Abstract
An 8‐week, multicenter, double‐blind, randomized, parallel‐group, forced‐titration study was conducted to evaluate the antihypertensive efficacy of candesartan vs. losartan in 654 hypertensive patients with a diastolic blood pressure between 95 and 114 mm Hg from 72 sites throughout the U.S. Eligible patients were randomized to candesartan cilexetil 16 mg once daily, or losartan 50 mg once daily. Two weeks following randomization, patients doubled the respective doses of their angiotensin receptor blockers for an additional 6 weeks. At week 8, candesartan cilexetil lowered trough systolic/diastolic blood pressure by a significantly greater amount than did losartan (13.3/10.9 mm Hg with candesartan cilexetil vs. 9.8/8.7 mm Hg with losartan; p< 0.001). At the same period, candesartan cilexetil also lowered peak blood pressure by a significantly greater amount than did losartan (15.2 to 11.6 mm Hg with candesartan cilexetil vs. 12.6 to 10.1 mm Hg with losartan; p< 0.05). There were statistically significantly (p< 0.05) higher proportions of responders and controlled patients in the candesartan cilexetil group (62.4% and 56.0%, respectively) than in the losartan group (54.0% and 46.9%, respectively). Both treatment regimens were well tolerated; 1.8% in the candesartan cilexetil group and 1.6% in the losartan group withdrew because of adverse events. In conclusion, this forced‐titration study confirms that candesartan cilexetil is more effective than losartan in lowering blood pressure when both are administered once daily at maximum doses. Both drugs were well tolerated.
Angiotensin II receptor blockers (ARBs) inhibit the renin‐angiotensin system by selectively blocking the AT1 subtype of angiotensin II receptor. Various studies have demonstrated their effectiveness in lowering blood pressure with an excellent tolerability and safety profile. 1 Further large‐scale studies are being conducted to determine whether the use of this class of drugs will result in end‐organ protection, as well as beneficial effects on morbidity and mortality. 2
Different ARBs vary in their binding characteristics to the AT1 subtype of angiotensin II receptor. Preclinical studies have demonstrated that candesartan is a highly selective, insurmountable ARB. 3 It has an in vitro affinity for the AT1 receptor 80 times greater than that of losartan and 10 times greater than that of EXP‐3174, the active metabolite of losartan. 4 However, it remains uncertain whether these differences in pharmacologic properties result in greater blood pressure (BP) lowering efficacy for candesartan, compared to that of other ARBs.
Clinically, candesartan is administered as candesartan cilexetil, an inactive prodrug that is hydrolyzed to candesartan during absorption from the gastrointestinal tract. Three previous studies have demonstrated greater antihypertensive efficacy of candesartan cilexetil when compared to losartan. However, these studies either evaluated the starting doses of both drugs or used a response titration design for comparison at once‐daily maximum doses. 5 , 6 , 7 The present study is one of two identically designed, concurrently conducted, forced‐titration studies that provide a direct comparison of the blood pressure lowering effects of these two ARBs at once‐daily maximum doses.
METHODS
In this 8‐week, multicenter, double‐blind, randomized, parallel‐group, forced‐titration study, candesartan cilexetil was compared to losartan in 654 hypertensive patients from 72 sites throughout the U.S. The study population consisted of men and women without childbearing potential between 18 and 80 years of age with moderate hypertension (a mean sitting diastolic BP [DBP] of 95–114 mm Hg). Major exclusion criteria included systolic BP (SBP of ≥180 mm Hg or DBP of ≥115 mm Hg, known hypersensitivity to ARBs, secondary hypertension, severely impaired liver function, significant renal impairment, hemodynamically significant valvular heart disease, angina pectoris requiring more than short‐acting nitrates, and a recent history of myocardial infarction, coronary revascularization procedures, stroke, or transient ischemic attack. Current use of an antihypertensive agent was cause for exclusion, unless it could be discontinued safely by the first week of the placebo run‐in period. The study protocol was approved by the Institutional Review Board at each site, and all patients provided written informed consent.
For each patient, visits were scheduled at the same time in the morning. Patients were instructed to refrain from taking the study medication on the morning of clinic visits until after BP was measured. All BP determinations were performed in the sitting position with a mercury sphygmomanometer under standardized conditions. Blood pressure was measured three times at 2‐minute intervals and the mean value computed. The differences in the DBP readings were required to be no more than 5 mm Hg, with additional readings performed if necessary until such consistency was obtained. To be eligible for the study, patients' DBP had to be in the range of 95–114 mm Hg measured on two visits during the single‐blind, 4‐ or 5‐week placebo run‐in period.
Once eligibility was confirmed, patients were randomized in a 1:1 ratio to candesartan cilexetil 16 mg once daily, or losartan 50 mg once daily. After 2 weeks of randomized treatment, all patients were required to double their dose of candesartan cilexetil (16 to 32 mg once daily), or losartan (50 to 100 mg once daily) for an additional 6 weeks. Patients were evaluated at weeks 1, 2, 4, and 8 during the 8‐week double‐blind period. Patients were also seen at follow up visits, 48 hours following their last dose of study medication and 2 weeks after they had discontinued therapy with the study medication. Post‐study treatment for hypertension was not instituted until after the 48‐hour assessment was completed. Trough sitting BP (24±3 hours after dose) and heart rate were recorded at each visit. In addition, peak BP (6±2.5 hours after dose) was measured at week 3 or 4 of the placebo run‐in period, and also at week 8 of the double‐blind period.
Compliance with the protocol‐defined treatment regimen was assessed by tablet and capsule counts derived from the drug accountability case report form. The actual number of tablets and capsules used (number of tablets and capsules dispensed minus number of tablets and capsules returned) was divided by the expected number of tablets and capsules used, then multiplied by 100 to obtain a compliance percentage. This compliance percentage was calculated for all randomized patients by treatment group for the placebo run‐in phase and for the randomized treatment period.
Statistical analyses were performed with an intent‐to‐treat approach, with the last observation carried forward (i.e., last available BP on treatment carried forward to week 8 for patients who withdrew). An analysis of covariance was employed for the primary efficacy parameter to ascertain whether candesartan cilexetil 16 mg titrated to 32 mg was different from losartan 50 mg titrated to 100 mg with respect to reducing trough DBP over an 8‐week treatment period. In order to accomplish this comparison, the generalized linear models procedure in SAS® was utilized, with the change from baseline to double‐blind week 8 in trough sitting DBP as the response variable; treatment, center, and treatment by center were fixed effects in the model and the baseline trough sitting DBP was the covariate. The appropriateness of employing an analysis of covariance was assessed by examining the linear model using the same response variable and including treatment, baseline value, and treatment by baseline interaction value as fixed effects in the model. This interaction term was assessed at the 0.10 level of significance to determine the parallelism of slopes between the treatment groups assumed in the covariate analysis. In all cases, the analysis was repeated with exclusion of the covariate.
The investigations of other secondary efficacy variables were identical to the aforementioned analyses. The secondary efficacy end points included the change from baseline to week 8 in trough SBP, the changes in peak SBP and DBP, and changes in trough SBP and DBP, 48 hours after the last dose of the study medication. In addition, the proportion of responders (either a sitting trough DBP at week 8 of <90 mm Hg or a decrease from baseline of ≥10 mm Hg) and controlled patients (a sitting trough DBP at week 8 of <90 mm Hg) at week 8 were analyzed across treatment groups by means of Fisher's exact test. All data analyses are presented using the least‐squares means. A p value of <0.05 was taken as statistically significant. All changes in BP are expressed as means with 95% confidence intervals (CI). Adverse events and laboratory data were compared descriptively between the two treatment groups. Laboratory data were evaluated according to predefined limits of change and mean change from baseline.
RESULTS
A total of 654 patients were randomized to either candesartan cilexetil (n=332) or losartan (n=322). Six hundred nineteen patients (95%) completed the entire 8‐week, double‐blind treatment period: 96% for candesartan cilexetil and 94% for losartan. The mean treatment compliance during the placebo run‐in phase was 95.6%. During the double‐blind portion of the study, compliance was similar between the two treatment groups, with the mean compliance for candesartan cilexetil at 102.3% and for losartan at 101.2%. The study population was 41.9% female and 17.3% black, with a mean age of 54.1 years and a mean baseline BP of 152/100 mm Hg. About 9% of patients had diabetes mellitus. Patient characteristics at baseline were similar in the two treatment groups (Table).
Table TABLE.
PATIENT CHARACTERISTICS AT BASELINE
| Candesartan Cilexetil n=322) | Losartan (n=332) | Overall (n=654) | |
| Age in years* | 54.2 (11.1) | 54.1 (10.4) | 54.1 (10.8) |
| Weight in pounds* | 205.6 (46.6) | 202.6 (42.1) | 204.2 (44.4) |
| Duration of hypertension in years * | 10.4 (8.9) | 10.0 (9.0) | 10.2 (9.0) |
| Sex** | |||
| Male | 192 (57.8) | 188 (58.4) | 380 (58.1) |
| Female | 140 (42.2) | 134 (41.6) | 274 (41.9) |
| Race** | |||
| Nonblack | 273 (82.2) | 268 (83.2) | 541 (82.7) |
| Black | 59 (17.8) | 54 (16.8) | 113 (17.3) |
| Baseline trough sitting DBP* | 100.1 (3.9) | 99.9 (4.2) | 100.0 (4.1) |
| Baseline trough sitting SBP* | 152.6 (12.3) | 152.0 (12.6) | 152.3 (12.4) |
| *Expressed as least squares mean (SD);**Expressed as number (%); DBP=diastolic blood pressure; SBP=systolic blood pressure; includes all formulations, strengths, and brands combined for each of the drugs. | |||
As shown in Fig. 1, candesartan cilexetil lowered mean sitting trough SBP/DBP by 13.3/10.9 mm Hg, compared to a mean reduction of 9.8/8.7 mm Hg by losartan at week 8 (p<0.001 for both DBP and SBP). Peak mean sitting SBP/DBP was reduced by 15.2/11.6 mm Hg with candesartan cilexetil treatment, and by 12.6/10.1 mm Hg with losartan treatment (Fig. 2; p<0.05 for both DBP and SBP). At 48 hours after dosing, candesartan cilexetil continued to produce reductions in mean SBP/DBP of 11.2/10.2 mm Hg, while losartan provided mean reductions of 5.3/6.0 mm Hg (Fig. 3; p<0.0001 for both DBP and SBP).
Figure 1.
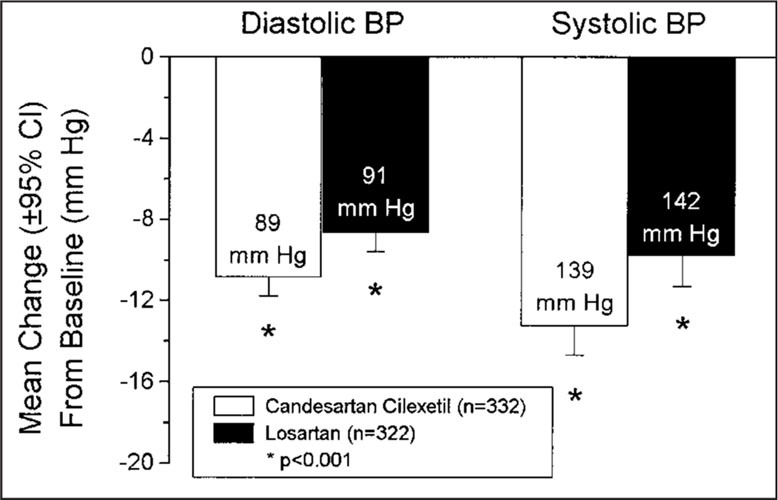
Effects of candesartan cilexetil and losartan on trough blood pressure (BP). Labels within bars are the trough sitting BP readings (24±3 hours after dosing) at week 8. CI=confidence interval.
Figure 2.
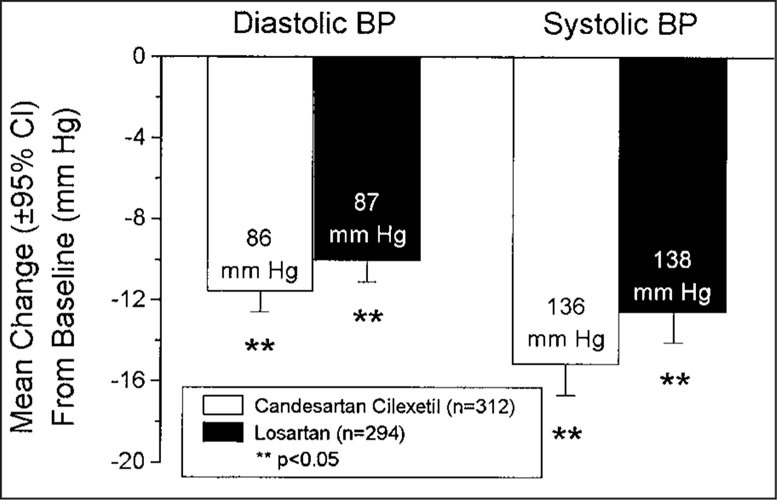
Effects of candesartan cilexetil and losartan on peak blood pressure (BP). Labels within bars are the peak sitting BP readings (6±2.5 hours after dose) at week 8. CI=confidence interval.
Figure 3.
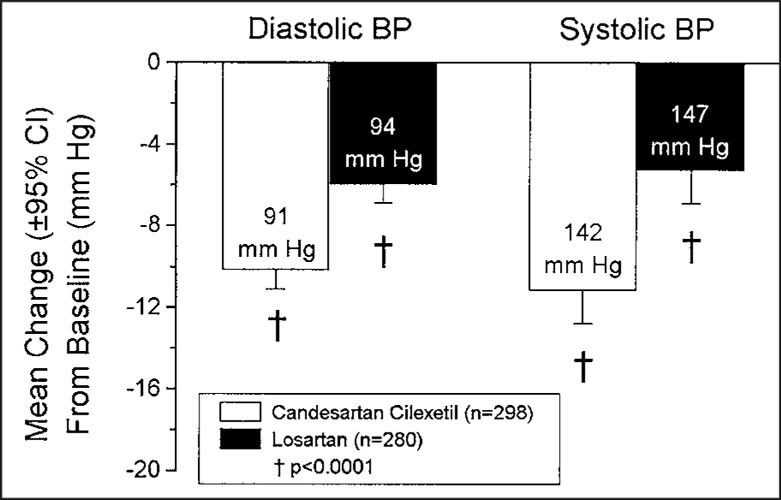
Effects of candesartan cilexetil and losartan on blood pressure (BP) 48 hours after the last dose of study medication. Labels within bars are the BP readings at the 48‐hour, post‐dosing clinic visit. CI=confidence interval.
The trough‐to‐peak ratio was 0.96 for the candesartan cilexetil group, and 0.88 for the losartan group. The proportion of patients who responded to treatment was significantly higher (p=0.033) in the candesartan group (62.4%) than in the losartan group (54.0%). Proportionately, more candesartan cilexetil patients than losartan patients attained control of DBP after treatment (56.0% compared to 46.9%; p=0.023)
Overall, the incidence and intensity of adverse events were similar in the two treatment groups. A total of 301 of 654 (46.0%) patients reported a treatment‐emergent adverse event—46.4% in the candesartan cilexetil group and 45.7% in the losartan group. Most adverse events were mild to moderate in intensity and resolved despite continued treatment, including dose escalation. The most common adverse events for the candesartan cilexetil group were respiratory infection (9.0%), dizziness (5.1%), headache (4.5%), and sinusitis (4.5%), whereas those for the losartan group were respiratory infection (9.9%), headache (5.3%), pharyngitis (3.7%), and back pain (3.4%). Eleven of the 654 (1.7%) patients withdrew from the study due to an adverse event, including six (1.8%) in the candesartan group and five (1.6%) in the losartan group. Four of the 654 (0.6%) patients reported treatment‐emergent events that were considered serious because they required hospitalization during the double‐blind treatment period; three were in the candesartan cilexetil group and one was in the losartan group. All events were considered by the investigators unlikely to be related to study medication. There were no deaths during this trial. Minor changes from baseline in laboratory values were observed in isolated patients. There were no clinically meaningful changes in mean laboratory values in either treatment group and no laboratory evidence of deterioration in renal, hepatic, or metabolic function.
DISCUSSION
This study demonstrates that candesartan had greater efficacy in lowering arterial pressure when compared to losartan. Moreover, the duration of effect and response/control rates were significantly better with candesartan than losartan. Side effect profiles were similar between the two groups. Data from this study, taken together with three other randomized, double‐blind studies comparing candesartan and losartan, demonstrate superior antihypertensive efficacy of candesartan over losartan. Andersson and Neldam 5 evaluated candesartan cilexetil 8 and 16 mg vs. losartan 50 mg and found that candesartan cilexetil 16 mg once daily (n=84) reduced trough DBP more effectively than losartan 50 mg once daily (n=83), by 3.7 mm Hg (p<0.05). Also, in the Candesartan Versus Losartan Efficacy Comparison Study (CANDLE), 6 candesartan cilexetil 16 mg, dose‐titrated if necessary to 32 mg once daily (n=160), reduced trough DBP more effectively than losartan 50 mg, dose‐titrated if necessary to 100 mg, once daily (n=169), by 2.1 mm Hg (p<0.05). Lastly, Lacourciere and Asmar 7 compared the effects of candesartan cilexetil 8 mg force‐titrated to 16 mg (n=116) and losartan 50 mg force‐titrated to 100 mg (n=115) once daily, as assessed by clinic and ambulatory blood pressure. They found that candesartan cilexetil 16 mg reduced ambulatory BP to a significantly greater extent than 100 mg of losartan, particularly systolic ambulatory BP during the daytime (p<0.05), nighttime (p<0.05), and 24‐hour period (p<0.01). In addition, candesartan cilexetil lowered both SBP and DBP after a missed dose to a greater extent than losartan (11.9/8.0 mm Hg and 6.1/4.5 mm Hg, respectively; p<0.05).
The net difference of candesartan cilexetil (CC) in lowering trough BP by 3.5/2.2 mm Hg more than losartan may appear too small to be of clinical significance, as it is common in clinical practice to encounter spontaneous BP variation of this magnitude in an individual patient. But in this study, precautions were taken to minimize spontaneous fluctuation of BP or recruitment of patients with labile BP. Blood pressure was measured under the same standardized conditions at each visit, and differences in serial DBP readings during each visit were required to be <5 mm Hg. The fact that CC consistently lowered trough, peak, and 48 hours post‐dose BP compared to losartan indicated true differences between the two drugs. It is interesting to note that the SBP/DBP differences between candesartan cilexetil and losartan widened at 48 hours post‐dose, i.e., 5.9/4.3 mm Hg, respectively. Thus, candesartan cilexetil produces an extended therapeutic antihypertensive effect that may confer additional protection to a patient with occasional missed doses. Furthermore, the CC group, compared with the losartan group, had statistically significantly higher rates of responders (62.4% and 54.0%, respectively) and controlled patients (56.0% and 46.9%, respectively).
The BP differences, although moderate, might result in clinically important benefits. Epidemiologic data show that cardiovascular risk increases with every mm Hg of BP above 110/70 mm Hg. 8 , 9 In the cohort of men screened for the Multiple Risk Factor Intervention Trial (MRFIT), 9 SBP increases of 20 mm Hg and 40 mm Hg increased the 11.6‐year risk of coronary heart disease deaths by 156% and 244%, respectively. Every SBP increment resulted in a 6%–8% increase in risk. Blood pressure reductions, even minor, assume clinical importance in high‐risk patients. About 9% of the study population had diabetes mellitus. It should be noted that the Hypertension Optimal Treatment (HOT) Study demonstrated that a mean decrease of 4.1 mm Hg in DBP was associated with a 51% reduction in major cardiovascular events in patients with diabetes mellitus. 10
Finally, only a moderate advantage over losartan in the treatment of hypertension could be expected from candesartan as monotherapy, despite the superior binding characteristics of this agent to angiotensin II. This pathway may be only one, rather than the sole, effector in the pathogenesis of hypertension. In general, hypertension is a multifactorial disease and a single antihypertensive agent targeting one mechanism provides long‐term BP control for only approximately 50% of hypertensive patients. 11 However, in situations in which the renin‐angiotensin system is activated, candesartan cilexetil may exert a greater antihypertensive effect. This occurs when candesartan cilexetil is used with a diuretic, such as hydrochlorothiazide. Ohman et al. 12 reported that candesartan cilexetil plus hydrochlorothiazide (16 and 12.5 mg, respectively) reduced DBP/SBP by 19.4/10.4 mm Hg (n=151), whereas losartan plus hydrochlorothiazide (50 and 12.5 mg, respectively) reduced DBP/SBP by 13.7/7.8 mm Hg (n=148). The difference of 5.7/2.6 mm Hg in SBP/DBP reduction was statistically significant (p<0.05). These findings suggest that ARBs with different binding characteristics may exert different degrees of antihypertensive efficacy, both as monotherapy and in combination with other agents.
In conclusion, the results of this randomized, double‐blind, parallel‐group, forced‐titration study in a diverse population of hypertensive patients in the U.S. indicates that 32 mg of candesartan cilexetil, given once daily, lowers the peak, trough, and 48‐hour post‐dose BP more effectively than 100 mg of losartan given in the same time course. There is no difference in their safety/tolerability profile. This study confirms that candesartan cilexetil is a more effective antihypertensive agent than losartan when compared at once‐daily maximum doses. Both drugs are well tolerated.
Acknowledgments: We gratefully acknowledge the assistance of Channeary McDowell, BS; Jeanine Parsons, BS; Melissa Grozinski, BS; Anne Kezer, BS; Conrad Tou, PhD; Terry Flanagan, MPH; James Gaddy, PhD; Oliver Yeh, BA; and Debbie Brangman, MBA in the conduct of the study and manuscript preparation, and the diligent efforts of the study coordinators at the 72 investigative sites. Supported by a grant from AstraZeneca LP, Wayne, PA
Appendix: CLAIM Study Investigators: Amin Abdelghany, MD, Panama City, FL; Allen B. Adolphe, MD, Albuquerque, NM; J. Richard Allison, MD, Columbia, SC; Norse Bear, MD, Lakewood, CO; Patricia Buchanan, MD, Eugene, OR; Michael Burleson, MD, Eureka, CA; Larry L. Carr, DO, Bay City, MI; Rogelio Cattan, MD, Miami, FL; Bronell Chandler, MD, Upper Darby, PA; Shane Christensen, MD, Salt Lake City, UT; Steven G. Chrysant, MD, Oklahoma City, OK; James L. Conrad, MD, Perkasie, PA; Lydia Corn, MD, Sarasota, FL; Robert E. Cronin, MD, Dallas, TX; Steven Curland, MD, Norwich, CT; William Dachman, MD, Mesa, AZ; Berge Dadourian, MD, Las Vegas, NV; D. Marty Denny, MD, Jeffersonville, IN; Margaret Drehobl, MD, San Diego, CA; Bonafacio Ferrer, MD, Cleveland, OH; David Fried, MD, Providence, RI; Stefan Gravenstein, MD, Norfolk, VA; Joe L. Hargrove, MD, Little Rock, AR; J. Freeman Harris, MD, Lake Oswego, OR; Terence Hart, MD, Muscle Shoals, AL; Lawrence Horwitz, MD, Denver, CO; Nazim A. Jaffer, MD, Youngstown, OH; Lois Anne Katz, MD, New York, NY; Robert Kipperman, MD, Oklahoma City, OK; Tom C. Klein, MD, Wichita, KS; Nelson Kopyt, DO, Allentown, PA; Julie A. Kovach, MD, Ann Arbor, MI; John Lafata, MD, Vista, CA; Bobby Quentin Lanier, MD, Fort Worth, TX; Kenneth Lasseter, MD, Miami, FL; Andrew J. Lewin, MD, Los Angeles, CA; Richard Maravel, MD, New Port Richey, FL; Clark D. McKeever, MD, Houston, TX; Mahendra Mirani, MD, Hamburg, NY; K. K. Mohan, MD, Essexville, MI; Jeffrey S. Morton, MD, Peoria, IL; William S. Mullican, MD, Evansville, IN; Gary Rabetoy, MD, Salt Lake City, UT; Edward Myers, DO, Warren, OH; Puneet Narayan, MD, Springfield, VA; Stephen Nash, MD, Syracuse, NY; Nicholas Nayak, MD, Normal, IL; Kuang Ou, MD, Pittsburgh, PA; John E. Pappas, MD, Lexington, KY; Jeffrey Passer, MD, Omaha, NE; Andres Patron, DO, Hollywood, FL; Robert A. Phillips, MD, New York, NY; Jacob Pinnas, MD, Tucson, AZ; Robert B. Rhoades, MD, Martinez, GA; Peter M. Ripley, MD, South Yarmouth, MA; Jeffrey S. Rosen, MD, Coral Gables, FL; Dennis Ruff, MD, San Antonio, TX; Douglas Schumacher, MD, Columbus, OH; L. Kent Smith, MD, Phoenix, AZ; David H.G. Smith, MD, Long Beach, CA; James W. Snyder, MD, Las Vegas, NV; Nina Stuccio‐White, DO, Marlton, NJ; Louise Taber, MD, Phoenix, AZ; Melvin Tonkon, MD, Anaheim, CA; Timothy S. Truitt, MD, Melbourne, FL; Nostratola D. Vizari, MD, Orange, CA; Heiner Vogelbach, MD, Arcadia, CA; Jonathan Wechsler, DO, Las Vegas, NV; Kenneth Williams, MD, Baltimore, MD; Leonard Wojnowich, MD, Atlanta, GA.
References
- 1. Bakris GL, Weber MA, Black HR, et al. Clinical efficacy and safety profiles of AT1 receptor antagonist. Cardiovasc Rev Rep. 1999;20:77–100. [Google Scholar]
- 2. Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. [DOI] [PubMed] [Google Scholar]
- 3. Morsing P, Adler G, Brandt‐Eliasson U, et al. Mechanistic differences of various AT1‐receptor blockers in isolated vessels of different origin. Hypertension. 1999;33:1406–1413. [DOI] [PubMed] [Google Scholar]
- 4. Nishikawa K, Naka T, Chatani F, et al. Candesartan cilexetil; a review of its preclinical pharmacology. J Hum Hypertens. 1997;11(suppl 2):S9–S17. [PubMed] [Google Scholar]
- 5. Andersson OK, Neldam S. The antihypertensive effect and tolerability of candesartan cilexetil, a new generation angiotensin II antagonist, in comparison with losartan. Blood Pressure. 1998;7:53–59. [DOI] [PubMed] [Google Scholar]
- 6. Gradman AH, Lewin A, Bowling BT, et al. Comparative effects of candesartan cilexetil and losartan in patients with systemic hypertension. Heart Dis. 1999;1:52–57. [PubMed] [Google Scholar]
- 7. Lacourciere Y, Asmar R, for the Candesartan/Losartan Study Investigators . A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients: A placebo‐controlled, forced titration study. Am J Hypertens. 1999;12:1181–1187. [DOI] [PubMed] [Google Scholar]
- 8. National High Blood Pressure Education Program Working Group . Report on primary prevention of hypertension. Arch Intern Med. 1993;153:186–208. [PubMed] [Google Scholar]
- 9. Stamler J, Stamler R, Neaton J. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch Intern Med. 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 10. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low dose aspirin in patients with hypertension: Principal results of the Hypertensive Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 11. Materson BJ, Reda DJ, Cushman WC, et al. Single‐drug therapy for hypertension in men: A comparison of six antihypertensive agents with placebo. N Engl J Med. 1993: 328: 914–921. [DOI] [PubMed] [Google Scholar]
- 12. Ohman KP, Milon H, Valnes K. Candesartan cilexetil‐HCT in primary hypertension insufficiently controlled on monotherapy—a comparison with losartan‐HCT. Am J Hypertens. 2000;13(4, pt 2):137A. [DOI] [PubMed] [Google Scholar]


