Abstract
Sleep‐disordered breathing, manifested by repetitive episodes of partial or complete cessation of breathing during sleep associated with brief arousal and autonomic activation, is estimated to affect as many as 4% of adult men and 2% of adult women. Studies conducted during the 1980s revealed a strong association between sleep‐disordered breathing and hypertension. The results of these early studies, which relied on relatively small samples of patients, have been confirmed in recent years by large‐scale epidemiologic studies that are controlled for all possible confounding factors. This paper reviews the evidence suggesting a causative relationship between hypertension and disordered breathing in sleep. The authors discuss the possible underlying mechanisms of the two entities and address the clinical implications of this relationship. They conclude by recommending a proactive approach to the diagnosis of breathing disorders in sleep, in order to prevent the cardiovascular sequelae of this syndrome.
Obstructive Sleep Apnea Syndrome (OSAS) is characterized by repeated upper airway obstructions during sleep, causing intermittent nocturnal hypoxemia, sleep fragmentation, and daytime sleepiness. It affects 2%–4% of the adult population 1 and has been described as a major public health problem. 2 The association of OSAS with hypertension (HT) has been noted since the first description of OSAS in the medical literature. 3 Approximately 40%–60% of OSAS patients seen in sleep laboratories have HT. 4 , 5 , 6 This HT rate is two‐ to three‐fold higher than that in the general population; although this is a highly select group of patients, early studies of random groups of hypertensives in the sleep laboratory also disclosed a very high prevalence of OSAS, ranging from 30%–80%. 7 , 8 , 9 , 10 Furthermore, habitual snoring, one of the hallmark symptoms of OSAS, has been reported to be associated with HT in several epidemiologic studies. 11 , 12 Since OSAS and HT share certain risk factors, i.e., male gender, obesity, middle age, and a sedentary lifestyle, questions have been raised as to how much of this association may be attributable to these confounding variables. This argument diminishes the importance of the syndrome as a major public health problem. 13 This paper reviews the evidence supporting a causal relationship between OSAS and HT, discusses some of the mechanisms that possibly underlie this relationship, and addresses their clinical implications.
CONTROLLING FOR CONFOUNDERS
As mentioned above, HT and OSAS share the same risk factors. Figure 1 and Figure 2 illustrate the relationships between HT and body mass index (BMI, expressed as the square of weight in kg/height in m) and between HT and the severity of OSAS, as indicated by the respiratory disturbance index (RDI, expressed as the total number of respiratory disturbances during sleep divided by hours of sleep) in 10,000 patients diagnosed in the Technion Sleep Disorders Center in Israel. The rates of HT linearly increase as a function of both BMI and RDI.
Figure 1.
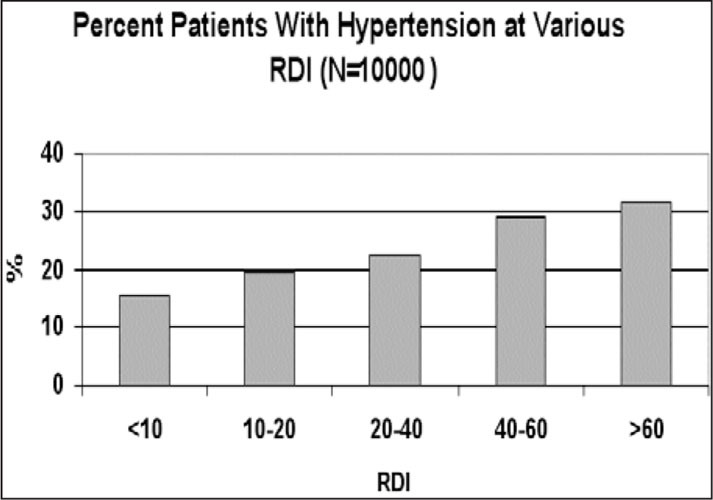
Relationship between severity of breathing disorders in sleep, as indicated by the respiratory disturbance index (RDI)—the total number of apneic plus hypopneic events divided by the total sleep time in hours—and self‐reported hypertension. Data of 10,000 patients diagnosed in the Technion Sleep Medicine Center in Israel during 1990–2000 are presented.
Figure 2.
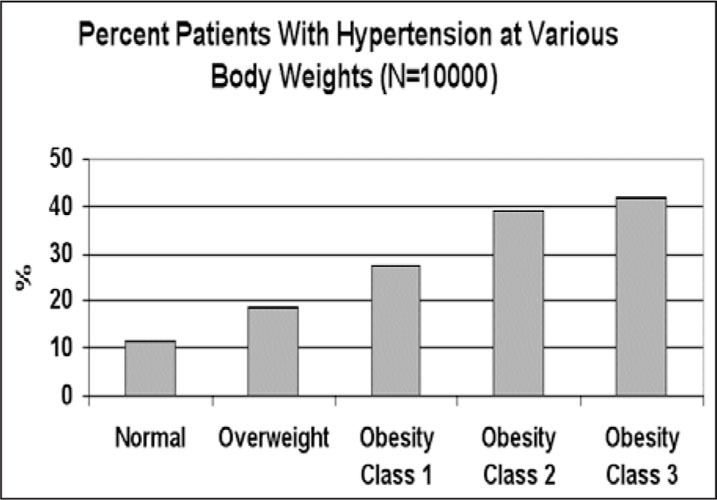
Relationship between self‐reported hypertension and the degree of overweight based on the World Health Organization classification of body mass index (normal, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; obesity class 1, 30.0–34.9 kg/m2; class 2, 35.0–39.9 kg/m2; class 3, >40 kg/m2). Data of 10,000 patients diagnosed in the Technion Sleep Medicine Center in Israel during 1990–2000 are presented.
How can the effects of confounding variables be disentangled from those of the repeated insult of multiple episodes of nocturnal apnea? This can be achieved either by comparing blood pressure (BP) in OSAS patients and controls of the same age, gender, and BMI, as well as all other potential confounders, or by using statistical techniques to control for confounding effects. So far, 47 studies have examined the association between HT and OSAS, with attempts to control for potential confounders. The majority of these studies (36/47; 76.6%) support an independent association between OSAS and HT. 14 Since disentangling the contribution of confounding factors from that of repeated apneic and hypoxemic events requires large populations, special attention should be given to studies that have involved exceptionally large populations of either sleep laboratory patients or randomly sampled subjects. Our group 15 analyzed BP data of 2677 patients recorded over a number of years in St. Michael's Hospital sleep laboratory in Toronto. Using either a history of known HT or a cut‐off BP of 140/90 mm Hg, we found that the risk of HT increased with increasing OSAS severity, as indicated by the RDI or the level of nocturnal arterial oxygen desaturation. This relationship was independent of all potential confounding factors, such as BMI, age, gender, and comorbidity. There was a 1 % increased risk of HT for each respiratory event per hour of sleep. Later, we reported 16 that the association between HT and OSAS was limited to patients aged 40–60 years. Grote et al. 17 similarly investigated 1190 patients consecutively referred for diagnosis of sleep‐related breathing disorders and reported that the relative risk of HT, defined as BP of 160/95 mm Hg, was 4.15 for an RDI of >40, in comparison with an RDI of <5, and 1.95 for HT defined as BP of >140/90 mm Hg, after controlling for confounding variables. As our own results indicated, 15 the relative risk of HT was greater for patients under 50 years of age.
In three recent studies, the association between HT and breathing disorders in sleep was investigated in large, random cohorts of the general population with wide age ranges. Young et al. 18 reported that in an unselected population of 1189 state employees in Wisconsin, breathing disorders in sleep were a risk factor for HT that was independent of age, BMI, or gender. Each apneic event per hour of sleep increased the HT risk in that population by approximately 4%. Importantly, their results show that the risk of HT was associated with the RDI, which was lower than the commonly employed cut‐off point for OSAS of five or 10 respiratory events per hour. In a 4‐year follow‐up study performed in a subsample of 709 of the original Wisconsin cohort, 19 the odds ratio (OR) for the presence of HT at follow‐up (compared to an OR of 1 for an RDI of 0) was 1.42 for an RDI of 0.1–4.9 at baseline, and 2.03 and 2.89 for RDIs of 5.0–14.9 and >15, respectively. This relationship was independent of known confounding factors.
Nieto et al. 20 investigated 6132 subjects aged >40 years who were recruited from an ongoing population‐based US study (the Sleep Heart Health Study, discussed below). Sleep was studied by unattended home sleep recordings, and the association between the RDI and HT (defined as BP of >140/90 mm Hg) was determined. They showed that after adjustment for confounding variables, the ORs for HT increased with escalating RDI categories in a graded dose‐response fashion. Comparison of the highest category of RDI (>30 per hour) with the lowest (<1.5 per hour) yielded an OR of 1.37.
Bixler et al. 21 studied the relationship between HT and sleep‐disordered breathing in 1000 women and 741 men sampled from a much larger population with specific risk factors for sleep‐disordered breathing. They reported an independent association between sleep‐disordered breathing and HT that was strongest in young individuals, especially those of normal weight. The association was not significant, or was inverted, among older individuals.
In addition to these large population studies, Davies et al., 22 in a case‐controlled study of 24‐hour ambulatory BP measurements in 45 patients with OSAS and 45 normal matched controls, recently found that the OSAS patients had higher diastolic BP during the day and night, and higher systolic BP at night.
EFFECTS OF OSAS TREATMENT ON HT
If there is a causative relationship between OSAS and HT, effective treatment of OSAS can be expected to reduce daytime or 24‐hour levels of BP in apneic patients. Several studies have provided support for this. Mayer et al. 23 reported that 6 months of treatment with nasal continuous positive airway pressure (nCPAP) significantly decreased BP, both during sleep and wakefulness, in 12 patients with severe OSAS. This change could not be explained by a change in BMI.
Suzuki et al. 24 performed ambulatory BP monitoring for 48 hours in normotensive and hypertensive OSAS patients before and after CPAP treatment and reported a significant decrease in daytime and nighttime BP, but only in hypertensive patients. A possible selective effect of nCPAP treatment on OSAS patients was also reported by Engelman et al., 25 who observed a significant improvement in mean daytime arterial BP only in a subgroup of patients defined as “nondippers” under placebo treatment conditions. Others also reported that nCPAP treatment restored the normal circadian pattern of nocturnal “dipping” in OSAS patients. 26 Wilcox et al., 27 on the other hand, showed a significant drop in mean 24‐hour BP in both normotensive and hypertensive patients after nCPAP treatment. Only diastolic BP, however, was decreased during the day. Akashiba et al. 28 reported that 2 weeks of nCPAP treatment was sufficient to reduce waking systolic and diastolic BP in 31 OSAS patients. Minemura et al. 29 reported that the daytime and nighttime decreases in BP after nCPAP treatment were accompanied by significant decreases in daytime and nighttime levels of urinary noradrenaline.
Interestingly, in comparing the effects of nCPAP treatment with those of a placebo (nCPAP administered at ineffective pressure) on 24‐hour BP in OSAS patients, Dimsdale et al. 30 found that both treatments reduced daytime BP levels to the same degree, while only effective nCPAP treatment significantly reduced nighttime BP. Voogel et al. 31 measured BP for 24 hours in OSAS patients before and after 3 weeks of CPAP treatment, in a tightly controlled environment and with a control group. They showed reductions in daytime and nighttime Bps only in the treated OSAS group.
Berger et al. 32 studied positional OSAS patients (those in whom most apneic events occurred in the supine position) and found that avoidance of the supine posture during sleep for a 1‐month period resulted in a significant fall in mean 24‐hour, sleeping, and waking Bps.
Perhaps the most convincing evidence that OSAS can cause HT is found in animal models. 33 Investigators in Toronto 34 mechanically produced OSAS in four dogs. Within a few weeks, the daytime and sleeping systemic Bps rose and, when they stopped the apneic events, the Bps reverted to normal within another few weeks. Here, obviously, there were no “confounding variables.” Persistent HT can also be produced in animals by intermittent exposure to hypoxia for several days. 34
POSSIBLE MECHANISMS
Acute Effects of OSAS on BP
A patient with severe OSAS can have 400–600 abnormal breathing events during the night. Most of these events are terminated by a brief “arousal” (lasting several seconds but unnoticed by the patient), which is associated with an increase in systemic BP. 35 Because these surges in BP occur so frequently during the night, the net result is an increase in mean overall BP in OSAS patients throughout the night.
In addition to the surges of systemic BP, each short arousal, which signals the end of an apneic or hypopneic event, is associated with an increase in heart rate, pulmonary artery BP, and sympathetic activity, all lasting for several seconds. There is still disagreement as to what is the major trigger of the arousal—the hypoxia, the hypercapnia, or the increased respiratory effort. It is likely that the arousal is multifactorial in origin and that all three of these factors, as well as others as yet unidentified, contribute to its occurrence.
Chronic Effects of OSAS on BP
What are the possible mechanisms by which these intermittent surges of BP that occur at the end of abnormal respiratory events eventually lead to permanently elevated BP during the waking hours?
One hypothesis put forward during the early 1990s to explain the persistence of high BP during the daytime concerns increased sympathetic activity, which frequently accompanies OSAS, both during sleep 36 and waking. 37 OSAS patients have reduced baroreceptor sensitivity and increased chemoreceptor sensitivity, 38 both of which could explain the persistent increase in sympathetic activity. 39 Systolic blood pressure is significantly correlated with muscle sympathetic nerve activity (MSNA). Somers et al. 40 confirmed these findings and also showed that sympathetic nerve activity further increased during sleep in OSAS patients. Interestingly, in 1998, Narkiewicz et al. 41 reported that obesity alone, in the absence of OSAS, is not accompanied by increased sympathetic activation and that treatment with nCPAP decreased sympathetic activation during sleep. Waradekar et al. 42 recorded waking MSNA in seven OSAS patients before and after 1 month of treatment with nCPAP, and showed a decrease in MSNA that was linearly related to the nightly use of nCPAP. Tonic activation of excitatory chemoreflex afferents appeared to contribute to increased daytime MSNA. 43 , 44 Thus, in addition to its effects on BP, effective nCPAP treatment has been shown to decrease daytime MSNA in OSAS patients. This effect, however, was evident only after an extended period of therapy. 45
Perhaps as a result of the combination of intermittent HT, increased sympathetic activity, and hypoxia that occurs with apneic events hundreds of times a night over a period of several years, there is an increase in systemic vascular reactivity in OSAS. The vasoconstrictor response to angiotensin II in the forearm vasculature of OSAS patients is enhanced. 46 Remsburg et al. 47 showed that OSAS patients have an abnormal peripheral vascular response to isocapnic hypoxia. Kato et al. 48 demonstrated impairment of resistance‐vessel endothelium‐dependent vasodilatation in OSAS patients. Duchna et al. 49 found blunted vasodilatory responsiveness to bradykinin in OSAS patients, which was reversed by nCPAP treatment.
Recent studies have also shown that OSAS patients have reduced levels of nitric oxide, the most potent naturally occurring vasodilator. 50 , 51 Circulating levels of nitric oxide were approximately one half those measured in controls, and significantly increased after nCPAP treatment. Stepwise multiple linear regression with systolic and diastolic BP as the dependent variables identified nitric oxide as the only significant correlate. Similar results were obtained in our laboratory (Lavie et al., manuscript in preparation); measurement of circulating levels of nitric oxide at hourly intervals during the night in OSAS patients and controls revealed that OSAS patients had consistently lower levels.
Thus, a cascade of events occurs in OSAS by which the peripheral vasculature is progressively damaged by chronic exposure to surges of hypertension, sympathetic activity, and hypoxia. The associated endothelial dysfunction promotes further vasoconstriction and this leads to chronic hypertension.
CLINICAL IMPLICATIONS
The accumulated findings from large‐scale studies of sleep laboratory‐diagnosed OSAS patients monitored with all‐night polysomnography, as well as studies of random cohorts of the general population, case‐controlled studies, and intervention studies, all suggest that OSAS constitutes a major contributor to the development of HT. In 1995, our group 52 reported that HT was a significant independent predictor of cardiopulmonary mortality in OSAS patients. The recently reported US Sleep Heart Health Study 53 demonstrated that OSAS is an independent risk factor not only for HT but also for heart failure, stroke, and coronary heart disease. These findings have important clinical implications concerning diagnosis and treatment of OSAS patients, as well as patients with essential HT. At the time of OSAS diagnosis, more than one quarter of the patients were already aware of having HT. This is probably an underestimation of the true prevalence of HT among OSAS patients, since a large number of patients have elevated BP without being aware of it. Figure 3 depicts the percentage of OSAS patients who reported having HT at the time of their diagnosis, and the percentage who were found to have BP levels of >140/90 mm Hg by objective measurements obtained just before sleep monitoring. Thus, the actual percentage of patients with elevated BP is much higher than the percentage of patients who report a history of HT.
Figure 3.
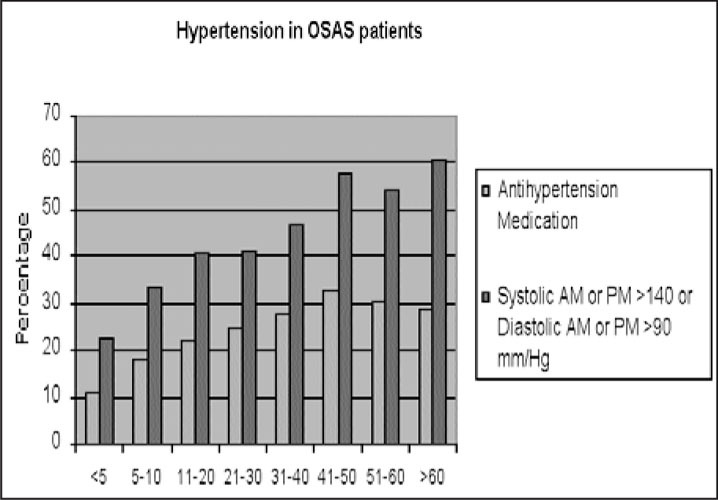
Comparison between the percentage of patients with breathing disorders in sleep who reported on hypertension or used antihypertensive medications and the percentage of patients who were found to have blood pressures >140/90 mm Hg either during morning or evening measurements. Data of 2677 adult patients diagnosed in St. Michael's Hospital Sleep Laboratory in Toronto are presented.
Another clue to the presence of OSAS may be refractoriness to antihypertensive therapy. There is evidence that hypertensive OSAS patients are more resistant to antihypertensive therapy than non‐OSAS patients with HT. 54 , 55 , 56 , 57 This phenomenon may extend to the sleeping as well as the waking BP. On the other hand, as was mentioned before, nCPAP effectively lowers BP in hypertensive OSAS patients, and in one study, 58 termination of CPAP treatment resulted in an immediate increase in BP.
Currently, most OSAS patients are referred to a sleep laboratory for diagnosis only after experiencing symptoms severe enough to affect their quality of life, or when they attract the attention of family members (usually because of loud snoring). Snorers themselves, even with clear daytime sleepiness, are reportedly passive in terms of seeking medical help for their symptoms. 59 The average age of patients diagnosed in sleep laboratories is approximately 50 years. The impact of the apnea on the cardiovascular system is gradual and cumulative. If we assume that in most patients the interval from the first appearance of frequent apneic events during sleep to the formal diagnosis of OSAS is about 10 years, by the time of diagnosis it may be too late to prevent the impact of the apnea on the cardiovascular system. The Wisconsin data and those of the Sleep Heart Health Study demonstrated that even a very mild breathing disorder in sleep is associated with a significant HT risk. Thus, a proactive attitude toward the diagnosis of OSAS should be taken. Instead of waiting for patients to complain about heavy snoring and excessive daytime sleepiness, physicians should actively seek out symptoms suggestive of OSAS in all patients. In view of the close associations between OSAS and obesity, HT, and a family history of OSAS, those with these three risk factors in particular should be questioned routinely about symptoms of sleep‐related breathing disorders. The three most important subjects on which to question patients are:
-
1
) Snoring. Do you snore a lot, and is the snoring independent of the body position? Is the snoring quiet and steady (which suggests that it is mild in nature) or loud, of variable intensity, and with many gasps or grunts (suggesting that it is more severe and accompanied by apneic episodes)?
-
2
) Excessive daytime sleepiness. Do you tend to fall asleep quickly during passive activities, such as reading an interesting book, watching an interesting television program, or driving a car? Sometimes the complaint may be “fatigue” or “tiredness” rather than “sleepiness.”
-
3
) Apneic episodes at night. Does your bed partner or other family member say that you stop breathing or gasp a lot during sleep?
Starting effective treatment at the earliest possible age may prevent the cumulative impact of the apnea on the cardiovascular system, as well as improve patients' wakefulness and quality of life.
References
- 1. Young T, Palta M, Dempsey J, et al. The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med. 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2. Phillipson EA. Sleep apnea—a major public health problem. N Engl J Med. 1993;328:1271–1273. [DOI] [PubMed] [Google Scholar]
- 3. Lavie P. Incidence of sleep apnea in a presumably healthy working population: a significant relationship with excessive daytime sleepiness. Sleep. 1983;6:312–318. [PubMed] [Google Scholar]
- 4. Millman RP, Redline S, Arlisle CC, et al. Daytime hypertension in obstructive sleep apnea. Prevalence and contributing risk factors. Chest. 1991;99:861–866. [DOI] [PubMed] [Google Scholar]
- 5. Silverberg DS, Oksenberg A, Radwan H, et al. Is obstructive sleep apnea a common cause of essential hypertension? Isr J Med Sci. 1995;31:527–535. [PubMed] [Google Scholar]
- 6. Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theory. Am J Med. 1995;98:118–128. [DOI] [PubMed] [Google Scholar]
- 7. Lavie P, Ben‐Yosef R, Rubin AE. Prevalence of sleep apnea syndrome among patients with essential hypertension. Am Heart J. 1984,108:373–376. [DOI] [PubMed] [Google Scholar]
- 8. Williams AJ, Houston D, Finberg S, et al. Sleep apnea syndrome and essential hypertension. Am J Cardiol. 1985;55:1019–1022. [DOI] [PubMed] [Google Scholar]
- 9. Fletcher EC, DeBehnke RD, Lovoi MS, et al. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med. 1985;103:190–195. [DOI] [PubMed] [Google Scholar]
- 10. Kales A, Bixler EO, Cadieux RJ, et al. Sleep apnea in a hypertensive population. Lancet. 1984;2:1005–1008. [DOI] [PubMed] [Google Scholar]
- 11. Gislason T, Aberg H, Taube A. Snoring and systemic hypertension—an epidemiological study. Acta Med Scand. 1987;222:415–421. [DOI] [PubMed] [Google Scholar]
- 12. Marrone O, Bonsignore MR, Fricano L, et al. Gender and the systemic hypertension‐snoring association: a questionnaire‐based case‐control study. Blood Press. 1998;7:11–17. [DOI] [PubMed] [Google Scholar]
- 13. Wright J, Johns R, Watt I, et al. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. BMJ. 1997;314:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silverberg DS, Oksenberg A, Iaina A. Sleep‐related breathing disorders as a major cause of essential hypertension: fact or fiction? Curr Opin Nephrol Hypertens. In press. [DOI] [PubMed] [Google Scholar]
- 15. Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavie P, Hoffstein V. Obstructive sleep apnoea syndrome and hypertension: moderating effects of age. Sleep. 2000;23(suppl 2):A60. [Google Scholar]
- 17. Grote L, Hedner J, Peter JH. Sleep‐related breathing disorder is an independent factor for uncontrolled hypertension. J Hypertens. 2000;18:679–685. [DOI] [PubMed] [Google Scholar]
- 18. Young T, Peppard PE, Palta M, et al. Population‐based study of sleep‐disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 19. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 20. Nieto FJ, Young TB, Lind BK, et al. Association of sleep‐disordered breathing, sleep apnea and hypertension in a large community‐based study. JAMA. 2000;283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 21. Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep‐disordered breathing. Arch Intern Med. 2000;160:2289–2295. [DOI] [PubMed] [Google Scholar]
- 22. Davies CW, Crosby JH, Mullins RL, et al. Case‐control study of 24‐hour ambulatory blood pressure in patients with obstructive sleep apnoea and non‐matched control subjects. Thorax. 2000;55:736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayer J, Becker H, Brandenburg U, et al. Blood pressure and sleep apnea: results of long‐term nasal continuous positive airway pressure therapy. Cardiology. 1991;79:84–92. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki M, Otsuka K, Guilleminault C. Long‐term nasal continuous positive airway pressure administration can normalize hypertension in obstructive sleep apnea patients. Sleep. 1993;16:545–549. [DOI] [PubMed] [Google Scholar]
- 25. Engleman HM, Gough K, Martin SE, et al. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non‐dippers. Sleep. 1996;19:378–381. [DOI] [PubMed] [Google Scholar]
- 26. Akashiba T, Minemura H, Yamamoto H, et al. Nasal continuous positive airway pressure changes blood pressure “non‐dippers” to “dippers” in patients with obstructive sleep apnea. Sleep. 1999;22:849–853. [DOI] [PubMed] [Google Scholar]
- 27. Wilcox I, Grunstein RR, Hedner JA, et al. Effect of nasal continuous positive airway pressure during sleep on 24‐hour blood pressure in obstructive sleep apnea. Sleep. 1993;16:539–544. [DOI] [PubMed] [Google Scholar]
- 28. Akashiba T, Kurashina K, Minemur H, et al. Daytime hypertension and the effects of short‐term nasal continuous positive airway pressure treatment in obstructive sleep apnea syndrome. Intern Med. 1995;34:528–532. [DOI] [PubMed] [Google Scholar]
- 29. Minemura H, Akashiba T, Yamamoto H, et al. Acute effects of nasal continuous positive airway pressure on 24‐hour blood pressure and catecholamines in patients with obstructive sleep apnea. Intern Med. 1998;37:1009–1013. [DOI] [PubMed] [Google Scholar]
- 30. Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension. 2000;35:144–147. [DOI] [PubMed] [Google Scholar]
- 31. Voogel AJ, Van Steenwijk RP, Karemaker JM, et al. Effects of treatment of obstructive sleep apnea on circadian hemodynamics. J Auton Nerv Syst. 1999;77:177–183. [DOI] [PubMed] [Google Scholar]
- 32. Berger M, Oksenberg A, Silverberg DS, et al. Avoiding the supine position during sleep lowers 24 hr blood pressure in obstructive obstructive sleep apnea (OSA) patients. J Hum Hypertens. 1997;11:657–664. [DOI] [PubMed] [Google Scholar]
- 33. Brooks D, Horner RL, Kozar LF, et al. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol. 2000;119:189–197. [DOI] [PubMed] [Google Scholar]
- 35. Schneider H, Schaub CD, Chen CA, et al. Effects of arousal and sleep state on systemic and pulmonary hemodynamics in obstructive apnea. J Appl Physiol. 2000;88:1084–1092. [DOI] [PubMed] [Google Scholar]
- 36. Leuenberger U, Jacob E, Sweer L, et al. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–588. [DOI] [PubMed] [Google Scholar]
- 37. Carlson JT, Hedner J, Elam M, et al. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. [DOI] [PubMed] [Google Scholar]
- 38. Narkiewicz K, Pesek CA, Kato M, et al. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. [DOI] [PubMed] [Google Scholar]
- 39. Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens. 1997;15:1613–1619. [DOI] [PubMed] [Google Scholar]
- 40. Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Narkiewicz K, Van De Borne PJ, Cooley RL, et al. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. [DOI] [PubMed] [Google Scholar]
- 42. Waradekar NV, Sinoway LI, Zwillich CW, et al. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996;153:1333–1338. [DOI] [PubMed] [Google Scholar]
- 43. Narkiewicz K, Van De Borne PJ, Montano N, et al. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. [DOI] [PubMed] [Google Scholar]
- 44. Narkiewicz K, Van De Borne PJ, Pesek CA, et al. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. [DOI] [PubMed] [Google Scholar]
- 45. Narkiewicz K, Kato M, Phillips BG, et al. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999; 100:2332–2335. [DOI] [PubMed] [Google Scholar]
- 46. Kraiczi H, Hedner J, Peker Y, et al. Increased vasoconstrictor sensitivity in obstructive sleep apnea. J Appl Physiol. 2000;89:493–498. [DOI] [PubMed] [Google Scholar]
- 47. Remsburg S, Launois SH, Weiss JW. Patients with obstructive sleep apnea have an abnormal peripheral vascular response to hypoxia. J Appl Physiol. 1999;87:1148–1153. [DOI] [PubMed] [Google Scholar]
- 48. Kato M, Roberts‐Thomson P, Phillips BG, et al. Impairment of endothelium‐dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. [DOI] [PubMed] [Google Scholar]
- 49. Duchna HW, Guilleminault C, Stoohs RA, et al. Vascular reactivity in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2000;161:187–191. [DOI] [PubMed] [Google Scholar]
- 50. Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. [DOI] [PubMed] [Google Scholar]
- 51. Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax. 2000;55:1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1995;18:149–157. [DOI] [PubMed] [Google Scholar]
- 53. Shahar E, Whitney CW, Redline S, et al. Sleep‐disordered breathing and cardiovascular disease. Cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 54. Pelttari LH, Hitanen EK, Salo TT, et al. Little effect of ordinary antihypertensive therapy on nocturnal high blood pressure in patients with sleep disordered breathing. Am J Hypertens. 1998;11:272–279. [DOI] [PubMed] [Google Scholar]
- 55. Isaksson H, Svanborg E. Obstructive sleep apnea syndrome in male hypertensives, refractory to drug therapy. Nocturnal automatic blood pressure measurements—an aid to diagnosis? Clin Exp Hypertens. 1991;13:1195–1212. [DOI] [PubMed] [Google Scholar]
- 56. Grote L, Wutkewicz K, Knaack L, et al. Association between blood pressure reduction with antihypertensive treatment and sleep apnea activity. Am J Hypertens. 2000;13:1280–1287. [DOI] [PubMed] [Google Scholar]
- 57. Lavie P, Hoffstein V. Sleep apnea syndrom: a possible contributing factor to resistant hypertension. Sleep. 2000;24:721–725. [DOI] [PubMed] [Google Scholar]
- 58. Stradling JR, Partlett J, Davis RJ, et al. Effect of short‐term graded withdrawal of nasal continuous positive airway pressure on systemic blood pressure in patients with obstructive sleep apnoea. Blood Press. 1996;5:2234–2240. [DOI] [PubMed] [Google Scholar]
- 59. Martikainen K, Pertinen M, Urponen H, et al. Natural evolution of snoring: a 5‐year follow‐up study. Acta Neurol Scand. 1994;90:437–42. [DOI] [PubMed] [Google Scholar]


