Helicobacter pylori infection and transmission routes
Helicobacter pylori is a gram-negative microaerophilic bacterium. The infection of H. pylori can increase the risk of gastric cancer which is the second leading cause of cancer death worldwide [1]. The World Health Organization’s International Agency for Research on Cancer (IARC) has classified H. pylori as a type I (definite) carcinogen since 1994. H. pylori infection is a global health problem. In developed countries, its infection rate is 20% to 50%, while in developing countries, the infection rate of middle-aged people has reached 80% [2]. The fecal–oral and the oral–oral routes are considered as the main transmission routes of H. pylori [3]. Nevertheless, only H. pylori genes have been detected in saliva and dental plaques, but culturable H. pylori has not been isolated yet in large quantities [4], indicating that there may be some new strategies of H. pylori to implement its transmission through oral cavity.
Candida albicans, a dimorphic fungus, is one of the most common fungi in the human body [5]. It was noteworthy that C. albicans and H. pylori were abundant in certain human niches, such as the root canal necrotic pulp, stomach, duodenum, and vagina [6], suggesting that C. albicans may interact with H. pylori to promote the growth, spread, and infection of H. pylori in some nonadaptive condition, such as the oral cavity and vagina.
The synergy between H. pylori and C. albicans in gastric diseases
H. pylori infection is positively correlated with yeast in gastric diseases [7]. A total of 36% gastric ulcers patients, 2% non-ulcerative dyspepsia patients, and 56% large-scale gastric ulcers (greater than 2 cm) patients with H. pylori have fungal co-colonization in the upper gastrointestinal tract, such as C. albicans and Candida krusei, indicating the strong relationship between fungi and H. pylori in ulcerative lesions [8]. C. albicans is highly correlated with H. pylori in gastric cancer, peptic ulcer, and chronic gastritis patients. The gastric ulcer patient with C. albicans and H. pylori in the stomach developed even larger ulcers. The presence of C. albicans was closely related to the prolongation of gastric diseases by the increase of healing time and persistence of clinical symptoms [9,10]. The strong positive correlation between C. albicans and H. pylori in the development of gastric diseases indicates their synergistic pathogenesis [7]. C. albicans may enhance the colonization, toxicity, and pathogenicity of H. pylori especially in gastrointestinal diseases through adhesion and the formation of a mixed species, like that C. albicans promotes the pathogenicity of other bacteria [11].
Potential for H. pylori residence within C. albicans vacuoles
H. pylori is an invading intracellular pathogen, and its entry into cells such as gastric epithelial cells, dendritic cells, and macrophages is one of the reasons for the failure of antibiotics treatment [12]. Interestingly, H. pylori was also found to enter C. albicans yeast cells. Moving “bacterial-like bodies” in the vacuoles of the C. albicans yeast cells from the stomach were observed, and these were identified as H. pylori by PCR and immunofluorescence [13–15]. These “invaded” C. albicans cells were able to survive from the exposure of high temperature, dryness, and antibiotics. The H. pylori in their vacuole showed an active state of motion under these conditions [13], suggesting that the internalization of H. pylori into C. albicans can protect H. pylori from strict conditions. Meanwhile, the invading H. pylori seems to be vertically transmitted to the daughter cells of C. albicans and continue to express its own proteins through its proliferation within the yeast cells [16]. The C. albicans containing H. pylori in this fast-moving state can also be observed from other body sites, such as the oral cavity and vagina [17]. The frequency of H. pylori–invaded C. albicans in the oral cavity of the normally born babies is higher than that of cesarean birth, indicating that C. albicans in the vagina may be the main reservoir for transmitting H. pylori to the newborns through their oral cavity [17]. It’s possible that C. albicans can act as a shelter and an oral transmission promoter for H. pylori [16,18,19]. Besides C. albicans, clinical isolates of Candida dubliniensis, C. krusei, and Candida tropicalis have also found the H. pylori internalization by amplifying the 16S rDNA of H. pylori [20,21], suggesting that this kind of interaction manner between H. pylori and yeast can occur in different species.
H. pylori–invaded C. albicans is not only widely distributed in the human body, but also abundantly in food, such as yogurt, grape juice, bread, preserves, fruits, and honey [22]. C. albicans may protect H. pylori against the environmental stresses in these habitats. The internalization into C. albicans could be a crucial strategy for H. pylori to survive and transmit in a variety of environments especially the nonadaptive conditions.
It’s still worthwhile to note that there are some moving volutin (polyphosphate) granules known as “dancing bodies” in the vacuoles of the C. albicans cells [23], which do not depend on the cell cycle phase, but on the growth stage, metabolic level, and stress responses [24]. The presence of H. pylori may induce a stress response that activate the formation of volutin granules in the vacuoles of C. albicans. Therefore, more evidence is needed to distinguish the “dancing bodies” in the vacuole and evaluate the cross-kingdom interaction mechanisms between C. albicans and H. pylori.
The pores of C. albicans cell wall may act as the channel which the H. pylori can pass through. The FITC-IgY-H. pylori can enter into C. albicans yeast cells through the cell wall and eventually accumulated in the vacuoles [25]. However, the specific mechanism still needs further investigation, such as the cell wall/membrane remodeling of C. albicans when cocultured with H. pylori.
The internalization of H. pylori into C. albicans is highly influenced by pH stress as the percentage of yeasts harboring bacteria at an acidic pH was nearly twice than that observed in the neutral environment [26]. But when the pH is lower than 4, the number of yeasts harbored bacteria falls sharply. This may be due to the change in the cell wall structure and the surface electric charge of C. albicans under acidic conditions, such as the significant loss of the fibrillar layer, the increased exposure of chitin and β-glucans [26], and the change of zeta potential of C. albicans [27].
Possible relationship between the interaction of C. albicans and H. pylori with gastrointestinal flora
The clinical outcomes of the H. pylori–infected patients were quite different due to the diversity of the gastric and intestinal microbiota [28]. H. pylori infection can reduce the diversity of gastrointestinal flora. The Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria showed a decreased abundance, while Spirochetes and Acidobacteria showed an increased abundance [29]. The eradication of H. pylori can significantly increase the abundance of the gastric and downstream intestinal flora [30], such as Clostridium, Bacillus, etc., while the abundance of fungi such as yeast was significantly increased [31]. Disorders of the intestinal microbiota from H. pylori–infected patients may lead to the destruction of the intestinal barrier, thereby increasing the susceptibility to inflammatory bowel diseases [28]. The colonization of C. albicans in the gastrointestinal tract can also influence gastrointestinal flora. C. albicans creates a niche to increase the growth and survival of various microorganisms through the formation of the poly-species biofilms with bacteria, such as Bacteroides spp. and Firmicutes spp. [32]. However, C. albicans can reduce intestinal colonization of Clostridium difficile, a pathogenic agent of inflammatory bowel diseases [33].
The “standard triple treatment” of H. pylori infection recommended a composition of a proton pump inhibitor plus clarithromycin, together with amoxicillin or metronidazole. However, the effectiveness of this treatment declined to unacceptably low levels due to the antibiotic resistance in less than a decade [34]. Moreover, the use of antibiotics from the “standard triple treatment” significantly reduced the alpha and beta diversity of gastrointestinal flora, mainly including Bifidobacterium bifidum, Lactobacillus acidophilus, and Escherichia coli. The surviving bacteria, such as E. coli, increased the resistant capability to these antibiotics [35]. The antibiotics-affected gastrointestinal flora became more vulnerable to the colonization of C. albicans [36]. C. albicans colonized in the gastrointestinal tract may protect H. pylori from antibiotics killing through endosymbiosis or biofilm formation, then disrupt gastrointestinal metabolism and immunity to increase the risk of other diseases.
Probiotics may serve as a potential treatment
Probiotics have some natural advantages, such as safety, immunomodulation, and anti-pathogen abilities. They are usually used alone or in combination with drugs to treat gastrointestinal diseases. Lactobacillus spp. resided in the human stomach can inhibit H. pylori by secreting antibacterial substances, competing for binding sites, or interfering with the adhesion process to prevent H. pylori colonization, enhance the mucus barrier function, and reduce the host’s inflammatory response [37]. The triple therapy supplied with probiotic, including Saccharomyces boulardii, Limosilactobacillus reuteri, and Lactobacillus casei, for the treatment of H. pylori infection has the best therapeutic effect with the least adverse events [34]. Meanwhile, probiotics, such as Lactobacillus sp., can inhibit the adherence, biofilms, hyphae formation, and virulence expression of C. albicans [38]. Lactobacillus rhamnosus L34 can attenuate local inflammation, severity of intestinal leakage, fecal malnutrition, and systemic inflammation in mice infected with C. albicans [39]. Accordingly, the application of probiotics may be served as a potential treatment to inhibit the synergistic infections caused by H. pylori and C. albicans.
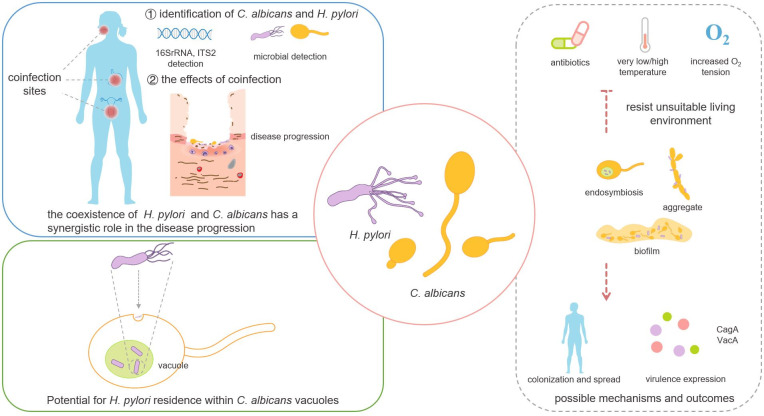
More than 50% of people were infected with H. pylori worldwide [2]. The triple or quadruple therapy with different antibiotics in clinical has gradually failed to eradicate the H. pylori infection mainly due to the increased drug resistance of H. pylori [40]. Moreover, these antibiotics disrupted the balance of the gastrointestinal flora, metabolism, and immunity and even increased the risk of other diseases [28,36]. The investigation of the interaction between H. pylori and other microorganisms can be one of the new ways to treat H. pylori infection. C. albicans may increase the expression of virulence factors and the growth and colonization of H. pylori in different environmental conditions to promote its pathogenicity and transmission (Fig 1), since C. albicans synergized with H. pylori to resist its unsuitable living environment and increase the infection. Their cross-kingdom interactions may be a new target for the prevention, diagnosis, and treatment of H. pylori infection.
Fig 1. The cross-kingdom interaction between Helicobacter pylori and Candida albicans and the possible mechanisms and outcomes.
There is a strong positive correlation between H. pylori and C. albicans in the colonization and synergistic pathogenesis. H. pylori potentially inhabit within C. albicans vacuoles through the endocytosis pathway. The interactions between H. pylori and C. albicans may increase the resistance to the killing effect of antibiotics and unfavorable living environment through endosymbiosis, adhesion, or formation of mixed biofilms, then promote the spread and colonization and increase the virulence factors to affect the occurrence and development of diseases.
Funding Statement
This research received funding from National Natural Science Foundation of China, Grant number: 81870778, 81600858 to BR, Applied Basic Research Programs of Sichuan Province, Grant number 2020YJ0227 to BR, National Natural Science Foundation of China, Grant number 81870759 to LC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48. 10.1016/S0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- 2.Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380(12):1158–65. 10.1056/NEJMcp1710945 [DOI] [PubMed] [Google Scholar]
- 3.Quaglia NC, Dambrosio A. Helicobacter pylori: A foodborne pathogen? World J Gastroenterol. 2018;24(31):3472–87. 10.3748/wjg.v24.i31.3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwai K, Watanabe I, Yamamoto T, Kuriyama N, Matsui D, Nomura R, et al. Association between Helicobacter pylori infection and dental pulp reservoirs in Japanese adults. BMC Oral Health. 2019;19(1):267. 10.1186/s12903-019-0967-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.d’Enfert C, Kaune AK, Alaban LR, Chakraborty S, Cole N, Delavy M, et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev. 2020. 10.1093/femsre/fuaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brito LC, Sobrinho AP, Teles RP, Socransky SS, Haffajee AD, Vieira LQ, et al. Microbiologic profile of endodontic infections from HIV- and HIV+ patients using multiple-displacement amplification and checkerboard DNA-DNA hybridization. Oral Dis. 2012;18(6):558–67. 10.1111/j.1601-0825.2012.01908.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karczewska E, Wojtas I, Sito E, Trojanowska D, Budak A, Zwolinska-Wcislo M, et al. Assessment of co-existence of Helicobacter pylori and Candida fungi in diseases of the upper gastrointestinal tract. J Physiol Pharmacol. 2009;60(Suppl 6):33–9. [PubMed] [Google Scholar]
- 8.Ramaswamy K, Correa M, Koshy A. Non-healing gastric ulcer associated with Candida infection. Indian J Med Microbiol. 2007;25(1):57–8. 10.4103/0255-0857.31064 [DOI] [PubMed] [Google Scholar]
- 9.Ince AT, Kocaman O, Ismailova M, Tozlu M, Gucin Z, Iraz M. A rare co-existence of helicobacter pylori, candida albicans and candida keyfr in a giant gastric ulcer. Turk J Gastroenterol. 2014;25(4):435–6. 10.5152/tjg.2014.3401 [DOI] [PubMed] [Google Scholar]
- 10.Khetsuriani S, Khetsuriani Z, Thanigaivasan M, Kulasekar C. Helicobacter pylori urease activity and spread of Candida spp in patients with gastric cancer. Bull Georg Natl Acad Sci. 2012;6(3). [Google Scholar]
- 11.Abrantes PMDS Africa CWJ. Measuring Streptococcus mutans, Streptococcus sanguinis and Candida albicans biofilm formation using a real-time impedance-based system. J Microbiol Methods. 2020;169:105815. 10.1016/j.mimet.2019.105815 [DOI] [PubMed] [Google Scholar]
- 12.Wang YH, Lv ZF, Zhong Y, Liu DS, Chen SP, Xie Y. The internalization of Helicobacter pylori plays a role in the failure of H. pylori eradication. Helicobacter. 2017;22(1). [DOI] [PubMed] [Google Scholar]
- 13.Saniee P, Siavoshi F, Nikbakht Broujeni G, Khormali M, Sarrafnejad A, Malekzadeh R. Localization of H.pylori within the vacuole of Candida yeast by direct immunofluorescence technique. Arch Iran Med. 2013;16(12):705–10. doi: 0131612/AIM.005 [PubMed] [Google Scholar]
- 14.Saniee P, Siavoshi F, Nikbakht Broujeni G, Khormali M, Sarrafnejad A, Malekzadeh R. Immunodetection of Helicobacter pylori-specific proteins in oral and gastric Candida yeasts. Arch Iran Med. 2013;16(11):624–30. doi: 0131611/AIM.003 [PubMed] [Google Scholar]
- 15.Siavoshi F, Heydari S, Shafiee M, Ahmadi S, Saniee P, Sarrafnejad A, et al. Sequestration inside the yeast vacuole may enhance Helicobacter pylori survival against stressful condition. Infect Genet Evol. 2019;69:127–33. 10.1016/j.meegid.2019.01.029 [DOI] [PubMed] [Google Scholar]
- 16.Siavoshi F, Saniee P. Vacuoles of Candida yeast as a specialized niche for Helicobacter pylori. World J Gastroenterol. 2014;20(18):5263–73. 10.3748/wjg.v20.i18.5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siavoshi F, Taghikhani A, Malekzadeh R, Sarrafnejad A, Kashanian M, Jamal AS, et al. The role of mother’s oral and vaginal yeasts in transmission of Helicobacter pylori to neonates. Arch Iran Med. 2013;16(5):288–94. doi: 013165/AIM.009 [PubMed] [Google Scholar]
- 18.Siavoshi F, Salmanian AH, Akbari F, Malekzadeh R, Massarrat S. Detection of Helicobacter pylori-specific genes in the oral yeast. Helicobacter. 2005;10(4):318–22. 10.1111/j.1523-5378.2005.00319.x [DOI] [PubMed] [Google Scholar]
- 19.Salmanian AH, Siavoshi F, Akbari F, Afshari A, Malekzadeh R. Yeast of the oral cavity is the reservoir of Heliobacter pylori. J Oral Pathol Med. 2008;37(6):324–8. 10.1111/j.1600-0714.2007.00632.x [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Alonzo K, Parra-Sepúlveda C, Vergara L, Bernasconi H, García-Cancino A. Detection of Helicobacter pylori in oral yeasts from students of a Chilean university. Rev Assoc Med Bras (1992). 2020;66(11):1509–14. 10.1590/1806-9282.66.11.1509 [DOI] [PubMed] [Google Scholar]
- 21.Huong DT, Zhao Y, Nguyet NT, Loan TT, Binh NT, Thinh NV, et al. Candida krusei colonization in patients with gastrointestinal diseases. Med Mycol. 2013;51(8):884–7. 10.3109/13693786.2013.804215 [DOI] [PubMed] [Google Scholar]
- 22.Siavoshi F, Sahraee M, Ebrahimi H, Sarrafnejad A, Saniee P. Natural fruits, flowers, honey, and honeybees harbor Helicobacter pylori-positive yeasts. Helicobacter. 2018;23(2):e12471. 10.1111/hel.12471 [DOI] [PubMed] [Google Scholar]
- 23.Puchkov EO. Brownian motion of polyphosphate complexes in yeast vacuoles: characterization by fluorescence microscopy with image analysis. Yeast. 2010;27(6):309–15. 10.1002/yea.1754 [DOI] [PubMed] [Google Scholar]
- 24.Kharchuk MS, Glushenkov AN, Gromozova EN. Analysis of the motion of vacuolar volutin granules in Saccharomyces cerevisiae. Folia Microbiol (Praha). 2019;64(2):207–13. 10.1007/s12223-018-0646-8 [DOI] [PubMed] [Google Scholar]
- 25.Saniee P. Siavoshi F. Endocytotic uptake of FITC-labeled anti-H pylori egg yolk immunoglobulin Y in Candida yeast for detection of intracellular H pylori. Front Microbiol. 2015;6:113. 10.3389/fmicb.2015.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez-Alonzo K, Parra-Sepúlveda C, Vega S, Bernasconi H, Campos VL, Smith CT, et al. In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress. Pathogens. 2020;9(6). 10.3390/pathogens9060489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Zhou Y, Zhou X, Liao B, Xu HHK, Chu CH, et al. Dimethylaminododecyl methacrylate inhibits Candida albicans and oropharyngeal candidiasis in a pH-dependent manner. Appl Microbiol Biotechnol. 2020;104(8):3585–95. 10.1007/s00253-020-10496-0 [DOI] [PubMed] [Google Scholar]
- 28.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alarcón T, Llorca L, Perez-Perez G. Impact of the Microbiota and Gastric Disease Development by Helicobacter pylori. Curr Top Microbiol Immunol. 2017;400:253–75. 10.1007/978-3-319-50520-6_11 [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Xu W, Lee A, He J, Huang B, Zheng W, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine. 2018;35:87–96. 10.1016/j.ebiom.2018.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ermolenko E, Varsin S, Baryshnikova N, Svarval A, Ferman R, Besedina N, et al. Gastrointestinal Dysbiosis Accompanied of Helicobacter Pylori Infection and its Correction by Probiotic. J Clin Gastroenterol Treatment. 2018;4(1). [Google Scholar]
- 32.Jiang TT, Shao TY, Ang WXG, Kinder JM, Turner LH, Pham G, et al. Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria. Cell Host Microbe. 2017;22(6):809–16.e4. 10.1016/j.chom.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapitan M, Niemiec MJ, Steimle A, Frick JS, Jacobsen ID. Fungi as Part of the Microbiota and Interactions with Intestinal Bacteria. Curr Top Microbiol Immunol. 2019;422:265–301. 10.1007/82_2018_117 [DOI] [PubMed] [Google Scholar]
- 34.Li BZ, Threapleton DE, Wang JY, Xu JM, Yuan JQ, Zhang C, et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: systematic review and network meta-analysis. BMJ. 2015;h4052:351. 10.1136/bmj.h4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liou JM, Chen CC, Chang CM, Fang YJ, Bair MJ, Chen PY, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis. 2019;19(10):1109–20. 10.1016/S1473-3099(19)30272-5 [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Covington A, Pamer EG. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279(1):90–105. 10.1111/imr.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji J, Yang H. Using Probiotics as Supplementation for Helicobacter pylori Antibiotic Therapy. Int J Mol Sci. 2020;21(3). 10.3390/ijms21031136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro FC, Rossoni RD, de Barros PP, Santos JD, Fugisaki LRO, Leão MPV, et al. Action mechanisms of probiotics on Candida spp. and candidiasis prevention: an update. J Appl Microbiol. 2020;129(2):175–85. 10.1111/jam.14511 [DOI] [PubMed] [Google Scholar]
- 39.Panpetch W, Hiengrach P, Nilgate S, Tumwasorn S, Somboonna N, Wilantho A, et al. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes. 2020;11(3):465–80. 10.1080/19490976.2019.1662712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Can F, Yilmaz Z, Demirbilek M, Bilezikci B, Kunefeci G, Atac FB, et al. Diagnosis of Helicobacter pylori infection and determination of clarithromycin resistance by fluorescence in situ hybridization from formalin-fixed, paraffin-embedded gastric biopsy specimens. Can J Microbiol. 2005;51(7):569–73. 10.1139/w05-035 [DOI] [PubMed] [Google Scholar]