Abstract
Awareness of an increased incidence of cardiovascular events shortly after awakening has heightened interest in the chronopathology of cardiovascular diseases. Blood pressure varies according to cycles characterized by a reduction during sleep and an increase on awakening. The surge in blood pressure coincides with the circadian nature of various endocrine and hematologic parameters that also have a putative role in triggering the onset of cardiovascular events. The nighttime decrease is absent or blunted in some hypertensive patients (termed “nondippers”), an effect associated with increased morbidity. Drugs can influence the effect of these circadian patterns. Research efforts are focused on clarifying an underlying pathophysiologic process that could be modified by pharmacologic or other means. Long‐acting angiotensin II receptor blockers have an effect on blood pressure over 24 hours due to their long half‐life, but could also limit the morning surge in blood pressure through an effect on the renin‐angiotensin‐aldosterone and noradrenergic systems.
The term “biologic rhythm” may be defined as the intrinsic, regular fluctuation of a physiologic process. A rhythm may not necessarily follow a rigid, oscillating curve, and not every type of repeated biologic behavior constitutes a rhythm. Biologic rhythms, however, are actively maintained and often follow a daily or circadian pattern, as exemplified by the endocrine system. Biologic rhythms are also intrinsic in many pathologic conditions.
Recognition of an increased incidence of myocardial infarction, angina, stroke, and sudden cardiac death in the early morning period over the first 4–6 hours after awakening has heightened interest in the chronopathology of cardiovascular diseases. 1 , 2 Further, epidemiologic data show that both systolic (SBP) and diastolic blood pressure (DBP) measured in the office environment correlate positively with the incidence of cardiovascular mortality. 3 Indeed, the higher the blood pressure (BP) measured, the greater the relative risk of cardiovascular mortality, such as stroke or coronary heart disease. 4
During recent years, the increased use of ambulatory blood pressure measurement over a 24‐hour period has enabled more thorough examination of the chronologic variation in BP and has provided further evidence of a link between BP and the incidence of cardiovascular events. This review details the association between BP and the chronopathology of cardiovascular diseases, particularly in terms of the nocturnal fall in BP.
CIRCADIAN RHYTHM: DIPPERS AND NONDIPPERS
BP generally varies according to a circadian rhythm characterized by a reduction during sleep and an increase during wakefulness. The same circadian fluctuation is observed in normotensive subjects and in both treated and untreated hypertensive patients. 5 All too often, however, the BP reduction in treated hypertensives observed during the day is not maintained throughout the night. This raises two important points regarding BP during sleep. First, BP in treated and untreated hypertensives continues to be higher than in normotensives during the night. Second, the duration of action of many antihypertensive agents is such that they do not provide efficacy through the night and the following morning, when the incidence of many cardiovascular events is at its highest. 1 , 2
The circadian fall in BP during sleep does not always occur (Figure 1), 6 , 7 , 8 , 9 , 10 , 11 leading to the tentative division of individuals into those who display a fall in BP during sleep (“dippers”) and those who do not (“nondippers”). Definitions of dippers and nondippers vary among authors, but a useful description of a dipper is someone who shows a reduction in BP of 10% or 10/5 mm Hg. 12 Those who have less than a 10% reduction in BP during sleep are defined as nondippers.
Figure 1.
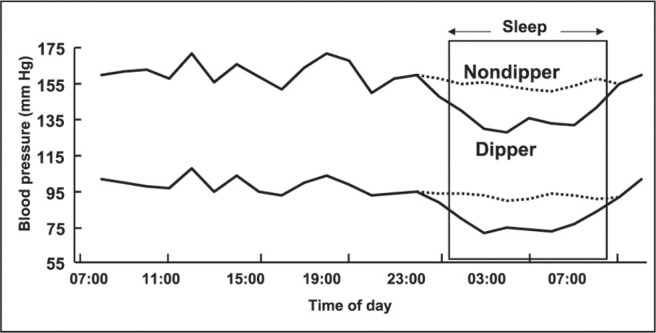
Twenty‐four‐hour blood pressure profile of dipper and nondipper hypertensive patients. 6, 7, 8, 9, 10, 11
The period for measurement of the decrease shows some variation, depending on the definition of night and day. A definition of daytime is the period from 6 a.m. to 10 p.m., with nighttime defined as 10 p.m. to 6 a.m. However, a narrow definition might define day as between 10 a.m. and 8 p.m. and nighttime as midnight through 6 a.m., allowing a 4‐hour transition period between night and day during which BP is not included in the calculation. Use of the narrow definition excludes the period when a variable proportion of subjects are awake or asleep and therefore provides a more accurate estimate of the actual BP values during sleep and wakefulness. 12
Irrespective of the definition used, a number of disorders and syndromes appear to be associated with a nondipper status, including malignant hypertension, pheochromocytoma, pre‐eclamptic toxemia, cardiac transplantation, idiopathic orthostatic hypotension, diabetes mellitus (without neuropathy), and sleep apnea syndrome.
ASSOCIATION OF A BP DECREASE WITH OTHER CIRCADIAN RHYTHMS
The circadian fall in BP during sleep and its rise upon awakening is temporally associated with several other circadian rhythms that have been described over a number of years, including fluctuations in hemodynamic, electrolyte, hormonal, and neuronal parameters. 13 Although the association is circumstantial, many of these rhythms have the potential to modulate the autonomous mechanisms controlling BP and have a putative role in triggering the onset of cardiovascular events.
Renin‐Angiotensin‐Aldosterone System (RAAS)
The activity of renin has long been known to exhibit diurnal variation. More than 30 years ago, Gordon et al. 14 demonstrated that plasma renin activity gradually decreases during the day, reaching its nadir at 4 p.m., followed by a gradual increase overnight and peaking at 8 a.m. Closer examination of the circadian pattern of plasma renin activity has demonstrated more complex variations over 24 hours, rather than a constant, protracted transition between its rise and fall. However, the trend is for diurnal variation to rise during the morning. 15 Levels of angiotensin II probably follow the same morning increase as plasma renin activity, though this is difficult to confirm because of the short duration of angiotensin II in blood. Plasma levels of aldosterone follow a diurnal pattern similar that of renin, with an increase during the early hours of the morning that peaks just before or during the waking hours. 15
Catecholamines and Vascular Resistance
Epinephrine and norepinephrine (NE) show a cyclical pattern similar to that of the RAAS. 16 However, much of the variation may be due to posture changes rather than circadian rhythm. The plasma epinephrine level gradually decreases during the night, which is to be expected during the relaxation of sleep. However, it rises again during the early hours of the morning and continues to rise during the waking period, following the same pattern as BP. Plasma NE levels vary similarly, except that the peak upon waking appears to be more pronounced, suggesting that this is a reflex effect rather than a circadian cycle effect. 16
A surge in BP and increased heart rate in the morning may be accompanied by vasoconstriction and reduction of flow in the coronary arteries, an increase in myocardial oxygen demand, and potentially an increase in arrhythmias, as this coincides with peak plasma catecholamine levels. In a study by Panza et al., 17 either the vasodilator nitroprusside or the α‐adrenoceptor antagonist phentolamine was infused into the forearm of volunteers. Reduction in vascular resistance was recorded in the morning, afternoon, and evening. The reduction in vascular resistance induced by nitroprusside was consistent at all times of the day, regardless of dose, indicating a pure vasodilator effect (Figure 2A). In contrast, the reduction in vascular resistance induced by antagonism of α‐adrenoceptors varied according to the time of day. Phentolamine, for example, was twice as effective in the morning than in the evening (Figure 2B), suggesting increased vasoconstriction in the morning due to α‐adrenoceptor agonism, probably by NE or epinephrine.
Figure 2.
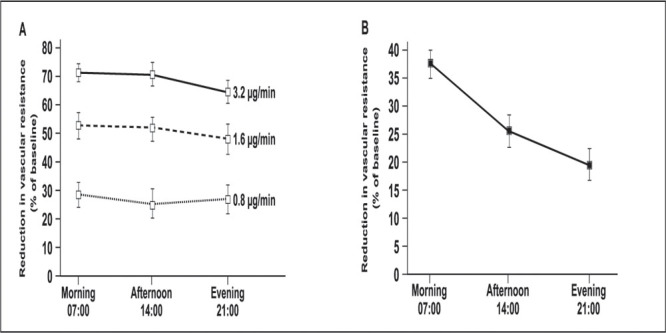
Reduction in vascular resistance by nitroprusside (A) and the α‐adrenoceptor antagonist phentolamine (B). Drugs were infused into the forearm of volunteers. 17 Reprinted with permission from Panza JA, et al. N Engl J Med. 1991;325:986–990. Copyright ©1991 Massachusetts Medical Society. All rights reserved.
Renin Activity and NE Release are Linked
Interaction between the RAAS and NE has been demonstrated. 18 For many years it has been known that angiotensin II type 1 (AT1) receptors are present on the presynaptic adrenergic nerve endings, and their stimulation increases NE release. Hence, increased renin activity induces production of angiotensin II, which stimulates the presynaptic AT1 receptors on the adrenergic nerve endings, resulting in increased NE release. Furthermore, NE stimulates further renin release, thereby forming a cycle of NE release and production of angiotensin II. Both sympathetic hyperactivity and elevated plasma renin activity are associated with increased risk of cardiovascular events. 19 Thus, drugs capable of reducing the effect of the morning increase in NE and angiotensin II could have considerable cardioprotective potential in addition to their BP‐lowering effect.
Hematologic Factors
Some hematologic factors vary according to a circadian pattern. Increases in blood viscosity and platelet aggregation, and a lesser increase in circulating‐type plasminogen activator activity, have been observed during the early morning period. 20
Interestingly, adenosine diphosphate‐stimulated platelet aggregation follows a pattern similar to the epinephrine cycle and increases when adrenergic activity is at its highest. 21 Epinephrine is known to increase the ability of platelets to aggregate. 21 Hence, coupled with the increased vasoconstriction observed during the early morning period, stimulation in adrenergic activity may be an important factor in the raised incidence of cardiovascular accidents, notably thrombosis, at this time of the day.
Activation of plasminogen is reduced during the early hours of the morning, which potentially reduces thrombolysis and increases the risk of thrombosis and embolism. Tissue plasminogen activator antigen and plasminogen activator inhibitor antigen follow reciprocal diurnal cycles. 20 Tissue plasminogen activator decreases during the night and begins to increase during the early hours of the morning. Conversely, plasminogen activator inhibitor increases during the night and decreases in the early hours of the morning.
Other Hormones and Peptides
The circadian rhythm of cortisol is one of the best described cycles and likely plays a role in the sensitivity of vessels to the noradrenergic system. The plasma cortisol level rises during the early morning until the waking hours, after which it gradually falls during the day. 22 Increased sensitivity of blood vessels to NE accompanies an increased plasma cortisol level, suggesting that cortisol is another contributory factor to the BP surge observed during the early hours of the morning. 17
A cycle for insulin has also been described. Infusion of insulin in the forearm of diabetic patients in a closed‐loop system designed to maintain a constant level of plasma glucose demonstrated the need for a greater insulin infusion rate during the early hours of the morning, corresponding to the surge in BP and increase in glucagon at this time. 23
A certain degree of circadian pattern has also been demonstrated for the activities or plasma concentrations of other hormones and peptides (such as atrial natriuretic peptide and calcitonin gene‐related peptides), but their cyclic function is not clear.
MORNING BP INCREASE—TEMPORAL ASSOCIATION WITH CARDIOVASCULAR EVENTS
The BP increase that takes place in the morning following the overnight fall in BP in dippers is temporally related to the increase in cardiovascular accidents, such as stroke and myocardial infarction. 1 , 24 In a typical hypertensive patient, SBP and DBP both rise by more than 10 mm Hg during the waking hours, normally between 6 a.m. and 10 a.m. 7 , 25 In association with this increase, the incidence of stroke dramatically rises by almost three‐fold between 8 a.m. and 10 a.m., 26 the incidence of myocardial infarction increases by up to two‐fold between 5 a.m. and noon, 1 and the incidence of sudden cardiac death increases by approximately 1.5‐fold between 6 a.m. and noon. 2
The increase in cardiovascular accidents appears to be associated with the waking hours rather than with the time of day. Adjusting the data on the incidence of sudden cardiac death to consider the number of hours after waking shows a transient doubling of the incidence of stroke within the first 3 hours of waking (Figure 3). 2
Figure 3.
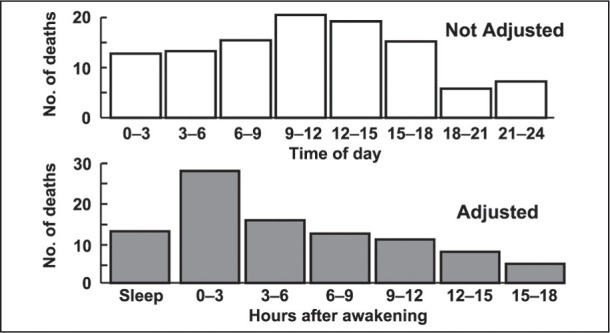
Circadian incidence of sudden cardiac death, adjusted for time of awakening. 2 Reprinted from Willich SN, et al. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68. © 1992, with permission from Excerpta Medica Inc.
Effect of Altered Sleep Pattern on BP Increase and Sudden Death
The circadian pattern of the BP decrease is dependent on activity rather than on time of day. This effect has been clearly demonstrated in an investigation of BP variations in those who do shift work requiring alternation between working days and nights. 27 In individuals who work during the night, DBP and SBP remain as expected during the activity of the day. BP does not decline until the period of sleep taken in the morning. Similarly, after the change to working during the day, the pattern of BP changes back to a fall during sleep at night, with an increase during the day. This change is rapid, suggesting that BP depends on the sleep pattern and not the circadian rhythm. Similarly, plasma renin activity follows the pattern of sleep, increasing during periods of sleep regardless of the time of day. 28
Interestingly, the incidence of sudden death contrasts with BP and plasma renin activity by not following the pattern of sleep. Investigation of the effects of altered sleep patterns due to changing time zones suggests that sudden cardiac death follows a circadian rhythm set in the time zone of origin. 29 The incidence of myocardial infarction and of sudden death in the overall population of the Hawaiian Islands shows a transient increase between 6 a.m. and noon, as expected. In contrast, the incidence of sudden death within the visiting population of Hawaii showed a much greater increase between noon and 6 p.m., which corresponded to sudden death in the morning in visitors who were from the continental United States.
BP DURING SLEEP AS A PROGNOSTIC INDICATOR
The existence of dippers and nondippers raises the question of the significance of the decrease in BP during sleep. Nondippers provide the opportunity to investigate the implications of BP remaining high during the night, as occurs in many untreated and treated hypertensive patients.
Association of Nondippers with Disease Severity
A number of studies have demonstrated an association of nondippers with an increase in severity of disease, such as an increased progression of renal disease and increased brain and cardiac complications. 30 , 31 A comparison of patients with hypertensive renal disease of over 3 years' duration showed a 1.7‐fold greater fall in creatine and a 1.7‐fold greater increase in the level of proteinuria in nondippers, relative to dippers matched for such characteristics as age, sex, and office BP. 30 This phenomenon suggests that being a nondipper is associated with a more rapid decline in renal function. Similarly, hypertensive nondippers have been associated with a greater average number of lacunae within the brain and a greater incidence of left ventricular hypertrophy, compared with hypertensive dippers and normotensive subjects. 31 More recently, increased intimal‐medial thickness of blood vessels and increased prevalence of plaques have been observed in hypertensive nondippers compared with hypertensive dippers. 32 Also, supraventricular and ventricular arrhythmias have been found to be more prevalent among nondippers than dippers. 33 Taken together, these data suggest that people in whom BP decreases during the night incur less damage to their brain, kidneys, heart, and blood vessels than people with elevated nocturnal BP.
Variation in Dipper Status
A person who may be categorized as a nondipper one night may change to become a dipper the following night. A study from Japan showed that nearly one third of untreated mildly to moderately hypertensive patients had changed their status the following night, 34 and a study conducted in the United Kingdom found that 44% of patients had a variable dipping status. 35 As dipper status varies within an individual, it is difficult to interpret data that rely on a single recording of ambulatory BP. Data from multiple recordings of nondipper/dipper intervals are required in order to draw any firm conclusions.
Dipper/Nondipper Survival Data
The probability of event‐free survival has been shown to differ between dipper and nondipper patients with essential hypertension. In a prospective study, 36 patients with essential hypertension were followed for up to 7.5 years. After adjustment for traditional risk markers for cardiovascular disease, the relative risk of cardiovascular morbidity in nondippers was almost double that of dippers (6.26 compared with 3.70). A retrospective study 37 compared hypertensive patients experiencing cardiovascular events with a similar group of hypertensive patients free from cardiovascular events. Nocturnal reductions of SBP and DBP were, on average, below 10% (nondipper) in women experiencing a cardiovascular event, compared with more than 10% (dipper) in women who remained event‐free, suggesting that the probability of cardiovascular events is greater in nondippers than in dippers. In a separate study, 38 the probability of cerebrovascular events was greater among nondippers than dippers.
More recently, analysis of the control group from the Systolic Hypertension in Europe (Syst‐Eur) Trial 39 showed that SBP at night (midnight to 6 a.m.) was more closely associated with the incidence of cardiovascular events than average daytime, 24‐hour, or conventional office‐measured BP. The relative hazard ratios predicted from a 10 mm Hg increase in nighttime SBP for cardiovascular, cardiac, and cerebrovascular events were 1.20, 1.16, and 1.31, respectively (increases of 20%, 16%, and 31%, respectively). Although these data are not specific to nondippers and dippers, they suggest that nondippers are more likely than dippers to experience cardiovascular events.
The Ohasama study 40 in Japan examined the relationship between nocturnal BP and mortality. Subjects aged 40 years or over from a rural community had their ambulatory BP measured and were then followed for a mean of 5.1 years, after which they were divided into groups according to the extremity of the nocturnal fall in SBP and DBP. Subjects with no nocturnal decline in SBP and DBP were termed inverted dippers, those with a decline of less than 10% were termed nondippers, those with a decline between 10% and 20% were termed dippers, and those with a decline greater than 20% were termed extreme dippers. The risk of mortality was highest in the inverted dippers (no nocturnal decline), followed by nondippers (Figure 4), and this association was more pronounced for cardiovascular mortality than for noncardiovascular mortality. Furthermore, there was no difference between dippers and extreme dippers, suggesting that extreme lowering of nocturnal BP has no detrimental effect on mortality rates, supporting the need for 24‐hour reduction in BP in hypertensives.
Figure 4.
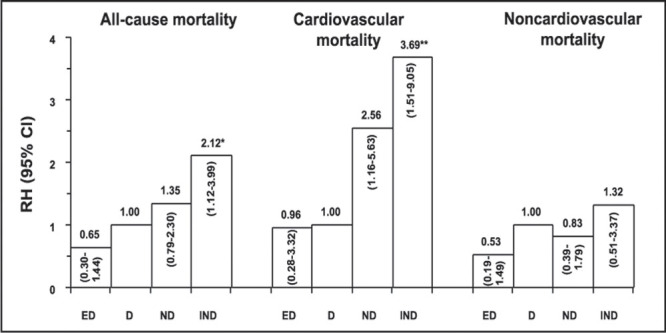
Comparison of the risk of mortality in extreme dippers (ED), dippers (D), nondippers (ND), and inverted dippers (IND) after an average follow‐up of 5.1 years. RH=relative hazard; *p=0.02; **p=0.004 vs. D Reprinted with permission of Elsevier Science from Am J Hypertens. 1997;10:1201–1207. 40
DISCUSSION
Much of the research into circadian rhythms is directed at clarifying an underlying pathophysiologic process that could be modified by pharmacologic or other means. Drugs can influence and sometimes block the effect of these circadian patterns. From a therapeutic point of view, the presence of a circadian rhythm with a morning surge in BP suggests that a drug given once daily should be able to cover this increase through a long half‐life as well as through its mechanisms of action. Drugs capable of also reducing the morning increase in NE and angiotensin II, in particular, could have a more cardioprotective effect and a better BP‐lowering effect. For example, the long‐acting angiotensin II receptor antagonist telmisartan not only has an effect on BP over a complete 24‐hour period because of its long half‐life, but it could also limit the morning surge in BP through an effect on the RAAS and noradrenergic system.
The dipper status may be influenced by drugs. For example, Uzu et al. 41 have shown a significant association between diuretic therapy (hydrochlorothiazide) and the nocturnal fall in SBP and DBP in nondippers who became dippers, but not in previously recorded dippers. In contrast, a blunting of the nocturnal fall in BP was recently suggested in a study to investigate the effects of the α1‐adrenergic antagoist doxazosin. 42 Exploitation of the knowledge gained from these and similar studies should prove useful in further understanding the significance of the dipper status relative to the incidence of cardiovascular disease and may lead to a better understanding of hypertensive therapy.
References
- 1. Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1323. [DOI] [PubMed] [Google Scholar]
- 2. Willich SN, Goldberg RJ, Maclure M, et al. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68. [DOI] [PubMed] [Google Scholar]
- 3. Neaton JD, Kuller L, Stamler J, et al. Impact of systolic and diastolic blood pressure on cardiovascular mortality. In: Laragh JH, Brenner BM, eds. Hypertension: Pathology, Diagnosis, and Management. 2nd ed. New York, NY: Raven Press; 1995:127–144. [Google Scholar]
- 4. MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Lancet. 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 5. Mancia G, Sega R, Milesi C, et al. Blood‐pressure control in the hypertensive population. Lancet. 1997;349:454–457. [DOI] [PubMed] [Google Scholar]
- 6. Redman CWG, Beilin LJ, Bonnar J. Reversed diurnal blood pressure rhythm in hypertensive pregnancies. Clin Sci Mol Med Suppl. 1976;3:687S–689S. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Ferrari A, Gregorini L, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104. [DOI] [PubMed] [Google Scholar]
- 8. Kobrin I, Oigman W, Kumar A, et al. Diurnal variation of blood pressure in elderly patients with essential hypertension. J Am Geriatr Soc. 1984;32:896–899. [DOI] [PubMed] [Google Scholar]
- 9. Baumgart P, Walger P, Gerke M, et al. Nocturnal hypertension in renal failure, haemodialysis and after renal transplantation. J Hypertens Suppl. 1989;7(6):S70–S71. [DOI] [PubMed] [Google Scholar]
- 10. Imai Y, Abe K, Munakata M, et al. Does ambulatory blood pressure monitoring improve the diagnosis of secondary hypertension? J Hypertens Suppl. 1990;8(6):S71–S75. [PubMed] [Google Scholar]
- 11. Portaluppi F, Montanari L, Massari M, et al. Loss of nocturnal decline of blood pressure in hypertension due to chronic renal failure. Am J Hypertens. 1991;4:20–26. [DOI] [PubMed] [Google Scholar]
- 12. Verdecchia P. Prognostic value of ambulatory blood pressure. Hypertension. 2000;35:844–851. [DOI] [PubMed] [Google Scholar]
- 13. Pickering TG. The clinical significance of diurnal blood pressure variations: dippers and non‐dippers. Circulation. 1990;81:700–702. [DOI] [PubMed] [Google Scholar]
- 14. Gordon RD, Wolfe LK, Island DP, et al. A diurnal rhythm in plasma renin activity in man. J Clin Invest. 1966; 45:1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stern N, Sowers JR, McGinty D, et al. Circadian rhythm of plasma renin activity in older normal and essential hypertensive men: relation with inactive renin, aldosterone, cortisol and REM sleep. J Hypertens. 1986;4:543–550. [DOI] [PubMed] [Google Scholar]
- 16. Linsell CR, Lightman SL, Mullen PE, et al. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. 1985;60:1210–1215. [DOI] [PubMed] [Google Scholar]
- 17. Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to a‐sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. [DOI] [PubMed] [Google Scholar]
- 18. Laflamme AK, Oster L, Cardinal R, et al. Effects of reninangiotensin blockade on sympathetic reactivity and β‐adrenergic pathway in the spontaneously hypertensive rat. Hypertension. 1997;30:278–287. [DOI] [PubMed] [Google Scholar]
- 19. Julius S. Corcoran Lecture. Sympathetic hyperactivity and coronary risk in hypertension. Hypertension. 1993;21:886–893. [DOI] [PubMed] [Google Scholar]
- 20. Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol. 1988;62:635–637. [DOI] [PubMed] [Google Scholar]
- 21. Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–1518. [DOI] [PubMed] [Google Scholar]
- 22. Weitzman ED, Fukushima D, Nogeire C, et al. Twenty‐four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. [DOI] [PubMed] [Google Scholar]
- 23. Bolli GB, Gerich JE. The “dawn phenomenon”—a common occurrence in both non‐insulin‐dependent and insulin‐dependent diabetes mellitus. N Engl J Med. 1984;310:746–750. [DOI] [PubMed] [Google Scholar]
- 24. Kelly‐Hayes M, Wolf PA, Kase CS, et al. Temporal patterns of stroke onset. The Framingham Study. Stroke. 1995; 26:1343–1347. [DOI] [PubMed] [Google Scholar]
- 25. Millar‐Craig MW, Bishop CN, Raftery EB. Circadian variation of blood pressure. Lancet. 1978;1:795–797. [DOI] [PubMed] [Google Scholar]
- 26. Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke. 1989;20:473–476. [DOI] [PubMed] [Google Scholar]
- 27. Baumgart P, Walger P, Fuchs G, et al. Twenty‐four‐hour blood pressure is not dependent on endogenous circadian rhythm. J Hypertens. 1989;7:331–334. [PubMed] [Google Scholar]
- 28. Brandenberger G, Follenius M, Goichot B. Twenty‐fourhour profiles of plasma renin activity in relation to the sleepwake cycle. J Hypertens. 1994;12:2 77–283. [PubMed] [Google Scholar]
- 29. Couch RD. Travel, time zones and sudden cardiac death. Am J Forensic Med Pathol. 1990;11:106–111. [DOI] [PubMed] [Google Scholar]
- 30. Timio M, Venanzi S, Lolli S, et al. ‘Non‐dipper’ hypertensive patients and progressive renal insufficiency: a 3‐year longitudinal study. Clin Nephrol. 1993;43:382–387. [PubMed] [Google Scholar]
- 31. Shimada K, Kawamoto A, Matsubayashi K, et al. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992; 10:875–878. [PubMed] [Google Scholar]
- 32. Roman MJ, Pickering TG, Schwartz JE, et al. Is the absence of a normal nocturnal fall in blood pressure (nondipping) associated with cardiovascular target organ damage? J Hypertens. 1997;15:969–978. [DOI] [PubMed] [Google Scholar]
- 33. Ijiri H, Khono I, Iwasaki H, et al. Cardiac arrhythmias and left ventricular hypertrophy in dipper and nondipper patients with essential hypertension. Jpn Circ J. 2000;64:499–504. [DOI] [PubMed] [Google Scholar]
- 34. Mochizuki Y, Okutani M, Donfeng Y, et al. Limited reproducibility of circadian variation in blood pressure dippers and nondippers. Am J Hypertens. 1998;11:403–409. [DOI] [PubMed] [Google Scholar]
- 35. Manning G, Rushton L, Donnelly R, et al. Variability of diurnal changes in ambulatory blood pressure and nocturnal dipping status in untreated hypertensive and normotensive subjects. Am J Hypertens. 2000;13:1035–1038. [DOI] [PubMed] [Google Scholar]
- 36. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 37. Verdecchia P, Schillaci G, Gatteschi C, et al. Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation. 1993;88:986–992. [DOI] [PubMed] [Google Scholar]
- 38. Phillips RA, Sheinart KF, Godbold JH, et al. The probability of cerebrovascular events also appears to be greater among nondippers than dippers. Am J Hypertens. 2000; 13:1250–1255. [DOI] [PubMed] [Google Scholar]
- 39. Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999; 282:539–546. [DOI] [PubMed] [Google Scholar]
- 40. Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality. Am J Hypertens. 1997;10:1201–1207. [DOI] [PubMed] [Google Scholar]
- 41. Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. [DOI] [PubMed] [Google Scholar]
- 42. Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of α‐adrenergic blocker, doxazosin. Hypertension. 2000;35:787–794. [DOI] [PubMed] [Google Scholar]


