Abstract
Obesity is a common disease with an ever‐increasing prevalence and usually with late‐onset consequences. If acquired during childhood, it tracks into adult life to some extent, and since the relationship between obesity and hypertension is well established in adults, obese children appear to be at particularly high risk of becoming hypertensive adults. In the authors'study, obese children seemed to have significantly higher casual and ambulatory blood pressure than nonobese children, except for nighttime diastolic blood pressure. The health effects of obesity may depend on the anatomic distribution of body fat, which in turn may be a better indicator of endocrinologic imbalance, environmental stress, or genetic factors than is fatness per se. Subjects with a higher waist‐to‐hip ratio or a larger waist, as an estimate of central obesity, tend to have higher blood pressure values even during childhood. Prevention of the onset of obesity in early life may be important to reducing the risk of coronary heart disease in later life.
The prevalence of obesity is high and increasing, and the disorder usually has late‐onset consequences. In westernized societies, it is currently estimated that approximately 35% of the population, and about 50% of those over 50 years of age, are overweight or obese. 1 Although many of the associated medical problems are deferred to adult life, an important proportion of adult obesity has its origins in childhood. 1 , 2 , 3 It appears that nearly 50% of overweight children become overweight adults. These figures are of great medical concern, as obesity is a risk factor for a wide variety of conditions and is associated with myriad comorbid diseases.
Substantial clinical and epidemiologic evidence supports the influence of obesity on blood pressure (BP) levels, even early in life. 4 The association between obesity and hypertension has been well documented. 5 Hypertension is one of the most important obesity‐related cardiovascular risk factors for two reasons: obesity‐induced hypertension is very prevalent, and the lowering of BP levels is the most cost‐effective way to reduce cardiovascular morbidity and mortality. Factors associated with childhood BP may provide important insights into adult hypertension. Obesity acquired during childhood tracks, to some extent, into adult life, 6 and since the relationship between obesity and hypertension is well established in adults, 7 obese children appear to be at particularly high risk of becoming hypertensive adults.
The type of obesity is an important determinant of the prevalence of hypertension, and subjects with central or abdominal obesity have the highest risk of developing hypertension, along with other cardiovascular risk factors. Although the waist/hip (W/H) ratio has been shown to be a useful marker of abdominal obesity in adults, 8 waist circumference is increasingly used as the best anthropometric alternative to the W/H ratio. How body fat distribution patterns develop in childhood, and which index is the more appropriate for measuring the relationship between body fat distribution and potential cardiovascular risk factors in children, are not clear. 9 , 10 , 11 , 12
METHODOLOGIC CONSIDERATIONS
To establish the relationship between body size and BP, we need to bear in mind some caveats pertaining to the parameters that are regularly used to estimate body size and the relationship between body size and BP in children and adolescents. Because body weight and height change during growth and development, the best parameters for defining obesity and for assessing the relationship of body size to BP during childhood are under debate. In our studies, and using local tables, we have defined childhood obesity in terms of the body mass index and tricipital and subscapular skinfold thickness above the age and sex‐specific 95th percentile. 13 Although there is no established cut‐off point for childhood obesity, we have used the most stringent criteria of those recommended in a number of previously published guidelines, and define obesity as higher than the 95th percentile of body mass index. 14 , 15 For a 10‐year‐old boy, the threshold for obesity is 21 kg/m2 (46 lbs), while for a 12‐year‐old girl, it is 24 kg/m2 (53 lbs).
At the time of our first study, the ponderal index (kg/m3)was used to establish a relationship between BP and the estimates of body size, because this index has less collinearity with height than does the body mass index. Any three‐dimensional structure that increases in size while its configuration and density remain the same will maintain a constant ratio of weight to height cubed. The ratio of weight to height squared, however, increases proportionally to height squared. Similar reasoning applies to the human body; therefore, the ratio of weight to height cubed was preferred. 16
Knowledge of the factors that affect the measurement of BP is important not only to the epidemiologist, who is primarily interested in identifying precursors of high blood pressure, but also to the clinician, who relies on epidemiologic data to define the normal range of BP in children. A major impediment to the standardization of BP is variation in cuff size. 17 The potential for error in measuring BP may be greater in children, whose wide range of arm sizes makes selection of the proper cuff critical. Cuff size is important in children because as the upper arm grows, cuff artifacts will cause systematic errors in measurement that can lead to wrongly labeling a child as hypertensive or falsely reassuring another whose BP is truly elevated. For an obese child with a 28‐cm arm circumference, the 12‐cm bladder width is the right size; the use of an 8‐cm bladder width overestimates systolic BP by at least 5 mm Hg. Consequently, it is important to closely follow the guidelines when selecting cuff size. 18
Measurement of BP is also influenced by the instrument and the individual taking the measurement, by the subject's physical activity and behavior, and by the setting in which the BP value is obtained. Most researchers agree that values obtained from 24‐hour monitoring are more accurate in indicating cardiovascular risk than are those obtained from casual measurements. 19 , 20 , 21 The use of ambulatory BP monitoring is feasible in a pediatric population, with the application of carefully standardized and specially adapted recording techniques. 22 Under these conditions, one feature of ambulatory BP values is their superior reproducibility relative to those obtained with casual measurement. 23 , 24 Moreover, automatic ambulatory BP monitoring provides more accurate BP values and records changes in BP whether subjects are active or asleep. 23 It is a useful research tool, although its use in clinical pediatric practice awaits more precise information.
BP AND GROWTH
Among all that is known about the levels and distribution of casual BP measurement in children and adolescents, it is well recognized that BP increases during growth and maturation, and that adolescence is a fast‐growth period during which body mass and BP change rapidly. During the last few decades, this was the main reason that reference BP values were referred to as specific values for age or height and sex in children up to 18 years of age. Recently, the Task Force for Blood Pressure in Children has become aware of the importance of considering age and height together when defining the reference values. 25 In children of the same age, the upper limit of normal systolic BP for the 5th percentile of height is 8–9 mm Hg lower than the limit for the 95th percentile (Table).
Table.
95th Percentiles of Blood Pressure According to Age, Sex, and Height
| Blood Pressure | Age (Years) | Height Percentiles (boys) | Height Percentiles (Girls) | ||
| 5th | 95th | 5th | 95th | ||
| Systolic | 3 | 104 | 113 | 104 | 110 |
| 6 | 109 | 117 | 108 | 114 | |
| 10 | 114 | 123 | 116 | 122 | |
| 13 | 121 | 130 | 121 | 128 | |
| 16 | 129 | 138 | 125 | 132 | |
| Diastolic | 3 | 63 | 67 | 65 | 68 |
| 6 | 72 | 76 | 71 | 75 | |
| 10 | 77 | 82 | 77 | 80 | |
| 13 | 79 | 84 | 80 | 84 | |
| 16 | 83 | 87 | 83 | 86 | |
Ambulatory BP in children and adolescents has not been sufficiently assessed, in part because ambulatory monitoring has only recently been introduced in this population. The distribution of ambulatory BP values in both healthy, normotensive children 22 and healthy, school‐based populations 26 was used to obtain an approach to reference values. The average systolic Bps progressively increased from the 50th to the 95th percentile during the daytime, nighttime, and 24‐hour periods in both boys and girls. The rate of BP increase across age and height ranges, however, was lower than that observed in the casual BP readings. In contrast, the average values for diastolic BP did not change with age or height, regardless of the time period considered.
OBESITY AND BLOOD PRESSURE
Our group studied the characteristics of ambulatory BP in obese children and adolescents. Ambulatory BP monitoring was performed to examine the impact of obesity and body fat distribution on BP in children and adolescents. Although the use of ambulatory BP monitoring is feasible in a pediatric population, 22 , 26 , 27 studies involving this technique in obese children have been scarce.
Except for nighttime diastolic BP, obese children had significantly higher casual and ambulatory BP than did nonobese children, regardless of the period analyzed. The systolic BP values for the obese and nonobese children are shown in Figure 1 (panel A). The obese children had higher BP values over 24 hours than did the nonobese subjects, and the magnitude of the differences was similar during the daytime and nighttime periods. In contrast, diastolic BP did not differ during the nighttime period, and only small differences were observed during the daytime (Figure 1, panel B). The physiologic BP fall that normally occurs during sleep was present in equal magnitude in the two groups, for both systolic and diastolic BP.
Figure 1.
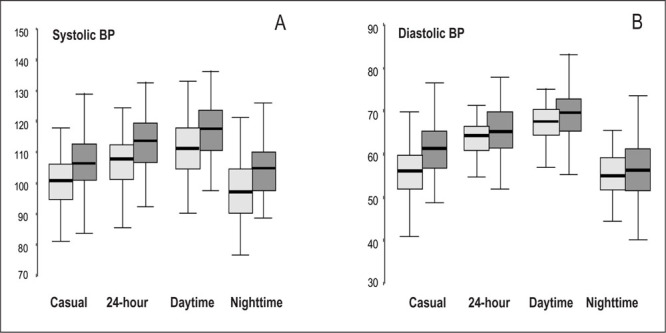
Box plot of casual blood pressure (BP) measurement and ambulatory monitoring of 24‐hour, daytime, and nighttime systolic BP (panel A) and diastolic BP (panel B) in obese (n=85, shaded boxes) and nonobese (n=88, filled boxes) children and adolescents. Casual systolic and diastolic BP were significantly higher in obese than in nonobese subjects (p<0.01 and p<0.05, respectively). Systolic BP over 24 hours was higher in obese than in nonobese children (p<0.01), and the magnitude of the differences was similar during the daytime (p<0.01) and nighttime periods (p<0.05). In contrast, diastolic BP did not significantly differ during the nighttime period, and only small differences were observed during the day (p<0.05).
The impact of obesity on BP is independent of other parameters, as demonstrated in a previous study. 4 Adjusting for height and sex had a minimal effect on the systolic BP differences between the obese and nonobese children. After further controlling for the ponderal index, the differences in casual and ambulatory BP between obese and nonobese children were no longer significant. An additional decrease in these differences was seen when tricipital skinfold thickness, one of the measurements for estimating obesity, was considered.
Casual and ambulatory systolic BP, day and night, were positively correlated with height, weight, the ponderal index, tricipital and subscapular skinfold thickness, and the W/H ratio. These correlations indicated that systolic BP was related to more than just body size parameters. All examined indices of obesity were positively related to systolic BP, and a significant relationship to body fat distribution was also found. In a multiple regression analysis, the W/H ratio, rather than obesity or body size parameters, was the main determinant of ambulatory systolic BP.
FAT DISTRIBUTION AND BP
The importance of body fat distribution derives from the association of obesity with the chronic diseases of well fed societies. Two individuals of similar body weight, with similar skinfold thickness and/or percent body fat, can have a very different anatomic distribution of subcutaneous fat. It is known that the health effects of obesity depend on the anatomic distribution of body fat, which in turn may be a better indicator of endocrinologic imbalance, environmental stress, or genetic factors than is fatness per se.
The W/H ratio has been shown to be a useful marker of abdominal obesity in adults, 7 and it is also informative in postpubertal boys and girls. However, the use of the W/H in younger children has been questioned. The discrepancies observed among studies may be attributable to the morphologic changes operating during pubertal development, 11 , 12 as well as the studies' subject inclusion criteria.
The impact of body fat distribution, as estimated from the W/H ratio, on ambulatory BP values has been studied in children by our group. 4 Our data indicate that subjects with a higher W/H ratio tended to have higher BP values even during childhood. Taking into account that the redistribution of body fat from leg to trunk occurs during adolescence and young adulthood, the presence of a high W/H ratio and high systolic BP during childhood can predispose one to develop cardiovascular risk later in life.
The use of waist circumference, rather than the W/H ratio, for estimating central obesity has been emphasized in recent years. The waist circumference can express abdominal fat accumulation better than the W/H ratio does, in part because in growing children the hip may reflect changes in bone and muscle more than changes in fat. 28 In our study group of obese and nonobese children and adolescents, waist dimension was independently related to the average of 24‐hour systolic BP when age, sex, height, and weight were taken into account. The increased relation of waist to 24‐hour systolic BP, as shown in Figure 2, emphasizes the relationship of waist to cardiovascular risk factors.
Figure 1.
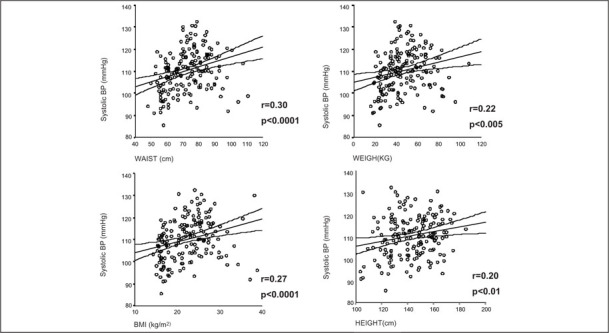
Regression lines and the 95% confidence interval between the average 24‐hour systolic blood pressure (BP) and several anthropometric parameters: waist measurement, weight, body mass index (BMI), and height. Bps tend to be higher with increased weight, waist, and height measurements. r=Pearson correlation coefficient
MECHANISMS OF OBESITY‐RELATED HIGH BP
When obesity‐associated hypertension is characterized principally by increased vascular volume, and when peripheral vascular resistance is “abnormally normal” or only slightly elevated, volume expansion appears to be a key determinant in the tendency toward higher BP values in obese children and adolescents. This relationship implies that the primary defect leading to increased vascular volume and cardiac output, and ultimately to hypertension, consists of some abnormality in kidney function that is responsible for a shift in pressor natriuresis toward higher BP values. Therefore, obesity‐induced hypertension should be considered a salt‐sensitive state. 29
Rocchini and colleagues 30 observed the shift in the pressure‐natriuresis curve in obese adolescents. Sixty obese and 18 nonobese adolescents were evaluated after successive 2‐week periods of a high‐salt diet of >250 mmol/day of sodium and a low‐salt diet of <30 mmol/day. The mean arterial pressures for the two groups were compared as they switched from high‐ to low‐sodium diets. Mean pressures underwent a significantly larger decrease in the obese than in the nonobese group. In addition, obese adolescents had a renal function plot (urinary sodium excretion as a function of arterial pressure) with a more gradual slope than that of nonobese adolescents. When the renal function relationship was normalized for weight loss, BP was no longer sensitive to dietary sodium intake.
In a previous study, 31 our group evaluated whether obesity influences the relationship between BP and urinary sodium excretion by using ambulatory BP monitoring and, simultaneously, split urine collections during the waking and sleeping periods. We compared the age‐ and sex‐adjusted mean BP between obese and nonobese children within each time‐specific quartile of sodium excretion rate. Obese children, at the same urinary sodium excretion levels as nonobese children, had a higher ambulatory systolic BP than the nonobese. Weight and sodium excretion were directly associated with sleep BP and 24‐hour systolic BP, and the effect of sodium excretion was modified by weight. The sodium excretion‐weight interaction, included in the linear regression model, allowed us to determine if the effect of obesity was the same at different sodium excretion rates. At the same urinary sodium excretion level, the obese children had higher ambulatory systolic BP. At the higher levels of sodium excretion, however, differences in BP between obese and nonobese children were smaller. This indicates that obese children may be heterogeneous in terms of the relationship between ambulatory BP and sodium excretion. Whether or not the different values observed in the obese children denote a subset whose risk for developing hypertension may be different is an attractive hypothesis to be explored in further studies.
An understanding of the mechanisms responsible for the resetting of pressor natriuresis, inducing a permanent expansion of extracellular fluid and blood volume, is essential for the elucidation of the pathophysiologic link between overweight and high BP. It is possible that some common mechanisms may affect renal sodium handling and peripheral vascular resistance, the pathophysiology of the abnormal tubular sodium retention, and the lack of a normal adaptation of peripheral vascular resistance to increased cardiac output. 32 Several common factors are involved in the establishment of both sodium retention and vascular resistance, and this may be, at least in part, critically influenced by the neurobiologic/genetic mechanisms producing obesity. 33
Three mechanisms appear to be especially important in initiating the increased sodium reabsorption, impaired renal pressure natriuresis, and hypertension associated with weight gain. These mechanisms are an increase in sympathetic activity, activation of the renin‐angiotensin system, and alteration of intrarenal physical forces due to compression of the kidneys. The effect of obesity on the relationship between BP and sodium excretion was evident during sleeping hours, not during waking hours, and it may reflect circadian rhythm alteration by several mechanisms. Sympathetic nerve activity or the renin‐angiotensin system can alter renal sodium handling and/or BP. Under normal conditions, sympathetic activity decreases during the night and, in the absence of demanding situations, basal overactivity can be detected more easily and may provide early evidence of pathophysiologic changes that predispose obese individuals to hypertension.
Obesity, and specifically abdominal obesity, is linked to the presence of insulin resistance and hyperinsulinemia. The role of insulin in the development of obesity‐induced hypertension has been greatly debated. Landsberg 32 hypothesized that high calorie intake increases thermogenesis by activating the sympathetic nervous system to act as a buffer against weight gain. However, insulin resistance is another mechanism the obese recruit to stabilize body weight and limit further gain. The resultant hyperinsulinemia stimulates sympathetic activity, driving thermogenic mechanisms that increase the metabolic rate. 34 Sympathetically mediated vasconstriction and cardiac stimulation and enhanced sodium reabsorption exert a prohypertensive effect. 35
The hemodynamic effects of insulin and its implication in the structural changes in arterial wall thickness and/or arterial compliance have been recently reviewed. 36 Insulin's vasodilator capacity is well established in healthy, normotensive subjects; however, in insulin‐resistant states, such as obesity, insulin‐mediated vasodilatation may be blunted, in part by endothelial dysfunction and the consequent lower nitric oxide production or by abnormal glucose uptake, which reduces the calcium influx to muscle cells.
CONCLUSIONS
Substantial clinical and epidemiologic evidence supports the influence of obesity in BP levels, even early in life. Ambulatory BP monitoring provides better assessment of BP and has allowed the observation that not only obesity but also body weight distribution are independent determinants of BP values. Since obesity is related to a clustering of risk factor variables in children and young adults, the prevention of the onset of obesity in early life may be important in reducing the risk of coronary heart disease in later life.
References
- 1. World Health Organization . Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. Presented at: The World Health Organization: June 3–5, 1997; Geneva, Switzerland. Publication WHO/NUT/NCD/98.1. [PubMed] [Google Scholar]
- 2. Tell GS. Cardiovascular disease risk factors related to sexual maturation: the Oslo youth study. J Chronic Dis. 1985;38:633–642. [DOI] [PubMed] [Google Scholar]
- 3. Voors AW, Webber LS, Frerichs RR, et al. Body height and body mass as determinants of basal blood pressure in children—the Bogalusa Heart Study. Am J Epidemiol. 1977;106:101–108. [DOI] [PubMed] [Google Scholar]
- 4. Lurbe E, Alvarez V, Liao Y, et al. The impact of obesity and body fat distribution on ambulatory blood pressure in children and adolescents. Am J Hypertens. 1998;11:418–424. [DOI] [PubMed] [Google Scholar]
- 5. Faloia E, Giacchetti G, Mantero F. Obesity and hypertensión. J Endocrinol Invest. 2000;23:54–62. [DOI] [PubMed] [Google Scholar]
- 6. Kroke A, Bergmann M, Klipstein‐Grobusch K, et al. Obesity, body fat distribution and body build: their relation to blood pressure and prevalence of hypertension. Int J Obes Related Metab Disord. 1998;22:1062–1070. [DOI] [PubMed] [Google Scholar]
- 7. Okosun IS, Prewitt TE, Cooper RS. Abdominal obesity in the United States: prevalence and attributable risk of hypertension. J Hum Hypertens. 1999;13:425–430. [DOI] [PubMed] [Google Scholar]
- 8. Gillum RF. The association of body fat distribution with hypertension, hypertensive heart disease, coronary heart disease, diabetes, and cardiovascular risk factors in men and women aged 18–79 years. J Chronic Dis. 1987;40:421–428. [DOI] [PubMed] [Google Scholar]
- 9. Weststrate JA, Deurenberg P, Van Tinteren H. Indices of body fat distribution and adiposity in Dutch children from birth to 18 years of age. Int J Obes. 1990;14:149–157. [PubMed] [Google Scholar]
- 10. Sangi H, Mueller WH. Which measure of body fat distribution is best for epidemiologic research among adolescents? Am J Epidemiol. 1991;133:870–883. [DOI] [PubMed] [Google Scholar]
- 11. Sangi H, Mueller WH, Harrist RB, et al. Is body fat distribution associated with cardiovascular risk factor in children? Ann Hum Biol. 1992;19:559–578. [DOI] [PubMed] [Google Scholar]
- 12. Zwiauer KF, Pakosta R, Meller T, et al. Cardiovascular risk factors in obese children in relation to weight and body fat distribution. J Am Coll Nutr. 1992;11(suppl):41S–50S. [DOI] [PubMed] [Google Scholar]
- 13. Hernandez M, Castellet J, Narvaiza JL, et al. Curvas y tablas de crecimiento. Instituto de investigaciones sobre crecimiento y desarrollo. Madrid, Spain: Fundación F. Orbegozo, Garsi; 1988. [Google Scholar]
- 14. Himes J, Dietz W. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. Am J Clin Nutr. 1994;59:307–316. [DOI] [PubMed] [Google Scholar]
- 15. Whitaker RC, Wright JA, Pepe MS, et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. [DOI] [PubMed] [Google Scholar]
- 16. Florey C. The use and interpretation of ponderal index and other weight‐height ratios in epidemiological studies. J Chronic Dis. 1970;23:93–103. [DOI] [PubMed] [Google Scholar]
- 17. Alpert BS. Cuff width and accuracy of measurement of blood pressure. Blood Press Monit. 2000;5:151–152. [DOI] [PubMed] [Google Scholar]
- 18. Report of the Second Task Force on Blood Pressure Control in Children . National Heart, Lung and Blood Institute. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 19. Redon J, Campos C, Narciso ML, et al. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension. A prospective study. Hypertension. 1998;31:712–718. [DOI] [PubMed] [Google Scholar]
- 20. Frattola A, Parati G, Cuspidi C, et al. Prognostic value of 24‐hour blood pressure variability. J Hypertens. 1993;11:1133–1138. [DOI] [PubMed] [Google Scholar]
- 21. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 22. Lurbe E, Redon J, Liao Y, et al. Ambulatory blood pressure monitoring in normotensive children. J Hypertens. 1994;12:1417–1423. [PubMed] [Google Scholar]
- 23. Lurbe E, Aguilar F, Gomez A, et al. Reproducibility of ambulatory blood pressure monitoring in children. J Hypertens. 1993;11(suppl 5):S288–S289. [PubMed] [Google Scholar]
- 24. Lurbe E, Thijs L, Redon J, et al. Diurnal blood pressure curve in children and adolescents. J Hypertens. 1996;14:41–46. [PubMed] [Google Scholar]
- 25. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents . Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics. 1996;98:649–658. [PubMed] [Google Scholar]
- 26. Soergel M, Kirschstein M, Busch C, et al. Oscillometric twenty‐four‐hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. [DOI] [PubMed] [Google Scholar]
- 27. O'Sullivan JJ, Derrick G, Griggs P, et al. Ambulatory blood pressure in schoolchildren. Arch Dis Child. 1999;80:529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. [DOI] [PubMed] [Google Scholar]
- 29. Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10(5 pt 2):49S–55S. [PubMed] [Google Scholar]
- 30. Rocchini AP, Key J, Bondie D, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–585. [DOI] [PubMed] [Google Scholar]
- 31. Lurbe E, Alvarez V, Liao Y, et al. Obesity modifies the relationship between ambulatory blood pressure and natriuresis in children. Blood Press Monit. 2000;5(5‐6):275–280. [DOI] [PubMed] [Google Scholar]
- 32. Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61(236):1081–1090. [PubMed] [Google Scholar]
- 33. Mark AL, Correia M, Morgan DA, et al. Obesity‐induced hypertension. New concepts from the emerging biology of obesity. Hypertension. 1999;33(1 pt 2):537–541. [DOI] [PubMed] [Google Scholar]
- 34. Biolo G, Toigo G, Ciocchi B, et al. Slower activation of insulin action in hypertension associated with obesity. J Hypertens. 1998;16(12 pt 1):1783–1788. [DOI] [PubMed] [Google Scholar]
- 35. Hall JE. Pathophysiology of obesity hypertension. Curr Hypertens Rep. 2000;2:139–147. [DOI] [PubMed] [Google Scholar]
- 36. Mikhail N, Tuck MI. Insulin and the vasculature. Curr Hypertens Rep. 2000;2:148–153. [DOI] [PubMed] [Google Scholar]


