Abstract
A large proportion of patients have both hypertension and hypercholesterolemia, two of the most important risk factors for cardiovascular diseases. Statins are the most widely used drugs for the treatment of plasma lipid abnormalities and have been reported to interact with elevated blood pressure. A reduction in blood pressure associated with the use of these agents has been reported in patients with untreated hypertension and in patients treated with antihypertensive drugs, particularly angiotensin‐converting enzyme inhibitors and calcium channel blockers. This effect on blood pressure control has also been observed in diabetic patients. The mechanism responsible for the hypotensive effect seems to be largely independent of the effect of statins on plasma cholesterol, and probably is related to the interaction of the medications with endothelial function or angiotensin II receptors. The capacity of statins to improve blood pressure control may represent a useful tool for improvement in the prevention of cardiovascular diseases.
Atherosclerotic disease and its complications, i.e., myocardial infarction and stroke, are one of the leading causes of morbidity and mortality among adults in Europe and North America. 1 Its overall prevalence is strongly related to that of different cardiovascular (CV) risk factors, including high blood pressure (BP), cigarette smoking, total plasma cholesterol (TC), low‐ and high‐density lipoprotein (LDL and HDL) cholesterol, and diabetes. 2 , 3 , 4 , 5 Risk factor modification is an integral part of the optimal care of patients with and without cardiovascular disease. Data supporting this concept have been published in the fields of basic science, epidemiology, clinical medicine, and cost effectiveness analyses. Effective management of risk factors for CV diseases should be viewed as an integrated strategy of intervention to correct as many as possible of the modifiable risk factors. A combined approach to CV risk has been adopted as part of the framework for hypertension management programs (Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and the World Health Organization/International Society for Hypertension) and high plasma cholesterol treatment (National Cholesterol Education Program), in both the United States and Europe. 6 , 7 , 8 Data from large population studies have demonstrated that the long‐term incidence of CV diseases can be significantly improved by using algorithms that take into account the impact of several risk factors, such as BP, smoking habits, TC and HDL cholesterol levels, diabetes, and left ventricular hypertrophy. 5 This suggests that CV risk could be better managed in the clinical setting by treatment strategies aimed at normalizing multiple CV risk factors.
ROLE OF HIGH PLASMA CHOLESTEROL IN HYPERTENSION
The concomitant presence of high BP and high plasma cholesterol can account for a large proportion of the cumulative risk of CV disease. A significant increase in plasma cholesterol levels has been observed in as many as 40% of the hypertensive population in the age range from 35–65 years. 9 The results of the Multiple Risk Factor Intervention Trial (MRFIT) 3 have demonstrated that for any level of BP, the risk of developing a CV complication is significantly increased in patients with higher cholesterol levels. The negative interaction between high BP and hypercholesterolemia can also be demonstrated in patients with a marginal increase of plasma cholesterol levels, ranging from 180–220 mg/dL, 3 suggesting that lipid‐lowering strategies may be effective even in the population of hypertensive patients with borderline elevations of plasma cholesterol levels.
From an epidemiologic point of view, many observational studies have described a high level of plasma cholesterol in patients with mild to moderate hypertension, and the same trend has been detected in young patients with borderline hypertension. 10 , 11 In this latter population of subjects, we have observed that an increase of serum cholesterol levels above the cut‐off value of 200 mg/dL has been associated with an exaggerated BP response to mental stress (Figure 1) and a significant increase in the 15‐year relative risk of developing more severe hypertension (Figure 2). This relationship persists after adjustment for the main confounding risk factors of age, sex, body weight, family history of hypertension, and urinary sodium excretion. 11 These data suggest that elevated plasma cholesterol levels can be directly or indirectly correlated to some of the mechanisms responsible for the BP increases that characterize patients with hypertension. Accordingly, it is possible that any measures resulting in a reduction of plasma cholesterol levels could improve BP control and might interfere with the onset and progression of hypertensive disease and the therapeutic impact of antihypertensive treatment in patients with hypertension. This additional benefit of lipid‐lowering strategies might contribute to an overall reduction of the risk of coronary artery disease and improve the clinical outcome of hypertensive patients. In a primary prevention study carried out in a population of Swedish patients with multiple risk factors, 12 the probability of an acute coronary event was reduced to a greater extent in patients in whom both BP and TC were reduced by a combination of antihypertensive and lipid‐lowering treatment (Figure 3). In the same study, a more detailed analysis of CV morbidity, as a function of the extent of control of BP and serum cholesterol, has clearly demonstrated that the magnitude of benefit that can be achieved with various degrees of BP reduction is significantly reduced in patients whose plasma cholesterol levels were unchanged or increased. This suggests that in patients with hypertension and plasma lipid abnormalities, a reduction of both risk factors should be recommended to achieve a substantial reduction in CV morbidity. This goal can be achieved with the use of combinations of different drugs acting on different risk factors.
Figure 1.
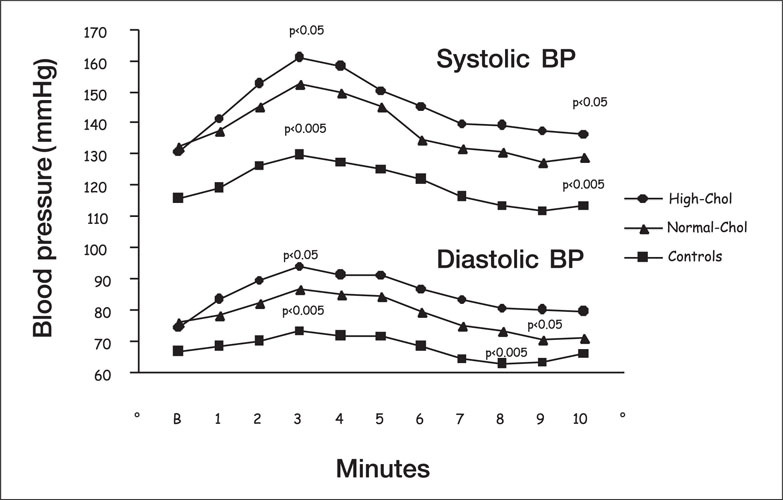
Systolic and diastolic blood pressure (BP) responses to mental stress in normotensive controls and in borderline hypertensive patients with average, normal, or high plasma cholesterol (chol) levels
Figure 2.
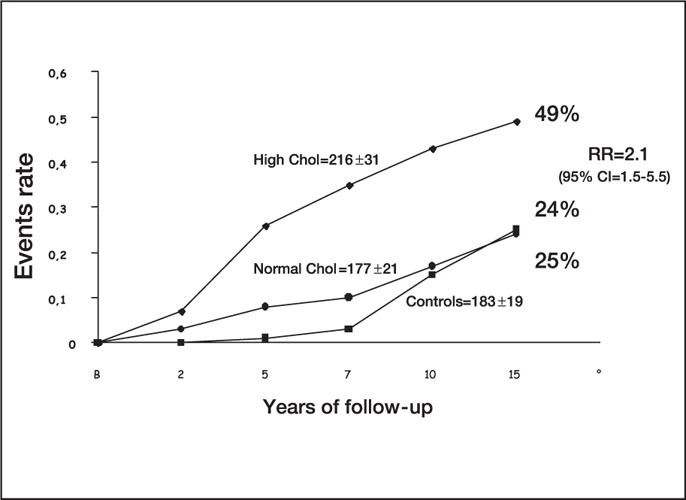
Proportion of patients and relative risk (RR) of developing persistent hypertension in normotensive controls and in borderline hypertensive patients with average, normal, or high plasma cholesterol (chol) levels. CI=confidence interval
Figure 3.
Three‐year coronary heart disease (CHD) morbidity in a Swedish population of patients with hypertension and hypercholesterolemia according to blood pressure and plasma cholesterol reduction SBP=systolic blood pressure; T=total Modified with permission from JAMA. 1987;258(13): 1768–1776. 12
Many drugs currently available for the treatment of hypertension and plasma lipid abnormalities have been reported to influence plasma lipids as well as BP. 13 In particular, some agents (i.e., angiotensin‐converting enzyme [ACE] inhibitors, calcium channel blockers, statins) have been reported to interact with the same targets at the vascular endothelial level, 14 which suggests the possibility that they might exert some additional benefits beyond their primary actions.
ROLE OF STATINS IN MANAGEMENT OF “OVERALL” CV RISK
3‐Hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase inhibitors (“statins”) are the most effective agents on the market for the treatment of plasma lipid abnormalities, and many clinical trials have demonstrated that the use of statins (lovastatin, simvastatin, and pravastatin) reduces morbidity and mortality in patients at risk of CV disease. 15 , 16 , 17 , 18 In particular, the benefit of statins has been demonstrated in a large population of patients (>30,000), with and without coronary artery disease and across a wide range of plasma cholesterol levels (from 210–310 mg/dL). This suggests the possibility that the use of HMG‐CoA reductase inhibitors could result in a reduction of CV risk that extends beyond the simple prevention of progression of cholesterol‐related atherosclerotic lesions.
During the last 2–3 years, some interesting evidence 19 has suggested the possibility that a significant proportion of the beneficial effects of HMG‐CoA reductase inhibitors may result from their capacity to exert some degree of “therapeutic crossover” in patients with multiple risk factors. In particular, this effect might be noted in patients with elevated cholesterol levels and hypertension.
ROLE OF STATINS IN BP CONTROL: EXPERIMENTAL EVIDENCE
The first demonstration of the ability of statins to influence BP control comes from experimental data obtained in Dahl salt‐sensitive rats, 20 where the use of lovastatin or pravastatin 21 was able to attenuate the onset and progression of hypertension as well as to reduce the degree of proteinuria and glomerular injury. These effects were not dependent on the direct inhibition of renal sodium reabsorption. Jiang and Roman 22 investigated the effects of statins in spontaneously hypertensive rats and demonstrated that chronic treatment with lovastatin (20 mg/kg/day) in this salt‐insensitive model of hypertension shifts the relation between renal perfusion pressure and sodium excretion toward lower BP values and attenuates the development of hypertension and renal vascular damage. The mechanism by which the use of statins attenuates the development of hypertension is still poorly understood. Spontaneously hypertensive treated rats 22 showed a 33% reduction in plasma cholesterol, but it remains unclear whether the effects of lovastatin in preventing the BP rise are directly or indirectly related to its hypolipidemic effects. Basically, the plasma lipid concentrations of spontaneously hypertensive rats are not elevated and it is not known whether a reduction in plasma cholesterol concentration from the normal baseline levels has any effect on the mechanisms involved in BP control. An intriguing hypothesis suggests the possibility that the antihypertensive effect of statins in experimental rat models is related to their ability to prevent renal vascular hypertrophy, thus improving the pressure‐natriuresis relationship. 22 Thus, the effect of statins may be largely independent of their lipid‐lowering activity and more directly related to their ability to alter the function of vascular smooth muscle cells. 23 , 24 This suggests a role for statins, per se, in the modulation of some of the mechanisms responsible for the progressive increase and maintenance of elevated BP and supports the possibility that statins may be involved in BP control irrespective of their capacity to modulate the plasma lipid profile.
ROLE OF STATINS AND BP CONTROL: CLINICAL EVIDENCE
In addition to experimental evidence, the possibility that the use of statins can exert a favorable effect on BP control in humans has been evaluated (Table). Unfortunately, none of the large trials carried out to assess the clinical relevance of the use of statins in patients with and without CV disease has revealed information about the extent of BP control. In addition, these studies have often been carried out in populations of patients who were not representative of the average hypertensive population.
Table.
Effects of Statins on Blood Pressure Control in Normotensives and Hypertensives: Clinical Trial Results
| Author | Patients | Variable | Effects of Statins |
| Sung et al., 1997 26 | NBP | SBP response to stress | Significantly reduced |
| Resting SBP | Not significantly reduced | ||
| Muramatsu et al., 1997 31 | NBP | Resting SBP, DBP | Unchanged |
| Antonicelli et al., 1990 32 | NBP | Resting SBP, DBP | Unchanged |
| Kool et al., 1995 33 | NBP | Resting SBP | Unchanged |
| Leibovitz et al., 2001 50 | NBP | Resting SBP, DBP | Significantly reduced |
| Jarai et al, 1996 27 | U‐HBP | Resting SBP, DBP | Significantly reduced |
| Straznicky et al., 1995 28 | U‐HBP | SBP and DBP response to | Significantly reduced |
| A‐II, NE | Unchanged | ||
| Abetel et al., 1998 29 | U‐HBP | Resting SBP, DBP | Significantly reduced |
| D'Agostino et al., 1993 30 | T‐HBP† | Resting SBP,DBP | Unchanged |
| Borghi et al., 2001 34 | T‐HBP*† | Resting SBP, DBP | Significantly reduced |
| Glorioso et al., 1999 35 | U‐HBP Resting | SBP, DBP, PP, | Significantly reduced |
| MBP response to CPT | Significantly reduced | ||
| Tonolo et al., 2000 36 | T‐HBP+NIDDM | Resting SBP, DBP | Significantly reduced |
| Velussi et al., 1999 37 | T‐HBP+NIDDM | Resting SBP, DBP | Significantly reduced SBP |
| Sposito et al., 1999 38 | T‐HBP* | Resting SBP, DBP | Significantly reduced |
| Borghi et al., 2000 39 | T‐HBP* | Resting SBP, DBP, MBP, PP | Significantly reduced |
| Nazzaro et al., 1999 40 | U‐HBP | Resting SBP, DBP | Significantly reduced |
| Mugen et al., 2000 48 | HBP | Resting SBP, DBP | Significantly reduced |
| Ferrari et al., 2001 49 | T‐HBP | 24‐hour SBP | Significantly reduced |
| NBP, HBP=normotensive, hypertensive patients; SBP/DBP=systolic/diastolic blood pressure; U=untreated; A‐II=angiotensin II; NE=norephinephrine; T=treated; PP=pulse pressure; MBP=mean blood pressure; CPT=cold pressor test; NIDDM=non‐insulin‐dependent diabetes; *uncontrolled; †well controlled | |||
In 1995, a retrospective review of different studies performed with a variety of different lipid‐lowering strategies 25 suggested that the reduction of plasma cholesterol in patients with hyperlipemia is associated with a reduction in BP that was clinically significant (3–5 mm Hg in diastolic BP [DBP]). This was directly related to the decrease in serum cholesterol and was larger in patients treated with statins. There is evidence, in some studies, supporting the concept that chronic treatment with statins can reduce systemic BP in the high‐risk population of patients with hypertension and hypercholesterolemia., 26 , 27 , 28 , 29 but this effect was not seen in other studies. 30 In normotensive populations with elevated cholesterol levels, available clinical data do not support a significant effect of statins on BP, 31 , 32 , 33 despite some significant changes in the systemic hemodynamic profile. 31 So far, no controlled clinical studies have been carried out in patients with hypertension and normal plasma cholesterol levels.
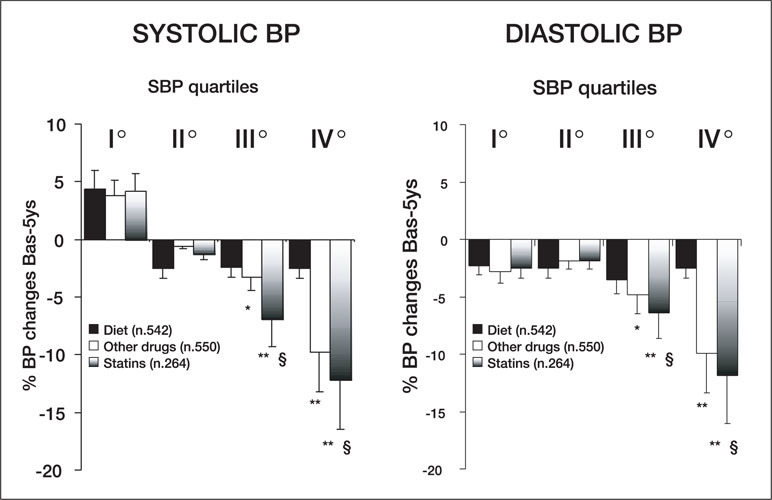
Some intriguing observations regarding statins and BP control can be derived from a retrospective analysis of a subpopulation of the Brisighella Heart Study, 34 which enrolled over 1500 patients with total cholesterol levels >239 mg/dL and at least one other CV risk factor. These patients were subdivided into four quartiles of systolic blood pressure (SBP) and treated with a low‐cholesterol diet for a period of 6 months. Patients considered nonresponders to the diet (TC levels still >239 mg/dL) were prospectively allocated to treatment with statins (mainly, simvastatin 20–40 mg/day) or other lipid‐lowering drugs (fibrates or cholestyramine) for a cumulative period of 5 years. After completion of the follow‐up period, the decrease in SBP and DBP was greater in patients with hypertension (third and fourth quartiles, SBP >150 mm Hg) who were treated with lipid‐lowering drugs (Figure 4). After 6 months of treatment, the BP reduction was significant (Figure 5) and was greater in the subgroup of patients on statin treatment than in patients maintained on a low‐cholesterol diet without medication (Figures 4 and 5). Despite obvious limitations due to the observational nature of the study design, the data from the Brisighella Heart Study suggest that long‐term treatment with lipid‐lowering drugs might improve BP control in a population of subjects with a high CV risk profile. Interestingly, the effect of statins on BP appears to be limited to patients with BPs in the hypertensive range, suggesting that, as with most antihypertensive agents, statins may decrease elevated but not normal BP. These data are in agreement with those reported for normotensive populations 31 , 32 , 33 and provide additional support to the possibility that statins might exert their favorable effects by counteracting some of the mechanisms responsible for the development and maintenance of hypertension.
Figure 4.

Five‐year changes in systolic (SBP) and diastolic blood pressure (BP) observed in patients enrolled in the Brisighella Heart Study and treated with a low‐cholesterol diet, statins, and other lipid‐lowering drugs (fibrates or cholestyramine) *p<0.05, **p<0.05 vs. Diet; §p<0.05 vs. other drugs
Figure 5.
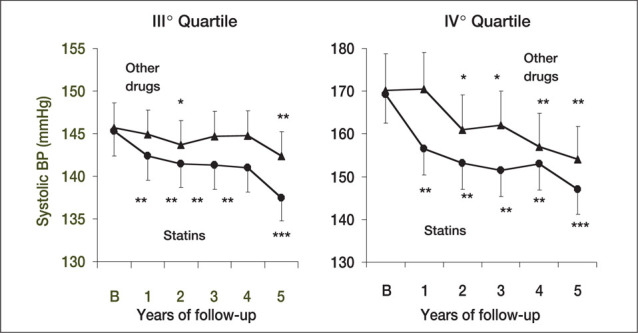
Time course of changes in systolic and diastolic blood pressure (BP) in response to statins or other lipid‐lowering strategies in patients in the last two quartiles for systolic BP in the Brisighella Heart Study *p<0.05, **p<0.005 vs. Diet; ***p<0.005 vs. B
PRESSOR RESPONSES, STATINS, AND BP
The effects of statins on BP have been evaluated in a study specifically designed to investigate resting and stimulated (cold pressor test) SBP and DBP changes in patients with untreated hypertension and elevated cholesterol levels. 35 After an 8‐week placebo and low‐cholesterol diet run‐in period, the hypertensive patients were randomly allocated to placebo or pravastatin (20–40 mg/day), according to a double‐blind, crossover design. Upon completion of the double‐blind phase of treatment, the statin group had lowered systolic, diastolic, and pulse pressures, (Figure 6) and the pressor response to the cold pressor test was blunted. These data are in agreement with the finding of Sung and coworkers 26 and suggest that the pressor response to mental stress can be significantly reduced after cholesterol reduction. The effects of pravastatin on BP control were independent of any adjustment carried out according to gender and age and were not related to baseline levels of plasma LDL and HDL cholesterol. The effects of statins on BP were also independent of plasma endothelin levels, 35 thus excluding the possibility that statins may improve BP by interfering with this important vasoconstrictive system.
Figure 6.
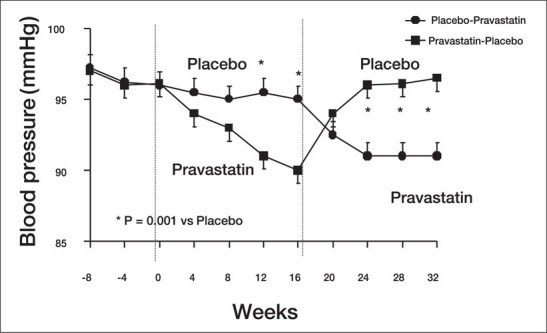
Diastolic blood pressure modifications in response to pravastatin treatment in patients with untreated arterial hypertension and hypercholesterolemia Modified with permission from Hypertension. 1999;34:1281–1286. 35
COMPARATIVE DATA
Results of a recent comparison of simvastatin and cholestyramine in type 2 diabetic patients 36 demonstrate that although the two drugs were equally effective in reducing total and LDL cholesterol, only statin treatment led to a reduction in diastolic BP and 24‐hour urinary albumin excretion. The relative independence of BP reduction from lipid‐lowering activity of statins may be of clinical importance in terms of CV protection.
The favorable effects of statins on BP have also been confirmed in the high‐risk population of patients with type 2 diabetes and hypertension. Treatment with atorvastatin at an average dose of 10 mg/day has been reported to improve diastolic BP control without modification of ongoing antihypertensive treatment. 37 BP reduction was associated with an improvement in the lipid profile (reduction in LDL cholesterol and an increase in HDL cholesterol), as well as with significant reductions of microalbuminuria and fibrinogen plasma levels. These changes contribute to the benefits reported in large‐scale trials with the use of statins in patients with diabetes. 16 , 17
In addition to the effects on BP in untreated hypertensive patients in comparison with either placebo or other lipid‐lowering strategies (cholestyramine and/or fibrates), the use of statins has been reported to improve BP control in patients currently on therapy with antihypertensive drugs. The first demonstration of a positive interaction between statins and antihypertensive agents was reported by Sposito et al., 38 who noted an enhanced antihypertensive effect of enalapril and lisinopril in a small population of patients with hypertension and elevated serum TC treated with pravastatin or lovastatin. Similar effects have been reported in a larger population of patients (n=127) with hypercholesterolemia and poorly controlled hypertension treated with various antihypertensive drugs. 39 The SBP and DBP response to antihypertensive treatment was significantly enhanced by the concomitant administration of pravastatin or simvastatin (Figure 7). A retrospective analysis of data showed that effects on BP control were enhanced in patients treated with statins in combination with ACE inhibitors and calcium channel blockers (Figure 7).
Figure 7.
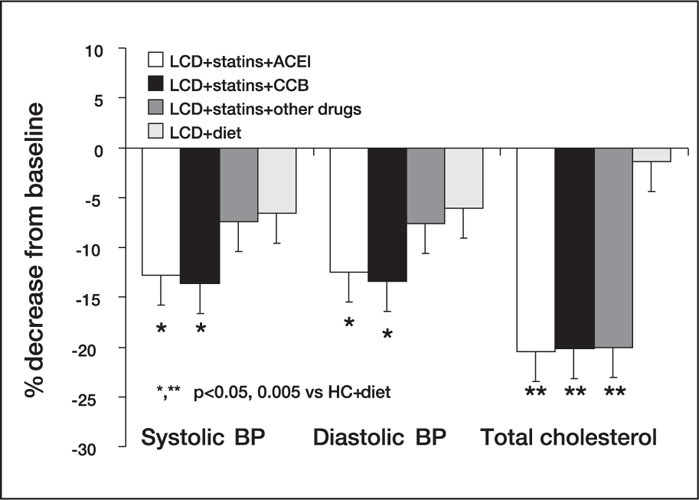
Changes in systolic and diastolic blood pressure (BP) and total cholesterol in patients with hypertension treated with a low‐cholesterol diet (LCD) alone and in combination with antihypertensive drugs and statins ACEI=angiotensin‐converting enzyme inhibitor; CCB=calcium channel blocker; *p<0.05, **p<0.005 vs. LCD+ diet Reproduced with permission from J Cardiovasc Pharmacol. 2000;35:549–555. 39
No significant interactions have been observed with β blockers and diuretics.
A slight but marginally significant correlation has been observed between the decrease in DBP and that of serum TC as well as LDL cholesterol (r=0.37; p=0.043 and r=0.39; p=0.041, respectively), whereas no relationship has been found with SBP changes. These findings largely confirm those demonstrated by many clinical studies and suggest the possibility that statins interact with some of the mechanisms responsible for BP increase. Nazzaro and coworkers 40 reported that the addition of simvastatin (10 mg/day) to baseline treatment with enalapril (20 mg/day) provides a significant additive effect on BP and forearm vascular resistance, which may result from their interaction in the control of vascular tone. This mechanism may be responsible for a kind of “synergism” between different drugs acting on the same target, with enhancement of the therapeutic impact of each single pharmacologic agent.
PROPOSED MECHANISMS OF INTERACTION BETWEEN STATINS AND BLOOD PRESSURE CONTROL
A possible explanation for the effect of statins on BP control involves a vasodilatory effect that may relate to their capacity to improve endothelium‐dependent vasorelaxation. 41 , 42 An impairment in peripheral arterial compliance has been described in patients with high serum cholesterol levels. 43 This might contribute to an increase in BP. Treatment with statins possibly increases arterial compliance, thereby improving the vasodilator capacity of the large arteries. This could contribute to the BP lowering and might explain the reduction in pulse pressure observed in patients treated with the combination of antihypertensive drugs and statins. In addition, treatment with statins may promote the up‐regulation of vascular nitric oxide synthase 44 , 45 that has been described in experimental conditions. This could contribute to modulation of peripheral vascular tone irrespective of the reduction in plasma cholesterol levels. An additional mechanism may reside in the capacity of statins to blunt the vasoconstrictive and pressor response to vascular agonists, such as angiotensin II and norepinephrine. 28 This might further contribute to the reduction of peripheral vascular tone. Interestingly, all of these conditions might increase the sensitivity of the vessel wall to the vasodilating effect of such drugs as ACE inhibitors, calcium channel blockers, and angiotensin II receptor inhibitors. This could provide a suitable explanation for the previously reported pharmacodynamic interaction between statins and some classes of antihypertensive drugs.
Recently, it was demonstrated that hypercholesterolemia is associated with an up‐regulation of vascular angiotensin II type 1 (AT1) receptors (vasoconstrictors), 46 which might explain the increased pressor reactivity to an angiotensin II infusion observed in this condition. 28 Experimental therapy with statins decreases the density of AT1 receptors and reduces vascular sensitivity to angiotensin II and plasma levels of aldosterone. 47 The down‐regulation of the number and density of AT1 receptors might have some important pathophysiologic and clinical implications in improving vascular relaxation, preventing progression of vascular hypertrophy, and increasing the therapeutic impact of any strategy to improve BP and plasma lipid abnormalities. These findings suggest a new approach to CV disease prevention based on the multiple interactions among drugs addressing the same target but acting through different pathways. Studies are currently ongoing to evaluate the clinical effectiveness of fixed combinations of statins and antihypertensive drugs in the treatment of patients with both hypertension and hypercholesterolemia.
CONCLUSION
Available information supports some role for statins in the regulation of arterial BP in patients with hypertension. The effects of statins on BP control seem to be partially independent of their lipid‐lowering activity and more strictly related to their capacity to directly interact with some mechanisms responsible for BP regulation. If confirmed by the ongoing large‐scale trials, these effects could be important in the prevention of CV morbidity and mortality.
References
- 1. American Heart Association. 2001 Heart and Stroke Statistical Update. Dallas, TX:American Heart Association; 2000. [Google Scholar]
- 2. Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275(20):1571–1576. [PubMed] [Google Scholar]
- 3. Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7): 1490–1500. [PubMed] [Google Scholar]
- 4. Alderman MH, Cohen H, Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension. 1999;33(5):1130–1134. [DOI] [PubMed] [Google Scholar]
- 5. Wilson PWF, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 6. The JNC‐VI Sub‐Committee . The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–2446. [DOI] [PubMed] [Google Scholar]
- 7. Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization‐International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub‐committee of the World Health Organization. Clin Exp Hypertens. 1999;21(5‐6):1009–1060. [DOI] [PubMed] [Google Scholar]
- 8. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel in Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult treatment Panel II). JAMA. 1993;269(23):3015–3033. [PubMed] [Google Scholar]
- 9. Laurenzi M, Cirillo M, Trevisan M. The Gubbio data. Epidemiology and pathophysiology. Gubbio Study Research Group. Clin Exp Hypertens A. 1992;14(1‐2):261–269. [DOI] [PubMed] [Google Scholar]
- 10. Julius S, Jamerson K, Mejia A, et al. The association of borderline hypertension with target organ changes and higher coronary risk. Tecumseh Blood Pressure study. JAMA. 1990; 264(3):354–358. [PubMed] [Google Scholar]
- 11. Borghi C, Bacchelli S, Degli Esposti D, et al. Pressor and metabolic correlates to long‐term development of stable hypertension in borderline hypertensive patients (abstact). J Hypertens. 1997;15(4):S111. [PubMed] [Google Scholar]
- 12. Samuelsson O, Wilhelmsen L, Andersson OK, et al. Cardiovascular morbidity in relation to change in blood pressure and serum cholesterol levels in treated hypertension. Results from the primary prevention trial in Goteborg, Sweden. JAMA. 1987;258(13):1768–1776. [PubMed] [Google Scholar]
- 13. Costa FV, Borghi C, Mussi A, et al. Hypolipidemic effects of long‐term antihypertensive treatment with captopril. A prospective study. Am J Med. 1988;84(3A):159–161. [DOI] [PubMed] [Google Scholar]
- 14. Taddei S, Virdis A, Ghiadoni L, et al. Antihypertensive drugs and reversing of endothelial dysfunction in hypertension. Curr Hypertens Rep. 2000;2(1):64–70. [DOI] [PubMed] [Google Scholar]
- 15. Maron DJ, Fazio S, Linton MF. Current perspectives on statin. Circulation. 2000;101:207–213. [DOI] [PubMed] [Google Scholar]
- 16. Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 17. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14): 1001–1009. [DOI] [PubMed] [Google Scholar]
- 18. Long‐Term Intervention with Pravastatin in Ischaemic Disease Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349–1357. [DOI] [PubMed] [Google Scholar]
- 19. Vaughan CJ, Gotto AM, Basson CT. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol. 1999;35:1–10. [DOI] [PubMed] [Google Scholar]
- 20. O'Donnell MP, Kasiske BL, Katz SA, et al. Lovastatin but not enalapril reduces glomerular injury in Dahl salt‐sensitive rats. Hypertension. 1992;651–658. [DOI] [PubMed] [Google Scholar]
- 21. Wilson TW, Alonso‐Galicia M, Roman RJ. Effects of lipid‐lowering agents in the Dahl salt‐sensitive rat. Hypertension. 1998;31(1 pt 2):225–231. [DOI] [PubMed] [Google Scholar]
- 22. Jiang J, Roman RJ. Lovastatin prevents development of hypertension in spontaneously hypertensive rats. Hypertension. 1997;30(4):968–974. [DOI] [PubMed] [Google Scholar]
- 23. Clarke S. Protein isoprenylation and methylation at carboxylterminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. [DOI] [PubMed] [Google Scholar]
- 24. Raiteri M, Arnaboldi L, McGeady P, et al. Pharmacological control of the mevalonate pathway: effect on arterial smooth muscle cell proliferation. J Pharmacol Exp Ther. 1997;281:1144–1153. [PubMed] [Google Scholar]
- 25. Goode GK, Miller JP, Heagerty AM. Hyperlipidaemia, hypertension, and coronary heart disease. Lancet. 1995;345(8946): 362–364. [DOI] [PubMed] [Google Scholar]
- 26. Sung BH, Izzo JL, Wilson MF. Effects of cholesterol reduction on BP response to mental stress in patients with high cholesterol. Am J Hypertens. 1997:10:592–599. [DOI] [PubMed] [Google Scholar]
- 27. Jarai Z, Kapocsi J, Farsang C, et al. Effect of fluvastatin on serum lipid levels in essential hypertension [in Hungarian]. Orv Hetil. 1996;137(34):1857–1859. [PubMed] [Google Scholar]
- 28. Straznicky NE, Howes LG, Lam W, et al. Effects of pravastatin on cardiovascular reactivity to norepinephrine and angiotensin II in patients with hypercholesterolemia and systemic hypertension. Am J Cardiol. 1995;75(8):582–586. [DOI] [PubMed] [Google Scholar]
- 29. Abetel G, Poget PN, Bonnabry JP. Hypotensive effect of an inhibitor of cholesterol synthesis (fluvastatin). A pilot study. [in French] Schweiz Med Wochenschr. 1998;128(7):272–277. [PubMed] [Google Scholar]
- 30. D'Agostino RB, Kannel WB, Stepanians MN, et al. Efficacy and tolerability of lovastatin in hypercholesterolemia in patients with systemic hypertension. Am J Cardiol. 1993;71:82–87. [DOI] [PubMed] [Google Scholar]
- 31. Muramatsu J, Kobayashi A, Hasegawa N, et al. Hemodynamic changes associated with reduction in total cholesterol by treatment with the HMG‐CoA reductase inhibitor pravastatin. Atherosclerosis. 1997;130:179–182. [DOI] [PubMed] [Google Scholar]
- 32. Antolicelli R, Onorato G, Pagelli P, et al. Simvastatin in the treatment of hypercholesterolemia in elderly patients. Clin Ther. 1990;12:165–171. [PubMed] [Google Scholar]
- 33. Kool M, Lustermans F, Kragten H, et al. Does lowering of cholesterol levels influence functional properties of large arteries? Eur J Clin Pharmacol. 1995;48:217–223. [DOI] [PubMed] [Google Scholar]
- 34. Borghi C, Gaddi A, Ambrosioni E, et al. Improved blood pressure control in hypertensive patients treated with statins. J Am Coll Cardiol. 2001;37(2)(suppl A): 233A–234A. [Google Scholar]
- 35. Glorioso N, Troffa C, Filigheddu F, et al. Effect of the HMG‐CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34:1281–1286. [DOI] [PubMed] [Google Scholar]
- 36. Tonolo G, Melis MG, Formato M, et al. Additive effects of Simvastatin beyond its effects on LDL cholesterol in hypertensive type 2 diabetic patients. Eur J Clin Invest. 2000;(11): 980–987. [DOI] [PubMed] [Google Scholar]
- 37. Velussi M, Cernigoi AM, Tortul C, et al. Atorvastatin for the management of Type 2 diabetic patients with dyslipidemia. A mid‐term (9‐month) treatment experience. Diabetes Nutr Metab. 1999;12:407–412. [PubMed] [Google Scholar]
- 38. Sposito AC, Mansur AP, Coelho OR, et al. Additional reduction in blood pressure after cholesterol‐lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin‐converting enzyme inhibitors (enalapril or lisinopril). Am J Cardiol. 1999;83(10): 1497–1499. [DOI] [PubMed] [Google Scholar]
- 39. Borghi C, Prandin MG, Costa FV, et al. Use of statins and blood pressure control in hypertensive patients with hypercholesterolemia. J Cardiovasc Pharmacol. 2000;35:549–555. [DOI] [PubMed] [Google Scholar]
- 40. Nazzaro P, Manzari M, Merlo M, et al. Distinct and combined vascular effects of ACE blockade and HMG‐CoA reductase inhibition in hypertensive subjects. Hypertension. 1999;33(2): 719–725. [DOI] [PubMed] [Google Scholar]
- 41. Egashira K, Hirooka Y, Kai H, et al. Reduction in serum cholesterol with pravastatin improves endothelium‐dependent coronary vasomotion in patients with hypercholesterolemia. Circulation. 1994;89(6):2519–2524. [DOI] [PubMed] [Google Scholar]
- 42. O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG‐coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95(5):1126–1131. [DOI] [PubMed] [Google Scholar]
- 43. Lewis TV, Cooper BA, Dart AM, et al. Responses to endothelium‐dependent agonists in subcutaneous arteries excised from hypercholesterolaemic men. Br J Pharmacol. 1998;124(1): 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–1135. [DOI] [PubMed] [Google Scholar]
- 45. Kaesemeyer WH, Caldwell RB, Huang J, et al. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol‐lowering actions. J Am Coll Cardiol. 1999; 33(1):234–241. [DOI] [PubMed] [Google Scholar]
- 46. Nickenig G, Bohm M. Regulation of the angiotensin AT1 receptor expression by hypercholesterolemia. Eur J Med Res. 1997;2(7):285–289. [PubMed] [Google Scholar]
- 47. Ide H, Fujiya S, Aanuma Y, et al. Effects of simvastatin, an HMG‐CoA reductase inhibitor, on plasma lipids and steroid hormones. Clin Ther. 1990;12(5):410–420. [PubMed] [Google Scholar]
- 48. Mugen E, Viskoper JR, Haiomeiser E, et al. Effects of low dose aspirin and simvastatin on blood pressure and endothelial dysfunction of hypertensive patients with and without hyperlipemia [abstract]. Hypertension. 2000;36(4):661. [Google Scholar]
- 49. Ferrari AU, Mircoli L, Terzoli L. Do statins lower blood pressure? An ambulatory monitoring assessment [abstract]. Eur Heart J. 2001;22;271. 11161941 [Google Scholar]
- 50. Leibovitz E, Hazanov N, Zimlichman R, et al. Treatment with atorvastatin improves small artery compliance in patients with severe hypercholesterolemia. Am J Hypertens. 2001;14:1096–1098. [DOI] [PubMed] [Google Scholar]


