Abstract
The Republic of Congo (RoC) is one of the African countries with the most histoplasmosis cases reported. This review summarizes the current status regarding epidemiology, diagnostic tools, and treatment of histoplasmosis in the RoC. A computerized search was performed from online databases Medline, PubMed, HINARI, and Google Scholar to collect literature on histoplasmosis in the RoC. We found 57 cases of histoplasmosis diagnosed between 1954 and 2019, corresponding to an incidence rate of 1–3 cases each year without significant impact of the AIDS epidemic in the country. Of the 57 cases, 54 (94.7%) were cases of Histoplasma capsulatum var. duboisii (Hcd) infection, African histoplasmosis. Three cases (5.3%) of Histoplasma capsulatum var. capsulatum infection were recorded, but all were acquired outside in the RoC. The patients’ ages ranged between 13 months to 60 years. An equal number of cases were observed in adults in the third or fourth decades (n = 14; 24.6%) and in children aged ≤15 years. Skin lesions (46.3%), lymph nodes (37%), and bone lesions (26%) were the most frequent clinical presentations. Most diagnoses were based on histopathology and distinctive large yeast forms seen in tissue. Amphotericin B (AmB) was first line therapy in 65% of the cases and itraconazole (25%) for maintenance therapy. The occurrence of African histoplasmosis in apparently normal children raises the possibility that African histoplasmosis is linked to environmental fungal exposure.
Author summary
The Republic of Congo (RoC) is one of the African countries with the most histoplasmosis cases reported. Here, we review what is published regarding epidemiology, diagnostic tools, and treatment of histoplasmosis in the RoC. We found 57 cases of histoplasmosis diagnosed between 1954 and 2019, corresponding to an incidence rate of 1–3 cases each year. There was no relationship with the increasing rates of HIV in the country. Most of the 57 cases we found (95%) were cases of African histoplasmosis caused by the fungus Histoplasma capsulatum var. duboisii. Those affected varied in age from 13 months to 60 years, with equal numbers observed in adults in their third or fourth decades and in children (approximately 25% each). Skin lesions (46%), enlarged lymph nodes (37%), and bone lesions (26%) were the most frequent clinical presentations. The diagnosis was usually based on histopathology with distinctive large yeast forms seen in tissue. Amphotericin B (AmB) and itraconazole (25%) were used for therapy. African histoplasmosis in apparently normal children raises the possibility that this disease is linked to environmental fungal exposure.
Introduction
Histoplasmosis is an endemic mycosis due to a dimorphic fungus named Histoplasma capsulatum [1]. Its distribution is worldwide [2,3], but Africa is unique in regard to this infection [4] as 2 clinical entities coexist due to Histoplasma capsulatum var. capsulatum (Hcc) and Histoplasma capsulatum var. duboisii (Hcd), the cause of “African Histoplasmosis.” Indeed, while the geographic distribution of the former encompasses South and North America, Asia, and Africa, the latter has only been reported in patients living or having lived in Africa [4,5].
While the HIV/AIDS pandemic and the increased use of immunosuppressive agents clearly demonstrated the opportunistic behavior of Hcc. Indeed, there were reports of cases in previously ‘‘non-endemic areas” revealing the global distribution of histoplasmosis in Africa [5] and throughout the world [2,3,6,7]. In countries where patients have limited access to diagnostic testing and antiretroviral therapies (ARTs), histoplasmosis is probably an important cause of mortality in persons living with HIV/AIDS. Histoplasmosis, particularly Hcd, has not been adopted by the World Health Organisation as a neglected tropical disease (NTD) (https://www.who.int/neglected_diseases/diseases/en/) despite early consideration [8] and was also rejected by G-Finder, despite a formal application (Global action fund for fungal infections, 2016).
In the Republic of Congo (RoC), the health epidemiological profile is characterized by the predominance of infectious diseases, mainly malaria, TB, and HIV/AIDS infections [9]. Many studies have been published since the description of the first case of Hcd infection reported in 1954 in the RoC [10]. However, the real problem of histoplasmosis in the RoC is underestimated, as the available information on this disease comes from case reports, case series, or old reviews published in the literature.
The knowledge gap of the local epidemiology of this disease is a serious limitation for effective infection control and treatment approaches. Therefore, we performed in this study an exhaustive review of the published cases from 1950 to 2019, including some cases published in non-English language, to provide the current status of histoplasmosis in the RoC regarding the epidemiology, diagnostic tools, and treatment.
Methods
Ethics statement
This analysis did not deal with individual patient data but with published data, which does not require regulatory approval.
Search strategy and eligibility criteria
Computerized literature searches for publications on histoplasmosis cases or series in the RoC were performed using online databases Medline, HINARI, and PubMed. The search engine used the key words and the detailed medical subject heading (MeSH) terms to identify all published papers: “Histoplasmosis,” “Congo,” “Brazzaville,” “HIV/AIDS,” “diagnosis,” “epidemiology,” “Africa,” or “Sub-Saharan Africa.” The Boolean operators “AND” and “OR” were used to combine 2 or 3 terms. All included studies were cases and series reports (n = 57) originating from the RoC. Although peer review articles published in the English language were included, most were in French language. No date limitation or any other search criteria were applied to avoid missing papers published in the RoC. Systematically, we searched Google Scholar and gray literature papers regarding the subject, to supplement Medline and PubMed searches. Some cases reports were excluded from some parts of our analysis due to the lack of information related to sex, age, clinical presentation, and treatment.
Further review of relevant individual cited references identified additional cases published in French-language journals such as the Journal of Medical Mycology (formerly Journal de Mycologie Médicale) et Armée, Médecine d’Afrique Noire, Médecine Tropicale, or Bulletin de la Société de Pathologie Exotique.
Results
Cases described from the Republic of Congo
Epidemiology
Our exhaustive literature search revealed a total of 57 diagnosed cases of histoplasmosis, including 54 cases (94.7%) of Hcd infection and 3 Hcc, were reported in the RoC between 1954 and 2019 (Tables 1 and 2). All were reported as single case report but 1 series of 11 cases reported by Carme and colleagues in 1990 [11]. The documentation of these cases varies from one study to the other and also from one case to another within the same study. For example, in 13 and 14 cases, sex or age, and clinical presentation (12 cases) were lacking. Also, the treatment of patients was not specified in 17 cases. Thus, those cases were excluded from some parts of our analysis. This total of cases represents a mean incidence of 1 to 3 cases each year, without significant change over the years, notably in regard to the AIDS epidemic (Fig 1).
Table 1. Description of 54 cases of Hcd infection in the RoC.
| Age/ Sex | No. of cases | Years | Origin areas | Occupation | HIV infection | Site of infection | Disseminated form | Treatment | Posology | Outcome | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| -/M | 1 | 1954 | Urban | NS | NT | Brain, Skin (Subcutaneous) | Yes | Terramycine, pentamidine | 1.5 g/day for 1 month | Good general condition. Chronic evolution of the disease |
Audebaud et al. [10] |
| 15/F | 1 | 1970 | Rural | NS | NT | Skin | NS | NS | NS | NS | Destombes et al. [12] |
| NS | 1 | 1967 | NS | NS | NT | Skin | NS | NS | NS | NS | Renoirte et al. [13] |
| 44/M | 1 | 1981 | Rural | Farmer | NT | Skin | No | AmB | 0.6 to 1 mg/kg to 1 mg/kg in 1 day over 2. Given as an infusion | Favorable outcome | Carme et al. [14] |
| 6/F | 1 | 1982 | Rural | Pupil | NT | Skin | No | ||||
| 36/M | 1 | 1983 | Rural | Farmer | NT | Skin, mucosa | No | ||||
| 13/F | 1 | 1983 | Rural | Pupil | NT | Mucosa, Bone | No | ||||
| 25/M | 1 | 1983 | Rural | Unemployed | NT | Skin | No | ||||
| 45/M | 1 | 1984 | Rural | NS | NT | Skin | No | ||||
| 11/M | 1 | 1984 | Urban | NS | NT | Skin, Bone | Yes | AmB | 1.6 g/24 h every 2 days | Favorable outcome | Griffet et al. [15] |
| 26/F | 1 | 1985 | NS | Unemployed | NT | Skin | No | AmB | 0.6 to 1 mg/kg to 1 mg/kg in 1 day over 2. Given as an infusion | Favorable clinical response | Carme et al. [14] |
| 27/M | 1 | 1986 | NS | Farmer | Negative | Skin | No | ||||
| 2/F | 1 | 1987 | NS | - | NT | Skin, Bone, Eye | Yes | AmB | |||
| 13/M | 1 | 1988 | NS | Pupil | NT | Skin, Bone, Shoulder | Yes | AmB | |||
| 17/M | 1 | 1989 | NS | Pupil | Negative | Skin | No | AmB | |||
| 17/M | 1 | 1989 | NS | Pupil | Negative | Skin, mucosa | No | ||||
| 26/M | 1 | 1990 | NS | Student | Positive | Skin | Yes | AmB KETO |
0.6 to 1 mg/kg to 1 mg/kg in 1 day over 2. Given as an infusion | Death | |
| 50/M | 1 | 1990 | NS | Farmer | Negative | Skin | Yes | AmB KETO |
0.6 to 1 mg/kg to 1 mg/kg in 1 day over 2. Given as an infusion | Death | |
| 13/M | 1 | 1991 | Rural | NS | NT | Bone | Yes | AmB, Surgery | 1.50 g Given as an infusion |
Favorable clinical response at months 3. No relapse at 1 year |
Moyikoua et al. [16] |
| NS | 11 | 1990 | NS | NS | Positive | NS | Yes | NS | NS | NS | Carme et al. [11] |
| 26/M | 1 | 1992 | NS | Student | Positive | Skin (cutaneous and subcutaneous) | Yes | NS | NS | Death | Carme et al. [17] |
| 4/M | 1 | 1995 | Urban | - | Negative | Lung, Skin, Bone | Yes | KETO AmB |
1/2 tablets/day 0.5 mg/kg × 2 days |
Favorable clinical response | Chandenier et al. [18] |
| 20/F | 1 | 1995 | Rural | NS | Positive | Skin | Yes | AmB ITRA |
1 mg/kg × 2 days 300 mg/days |
Death | |
| 44/M | 1 | 1995 | Urban | Driver | Positive | Skin | Yes | KETO AmB |
600 mg/days for 2 months and half. 1 mg/kg, 3 days/week. Given as an infusion |
Death | |
| 45/M | 1 | 1995 | Urban | NS | NT | Skin | - | NS | NS | Death | |
| 41/M | 1 | 1995 | Urban | NS | Positive | Skin | Yes | AmB | 1mg/kg/week | NS | |
| 32/M | 1 | 1995 | Urban | Nurse | Positive | Skin, Bone | Yes | AmB | 1mg/kg/week | NS | |
| NS | 1 | 2004 | Urban | NS | Positive | NS | Yes | NS | NS | Ondzotto et al. [19] | |
| 17/M | 1 | 2004 | Urban | NS | Negative | Skin, Bone | Yes | AmB | 0.25 mg/kg. Given as intravenous infusion. Increased by 5mg/days to a maximum of 1 mg/kg/day |
Favorable clinical response at months 11 | N’Golet et al. [20] |
| 33/F | 1 | 2006 | Urban | NS | Positive | Skin | Yes | Liposomal AmB, ITRA |
3 mg/kg per day 400 mg/day |
Death | Therby et al. [21] |
| 60/F | 1 | 2006 | Rural | Farmer | Negative | Skin, Bone | Yes | AmB, Terbinafine |
1mgkg/day for 30 days 250 mg/day |
Favorable clinical response at months 8 | Ngatse-Oko et al. [22] |
| 41/M | 1 | 2006 | Urban | NS | Positive | Skin | Yes | AmB ITRA |
NS | Favorable outcome. No relapse at 6.5 years. |
Breton et al. [23] |
| 15/M | 1 | 2010 | Urban | NS | Negative | Skin, Lung | Yes | None | - | Death | Okoko et al. [24] |
| 1/M | 1 | 2011 | Urban | - | Negative | Eye | No | AmB | 0.5 mg/kg Given as intravenous |
Favorable outcome at months 6 | Eboulabeka et al. [25] |
| 9/F | 1 | 2017 | Urban | NS | Negative | Skin | Yes | KETO | 200 mg × 2 /days | Insidious outcome at month 1. Death at months 3 |
Babela et al [26] |
| 3/M | 1 | 2017 | Urban | - | Negative | Skin | Yes | ITRA | 200 mg/day in per os | Insidious outcome at day 21. Death at months 2 |
|
| 4/M | 1 | 2017 | Urban | - | Negative | Bone | Yes | ITRA | 200 mg/day in per os | Insidious outcome at day 28. Death at months 3 |
|
| 7/F | 1 | 2014 | Urban | NS | Negative | Skin, Bone | Yes | Surgery, ITRA | 400 mg × 2/days. Given orally | Favorable clinical response at months 3 | Paugam et al. [27] |
| 30/F | 1 | 2019 | Urban | Unemployed | Negative | Skin | No | Surgery, ITRA | 800 mg/days for 12 weeks | Favorable outcome | Boukassa et al. [28] |
| 29/F | 1 | 2019 | Urban | Secretary | Negative | Skin | No | Surgery, ITRA | 800 mg/days for 12 weeks | ||
| 60/M | 1 | 2019 | Urban | Administrator | Negative | Skin, lung | Yes | Surgery, ITRA | 800 mg/days for 12 weeks | Death | |
| 52/M | 1 | 2019 | Urban | Construction worker | Negative | Skin, lung | Yes | Surgery, ITRA | 800 mg/days for 12 weeks | Death | |
| 34/M | 1 | 2019 | Urban | Sawyer | Negative | Skin, lung | Yes | None | - | Death | |
| 30/F | 1 | 2018 | Urban | NS | Negative | Skin, bone | Yes | ITRA | 800 mg/days for 12 weeks | Favorable outcome | Boukassa et al. [29] |
AmB, Amphotericin B; ITRA, Itraconazole; KETO, Ketoconazole; NS, not specified; NT, not tested.
Urban referred to the capital city, Brazzaville.
Table 2. Description of 3 cases of Hcc infection in the RoC.
| Age/ Sex | No. of cases | Years | Origin areas | Occupation | HIV infection | Site of infection | Disseminated form | Treatment | Posology | Outcome | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 49/M | 1 | 1978 | Urban | NS | NT | Mouth, liver | Yes | AmB | NS | Lost to follow-up | Lapèze et al. [30] |
| 22/F | 1 | 1984 | Urban | NS | Negative | Skin | Yes | AmB | NS | Death | Carme et al. [31] |
| 46/M | 1 | 1991 | Urban | NS | Positive | Skin | Yes | AmB | NS | Favorable outcome | Jaussaud et al. [32] |
AmB, Amphotericin B; NS, not specified; NT, not tested; RoC, Republic of Congo.
Fig 1. Cases of histoplasmosis reported from the Republic of Congo (1954–2019).
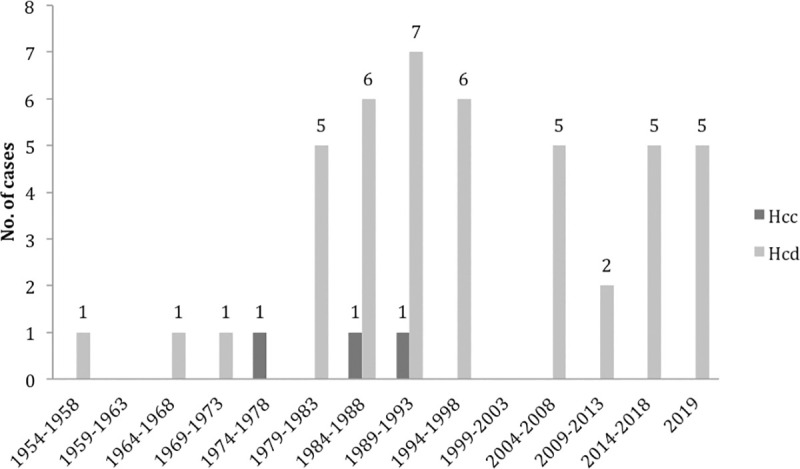
Light grey bars: H. capsulatum var duboisii. Dark grey bars: H. capsulatum var capsulatum.
Of the 57 cases of histoplasmosis, we found males (n = 30; 52.6%) to be as frequently infected as females (n = 14; 24.6%) with the sex ratio (M/F) at 2.1. The mean age and the median age were calculated at 24 ± 17.6 and 22 years, respectively. The patient’s age ranged between 13 months and 60 years.
An equal number of cases were observed in adults in the third or fourth decades (n = 14; 24.6%) and in children aged ≤15 years (Tables 1 and 2). Eight patients were under 10 years. The HIV testing results were mentioned for 7 children and none were HIV–positive. In 10 children, Hcd infection was disseminated.
Of the 54 Hcd cases, 2 cases were diagnosed in Congolese (from the RoC) expatriates living in France, a man arrived 15 years previously [23] and a woman for some months [21]. A single case of Hcd-disseminated infection was diagnosed in the RoC in a foreign national, a Chadian man living in the RoC for an indeterminate time [10]. In contrast, 51 (94.4%) of the 54 cases were definitely acquired in the RoC, as these patients had never traveled outside the country. No documented exposure to caves was reported in those cases and no clustering of cases was evident. Only 5 cases (9.3%) reported being farmers as their occupation in our series. The result for HIV testing in 54 cases of Hcd infection is mentioned for 39 patients of whom 20 were HIV–infected (51.3%). In adults, 9 cases (15.8%) of Hcd infection were associated with HIV/AIDS. In 38 cases (70.4%), patients had Hcd-disseminated infection indicated by infection of at least 2 noncontiguous body sites.
We found 3 cases (5.3%) of Hcc-disseminated infection out of the 57 cases. From these, 2 cases were diagnosed in patients of foreign nationality living in the RoC [30,31] and 1 case in a Congolese (from the RoC) patient living in France [32]. One of those cases had a disseminated infection in the context of HIV infection [30]. The first case was diagnosed in 1984 in a 22-year-old young Zairean woman (people of the Democratic Republic of Congo, formerly Belgian Congo or Zaïre) living in the RoC for an indeterminate period [31]. The second case was reported in 1991 in a Congolese AIDS patient living in France with an infection suspected to be acquired in Central Africa [32]. In the last case, Lapèze and colleagues reported a case of Hcc-disseminated infection occurring in a French patient living in the RoC for 3 years with a history of traveling and living in Indonesia (6 years), Chad (6 years), and Gabon (2 years).
Although there is no particular regional distribution, the possible origin of infection was notified for 29 patients (50.9%) out of the 57 cases of histoplasmosis reported in the RoC. Patients originated from urban areas (Brazzaville, the capital city) in 26 cases (45.6%) and only 3 cases have mentioned the rural origin of patients’ regions (Gamboma, Owando, Kinkala). Further related areas of rural origin were not mentioned in 28 cases (49.1%) and these cases were excluded from the map (Fig 2).
Fig 2. Distribution of reported cases of histoplasmosis in the RoC (1954–2019).
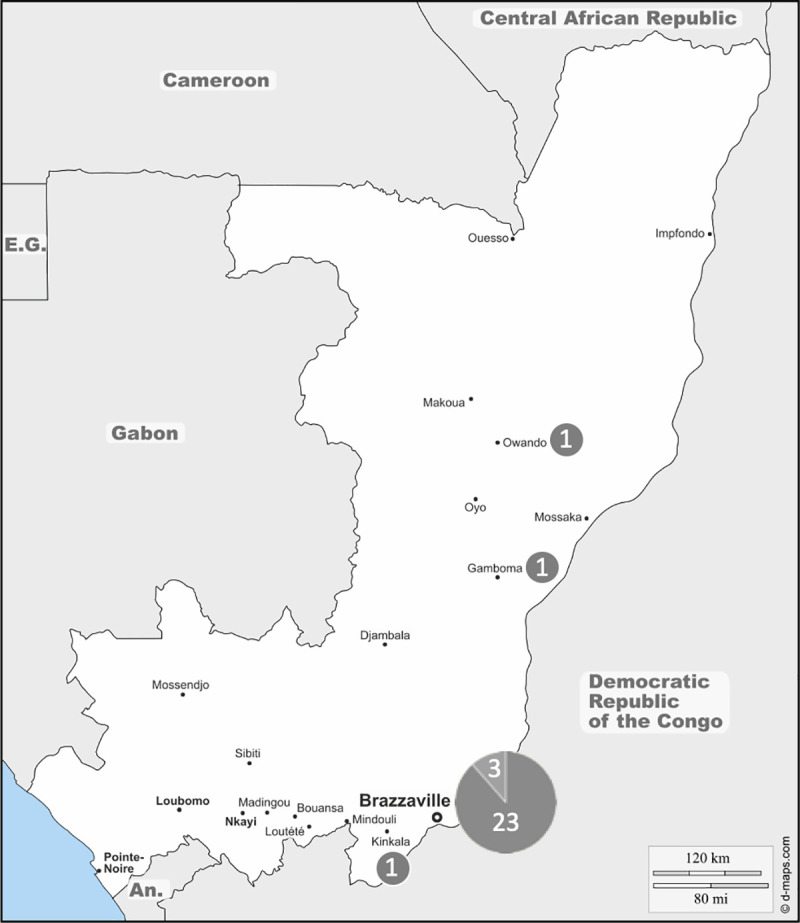
Note that the size of the pastilles is not correlated with the number of cases indicated within. (https://d-maps.com/carte.php?num_car=3378&lang=fr.) Dark gray: Histoplasma capsulatum var. duboisii. Light gray: Histoplasma capsulatum var. capsulatum.
Clinical presentation
The clinical features of the 54 cases of Hcd infection are presented in Table 3. Skin lesions (46.3%) including cutaneous and subcutaneous lesions, lymphadenopathy or lymph nodes (37%), and bone lesions (26%) were the most reported clinical presentations. As the most frequent type of lesion, cutaneous lesions resemble Molluscum contagiosum (15%) and usually take the form of umbilicated papules or nodules on the skin of limbs and face. Liver and spleen involvement was reported in 5 cases (9.3%). Lung and mucosal infection were reported with equal frequency (7.4%). Of the 14 pediatric cases of Hcd infection, the patients presented with bone lesions in 3 cases [14] of which 2 patients had major bone damage [16,27]. On X-ray, the images of bone lysis are observed, often resulting in the form of poorly defined bone cyst.
Table 3. Clinical presentation in 54 cases of Hcd infection in the RoC.
| Sex ratio (M/F) | 2.2 | |
|---|---|---|
| Mean age ± SD | 23 ± 17.4 | |
| Clinical manifestations | No. of cases | % |
| Skin lesions | 25 | 46.3 |
| Lymph nodes | 20 | 37 |
| Bone lesions | 14 | 25.9 |
| Molluscum contagiosum-like lesions | 8 | 14.8 |
| Liver/spleen involvement | 5 | 9.3 |
| Mucosal lesion | 4 | 7.4 |
| Lung disease | 4 | 7.4 |
| Adrenal enlargement | 1 | 1.9 |
Laboratory diagnosis
The diagnostic methods of Hcd infection are summarized in Fig 3. Mostly diagnosis was based on histopathology examination of biopsy specimen (25.9%) and mycological examination (Fig 4) of different fluids or skin scrapings in combination with histopathology (24.1%). A positive Histoplasma antigen was reported in a single case of Hcd-disseminated infection diagnosed, in France, in an HIV–positive woman originated from the RoC [21]. Diagnosis of Hcc infection was based on direct examination of mucopurulent serosities in 2 cases out of 3. Bone marrow revealed histiocytes with small oval elements surrounded by a clear halo in a single case of a Hcc-disseminated infection in an HIV–negative foreign woman living in the RoC [31].
Fig 3. Diagnostic methods used for the diagnosis of Hcd cases in the RoC since 1954.
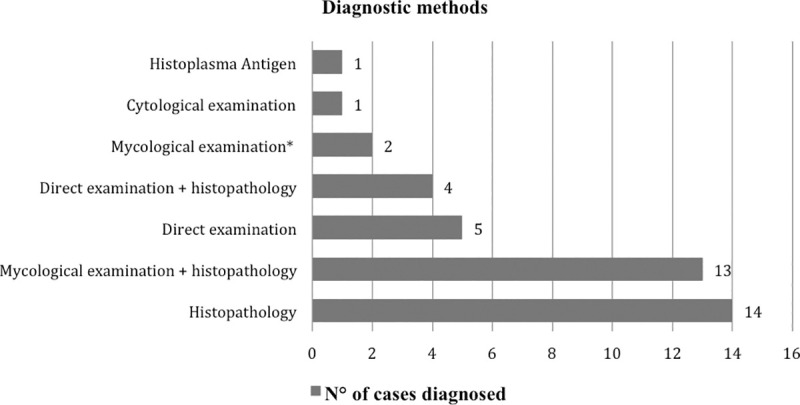
Mycological examination* = Direct examination and culture.
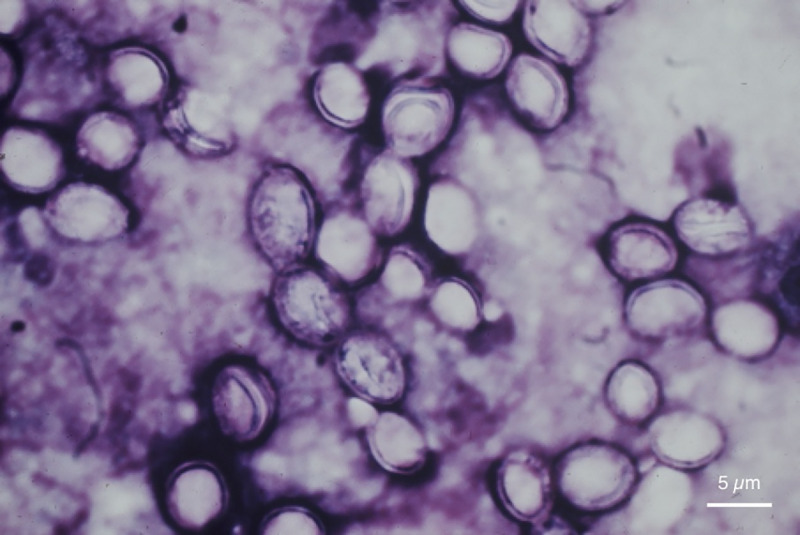
Fig 4. Direct examination of pus from a mesenteric adenomegaly showing large yeast characterized by a large budding site (Giemsa staining, original magnification ×400).
Treatment
Treatment information was described in 40 (70.2%) of 57 cases of histoplasmosis. Twenty-six patients (65%) were treated with intravenous amphotericin B (AmB) as first-line therapy. The use of itraconazole (ITRA) was reported only in cases of Hcd infection (n = 10) while 5 patients were treated with ketoconazole (KETO). In 6 cases of Hcd infection, the antifungal drug was combined with surgical treatment for example excision of necrosis and paravertebral and epidural abscesses. Terbinafine was used as a maintenance therapy after AmB in 1 case of Hcd infection with bone lesion [22]. All patients presented with Hcc infection (n = 3) were treated with AmB. Two patients did not receive any antifungal treatment, either due to the misdiagnosis of the disease in favor of tuberculosis or delayed diagnosis of a severe case leading to the rapid death of the patient.
Clinical outcome depended on a number of factors, such as the type of disease (Hcd or Hcc infection), early or prompt diagnosis, and accessibility to the effective drugs. Most cases with skin lesions (n = 20) including those with cutaneous and subcutaneous lesions and disseminated diseases (n = 10) resolved with AmB and ITRA, while response to KETO was poor leading to the death of patients (n = 4).
Discussion
Human histoplasmosis is due to 2 different fungal varieties in which geographical distribution differs, and that lead to different clinical presentations. However, molecular analysis reveals that strains of both varieties isolated in Africa belong to the same clade, suggesting that the orientation toward one or the other is driven by environmental conditions or mode of contamination rather than by intrinsic genetic characteristics [33]. To the best of our knowledge, no animal model of Hcd infection has yet been set up, that could support different virulence between the varieties. A major result of our study is the large dominance of Hcd infection in contrast to Hcc infection in the RoC. The first case of Hcd has been described in 1952 by Dubois and colleagues in a European man coming from the Democratic Republic of Congo (formerly, Belgian Congo or Zaïre), a border country of the RoC [10,34].
In regard to the total population of the country (5.2 million inhabitants), the number of Hcd infection (54 cases) gives a higher prevalence of this infection, unlike other countries in central Africa [5]. Considering the difficulties in diagnosis in our country, it is likely that this prevalence is underestimated. In contrast, the number of Hcc was few, as only 3 cases of uncertain geographical origin have been described from the RoC. In addition to a limitation in the availability of the most key diagnostic tools, one can imagine particular geoclimatic local conditions can play a role. Indeed, a remarkable annual rainfall, a high degree of humidity, and a reduced variation in diurnal temperature characterize the RoC climate. We did not observe any particular geographical distribution within the country in the cases reviewed. The possible source of infection was notified for 29 patients (50.9%), and a rural origin of the patients was only notified for 3. Whether or not local environments favor the “expression” of Hcd infection rather than Hcc infection required further studies. Finally, it should also be mentioned that facilities in the diagnosis of histoplasmosis are limited mainly to Brazzaville, the capital and largest city. Indeed, most of infected patients were from urban areas (Brazzaville) but there might be a bias because the population is predominantly urban (62.2%), and 56.5% of the total population lives in Brazzaville [35]. Also, the diagnosis can only be achieved in medical centers from urban areas. This contrasts with previous reports suggesting that Hcd infection is mainly a rural mycosis in other countries [26,36]. However, the increase in HIV–positive patients in the RoC probably promotes cases of histoplasmosis being seen in Brazzaville. Nevertheless, it should be noted that in central Africa, Zaïre was the main focus of Hcd infection with 26 cases. In contrast, all of the cases (n = 7) described before 1984 originated from a rural area (the plateaux and Pool region) of the RoC [15]. Although 5 cases were reported in farmers, the overall risk of Hcd infection is not well understood. Moreover, Carme and colleagues [14] reported that most patients with Hcd infection are farmers, most consistent with a soil-related acquisition. In the study from Democratic Republic of the Congo, which shares a border with the RoC, Pakasa and colleagues reported that 4 patients infected with Hcd had collected guano from bat roosts to fertilize gardens [37]. In the environment, Histoplasma may be found in so-called microfoci in endemic areas for histoplasmosis. The main characteristic of these microfoci is contamination with bird/or bat guano, notably caves, but the extent of its natural occurrence remains largely unexplored. Of note, in a recent review, no difference was found between the 2 varieties in terms of environmental and wilderness-related risk factors [38].
Interestingly, only 3 cases of Hcc infection were recorded and none of these cases seemed to be acquired in the RoC. Additional studies are warranted to explore more precisely the conditions leading to infection with one variety rather than the other.
Moreover, 14 cases of Hcd infection were recorded in children and none of them was HIV–positive. This finding agrees with that of previous reports [18,24–26]. A review of the risk factors by Lopez and colleagues revealed that environmental fungal exposure was the most important contributing risk factors to the acquisition of Hcd infection in children [39].
In Hcd infection cases from the RoC, skin lesion including cutaneous or subcutaneous and mucosa involvement and lymph nodes were the most common as noted by Carme and colleagues [14] and Develoux and colleagues [5]. The clinical manifestations of Hcd infection are more commonly the skin, subcutaneous tissues, lymph nodes and bones unlike Hcc infection which generally involves lungs and mucosa and rarely shows cutaneous or bone lesions. The cutaneous manifestations in Hcd infection are papular, nodular, ulcerative, eczematoid lesions [40]. However, the severity of the disease is related to the immunity status of patients. Nine Hcd infection cases (51.3%) in adults were associated with HIV/AIDS in the RoC. Similarly, Oladele and colleagues reported that the disease manifestations vary depending on immune status of patients, the number of fungal particles inhaled (particularly for Hcc), and the virulence of the infective strain [4]. A single RoC case of Hcc-disseminated infection was reported with a painful oral lesion on a background of leukoplakic mucosa [30].
Oral and laryngeal lesions are possible with Hcc infection. Most oral lesions occur in the Hcc-disseminated form of the disease and may affect any area of the oral cavity but are most commonly found on the tongue, palate, and buccal mucosa [41,42]. Patients may present with painful ulcers that persist for several weeks [30,41]. Hcd-disseminated forms (51.3%) were not frequently reported in HIV–infected patients. In the current decades, the rate of disseminated forms (60.6%) has significantly increased without correlation with HIV infection [5]. The bone lesions in Hcd infection are of a lytic type found at cranial, maxillary, femoral, tibial, and spinal bones where they simulated Pott disease (tuberculous osteomyelitis) [28]. All organs and tissues can be involved [43]; indeed, we found that skin, mucosa, bone, and lung were mostly involved in the Hcd infection.
The WHO-endorsed essential diagnostic tests (2019) for fungal diseases are direct microscopy and histopathology, fungal culture, blood culture, and Histoplasma antigen [44]. In the RoC, most of the reported cases were diagnosed by histopathology [20,21,45] and mycological examination (direct microscopy and culture) [14,21]. It is important to recall that due to the coexistence of the two H. capsulatum varieties in Africa, differential diagnosis cannot be made only based on culture, as it is similar for both. Only the demonstration of the ovoid budding yeast (Fig 4) with thick cell walls and much larger (6 to 12 μm in diameter) and intracellular fat droplets supports the identification of Hcd [46]. In addition, Hcc appears as a 2 to 4-μm narrow-based budding yeast on histopathology of a tissue specimen [46]. Thus, the histological appearances of Hcc must be differentiated from other microorganisms, principally Candida glabrata, Cryptococcus neoformans, Leishmania spp., Blastomyces dermatitis, Pneumocystis jirovecii, Talaromyces (formerly Penicillium) marneffei, Toxoplasma gondii, and Trypanosoma cruz [46]. Although, many of these organisms are not endemic and would only be seen in travelers abroad. Mostly, the characteristics that help distinguish these yeasts include predominant cellular location (intracellular for H. capsulatum, predominantly extracellular for C. glabrata), shape and size variation (uniform versus heterogenous), histopathologic response (granulomatous versus suppurative), and culture. Also, the use of specific histochemical stains such as Giemsa, hematoxylin and eosin, Gomori methenamine silver, and periodic acid-Schiff facilitates the differentiation of these pathogens [46]. Detection of Hcc is also possible in approximately 40% of blood smears of patients with disseminated histoplasmosis [43–45], though this was not yet reported in the RoC. While antigen detection and PCR emerge as valuable adjunctive diagnostic tools, those are not yet available in the RoC.
The treatment of histoplamosis is a challenge for physicians in most low- and middle-income countries including the RoC, where WHO-listed essential systemic antifungal drugs namely itraconazole, voriconazole, flucytosine, and AmB are not always available. Currently, only fluconazole, ketoconazole (withdrawn across most of the world because of toxicity), topical miconazole, griseofulvin, and nystatin are available in the RoC.
In our review, most patients were treated with intravenous AmB. The lack of itraconazole availability is a serious limitation as only fluconazole can be given for maintenance therapy in the RoC. Fluconazole has a lower efficacy compared to itraconazole in the treatment of histoplasmosis due to its lower bone penetration and lower intrinsic activity [47]. Symptoms of Hcd infection in children are similar to those that occur in adults, with some exceptions, thus treatment recommended for adults is considered for children.
Conclusions
Our study shows that histoplasmosis appears to have a higher rate of prevalence in the RoC compared with other countries in central Africa. Surprisingly, 94.7% of the cases were due to Hcd, and Hcc cases may have been infected out of the RoC. While both varieties cannot be distinguished based on molecular analysis, there is no clear explanation for the reasons leading infection with one or other. Currently the differentiation between the two varieties only relies on direct examination of biopsy specimens or pathologic fluids, and set-up of more standardized techniques allow this differentiation is desirable. This review also highlighted the importance of pediatric cases of histoplasmosis due to Hcd. This observation should also drive future studies to better understand the mode of contamination, which remains uncertain.
While most of the patients were treated with intravenous AmB, itraconazole should be more widely available in Africa as it represents a valuable alternative for ambulatory treatment.
Acknowledgments
The authors are grateful to Prof. Chandenier Jacques of the Centre Hospitalier Universitaire Bretonneau, Tours, France for providing the old published articles on histoplasmosis in the RoC, which were lacking for this review.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Histoplasmosis HC. Infect Dis Clin N Am. 2016;30(1):207–27. [DOI] [PubMed] [Google Scholar]
- 2.Bahr NC, Antinori S, Wheat LJ, Sarosi GA. Histoplasmosis Infections Worldwide: Thinking Outside of the Ohio River Valley. Curr Trop Med Rep. 2015;2(2):70–80. 10.1007/s40475-015-0044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker J, Setianingrum F, Wahyuningsih R, Denning DW. Mapping histoplasmosis in South East Asia–implications for diagnosis in AIDS. Emerg Microbes Infect. 2019;8(1):1139–45. 10.1080/22221751.2019.1644539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oladele RO, Ayanlowo OO, Richardson MD, Denning DW. Histoplasmosis in Africa: An emerging or a neglected disease? PLoS Negl Trop Dis. 2018;12(1):e0006046. 10.1371/journal.pntd.0006046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Develoux M, Amona FM, Hennequin C. Histoplasmosis caused by Histoplasma capsulatum var. duboisii: a comprehensive review of cases from 1993 to 2019. Clin Infect Dis. 2020;ciaa1304. 10.1093/cid/ciaa1304 [DOI] [PubMed] [Google Scholar]
- 6.Pan B, Chen M, Pan W, Liao W. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses. 2013;56(3):212–21. 10.1111/myc.12029 [DOI] [PubMed] [Google Scholar]
- 7.Randhawa H. Occurrence of histoplasmosis in Asia. Mycopathol Mycol Appl 1970;41(1–2):75–89. 10.1007/BF02051485 [DOI] [PubMed] [Google Scholar]
- 8.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of Neglected Tropical Diseases. N Engl J Med. 2007;357(10):1018–27. 10.1056/NEJMra064142 [DOI] [PubMed] [Google Scholar]
- 9.UNAIDS Country factsheets CONGO | 2018 HIV and AIDS Estimates 2018; Available from: https://www.unaids.org/en/regionscountries/countries/congo
- 10.Audebaud G, Merveille P, Laluque P, Depoux R. On the first case of histoplasmosis in AEF. Bull Soc Pathol Exot. 1954;47(6):803–8. [PubMed] [Google Scholar]
- 11.Carme B, Ngolet A, Ebikili B, Itoua AI. African histoplasmosis an opportunistic fungal infection in AIDS? Trans R Soc Trop Med Hyg. 1990;84(2):293. 10.1016/0035-9203(90)90292-m [DOI] [PubMed] [Google Scholar]
- 12.Destombes P, Ravisse P, Nazimoff O. Assessment of deep mycoses established in twenty years of histopathology at the Pasteur Institute of Brazzaville. Bull Soc Pathol Exot. 1970;63:315–23. [PubMed] [Google Scholar]
- 13.Renoirte R, Michaux JL, Gatti F, Vanbreuseghem R, Bastin JP, Drexler L, et al. New cases of African histoplasmosis and cryptococcosis observed in the Republic of the Congo. Bull Acad R Med Belg. 1967;7(5):465–527. [PubMed] [Google Scholar]
- 14.Carme B, Hayette M, Ngaporo AI, Ngolet A, Darozzin F, Moyikoua A, et al. African histoplasmosis due to Histoplasma duboisii (Histoplasma capsulatum var. duboisii): fourteen Congolese cases observed in 10 years (1981–1990). J Mycol Med. 1993;1991(40):67–73. [Google Scholar]
- 15.Griffet P, Poaty-Mapakou C, Bouyou-Mananga E, Samson P. African histoplasmosis due to Histoplasma duboisii: report of an exemplary case with cutaneous and bone localization. Med armee. 1984;12(1):679. [Google Scholar]
- 16.Moyikoua A, Carme B, Ngolet A, Pena-Pitra B. African histoplasmosis: report of a case of osteoarthritis of the shoulder. Med Afr Noire. 1991;3S:372–6. [Google Scholar]
- 17.Carme B, Ngaporo AI, Ngolet A, Ibara JR, Ebikili B. Disseminated African histoplasmosis in a Congolese patient with AIDS. J Med Vet Mycol. 1992;30(3):245–8. 10.1080/02681219280000301 [DOI] [PubMed] [Google Scholar]
- 18.Chandenier J, Goma D, Moyen G, Samba-Lefèbvre MC, Nzingoula S. Obengui, et al. African histoplasmosis due to Histoplasma capsulatum var. duboisii: relationship with AIDS in recent Congolese cases. Sante. 1995;5(4):227–34. [PubMed] [Google Scholar]
- 19.Ondzotto G, JR I, Mowondabeka P, Galiba J. Cervico-facial and ENT symptoms due to HIV infection in tropical area. About 253 Congolese cases. Bull Soc Pathol Exot. 2004;97(1):59–63. [PubMed] [Google Scholar]
- 20.N’Golet A, N’Gouoni BG, Moukassa D, Nkoua-Mbon JB. Maxillary African histoplasmosis: unusual diagnostic problems of an unusual presentation. Pathol Res Pract. 2005;200(11–12):841–4. 10.1016/j.prp.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 21.Therby A, Polotzanu O, Khau D, Monnier S, Greder Belan A, Eloy O. Aspergillus galactomannan assay for the management of histoplasmosis due to Histoplasma capsulatum var. duboisii in HIV-infected patients: education from a clinical case. J Mycol Med. 2014;24(2):166–70. 10.1016/j.mycmed.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 22.Ngatse-Oko A, Péko JF, Ntsiba H, Ngolet A, Kokolo J, Ondzoto M, et al. Pathological fracture revealing an osseous histoplasmosis. A case report on a 60-year patient. Bull Soc Pathol Exot. 2006;99(4):227–9. [PubMed] [Google Scholar]
- 23.Breton G, Adle-biassette H, Audrey Therby, Ramanoelina J, Choudat L, Bissuel F, et al. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. AIDS 2006;20(1):119–32. 10.1097/01.aids.0000199014.66139.39 [DOI] [PubMed] [Google Scholar]
- 24.Okoko A, Bowassa GE, Oko A. Generalized histoplasmosis in a child immunocompetent to HIV. Med Afr Noire 2010;57:590–2. [Google Scholar]
- 25.Eboulabeka GK, Ngolet A. Fronto-orbital-palpebral swelling indicative of African histoplasmosis caused by Histoplasma capsulatum var duboisii in a 13-month-old infant. About a clinical observation and review of the literature. Med Afr Noire. 2011;58:144–8. [Google Scholar]
- 26.Babela J. M, Mandavo CM, Evrard RN, Ibara BO, Lamah L. African histoplamosis. A report of three pediatric cases. J Mycol Med 2017;27(2):133–8. 10.1016/j.mycmed.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 27.Paugam A, Pécoulas PE de, Bourée P. African young girl with sores of the elbow, cold abcesses and Molluscum-like cutaneous lesions. Lett Infect 2014;7(4):126–9. [Google Scholar]
- 28.Boukassa L, Ngackosso OB, Bambino SBK, Mbaki HBE, Monianga SN, Biatsi KM, et al. Cranial and Spinal Locations of Histoplasma capsulatum var. duboisii in Brazzaville, Congo. Iran J Neurosurg. 2019;5(2):63–9. [Google Scholar]
- 29.Boukassa L. S KB, HB E-M, OB N, JF P. Boukassa L, Kinata BS, Ekouélé-Mbaki HB, Ngackosso OB, Péko JF. Histoplasmosis caused by Histoplasma capsulatum var. duboisii: A rare cause of isolated scalp abscess. Heal Sci Dis. 2018;19(1):99–100. [Google Scholar]
- 30.Lapeze M, Bourgarel J. A case of histoplasmosis caused by Histoplasma capsulatum observed in Brazzaville. Bull Soc Med Afr Lgue Frse. 1973;23(3):214–8. [PubMed] [Google Scholar]
- 31.Carme B, Itoua-Ngaporo A, Bourgarel J, Poste B. Histoplasmosis caused by Histoplusma capsulatum. About a disseminated form with cutaneous localization in a Zairian woman. Bull Soc Pathol Exot. 1984;77:653–7. [PubMed] [Google Scholar]
- 32.Jaussaud R, Riche O, Puygauthier-toubas D, Rouger C, Strady A, Remy G, et al. Disseminated histoplamosis in AIDS: Three observations. Med Mal Infect. 1991;21(12):746–9. [Google Scholar]
- 33.Valero C, Gago S, Monteiro MC, A-I, Buitrago MJ. African histoplasmosis: new clinical and microbiological insights. Med Mycol. 2017;56(1):51–9. [DOI] [PubMed] [Google Scholar]
- 34.Dubois A, Janssens P, Brutsaert P, Vanbreuseghem R. A case of African histoplasmosis; with a mycological note on Histoplasma duboisii n.sp. Ann Soc Belg Med Trop (1920). 1952;32(6):569–84. [PubMed] [Google Scholar]
- 35.United Nations, Department of Economic and Social Affairs PD. Total population—both sexes. World Population Prospects 2019, Online Edition Rev 1. 2019; Available from: https://population.un.org/wpp/Download/Files/1_Indicators(Standard)/EXCEL_FILES/1_Population/WPP2019_POP_F01_1_TOTAL_POPULATION_BOTH_SEXES.xlsx
- 36.Chandenier J, Renard J, Goma D. Tropical mycoses diagnosed at the university hospital of Brazzaville (Congo), November 1991-July 1995, clinical, mycological and therapeutic aspects. J Mycol Med. 2000;10:67–77. [Google Scholar]
- 37.Pakasa N, Biber A, Nsiangana S, Imposo D, Sumaili E, Muhindo H, et al. African histoplasmosis in HIV-negative patients, Kimpese, Democratic Republic of the Congo. Emerg Infect Dis. 2018;24(11):7–9. 10.3201/eid2411.180236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz JH. Environmental and wilderness-related risk factors for histoplasmosis: More than bats in caves. Wilderness Environ Med 2018;29(4):531–40. 10.1016/j.wem.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 39.Lopez LF, Valencia Y, Tobon AM, Velasquez O, Santa CD, Caceres DH, et al. Childhood histoplasmosis in Colombia: Clinical and laboratory observations of 45 patients. Med Mycol. 2016;54(7):677–83. 10.1093/mmy/myw020 [DOI] [PubMed] [Google Scholar]
- 40.Gugnani HC, Muotoe-okafor F. African histoplasmosis: a review. Rev Iberoam Micol. 1997;14(4):155–9. [PubMed] [Google Scholar]
- 41.Folk GA, Oral Histoplasmosis NBL. Head Neck Pathol. 2017;11(4):513–6. 10.1007/s12105-017-0797-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Souza BC, Munerato MC. Oral manifestation of histoplasmosis on the palate. An Bras Dermatol. 2017;92(5 suppl 1):107–9. 10.1590/abd1806-4841.20175751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inojosa W, Rossi MC, Laurino L, Giobbia M, Fuser R, Carniato A, et al. Progressive disseminated histoplasmosis among human immunodeficiency virus-infected patients from West-Africa: report of four imported cases in Italy. Infez Med. 2011;19(1):49–55. [PubMed] [Google Scholar]
- 44.Hay R, Denning DW, Bonifaz A, Queiroz-telles F, Beer K, Bustamante B, et al. The diagnosis of fungal neglected tropical diseases (Fungal NTDs) and the role of investigation and laboratory tests: an expert consensus report. Trop Med Infect Dis. 2019;4(4):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borges-Costa J, Marques T, Soares-Almeida L, Sacramento-Marques M. Progressive disseminated histoplasmosis as a presentation of AIDS in a patient from the Congo: the role of skin biopsy. Trop Dr. 2011;41(4):251–2. [DOI] [PubMed] [Google Scholar]
- 46.Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55(6):1612–20. 10.1128/JCM.02430-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev. 2014;27(1):68–88. 10.1128/CMR.00046-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


