Abstract
Objective
Although the adaptive immune response to SARS‐CoV‐2 has been characterised in the acute and early convalescent phase of the disease, few studies explore whether natural infection elicits long‐lasting immunological memory in recovered individuals. In this work, we aimed to assess the maintenance of immunological memory to SARS‐CoV‐2.
Methods
We evaluated the long‐term virus‐specific cellular and humoral immune response in the members of an Italian Serie A football team, who experienced a cluster of COVID‐19 in March 2020, which was strictly evaluated in the following months.
Results
Our results highlight a heterogeneous magnitude of immunological memory at 5 months after infection. Indeed, 20% of the subjects displayed a weak cellular and humoral memory to SARS‐CoV‐2, suggesting that they may be at higher risk of reinfection. In addition, a history of symptomatic COVID‐19 was associated with higher levels of SARS‐CoV‐2‐reactive CD4+ T cells and specific antibody levels than in asymptomatic individuals.
Conclusion
Collectively, these data demonstrate that immunity to SARS‐CoV‐2 is maintained five months postinfection even if the magnitude of response is heterogeneous among individuals. This finding suggests that some COVID‐19‐recovered subjects may benefit from vaccination.
Keywords: immunoglobulins, immunological memory, SARS‐CoV‐2, T cells
It is currently unknown whether SARS‐CoV‐2 elicits long‐lasting immunological memory. We evaluated the long‐term immunity in an Italian football team, who experienced a cluster of SARS‐CoV‐2 infections in March 2020. Our results highlight a heterogeneous magnitude of immunological memory, suggesting that some individuals may be at risk of reinfection.
Introduction
As of 1 April 2021, SARS‐CoV‐2 has infected more than 128 million people worldwide, with more than 2.8 million deaths. 1 It is still poorly understood whether COVID‐19‐recovered individuals are protected from reinfection, and how long the protection is maintained. Immunological memory plays a primary role in host protection from secondary infections. Indeed, long‐lived memory T and B cells are crucial to provide a fast response in case of pathogen re‐encounter, favoring its rapid elimination. 2 Regarding SARS‐CoV‐2 infection, several data sets have shown that the virus‐specific adaptive immune response develops in the acute phase of the disease. Indeed, it has been demonstrated that CD4+ and CD8+ SARS‐CoV‐2‐specific T cells are present in the circulation of recovered individuals, 3 , 4 and within 19 days after symptom onset, all COVID‐19 patients test positive for antivirus IgG. 5 Moreover, a significant correlation between the frequency of SARS‐CoV‐2‐specific T cells and the levels of specific antibody titres has been demonstrated, 6 suggesting a coordinated activity of both branches of adaptive immunity. The strength of SARS‐CoV‐2‐specific immune response is strictly correlated with clinical disease manifestations. Subjects with asymptomatic SARS‐CoV‐2 infection display lower levels of both humoral and cellular adaptive responses than those with symptomatic infection. 6 , 7 Moreover, a dysregulated immune response has been observed in critically ill COVID‐19 patients. 8 , 9 While some data are now available regarding the characterisation of virus‐specific immune response in the acute or early convalescent phase of the disease, there are instead a few studies investigating long‐term immunity, because of the recent emergence of this infectious disease. Data available on human endemic coronaviruses show that these pathogens elicit a short‐lasting memory, thus leading to possible reinfections. 10 This is a matter of concern, highlighting the possibility that reinfections by SARS‐CoV‐2 may also occur. Therefore, the characterisation of long‐term immunological memory to SARS‐CoV‐2 is urgently need. This is of importance also because vaccines against SARS‐CoV‐2 are now available, and mass immunisation strategies have begun worldwide. Since we are now facing vaccine shortage and it will require several months to complete the immunisation process, it will be important to understand whether vaccination of COVID‐19‐recovered individuals can be postponed because of the protection given by their natural antiviral immunity.
For these reasons, we evaluated the immunological memory to SARS‐CoV‐2 in a group of players and staff members of an Italian Serie A football team, 5 months after the occurrence of a cluster of COVID‐19. Our results show that, although the majority of previously infected subjects maintain elevated levels of circulating specific CD4+ T cells and antibodies, a significant fraction displays reduced immunity. This raises the possibility that some individuals may be at higher risk of reinfection, thus needing vaccination.
Results and discussion
We investigated the transmission of SARS‐CoV‐2 in a football team of the Italian Serie A from March to August 2020. On 8 March 2020, the team played the last official match before the Italian Football Association (FIGC) decided to stop the Serie A to contain COVID‐19 diffusion. On 13 March, the team reported the first case of a SARS‐CoV‐2‐infected player. Within few days, 15 additional cases of SARS‐CoV‐2 infection were diagnosed among members of the team group. At the beginning of May 2020, individual workouts for professional athletes, including football players, were resumed under strict health monitoring rules. For this reason, all team group members were tested for SARS‐CoV‐2 infection by nasopharyngeal swab PCR and serologic evaluation on 6 May 2020. All the 16 subjects who had tested positive by PCR analysis of nasopharyngeal swabs in March (exposed group 1) displayed serum SARS‐CoV‐2‐specific IgG (Figure 1). Of note, 19 additional individuals showed the presence of SARS‐CoV‐2‐specific IgG, while resulted negative to nasopharyngeal swab PCR, indicating a prior exposure to the virus (exposed group 2) (Figure 1). Indeed, 8 subjects in this group referred non‐specific clinical symptoms compatible with COVID‐19 in March 2020, although they had not been tested, but making probable the disease onset at that time. 19 additional team group members had negative SARS‐CoV‐2‐specific IgG, had negative nasopharyngeal swab and did not report any symptom; thus, they were included in the uninfected group (Figure 1). Team group members were routinely screened until the beginning of August 2020 to confirm the absence of any new cases of infection, thus allowing the conclusion of the sport season. On 25 August 2020, at the beginning of the new sport season, all team members were enrolled with the exclusion of 1 individual from the uninfected group whose nasopharyngeal swab tested positive for SARS‐CoV‐2 on enrolment date. 10 other subjects were not included because they were transferred to other teams before the enrolment date. In total, the study included 44 subjects: 15 in exposed group 1 (mean age, 42 ± 14; range, 20–66 years), 15 in exposed group 2 (mean age, 30 ± 11; range, 21–55 years) and 14 in the uninfected group (mean age, 41 ± 14; range, 21–66 years). Main clinical features are summarised in Supplementary table 1. 2a, both exposed group 1 and exposed group 2 displayed significantly higher levels of IgG specific for a virion extract than in the uninfected group. Similar findings were obtained when evaluating the levels of spike (S)‐specific IgG or nucleoprotein (N)‐specific total Ig (Figure 2b, c). SARS‐CoV‐2‐specific IgM and IgA for a virion extract were not detected in all groups of individuals (data not shown). We also evaluated CD4+ T‐cell reactivity to S, membrane (M) and N viral antigens. As shown in Figure 2d, group 1‐ and group 2‐exposed individuals showed a higher frequency of virus‐specific CD4+ T cells, defined by CD154 expression and production of at least one cytokine among IFN‐γ, IL‐2 and TNF‐α, than that of the uninfected group. When considering single cytokine production, we observed an increased percentage of CD4+ T cells producing IFN‐γ (Figure 2e), IL‐2 (Figure 2f) and TNF‐α (Figure 2g) in both groups of exposed subjects compared to uninfected individuals. The gating strategy for the identification of SARS‐CoV‐2‐specific CD4+ T cells is reported in Supplementary figure 1. Of note, we did not observe any differences in humoral and cellular adaptive immune responses between exposed group 1 and exposed group 2. Collectively, these data suggest that immunological memory to SARS‐CoV‐2 is maintained for at least 5 months after infection. Moreover, total virion‐specific IgG and S‐specific IgG serum levels were positively correlated with the frequency of specific CD4+ T cells (Figure 2h, i), suggesting a similar strength of humoral and cellular adaptive immunity. Although both groups of exposed individuals collectively displayed a higher magnitude of humoral and cellular immune responses than in unexposed subjects, it should be noted that within the two exposed groups some individuals showed antibody levels and/or specific CD4+ T‐cell frequencies below reference ranges. For this reason, we deeply investigated the variability sources affecting our data set, performing a principal component analysis (PCA) and then visualising the data in a 2D space through the uniform manifold approximation and projection (UMAP) algorithm. As shown in Figure 2l, a cluster of exposed individuals could be clearly separated from unexposed subjects. However, 6 exposed subjects clustered in proximity to, although separated from, the unexposed group. This finding suggests that in some individuals a weak immune response is present at 5 months postinfection. In agreement, these 6 individuals displayed reduced levels of both humoral (Figure 3a–c) and cellular (Figure 3d–g) immune responses as compared to all other exposed subjects. It has been previously shown that COVID‐19 patients with moderate disease display higher specific antibody titres and frequency of SARS‐CoV‐2‐reactive CD4+ T cells than asymptomatic cases. 6 , 7 These data were obtained in the early convalescence phase; thus, we decided to evaluate whether this difference is maintained also in the memory phase. Consequently, we divided exposed individuals into two groups, asymptomatic (n = 10) and symptomatic (n = 20), based on the absence or presence, respectively, of clinical symptoms compatible with COVID‐19 in March 2020. Subjects with a history of symptomatic COVID‐19 displayed higher levels of serum IgG specific for a total virion extract (Figure 4a) and spike protein (Figure 4b) than in asymptomatic ones. No differences were observed regarding the levels of total Ig specific for the viral nucleoprotein (Figure 4c). Regarding the cellular immune response, symptomatic subjects showed significantly higher frequencies of CD4+ T cells expressing CD154 and producing at least one cytokine among IFN‐γ, IL‐2 and TNF‐α upon in vitro stimulation with viral antigens (Figure 4d). Similar differences were observed when evaluating the frequency of CD4+ T cells expressing CD154 and secreting only IFN‐γ (Figure 4e), IL‐2 (Figure 4f) or TNF‐α (Figure 4g). Of note, among the 24 subjects with a strong immunological memory to SARS‐CoV‐2, 18 (75%) had a history of symptomatic infection. On the contrary, among the 6 subjects with a low magnitude of SARS‐CoV‐2‐specific immunological memory, 2 (33%) had a previous symptomatic COVID‐19 (Figure 4h). Finally, we evaluated the polyfunctional properties of SARS‐CoV‐2‐reactive CD4+ T cells, given that it has been demonstrated that T cells secreting more than one cytokine are functionally superior in host protection against viral pathogens. 11 Although the percentages of CD4+ T cells producing all the three cytokines were comparable, subjects with a history of symptomatic COVID‐19 exhibited an increased frequency of cells producing 2 cytokines in all possible combinations, when compared with asymptomatic individuals (Figure 4i). Contemporarily, a decrease in cells expressing only one cytokine was observed in symptomatic compared to asymptomatic subjects (Figure 4i).
Figure 1.
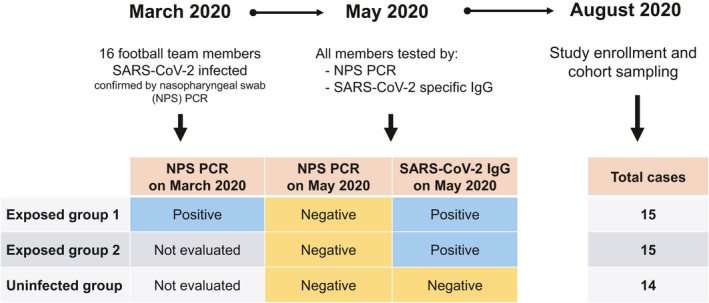
Characterisation of the study cohort.
Figure 2.
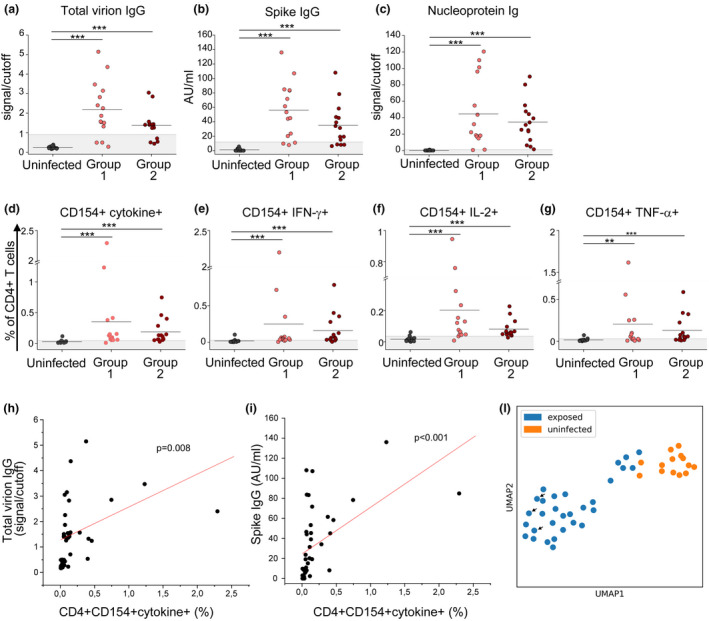
Characterisation of the immunological memory to SARS‐CoV‐2 in exposed individuals. Evaluation of IgG specific for a total virion extract (a) or spike protein (b) in uninfected individuals (n = 14), exposed group 1 (n = 15) and exposed group 2 (n = 15). (c) Evaluation of nucleoprotein‐specific total Ig in uninfected individuals (n = 14), exposed group 1 (n = 15) and exposed group 2 (n = 15). (d) Frequency of CD4+ T cells reactive to SARS‐CoV‐2, defined by the expression of CD154 and at least one cytokine among IFN‐γ, IL‐2 and TNF‐α (CD4+CD154+cytokine+) in uninfected individuals (n = 14), exposed group 1 (n = 15) and exposed group 2 (n = 15). Frequency of SARS‐CoV‐2‐specific CD4+ T cells expressing CD154 and IFN‐γ (e), IL‐2 (f) or TNF‐α (g) in uninfected individuals (n = 14), exposed group 1 (n = 15) and exposed group 2 (n = 15). Data in d–g are presented as percentages of CD4+ T cells, subtracted of background unstimulated negative control. Horizontal lines in a–g represent mean values. Grey area represents the cut‐off value. Correlation between total virion‐specific IgG (h) or spike‐specific IgG levels (i) with the frequency of SARS‐CoV‐2‐reactive CD4+CD154+cytokine+ T cells. (l) Uniform manifold approximation projection (UMAP) visualisation of uninfected (orange) and exposed (blue) individuals. Arrows indicate the three subjects hospitalised for COVID‐19. **P < 0.01; ***P < 0.001.
Figure 3.
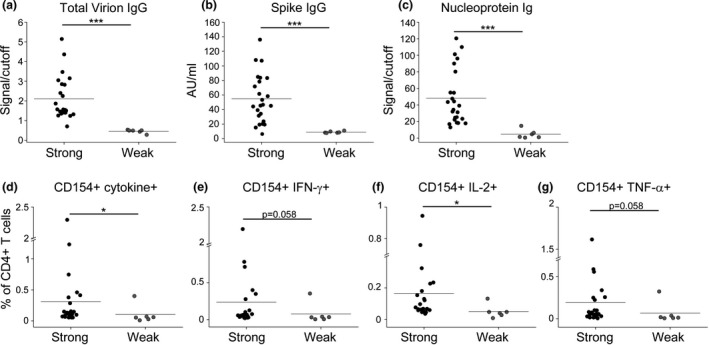
Different strength of immunological memory to SARS‐CoV‐2 in exposed individuals. Evaluation of IgG specific for a total virion extract (a) or spike protein (b) in individuals with a strong (n = 24) or weak (n = 6) signature of immunological memory to SARS‐CoV‐2. (c) Evaluation of nucleoprotein‐specific total Ig in individuals with a strong (n = 24) or weak (n = 6) signature of immunological memory to SARS‐CoV‐2. (d) Frequency of CD4+ T cells reactive to SARS‐CoV‐2, defined by the expression of CD154 and at least one cytokine among IFN‐γ, IL‐2 and TNF‐α (CD4+CD154+cytokine+) in individuals with a strong (n = 24) or weak (n = 6) signature of immunological memory to SARS‐CoV‐2. Frequency of SARS‐CoV‐2‐specific CD4+ T cells expressing CD154 and IFN‐γ (e), IL‐2 (f) or TNF‐α (g) in individuals with a strong (n = 24) or weak (n = 6) signature of immunological memory to SARS‐CoV‐2. Data in d–g are presented as percentages of CD4+ T cells, subtracted of background unstimulated negative control. Horizontal lines in a–g represent mean values. *P < 0.05; ***P < 0.001.
Figure 4.
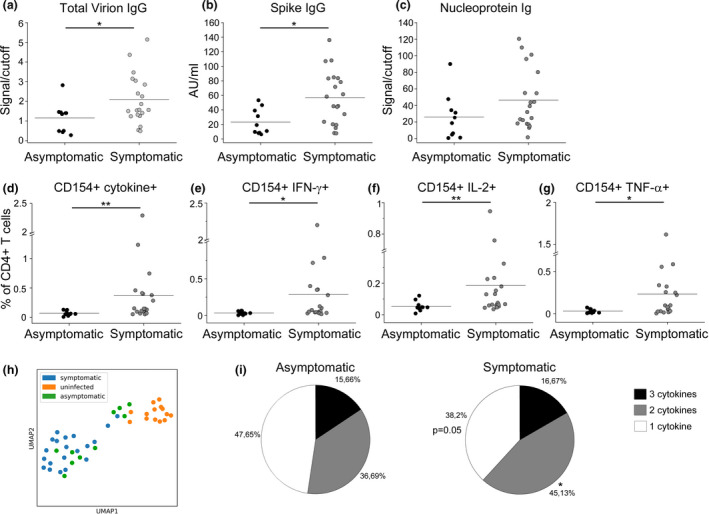
Immunological memory to SARS‐CoV‐2 in subjects with a history of symptomatic or asymptomatic COVID‐19. Evaluation of IgG specific for a total virion extract (a) or spike protein (b) in exposed individuals with a history of symptomatic (n = 20) or asymptomatic (n = 10) COVID‐19. (c) Evaluation of nucleoprotein‐specific total Ig in exposed individuals with a history of symptomatic (n = 20) or asymptomatic (n = 10) COVID‐19. (d) Frequency of CD4+ T cells reactive to SARS‐CoV‐2, defined by the expression of CD154 and at least one cytokine among IFN‐γ, IL‐2 and TNF‐α (CD4+CD154+cytokine+) in individuals with a history of symptomatic (n = 20) or asymptomatic (n = 10) COVID‐19. Frequency of SARS‐CoV‐2‐specific CD4+ T cells expressing CD154 and IFN‐γ (e), IL‐2 (f) or TNF‐α (g) in individuals with a history of symptomatic (n = 20) or asymptomatic (n = 10) COVID‐19. Data in d–g are presented as percentages of CD4+ T cells, subtracted of background unstimulated negative control. Horizontal lines in a–g represent mean values. (h) Uniform manifold approximation projection (UMAP) visualisation of uninfected (orange), symptomatic (blue) and asymptomatic (green) individuals. (i) Characterisation of SARS‐CoV‐2‐specific CD4+ T‐cell polyfunctionality in individuals with a history of symptomatic (n = 20) or asymptomatic (n = 10) COVID‐19. **P < 0.01.
Several groups have demonstrated that an effective adaptive immune response to SARS‐CoV‐2 generates in recovered subjects. However, it is still unclear whether immunological memory to this virus is long‐lasting. Differently from other viruses, endemic coronaviruses induce a short‐lasting immunological memory, exposing individuals to possible reinfections. 10 In agreement, few cases of reinfection by SARS‐CoV‐2 have been demonstrated so far. 12 , 13 , 14 Contradicting reports have been produced regarding the stability of serum‐specific antibody levels, 15 , 16 , 17 , 18 while regarding T cells it has been shown that they persist for at least 3 months after infection in a group of individuals with a history of mild COVID‐19. 19 More recently, Dan et al. described the dynamics of SARS‐CoV‐2 memory B cells, CD8+ T cells and CD4+ T cells for more than 6 months after infection and found a high degree of heterogeneity in the magnitude of response in the immune memory phase to the virus, although most individuals showed durable immunity 5–8 months post symptom onset. 20 Our findings show that at 5 months after infection, 80% (24 of 30 exposed individuals) of the subjects show an immunological signature compatible with persistent immunity to SARS‐CoV‐2. Moreover, a history of symptomatic COVID‐19 is associated with the maintenance of a higher magnitude of specific adaptive response. In our study, subjects with a history of symptomatic COVID‐19 exhibited an increased frequency of cells producing 2 cytokines in all possible combinations as compared with asymptomatic individuals. However, Le Bert et al. have also shown that asymptomatic individuals mount a highly functional virus‐specific cellular immune response. 21
Nevertheless, 20% of the subjects seem to display a weak immunological memory, but we do not know whether this feature will lead to an increased chance of reinfection. Thus, additional studies on bigger cohorts are urgently needed. Understanding the heterogeneity of the immunological memory against SARS‐CoV‐2 is crucial to define the real need for vaccinating people who recovered from COVID‐19. This is of importance to design optimal vaccination strategies, given that vaccine doses are currently limited.
Methods
Patients
A total of 44 team group members (players and staff members) of a football team of the Italian Serie A were enrolled in this study (Figure 1). Sera were obtained from peripheral blood collected in tubes containing clot activators. For PBMNC recovery, blood was collected using EDTA as anticoagulant.
Evaluation of SARS‐CoV‐2‐reactive T cells
PBMNCs were obtained following density gradient centrifugation of blood samples using Lymphoprep (Axis Shield Poc As™; Dundee, Scotland). For cell stimulation in vitro, 1.5 millions of freshly isolated PBMNCs were cultured in 96‐well flat‐bottom plates in the presence of medium alone (background, negative control) or a mixture of nucleoprotein, membrane and spike SARS‐CoV‐2 peptide pools (0.6 µM per peptide, according to the manufacturer’s instructions; Miltenyi Biotec, Bergisch Gladbach, Germany). The peptide pools for N and M proteins cover the entire sequence of the proteins, while the S peptide pool contains the sequence domains aa 304–338, 421–475, 492–519, 683–707, 741–770, 785–802 and 885–1273. After 2 h of incubation at 37°C, 5% CO2, Brefeldin A (Sigma‐Aldrich, Darmstadt, Germany) (5 µg mL–1) was added, followed by an additional 4‐h incubation. Finally, cells were fixed for 15 min with 2% formaldehyde and stained using fluorochrome‐conjugated antibodies listed in Supplementary table 2. All samples were acquired on a BD LSR II Flow Cytometer (BD Biosciences, San Jose). The gating strategy is reported in Supplementary figure 1. Cut‐off values were calculated as mean values of the unexposed group +2SD. Outliers were removed for this calculation.
Evaluation of SARS‐CoV‐2‐specific IgM, IgA and IgG
Serum levels of anti‐SARS‐CoV‐2‐specific antibodies were evaluated by three commercial kits: an enzyme‐linked immunosorbent assay (ELISA) using inactivated native viral proteins (Enzywell SARS‐CoV‐2; Diesse, Siena, Italy); a chemiluminescent immunoassay (CLIA) (LIAISON® SARS‐CoV‐2 S1/S2 IgG; DiaSorin, Saluggia, Italy), detecting IgG antibodies against recombinant S1 and S2 SARS‐CoV‐2 proteins; and an electrochemiluminescence immunoassay (ECLIA) (Roche Elecsys Anti‐SARS‐CoV‐2; Roche Diagnostics, Monza, Italy), using a recombinant protein representing the nucleocapsid (N) antigen. All the tests were performed according to the manufacturer’s instructions.
Bioinformatic analysis
Flow cytometry data and immunoglobulin levels were used to investigate the relations among samples. To this aim, we scaled the different data sets to make them comparable and to build a matrix, later dimensionally reduced through a principal component analysis (PCA). The first 2 principal components (PCs) were used to construct a neighbourhood graph of observations embedded in a bidimensional space through the uniform manifold approximation and projection (UMAP) algorithm.
Statistics
All data were analysed with the non‐parametric Mann–Whitney U‐test for unpaired samples. In all cases, P‐values ≤ 0.05 were considered significant. Pearson’s correlation coefficients were used to calculate the correlations.
Study approval
The procedures followed in the study were approved by the Careggi University Hospital Ethical Committee (Protocol 16859). Written informed consent was obtained from recruited patients.
Author contribution
Alessio Mazzoni: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Writing‐original draft. Laura Maggi: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Writing‐original draft. Manuela Capone: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Writing‐original draft. Anna Vanni: Conceptualization; Data curation; Formal analysis; Methodology; Project administration. Michele Spinicci: Conceptualization; Data curation; Project administration; Writing‐original draft. Lorenzo Salvati: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Writing‐original draft. Seble Tekle Kiros: Data curation; Methodology. Roberto Semeraro: Data curation; Formal analysis; Methodology; Software. Luca Pengue: Conceptualization; Methodology; Project administration. Maria Grazia Colao: Data curation; Methodology. Alberto Magi: Data curation; Formal analysis; Methodology; Software. Gian Maria Rossolini: Conceptualization; Data curation; Methodology; Project administration. Francesco Liotta: Conceptualization; Investigation; Project administration; Writing‐original draft. Lorenzo Cosmi: Conceptualization; Investigation; Project administration; Writing‐original draft. Alessandro Bartoloni: Conceptualization; Data curation; Investigation; Methodology; Project administration; Writing‐original draft. Francesco Annunziato: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Writing‐original draft.
Supporting information
Acknowledgments
This study was supported by funds to the Department of Experimental and Clinical Medicine, University of Florence, derived from Ministero dell’Istruzione, dell’Università e della Ricerca (Italy) (Project Excellence Departments 2018‐2022), by University of Florence, Project RICTD2122, by the Italian Ministry of Health (COVID‐2020‐12371849) and by Tuscany Region (TagSARS CoV 2).
References
- 1. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard. https://covid19.who.int/. Accessed on 1 April 2021.
- 2. Beverley PC. Kinetics and clonality of immunological memory in humans. Semin Immunol 2004; 16: 315–321. [DOI] [PubMed] [Google Scholar]
- 3. Grifoni A, Weiskopf D, Ramirez SI et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell 2020; 181: 1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Bert N, Tan AT, Kunasegaran K et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature 2020; 584: 457–462. [DOI] [PubMed] [Google Scholar]
- 5. Long QX, Liu BZ, Deng HJ et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med 2020; 26: 845–848. [DOI] [PubMed] [Google Scholar]
- 6. Mazzoni A, Maggi L, Capone M et al. Cell‐mediated and humoral adaptive immune responses to SARS‐CoV‐2 are lower in asymptomatic than symptomatic COVID‐19 patients. Eur J Immunol 2020; 50: 2013–2024. [DOI] [PubMed] [Google Scholar]
- 7. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell 2020; 183: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sattler A, Angermair S, Stockmann H et al. SARS‐CoV‐2‐specific T cell responses and correlations with COVID‐19 patient predisposition. J Clin Invest 2020; 130: 6477–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rydyznski Moderbacher C, Ramirez SI, Dan JM et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell 2020; 183: 996–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edridge AWD, Kaczorowska J, Hoste ACR et al. Seasonal coronavirus protective immunity is short‐lasting. Nat Med 2020; 26: 1691–1693. [DOI] [PubMed] [Google Scholar]
- 11. Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T‐cell immunity in human virus infections. Immunol Rev 2006; 211: 236–254. [DOI] [PubMed] [Google Scholar]
- 12. Tillett RL, Sevinsky JR, Hartley PD et al. Genomic evidence for reinfection with SARS‐CoV‐2: a case study. Lancet Infect Dis 2020; 21: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. To KK, Hung IF, Ip JD et al. COVID‐19 re‐infection by a phylogenetically distinct SARS‐coronavirus‐2 strain confirmed by whole genome sequencing. Clin Infect Dis 2020; ciaa1275. 10.1093/cid/ciaa1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Elslande J, Vermeersch P, Vandervoort K et al. Symptomatic SARS‐CoV‐2 reinfection by a phylogenetically distinct strain. Clin Infect Dis 2020; ciaa1330. 10.1093/cid/ciaa1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D et al. Rapid decay of anti–SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med 2020; 383: 1085–1087.PMID: 32706954; PMCID: PMC7397184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long QX, Tang XJ, Shi QL et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med 2020; 26: 1200–1204. [DOI] [PubMed] [Google Scholar]
- 17. Lyer AS, Jones FK, Nodoushani A et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS‐CoV‐2 spike protein in COVID‐19 patients. Sci Immunol 2020; 5: eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ripperger TJ, Uhrlaub JL, Watanabe M et al. Orthogonal SARS‐CoV‐2 serological assays enable surveillance of low‐prevalence communities and reveal durable humoral immunity. Immunity 2020; 53: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodda LB, Netland J, Shehata L et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. Cell 2020; 184: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dan JM, Mateus J, Kato Y et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science 2021; 371: eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le Bert N, Clapham HE, Tan AT et al. Highly functional virus‐specific cellular immune response in asymptomatic SARS‐CoV‐2 infection. J Exp Med 2021; 218: e20202617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


