Abstract
In a multicenter, randomized, double‐blind trial, the authors compared the antihypertensive efficacy of once‐daily treatment with the new angiotensin II type 1 receptor blocker (ARB) olmesartan (20 mg) with recommended starting doses of losartan (50 mg), valsartan (80 mg), and irbesartan (150 mg) in 588 patients with a cuff diastolic blood pressure (DBP) of ≥100 and ≥115 mm Hg and a mean daytime DBP of ≥90 mm Hg and <120 mm Hg, as measured by ambulatory blood pressure monitoring. Cuff and ambulatory blood pressures were monitored at baseline and after 8 weeks of treatment. All groups were predominantly white and approximately 62% male, and their mean age was approximately 52 years. In all groups, mean baseline DBP and systolic blood pressure (SBP) were approximately 104 and 157 mm Hg, respectively. The reduction of sitting cuff DBP with olmesartan (11.5 mm Hg), the primary efficacy variable of this study, was significantly greater than with losartan, valsartan, and irbesartan (8.2, 7.9, and 9.9 mm Hg, respectively). Reductions of cuff SBP with the four ARBs ranged from 8.4–11.3 mm Hg and were not significantly different. The reduction in mean 24‐hour DBP with olmesartan (8.5 mm Hg) was significantly greater than reductions with losartan and valsartan (6.2 and 5.6 mm Hg, respectively) and showed a trend toward significance when compared to the reduction in DBP with irbesartan (7.4 mm Hg; p=0.087). The reduction in mean 24‐hour SBP with olmesartan (12.5 mm Hg) was significantly greater than the reductions with losartan and valsartan (9.0 and 8.1 mm Hg, respectively) and equivalent to the reduction with irbesartan (11.3 mm Hg). All drugs were well tolerated. The authors conclude that olmesartan, at its starting dose, is more effective than the starting doses of the other ARBs tested in reducing cuff DBP in patients with essential hypertension.
Angiotensin II receptor blockers (ARBs) are the newest class of approved antihypertensive agents and the second class of drugs to exert their primary antihypertensive action by interrupting the renin‐angiotensin system. ARBs prevent the hypertensive effects of angiotensin II by selective blockade of the angiotensin II type 1 (AT1) receptor. 1 The success of ARBs in the treatment of hypertension is reflected in the fact that six of these agents have been approved for this use since 1994.
Olmesartan medoxomil is a new ARB that was discovered during a systematic survey of the AT1 binding actions of substituted imidazole‐5‐carboxylic acids. 2 It is a prodrug that, following oral administration, is rapidly and completely de‐esterified in the gut to its active form, in a reaction that is not cytochrome P‐450‐dependent. 3 This active metabolite, olmesartan, is a potent and selective AT1 receptor antagonist, with no agonist activity. 3
In healthy subjects, olmesartan has an elimination half‐life of 12–18 hours, 4 a value that is comparable to the longest half‐lives of ARBs currently in clinical use. 5 In a dose‐ranging study, olmesartan was shown to be an effective once‐per‐day drug for the treatment of hypertension on the basis of ambulatory blood pressure measurements, and to have a safety profile similar to that of placebo. 6
Although the pharmacokinetic and pharmacodynamic properties of olmesartan suggest that at its starting dose, it should compare favorably with ARBs already in clinical use for the treatment of hypertension, a direct comparison of the efficacy of these agents can be determined only in a head‐to‐head trial. Although several previous studies have compared the antihypertensive efficacy of ARBs on the basis of cuff blood pressure change, 7 , 8 , 9 such comparisons have largely been against losartan only. Losartan is the first drug to be marketed within the ARB class and has been shown to be relatively ineffective for 24‐hour control of blood pressure. 9 In the present study, we compared the efficacy of once‐daily olmesartan with that of losartan, valsartan, and irbesartan in patients with uncomplicated essential hypertension. All drugs were given at their recommended initial dosages. Blood pressure was evaluated with both cuff and ambulatory blood pressure monitoring (ABPM).
METHODS
Patients
Male and female patients 18 years of age or older with essential hypertension were eligible for participation in this study. To be included, patients were required to have an average cuff diastolic blood pressure (DBP) of ≥100 and ≤115 mm Hg and a mean daytime DBP of ≥90 mm Hg and <120 mm Hg, as measured by an ABPM device, after successful completion of a 4‐week placebo run‐in period. Women were excluded from the study if they were nursing or were of child‐bearing age and were not using a reliable means of birth control. Other exclusion criteria included any serious disorder that could limit the ability of the patient to participate in the trial, significant cardiovascular disease within the previous 6 months, and secondary hypertension.
No antihypertensive medications, other than the drugs used in the study, were allowed during the placebo run‐in and active treatment phases of this trial. Patients were required to stop taking such medications at least 24 hours prior to receiving the first dose of placebo in the run‐in phase of the study.
Study Design
This randomized, double‐blind, parallel‐group, multicenter clinical trial was conducted at 68 sites in the United States. The study protocol was approved by an institutional review board at each site. The study was divided into three phases: initial screening; 4‐week single‐blind placebo run‐in; and 8‐week double‐blind active treatment. During the screening phase, patients signed an informed consent agreement and a medical history was taken. A physical examination, 12‐lead electrocardiography, and laboratory tests were performed. Patients fasted for a minimum of 8 hours prior to collection of blood and urine samples for laboratory testing. Sitting cuff blood pressure was measured with a mercury sphygmomanometer. For all cuff blood pressure measurements, patients were seated for a minimum of 5 minutes before the first measurement. Three recordings were taken, each separated by a minimum period of 1 minute. The pulse rate was measured once at the time of the second blood pressure reading.
Patients who met the entry criteria for the study during screening entered the 4‐week single‐blind placebo run‐in phase of the study. Blood pressure and heart rate were measured at the end of each week of the run‐in period (designated visits 1–4). If the daily average cuff DBP at both visits 3 and 4 was ≥100 mg Hg and ≤115 mm Hg, and if the difference between these two daily averages was ≤10 mm Hg, the patient was considered eligible for ABPM (model #90207, Spacelabs Medical, Redmond, WA). ABPM was started in eligible patients immediately after the cuff blood pressure measurement at visit 4 and was continued for 24 hours. Patients with a mean daytime DBP of ≥90 mm Hg and <120 mm Hg by ABPM were eligible for randomization to treatment.
Patients entering the active treatment phase of the study were randomly assigned to receive a once‐daily dose of one of the following ARBs: 20 mg olmesartan; 50 mg losartan; 80 mg valsartan; or 150 mg irbesartan. All drugs were provided at the starting dose recommended by the manufacturer and were placed in identical capsules that matched the placebo capsules administered during the run‐in phase of the study. All drugs were taken at breakfast except on examination days, when medication was not taken until after blood pressure had been measured.
Patients in the active treatment phase of the study were required to visit the clinic prior to taking their daily dose of medication 2, 4, and 8 weeks after commencing active treatment. At each visit, sitting cuff blood pressure was measured in triplicate, heart rate was measured, compliance was assessed by pill count, and patients were queried for adverse events. The ABPM measurement was repeated at week 8 only. If, at any visit, a patient had a mean daytime or average sitting cuff DBP that was ≥120 mm Hg, or if the average sitting cuff systolic blood pressure (SBP) was =200 mm Hg, the patient was removed from the study and treated with appropriate antihypertensive medication.
Acceptance Criteria for ABPM Data
The ABPM devices were programmed to record blood pressure every 15 minutes throughout a 24‐hour period. Data acquired using ABPM were acceptable only if administration of medication occurred between 6:30 a.m. and 9:30 a.m. and were collected for a minimum period of 24 hours after administration of drugs. Within the 24‐hour period, only hours with at least one reading were considered to be valid. Data from the entire 24‐hour collection period were rejected if there were 6 or more nonconsecutive hours with no readings or 2 or more consecutive hours with no readings.
Statistical Design
The primary objective of this study was to assess the comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in terms of the reduction of elevated blood pressure. The primary efficacy variable was the change in sitting cuff DBP from baseline to the week 8 visit of the active treatment phase. The following parameters were secondary efficacy variables: change in sitting cuff DBP from baseline to the week 2 and 4 visits; change in sitting cuff SBP from baseline to the week 2, 4, and 8 visits; and change in mean 24‐hour ambulatory DBP and SBP from baseline to week 8.
The duration and consistency of 24‐hour blood pressure control were estimated by determining the DBP and SBP trough‐to‐peak ratios after 8 weeks of treatment. These ratios were calculated by determining the difference between the baseline and week 8 measurements for each hour of ABPM recording. The resultant data followed the typical curves representative of circadian variation in blood pressure. Plots of the hourly mean values from each treatment group were fitted by application of a seven‐term Fourier series. 10 The trough‐to‐peak ratio was defined as the ratio of the lowest value of the fitted curve divided by the highest value of the fitted curve.
The required sample size of the treatment groups was estimated by assuming that the decrease in cuff sitting DBP during treatment with olmesartan would be 4.4, 3.8, and 3.0 mm Hg greater than the decreases during treatment with losartan, valsartan, and irbesartan, respectively. The values used in these calculations were taken from the results of parallel‐design studies of similar duration to the present study, as presented in the Food and Drug Administration Summary Basis of Approval documents for losartan, valsartan, and irbesartan. Values for olmesartan were taken from previous registrational trials performed by Sankyo Pharma (Sankyo Pharma, data on file, 2001). Given expected differences between drugs and standard deviations, and assuming an overall one‐sided significance level of 0.05 and 90% power, 135 patients per treatment group were calculated to be required for this trial.
All efficacy analyses were performed on the intent‐to‐treat population, defined as any patient who had received at least one dose of study medication after randomization, and for whom baseline data and at least one postbaseline measurement were available. If a patient discontinued treatment before the end of the study, the last measurement prior to removal from the trial was carried forward for analysis.
Baseline demographic characteristics were summarized and compared among treatment groups. Categorical variables were analyzed by the X 2 test and continuous variables were tested with analysis of variance (ANOVA), with treatment used as a factor. The changes in blood pressure that occurred within each treatment group during the study were analyzed with paired t‐tests. A probability (p) of =0.05 was considered significant for these analyses.
Differences among treatment groups in the primary efficacy variable (change in cuff DBP over the 8 weeks of treatment) were analyzed with an analysis of covariance (ANCOVA) model, with baseline as the covariate and treatment and center as factors. The primary statistical comparisons were between olmesartan and each of the three comparison drugs. One‐sided tests were used to compare the least squared means computed from ANCOVA models. To ensure that the overall significance level remained at 5%, p values were adjusted with a multiple‐test procedure. 11 A similar ANCOVA model was used for all other comparisons of cuff blood pressure, and for comparisons of ambulatory blood pressure. All subsequent references to means refer to least squared means rather than unadjusted raw means.
Safety
All adverse events reported by patients or observed by investigators during any stage of the trial were recorded and assessed for seriousness and relation to the study drug. The results of all laboratory tests were assessed by the investigators for clinical significance and for possible relationship to the study drug. Adverse event data are presented for the period of active treatment only and all randomized patients are included. The clinical and laboratory adverse event data were examined by Fisher's exact test for differences among treatment groups. Clinically significant changes in physical examination findings that occurred between screening and the end of the study were also recorded.
RESULTS
Patient Disposition
A total of 1257 patients were screened for participation in the trial and 1090 were enrolled in the placebo run‐in phase of the study. Of these, 588 patients entered the treatment phase of the study and were randomized to olmesartan (n=147), losartan (n=150), valsartan (n=145), or irbesartan (n=146). The most common reasons for discontinuation prior to randomization were failure to meet the blood pressure entry criteria (70%) and patient request (9%). The percentage of patients in each group who completed the entire 8 weeks of the study were 93.2%, 91.3%, 91.0%, and 95.9% for olmesartan, losartan, valsartan, and irbesartan, respectively.
Baseline Demographics
The demographic characteristics of the intent‐to‐treat population for cuff analysis of blood pressure are shown in Table I. There were no significant differences in the demographics of the different treatment groups. All groups were predominantly white, approximately 62% male, and the mean age of all groups was approximately 52 years. The average patient had stage 2 hypertension according to DBP. In all treatment groups, baseline DBP was approximately 104 mm Hg and baseline SBP approximately 157 mm Hg.
Table I.
Summary of Baseline Demographic Characteristics and Blood Pressure of Patients in the Intent‐to‐Treat Population
| Olmesartan (20 mg) | Losartan (50 mg) | Valsartan (80 mg) | Irbesartan (150 mg) | |
|---|---|---|---|---|
| N | 145 | 146 | 142 | 145 |
| Age (years) | 52.4±8.95 | 51.6±9.30 | 51.7±9.62 | 51.9±9.63 |
| Race(%) | ||||
| White | 75.2 | 69.2 | 76.1 | 66.9 |
| Black | 13.8 | 13.0 | 10.6 | 16.6 |
| Other | 11.0 | 17.8 | 13.3 | 16.5 |
| Gender (%) | ||||
| Male | 66.9 | 62.3 | 57.7 | 58.6 |
| Female | 33.1 | 37.7 | 42.3 | 41.4 |
| Baseline blood pressure | ||||
| Cuff DBP | 104±3.5 | 104±3.5 | 104±3.3 | 104±3.6 |
| Cuff SBP | 157±13.3 | 157±11.9 | 155±12.1 | 156±12.8 |
| All values are means±SD. DBP=diastolic blood pressure; SBP=systolic blood pressure | ||||
Cuff Blood Pressure and Heart Rate
Treatment with all four ARBs resulted in significant decreases in both cuff DBP and SBP from baseline after 8 weeks of treatment (p<0.001 for all groups). The mean reduction in cuff DBP achieved with olmesartan (11.5 mm Hg) was significantly greater than that with losartan (8.2 mm Hg; p=0.0002), valsartan (7.9 mm Hg; p<0.0001), or irbesartan (9.9 mm Hg; p=0.0412) (Figure 1). Over the 8‐week treatment period, therapy with olmesartan also resulted in a mean reduction of SBP of 11.3 mm Hg. Patients treated with losartan, valsartan, and irbesartan achieved mean SBP reductions of 9.5, 8.4, and 11.0 mm Hg, respectively, over the same period. These differences were not statistically significant at 8 weeks.
Figure 1.
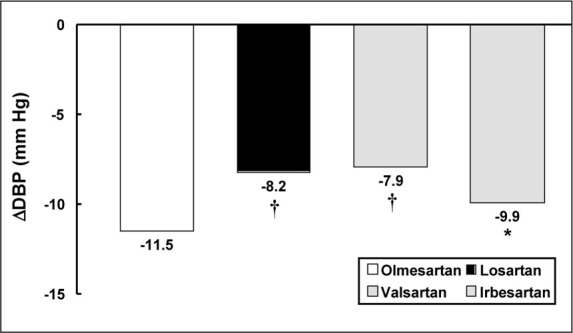
Least squares mean change from baseline in cuff diastolic blood pressure (DBP) after 8 weeks of treatment with olmesartan, losartan, valsartan, and irbesartan. *p<0.05 vs. olmesartan; †p<0.0005 vs. olmesartan
The differences in cuff blood pressure reduction after treatment with olmesartan and each of the three comparison drugs were apparent within 2 weeks (Table II). At this time, the mean DBP of the olmesartan‐treated group had decreased by 10.7 mm Hg, while treatment with losartan had resulted in a mean decrease of 7.6 mm Hg, and both the valsartan‐ and irbesartan‐treated patients showed a mean decrease of 9.0 mm Hg. Similar differences in DBP reduction among the treatment groups were evident in the week 4 data (Table II). The differences in DBP response between olmesartan and the comparison drugs were significant for all comparisons at both 2 and 4 weeks. Olmesartan was also significantly more effective than all three comparison drugs in reducing SBP after 2 weeks but not at 4 weeks of treatment (Table II). At 2 weeks mean SBP was reduced by 13.0 mm Hg in the olmesartan‐treated group, compared with 8.9 mm Hg in the losartan group (p=0.001), 9.2 mm Hg in the valsartan group (p=0.003), and 10.8 mm Hg in the irbesartan group (p=0.050). At week 4, the changes in SBP with olmesartan and the comparison drugs were not significantly different. None of the ARBs used in this study resulted in any significant change in heart rate.
Table II.
Change in Cuff DBP and SBP After 2 and 4 Weeks of Treatment
| Olmesartan | Losartan | Valsartan | Irbesartan | |
|---|---|---|---|---|
| 2 Weeks | ||||
| Δ DBP | −10.7 | −7.6† | −9.0* | −9.0* |
| Δ SBP | −13.0 | −8.9** | −9 2** | −10.8* |
| 4 Weeks | ||||
| Δ DBP | −11.4 | −8.9† | −9.7* | −9.9* |
| Δ SBP | −13.4 | −11.4 | −10.6 | −13.2 |
| Least squares mean change from baseline in cuff diastolic blood pressure (DBP) and systolic blood pressure (SBP) after 2 and 4 weeks of treatment with olmesartan, losartan, valsartan, and irbesartan. *p=0.05 vs. olmesartan; **p=0.005 vs. olmesartan; †p=0.0005 vs. olmesartan | ||||
Ambulatory Blood Pressure Monitoring
The results of the 24‐hour ABPM measurements after 8 weeks of treatment are shown in Figure 2. The overall results were similar to those obtained with cuff blood pressure measurements. The reduction in mean 24‐hour DBP with olmesartan (8.5 mm Hg) was significantly greater than the reduction obtained with losartan and valsartan (6.2 and 5.6 mm Hg, respectively) and showed a trend toward significance when compared to the reduction in DBP seen with irbesartan (7.4 mm Hg; p=0.087).
Figure 2.

Change in least squares mean 24‐hour diastolic (DBP) and systolic blood pressure (SBP) from baseline after 8 weeks of treatment with olmesartan, losartan, valsartan, and irbesartan. *p<0.05 vs. olmesartan
A similar pattern of difference was evident in the ambulatory SBP data. Olmesartan reduced mean 24‐hour SBP by 12.5 mm Hg after 8 weeks. This decrease was significantly greater than the reduction achieved by losartan and valsartan (9.0 and 8.1 mm Hg, respectively) but not statistically different from the reduction with irbesartan (11.3 mm Hg).
Changes in mean daytime and nighttime DBP and SBP, as measured by ABPM after 8 weeks of treatment with the various ARBs, are shown in Table III For purposes of these measurements, daytime was defined as 8:00 a.m. to 7:59 p.m. and nighttime as 8:00 p.m. to 7:59 a.m. Treatment with olmesartan for 8 weeks resulted in a reduction of both mean daytime DBP and SBP (10.2 and 14.7 mm Hg, respectively) that was significantly larger than the reductions seen with losartan and valsartan but not significantly different from that seen with irbesartan.
Table III.
Change in Mean Daytime and Nighttime ABPM, DBP, and SBP After 8 Weeks of Treatment With Olmesartan, Losartan, Valsartan, or Irbesartan
| Olmesartan | Losartan | Valsartan | Irbesartan | |
|---|---|---|---|---|
| Day | ||||
| Δ DBP | −10.2 | −7.2** | −7.0† | −8.8 |
| Δ SBP | −14.7 | −10.9** | −10.2** | −13.8 |
| Night | ||||
| Δ DBP | −6.8 | −5.2 | −4.2** | −5.9 |
| Δ SBP | −10.3 | −7.3* | −6.1** | −8.8 |
| ABPM=ambulatory blood pressure monitoring; DBP=diastolic blood pressure; SBP=systolic blood pressure *p=0.05 vs. olmesartan; **p=0.005 vs. olmesartan; †p=0.0005 vs. olmesartan | ||||
All of the ARBs in this study had less effect on blood pressure during the night than during the day. The drop in mean nighttime DBP with olmesartan treatment (6.8 mm Hg) was statistically greater than the nighttime DBP reduction with valsartan and similar to the reductions with losartan and irbesartan. The reduction from baseline in nighttime SBP after 8 weeks of olmesartan (10.3 mm Hg) was significantly greater than the reductions with losartan (7.3 mm Hg) and valsartan (6.1 mm Hg) and similar to the drop in nighttime SBP with irbesartan (8.8 mm Hg).
Trough‐to‐Peak Ratios
The stability of blood pressure control achieved with each treatment during the 24‐hour between‐doses period was also assessed by determination of the systolic and diastolic trough‐to‐peak ratios from the week 8 ABPM data. For SBP, this ratio was highest for olmesartan (0.69). Losartan, valsartan, and irbesartan achieved SBP trough‐to‐peak ratios of 0.64, 0.55, and 0.62, respectively. For DBP, the trough‐to‐peak ratios of olmesartan and losartan were similar (0.68 and 0.69, respectively), and higher than those for valsartan (0.48) and irbesartan (0.60). Trough‐to‐peak ratios from the four treatment groups were not compared statistically.
Safety
The overall incidence of adverse events was comparable among the four treatment groups. In this study, 30.6% (n=45) of the patients treated with olmesartan experienced at least one clinical adverse event. This compares with 32.0% (n=48) of the losartan group, 44.8% (n=65) of the valsartan group, and 35.6% (n=52) of the irbesartan group (Table IV). Upper respiratory infection, headache, fatigue, back pain, and dizziness were the most common complaints. Serious adverse events occurred in a total of four patients after randomization (olmesartan, n=1; losartan, n=1; valsartan, n=2). In the opinion of the investigator, these events were not related to the study drugs.
Table IV.
Adverse Events During the Active Treatment Period
| Olmesartan | Losartan | Valsartan | Irbesartan | |
|---|---|---|---|---|
| n=147 | n=150 | n=145 | n=146 | |
| n (%) | ||||
| Patients with ≥1 AE during active treatment | ||||
| Total AEs | 45 (30.6) | 48 (32.0) | 65 (44.8) | 52 (35.6) |
| Drug‐related AEs* | 12 (8.2) | 14(9.3) | 13 (9.0) | 11 (7.5) |
| Serious AEs (total) | 1 (0.7) | 1(0.7) | 2(1.4) | 0(0.0 |
| Severe AEs (total) | 4 (2.7) | 2(1.3) | 3(2.1) | 3(2.1) |
| Total AES in ≥2% of patients in any treatment group | ||||
| URT infection | 4 (2.7) | 4 (2.7) | 12(8.3) | 8(5.5) |
| Headache | 7(4.8) | 6 (4.0) | 6(4.1) | 8(5.5) |
| Fatigue | 3 (2.0) | 5 (3.3) | 3 (2.1) | 2(1.4) |
| Back pain | 1 (0.7) | 5 (3.3) | 3 (2.1) | 2(1.4) |
| Dizziness | 2(1.4) | 1 (0.7) | 2(1.4) | 5 (3.4) |
| Diarrhea | 2(1.4) | 1 (0.7) | 1 (0.7) | 5 (3.4) |
| Arthralgia | 1 (0.7) | 3 (2.0) | 3 (2.1) | 1 (0.7) |
| Coughing | 3 (2.0) | 1 (0.7) | 2(1.4) | 1 (0.7) |
| Pharyngitis | 0 (0.0) | 4 (2.7) | 1 (0.7) | 1 (0.7) |
| Influenza‐like symptoms | 1 (0.7) | 0 (0.0) | 1 (0.7) | 4 (2.7) |
| Myalgia | 0 (0.0) | 1 (0.7) | 4 (2.8) | 0 (0.0) |
| Toothache | 0 (0.0) | 0 (0.0) | 4 (2.8) | 1 (0.7) |
| Peripheral edema | 1 (0.7) | 0 (0.0) | 3(2.1) | 1 (0.7) |
| Migraine | 0 (0.0) | 3 (2.0) | 0 (0.0) | 0 (0.0) |
| AE=adverse event; URT=upper respiratory tract; *adverse events considered by the investigator to be definitely, probably, or possibly related to study drug administration | ||||
Laboratory adverse events occurred in a total of 21 randomized patients during the period of active treatment. Eight of these patients received olmesartan (5.4%), five losartan (3.3%), five valsartan (3.4%), and three irbesartan (2.1%). There were no significant differences among groups in the overall incidence of laboratory adverse events, or in the incidence of adverse events within any body system. Four patients (two losartan, two valsartan) had elevations of alanine aminotransferase or aspartate aminotransferase of >3x the upper limit of normal or >3x the baseline value, if the baseline value was above the normal range. One of these patients had elevated alanine aminotransferase and γ‐glutamyl transferase levels prior to study treatment; the elevations in two patients decreased after the end of study treatment; and one patient did not have follow‐up levels tested (the investigator did not consider the elevations to be significant).
A total of seven patients discontinued the study after randomization as a result of clinical or laboratory adverse events (olmesartan, n=2; valsartan, n=4; irbesartan, n=1). Two of these adverse events were deemed possibly related to treatment (fatigue and malaise [olmesartan] and cough [valsartan]).
DISCUSSION
Cuff Blood Pressure
Although several previous head‐to‐head comparisons of ARBs in which cuff blood pressure was used as the primary efficacy variable have been published, 7 , 8 , 12 , 13 all of the previous studies were comparisons with only losartan, the first ARB marketed. The present study is the first to include more than two ARBs at recommended starting doses and to directly compare the antihypertensive efficacy of more recently introduced ARBs. The principal finding of this study is that treatment with a starting dose of olmesartan results in a significantly greater reduction of cuff DBP, the primary efficacy variable of this trial, than treatment with starting doses of losartan, valsartan, and irbesartan. The superior efficacy of olmesartan in reducing cuff DBP was evident 2 weeks after the initiation of treatment, and was maintained for the duration of the trial.
As with the change in DBP, the olmesartan‐induced reduction in SBP was rapid in onset. Patients treated with olmesartan experienced a mean reduction in cuff SBP of 13.0 mm Hg after 2 weeks of treatment. Mean reductions achieved in the three comparison groups at 2, but not 4 weeks, were significantly lower, ranging from 8.9 mm Hg (losartan) to 10.8 mm Hg (irbesartan). The efficacy of olmesartan was maintained at 4 and 8 weeks (reductions of 13.4 and 11.3 mm Hg, respectively), although the comparisons with losartan, valsartan, and irbesartan did not achieve statistical significance at these time periods.
The greater efficacy of olmesartan in reducing trough cuff DBP may be related to its relatively long half‐life (12–18 hours). 4 Of the three comparison drugs used in the current study, irbesartan has the longest half‐life (11–15 hours) 14 ; the half‐lives of losartan (2 hours), 15 the active metabolite of losartan, EXP3174 (4–5 hours), 15 and valsartan (6 hours) 16 are all substantially shorter. Since a longer half‐life is associated with a longer duration of action, 17 this difference in pharmacokinetics may partially explain the differences in efficacy among these four ARBs. As a corollary, the long half‐life of drugs such as olmesartan and irbesartan may minimize the effect of missed or delayed dosing of medication. A substantial proportion of patients are erratic in the time of day at which they take once‐daily antihypertensive medication, and this inconsistency in dosing interval is associated with less effective control of blood pressure. 18
Ambulatory Blood Pressure
ABPM is the most reliable way to test the 24‐hour efficacy of an antihypertensive agent. The use of ABPM criteria for diagnosis of hypertension permits elimination of patients with white‐coat hypertension from clinical trials of hypertension and provides a continuous record of blood pressure during the normal daily activities of the patient. 19
Ambulatory blood pressure has been used as a primary efficacy variable in several previous head‐to‐head comparisons of the antihypertensive effectiveness of ARBs. 9 , 20 , 21 , 22 All of these studies involved direct comparison of the effects of two ARBs on ambulatory blood pressure and in all but one of these studies, 22 one of the ARBs was losartan. The present study, by contrast, is the first to compare antihypertensive efficacy as measured by ABPM in more than two ARBs in head‐to‐head fashion.
The results of the present study demonstrated that olmesartan is more effective than valsartan and losartan in reducing mean 24‐hour ambulatory DBP and SBP after 8 weeks of treatment. Similar reductions in mean ambulatory DBP and SBP were seen after treatment with olmesartan and irbesartan. This pattern of antihypertensive superiority to losartan and valsartan, and similarity to irbesartan, was also seen in both the daytime and nighttime ABPM measurements.
Magnitude of Blood Pressure Differences among Treatments: Relationship with Outcome
Available data suggest that the small differences in DBP reduction between olmesartan and the other ARBs in this study (approximately 2–4 mm Hg), sustained over time, may be associated with reductions in the risk of cardiovascular events. In a comprehensive overview of nine prospective observational studies involving 420,000 individuals, MacMahon et al. 23 concluded that a reduction in DBP of 5 mm Hg is associated with reductions of at least 21% in the incidence of coronary heart disease and at least 34% in the incidence of stroke. More recently, in the Hypertension Optimal Treatment (HOT) trial, 24 there were 28% fewer myocardial infarctions in the treatment group with a target DBP of =80 mm Hg than in the group with a target DBP of =90 mm Hg, although the actual difference in mean DBP achieved by these two groups was only 4.1 mm Hg. A similarly strong association between the risk of adverse cardiovascular events and both DBP and SBP has also been demonstrated in special populations, such as patients with diabetes. 24 , 25 Observations such as these suggest that the significant differences in DBP reduction with olmesartan compared to the other ARBs in the present study may be of clinical value.
As with DBP, elevations in SBP are associated with increased risk of coronary heart disease, stroke, myocardial infarction, occlusive peripheral arterial disease, and congestive heart failure. 26 , 27 , 28 , 29
A number of studies have quantified the change in risk of adverse cardiovascular outcomes associated with specific changes in SBP. Kannel 30 found that men with SBP of 140–159 mm Hg were at 50%–75% greater risk of cardiovascular disease than men with SBP of 120–139 mm Hg. In a meta‐analysis of eight trials carried out in elderly patients with isolated systolic hypertension, Staessen et al. 31 found that the relative risks of cardiovascular events, cardiovascular deaths, stroke, and all‐cause mortality increased by 15%, 22%, 22%, and 26%, respectively, for each 10 mm Hg increase in initial SBP. These observations suggest that the ARB‐induced reductions in cuff SBP of the magnitude seen in the present study are very likely to be of clinical significance.
Trough‐to‐Peak Ratio
The trough‐to‐peak ratio is a measure of the consistency of the antihypertensive efficacy of a drug during the entire dosing interval. It is an important parameter because increased blood pressure variability is associated with increased risk of end‐organ damage in hypertensive patients. 32 An optimal antihypertensive formulation should provide 24‐hour efficacy with a once‐daily dose, with at least 50% of the peak effect remaining after 24 hours. 33 Lower ratios may reflect excessive and potentially detrimental decreases in blood pressure at peak, poor control of hypertension at trough, or excessive variability of pharmacologic effect. 34 This parameter is also of therapeutic importance if patients miss a dose of medication. 35 All of the agents assessed in this study had trough‐to‐peak ratios for both DBP and SBP that were well above 0.5, with the exception of valsartan, which had a diastolic trough‐to‐peak ratio of 0.48.
Safety
There were no differences among treatment groups in the incidence of clinical or laboratory adverse events. Serious and severe adverse events were rare in all groups. As a class, ARBs are noted for having a side effect profile similar to that of placebo. 36 A placebo group was not included in the current study, but the total adverse event rate (which ranged from 31% for olmesartan to 45% for valsartan) is similar to that reported for the placebo group in several placebo‐controlled trials carried out in hypertensive patients. 7 , 8 , 12 , 20 Headache, which is often one of the most common adverse events in studies involving hypertensive patients, frequently has a lower incidence in patients treated with ARBs than in those treated with placebo. 8 , 9 , 12 Wiklund et al. 37 showed that the incidence of headache was reduced after 6 months of antihypertensive treatment in all three target groups in the HOT trial, a finding that supports the conclusion that lowering elevated blood pressure reduces the incidence of headache in hypertensive patients.
CONCLUSION
This study has shown that the reduction in cuff DBP resulting from 8 weeks of treatment with olmesartan is greater than that seen following treatment with losartan, valsartan, or irbesartan. Olmesartan also produced a reduction in cuff SBP that was numerically greater than, but not statistically significantly different from, that achieved by the three comparison drugs. The observation made in several clinical trials that small differences in both DBP and SBP are associated with substantial reductions in the incidence of major cardiovascular events suggests that small differences in blood pressure reduction between ARBs may have important long‐term effects.
References
- 1. Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. [DOI] [PubMed] [Google Scholar]
- 2. Yanagisawa H, Amemiya Y, Kanazaki T, et al. Nonpeptide angiotensin II receptor antagonists: synthesis, biological activities, and structure‐activity relationships of imidazole‐5‐carboxyliv acids bearing alkyl, alkenyl, and hydroxyalkyl substituents at the 4‐position and their related compounds. J Med Chem. 1996;39:323–338. [DOI] [PubMed] [Google Scholar]
- 3. Mizuno M, Sada T, Ikeda M, et al. Pharmacology of CS‐866, a novel nonpeptide angiotensin‐II receptor antagonist. Eur J Pharmacol. 1995;285:181–188. [DOI] [PubMed] [Google Scholar]
- 4. Schwocho LR, Masonson HN. Pharmacokinetics of CS‐866, a new angiotensin‐II receptor blocker, in healthy subjects. J Clin Pharmacol. 2001;41:515–527. [DOI] [PubMed] [Google Scholar]
- 5. Israili ZH. Clinical pharmacokinetics of angiotensin‐II (AT1) receptor blockers in hypertension. J Hum Hypertens. 2000;14(suppl 1):S73–S86. [DOI] [PubMed] [Google Scholar]
- 6. Masonson HN, Punzi HA, Neutel JM, et al. CS‐866 (angiotensin‐II receptor antagonist): a double‐blind study using ambulatory blood pressure monitoring in hypertensive patients [abstract]. Am J Hypertens. 1998;11:77A. [Google Scholar]
- 7. Kassler‐Taub K, Littlejohn T, Elliott W, et al. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan in mild‐to‐moderate hypertension. Irbesartan/Losartan Study Investigators. Am J Hypertens. 1998;11:445–453. [DOI] [PubMed] [Google Scholar]
- 8. Hedner T, Oparil S, Rasmussen K, et al. A comparison of the angiotensin II antagonists valsartan and losartan in the treatment of essential hypertension. Am J Hypertens. 1999;12:414–417. [DOI] [PubMed] [Google Scholar]
- 9. Mallion JM, Siche JP, Lacourcière Y, for the Telmisartan Blood Pressure Monitoring Group . ABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists telmisartan and losartan in patients with mild‐to‐moderate hypertension. J Hum Hypertens. 1999;13:657–664. [DOI] [PubMed] [Google Scholar]
- 10. Diamant M, Idema RN, Vincent HH. The use of Fourier analysis in the calculation of trough‐to‐peak ratio from ambulatory blood pressure measurements. J Hum Hypertens. 1998;12:61–67. [DOI] [PubMed] [Google Scholar]
- 11. Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–386. [Google Scholar]
- 12. Andersson OK, Neldam S. The antihypertensive effect and tolerability of candesartan cilexetil. A new generation angiotensin II antagonist, in comparison with losartan. Blood Press. 1998;7:53–59. [DOI] [PubMed] [Google Scholar]
- 13. Oparil S, Guthrie R, Lewin AJ, et al. An elective‐titration study of the comparative effectiveness of two angiotensin II‐receptor blockers, irbesartan and losartan. Irbesartan/Losartan Study Investigators. Clin Ther. 1998;20:398–409. [DOI] [PubMed] [Google Scholar]
- 14. Marino MR, Langenbacher K, Ford NF, et al. Pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Clin Pharmacol. 1998;38:246–255. [DOI] [PubMed] [Google Scholar]
- 15. Ohtawa M, Takayama F, Saitoh K, et al. Pharmacokinetics and biochemical efficacy after single and multiple oral administration of losartan, an orally active nonpeptide angiotensin II receptor antagonist, in humans. Br J Clin Pharmacol. 1993;35:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muller P, Flesch G, De Gasparo M, et al. Pharmacokinetics and pharmacodynamics effects of the angiotensin II antagonist valsartan at steady state in healthy, normotensive subjects. Eur J Clin Pharmacol. 1997;52:441–449. [DOI] [PubMed] [Google Scholar]
- 17. Leenen FH, Fourney A, Notman G, et al. Persistence of antihypertensive effect after “missed doses” of calcium antagonist with long (amlodipine) vs. short (diltiazem) elimination of half‐life. Br J Clin Pharmacol. 1996;41:83–88. [DOI] [PubMed] [Google Scholar]
- 18. Lee JY, Kusek JW, Greene PG, et al. Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Am J Hypertens. 1996;9:719–725. [DOI] [PubMed] [Google Scholar]
- 19. Staessen JA, Beilin L, Parati G, et al. Task force IV: clinical use of ambulatory blood pressure monitoring. Participants of the 1999 Consensus Conference on Ambulatory Blood Pressure Monitoring. Blood Press Monit. 1999;4:319–331. [DOI] [PubMed] [Google Scholar]
- 20. Lacourciere Y, Asmar R. A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients. A placebo‐controlled, forced titration study. Am J Hypertens. 1999;12:1181–1187. [DOI] [PubMed] [Google Scholar]
- 21. Monterroso VH, Rodriguez Chavez V, Carbajal ET, et al. Use of ambulatory blood pressure monitoring to compare antihypertensive efficacy and safety of two angiotensin II receptor antagonists, losartan and valsartan. Losartan Trial Investigators. Adv Ther. 2000;17:117–131. [DOI] [PubMed] [Google Scholar]
- 22. Littlejohn T, Mroczek W, Marbury T, et al. A prospective, randomized, open‐label trial comparing telmisartan 80 mg with valsartan 80 mg in patients with mild to moderate hypertension using ambulatory blood pressure monitoring. Can J Cardiol. 2000;16:1123–1132. [PubMed] [Google Scholar]
- 23. MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease; part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 24. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 25. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kannel WB, Schwartz MJ, McNamara PM. Blood pressure and risk of coronary heart disease: the Framingham study. Dis Chest. 1969;56:43–52. [DOI] [PubMed] [Google Scholar]
- 27. Kannel WB, Wolf PA, Verter J, et al. Epidemiologic assessment of the role of blood pressure in stroke (The Framingham Study). JAMA. 1970;214:301–310. [PubMed] [Google Scholar]
- 28. Kannel WB. Role of blood pressure in cardiovascular morbidity and mortality. Prog Cardiovasc Dis. 1974;17:5–24. [DOI] [PubMed] [Google Scholar]
- 29. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the systolic hypertension in the elderly program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 30. Kannel WB. Epidemiology of essential hypertension: the Framingham experience. Proc R Coll Physicians. 1991;21:273–287. [Google Scholar]
- 31. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet. 2000;355:865–872. [DOI] [PubMed] [Google Scholar]
- 32. Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24‐hour blood pressure variability. J Hypertens. 1993;11:1133–1137. [DOI] [PubMed] [Google Scholar]
- 33. Joint National Committee on Prevention, Detection, and Treatment of High Blood Pressure . The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 34. Mancia G, Dell'Oro R, Turri C, et al. Comparison of angiotensin II receptor blockers: impact of missed doses of candesartan cilexetil and losartan in systemic hypertension. Am J Cardiol. 1999;84:28S–34S. [DOI] [PubMed] [Google Scholar]
- 35. Elliot HL. Trough:peak ratio and twenty‐four hour blood pressure control. J Hypertens. 1994;12(suppl 5):29S–33S. [PubMed] [Google Scholar]
- 36. Mazzolai L, Burnier M. Comparative safety and tolerability of angiotensin II receptor antagonists. Drug Saf. 1999;21:23–33. [DOI] [PubMed] [Google Scholar]
- 37. Wiklund I, Halling K, Ryden‐Bergsten T, et al. Does lowering the blood pressure improve the mood? Quality‐of‐life results from the Hypertension Optimal Treatment (HOT) study. Blood Press. 1997;6:357–364. [DOI] [PubMed] [Google Scholar]


