Abstract
Microalbuminuria (MA) is defined as persistent elevation of albumin in the urine, of 30–300 mg/day (20–200 μg/min). These values are less than the values detected by routine urine dipstick testing, which does not become positive until protein excretion exceeds 300–500 mg/day. Use of the albumin‐to‐creatinine ratio is recommended as the preferred screening strategy for all diabetic patients. MA is measured in spot morning urine obtained from the patient in the office and sent for measurement of both albumin and creatinine. A value above 0.03 mg/mg suggests that albumin excretion is above 30 mg/day and therefore MA is present. MA should be checked annually in everyone, and every 6 months within the first year of treatment to assess the impact in patients started on antihypertensive therapy. MA is an established risk factor for renal disease progression in type 1 diabetes and its presence is the earliest clinical sign of diabetic nephropathy. In addition, a number of studies suggest that MA is an important risk factor for cardiovascular disease and defines a group at high risk for early cardiovascular mortality in both type 2 diabetes and essential hypertension. MA also signifies abnormal vascular permeability and the presence of atherosclerosis. Among nondiabetic patients with essential hypertension, MA is associated with higher blood pressures, increased serum total cholesterol, and reduced serum high‐density lipoprotein cholesterol. Thus, taken together these data support the concept that the presence of MA is the kidney's notice to the physician/patient that there is a problem with the vasculature. MA can be reduced, and progression to overt proteinuria prevented, by aggressive blood pressure reduction. The National Kidney Foundation recommends that blood pressure levels be maintained at or below 130/80 mm Hg in anyone with diabetes or renal disease. This should be accomplished with antihypertensive agents that prevent the rise in MA and hence prevent development of proteinuria. Such agents are angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers and, to a lesser extent, β blockers, non‐dihydropyridine calcium antagonists, and diuretics. In summary, the presence of MA is a marker of endothelial dysfunction and a harbinger of markedly enhanced cardiovascular risk. All patients with diabetes and/or hypertension should be screened for the presence of microalbuminuria with use of spot morning urine. To maximize prevention of MA development, the following goals should be instituted: 1) blood pressure should be maintained at <130/80 mm Hg and a low‐salt, moderate‐potassium diet instituted; 2) in diabetics, HbA1c should be kept at <7%; 3) in obese patients, a weight loss program should be implemented, with a goal BMI of <30; and 4) the physician and patient, working together, should maintain low‐density lipoprotein cholesterol at <120 mg/dL, and <100 mg/dL if diabetes is present.
The normal rate of albumin excretion is less than 30 mg/day; values above 300 mg/day (200 μg/min) are considered to represent overt proteinuria. Microalbuminuria is defined as persistent values of albumin in the urine between 30 and 300 mg/day (20–200 μg/min). 1 These values are less than the values detected by routine urine dipstick, which does not become positive, even for trace amounts, until protein excretion exceeds 300–500 mg/day. Thus, the routine urine dipstick is relatively insensitive for detection of microalbuminuria.
MEASUREMENT OF MICROALBUMINURIA
For many years, 24‐hour urine collection was the gold standard for the detection of microalbuminuria. However, recent studies have shown strong correlations between spot morning urine samples and 24‐hour collections. 2 A spot morning urine may be collected from the patient in the office and sent for measurement of both albumin and creatinine. The reason for measuring both albumin and creatinine is that measuring the albumin concentration alone can give false negative results, since the albumin concentration, but not the rate of albumin excretion, is influenced by urine volume. Creatinine levels provide a true estimate of an adequate urine collection, since they are dependent on the muscle mass of a given person. A value above 0.02 suggests that albumin excretion is above 30 mg/day and therefore microalbuminuria is present. For example, if urine has 30 mg of albumin and 140 mg of creatine, the ratio is 30 mg/150 mg, or 0.2. This about 300 mg/day of albuminaria (dipstick positive); the microalbuminuria is 3 mg albumin with creatine of 150 mg, or 3/150=0.02. Dehydration, fever, exercise, heart failure, and poor glycemic control are among the factors that can cause transient microalbuminuria. 3
Use of the albumin:creatinine ratio is recommended as the preferred screening strategy for all diabetic patients. 4 , 5 In patients started on antihypertensive therapy, microalbuminuria should be checked every 6 months within the first year, and annually thereafter, to assess the effect of treatment. The physician should consider the following, however, to maximize the reliability of this test: 1) Vigorous exercise may cause a transient increase in albumin excretion, so patients should refrain from vigorous exercise in the 24 hours prior to the test. 2) The slope of the relationship between the spot urine and the 24‐hour collection varies throughout the day, and the best correlation occurs in the mid‐morning.
Cost and Convenience of Measurement
Microalbuminuria can be measured in the office by one of two methods, Micral II® sticks and the spot albumin:creatinine ratio. The Micral II® is a dipstick method specifically designed to detect the lower levels of albumin in the spot urine. The sensitivity of the test ranges from 88%–95%, with a specificity of 92%–95%.6 The cost of microalbumin determination using the Micral II® stick ranges from $4.00–$7.00 per strip used.
Use of a morning spot urine for the albumin:creatinine ratio has a sensitivity and a specificity of 93% and 97%, respectively. An advantage over the Micral II® stick is that it provides a quantitative value rather than a qualitative “one or two plus” value. The cost, however, is more than that of the Micral II® method, ranging from $12.00–$24.00 per sample tested. Additionally, using the albumin:creatinine ratio, one must wait 24–48 hours for a result.
WHY MEASURE MICROALBUMINURIA?
Microalbuminuria is an established risk factor for renal disease progression in type 1 diabetes. Its presence is the earliest clinical sign of diabetic nephropathy. 1 , 4 , 5 However, fewer than 50% of patients with type 1 diabetes and microalbuminuria are at risk for renal disease progression. These disparate findings may be due in part to the time at which microalbuminuria begins. Most patients who develop microalbuminuria within the first 10 years of having type 1 diabetes have progression to macroalbuminuria (>300 mg/day—dipstick‐positive). Once macroalbuminuria is present in these patients, more than one half will progress to end‐stage renal disease and require dialysis, if they do not suffer a fatal cardiovascular event prior to requiring dialysis.
More importantly, however, a number of studies 4 , 5 , 7 , 8 , 9 suggest that microalbuminuria is also a significant risk factor for cardiovascular disease and defines a group at high risk for early cardiovascular mortality in both type 2 diabetes and essential hypertension. In one study, 10 for example, 141 nonproteinuric patients with type 2 diabetes were followed for a mean of 3.4 years. The mortality rate was 28% in those with microalbuminuria and 4% in those with normal albumin excretion. This increase in risk was independent of other cardiovascular risk factors. A similar increase in mortality risk has been described in nondiabetic patients with essential hypertension. 11 In addition, a 10‐year follow‐up study 12 of over 2000 people indicated that the presence of microalbuminuria more than doubled the risk of development of ischemic heart disease. Moreover, in those with blood pressures of <140/90 mm Hg, microalbuminuria doubled the risk of ischemic disease development relative to the group with normoalbuminuria.
The apparent association between microalbuminuria and atherosclerosis is related, in part, to an adverse risk factor profile. Among nondiabetic patients with essential hypertension, microalbuminuria is associated with higher blood pressures, increased serum total cholesterol, and reduced serum high‐density lipoprotein cholesterol. Generalized vascular wall dysfunction also may be observed in nondiabetic patients with essential hypertension. 13 , 14 Thus, taken together, these data support the notion that the kidney is the “sentinal of the vasculature.” The presence of microalbuminuria signals increased permeability of the endothelial cells and signifies that some level of injury is present and vascular responsiveness is compromised.
The enhanced risk of cardiovascular disease with microalbuminuria may also be due in part to an association with hyperhomocysteinemia, a risk factor for atherosclerosis, or hypercholesterolemia. In addition, a cross‐sectional analysis of 1160 type 1 diabetic subjects in the Diabetes Control and Complications Trial 15 showed that progressive degrees of albuminuria were associated with an increase in intermediate‐density lipoprotein and small, dense low‐density lipoprotein (LDL) particles. This suggests that the associated dyslipidemia may account for the increased cardiovascular risk in diabetic patients with microalbuminuria.
WHEN TO TREAT, AND HOW?
The current recommendation is for an annual screening of microalbuminuria in all patients with diabetes. 4 , 5 Screening can be deferred for 5 years after the onset of disease in type 1 diabetics because microalbuminuria is uncommon before this time.
Albumin excretion can be reduced and progression to overt proteinuria prevented by aggressive blood pressure reduction. The National Kidney Foundation recommends that blood pressure levels be maintained at or below 130/80 mm Hg in anyone with diabetes or renal disease. 4 This should be accomplished with antihypertensive agents that prevent the rise in microalbuminuria and hence prevent development of proteinuria. Such agents include angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), given either alone or with a diuretic to achieve blood pressure goals of <130/80 mm Hg in the diabetic and <140/90 in the general population. A paradigm that exemplifies the approach is shown in the Figure. Whether other antihypertensive drugs will be as effective as ACE inhibitors in preventing progressive proteinuria is unclear; studies in patients with overt proteinuria suggest that some β blockers (such as carvedilol), non‐dihydropyridine calcium channel blockers (such as diltiazem and verapamil), and thiazide diuretics may have prominent antiproteinuric activity. 16 , 17
Figure.
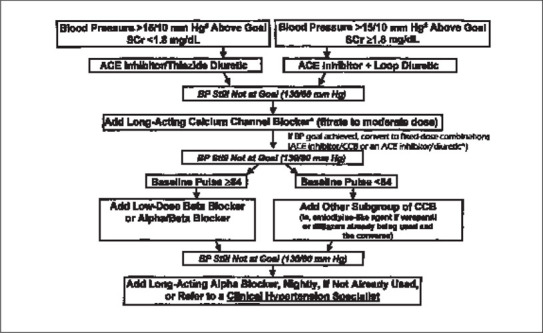
A suggested paradigm by which blood pressure goals in people with renal insufficiency and/or diabetes can be achieved by the least intrusive means possible. Everyone with diabetes and/or renal insufficiency should be instructed on lifestyle modifications, as per the JNC VI. Everyone, however, should be started on therapy if blood pressure is greater than 130/85 mm Hg. In patients with blood pressures >15/10 mm Hg above goal, two drugs should be used; in patients with blood pressures above goal but not >15/10 mm Hg above goal, one drug may be used alone. #Note: If blood pressure is <15/10 mm Hg above goal (130/80 mm Hg), an angiotensin‐converting enzyme (ACE) inhibitor alone may be used. The ACE inhibitor should be the same if two different fixed dose combinations are used. *Non‐dihydropyridine calcium channel blockers (verapamil and diltiazem) have been shown to reduce both cardiovascular mortality and progression of diabetic nephropathy independent of an ACE inhibitor). Adapted with permission from Bakris et al. Am J Kidney Dis. 2000;36:646–661. 4
SUMMARY
It has become clear that the presence of microalbuminuria is a marker of endothelial dysfunction and a harbinger of markedly enhanced cardiovascular risk. All patients with diabetes and/or hypertension should be screened for the presence of microalbuminuria with the use of spot morning urine for albumin and creatinine determinations. To maximize prevention of microalbuminuria, the following factors should be instituted. First, blood pressure should be maintained at <130/80 mm Hg and the patient should be on a low‐salt/moderate‐potassium diet. Second, if the patient is diabetic, HbA1c should be maintained at <7%. Third, for obese patients a weight loss program should be instituted to achieve a body mass index of <30. Last, the physician and patient, working together, should maintain LDL cholesterol at <120 mg/dL, and at <100 mg/dL if diabetes is present.
References
- 1. Bakris GL. Microalbuminuria: Prognostic implications. Curr Opin Nephrol Hypertens. 1996;5:219–223. [DOI] [PubMed] [Google Scholar]
- 2. Ruggenenti P, Gaspari F, Perna A, et al. Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ. 1998;316:504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mogensen CE, Vestbo E, Poulsen PL, et al. Microalbuminuria and potential confounders. A review and some observations on variability of urinary albumin excretion. Diabetes Care. 1995;18:572. [DOI] [PubMed] [Google Scholar]
- 4. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: A consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. [DOI] [PubMed] [Google Scholar]
- 5. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez I, Paez PJM, Hermosin BT, et al. Rapid screening test evaluation for microalbuminuria in diabetes mellitus. Acta Diabetol. 1998;35(4):199–202. [DOI] [PubMed] [Google Scholar]
- 7. Mattock MB, Morrish NJ, Viberti G, et al. Prospective study of microalbuminuria as a predictor of mortality in NIDDM. Diabetes. 1992;41:736–741. [DOI] [PubMed] [Google Scholar]
- 8. Mogensen CE. Natural history of cardiovascular and renal disease in patients with type 2 diabetes: Effect of therapeutic interventions and risk modification. Am J Cardiol. 1998;82:4R–8R. [DOI] [PubMed] [Google Scholar]
- 9. Haffner SM, Lehto S, Ronnernaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 10. Valmadred CT, Klein R, Moss SE, et al. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria with older‐onset diabetes mellitus. Arch Intern Med. 2000;160:1093–1099. [DOI] [PubMed] [Google Scholar]
- 11. Bianchi S, Bigazzi R, Campese VM. Microalbuminuria in essential hypertension: Significance, pathophysiology, and therapeutic implications. Am J Kidney Dis. 1999;34:973–979. [DOI] [PubMed] [Google Scholar]
- 12. Borch‐Johnsen K, Feldt‐Rasmussen B, Strandgaard S, et al. Urinary albumin excretion. An independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol. 1999;19:1992–1997. [DOI] [PubMed] [Google Scholar]
- 13. Calvino J, Calvo C, Romero R, et al. Atherosclerosis profile and microalbuminuria in essential hypertension. Am J Kidney Dis. 1999;34:996–1002. [DOI] [PubMed] [Google Scholar]
- 14. Pedrinelli R, Giampietro O, Carmassi F, et al. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet. 1994;344:14–19. [DOI] [PubMed] [Google Scholar]
- 15. Sibley SD, Hokanson JE, Steffes MW, et al. Increased small dense LDL and intermediate‐density lipoprotein with albuminuria in type 1 diabetes. Diabetes Care. 1999;22:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koshy S, Bakris GL. Therapeutic approaches to achieve desired blood pressure goals: Focus on calcium channel blockers. Cardiovasc Drugs Ther. 2000;14:295–301. [DOI] [PubMed] [Google Scholar]
- 17. Makrilakis K, Bakris GL. New therapeutic approaches to achieve the desired blood pressure goal. Cardiovasc Rev Rep. 1997;18:10–16. [Google Scholar]


