Abstract
Uncontrolled hypertension leads to an increased risk of cardiovascular disease and stroke. Hypertensive patients with concomitant type 2 diabetes are at even greater risk of cardiovascular complications; also, this high‐risk patient population is at increased risk of renal disease and, ultimately, renal failure. Prospective morbidity and mortality trials have demonstrated that tight blood pressure control improves the cardiovascular prognosis and provides target organ protection. Current treatment guidelines recommend a target blood pressure of <130/85 mm Hg for patients with hypertension and diabetes. Angiotensin II (A‐II), a major component of the renin‐angiotensin system, plays an essential role in the pathophysiology of hypertension and diabetes‐related renal disease. Currently, the treatment of choice for hypertensive patients with diabetes is angiotensin‐converting enzyme (ACE) inhibition, but most of the data are limited to patients with type 1 diabetes. Although ACE inhibition is clearly a mechanism for blocking A‐II formation, inhibition at this site may not be complete, as alternate pathways exist for A‐II formation. Thus, for interrupting the renin‐angiotensin system, A‐II receptor antagonists theoretically provide advantages over ACE inhibitors in that they directly inhibit A‐II by binding to the AT1 receptor subtype. The objectives of this review are to: 1) provide an overview of the associated risk of cardiovascular complications with concomitant hypertension and diabetes; 2) demonstrate the cardiovascular benefits of effective blood pressure control in this patient population; 3) review the current treatment guidelines for managing high‐risk hypertensive patients; and 4) discuss major, ongoing clinical studies with A‐II receptor antagonists in patients with concomitant hypertension, type 2 diabetes, and renal disease.
Hypertension, one of the most prevalent chronic disease states worldwide, remains a major health care issue despite numerous readily available treatments. 1 , 2 , 3 Only about one fourth of the total hypertensive population in the United States achieves adequately controlled blood pressure (<140/90 mm Hg) with currently available medications, 4 , 5 and even lower rates are reported outside the United States. 3 Although awareness, prevention, treatment, and control of hypertension increased between the National Health and Nutrition Examination Surveys (NHANES) of 1976–1980 and 1988–1991, there was no further improvement in the NHANES of 1988–1994. 2 Patients with uncontrolled hypertension are at increased risk of cardiovascular disease and stroke. 2 Many patients with hypertension also have concomitant type 2 diabetes mellitus, 6 , 7 which places them at even greater risk of cardiovascular complications, including myocardial infarction (MI), stroke, transient ischemic attacks, 8 and cardiovascular death. 9 To further complicate this issue, patients with diabetes mellitus are also at increased risk of renal disease. Microalbuminuria, defined as an albumin excretion rate (AER) of 30–300 mg/24 hours, generally begins 5–10 years after diabetes mellitus has been diagnosed and is often the first clinical sign of diabetic damage to the kidney. 10 It has been demonstrated 11 , 12 , 13 , 14 that once microalbuminuria is present, it progresses to proteinuria (AER of >300 mg/24 hours) in 22%–50% of patients over a 5‐ to 10‐year period. Further decline of renal function is inevitable after the development of proteinuria. 15 , 16 The simultaneous presence of either microalbuminuria or proteinuria and hypertension sharply exaggerates the mortality risk in patients with type 2 diabetes mellitus. 17 , 18
Control of hypertension and type 2 diabetes mellitus improves the cardiovascular prognosis of these patients and provides target organ protection, as demonstrated by surveys 4 and prospective morbidity and mortality trials. 19 , 20 , 21 Angiotensin II (A‐II), a major component of the renin‐angiotensin system, is a powerful vasoconstrictor 22 and is therefore important in the pathophysiology of essential hypertension. In addition, A‐II has been implicated in the progression of renal failure in patients with diabetic nephropathy, through such effects as its selective constriction of efferent glomerular arterioles, which increases intraglomerular pressure. 23 Inhibition of A‐II may have physiologic and mechanistic effects that reduce the risk of cardiovascular and diabetic complications. 24
Several randomized clinical trials have shown that angiotensin‐converting enzyme (ACE) inhibitors may decrease cardiovascular morbidity and mortality in patients with concomitant hypertension and diabetes mellitus to a greater extent than such agents as β blockers, diuretics, and calcium channel blockers. 20 , 25 , 26 It is important to note, though, that the numbers of cardiovascular events in these studies were generally small, and cardiovascular morbidity and mortality were often secondary end points. Furthermore, in another comparison of ACE inhibition and β‐blocker therapy in patients with hypertension and diabetes mellitus, both treatments were similarly effective in reducing diabetic and cardiovascular complications. 27 Thus, while ACE inhibition is currently the treatment of choice for these patients (both types 1 and 2 diabetes), 2 further prospective studies designed to compare different antihypertensive classes are needed before definitive conclusions about optimal treatment strategies can be made, particularly for type 2 diabetes. 28
A‐II receptor antagonists may also offer benefit in this patient population because of their direct inhibition of A‐II at the site of the receptor (AT1); clinical‐event trials with these agents are ongoing. Because of their effectiveness in selectively blocking the AT1 receptor, these agents may be particularly valuable for the prevention of cardiovascular complications in hypertensive patients with diabetes mellitus. The objectives of this review are to: 1) provide an overview of the associated risk of cardiovascular complications with concomitant hypertension and diabetes mellitus; 2) demonstrate the cardiovascular benefits of effective blood pressure control in this patient population; 3) review the current treatment guidelines for managing high‐risk hypertensive patients; and 4) discuss major, ongoing clinical end point studies of A‐II receptor antagonists in patients with concomitant hypertension, type 2 diabetes, and renal disease.
INCIDENCE OF CARDIOVASCULAR COMPLICATIONS OF HYPERTENSION AND TYPE 2 DIABETES MELLITUS
The 7‐year incidences of fatal or nonfatal MI, fatal or nonfatal stroke, and death from cardiovascular causes were compared between 1373 nondiabetic subjects and 1059 patients with type 2 diabetes mellitus in a Finnish population‐based cohort study. 29 Cardiovascular risk was as high in patients with diabetes mellitus as in nondiabetic subjects who had a history of MI. Once patients with diabetes mellitus develop overt coronary heart disease, they have a particularly poor prognosis, as shown in a 35‐month comparison of the course of acute MI in 85 patients with diabetes mellitus and 415 nondiabetic subjects. 30 Patients with diabetes mellitus experienced a more complicated in‐hospital and postdischarge course than did nondiabetic subjects, including higher incidences of postinfarction angina, infarct extension, heart failure, and death.
A 12‐year follow‐up study of 4714 patients with diabetes mellitus participating in the World Health Organization Multinational Study of Vascular Disease in Diabetes 17 showed that coronary heart disease and stroke were major contributors to the excess mortality rates seen in this study. The combination of hypertension and diabetes mellitus gives these patients an approximate fourfold increase in cardiovascular risk over the normotensive, nondiabetic population. 6 , 31 It has been demonstrated that systolic blood pressure (SBP) is a powerful determinant of cardiovascular risk for patients with diabetes mellitus (Fig. 1). 9 The relationships of SBP and other cardiovascular risk factors to cardiovascular mortality were compared in men with diabetes mellitus (n=5163) and without diabetes mellitus (n=342,815) in a large cohort study. 9 SBP was positively related to the risk of cardiovascular death, with a significant trend in both nondiabetic subjects and patients with diabetes mellitus. Moreover, with higher SBP levels, the cardiovascular mortality rate increased more steeply among those with diabetes mellitus than those without diabetes mellitus. The absolute risk of cardiovascular death was three times higher for men with diabetes mellitus than for nondiabetic men, after adjustment for age, race, income, serum cholesterol, SBP, and cigarette smoking (p<0.0001).
Figure 1.
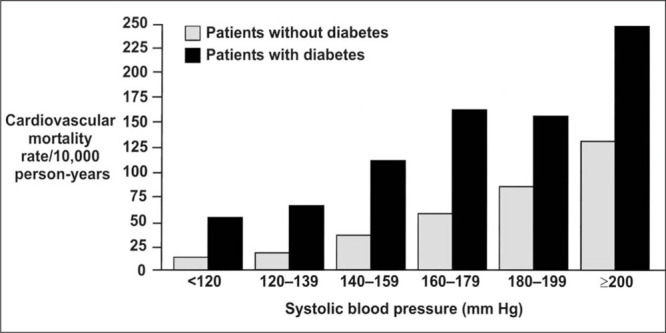
Age‐adjusted cardiovascular disease mortality rates by systolic blood pressure in men with and without diabetes mellitus 9
A cross‐sectional study 8 of 3648 newly diagnosed patients with type 2 diabetes mellitus was performed to determine the association between hypertension and other risk factors for cardiovascular complications. Patients with hypertension and diabetes mellitus had a higher prevalence of prior cardiovascular events, including MI, stroke, left ventricular hypertrophy, and electrocardiographic signs of ischemic heart disease than did patients who were normotensive with type 2 diabetes mellitus (Fig. 2). Thus, the association between blood pressure and cardiovascular complications is already apparent at the time of diagnosis of diabetes mellitus. Proteinuria and hypertension were the most important risk factors for mortality in the World Health Organization Multinational Study of Vascular Disease in Diabetes. 17 In a 7‐year follow‐up of 1056 patients with type 2 diabetes mellitus, 18 clinical proteinuria significantly predicted mortality and the incidence of stroke and other atherosclerotic vascular disease events, indicating that proteinuria adds to the risk associated with diabetes and hypertension. 6 , 31
Figure 2.
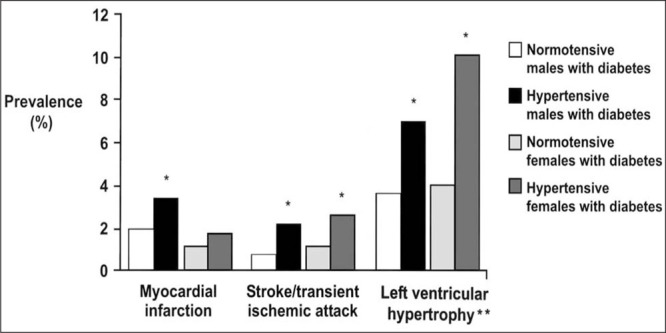
Prevalence of cardiovascular disease in hypertensive and normotensive patients at diagnosis of type 2 diabetes mellitus. *Statistically significant, hypertensive vs. normotensive; **left ventricular hypertrophy on electrocardiograph 8
BENEFITS OF BLOOD PRESSURE CONTROL
Recent pivotal studies have shown that blood pressure control leads to a substantial reduction in cardiovascular morbidity and mortality in hypertensive patients with diabetes mellitus.
The Systolic Hypertension in the Elderly Program (SHEP)
This landmark study in 4736 patients with isolated systolic hypertension (SBP of 160–219 mm Hg and diastolic blood pressure [DBP] of <90 mm Hg) revealed that diuretic‐based antihypertensive therapy reduced the incidence of total stroke by 36%; p=0.0003). 32 , 33 Major cardiovascular events were reduced by 32% in patients receiving active antihypertensive therapy, compared with patients receiving placebo. Among patients with type 2 diabetes mellitus, the 5‐year major cardiovascular disease rate was reduced by 34% by active treatment (Fig. 3). A similar relative reduction was observed in the nondiabetic subjects, but since events were more common in patients with diabetes mellitus, the absolute risk reduction with active treatment was greater than in the nondiabetics. 32 , 33
Figure 3.
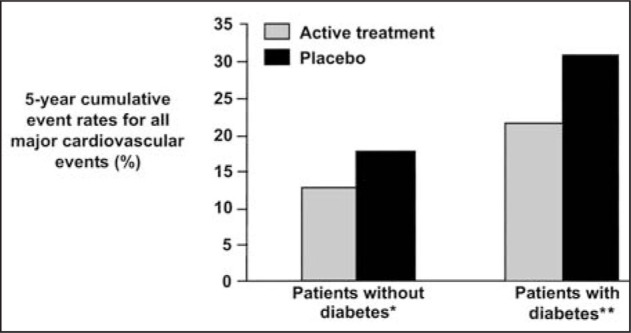
Cumulative 5‐year cardiovascular disease event rates for hypertensive patients with and without diabetes mellitus by treatment group (diuretic‐based treatment vs. placebo). *Relative risk, 0.66; 95% confidence interval, 0.55–0.79; **relative risk, 0.66; 95% confidence interval, 0.46–0.94 33
Systolic Hypertension in Europe (Syst‐Eur) Trial
This European trial 34 was conducted to determine whether treatment with a dihydropyridine calcium channel blocker would prevent strokes in patients with isolated systolic hypertension. Active treatment reduced the rate of stroke by 42% (p=0.003), all cardiac end points by 26% (p=0.03), and all cardiovascular end points by 31% (p<0.001). These powerful results were observed even though the trial was ended earlier than planned. In a post hoc analysis of 492 patients with diabetes mellitus, the treatment reduced overall mortality by 55%, cardiovascular mortality by 76%, all cardiovascular events by 69%, fatal and nonfatal strokes by 73%, and all cardiac events by 63%. 35 These reductions were greater than those in the nondiabetic patients (Fig. 4). Thus, both the SHEP and Syst‐Eur trials have demonstrated the benefit of blood pressure control on clinical events and mortality in patients with diabetes mellitus.
Figure 4.
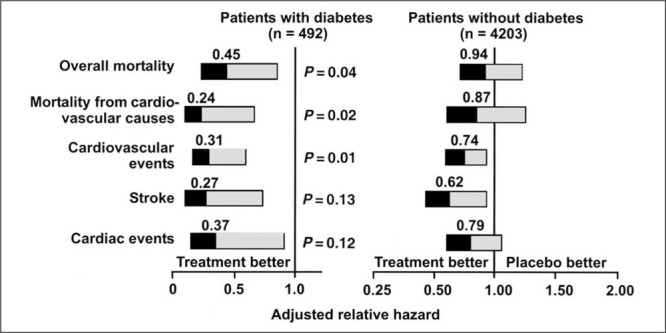
Adjusted relative hazard of cardiovascular morbidity and mortality based on treatment (active hypertensive treatment vs. placebo) in hypertensive patients with and without diabetes mellitus. Reprinted with permission from N Engl J Med. 1999;34D:677–684. 35
The Hypertension Optimal Treatment (HOT) Study
The HOT study 36 was originally designed to learn whether three different target DBP levels (≤90 mm Hg, ≤85 mm Hg, ≤80 mm Hg) might influence clinical outcomes in hypertensive patients. Antihypertensive therapy was started with the long‐acting calcium channel blocker felodipine. Additional therapy with ACE inhibitors, β blockers, and diuretics was prescribed in order to reach blood pressure goals. The greatest reduction (30%) in risk of a major cardiovascular event was associated with a mean DBP of 83 mm Hg. In the 1501 patients with concomitant hypertension and diabetes mellitus, there was a 51% reduction in major cardiovascular events in patients assigned to the target DBP of ≤80 mm Hg (achieved DBP of 81 mm Hg) compared with those assigned to the target DBP of ≤90 mm Hg (achieved DBP of 85 mm Hg) (p=0.005 for trend). Figure 5 demonstrates the clear clinical benefit associated with this blood pressure difference of only 4 mm Hg. This finding suggests that intensive blood pressure reduction may be beneficial in reducing cardiovascular events in patients with diabetes mellitus and hypertension.
Figure 5.
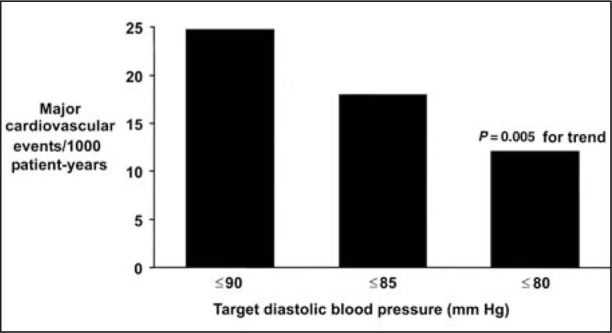
Major cardiovascular events/1000 patient‐years by target blood pressure group in patients with hypertension and diabetes mellitus 36
The United Kingdom Prospective Diabetes Study (UKPDS)
A multicenter, randomized, interventional trial 19 was designed to determine the effect of intensive blood glucose control in 3867 patients with newly diagnosed type 2 diabetes mellitus. Over 10 years, glycosylated hemoglobin was reduced by 11% in patients receiving antidiabetic medications, compared with those treated only with diet. This resulted in a 12% reduction in the risk of any diabetes‐related end point (p=0.029), mainly due to a 25% risk reduction in microvascular end points (p=0.01). Embedded within the UKPDS was a randomized, controlled trial 21 designed to compare a captopril‐ or atenolol‐based regimen of tight blood pressure control (<150/85 mm Hg) to less tight control (<180/105 mm Hg, avoiding ACE inhibitor and β‐blocker therapy) in 1148 diabetic patients with concurrent hypertension. The mean blood pressure during follow‐up was 154/87 mm Hg in the less tightly controlled group and 144/82 mm Hg in the tightly controlled group. Compared with less tight blood pressure control, tight control reduced the risk of diabetes‐related mortality by 32% (p=0.019), stroke by 44% (p=0.013), and MI by 21% (p=0.13). The captopril‐ and atenolol‐based regimens were similarly effective (Fig. 6). Tight blood pressure control appears to be even more effective than tight glucose control in protecting against cardiovascular events in hypertensive patients with diabetes mellitus.
Figure 6.
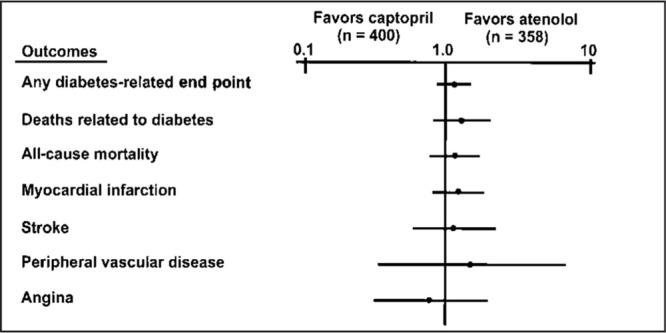
Relative risks of attaining clinical end points based on treatment group in patients with hypertension and diabetes mellitus. Adapted with permission from BMJ. 1998;317:713–720. 27
Rationale for Renin‐Angiotensin System Blockade.
The Captopril Prevention Project (CAPPP). 20
This trial was designed to compare the effects of the ACE inhibitor captopril (given only once daily) with conventional antihypertensive therapy (β blockers, diuretics, or the combination) and included 10,985 patients with hypertension. The primary end point was a composite of fatal and nonfatal MI, stroke, and other cardiovascular deaths. Secondary end points included new or worsened ischemic heart disease and congestive heart failure, atrial fibrillation, diabetes mellitus, transient ischemic attacks, and death from all causes. The main findings of the study were that cardiovascular morbidity and mortality were the same in the captopril and conventional therapy groups. However, analysis of the 572 patients with hypertension and diabetes mellitus showed that patients treated with captopril experienced a 41% reduction in the risk of fatal and nonfatal MI, stroke, and other cardiovascular deaths (p=0.019), as well as significant reductions in other end points, compared with patients treated with conventional antihypertensive therapy (Fig. 7).
Figure 7.
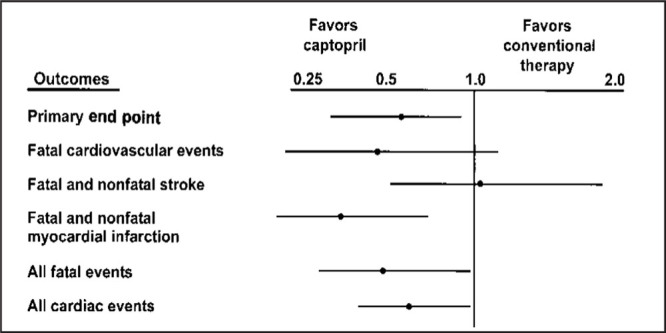
Relative risks of attaining clinical end points (adjusted for age, sex, systolic blood pressure, and previous treatment) according to treatment in patients with hypertension and diabetes mellitus at baseline. Adapted with permission from Lancet. 1999;53:611–616. 20
The Appropriate Blood Pressure Control in Diabetes (ABCD) Trial. 25
A randomized, blinded study was performed in 950 patients with type 2 diabetes mellitus and a DBP of =80 mm Hg who were not taking any antihypertensive medications. A subgroup of 470 patients with hypertension and type 2 diabetes mellitus was randomized to achieve intensive blood pressure control (target DBP, 75 mm Hg) or moderate control (target DBP, 80–89 mm Hg). The primary end point was the effect of blood pressure control on the change in 24‐hour creatinine clearance. Secondary end points included the effect of blood pressure control on cardiovascular events, retinopathy, neuropathy, urinary albumin excretion, and left ventricular hypertrophy. Patients were randomized to receive treatment with the ACE inhibitor enalapril or the calcium channel blocker nisoldipine. After 67 months, the Data and Safety Monitoring Board recommended discontinuation of nisoldipine among the patients with hypertension because there were significantly more cardiovascular events in the calcium channel blocker arm than in the ACE inhibitor arm of the hypertensive cohort. In particular, patients receiving nisoldipine experienced a somewhat higher incidence of MI than patients receiving enalapril (25 vs. five, respectively). In addition, there appeared to be more overall cardiovascular events in the nisoldipine group than in the enalapril group. Since calcium channel blockers have been shown to be safe, and in all likelihood are superior to placebo in preventing cardiovascular events, the findings in this study suggest that ACE inhibitors may be especially valuable in the setting of diabetes. However, since these findings are based on a secondary end point and there were small absolute numbers of cardiovascular events in this trial, further evidence is required to allow firm conclusions.
The Fosinopril Versus Amlodipine Cardiovascular Events Trial (FACET).
26 An open‐label, randomized, prospective trial was conducted to compare the effects of fosinopril, an ACE inhibitor, with those of amlodipine, a calcium channel blocker, in 380 patients with hypertension and type 2 diabetes mellitus. In order to control blood pressure, amlodipine was added in 31% of the fosinopril‐treated group, and fosinopril was added in 26% of the amlodipine‐treated group. There were no significant differences between the two groups with regard to primary efficacy end points, including serum lipids and diabetes control. As to secondary end points, fosinopril‐treated patients, compared with patients receiving amlodipine, had a combined 51% reduction in the risk of acute MI, stroke, or angina requiring hospitalization (14 vs. 27 events, respectively; p=0.03). Interestingly, patients on the combination of fosinopril and amlodipine had the fewest events of all, suggesting that both aggressive blood pressure control and blockade of the renin‐angiotensin system are required for optimal results in patients with diabetes. These results, however, should be interpreted with caution, since the trial was not designed and powered to assess a difference in cardiovascular events between the two treatments and there were only small numbers of observed cardiovascular events.
The Heart Outcomes Prevention Evaluation (HOPE) Study.
37 In this double‐blind, randomized, placebo‐controlled study, the effects of the ACE inhibitor ramipril were evaluated in 9541 patients at high risk for cardiovascular events. Patients were enrolled if they had evidence of vascular disease (history of coronary artery disease, stroke, or peripheral vascular disease) or diabetes plus one other cardiovascular risk factor (hypertension, elevated total cholesterol or low high‐density lipoprotein cholesterol [HDL‐C], cigarette smoking, or microalbuminuria). Compared with placebo, treatment with ramipril significantly lowered the risk of death from cardiovascular causes (p<0.001), stroke (p<0.001), revascularization procedures (p=0.002), and heart failure (p<0.001), and it reduced total mortality (p=0.005). In the 3577 patients with diabetes mellitus, ramipril reduced the risk of the primary outcome (a composite of MI, stroke, and cardiovascular death) by 25% (p=0.004), MI by 22% (p=0.01), stroke by 33% (p=0.0074), cardiovascular death by 37% (p=0.0001), total mortality by 24% (p=0.004), revascularization by 17% (p=0.031), and overt nephropathy by 24% (p=0.027). 38 The independent Data and Safety Monitoring Board stopped the study 6 months early because of consistent benefit with ramipril therapy relative to placebo.
TREATMENT GUIDELINES FOR HIGH‐RISK HYPERTENSIVE PATIENTS
The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) 2 was designed to assist practitioners in reducing hypertension‐related morbidity and mortality. Hypertension is defined as SBP of ≥140 mm Hg and DBP of ≥90 mm Hg in patients not taking any antihypertensive agents, although optimal blood pressure with respect to cardiovascular prognosis is considered to be <120/80 mm Hg. The risk of cardiovascular disease in patients with hypertension is determined not only by the level of blood pressure, but also by the presence or absence of target organ damage or other risk factors, such as smoking, dyslipidemia, and diabetes mellitus. The presence of diabetes or nephropathy automatically stratifies a hypertensive patient into the highest risk group, for whom immediate initiation of antihypertensive drug therapy (with adjunctive lifestyle modification) is recommended for those with blood pressure above 130/85 mm Hg.
The JNC VI recommends an SBP of <140 mm Hg and a DBP of <90 mm Hg, along with control of other modifiable cardiovascular risk factors, as the general goal in hypertensive patients. For patients with hypertension and concomitant diabetes mellitus, the goal is <130/85 mm Hg. Hypertensive patients with renal insufficiency and proteinuria of >1 g/day should be treated to achieve a target blood pressure of <125/75 mm Hg. The 1999 World Health Organization‐International Society of Hypertension 3 and the American Diabetes Association guidelines 28 are in agreement with those of the JNC VI for the treatment of patients with concomitant hypertension and diabetes mellitus.
A‐II Receptor Antagonists
A‐II is the principal active component of the renin‐angiotensin system. It is produced through the hydrolysis of angiotensin I by ACEs. However, while ACE inhibition is clearly a mechanism for blocking the formation of A‐II, inhibition by this means may not be fully effective, since alternate pathways of A‐II formation exist. A‐II receptor antagonists selectively block the AT1 receptor, thus providing a more direct interruption of the renin‐angiotensin system that is independent of A‐II production. 39 A‐II receptor antagonists have been shown to be as effective as ACE inhibitors in reducing blood pressure 40 , 41 and proteinuria. 42 A pilot study 43 in patients with hypertension, type 2 diabetes mellitus, and proteinuria demonstrated that the A‐II receptor antagonist irbesartan reduced mean urinary protein excretion from baseline, while the dihydropyridine calcium channel blocker amlodipine did not do so. In addition, irbesartan increased creatinine clearance (8.6 ml/min/1.73 m2) (Fig. 8).
Figure 8.
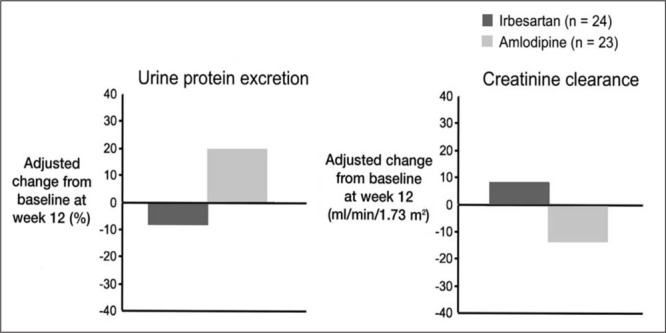
Effect of irbesartan on renal function in type 2 diabetic nephropathy at week 12 43
Large‐scale trials involving different antihypertensive regimens have demonstrated reductions in cardiovascular events in hypertensive patients with type 2 diabetes mellitus. 20 , 21 , 25 , 33 , 35 , 36 , 38 Agents that block the renin‐angiotensin system may be the most beneficial, although definitive prospective, head‐to‐head comparative data are lacking. Two large clinical programs evaluating the effects of A‐II receptor antagonists on renal function and cardiovascular end points have been recently completed: the Losartan Diabetic Nephropathy Study (RENAAL) 44 and the Program for Irbesartan Mortality and Morbidity Evaluations (PRIME).
RENAAL.
The primary composite end point of RENAAL, a multinational, double‐blind, placebo‐controlled trial to evaluate the renal‐protective effects of the A‐II receptor antagonist losartan, was a doubling of serum creatinine, end‐stage renal disease (dialysis or transplantation), and death in patients with type 2 diabetes and renal disease. The study design is depicted in Figure 9. A total of 1513 patients with a mean age of 60 years were enrolled. Target blood pressure was < 140/90 mm Hg. Baseline characteristics included a sitting trough blood pressure of 153/82 mm Hg, serum creatinine of 1.9 mg/dL, glycosylated hemoglobin of 8.5%, and a body mass index of 29.7 kg/m2.
Figure 9.
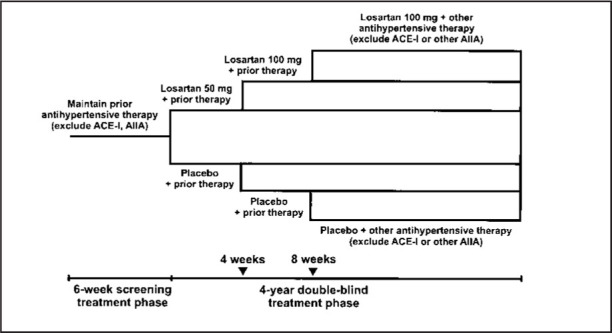
Study design of the Losartan Diabetic Nephropathy Study (RENAAL). ACE‐I=angiotensin‐converting enzyme inhibitor; AIIA=angiotensin II antagonist. Reprinted with permission from J Renin Angiotensin Aldosterone Syst. 2000;1:328–335. 44
PRIME.
PRIME is a research program composed of two trials (the Irbesartan Diabetic Nephropathy Trial [IDNT] 45 and the Irbesartan Microalbuminuria [IRMA] II trial), designed to evaluate the morbidity and mortality benefits of irbesartan in high‐risk hypertensive patients with type 2 diabetes mellitus. Both trials were completed in December, 2000. IDNT was designed to examine the cardiovascular and renal effects of irbesartan in high‐risk patients with hypertension and type 2 diabetes mellitus at both early and late stages of diabetic nephropathy.
IDNT, a multinational, double‐blind, placebo‐controlled trial, was designed to assess the effects of irbesartan and the calcium channel blocker amlodipine on cardiovascular morbidity and mortality and on the progression of renal disease in high‐risk hypertensive patients with type 2 diabetes mellitus and proteinuria (≥900 mg/day). A total of 1715 patients were randomized to receive once‐daily irbesartan, amlodipine, or placebo, with equal blood pressure goals in all three arms (seated SBP of ≤135 mm Hg or a reduction of ≥10 mm Hg in seated SBP in patients with seated SBP of >145 mm Hg at screening). The study design of IDNT is depicted in Figure 10a. Doses could be titrated over 8 weeks until target blood pressure goals were reached, up to maximum doses of 300 mg of irbesartan or 10 mg of amlodipine. Adjunctive antihypertensive medications could be added (excluding ACE inhibitors, calcium channel antagonists, and other A‐II receptor antagonists) if mono‐ therapy did not achieve target blood pressure. Treatment was for a minimum of approximately 2 years, with patients returning for follow‐up every 3 months. The primary comparison was irbesartan with placebo, while the secondary comparison was irbesartan with amlodipine. The primary outcome was time to a composite end point consisting of doubling of the baseline serum creatinine level, end‐stage renal disease, and death (all‐cause mortality). The secondary outcome measures included time from randomization until the first occurrence of cardiovascular death, nonfatal MI, hospitalization for heart failure, permanent neurologic deficit attributed to stroke, or above‐the‐ankle amputation.
Figure 10.
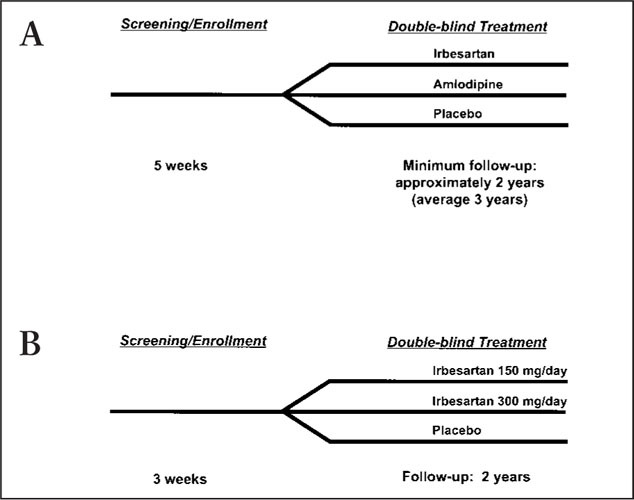
Study designs of (A) the Irbesartan Diabetic Nephropathy Trial (IDNT) 45 and (B) the Irbesartan Microalbuminuria (IRMA) II trial.
ASH Update
Preliminary results of the RENAAL, and final results of IRMA‐II, and IDNT studies were reported at the American Society of Hypertension meeting in San Francisco in May, 2001. These trials demonstrated a significant reduction in the composite end points of time to doubling of serum creatinine, end‐stage renal disease, and all‐cause mortality in the group treated with an angiotensin II (A‐II) receptor blocker compared to patients treated with other medication regimens that did not include an angiotensin‐converting enzyme (ACE) inhibitor or another A‐II receptor blocker. Specifically, the RENAAL data showed that the use of a losartan‐based regimen delayed progression of renal disease relative to a regimen that did not contain an A‐II receptor blocker or an ACE inhibitor. A‐II antagonism also reduced the occurrence of heart failure. The blood pressures achieved in the losartan group were similar to those achieved with treatment other than A‐II antagonism or placebo.
In the IRMA‐II trial, there was a 70% reduction in progression to more severe renal disease in the group of patients treated with irbesartan compared with the non‐A‐II receptor blocker‐ or ACE inhibitor‐treated patients. Higher dosages (300 mg) proved to be more effective than the 150‐mg dose. In the IDNT study, patients on irbesartan experienced a 20% reduction in risk of the composite end point of end‐stage renal disease, doubling of creatinine or death compared with patients in the placebo group (p=0.024) and a 23% risk reduction compared with those on amlodipine (p=0.006). No difference in all‐cause mortality was noted among the different groups, nor was there a statistically significant difference in reaching the secondary end points among patients receiving amlodipine, irbesartan, or other medications.
The results of these trials have treatment implications. It appears that the use of A‐II receptor blockers, when compared to treatment regimens that do not include an ACE inhibitor, will prevent progression of renal disease and reduce evidence of glomerular injury (proteinuria) in type 2 diabetic subjects.
—J Clin Hypertens
The baseline characteristics of the first 1554 patients enrolled in IDNT are summarized in Table I. Most patients were male (67%) and had had type 2 diabetes mellitus for a mean of 15 years. The screening mean seated blood pressure was 156/85 mm Hg. The mean urinary protein excretion rate and serum creatinine level were 4.2 g/24 hours and 1.7 mg/dL, respectively. The mean glycosylated hemoglobin was 8.1%.
Table I.
Mean Demographic and Baseline Characteristics of IDNT 45 : Interim Results
| Total number of patients | 1554 |
| Age in years | 59 |
| Male/female | 67%/33% |
| Duration of diabetes | 15 years |
| Screening seated blood pressure | 156/85 mm Hg |
| Urinary protein excretion rate | 4.2 g/24 hours |
| Serum creatinine | 1.7mg/dL |
| Glycosylated hemoglobin (HbA1c) | 8.1% |
| Body mass index | 31 kg/m2 |
| Previous coronary event | 16.5% |
| IDNT=Irbesartan Type 2 Diabetic Nephropathy Trial | |
IRMA II, a multicenter, randomized, double‐blind, placebo‐controlled trial, was designed to assess the effect of irbesartan on the progression of microalbuminuria (AER of 20–200 µg/minute) to overt nephropathy (AER of >200 µg/minute at two successive evaluations and an increase in AER of at least 30% over baseline) in hypertensive patients with type 2 diabetes mellitus, microalbuminuria, and normal renal function (protocol on file, Sanofi‐Synthelabo). Patients were randomized to receive irbesartan 150 mg once daily, irbesartan 300 µg once daily, or placebo, with equal degrees of blood pressure control being the goal for all three treatment groups (seated SBP of ≤135 mm Hg and seated DBP of ≤85 mm Hg; Fig. 10b). If the maximally titrated dose did not result in the target blood pressure levels, adjunctive antihypertensive medications were permissible, with the exception of ACE inhibitors and other A‐II receptor antagonists. The follow‐up period was 2 years. The primary comparison was time to occurrence of clinical proteinuria. Secondary end points included changes from baseline in overnight AER, calculated creatinine clearance, and lipid profile. Renal hemodynamics will be evaluated in a substudy.
Table II summarizes the baseline characteristics of enrolled patients. A total of 611 patients were enrolled, with a mean age of 58 years; 132 patients participated in the glomerular filtration substudy. The mean baseline blood pressure and glycosylated hemoglobin were 153/90 mm Hg and 7.2%, respectively. The mean baseline AER and serum creatinine were 64 mg/minute and 1.07 mg/dL, respectively.
Table II.
Mean Demographic and Baseline Characteristics of IRMAII*
| Total number of patients | 611 |
| Age in years | 59 |
| Male/female | 68%/32% |
| Duration of diabetes | 10 years |
| Screening seated blood pressure | 153/90 mm Hg |
| Duration of hypertension | 7 years |
| Albumin excretion rate | 71 µg/min |
| Serum creatinine | 1.2mg/dL |
| Glycosylated hemoglobin (HbA1c) | 7.2% |
| Body mass index | 30 kg/m2 |
| Substudy Analysis | |
| Number of patients | 132 |
| Glomerular filtration rate | 131 ml/min |
| Glomerular filtration rate | 112 ml/min/1.73 m2 |
| Extracellular volume† | 17.58 L |
| *Based on all randomized patients; †based on 121 patients. IRMA II=Irbesartan Microalbuminuria II trial | |
CONCLUSIONS
Hypertension and diabetes mellitus are chronic disease states that can have serious consequences if not treated intensively. The risk of cardiovascular events is four‐fold higher in patients with these concomitant disease states than in normotensive, nondiabetic subjects. 6 , 31 Clearly, effective management of blood pressure reduces the risk of cardiovascular morbidity and mortality. Various antihypertensive regimens, including diuretics, calcium channel blockers, and ACE inhibitors, have demonstrated significant reductions in cardiovascular morbidity and mortality in high‐risk patients with hypertension and diabetes mellitus. Data suggest that agents that block the renin‐angiotensin system may provide the greatest organ protection, although definitive proof as to which is more important—blood pressure control or the class of antihypertensive agent used—is still lacking. Current hypertension guidelines recommend renin‐angiotensin system blockade with ACE inhibitors as first‐line therapy for the management of hypertensive patients with diabetes mellitus. Because A‐II receptor antagonists effectively interrupt the renin‐angiotensin system, they may provide benefits in terms of preventing cardiovascular and renal events. It is unlikely that any head‐to‐head clinical trials comparing A‐II receptor antagonists with ACE inhibitors in the progression of renal disease will be conducted, as both classes of agents have been shown to similarly protect kidney function in experimental and small‐scale clinical trials with markers of progression. Of note, a recent study 46 has demonstrated that the combination of ACE inhibitors and A‐II receptor antagonists may have additive antiproteinuric effects. A combination of the two may be a rational antihypertensive, antiproteinuric approach in the patient with diabetes and hypertension.
The two clinical programs, RENAAL and PRIME, which have just been completed, have evaluated the effects of A‐II receptor antagonists on renal disease progression and cardiovascular morbidity and mortality. Such large studies are necessary, particularly in patients with hypertension, type 2 diabetes, and renal disease, since there are scant data concerning ACE inhibitors or any other antihypertensive classes. Moreover, these studies will add to the understanding of optimal cardiovascular risk reduction strategies in patients with hypertension and type 2 diabetic kidney disease, and will provide information as to benefits of angiotensin II receptor antagonists beyond blood pressure reduction.
References
- 1. Williams GH. Assessing patient wellness: new perspectives on quality of life and compliance. Am J Hypertens. 1998;11:186S–191S. [DOI] [PubMed] [Google Scholar]
- 2. Joint National Committee on Prevention, Detection , Evaluation, and Treatment of High Blood Pressure: the sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization‐International Society of Hypertension (WHO‐ISH) Mild Hypertension Liaison Committee. 1999 World Health Organization‐International Society of Hypertension guidelines for the management of hypertension . J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 4. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. [DOI] [PubMed] [Google Scholar]
- 5. Stockwell DH, Madhavan S, Cohen H, et al. The determinants of hypertension awareness, treatment, and control in an insured population. Am J Public Health. 1994;84:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Epstein M, Sowers JR. Diabetes mellitus and hypertension. Hypertension. 1992;19:403–418. [DOI] [PubMed] [Google Scholar]
- 7. Tarnow L, Rossing P, Gall MA, et al. Prevalence of arterial hypertension in diabetic patients before and after the JNC‐V. Diabetes Care. 1994;17:1247–1251. [DOI] [PubMed] [Google Scholar]
- 8. Hypertension in Diabetes Study (HDS): I . Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11:309–317. [DOI] [PubMed] [Google Scholar]
- 9. Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. [DOI] [PubMed] [Google Scholar]
- 10. Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. [DOI] [PubMed] [Google Scholar]
- 11. Cooper ME, Frauman A, O'Brien RC, et al. Progression of proteinuria in type 1 and type 2 diabetes. Diabetes Med. 1988;5:361–368. [DOI] [PubMed] [Google Scholar]
- 12. John L, Rao PSS, Kanagasabapathy AS. Rate of progression of albuminuria in type II diabetes. Five‐year prospective study from South India. Diabetes Care. 1994;17:888–890. [DOI] [PubMed] [Google Scholar]
- 13. Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity‐onset diabetes. N Engl J Med. 1984;310:356–360. [DOI] [PubMed] [Google Scholar]
- 14. Ravid M, Savin H, Lang R, et al. Proteinuria, renal impairment, metabolic control, and blood pressure in type 2 diabetes mellitus. Arch Intern Med. 1992;152:1225–1229. [PubMed] [Google Scholar]
- 15. Hasslacher C, Bostedt‐Kiesel A, Kempe HP, et al. Effect of metabolic factors and blood pressure on kidney function in proteinuric type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia. 1993;36:1051–1056. [DOI] [PubMed] [Google Scholar]
- 16. Gall M‐A, Nielsen FS, Smidt UM, et al. The course of kidney function in type 2 (non‐insulin‐dependent) diabetic patients with diabetic nephropathy. Diabetologia. 1993;36:1071–1078. [DOI] [PubMed] [Google Scholar]
- 17. Wang SL, Head J, Stevens L, et al. Excess mortality and its relation to hypertension and proteinuria in diabetic patients. The World Health Organization Multinational Study of Vascular Disease in Diabetes. Diabetes Care. 1996;19:305–312. [DOI] [PubMed] [Google Scholar]
- 18. Miettinen H, Haffner SM, Lehto S, et al. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non‐insulin‐dependent diabetic subjects. Stroke. 1996;27:2033–2039. [DOI] [PubMed] [Google Scholar]
- 19. UK Prospective Diabetes Study Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 20. Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin‐converting‐enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353:611–616. [DOI] [PubMed] [Google Scholar]
- 21. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 22. Lonn EM, Yusuf S, Jha P, et al. Emerging role of angiotensin‐converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994;90:2056–2068. [DOI] [PubMed] [Google Scholar]
- 23. Zatz R, Dunn BR, Meyer TW, et al. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77:1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weir MR. Diabetes and hypertension: blood pressure control and consequences. Am J Hypertens. 1999;12:170S–178S. [DOI] [PubMed] [Google Scholar]
- 25. Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. N Engl J Med. 1998;338:645–652. [DOI] [PubMed] [Google Scholar]
- 26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the fosinopril versus amlodipine cardiovascular events randomized trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603. [DOI] [PubMed] [Google Scholar]
- 27. UK Prospective Diabetes Study Group . Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998;317:713–720. [PMC free article] [PubMed] [Google Scholar]
- 28. American Diabetes Association . Diabetic nephropathy. Diabetes Care. 1998;21(suppl 1):S50–S53. [Google Scholar]
- 29. Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 30. Stone PH, Muller JE, Hartwell T, et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. 1989;14:49–57. [DOI] [PubMed] [Google Scholar]
- 31. Hypertension in Diabetes Study (HDS): II . Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients. J Hypertens. 1993;11:319–325. [DOI] [PubMed] [Google Scholar]
- 32. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the systolic hypertension in the elderly program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 33. Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic‐based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886–1892. [PubMed] [Google Scholar]
- 34. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 35. Tuomilehto J, Rastenyte D, Birkenhager WH, et al., for the Systolic Hypertension in Europe Trial Investigators . Effects of calcium‐channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999;340:677–684. [DOI] [PubMed] [Google Scholar]
- 36. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 37. Yusuf S, Sleight P, Pogue J, et al., for the Heart Outcomes Prevention Evaluation Study Investigators . Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 38. Heart Outcomes Prevention Evaluation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 39. Timmermans PB, Wong CM, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 40. Mimran A, Ruilope L, Kerwin L, et al. A randomised, double‐blind comparison of the angiotensin II receptor antagonist, irbesartan, with the full dose range of enalapril for the treatment of mild‐to‐moderate hypertension. J Hum Hypertens. 1998;12:203–208. [DOI] [PubMed] [Google Scholar]
- 41. Larochelle P, Flack JM, Marbury TC, et al. Effects and tolerability of irbesartan versus enalapril in patients with severe hypertension. Am J Cardiol. 1997;80:1613–1615. [DOI] [PubMed] [Google Scholar]
- 42. Gansevoort RT, De Zeeuw D, De Jong PE. Is the antiproteinuric effect of ACE inhibition mediated by interference in the renin‐angiotensin system? Kidney Int. 1994;45:861–867. [DOI] [PubMed] [Google Scholar]
- 43. Pohl M, Cooper M, Ulrey J, et al. Safety and efficacy of irbesartan in hypertensive patients with type II diabetes and proteinuria [abstract]. Am J Hypertens. 1997;10:105A. [Google Scholar]
- 44. Brenner BM, Cooper M, De Zeeuw D, et al. The losartan renal protection study—rationale, study design and baseline characteristics of RENAAL (reduction of end points in NIDDM with the angiotensin II antagonist losartan). J Renin Angiotensin Aldosterone Syst. 2000;1:328–335. [DOI] [PubMed] [Google Scholar]
- 45. Rodby RA, Rohde RD, Clarke WR, et al. The irbesartan type II diabetic nephropathy trial: study design and baseline patient characteristics. Nephrol Dial. 2000;15:487–497. [DOI] [PubMed] [Google Scholar]
- 46. Russo D, Pisani A, Balletta MM, et al. Additive antiproteinuric effect of converting enzyme inhibitor and losartan in normotensive patients with IgA nephropathy. Am J Kidney Dis. 1999;33:851–856. [DOI] [PubMed] [Google Scholar]


