not possible to pool the results of these two trials because the comparator interventions were different and the rapidity of symptom relief was measured and reported in different ways in the two studies. Beltrame 1998 reported no significant difference in the dyspnoea score (0 to 3) between intravenous nitroglycerin/N‐acetylcysteine and intravenous frusemide/morphine at 30 minutes (fixed‐effect mean difference (MD) ‐0.30, 95% confidence interval (CI) ‐0.65 to 0.05), 60 minutes (fixed‐effect MD ‐0.20, 95% CI ‐0.65 to 0.25), three hours (fixed‐effect MD 0.20, 95% CI ‐0.27 to 0.67) and 24 hours (fixed‐effect MD 0.00, 95% CI ‐0.31 to 0.31). VMAC 2002 reported the patient's self evaluation of dyspnoea (all patients) and global clinical status at three, six and 24 hours, respectively, after the start of the study drug. Global clinical status was rated by the patient on a five‐category scale (markedly better, better, no change, worse or markedly worse). Dyspnoea was rated by the patient on a three‐category scale (improved, no change or worse). However, the results were reported graphically and sufficient detail about the exact scores for these end points was not published (VMAC 2002).
Secondary outcomes
1. Requirement for mechanical ventilation
One study reported the need for mechanical ventilation (Beltrame 1998). The study reported that only seven patients required respiratory assistance (continuous positive airway pressure in four patients and intubation with ventilation in three patients), with no difference between the study groups (nitroglycerin/N‐acetylcysteine and frusemide/morphine). However, the study did not provide sufficient detail regarding the exact number of participants who needed mechanical ventilation in each comparator group (Beltrame 1998).
2. Changes in haemodynamic variables (systolic blood pressure, diastolic blood pressure, heart rate, pulmonary artery occlusion pressure, cardiac output)
The changes in many haemodynamic outcome measures at specific time points after drug initiation may be imprecise because they were reported by only one trial (Table 1).
1. Haemodynamic outcome measures for comparator interventions reported by only one trial.
| Change in SBP after 30 minutes | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Nelson 1983 | 28 | ISDN versus frusemide | Fixed‐effect MD ‐6.00 mmHg, 95% CI ‐10.51 to ‐1.49 |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | Fixed‐effect MD ‐2.00 mmHg, 95% CI ‐21.49 to 17.49 |
| Change in SBP after 60 minutes | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Nelson 1983 | 28 | ISDN versus frusemide | Fixed‐effect MD ‐10.00 mmHg, 95% CI ‐12.62 to ‐7.38 |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | Fixed‐effect MD ‐4.00 mmHg, 95% CI ‐23.75 to 15.75 |
| Change in SBP after 3 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| VMAC 2002 | 285 | NTG versus placebo | Fixed‐effect MD ‐3.20 mmHg, 95% CI ‐6.19 to ‐0.21 |
| VMAC 2002 | 347 | NTG versus nesiritide | Fixed‐effect MD ‐3.05 mmHg, 95% CI ‐3.05 to 2.85 |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | Fixed‐effect MD ‐2.00 mmHg, 95% CI ‐21.32 to 17.32 |
| Change in SBP after 24 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | Fixed‐effect MD ‐3.00 mmHg, 95% CI ‐21.94 to 15.94 |
| Change in DBP after 30 minutes | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Nelson 1983 | 28 | ISDN versus frusemide | Fixed‐effect MD ‐3.00 mmHg, 95% CI ‐5.22 to ‐0.78 |
| Change in DBP after 60 minutes | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Nelson 1983 | 28 | ISDN versus frusemide | Fixed‐effect MD ‐5.00 mmHg, 95% CI ‐7.22 to ‐2.78 |
| Change in HR after 30 minutes | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Nelson 1983 | 28 | ISDN versus frusemide | Fixed‐effect MD 0.00 bpm, 95% CI ‐3.35 to 3.35 |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | No significant difference reported |
| Change in HR after 60 minutes | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Nelson 1983 | 28 | ISDN versus frusemide | Fixed‐effect MD 1.00 bpm, 95% CI ‐2.35 to 4.35 |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | No significant difference reported |
| Change in HR after 3 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | Fixed‐effect MD ‐2.00 bpm, 95% CI ‐15.54 to 11.54 |
| Change in HR after 24 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Beltrame 1998 | 69 | NTG/NAC versus frusemide/morphine | Fixed‐effect MD 10.00 bpm, 95% CI ‐2.78 to 22.78 |
| Change in PAOP after 3 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| VMAC 2002 | 122 | NTG versus placebo | Fixed‐effect MD ‐1.80 mmHg, 95% CI ‐3.50 to ‐0.10 |
| VMAC 2002 | 184 | NTG versus nesiritide | Fixed‐effect MD 2.00 mmHg, 95% CI 0.24 to ‐3.76 |
| Change in PAOP after 18 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| Verma 1987 | 24 | ISDN versus frusemide | Fixed‐effect MD 2.00 mmHg, 95% CI ‐3.34 to 7.34 |
| Change in PAOP after 24 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| VMAC 2002 | 246 | NTG versus nesiritide | Mean reduction in PAOP was significantly greater with nesiritide (‐8.2 mmHg) than with NTG (‐6.3; P = 0.04) |
| Change in CO after 3 hours | |||
| Study ID | Number of participants | Comparator interventions | Measure of effects |
| VMAC 2002 | 184 | NTG versus placebo | Fixed‐effect MD 0.20 L/min/m2, 95% CI 0.00 to 0.40 |
| VMAC 2002 | 184 | NTG versus nesiritide | Fixed‐effect MD 0.10 L/min/m2, 95% CI ‐0.05 to ‐0.25 |
bpm: beats per minute CI: confidence interval CO: cardiac output HR: heart rate ISDN: isosorbide dinitrate MD: mean difference NAC: N‐acetylcysteine NTG: nitroglycerin PAOP: pulmonary artery occlusion pressure SBP: systolic blood pressure
Change in systolic blood pressure (SBP)
Change in SBP after 90 minutes
Two of the included trials (52 participants) comparing isosorbide dinitrate with frusemide reported change in systolic blood pressure after 90 minutes (Nelson 1983; Verma 1987). The pooled analysis for two trials revealed a significantly greater SBP reduction with isosorbide dinitrate compared with frusemide (fixed‐effect MD ‐8.97 mmHg, 95% CI ‐13.00 to ‐4.94; Analysis 1.1).
1.1. Analysis.

Comparison 1: Changes in systolic blood pressure, Outcome 1: Change in SBP after 90 mins (nitrates vs frusemide)
Change in diastolic blood pressure (DBP)
Change in DBP after 90 minutes
Two trials (52 participants) reported DBP as an outcome measure (Nelson 1983; Verma 1987). In the pooled analysis of the two trials regarding DBP change after 90 minutes, there was no significant difference between isosorbide dinitrate and frusemide (fixed‐effect MD ‐2.08, 95% CI ‐3.71 to ‐0.45; Analysis 2.1).
2.1. Analysis.

Comparison 2: Changes in diastolic blood pressure after 90 minutes, Outcome 1: Change in DBP (nitrates vs frusemide)
Change in heart rate
Change in heart rate after 90 minutes
Two trials involving 52 participants (Nelson 1983; Verma 1987) comparing isosorbide dinitrate with frusemide reported no significant difference in heart rate change at 90 minutes between the two interventions (fixed‐effect MD 2.00, 95% CI ‐0.57 to 4.57; Analysis 3.1).
3.1. Analysis.

Comparison 3: Changes in heart rate, Outcome 1: Change in heart rate at 90 minutes (nitrates vs frusemide)
Change in pulmonary artery occlusion pressure (PAOP)
Change in PAOP after 90 minutes
Two trials involving 52 participants (Nelson 1983; Verma 1987), comparing isosorbide dinitrate with frusemide, reported no significant difference in PAOP change at 90 minutes between the two interventions (fixed‐effect MD ‐2.00, 95% CI ‐2.80 to ‐1.20; Analysis 5.1).
5.1. Analysis.

Comparison 5: Changes in pulmonary artery occlusion pressure, Outcome 1: Change in pulmonary artery occlusion pressure at 90 minutes (nitrates vs frusemide)
Change in cardiac output
Change in cardiac output after 90 minutes
Two trials involving 52 participants (Nelson 1983; Verma 1987), comparing isosorbide dinitrate with frusemide, reported no significant difference in cardiac output change at 90 minutes between the two interventions (fixed‐effect MD 0.15 L/min/m2, 95% CI 0.07 to 0.22; Analysis 4.1).
4.1. Analysis.

Comparison 4: Changes in cardiac output, Outcome 1: Change in cardiac index at 90 minutes (nitrates vs frusemide)
3. Progression to myocardial infarction
One trial involving 69 participants (Beltrame 1998), comparing nitroglycerin/N‐acetylcysteine with frusemide/morphine (F/M), reported that acute myocardial infarction occurred in 10 patients (14%), of whom four (12%) were treated with F/M and six (16%) were treated with nitroglycerin/N‐acetylcysteine (Beltrame 1998). No further data is given regarding these patients.
There were no significant differences reported in one trial in the frequency or severity of ischaemic events between nitroglycerin and nesiritide groups in the first 24 hours (VMAC 2002). In the same study, through 30 days, there were three myocardial infarctions reported in nitroglycerin patients and two in nesiritide patients.
4. Immediate adverse events
Two studies reported that no untoward adverse events occurred in any patient during the studies (Nelson 1983; Verma 1987).
In one trial with three comparison arms, during the initial three‐hour placebo‐controlled period, any adverse event occurred in 39 (27%) nitroglycerin, 36 (18%) nesiritide and 20 (14%) placebo patients (Fisher exact test, P = 0.02) (VMAC 2002). Specifically, headache occurred in 17 (12%) patients administered nitroglycerin, 11 (5%) administered nesiritide and three (2%) placebo patients (P = 0.003). Abdominal pain occurred in four (3%) nitroglycerin patients only (P = 0.01) (VMAC 2002).
In one trial involving 69 participants, no adverse events (other than requirement for assisted ventilation and acute myocardial infarction discussed above) were documented (Beltrame 1998).
5. Adverse events during hospitalisation
Two studies reported that no untoward adverse events occurred to any patient during the studies (Nelson 1983; Verma 1987). In the trial involving 69 participants, no adverse events (other than requirement for assisted ventilation and acute myocardial infarction discussed above) were documented (Beltrame 1998).
In one trial with three comparison arms, during the first 24 hours of treatment with nesiritide, headache (8%) occurred significantly less frequently than with nitroglycerin (Fisher exact test, P = 0.001) (VMAC 2002).
Adverse events after three hours
One trial involving 285 participants (VMAC 2002), comparing nitroglycerin with placebo, reported a significantly lower incidence of adverse events after three hours with placebo (odds ratio 2.29, 95% CI 1.26 to 4.16). One trial involving 347 participants (VMAC 2002), comparing nitroglycerin with nesiritide, reported a lower incidence of adverse events after three hours with nesiritide (odds ratio 1.75, 95% CI 1.05 to 2.93).
There were no significant differences in the frequency or severity of asymptomatic or symptomatic hypotension or arrhythmias between nitroglycerin and nesiritide groups in the first 24 hours (VMAC 2002).
6. Duration of hospitalisation
Two of the studies did not report any data regarding duration of hospitalisation (Nelson 1983; Verma 1987).
One of the studies reported no variation in duration of hospitalisation (overall mean 5.6 + 3.0 days) between treatment groups (Beltrame 1998).
In one trial with three comparison arms involving 489 participants, through 30 days, 48 (23%) of patients administered nitroglycerin and 50 (20%) administered nesiritide were readmitted to the hospital for any cause (Fisher exact test, P = 0.36) (VMAC 2002). The investigators reported that readmission for acutely decompensated congested heart failure (CHF) occurred in 27 (13%) nitroglycerin and 20 (7%) nesiritide patients (VMAC 2002).
7. Cost
None of the included studies reported healthcare costs as an outcome measure or made any reference to the cost of treatment.
8. Mortality
Two trials reported no mortality associated with use of nitrates (Nelson 1983; Verma 1987).
One trial reported that there were three in‐hospital deaths during the study but the authors did not attribute the deaths to acute cardiogenic pulmonary oedema (Beltrame 1998). In one trial comparing nitroglycerin with nesiritide in 347 patients, reported through seven days, deaths occurred in one (0.5%) nitroglycerin and four (1.5%) nesiritide patients (VMAC 2002). None of these deaths was believed to be due to either study drug. The trial further demonstrated that there was no significant difference in six‐month mortality for nitroglycerin 20.8% (95% CI 15.5% to 26.5%) versus nesiritide patients 25.1% (95% CI 20.0% to 30.5%; P = 0.32) (VMAC 2002).
One trial involving 489 participants (VMAC 2002), comparing nitroglycerin with nesiritide, reported no significant difference between the two interventions in 30‐day mortality rate (odds ratio 1.91, 95% CI 0.32 to 11.52).
The trials reporting mortality as an outcome measure could not be pooled because the comparator interventions employed were different.
Subgroup analysis
Two trials reported the effects of nitrates on the subset of patients with acute heart failure syndromes (AHFS) complicating acute myocardial infarction (Nelson 1983; Verma 1987). The pooled analysis for the two trials revealed a significantly greater SBP reduction with isosorbide dinitrate compared with frusemide after 90 minutes (fixed‐effect MD ‐8.97 mmHg, 95% CI ‐13.00 to ‐4.94; Analysis 1.1). In the pooled analysis of the two trials, there was no significant difference in terms of change in haemodynamic parameters after 90 minutes between isosorbide dinitrate and frusemide (Analysis 2.1 to Analysis 4.1). In terms of immediate adverse events and adverse events during hospitalisation, both trials reported that no untoward event occurred to any patient during the studies. Both trials reported no mortality associated with the use of nitrates. However, neither trial reported the primary outcome measure of this review, rapidity of symptom relief, or cost as outcome measures.
Discussion
This review assessed the randomised controlled trial (RCT) evidence comparing the outcomes of nitrate vasodilator therapy and alternative interventions in the management of acute heart failure syndromes (AHFS).
Our review found no evidence to support a difference in the primary outcome measure, rapidity of symptom relief, between intravenous nitrate vasodilator therapy and alternative interventions. Specifically, only two studies reported the rapidity of symptom relief as an outcome measure. The two trials involved 69 and 285 participants, respectively. Both studies recruited both male and female participants (Beltrame 1998; VMAC 2002), but only one study detailed the ethnicity of the trial participants (VMAC 2002). Only the trial involving 69 patients presented numerical measures of rapidity of symptom relief; sufficient detail regarding this outcome measure was not provided by the study involving 285 patients because the results were presented graphically. It was not possible to pool the results of these two trials because the comparator interventions were different and the rapidity of symptom relief was measured and reported in different ways in the respective studies, and it was not possible to obtain the individual patient data from the trialists. The estimates yielded by the trial which reported numerical measures of rapidity of symptom relief may be imprecise due to the relatively small sample size.
This review found no evidence to support a difference in AHFS patients receiving intravenous nitrate vasodilator therapy or alternative interventions with regard to the following outcome measures: requirement for mechanical ventilation, systolic blood pressure (SBP) change after three hours and 24 hours, diastolic blood pressure (DBP) change after 30, 60 and 90 minutes, heart rate change at 30 minutes, 60 minutes, three hours and 24 hours, pulmonary artery occlusion pressure (PAOP) change after three hours and 18 hours, cardiac output change at 90 minutes and three hours, and progression to myocardial infarction. Regarding adverse events after three hours, our review found a higher incidence of adverse events with intravenous nitrate therapy compared with placebo and compared with nesiritide; however, both of these findings were based on one trial respectively, therefore the estimates yielded may be imprecise.
This review found no consistent evidence to support a difference in AHFS patients receiving intravenous nitrate vasodilator therapy or alternative interventions with regard to the following secondary outcome measures: SBP change after 30 and 60 minutes, heart rate change after 90 minutes, PAOP change after 90 minutes. The inconsistency in the evidence is due to the existence of RCT evidence both for and against a difference in AHFS patients receiving intravenous nitrate vasodilator therapy or alternative interventions.
Two of the four included studies in this review recruited only patients with AHFS following myocardial infarction (Nelson 1983; Verma 1987); these studies therefore had a different, and narrower, focus than that of this review. Three of the four included studies in this review enrolled inpatients with AHFS, bypassing the emergency department phase of management. Depending on the drug's pharmacodynamic properties, it is possible that a therapeutic window exists beyond which apparent efficacy is diminished (Weintraub 2010). This may be particularly true for relief of dyspnoea, one of the key end points in AHFS management (Mebazaa 2010; Pang 2008; Weintraub 2010). Furthermore, there is currently no consensus on reasonable end points in relation to AHFS management (Weintraub 2010). These factors may contribute to the inconsistent evidence found by this review regarding many of the outcomes studied.
The eligible RCTs for this review do not have comparisons which permit us to address two of the a priori objectives of this review. First, based on the eligible RCTs for this review, it is not possible to determine the effect of route of administration of nitrates on clinical outcome in AHFS in adults, because all the studies which met the a priori inclusion criteria only employed the intravenous route for nitrate administration. Second, it also not possible to quantify validly the effect of different nitrate preparations on clinical outcome in AHFS in adults based on the eligible RCTs for this review.
We were unable to perform a valid sensitivity analysis as planned a priori because all the included RCTs in this review were randomised (there were no included quasi‐randomised trials eligible for inclusion) and none of the included RCTs met the criteria of a good‐quality study (one which meets all of the following criteria: adequate allocation concealment, blinding of outcome assessment and data analysis performed according to the intention‐to‐treat principle).
Regarding the a priori subgroup analysis, this review found that nitrates (isosorbide dinitrate) cause a significantly greater SBP reduction after 90 minutes compared with frusemide in patients with AHFS complicating acute myocardial infarction. However, it found no significant difference between nitrates (isosorbide dinitrate) and frusemide in terms of other haemodynamic parameters (DBP, heart rate, PAOP and cardiac output). The findings of the subgroup analysis are limited and imprecise because it is based on two small RCTs which did not report the primary outcome measure of this review and employed only one comparison (isosorbide dinitrate versus frusemide).
There is no previous non‐Cochrane systematic review based on RCT evidence comparing outcomes of nitrate vasodilator therapy and alternative interventions in the management of AHFS. It is therefore not possible to contextualise the findings of this review in relation to evidence from an existing systematic review. Meanwhile, like all systematic reviews, the findings of our review are limited by the quality of existing studies (Khan 1996): the small number of studies, the relatively small sample sizes in the respective studies and the different comparator interventions employed. Only two included studies in this review shared the same comparator interventions. Additionally, three of the four included studies had a high or unclear risk of bias. In terms of observable statistical heterogeneity in the five pooled analysis on haemodynamic parameters, considerable heterogeneity is present in one haemodynamic parameter and moderate heterogeneity may be present in another haemodynamic parameter. Due to these limitations, the estimates yielded by the review are imprecise and firm conclusions cannot be drawn regarding the effects of the interventions.
The relatively low methodological quality of existing RCT evidence is probably due to the diverse pathophysiological precipitants (for example, acute coronary syndromes, arrhythmias, infection, poor compliance with drug therapy or dietary requirements) of AHFS and the heterogenous patient population presenting with AHFS. Small studies, but in multiple, tightly controlled, relatively homogeneous AHFS patient subgroups (to limit phenotypic variability), may have better internal and external validity (Pang 2010).
Authors' conclusions
Implications for practice.
Our review found no evidence to support a difference in the rapidity of symptom relief between intravenous nitrate vasodilator therapy and alternative interventions in patients with acute heart failure syndromes (AHFS). However, randomised controlled trial (RCT) evidence comparing clinical outcomes of nitrate vasodilator therapy and alternative interventions in the management of AHFS is limited and of relatively low methodological quality. The risk of bias in existing RCT evidence is therefore difficult to ascertain. The existing RCT evidence is derived from trials conducted in Western developed countries and they were mainly conducted in males; the evidence may not be generalisable in other healthcare settings or females.
Implications for research.
More adequately powered studies of high methodological quality are required to determine whether there is any difference in clinical outcomes between nitrate vasodilator therapy and alternative interventions in patients with AHFS. Due to the diverse pathophysiological processes seen in AHFS, future research should focus on conducting studies in relatively homogeneous AHFS patient subgroups to limit phenotypic variability.
Patients enrolled in inpatient acute heart failure studies are often enrolled after they have reached the inpatient unit some 12 to 24 hours after presentation. As most patients have symptom relief with either diuretic or nitroglycerin given in the emergency setting, enrolling patients in trials after they have received acute therapy is likely to be significantly confounded. Testing of new therapies for AHFS should include collaboration among emergency physicians and cardiologists to enrol participants early in the course of their exacerbation.
Currently, there are a lack of validated measures in the management of AHFS that assess long‐term outcomes. It is therefore clear that future studies should employ appropriate end points that are based on the mechanism of action and the principles of AHFS management.
What's new
| Date | Event | Description |
|---|---|---|
| 8 August 2018 | Review declared as stable | Another review published in 2016 (DOI: 10.1177/1074248416644345) has shown neutral results too and we are not aware of any more recent trials that could change the findings of this review. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 8, 2013
| Date | Event | Description |
|---|---|---|
| 29 March 2014 | Amended | The following statement in the Discussion section of the review (paragraph 3) has been amended: "Regarding adverse events after three hours, our review found a lower incidence of adverse events with intravenous nitrate therapy compared with placebo, but a higher incidence of adverse events compared with nesiritide; however, both of these findings were based on one trial respectively, therefore the estimates yielded may be imprecise." The statement has been amended to read as follows: "Regarding adverse events after three hours, our review found a higher incidence of adverse events with intravenous nitrate therapy compared with placebo and compared with nesiritide; however, both of these findings were based on one trial respectively, therefore the estimates yielded may be imprecise." |
| 27 June 2013 | Amended | Since the publication of the protocol for this review, the term 'acute heart failure syndromes' has emerged, by international consensus, as the encompassing term for the disease entities of interest in this review. The title of this review has therefore been changed from 'acute heart failure' to 'acute heart failure syndromes', to be consistent with the new international consensus terminology. |
Acknowledgements
We would like to thank the Cochrane Heart Group for their help and editorial advice during the preparation of this systematic review. We would also like to thank Dr. Geraldine McMahon for her help in developing the protocol for this review.
Appendices
Appendix 1. Search strategies 2011
CENTRAL
#1 MeSH descriptor Heart Failure explode all trees #2 heart next failure #3 cardiac next failure #4 (#1 OR #2 OR #3) #5 MeSH descriptor Nitroglycerin, this term only #6 MeSH descriptor Nitrates explode all trees #7 nitrat* #8 nitroglycerin* #9 trinitrate* #10 dinitrate* #11 mononitrate* #12 nitroprusside* #13 trinitrin #14 glyceryltrinitrate* #15 isosorbide #16 gtn #17 MeSH descriptor Isosorbide Dinitrate, this term only #18 MeSH descriptor Nitroprusside, this term only #19 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #20 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #21 (#19 OR #20) #22 (#4 AND #21)
MEDLINE
1. exp Heart Failure/ 2. heart failure.tw. 3. cardiac failure.tw. 4. or/1‐3 5. Nitroglycerin/ 6. exp Nitrates/ 7. Isosorbide Dinitrate/ 8. Nitroprusside/ 9. nitrate*.tw. 10. nitroglycerin*.tw. 11. trinitrate*.tw. 12. dinitrate*.tw. 13. mononitrate*.tw. 14. nitroprusside*.tw. 15. trinitrin.tw. 16. glyceryltrinitrate*.tw. 17. isosorbide.tw. 18. gtn.tw. 19. or/5‐18 20. 4 and 19 21. randomized controlled trial.pt. 22. controlled clinical trial.pt. 23. randomized.ab. 24. placebo.ab. 25. clinical trials as topic.sh. 26. randomly.ab. 27. trial.ti. 28. 21 or 22 or 23 or 24 or 25 or 26 or 27 29. exp animals/ not humans.sh. 30. 28 not 29 31. 20 and 30
EMBASE 2011
1. exp heart failure/ 2. heart failure.tw. 3. cardiac failure.tw. 4. or/1‐3 5. glyceryl trinitrate/ 6. nitrate/ 7. exp nitric acid derivative/ 8. nitroprusside sodium/ 9. nitrate*.tw. 10. nitroglycerin*.tw. 11. trinitrate*.tw. 12. dinitrate*.tw. 13. mononitrate*.tw. 14. nitroprusside*.tw. 15. trinitrin.tw. 16. glyceryltrinitrate*.tw. 17. isosorbide.tw. 18. gtn.tw. 19. or/5‐18 20. 4 and 19 21. random$.tw. 22. factorial$.tw. 23. crossover$.tw. 24. cross over$.tw. 25. cross‐over$.tw. 26. placebo$.tw. 27. (doubl$ adj blind$).tw. 28. (singl$ adj blind$).tw. 29. assign$.tw. 30. allocat$.tw. 31. volunteer$.tw. 32. crossover procedure/ 33. double blind procedure/ 34. randomized controlled trial/ 35. single blind procedure/ 36. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 37. (animal/ or nonhuman/) not human/ 38. 36 not 37 39. 20 and 38 40. (200807* or 200808* or 200809* or 20081* or 2009* or 2010* or 2011*).dd. 41. 39 and 40
Data and analyses
Comparison 1. Changes in systolic blood pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in SBP after 90 mins (nitrates vs frusemide) | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐8.97 [‐13.00, ‐4.94] |
Comparison 2. Changes in diastolic blood pressure after 90 minutes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Change in DBP (nitrates vs frusemide) | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐3.71, ‐0.45] |
Comparison 3. Changes in heart rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Change in heart rate at 90 minutes (nitrates vs frusemide) | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐0.57, 4.57] |
Comparison 4. Changes in cardiac output.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Change in cardiac index at 90 minutes (nitrates vs frusemide) | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.07, 0.22] |
Comparison 5. Changes in pulmonary artery occlusion pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Change in pulmonary artery occlusion pressure at 90 minutes (nitrates vs frusemide) | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.80, ‐1.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beltrame 1998.
| Study characteristics | ||
| Methods | Randomised, open‐label trial | |
| Participants | 87 consecutive patients with acute pulmonary oedema presenting to an ED of a University teaching hospital in Adelaide, Australia 69 patients enrolled (32 frusemide/morphine, 37 nitroglycerin/N‐acetylcysteine) presenting with: 1) Acute onset of dyspnoea within the preceding 6 hours 2) Clinical findings consistent with pulmonary oedema including tachypnoea, signs of increased respiratory work, gallop rhythm, widespread crepitations in the absence of a history of chest infection or aspiration 3) Radiological evidence of pulmonary oedema |
|
| Interventions | Intervention 1: Frusemide/morphine therapy: morphine by slow IV injection (1 to 2 mg/5 minutes) to a maximum dose of 10 mg + frusemide 40 mg IV bolus (or twice the patient's daily maintenance dose if appropriate) with a second bolus at 60 minutes if there was an inadequate response Intervention 2: Nitroglycerin/N‐acetylcysteine: N‐acetylcysteine 6.6 µg/minute as a continuous IV infusion over 24 hours + nitroglycerin 2.5 µg/minute as a continuous simultaneous infusion over 24 hours; if clinical response was considered inadequate and systolic BP was stable, the dose of the nitroglycerin infusion could be increased to 5 µg/minute after 15 minutes and/or 10 µg/minute after 60 minutes |
|
| Outcomes | Primary end point: change in the PaO2/FiO2 ratio over the first hour of therapy Secondary end points: Clinical status assessment by measuring: respiratory rate, pulse rate, blood pressure, Flammang dyspnoea score, pulmonary crepitation score, sweating score Rate of mechanical ventilatory assistance Duration of hospital admission |
|
| Notes | Clinical parameters improved significantly after 60 minutes of medical therapy although the PaO2/FiO2 ratio did not improve significantly until 3 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "On arrival...patients were...if suitable, randomised to F/M or NTG/NAC therapy. Trial therapy was then instituted..." Comment: method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "On arrival...patients were...if suitable, randomised to F/M or NTG/NAC therapy. Trial therapy was then instituted..." Comment: likely to have been done as baseline characteristics, including age, sex, history of heart disease, cardiovascular risk factors and drug therapy, are similar between both groups; however, there is no description of the randomisation process, with potential for selection bias |
| Blinding (performance bias and detection bias) | High risk | Comment: conducted as an open‐label study, with different administration protocols between the 2 types of drugs used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 69 patients enrolled, 4 were subsequently shown not to have acute pulmonary oedema...However, all were included in the intention to treat analysis". |
| Selective reporting (reporting bias) | Low risk | Comment: likely, with data being analysed on an intention‐to‐treat basis |
| Other bias | Unclear risk | Comment: as this was an open‐label study with no description of the randomisation process there is a potential for selection bias |
Nelson 1983.
| Study characteristics | ||
| Methods | Prospective, randomised, between‐group study | |
| Participants | Men aged between 35 and 65 years who fulfilled the study criteria were evaluated in a coronary care unit between 5 and 14 hours of the onset of symptoms of myocardial infarction | |
| Interventions | Group I: intravenous bolus of frusemide (1 mg/kg) Group II: isosorbide dinitrate by intravenous infusion, commencing at 50 μg/kg and doubled every 30 minutes to a maximum of 200 μg/kg/h or until the mean systemic arterial pressure has been reduced by approximately 10 mmHg; the infusion was then continued at this dose |
|
| Outcomes | Systemic arterial pressure, pulmonary artery occluded pressure, heart rate, cardiac output, stroke volume and systemic vascular resistance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients in the two randomised groups were well matched for age, site of infarct, plasma level of cardiac enzymes, and radiological evidence of left ventricular failure." Comment: method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients in the two randomised groups were well matched for age, site of infarct, plasma level of cardiac enzymes, and radiological evidence of left ventricular failure." Comment: baseline characteristics, including age, site of infarct, plasma level of cardiac enzymes are similar between both groups; however, there is no description of the randomisation process or concealment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study was designed as a single‐blind between‐group comparison." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No untoward incident occurred in any patient." Comment: no missing data from any treatment groups |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Verma 1987.
| Study characteristics | ||
| Methods | Randomised, single‐blind, parallel trial | |
| Participants | Men aged 35 to 68 years were studied in a coronary care unit within 18 hours of the onset of symptoms of acute myocardial infarction | |
| Interventions | Patients received 1 of 4 drugs:
|
|
| Outcomes | Systemic arterial pressure, pulmonary artery occluded pressure, heart rate, cardiac index, stroke volume, systemic vascular resistance index and stroke work index | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 48 patients were equally allocated, 12 to each of the four treatment groups according to predetermined randomisation." Comment: method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The 48 patients were equally allocated, 12 to each of the four treatment groups according to predetermined randomisation". Comment: Method of concealment is not described. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study was a randomised single‐blind parallel‐group comparison of ..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The study was accomplished without any untoward incident in any patient". Comment: no missing data from any treatment groups |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are of interest to this review have been reported in the pre‐specified way |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
VMAC 2002.
| Study characteristics | ||
| Methods | Prospective, multi‐centre trial | |
| Participants | Patients were randomised, of which 489 were treated with study drug (143 nitroglycerin, 204 nesiritide and 142 placebo) at 55 centres. All patients had dyspnoea at rest (or New York Heart Association class IV symptoms) at study entry, 84% had chronic decompensated CHF that was classified as class III or class IV prior to decompensation, and most had clinical evidence of fluid overload) | |
| Interventions | Intravenous nesiritide (n = 204), intravenous nitroglycerin (n = 143) or placebo (n = 142) added to standard medications for 3 hours, followed by nesiritide (n = 278) or nitroglycerin (n = 216) added to standard medication for 24 hours | |
| Outcomes | Primary outcome measure: change in pulmonary capillary wedge pressure (PCWP) among catheterised patients and patient self evaluation of dyspnoea at 3 hours after initiation of study drug among all patients Secondary outcomes included comparisons of haemodynamic and clinical effects between nesiritide and nitroglycerin at 24 hours |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization occurred after patients were confirmed to meet all inclusion and exclusion criteria and informed consent was obtained. ... Non catheterized patients were randomly assigned to receive either placebo, nitroglycerin… Catheterized patients were randomly assigned to these same 3 treatment groups or to the adjustable‐dose nesiritide group. For placebo patients in both strata, the randomization included a crossover to double blind treatment with either titratable dose or to fixed‐dose nesiritide at 3 hours…” |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed using random permuted blocks within strata (catherized or non‐catherized), with a block size of 8 for the catheterized strata and of 6 for the noncathereterized strata. Non catheterized patients were randomly assigned to receive either placebo, nitroglycerin… Catheterized patients were randomly assigned to these same 3 treatment groups or to the adjustable‐dose nesiritide group. For placebo patients in both strata, the randomization included a crossover to double blind treatment with either titratable dose or to fixed‐dose nesiritide at 3 hours…” |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study used a double‐blind, double‐dummy study drug administration design in which each patient received simultaneous infusions of nitroglycerin/placebo and nesiritide/placebo.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: small numbers 2 to 4 (per group) did not receive the study drug as assigned |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other major sources of bias |
BP: blood pressure CHF: congestive heart failure ED: emergency department IV: intravenous NAC: N‐acetylcysteine NTG: nitroglycerin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cotter 1998 | Both arms of the study contain nitrates |
| Elkayam 2004 | This is a subgroup analysis of another study (VMAC 2002) |
Differences between protocol and review
Since the publication of the protocol for this review, the term 'acute heart failure syndromes' has emerged, by international consensus, as the encompassing term for the disease entities of interest in this review. The title of this review has therefore been changed from 'acute heart failure' to 'acute heart failure syndromes', to be consistent with the new international consensus terminology.
Contributions of authors
Conceiving the review: Abel Wakai (AW)
Co‐ordinating the review: AW
Undertaking manual searches: AW
Screening search results: Rachel Kidney (RK), Caroline Pospisil (CP)
Organising retrieval of papers: AW
Screening retrieved papers against inclusion criteria: AW and Gregory J Fermann (GJF)
Appraising quality of papers: Aileen McCabe (AM) and Nigel Salter (NS)
Abstracting data from papers: Steven C Brooks (SCB) and RK
Writing to authors of papers for additional information: AW
Providing additional data about papers: AW and NS
Obtaining and screening data on unpublished studies: AW
Data management for the review: AW
Entering data into Review Manager (RevMan 5.2): Rawle A Seupal (RAS) and Deborah B Diercks (DBD)
RevMan statistical data: AW
Other statistical analysis not using RevMan: N/A
Double entry of data: (data entered by person one: RAS; data entered by person two: DBD)
Interpretation of data: AW
Statistical inferences: AW
Writing the review: AW
Securing funding for the review: N/A
Performing previous work that was the foundation of the present study: AW and Dr. Geraldine McMahon
Guarantor for the review (one author): AW
Person responsible for reading and checking review before submission: AW
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Beltrame 1998 {published data only}
- Beltrame J, Zeitz C, Unger S, Brennan R, Moran J, Horowitz J. Nitrate therapy is an alternative to furosemide/morphine therapy in the management of acute cardiogenic pulmonary oedema. Journal of Cardiac Failure 1998;4(4):271-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nelson 1983 {published data only}
- Nelson GIC, Silke B, Ahuja RC, Hussain M, Taylor SH. Haemodynamic advantages of isosorbide dinitrate over frusemide in acute heart failure following myocardial infarction. Lancet 1983;321:730-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Verma 1987 {published data only}
- Verma SP, Silke B, Hussain M, Nelson G, Reynolds GW, Richmond A, et al. First-line treatment of left ventricular failure complicating acute myocardial infarction: a randomised evaluation of immediate effects of diuretic, venodilator, arteriodilator, and positive inotropic drugs on left ventricular function. Journal of Cardiovascular Pharmacology 1987;10:38-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
VMAC 2002 {published data only}
- Publication committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure. JAMA 2002;287(12):1531-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cotter 1998 {published data only}
- Cotter G, Metzkor E, Kaluski E, Faigenberg Z. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet 1998;351:389-93. [PMID: 9482291] [DOI] [PubMed] [Google Scholar]
Elkayam 2004 {published data only}
- Elkayam U, Akhter MW, Singh H, Khan S, Usman A. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high-dose nitroglycerin in patients with decompensated heart failure. American Journal of Cardiology 2004;93:237-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Adamson 2011
- Adamson PB, Zile MR, Cho YK, Bennett TD, Bourge RC, Aaron MF, et al. Hemodynamic factors associated with acute decompensated heart failure: part 2 - use in automated detection. Journal of Cardiac Failure 2011;17(5):366-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
ADHERE 2003
- The ADHERE Registry. First Quarter 2003 National Benchmark Report. The ADHERE Registry 2003.
AHA 2001
- Heart and stroke statistical update. American Heart Association 2001.
Ambrosioni 1995
- Ambrosioni E, Borghi C, Magnani B (The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators). The effect of the angiotensin-converting enzyme inhibitor zofenopril on mortality and morbidity after myocardial infarction. New England Journal of Medicine 1995;332:80-5. [DOI] [PubMed] [Google Scholar]
Berry 2001
- Berry C, Murdoch DR, McMurray JJ. Economics of chronic heart failure. European Journal of Heart Failure 2001;3:283-91. [DOI] [PubMed] [Google Scholar]
Bueno 1995
- Bueno H, Vidan MT, Almazan A, Lopez-Sendon JL, Delcan JL. Influence of sex on the short-term outcome of elderly patients with a first acute myocardial infarction. Circulation 1995;92:1133-40. [DOI] [PubMed] [Google Scholar]
Campana 1993
- Campana C, Gavazii A, Berzuini C, Larizza C, Marioni R, D'Armini A, et al. Predictors of prognosis in patients awaiting heart transplantation. Journal of Heart and Lung Transplantation 1993;12:756-65. [PubMed] [Google Scholar]
Cleland 2001
- Cleland JGF, Khand A, Clark A. The heart failure epidemic: exactly how big is it? European Heart Journal 2001;22:623-6. [DOI] [PubMed] [Google Scholar]
Cleland 2003
- Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme - a survey of the quality of care among patients with heart failure in Europe. Part 1: Patient characteristics and diagnosis. European Heart Journal 2003;24:442-63. [DOI] [PubMed] [Google Scholar]
Cotter 2000
- Cotter G, Kaluski E, Blatt A, Milovanov O, Moshkovitz Y, Zaidenstein R et al. L-NMMA (a nitric oxide synthase inhibitor) is effective in the treatment of cardiogenic shock. Circulation 2000;101:1358-61. [DOI] [PubMed] [Google Scholar]
Cotter 2003
- Cotter G, Moshkovitz Y, Kaluski E, Milo O, Nobikov Y, Schneeweiss A, et al. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure. European Journal of Heart Failure 2003;5:443-51. [DOI] [PubMed] [Google Scholar]
Cowie 1997
- Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Suresh V, et al. The epidemiology of heart failure. European Heart Journal 1997;18:208-25. [DOI] [PubMed] [Google Scholar]
Crane 2002
- Crane SD. Epidemiology, treatment and outcome of acidotic, acute, cardiogenic pulmonary oedema presenting to an emergency department. European Journal of Emergency Medicine 2002;9:320-4. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses . In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. The Cochrane Collaboration, 2011. [Google Scholar]
DiDomenico 2004
- DiDomenico RJ, Park HY, Southworth MR, Eyrich HM, Lewis RK, Finley JM et al. Guidelines for acute decompensated heart failure treatment. Annals of Pharmacotherapy 2004;38:646-60. [DOI] [PubMed] [Google Scholar]
DiGuili 2001
- DiGuili F, Anand I. Current issues in heart failure. Acute Heart Failure 2001.
Doughty 1995
- Doughty R, Yee T, Sharpe N, MacMahon S. Hospital admissions and deaths due to congestive heart failure in New Zealand, 1981-1991. New Zealand Medical Journal 1995;108:473-5. [PubMed] [Google Scholar]
Eriksson 1991
- Eriksson H, Wilhelmsen L, Caidahl K, Svardsudd K. Epidemiology and prognosis of heart failure. Zeitschrift fur Kardiologie 1991;80:1-6. [PubMed] [Google Scholar]
Fonarow 2001
- Fonarow GC. The treatment targets in acute decompensated heart failure. Reviews in Cardiovascular Medicine 2001;2:S7-S12. [PubMed] [Google Scholar]
Fonarow 2002
- Fonarow GC. Pharmacologic therapies for acutely decompensated heart failure. Reviews in Cardiovascular Medicine 2002;3:S18-S27. [PubMed] [Google Scholar]
Fonarow 2003
- Fonarow GC (ADHERE Scientific Advisory Committee). The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Reviews in Cardiovascular Medicine 2003;4:S21-S30. [PubMed] [Google Scholar]
Fonarow 2008
- Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Archives of Internal Medicine 2008;168:847-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghali 1990
- Ghali JK, Cooper R, Ford E. Trends in hospitalisation rates for heart failure in the United States 1973-1986: evidence for screening population prevalence. Archives of Internal Medicine 1992;150:769-73. [PubMed] [Google Scholar]
Gheorghiade 2005
- Gheorghiade M, Zannad F, Sopko G, Klein L, Piña IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation 2005;20(112(25)):3958-68. [DOI] [PubMed] [Google Scholar]
GISSI‐3 1994
- GISSI-3. Effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet 1994;343:1115-23. [PubMed] [Google Scholar]
Glasziou 2001
- Glasziou P, Irwig L, Bain C, Colditz G. Systematic Reviews in Health Care: a Practical Guide. Cambridge: Cambridge University Press, 2001. [Google Scholar]
Hellermann 2002
- Hellermann JP, Jacobsen SJ, Gersh BJ, Rodeheffer RJ, Reeder GS, Roger VL. Heart failure after myocardial infarction: a review. American Journal of Medicine 2002;113:324-30. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JD, Altman G. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hochman 1999
- Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. New England Journal of Medicine 1999;341:625-34. [DOI] [PubMed] [Google Scholar]
Hunt 2001
- Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. Journal of the American College of Cardiology 2001;38:2101-13. [DOI] [PubMed] [Google Scholar]
Imhof 1980
- Imhof PR, Ott B, Frankhauser P, Chu LC, Hodler J. Difference in nitroglycerin dose response in the venous and arterial beds. European Journal of Clinical Pharmacology 1980;18:455-60. [DOI] [PubMed] [Google Scholar]
ISIS‐4 1995
- Anonymous. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet 1995;345:669-85. [PubMed] [Google Scholar]
Kaluski 2003
- Kaluski E, Kobrin I, Zimlichman R, Marmov A, Krakov O, Milo O, et al. RITZ-5: Randomized intravenous tezosentan (an endothelin-A/B antagonist) for the treatment of pulmonary edema. A prospective, multicenter, double-blind, placebo-controlled study. Journal of the American College of Cardiology 2003;41:204-10. [DOI] [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Jadad A. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156:661-6. [PMID: ] [PubMed] [Google Scholar]
Khand 1999
- Khand A, Gemmel I, Clark AL, Cleland JGF. Is the prognosis of heart failure improving? European Journal of Heart Failure 1999;1:229-41. [DOI] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. In: Mulrow C, Cook D, editors(s). Systematic Reviews: Synthesis of Best Evidence for Health Care Decisions. 1st edition. Philadelphia: American College of Physicians, 1998:91-101. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Using the Cochrane sensitivity-maximising RCT filter. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Lucas 2000
- Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. American Heart Journal 2000;140:840-7. [DOI] [PubMed] [Google Scholar]
McMurray 2012
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure 2012;14(8):803-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mebazaa 2010
- Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT- dyspnoea study. European Heart Journal 2010;31(7):832-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nohria 2002
- Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA 2002;287:628-40. [DOI] [PubMed] [Google Scholar]
Pang 2008
- Pang PS, Cleland JG, Teerlink JR, Collins SP, Lindsell CJ, Sopko G, et al. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. European Heart Journal 2008;29(6):816-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pang 2010
- Pang PS, Komajda M, Gheorghiade M. The current and future management of acute heart failure syndromes. European Heart Journal 2010;31(7):784-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peacock 2004
- Peacock WF, Emerman CL, Young J. Nesiritide in congestive heart failure associated with acute coronary syndromes: a pilot study of safety and efficacy. Journal of Cardiac Failure 2004;10:120-5. [DOI] [PubMed] [Google Scholar]
Reitsma 1996
- Reitsma JB, Mosterd A, Craen AJ, Koster RW, Capelle FJ, Grobbee DE, et al. Increase in hospital admission rates for heart failure in the Netherlands, 1980-1993. Heart 1996;76:388-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Centre, The Cochrane Collaboration, 2012.
Rodriguez 1997
- Rodriguez-Artalejo F, Guallar-Castillon P, Banegas Banegas JR, Rey Calero J. Trends in hospitalization and mortality for heart failure in Spain, 1980-1993. European Heart Journal 1997;18:388-92. [DOI] [PubMed] [Google Scholar]
Roger 2011
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association.. Circulation 2011;1(123(4)):e18-e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schappert 2008
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2006. National Health Statistics Reports 2008;6(8):1-29. [PubMed] [Google Scholar]
Sharon 2000
- Sharon A, Shpirer I, Kalunski E, Moshkovitz Y, Milovanov O, Polak R, et al. High-dose intravenous isosorbide-dinitrate is safer and better than Bi-PAP ventilation combined with conventional treatment for severe pulmonary edema. Journal of the American College of Cardiology 2000;36:832-7. [DOI] [PubMed] [Google Scholar]
Spencer 1999
- Spencer FA, Meyer TE, Goldberg RJ, Yarzebski J, Hatton M, Lessard D, et al. Twenty year trends (1975-1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. Journal of the American College of Cardiology 1999;34:1378-87. [DOI] [PubMed] [Google Scholar]
Spencer 2002
- Spencer FA, Meyer TE, Gore JM, Goldberg RJ. Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure: the National Registry of Myocardial Infarction. Circulation 2002;105:2605-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Steg 2004
- Steg PG, Dabbous OH, Feldman LJ, Cohen-Solal A, Aumont MC, Lopez-Sendon J, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004;109:494-9. [DOI] [PubMed] [Google Scholar]
Stevenson 1999
- Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. European Journal of Heart Failure 1999;1:251-7. [DOI] [PubMed] [Google Scholar]
Stewart 2001
- Stewart S, MacIntyre K, Hole DJ. More 'malignant' than cancer? 5-year survival following a first admission with heart failure. European Journal of Heart Failure 2001;3:315-22. [DOI] [PubMed] [Google Scholar]
Stewart 2002
- Stewart S, Jenkins A, Buchan A, McGuire A, Capewell S, McMurray JJ. The current cost of heart failure to the National Health Service in the UK. European Journal of Heart Failure 2001;3:361-71. [DOI] [PubMed] [Google Scholar]
Stewart 2003
- Stewart S, MacIntyre K, Capewell S, McMurray JJV. Heart failure and the aging population: an increasing burden in the 21st century? Heart 2003;89(1):49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weintraub 2010
- Weintraub NL, Collins SP, Pang PS, Levy PD, Anderson AS, Arslanian-Engoren C, et al. Acute heart failure syndromes: emergency department presentation, treatment and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation 2010;122(19):1975-96. [DOI] [PubMed] [Google Scholar]
Whellan 2013
- Whellan DJ, Sarkar S, Koehler J, Small RS, Boyle A, Warman EN, et al. Development of a method to risk stratify patients with heart failure for 30-day readmission using implantable device diagnostics. American Journal of Cardiology 2013;111(1):79-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2002
- Wu AH, Parsons L, Every NR, Bates ER. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2). Journal of the American College of Cardiology 2002;40:1389-94. [DOI] [PubMed] [Google Scholar]
Zile 2011
- Zile MR, Adamson PB, Cho YK, Bennett TD, Bourge RC, Aaron MF, et al. Hemodynamic factors associated with acute decompensated heart failure: part 1 - insights into pathophysiology. Journal of Cardiac Failure 2011;17(4):282-91. [PMID: ] [DOI] [PubMed] [Google Scholar]


