Abstract
The endosperm is a developmental innovation of angiosperms that supports embryo growth and germination. Aside from this essential reproductive function, the endosperm fuels angiosperm evolution by rapidly establishing reproductive barriers between incipient species. Specifically, the endosperm prevents hybridization of newly formed polyploids with their non-polyploid progenitors, a phenomenon termed the triploid block. Furthermore, recently diverged diploid species are frequently reproductively isolated by endosperm-based hybridization barriers. Current genetic approaches have revealed a prominent role for epigenetic processes establishing these barriers. In particular, imprinted genes, which are expressed in a parent-of-origin-specific manner, underpin the interploidy barrier in the model species Arabidopsis. We will discuss the mechanisms establishing hybridization barriers in the endosperm, the driving forces for these barriers and their impact for angiosperm evolution.
This article is part of the theme issue ‘How does epigenetics influence the course of evolution?’
Keywords: polyploidy, endosperm, hybridization barrier, speciation, genomic imprinting, triploid block
1. Introduction
Flowering plants, or angiosperms, are the most recently diverged clade of vascular plants, but with more than 300 000 species, they form the dominant group of plants on our planet [1,2]. The rise of flowering plants to ecological dominance in the early to Mid-Cretaceous has been intensively discussed and connected to the evolution of novel functional and physiological traits, including flowers and fruits, xylem vessels and faster growth rates [3–9]. One major innovation of flowering plants that has been largely neglected in this discussion is the evolution of the endosperm, an embryo-nourishing tissue that develops after fertilization [10]. In this review, we will focus on the potential role of the endosperm in promoting speciation by establishing hybridization barriers and illuminate the underlying molecular mechanisms as far as they are known to date.
The endosperm is the product of a double fertilization event, where one of the two sperm cells fertilizes the central cell, while the other sperm cell fertilizes the egg cell, initiating embryo formation. The formation of the endosperm is a distinctive feature of angiosperms; embryo nourishment in gymnosperms is mediated by the large female gametophyte [10]. Most higher-order flowering plants have a homodiploid central cell and form a triploid endosperm upon fertilization; however, the ancestral state is likely a haploid central cell and a diploid endosperm, as found in Nymphaeaceae and other basal angiosperms [10,11]. Increased maternal copy number in the endosperm has been proposed to facilitate maternal control over resource allocation to the developing progeny [12]. In support of this view, families with diploid endosperm, like Nymphaeaceae and Illiciaceae, have a very rudimentary endosperm and main resource accumulation occurs in the perisperm, a nutritive tissue derived from sporophytic tissues of the ovule [11,13].
Endosperm development of most flowering plants follows the nuclear type of development, where nuclear divisions are initially not followed by cell wall formation, leading to the formation of a coenocyte [14]. After a defined number of mitotic cycles, the endosperm cellularizes, followed by the differentiation of distinct tissue types [15–17]. The transition from the coenocytic to the cellular stage of endosperm development is an important transition and essential for embryo survival, for reasons that remain to be fully explored [18,19].
Aside from the most prominent nuclear type of endosperm development, some genera such as Solanum and Mimulus follow the cellular type of endosperm development, where mitosis and cytokinesis occur after the first division of the primary endosperm nucleus [14]. A minor fraction of families like Cabombaceae, Sabiaceae and Saxifragaceae follow the helobial type of endosperm development, where after an initial division of the fertilized central cell one cell follows the nuclear type of development while the other cell either remains undivided or also follows the nuclear type of development [14,20].
Failure in endosperm development is a frequent cause of seed arrest in response to hybridizations of related plant species and species that differ in ploidy [21–23]. The phenomenon of endosperm-based hybrid seed lethality is widespread among flowering plants. It is present in diverse taxa, evolves rapidly and manifests the key role of the endosperm in establishing hybridization barriers [22,24–30]. In the following, we will discuss the underlying mechanisms establishing endosperm-based hybridization barriers and their potential drivers.
2. The endosperm is a dosage-sensitive tissue
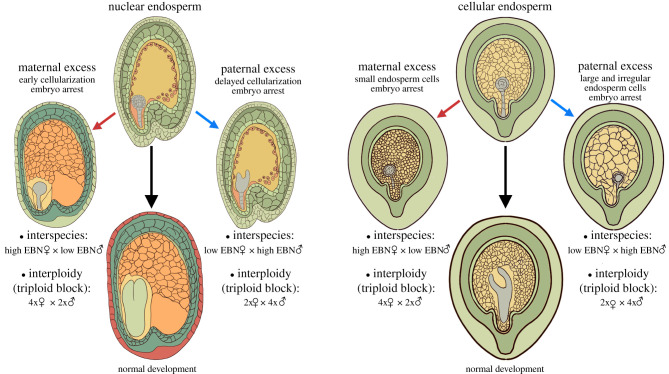
In most flowering plants, the endosperm is a triploid tissue, having two maternal and one paternal genome copies. This particular genome dosage is essential in many, if not most flowering plants to ensure viable embryo development [31–34]. Hybridizations of plants that differ in number of chromosome sets (i.e. ploidy levels) frequently result in seed arrest, a phenomenon termed the ‘triploid block’ [24,35–37]. In species with the nuclear mode of endosperm development, interploidy hybridizations affect the timing of endosperm cellularization. Crosses of maternal plants with higher ploidy pollen donors (referred to as paternal excess) cause a delay in endosperm cellularization, while reciprocal crosses (referred to as maternal excess) cause the opposite phenotype and lead to precocious cellularization [32,38,39] (figure 1). Also, species with the cellular mode of endosperm development show non-reciprocal effects on endosperm development, differing in number and size of endosperm cells [37,40] (figure 1).
Figure 1.
Endosperm defects in response to interploidy and interspecies hybridizations. Interploidy and interspecies hybridizations cause endosperm defects leading to embryo arrest. In species forming nuclear endosperm, paternal excess crosses delay endosperm cellularization, while maternal excess crosses lead to precocious endosperm cellularization. In species with the cellular type of endosperm development, paternal excess crosses lead to the formation of fewer and enlarged cells, while maternal excess crosses cause the formation of small endosperm cells. EBN, Endosperm balance number.
Similar to interploidy hybridizations, interspecies hybridizations also cause defects in endosperm development leading to seed lethality [22,23,25–28,41–44]. Depending on which species is used as maternal plant or pollen donor, non-reciprocal endosperm defects have been observed, with some species behaving like higher ploidy plants despite being diploid [42,43,45] (figure 1). This has led to the establishment of the endosperm balance number (EBN) concept, based on which every species has an effective ploidy that potentially differs from its actual ploidy. This effective ploidy is based on test crosses with defined species and is used to assess cross-compatibility with other species [46]. The EBN must be in a 2 : 1 maternal to paternal ratio in the endosperm for viable crosses.
One implication of the EBN concept is that interspecies and interploidy crosses likely have a similar molecular basis, an idea that is supported by findings showing that increasing the ploidy of one parent allows the generation of viable interspecies hybrids [27,43,47–49] (figure 2). This phenomenon likely explains the presence of gene flow between species that have strong hybridization barriers when crossed as diploids. For example, natural tetraploid Arabidopsis lyrata is able to form viable hybrid seeds with diploid Arabidopsis arenosa, while crosses between diploid species result in inviable seeds [43].
Figure 2.
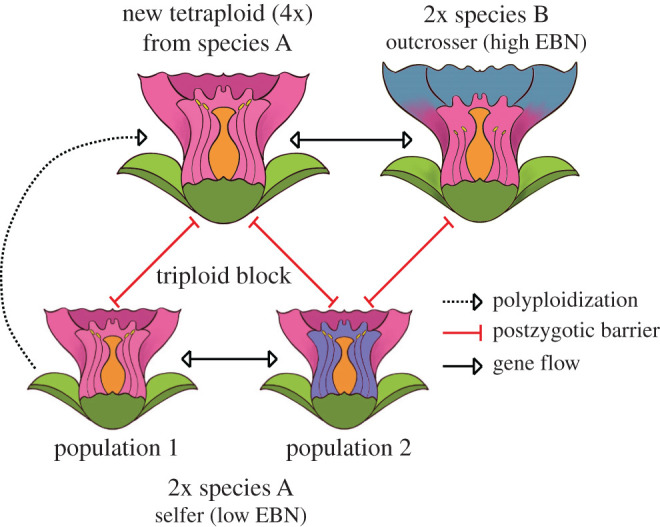
Endosperm-based postzygotic barriers shape gene flow by building interploidy and interspecies postzygotic barriers. Recent polyploidization of species A (low endosperm balance number, EBN) results in a new tetraploid population (with increased EBN) that is reproductively isolated by the triploid block from the ancestral population. Diploid populations of species A are reproductively isolated from the outcrossing species B (high EBN), while the newly formed tetraploid species A may successfully hybridize with species B as a result of increased EBN.
3. Role of genomic imprinting in establishing hybridization barriers
Interploidy and interspecies crosses both cause abnormal seed phenotypes, which are dependent on the direction of the hybridization. This cross-direction dependency raised the hypothesis that imprinted genes could be involved in establishing hybridization barriers in the endosperm [12,50,51]. Genomic imprinting is an epigenetic phenomenon that modifies the expression of genes depending on their parent-of-origin. Imprinted genes are epigenetically modified in the gametes, mainly by DNA methylation and histone modifications. The established epigenetic pattern is maintained after fertilization, leading to parent-specific gene expression. In flowering plants, genomic imprinting is mainly confined to the endosperm and affects several hundreds of genes that are preferentially expressed either maternally or paternally (MEGs and PEGs, respectively) [52–54].
Genetic support for the connection between deregulated imprinted genes and interploidy barriers came with the discovery that mutants in several PEGs could suppress the triploid block in Arabidopsis [55–61]. Imprinted expression of PEGs depends on the Polycomb Repressive Complex2 (PRC2), a chromatin-modifying complex that silences target genes by applying a repressive histone modification. The maternal alleles of PEGs are specifically targeted and silenced by the PRC2, while the paternal alleles remain active [62–64]. The activity of the paternal allele of PEGs is likely a consequence of mechanisms causing resetting of repressive epigenetic modifications in sperm, allowing transcription factors to activate the paternal alleles of PEGs after fertilization [58,65]. Loss of PRC2 function in the endosperm causes the breakdown of PEG imprinting and a phenotypic mimic of paternal excess Arabidopsis seeds, supporting a central role of deregulated PEGs in the triploid block [55,66,67].
Thus far, a role for MEGs in establishing interploidy or interspecies barriers remains to be identified. However, circumstantial evidence suggests that MEGs have a role in both types of hybridization barriers. Mutations in the MEG MEDEA, which encodes a subunit of the PRC2, normalizes seed size in maternal excess interploidy crosses in Arabidopsis [68]. Furthermore, genetic loci with maternal parent-of-origin effects underpin hybrid seed lethality in crosses between Mimulus species, suggesting that MEGs are causally involved [30].
Genomic imprinting has likely evolved as a mechanism to silence transposable elements (TEs) [69–71]; therefore, parent-of-origin-specific expression of many genes is not necessarily functionally relevant. Nevertheless, for some genes, genomic imprinting confers an advantage and maintenance of imprinted expression is likely to be under selection. This molecular scenario of TEs driving genomic imprinting can explain the rapid turnover of imprinted genes over evolutionary time and the low number of conserved imprinted genes among flowering plants [72–75]. The rapid evolution of imprinted genes provides a rationale for the rapid establishment of hybridization barriers between species, as demonstrated in Capsella, Mimulus and Solanum, where closely related sympatric species are separated by strong endosperm-based barriers [28,30,42,76,77].
4. Genetics of the interploidy barrier in Arabidopsis
The PEG PHERES1 (PHE1) encodes for a type I AGAMOUS-LIKE (AGL) MADS-box transcription factor that when mutated can suppress triploid seed inviability. PHE1 binds to the promoter region of many other PEGs, including many suppressors of the triploid block [58], suggesting that PHE1 acts upstream of the triploid block. Supporting this notion, increased dosage of PHE1 correlates with hyperactivation of suppressors of the triploid block [55,58,78]. Interestingly, the majority of suppressors that have been identified in Arabidopsis encode chromatin regulators that have functional roles in TE silencing or heterochromatin establishment [56,59,60,78–80]. This bears striking similarities to hybrid incompatibility in Drosophila, where hybrid incompatibility genes were found to encode dosage-sensitive heterochromatin-interacting proteins or components of the PIWI-interacting RNA pathway, which silences TEs [81–84]. Nevertheless, whether indeed TE derepression is causal for hybrid lethality remains to be established. In Drosophila, hybrid lethality caused by the heterochromatin-interacting proteins hybrid male rescue (Hmr) and lethal hybrid rescue (Lhr) is connected with TE derepression [81,82]; however, whether this is causal for the phenotype has been questioned [85]. Similarly, in Arabidopsis, the role of deregulated TEs in establishing the triploid block remains controversial and requires further investigation [60,78,80]. Increased dosage of the triploid block suppressor ADMETOS causes ectopic application of a heterochromatic histone modification on TEs in the endosperm of triploid Arabidopsis seeds. Genes flanking those TEs become highly overexpressed, possibly leading to triploid seed arrest [79]. Thus, dosage-sensitive chromatin-modifying complexes are causally involved in establishing postzygotic hybridization barriers in Arabidopsis and Drosophila, supporting the idea that the continuous arms race between TEs and their suppressors is a strong source for hybrid incompatibilities [86–88]. However, by which mechanism deregulated chromatin regulators cause lethality remains to be established.
5. Mechanistic similarities between interploidy and interspecies barriers
Interploidy and interspecies hybridizations cause similar developmental abnormalities of the endosperm, suggesting a common mechanistic basis. Notably, interspecies crosses resulting in paternal excess-like phenotypes in Arabidopsis, Capsella, Brassica, Solanum section Lycopersicon (wild tomatoes) and Oryza (rice) are accompanied by overexpression of several AGL Type I MADS-box genes in the developing endosperm [38,42,89–92], mimicking a pattern described for interploidy paternal excess crosses in Arabidopsis and rice [38,55,61,66,92,93]. Interestingly, deregulated AGLs are a common feature of incompatibilities between species having nuclear and cellular modes of endosperm development. The AGL PHE1 acts upstream of known suppressors of the triploid block [58], indicating that deregulated AGLs act on top of a cascade that establishes hybrid incompatibility. Furthermore, downstream pathways affecting cell-wall-modifying activities are similarly affected in Arabidopsis interploidy hybrid seeds and interspecies hybrid seeds of Arabidopsis, Capsella and wild tomatoes [42,55,90], arguing for a signalling pathway converging on similar downstream targets. This pathway likely involves auxin, since auxin signalling is similarly affected in interploidy and interspecies paternal excess seeds in Arabidopsis, as manifested by increased auxin response factor (ARF) expression levels [94,95].
Interestingly, auxin signalling is decreased in paternal excess interspecies hybrid seeds of wild tomatoes, consistent with decreased endosperm proliferation in paternal excess wild tomato seeds [44,90]. Similarly, decreased endosperm proliferation was reported for paternal excess interspecies hybridizations in Mimulus and paternal excess interploidy hybridizations in wild potato species [28,40], which like tomato have a cellular mode of endosperm development. It thus seems that in species with cellular mode of endosperm development, paternal excess interploidy and interspecies hybridizations suppress auxin signalling and reduce endosperm proliferation.
6. Role of auxin in building reproductive barriers
The Arabidopsis auxin biosynthesis genes YUC10 and TAR1 are PEGs and direct targets of PHE1, implying that increased auxin biosynthesis is a direct consequence of PHE1 overexpression [58]. Similarly, in rice, increased expression of the PHE1 orthologues MADS78 and MADS79 causes perturbed auxin homeostasis and delayed endosperm cellularization, suggesting similar regulatory circuits act in monocots [96].
Auxin biosynthesis is required for endosperm development by promoting the proliferation of nuclei [97]. Auxin levels furthermore determine the transition from the coenocytic to the cellular phase of endosperm development [95,98], a transition also defective in paternal excess interploidy and interspecies hybrid seeds [32,38,39,42]. Overexpression of auxin biosynthesis genes in the inner layer of the seed coat causes a similar paternal excess phenotype to overexpression of auxin biosynthesis genes in the endosperm, suggesting a negative feedback of auxin-induced seed coat growth on endosperm cellularization [95]. In support of this notion, the transparent testa glabra2 (ttg2) mutant has reduced integument cell elongation and precocious endosperm cellularization and acts as maternal suppressor of the triploid block [99,100]. Similarly, ttg4, defective in the enzyme chalcone synthase (CHS), is a maternal triploid block suppressor [101]. Both TTG2 and TTG4 are part of the flavonoid pathway, which produces flavonoids that, after oxidation, confer the brown colour of the seed coat in Arabidopsis and other angiosperms [102]. Flavonoids have been proposed to regulate auxin transport [103], linking flavonoids, auxin and the triploid block. Thus, altered auxin biosynthesis in the endosperm of triploid seeds causes altered auxin accumulation and growth in the seed coat, which affects endosperm cellularization. This scenario provides a possible explanation for the observed non-reciprocal effects of interploidy and interspecies crosses on seed coat development in Primula, Brassica and wild tomatoes [24,44,45,91].
7. Drivers of postzygotic barriers in the endosperm
Hybrid incompatibilities have been proposed to evolve as a consequence of interspecies divergence between selfish DNA elements and their regulators [86–88]. Thus, the genomic conflict between TEs and their repressors is considered a potent driver of postzygotic barriers [86–88]. Reduced DNA methylation in the endosperm [104–106] may render the endosperm particularly vulnerable for genomic conflict, providing an explanation for the preference of chromatin regulators among suppressors of the triploid block [56,59,60,78–80].
The conflict between maternally and paternally derived alleles (referred to as parental conflict, or kin conflict) is another potential driver of postzygotic barriers manifested in nourishing tissues of plants and animals [28,90,107–109]. Parental conflict can arise in polyandrous species because maternal and paternal parents differ in the investments of resources allocated to the offspring. Since only the maternal parent provides nutrients to the developing progeny, while there are no costs on the paternal side, genes of paternal origin are selected to increase resource allocation to the offspring. By contrast, the same or different genes when maternally inherited are under selection to equalize nutrient transfer [12,108,110]. In consequence, a co-evolutionary arms race initiates between paternally expressed loci promoting the nutrient acquisition and maternally expressed loci suppressing the growth of the progeny. If in different populations different genes have evolved to control this process, hybridizations between these populations can result in hybrid growth defects and lethality. There are several examples showing that seed size is affected by the paternal genotype and that seed size increases with the grade of outcrossing of the pollen parent [111–113]. Furthermore, several examples have shown that crosses between self-pollinating (selfers) and outcrossing plants (outcrossers) lead to seed lethality; the defects manifested in the endosperm correspond to the expected direction assuming that outcrossers behave like parents with increased ploidy or high EBN [28,42,47,114] (figures 1 and 2). This has been conceptualized in the weak inbreeder/strong outbreeder (WISO) hypothesis, which states that crosses between selfers and outcrossers cause dosage imbalance in the hybrid endosperm, resulting in seed lethality [107]. Nevertheless, there are exceptions to this rule, where outcrossers have low EBNs, which is possibly a consequence of small population size and low genetic diversity [28,90,115,116]. The parental conflict could drive the evolution of hybridization barriers by enforcing the evolution of imprinted genes with nutrient-acquiring functions as well as genes limiting nutrient acquisition. Thus, one can postulate that imprinted genes involved in establishing hybridization barriers impact endosperm growth. There is indeed supportive evidence for several PEGs having growth-promoting functions in the endosperm. Triploid seeds derived from paternal excess crosses show increased endosperm growth and delayed endosperm cellularization, connected with increased PEG expression [32,38,39,55,117]. Mutants in several PEGs can suppress endosperm overgrowth and restore endosperm cellularization in Arabidopsis paternal excess seeds, supporting a role of PEGs as growth promoters in the endosperm [55–58,60,79]. PEGs are controlled by the PRC2, and interestingly, in Arabidopsis, two subunits of this complex are encoded by MEGs [118–120], supporting the concept of MEGs having growth-suppressing functions. Similarly in maize and rice, components of the endosperm-expressed PRC2 are MEGs [121,122]. Nevertheless, further functional studies of MEGs are required to test whether this concept holds.
8. Formation of polyploids and relevance of endosperm-based hybridization barriers
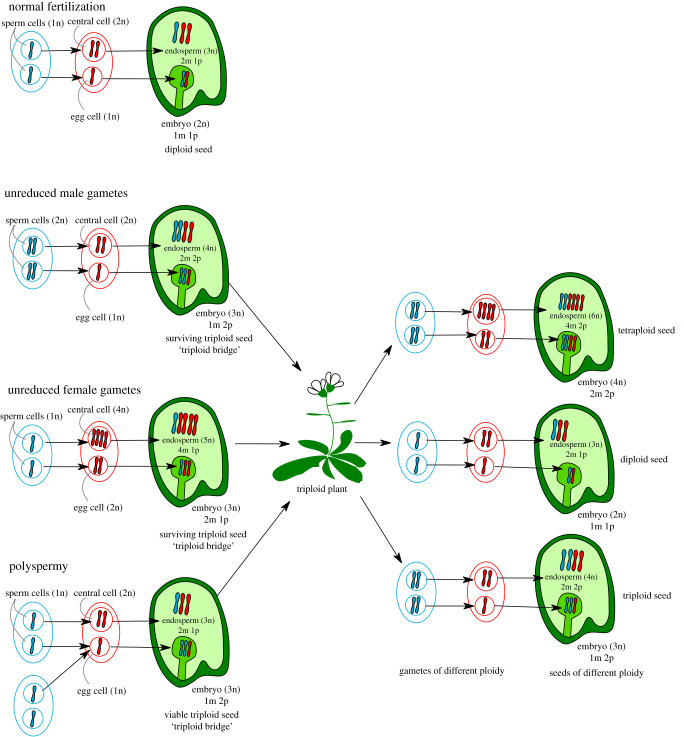
There are several pathways leading to the formation of polyploids; among those, the formation via unreduced diploid gametes is considered the most frequent route to polyploidy [29,123] (figure 3). The frequency of unreduced gamete formation differs between species and was shown to increase in response to heat and cold stress, which may explain the increased occurrence of polyploids within the Arctic [124–128]. The formation of polyploids has been proposed to occur via unstable triploid intermediates: a phenomenon termed the triploid bridge [29,129–131]. This path of polyploidy formation rests on the fact that a fraction of formed triploids can survive, as reported in many species [132–134]. Furthermore, in addition to the increased incidence of unreduced gamete formation under cold conditions [124,128], lower temperatures were also shown to alleviate postzygotic endosperm barriers [135], suggesting that specific climatic conditions promote the formation of polyploids via triploid intermediates. Another mechanism that has been proposed to give rise to polyploids is polyspermy, whereby two sperm cells fertilize the egg and thus bypass the triploid block [136–139] (figure 3). Nevertheless, the reported frequency of polyspermy-induced triploids in Arabidopsis is about 100-fold lower than the frequency of unreduced male gamete formation reported in Brassicaceae [136,140]. Furthermore, unreduced gamete formation is not restricted to pollen but also occurs in the egg at comparable frequency [29,123]; therefore, the frequency of potential unreduced gametes that can give rise to triploids is likely to be higher than currently estimated. Yet, comprehensive studies are required to establish the path and frequency of triploid formation in nature.
Figure 3.
Different routes giving rise to polyploid plants. The formation of polyploids can occur via the formation of unreduced male and female gametes, leading to the formation of triploid seeds that when overcoming the triploid block give rise to triploid plants. Triploids can also arise in consequence of polyspermy, which, if only the egg cell is fertilized by two sperm cells, will give rise to a viable triploid seed with a balanced triploid endosperm and a triploid embryo. Triploids can give rise to swarms of gametes with different grades of ploidy and thus act as a bridge to the formation of stable polyploids. m, maternal; p, paternal.
While triploids suffer from meiotic problems and are mainly sterile, they nonetheless can form gametes of varying ploidy grades, among them diploid gametes which when fused with each other can give rise to stable tetraploids [134] (figure 3). Reproductive isolation of newly established tetraploids prevents generating reproductively unfit triploids by backcrossing with diploid progenitors [130]. Niche separation, local pollen and seed dispersal and the transition to selfing are important factors facilitating tetraploid establishment [130,141,142]. Selfing increases the probability of successful matings during early stages of polyploid species establishment; however, enforcement mechanisms like the triploid block are likely required to ensure that predominantly selfing progeny is produced and unstable triploids aborted. The transition to selfing is generally followed by changes in flower morphology, enforcing selfing [143]. Nevertheless, before these changes are established, additional barriers preventing hybridizations of newly emerged self-fertilizers with their outcrossing relatives are likely promoting their establishment: a hypothesis that remains to be experimentally validated.
9. Conclusion
Accumulating evidence over the last century points that endosperm-based postzygotic hybridization barriers have a strong impact as drivers of angiosperm diversification. The formation of endosperm-based hybridization barriers is propelled by different conflicts, which promote the rapid evolution of speciation genes acting in the endosperm. Important gaps in our current knowledge that remain to be closed are the nature of the genes underpinning these barriers, their evolution and mode of action establishing these barriers. Furthermore, functionally connecting interploidy and interspecies barriers and testing the concept of a shared genetic basis are interesting avenues to be explored. Finally, assessing the contribution of these barriers to species divergence and the time of their establishment are areas of research that hold much promise for important discoveries.
Acknowledgement
We thank Marion Orsucci, Nicolas Butel and Lauriane Simon for critical comments on the manuscript. This work was supported by the Knut and Alice Wallenberg Foundation (grant no. 2018-0206 to C.K.), and the Göran Gustafsson Foundation for Research in Natural Sciences and Medicine (to C.K.).
Data accessibility
This article has no additional data.
Authors' contributions
C.K. wrote major parts of the manuscript with the support of G.D.T.-D.L. and K.D. G.D.T.-D.L. and K.D. generated the figures.
Competing interests
We declare we have no competing interests.
References
- 1.Silvestro D, Cascales-Miñana B, Bacon CD, Antonelli A. 2015. Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytol. 207, 425-436. ( 10.1111/nph.13247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimm SL, Raven PH. 2017. The fate of the world's plants. Trends Ecol. Evol. 32, 317-320. ( 10.1016/j.tree.2017.02.014) [DOI] [PubMed] [Google Scholar]
- 3.Berendse F, Scheffer M. 2009. The angiosperm radiation revisited, an ecological explanation for Darwin's ‘abominable mystery’. Ecol. Lett. 12, 865-872. ( 10.1111/j.1461-0248.2009.01342.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodribb TJ, Feild TS. 2010. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 13, 175-183. ( 10.1111/j.1461-0248.2009.01410.x) [DOI] [PubMed] [Google Scholar]
- 5.Labandeira CC. 2010. The pollination of mid Mesozoic seed plants and the early history of long-proboscid insects. Ann. MO Bot. Gard. 97, 469-513, 445. ( 10.3417/2010037) [DOI] [Google Scholar]
- 6.Feild TS, et al. 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl Acad. Sci. USA 108, 8363-8366. ( 10.1073/pnas.1014456108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augusto L, Davies TJ, Delzon S, De Schrijver A. 2014. The enigma of the rise of angiosperms: can we untie the knot? Ecol. Lett. 17, 1326-1338. ( 10.1111/ele.12323) [DOI] [PubMed] [Google Scholar]
- 8.Bond WJ. 1989. The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. 36, 227-249. ( 10.1111/j.1095-8312.1989.tb00492.x) [DOI] [Google Scholar]
- 9.Fawcett JA, Maere S, Van de Peer Y. 2009. Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event. Proc. Natl Acad. Sci. USA 106, 5737-5742. ( 10.1073/pnas.0900906106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baroux C, Spillane C, Grossniklaus U. 2002. Evolutionary origins of the endosperm in flowering plants. Genome Biol. 3, 1026. ( 10.1186/gb-2002-3-9-reviews1026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JH, Friedman WE. 2002. Identification of diploid endosperm in an early angiosperm lineage. Nature 415, 522-526. ( 10.1038/415522a) [DOI] [PubMed] [Google Scholar]
- 12.Haig D, Westoby M. 1989. Parent-specific gene expression and the triploid endosperm. Am. Nat. 134, 147-155. ( 10.1086/284971) [DOI] [Google Scholar]
- 13.Floyd SK, Friedman WE. 2001. Developmental evolution of endosperm in basal angiosperms: evidence from Amborella (Amborellaceae), Nuphar (Nymphaeaceae), and Illicium (Illiciaceae). Plant Syst. Evol. 228, 153-169. ( 10.1007/s006060170026) [DOI] [Google Scholar]
- 14.Lopes MA, Larkins BA. 1993. Endosperm origin, development and function. Plant Cell 5, 1383-1399. ( 10.1105/tpc.5.10.1383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, Dumas C, Haseloff J, Berger F. 2001. Dynamic analyses of the expression of the histone::YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13, 495-509. ( 10.1105/tpc.13.3.495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RC, Lemmon BE, Nguyen H, Olsen OA. 1999. Development of the endosperm in Arabidopsis thaliana. Sex. Plant Reprod. 12, 32-42. ( 10.1007/s004970050169) [DOI] [Google Scholar]
- 17.Sorensen MB, Mayer U, Lukowitz W, Robert H, Chambrier P, Jürgens G, Somerville C, Lepiniec L, Berger F. 2002. Cellularisation in the endosperm of Arabidopsis thaliana is coupled to mitosis and shares multiple components with cytokinesis. Development 129, 5567-5576. ( 10.1242/dev.00152) [DOI] [PubMed] [Google Scholar]
- 18.Hehenberger E, Kradolfer D, Köhler C. 2012. Endosperm cellularization defines an important developmental transition for embryo development. Development 139, 2031-2039. ( 10.1242/dev.077057) [DOI] [PubMed] [Google Scholar]
- 19.Lafon-Placette C, Kohler C. 2014. Embryo and endosperm, partners in seed development. Curr. Opin. Plant Biol. 17, 64-69. ( 10.1016/j.pbi.2013.11.008) [DOI] [PubMed] [Google Scholar]
- 20.Geeta R. 2003. The origin and maintenance of nuclear endosperms: viewing development through a phylogenetic lens. Proc. R. Soc. Lond. B 270, 29-35. ( 10.1098/rspb.2002.2206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brink RA, Cooper DC. 1947. The endosperm in seed development. Bot. Rev. 132, 423-477. ( 10.1007/BF02861548) [DOI] [Google Scholar]
- 22.Sukno S, Ruso J, Jan CC, Melero-Vara JM, Fernández-Martínez JM. 1999. Interspecific hybridization between sunflower and wild perennial Helianthus species via embryo rescue. Euphytica 106, 69-78. ( 10.1023/A:1003524822284) [DOI] [Google Scholar]
- 23.Dinu II, Hayes RJ, Kynast RG, Phillips RL, Thill CA. 2005. Novel inter-series hybrids in Solanum, section Petota. Theor. Appl. Genet. 110, 403-415. ( 10.1007/s00122-004-1782-x) [DOI] [PubMed] [Google Scholar]
- 24.Cooper DC, Brink RA. 1945. Seed collapse following matings between diploid and tetraploid races of Lycopersicon pimpinellifolium. Genetics 30, 371-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams E, White DWR. 1976. Early seed development after crossing of Trifolium ambiguum and T. repens. N. Z. J. Bot. 14, 307-314. ( 10.1080/0028825X.1976.10428903) [DOI] [Google Scholar]
- 26.Gill BS, Waines JG. 1978. Paternal regulation of seed development in wheat hybrids. Theor. Appl. Genet. 51, 265-270. ( 10.1007/BF00274813) [DOI] [PubMed] [Google Scholar]
- 27.Johnston SA, Hanneman RE Jr. 1982. Manipulations of endosperm balance number overcome crossing barriers between diploid Solanum species. Science 217, 446-448. ( 10.1126/science.217.4558.446) [DOI] [PubMed] [Google Scholar]
- 28.Coughlan JM, Wilson Brown M, Willis JH. 2020. Patterns of hybrid seed inviability in the Mimulus guttatus sp. complex reveal a potential role of parental conflict in reproductive isolation. Curr. Biol. 30, 83-93.e85. ( 10.1016/j.cub.2019.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey J, Schemske DW. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 29, 467-501. ( 10.1146/annurev.ecolsys.29.1.467) [DOI] [Google Scholar]
- 30.Garner AG, Kenney AM, Fishman L, Sweigart AL. 2016. Genetic loci with parent-of-origin effects cause hybrid seed lethality in crosses between Mimulus species. New Phytol. 211, 319-331. ( 10.1111/nph.13897) [DOI] [PubMed] [Google Scholar]
- 31.Lin BY. 1984. Ploidy barrier to endosperm development in maize. Genetics 107, 103-115. ( 10.1093/genetics/107.1.103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott RJ, Spielman M, Bailey J, Dickinson HG. 1998. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125, 3329-3341. [DOI] [PubMed] [Google Scholar]
- 33.Leblanc O, Pointe C, Hernandez M. 2002. Cell cycle progression during endosperm development in Zea mays depends on parental dosage effects. Plant J. 32, 1057-1066. ( 10.1046/j.1365-313x.2002.01491.x) [DOI] [PubMed] [Google Scholar]
- 34.Birchler JA. 1993. Dosage analysis of maize endosperm development. Annu. Rev. Genet. 27, 181-204. ( 10.1146/annurev.ge.27.120193.001145) [DOI] [PubMed] [Google Scholar]
- 35.Marks GE. 1966. The origin and significance of intraspecific polyploidy: experimental evidence from Solanum chacoense. Evolution 20, 552-557. ( 10.2307/2406589) [DOI] [PubMed] [Google Scholar]
- 36.Muntzing A. 1933. Hybrid incompatibility and the origin of polyploidy. Hereditas 18, 33-55. ( 10.1111/j.1601-5223.1933.tb02596.x) [DOI] [Google Scholar]
- 37.Woodell SRJ, Valentine DH. 1961. Studies in British primulas. IX. Seed incompatibility in diploid-autotetraploid crosses. New Phytol. 60, 282-294. ( 10.1111/j.1469-8137.1961.tb06256.x) [DOI] [Google Scholar]
- 38.Sekine D, Ohnishi T, Furuumi H, Ono A, Yamada T, Kurata N, Kinoshita T. 2013. Dissection of two major components of the post-zygotic hybridization barrier in rice endosperm. Plant J. 76, 792-799. ( 10.1111/tpj.12333) [DOI] [PubMed] [Google Scholar]
- 39.Pennington PD, Costa LM, Gutierrez-Marcos JF, Greenland AJ, Dickinson HG. 2008. When genomes collide: aberrant seed development following maize interploidy crosses. Ann. Bot. 101, 833-843. ( 10.1093/aob/mcn017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wangenheim K-HFV. 1957. Untersuchungen über den Zusammenhang zwischen Chromosomenzahl und Kreuzbarkeit bei Solanum-Arten [Investigations on the connections between chromosome number and crossability in Solanum species]. Z. Indukt. Abstamm. Vererbungslehre 88, 21-37. [In German.] ( 10.1007/BF00593652) [DOI] [Google Scholar]
- 41.Cooper DC, Brink RA. 1942. The endosperm as a barrier to interspecific hybridization in flowering plants. Science 95, 75-76. ( 10.1126/science.95.2455.75) [DOI] [PubMed] [Google Scholar]
- 42.Rebernig CA, Lafon-Placette C, Hatorangan MR, Slotte T, Kohler C. 2015. Non-reciprocal interspecies hybridization barriers in the Capsella genus are established in the endosperm. PLoS Genet. 11, e1005295. ( 10.1371/journal.pgen.1005295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lafon-Placette C, et al. 2017. Endosperm-based hybridization barriers explain the pattern of gene flow between Arabidopsis lyrata and Arabidopsis arenosa in Central Europe. Proc. Natl Acad. Sci. USA 114, E1027-E1035. ( 10.1073/pnas.1615123114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth M, Florez-Rueda AM, Griesser S, Paris M, Städler T. 2018. Incidence and developmental timing of endosperm failure in post-zygotic isolation between wild tomato lineages. Ann. Bot. 121, 107-118. ( 10.1093/aob/mcx133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valentine DH, Woodell SRJ. 1963. Studies in British primulas. X. Seed incompatibility in intraspecific and interspecific crosses at diploid and tetraploid levels. New Phytol. 62, 125-143. ( 10.1111/j.1469-8137.1963.tb06321.x) [DOI] [Google Scholar]
- 46.Johnston SA, Nijs TPM, Peloquin SJ, Hanneman RE Jr. 1980. The significance of genic balance to endosperm development in interspecific crosses. Theor. Appl. Genet. 57, 5-9. ( 10.1007/BF00276002) [DOI] [PubMed] [Google Scholar]
- 47.Lafon-Placette C, Hatorangan MR, Steige KA, Cornille A, Lascoux M, Slotte T, Kohler C. 2018. Paternally expressed imprinted genes associate with hybridization barriers in Capsella. Nat. Plants 4, 352-357. ( 10.1038/s41477-018-0161-6) [DOI] [PubMed] [Google Scholar]
- 48.Tonosaki K, Sekine D, Ohnishi T, Ono A, Furuumi H, Kurata N, Kinoshita T. 2018. Overcoming the species hybridization barrier by ploidy manipulation in the genus Oryza. Plant J. 93, 534-544. ( 10.1111/tpj.13803) [DOI] [PubMed] [Google Scholar]
- 49.Bushell C, Spielman M, Scott RJ. 2003. The basis of natural and artificial postzygotic hybridization barriers in Arabidopsis species. Plant Cell 15, 1430-1442. ( 10.1105/tpc.010496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore T. 2001. Genetic conflict, genomic imprinting and establishment of the epigenotype in relation to growth. Reproduction 122, 185-193. ( 10.1530/rep.0.1220185) [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez-Marcos JF, Pennington PD, Costa LM, Dickinson HG. 2003. Imprinting in the endosperm: a possible role in preventing wide hybridization. Phil. Trans. R. Soc. Lond. B 358, 1105-1111. ( 10.1098/rstb.2003.1292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodrigues JA, Zilberman D. 2015. Evolution and function of genomic imprinting in plants. Genes Dev. 29, 2517-2531. ( 10.1101/gad.269902.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gehring M, Satyaki PR. 2017. Endosperm and imprinting, inextricably linked. Plant Physiol. 173, 143-154. ( 10.1104/pp.16.01353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batista RA, Kohler C. 2020. Genomic imprinting in plants—revisiting existing models. Genes Dev. 34, 24-36. ( 10.1101/gad.332924.119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolff P, Jiang H, Wang G, Santos-Gonzalez J, Köhler C. 2015. Paternally expressed imprinted genes establish postzygotic hybridization barriers in Arabidopsis thaliana. eLife 4, e10074. ( 10.7554/eLife.10074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erdmann RM, Satyaki PR, Klosinska M, Gehring M. 2017. A small RNA pathway mediates allelic dosage in endosperm. Cell Rep. 21, 3364-3372. ( 10.1016/j.celrep.2017.11.078) [DOI] [PubMed] [Google Scholar]
- 57.Huang F, et al. 2017. Mutants in the imprinted PICKLE RELATED 2 gene suppress seed abortion of fertilization independent seed class mutants and paternal excess interploidy crosses in Arabidopsis. Plant J. 90, 383-395. ( 10.1111/tpj.13500) [DOI] [PubMed] [Google Scholar]
- 58.Batista RA, Moreno-Romero J, Qiu Y, van Boven J, Santos-Gonzalez J, Figueiredo DD, Kohler C. 2019. The MADS-box transcription factor PHERES1 controls imprinting in the endosperm by binding to domesticated transposons. eLife 8, e50541. ( 10.7554/eLife.50541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang G, Jiang H, Del Toro-De León G, Martinez G, Kohler C. 2018. Sequestration of a transposon-derived siRNA by a target mimic imprinted gene induces postzygotic reproductive isolation in Arabidopsis. Dev. Cell 46, 696-705. ( 10.1016/j.devcel.2018.07.014) [DOI] [PubMed] [Google Scholar]
- 60.Martinez G, Wolff P, Wang Z, Moreno-Romero J, Santos-Gonzalez J, Conze LL, DeFraia C, Slotkin RK, Köhler C. 2018. Paternal easiRNAs regulate parental genome dosage in Arabidopsis. Nat. Genet. 50, 193-198. ( 10.1038/s41588-017-0033-4) [DOI] [PubMed] [Google Scholar]
- 61.Kradolfer D, Wolff P, Jiang H, Siretskiy A, Köhler C. 2013. An imprinted gene underlies postzygotic reproductive isolation in Arabidopsis thaliana. Dev. Cell 26, 525-535. ( 10.1016/j.devcel.2013.08.006) [DOI] [PubMed] [Google Scholar]
- 62.Mozgova I, Hennig L. 2015. The polycomb group protein regulatory network. Annu. Rev. Plant Biol. 66, 269-296. ( 10.1146/annurev-arplant-043014-115627) [DOI] [PubMed] [Google Scholar]
- 63.Moreno-Romero J, Jiang H, Santos-Gonzalez J, Kohler C. 2016. Parental epigenetic asymmetry of PRC2-mediated histone modifications in the Arabidopsis endosperm. EMBO J. 35, 1298-1311. ( 10.15252/embj.201593534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, et al. 2014. Genome-wide high resolution parental-specific DNA and histone methylation maps uncover patterns of imprinting regulation in maize. Genome Res. 24, 167-176. ( 10.1101/gr.155879.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borg M, et al. 2020. Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat. Cell Biol. 22, 821-829. ( 10.1038/s41556-020-0515-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erilova A, Brownfield L, Exner V, Rosa M, Twell D, Mittelsten Scheid O, Hennig L, Köhler C. 2009. Imprinting of the Polycomb group gene MEDEA serves as a ploidy sensor in Arabidopsis. PLoS Genet. 5, e1000663. ( 10.1371/journal.pgen.1000663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsieh TF, et al. 2011. Regulation of imprinted gene expression in Arabidopsis endosperm. Proc. Natl Acad. Sci. USA 108, 1755-1762. ( 10.1073/pnas.1019273108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kradolfer D, Hennig L, Köhler C. 2013. Increased maternal genome dosage bypasses the requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis seed development. PLoS Genet. 9, e1003163. ( 10.1371/journal.pgen.1003163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim MY, Zilberman D. 2014. DNA methylation as a system of plant genomic immunity. Trends Plant Sci. 19, 320-326. ( 10.1016/j.tplants.2014.01.014) [DOI] [PubMed] [Google Scholar]
- 70.Köhler C, Weinhofer-Molisch I. 2010. Mechanisms and evolution of genomic imprinting in plants. Heredity 105, 57-63. ( 10.1038/hdy.2009.176) [DOI] [PubMed] [Google Scholar]
- 71.Bestor TH, Bourc'his D. 2004. Transposon silencing and imprint establishment in mammalian germ cells. Cold Spring Harb. Symp. Quant. Biol. 69, 381-387. ( 10.1101/sqb.2004.69.381) [DOI] [PubMed] [Google Scholar]
- 72.Hatorangan MR, Laenen B, Steige KA, Slotte T, Kohler C. 2016. Rapid evolution of genomic imprinting in two species of the Brassicaceae. Plant Cell 28, 1815-1827. ( 10.1105/tpc.16.00304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waters AJ, Bilinski P, Eichten SR, Vaughn MW, Ross-Ibarra J, Gehring M, Springer NM. 2013. Comprehensive analysis of imprinted genes in maize reveals allelic variation for imprinting and limited conservation with other species. Proc. Natl Acad. Sci. USA 110, 19 639-19 644. ( 10.1073/pnas.1309182110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C, et al. 2018. Characterization of imprinted genes in rice reveals conservation of regulation and imprinting with other plant species. Plant Physiol. 177, 1754-1771. ( 10.1104/pp.17.01621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roth M, Florez-Rueda AM, Paris M, Städler T. 2018. Wild tomato endosperm transcriptomes reveal common roles of genomic imprinting in both nuclear and cellular endosperm. Plant J. 95, 1084-1101. ( 10.1111/tpj.14012) [DOI] [PubMed] [Google Scholar]
- 76.Baek YS, et al. 2016. Interspecific reproductive barriers between sympatric populations of wild tomato species (Solanum section Lycopersicon). Am. J. Bot. 103, 1964-1978. ( 10.3732/ajb.1600356) [DOI] [PubMed] [Google Scholar]
- 77.Oneal E, Willis JH, Franks RG. 2016. Disruption of endosperm development is a major cause of hybrid seed inviability between Mimulus guttatus and Mimulus nudatus. New Phytol. 210, 1107-1120. ( 10.1111/nph.13842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satyaki PR, Gehring M. 2019. Paternally acting canonical RNA-directed DNA methylation pathway genes sensitize Arabidopsis endosperm to paternal genome dosage. Plant Cell 31, 1563-1578. ( 10.1105/tpc.19.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang H, Moreno-Romero J, Santos-Gonzalez J, De Jaeger G, Gevaert K, Van De S, Kohler C. 2017. Ectopic application of the repressive histone modification H3K9me2 establishes post-zygotic reproductive isolation in Arabidopsis thaliana. Genes Dev. 31, 1272-1287. ( 10.1101/gad.299347.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borges F, Parent JS, van Ex F, Wolff P, Martinez G, Köhler C, Martienssen RA. 2018. Transposon-derived small RNAs triggered by miR845 mediate genome dosage response in Arabidopsis. Nat. Genet. 50, 186-192. ( 10.1038/s41588-017-0032-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brideau NJ, Flores HA, Wang J, Maheshwari S, Wang X, Barbash DA. 2006. Two Dobzhansky-Muller genes interact to cause hybrid lethality in Drosophila. Science 314, 1292-1295. ( 10.1126/science.1133953) [DOI] [PubMed] [Google Scholar]
- 82.Thomae AW, Schade GO, Padeken J, Borath M, Vetter I, Kremmer E, Heun P, Imhof A. 2013. A pair of centromeric proteins mediates reproductive isolation in Drosophila species. Dev. Cell 27, 412-424. ( 10.1016/j.devcel.2013.10.001) [DOI] [PubMed] [Google Scholar]
- 83.Parhad SS, Tu S, Weng Z, Theurkauf WE. 2017. Adaptive evolution leads to cross-species incompatibility in the piRNA transposon silencing machinery. Dev. Cell 43, 60-70.e65. ( 10.1016/j.devcel.2017.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bayes JJ, Malik HS. 2009. Altered heterochromatin binding by a hybrid sterility protein in Drosophila sibling species. Science 326, 1538-1541. ( 10.1126/science.1181756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Satyaki PR, Cuykendall TN, Wei KH, Brideau NJ, Kwak H, Aruna S, Ferree PM, Ji S, Barbash DA. 2014. The Hmr and Lhr hybrid incompatibility genes suppress a broad range of heterochromatic repeats. PLoS Genet. 10, e1004240. ( 10.1371/journal.pgen.1004240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson NA. 2010. Hybrid incompatibility genes: remnants of a genomic battlefield? Trends Genet. 26, 317-325. ( 10.1016/j.tig.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 87.Presgraves DC. 2010. The molecular evolutionary basis of species formation. Nat. Rev. Genet. 11, 175-180. ( 10.1038/nrg2718) [DOI] [PubMed] [Google Scholar]
- 88.Maheshwari S, Barbash DA. 2011. The genetics of hybrid incompatibilities. Annu. Rev. Genet. 45, 331-355. ( 10.1146/annurev-genet-110410-132514) [DOI] [PubMed] [Google Scholar]
- 89.Walia H, Josefsson C, Dilkes B, Kirkbride R, Harada J, Comai L. 2009. Dosage-dependent deregulation of an AGAMOUS-LIKE gene cluster contributes to interspecific incompatibility. Curr. Biol. 19, 1128-1132. ( 10.1016/j.cub.2009.05.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roth M, Florez-Rueda AM, Städler T. 2019. Differences in effective ploidy drive genome-wide endosperm expression polarization and seed failure in wild tomato hybrids. Genetics 212, 141-152. ( 10.1534/genetics.119.302056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoute AI, Varenko V, King GJ, Scott RJ, Kurup S. 2012. Parental genome imbalance in Brassica oleracea causes asymmetric triploid block. Plant J. 71, 503-516. ( 10.1111/j.1365-313X.2012.05015.x) [DOI] [PubMed] [Google Scholar]
- 92.Lu J, Zhang C, Baulcombe DC, Chen ZJ. 2012. Maternal siRNAs as regulators of parental genome imbalance and gene expression in endosperm of Arabidopsis seeds. Proc. Natl Acad. Sci. USA 109, 5529-5534. ( 10.1073/pnas.1203094109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tiwari S, Spielman M, Schulz R, Oakey RJ, Kelsey G, Salazar A, Zhang K, Pennell R, Scott RJ. 2010. Transcriptional profiles underlying parent-of-origin effects in seeds of Arabidopsis thaliana. BMC Plant Biol. 10, 72. ( 10.1186/1471-2229-10-72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burkart-Waco D, Ngo K, Dilkes B, Josefsson C, Comai L. 2013. Early disruption of maternal–zygotic interaction and activation of defense-like responses in Arabidopsis interspecific crosses. Plant Cell 25, 2037-2055. ( 10.1105/tpc.112.108258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Batista RA, Figueiredo DD, Santos-Gonzalez J, Kohler C. 2019. Auxin regulates endosperm cellularization in Arabidopsis. Genes Dev. 33, 466-476. ( 10.1101/gad.316554.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paul P, et al. 2020. MADS78 and MADS79 are essential regulators of early seed development in rice. Plant Physiol. 182, 933-948. ( 10.1104/pp.19.00917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Figueiredo DD, Batista RA, Roszak PJ, Kohler C. 2015. Auxin production couples endosperm development to fertilization. Nat. Plants 1, 15184. ( 10.1038/nplants.2015.184) [DOI] [PubMed] [Google Scholar]
- 98.Ishimaru K, et al. 2013. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 45, 707-711. ( 10.1038/ng.2612) [DOI] [PubMed] [Google Scholar]
- 99.Dilkes BP, Spielman M, Weizbauer R, Watson B, Burkart-Waco D, Scott RJ, Comai L. 2008. The maternally expressed WRKY transcription factor TTG2 controls lethality in interploidy crosses of Arabidopsis. PLoS Biol. 6, e308. ( 10.1371/journal.pbio.0060308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia D, Fitz Gerald JN, Berger F. 2005. Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis. Plant Cell 17, 52-60. ( 10.1105/tpc.104.027136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scott RJ, Tratt JL, Bolbol A. 2013. Seed development in interploidy hybrids. In Polyploid and hybrid genomics (eds Chen ZJ, Birchler JA), pp. 271-290. Oxford, UK: Wiley. [Google Scholar]
- 102.Johnson CS, Kolevski B, Smyth DR. 2002. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 14, 1359-1375. ( 10.1105/tpc.001404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK. 2001. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 126, 524-535. ( 10.1104/pp.126.2.524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hsieh TF, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, Zilberman D. 2009. Genome-wide demethylation of Arabidopsis endosperm. Science 324, 1451-1454. ( 10.1126/science.1172417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zemach A, Kim MY, Silva P, Rodrigues JA, Dotson B, Brooks MD, Zilberman D. 2010. Local DNA hypomethylation activates genes in rice endosperm. Proc. Natl Acad. Sci. USA 107, 18 729-18 734. ( 10.1073/pnas.1009695107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lauria M, Rupe M, Guo M, Kranz E, Pirona R, Viotti A, Lund G. 2004. Extensive maternal DNA hypomethylation in the endosperm of Zea mays. Plant Cell 16, 510-522. ( 10.1105/tpc.017780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brandvain Y, Haig D. 2005. Divergent mating systems and parental conflict as a barrier to hybridization in flowering plants. Am. Nat. 166, 330-338. ( 10.1086/432036) [DOI] [PubMed] [Google Scholar]
- 108.Trivers RL. 1974. Parent–offspring conflict. Am. Zool. 14, 249-264. ( 10.1093/icb/14.1.249) [DOI] [Google Scholar]
- 109.Haig D. 1987. Kin conflict in seed plants. Trends Ecol. Evol. 2, 337-340. ( 10.1016/0169-5347(87)90110-8) [DOI] [PubMed] [Google Scholar]
- 110.Queller DC. 1983. Kin selection and conflict in seed maturation. J. Theor. Biol. 100, 153-172. ( 10.1016/0022-5193(83)90099-1) [DOI] [Google Scholar]
- 111.Raunsgard A, Opedal ØH, Ekrem RK, Wright J, Bolstad GH, Armbruster WS, Pélabon C. 2018. Intersexual conflict over seed size is stronger in more outcrossed populations of a mixed-mating plant. Proc. Natl Acad. Sci. USA 115, 11 561-11 566. ( 10.1073/pnas.1810979115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Willi Y. 2013. The battle of the sexes over seed size: support for both kinship genomic imprinting and interlocus contest evolution. Am. Nat. 181, 787-798. ( 10.1086/670196) [DOI] [PubMed] [Google Scholar]
- 113.Cailleau A, Grimanelli D, Blanchet E, Cheptou P-O, Lenormand T. 2018. Dividing a maternal pie among half-sibs: genetic conflicts and the control of resource allocation to seeds in maize. Am. Nat. 192, 577-592. ( 10.1086/699653) [DOI] [PubMed] [Google Scholar]
- 114.Lafon-Placette C, Kohler C. 2016. Endosperm-based postzygotic hybridization barriers: developmental mechanisms and evolutionary drivers. Mol. Ecol. 25, 2620-2629. ( 10.1111/mec.13552) [DOI] [PubMed] [Google Scholar]
- 115.Hardigan MA, Bamberg J, Buell CR, Douches DS. 2015. Taxonomy and genetic differentiation among wild and cultivated germplasm of Solanum sect. Petota. Plant Genome 8, plantgenome2014.06.0025. ( 10.3835/plantgenome2014.06.0025) [DOI] [PubMed] [Google Scholar]
- 116.Brandvain Y, Van Cleve J, Ubeda F, Wilkins JF. 2011. Demography, kinship, and the evolving theory of genomic imprinting. Trends Genet. 27, 251-257. ( 10.1016/j.tig.2011.04.005) [DOI] [PubMed] [Google Scholar]
- 117.Wang L, Yuan J, Ma Y, Jiao W, Ye W, Yang DL, Yi C, Chen ZJ. 2018. Rice interploidy crosses disrupt epigenetic regulation, gene expression, and seed development. Mol. Plant. 11, 300-314. ( 10.1016/j.molp.2017.12.006) [DOI] [PubMed] [Google Scholar]
- 118.Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. 1998. Maternal control of embryogenesis by MEDEA a Polycomb group gene in Arabidopsis. Science 280, 446-450. ( 10.1126/science.280.5362.446) [DOI] [PubMed] [Google Scholar]
- 119.Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL. 1999. Imprinting of the MEDEA Polycomb gene in the Arabidopsis endosperm. Plant Cell 11, 1945-1952. ( 10.1105/tpc.11.10.1945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A. 2000. Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc. Natl Acad. Sci. USA 97, 10 637-10 642. ( 10.1073/pnas.170292997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Danilevskaya ON, Hermon P, Hantke S, Muszynski MG, Kollipara K, Ananiev EV. 2003. Duplicated fie genes in maize: expression pattern and imprinting suggest distinct functions. Plant Cell 15, 425-438. ( 10.1105/tpc.006759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo M, Platten D, Chaudhury A, Peacock WJ, Dennis ES. 2009. Expression, imprinting, and evolution of rice homologs of the Polycomb group genes. Mol. Plant 2, 711-723. ( 10.1093/mp/ssp036) [DOI] [PubMed] [Google Scholar]
- 123.Bretagnolle F, Thompson JD. 1995. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 129, 1-22. ( 10.1111/j.1469-8137.1995.tb03005.x) [DOI] [PubMed] [Google Scholar]
- 124.De Storme N, Copenhaver GP, Geelen D. 2012. Production of diploid male gametes in Arabidopsis by cold-induced destabilization of postmeiotic radial microtubule arrays. Plant Physiol. 160, 1808-1826. ( 10.1104/pp.112.208611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Storme N, Geelen D. 2014. The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant Cell Environ. 37, 1-18. ( 10.1111/pce.12142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pécrix Y, Rallo G, Folzer H, Cigna M, Gudin S, Le Bris M. 2011. Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. J. Exp. Bot. 62, 3587-3597. ( 10.1093/jxb/err052) [DOI] [PubMed] [Google Scholar]
- 127.Brochmann C, Brysting AK, Alsos IG, Borgen L, Grundt HH, Scheen A-C, Elven R. 2004. Polyploidy in arctic plants. Biol. J. Linn. Soc. 82, 521-536. ( 10.1111/j.1095-8312.2004.00337.x) [DOI] [Google Scholar]
- 128.Mason AS, Nelson MN, Yan G, Cowling WA. 2011. Production of viable male unreduced gametes in Brassica interspecific hybrids is genotype specific and stimulated by cold temperatures. BMC Plant Biol. 11, 103. ( 10.1186/1471-2229-11-103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Comai L. 2005. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 6, 836-846. ( 10.1038/nrg1711) [DOI] [PubMed] [Google Scholar]
- 130.Rieseberg LH, Willis JH. 2007. Plant speciation. Science 317, 910-914. ( 10.1126/science.1137729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schinkel CCF, Kirchheimer B, Dullinger S, Geelen D, De Storme N, Hörandl E. 2017. Pathways to polyploidy: indications of a female triploid bridge in the alpine species Ranunculus kuepferi (Ranunculaceae). Plant Syst. Evol. 303, 1093-1108. ( 10.1007/s00606-017-1435-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kovalsky IE, Roggero Luque JM, Elías G, Fernández SA, Solís Neffa VG. 2018. The role of triploids in the origin and evolution of polyploids of Turnera sidoides complex (Passifloraceae, Turneroideae). J. Plant Res. 131, 77-89. ( 10.1007/s10265-017-0974-9) [DOI] [PubMed] [Google Scholar]
- 133.Husband BC. 2004. The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol. J. Linn. Soc. 82, 537-546. ( 10.1111/j.1095-8312.2004.00339.x) [DOI] [Google Scholar]
- 134.Henry IM, Dilkes BP, Young K, Watson B, Wu H, Comai L. 2005. Aneuploidy and genetic variation in the Arabidopsis thaliana triploid response. Genetics 170, 1979-1988. ( 10.1534/genetics.104.037788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bjerkan KN, et al. 2020. Genetic variation and temperature affects hybrid barriers during interspecific hybridization. Plant J. 101, 122-140. ( 10.1111/tpj.14523) [DOI] [PubMed] [Google Scholar]
- 136.Nakel T, Tekleyohans DG, Mao Y, Fuchert G, Vo D, Groß-Hardt R. 2017. Triparental plants provide direct evidence for polyspermy induced polyploidy. Nat. Commun. 8, 1033. ( 10.1038/s41467-017-01044-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grossniklaus U. 2017. Polyspermy produces tri-parental seeds in maize. Curr. Biol. 27, R1300-R1302. ( 10.1016/j.cub.2017.10.059) [DOI] [PubMed] [Google Scholar]
- 138.Toda E, Okamoto T. 2020. Polyspermy in angiosperms: its contribution to polyploid formation and speciation. Mol. Reprod. Dev. 87, 374-379. ( 10.1002/mrd.23295) [DOI] [PubMed] [Google Scholar]
- 139.Mao Y, Gabel A, Nakel T, Viehöver P, Baum T, Tekleyohans DG, Vo D, Grosse I, Groß-Hardt R. 2020. Selective egg cell polyspermy bypasses the triploid block. eLife 9, e52976. ( 10.7554/eLife.52976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kreiner JM, Kron P, Husband BC. 2017. Frequency and maintenance of unreduced gametes in natural plant populations: associations with reproductive mode, life history and genome size. New Phytol. 214, 879-889. ( 10.1111/nph.14423) [DOI] [PubMed] [Google Scholar]
- 141.Rausch JH, Morgan MT. 2005. The effect of self-fertilization, inbreeding depression, and population size on autopolyploid establishment. Evolution 59, 1867-1875. ( 10.1111/j.0014-3820.2005.tb01057.x) [DOI] [PubMed] [Google Scholar]
- 142.Baack EJ. 2005. To succeed globally, disperse locally: effects of local pollen and seed dispersal on tetraploid establishment. Heredity 94, 538-546. ( 10.1038/sj.hdy.6800656) [DOI] [PubMed] [Google Scholar]
- 143.Sicard A, Lenhard M. 2011. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Ann. Bot. 107, 1433-1443. ( 10.1093/aob/mcr023) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.