Abstract
Metabolic Syndrome (MetS) is associated with increased rates of mortality and increased risk for developing dementia. Changes in brain structure and cognitive functioning have been reported within the literature. However, research examining cognitive performance in individuals with MetS is limited, inconclusive, and focuses primarily on older cohorts. As such, the effect of MetS on cognitive functioning earlier in the lifespan is unclear. This study aimed to investigate cognitive performance in young, middle-aged, and older adults with multiple metabolic and vascular risk factors in a sample of community dwelling participants (N = 128). Participants were administered a comprehensive neuropsychological battery and self-report measures. As expected, older adults performed more poorly than young and middle-aged adults across most assessments. Relative to controls, individuals with MetS reported greater hunger and disinhibited eating. MetS participants performed more poorly on Color-Word Interference: Inhibition. Additionally, when weight was accounted for, there was a significant relationship between MetS and select executive functioning tasks in middle-aged adults. These findings suggest that aspects of executive functioning may be impaired in MetS and could be further impacted by excess weight in middle-age. Future studies aimed at investigating potential causal relationships between metabolic and vascular risk factors, disinhibited eating, and executive dysfunction may provide insight into effective intervention targets to prevent MetS.
Introduction
Metabolic syndrome (MetS) is a constellation of vascular and metabolic risk factors that are directly related to the development of Cardiovascular Disease (CVD) and increase the risk of developing type 2 diabetes mellitus (DM) [1–3]. These vascular and metabolic risk factors frequently occur in combination, and taken together, increase CVD morbidity rates more than the individual components alone [4, 5]. Middle-aged and older adults with MetS are three to four times more likely to have coronary heart disease, stroke, and higher mortality [4–7].
The prevalence rate of MetS within the United States was estimated to be 33% of the population between 2003 and 2012 [8]. Older adults (>65 years of age) are at an increased risk for CVD, type 2 DM, and MetS [9, 10]; however, these conditions have also been documented in young adulthood [11, 12]. While the long-term deleterious effects of developing these conditions in young adulthood are not well-established, the presence of multiple vascular risk factors and MetS in middle-aged and older adults, increases the risk of CVD and mortality [5, 10], the risk of developing dementia [13–16], and has been reported to be associated with impairments in executive functioning [17–19].
Obesity—a crucial component of MetS—is also highly prevalent, with approximately a third of the world’s population classified as overweight or obese [20]. Obesity is associated with increased risk for the development of MetS over the lifespan due its significant cardiovascular impact [21]. Furthermore, there is evidence suggesting that obesity is associated with cognitive deficits across the lifespan [22–25]. More specifically, significant deficits in decision making, cognitive flexibility, and inhibition have been linked to excess weight or obesity [22, 23, 26–28]. One study found deficits in inhibitory control and cognitive flexibility significantly predicted body weight in primary school children, though the direction of the effect warrants further study [29]. Interestingly, a review by Smith and colleagues [30], noted two studies that suggested that in older adults, obesity predicted better cognitive abilities or less decline in function; these findings may indicate a protective role of weight or a survival effect in older ages (i.e., middle-aged obesity-related mortality). Thus, components of executive functioning may represent the earliest domains of cognitive change associated with metabolic and vascular risk factors.
MetS has been associated with poorer cognitive performance [31–34]. Focal deficits in executive functioning (specifically cognitive flexibility and inhibition) have been repeatedly demonstrated in MetS [17–19]. However, specific neuropsychological domains of impairment have largely been inconsistent across the literature [35, 36]. Although cross-sectional studies examining domain-specific aspects of cognitive function have revealed poorer performance in MetS relative to controls on measures of information processing speed [19, 37, 38], attention [38], verbal memory [32, 37], executive functioning [19, 38], and fluid intelligence [37], there is considerable variability in the pattern of cognitive decline [39] and type of assessment used to measure each domain [35, 36].
To date, the majority of these studies have focused primarily on middle-aged and older adults; as such, information regarding the effect of MetS on cognition across the lifespan is limited. For instance, despite the increased risk of multiple cardiovascular risk factors in young adulthood [11], the effect of MetS on cognitive function in young adults has hardly been examined. Regarding middle-aged adults with MetS, those who consistently met criteria over a 10-year period, performed significantly poorer than those with non-persistent MetS and those without any history of MetS on measures of memory, verbal fluency, reasoning, and vocabulary [40]. In the oldest age group (85+ years of age), MetS has not been associated with significant declines in cognitive performance [35, 41], which might suggest that some aspects of MetS may be protective against cognitive decline later in life.
In the current study, we used the Color-Word Inhibition, Trails, Verbal Fluency and Design Fluency subtests of the Delis-Kaplan Executive Function System (D-KEFS) [42]; the California Verbal Learning Test-II (CVLT-II) [43]; Brief Visuospatial Memory Test-Revised (BVMT-R) [44]; and Conners’ Continuous Performance Test-2 (CPT-2) [45] to examine cognitive differences in young, middle-aged and older adults with and without vascular and metabolic risk factors. Investigating the effects of MetS on cognition in young, middle-aged, and older adults would help elucidate the age group in which changes in cognition first appear in MetS and may provide support for initiating targeted interventions earlier in the lifespan.
Method
Participants
Participants were part of a larger research study aimed at investigating the relationship among chemosensory and cognitive processes in healthy aging and metabolic disease. Participants received monetary compensation. This study included young adults (18–35 years of age, n = 42), middle-aged adults (45–54 years of age, n = 41), and older adults (65–86 years, n = 45), totaling at 128. Participants were excluded if they were left-handed, had a positive history of head injury with loss of consciousness > 5 minutes, substance use disorders, neurological or psychiatric diseases, or if they scored less than 24 on the MMSE, or less than 130 on the DRS. The research was approved by the San Diego State University Human Research Protections Program (1633) and the University of California, San Diego Human Research Protections Program (170289). Subjects provided written consent.
The following inclusion criteria were used to determine metabolic status. According to the International Diabetes Federation [3] and subsequent modification [46], the diagnosis of MetS requires ≥ 3 of 5 of the following risk factors: central obesity, operationally defined as body mass index (BMI) >30kg/m2 or waist circumference ≥ 94 cm for males and 80 cm for females; raised triglycerides (≥ 150 mg/dL) or currently receiving treatment for dyslipidemia; reduced HDL cholesterol (< 40 mg/dL in males and < 50 mg/dL in females) or currently receiving treatment for dyslipidemia; raised blood pressure (BP; systolic BP ≥ 130 or diastolic BP ≥ 85 mm Hg) or treatment of diagnosed hypertension; and raised fasting plasma glucose (≥ 100 mg/dL) or previous diagnosis of type 2 diabetes. Ethnic specific values of waist circumference were employed as outlined by the IDF (IDF, 2006). Blood pressure, height, weight, waist circumference, and systolic/diastolic blood pressures were measured. Calculations were performed for pulse pressure (systolic—diastolic blood pressure) and BMI (kg/m2). Participants’ self-reported a diagnosis and/or current treatment for raised triglycerides, reduced HDL, and type 2 DM.
Based on the MetS criteria outlined above, individuals were classified as either having MetS or as normal controls (Table 1). For the young adult metabolic cohort, all participants met criteria for obesity. Prevalence of MetS in young adults is estimated to be 20.3% and 15.6% for male and females, respectively [10]. For the present young adult metabolic cohort, 59% of participants met full criteria (3 out of 5 risk factors), 18% met partial criteria (2 out of 5 risk factors), and 23% were classified as only obese. Obesity is associated with increased risk for the development of MetS over the lifespan [47]. As such, for the purpose of the present manuscript, the metabolic young cohort will be operationally defined as obese with additional risk factors.
Table 1. Demographic characteristics of participants.
| Age Group & Metabolic Status: Mean (Standard Error) | ||||||
|---|---|---|---|---|---|---|
| Variable | Young Control (n = 20) | Young Metabolic (n = 22) | Middle-age Control (n = 18) | Middle-age Metabolic (n = 23) | Older Control (n = 22) | Older Metabolic (n = 23) |
| Age | 23.80 (.90) | 25.27 (.90) | 49.33 (.76) | 50.35 (.65) | 72.23 (1.75) | 69.57 (1.45) |
| Education | 15.35 (.35) | 15.32 (.54) | 15.11 (.59) | 14.74 (.48) | 14.73 (.54) | 14.52 (.55) |
| Gender (% Male) | 35 | 36.4 | 44.4 | 30.4 | 59.1 | 39.1 |
| MMSE | 29.63 (.22) | 29.44 (.17) | 29.39 (.20) | 28.83 (.35) | 28.52 (.36) | 28.43 (.27) |
| DRS | 141.22 (.46) | 141.71 (1.08) | 141.39 (.56) | 140.74 (.48) | 140.41 (.86) | 141.39 (.63) |
| Body Measurements | ||||||
| Weight (lbs) | 147.98 (6.71) | 223.56 (7.75) | 163.47 (3.25) | 254.14 (6.79) | 156.25 (6.13) | 201.87 (8.71) |
| Height (cm) | 171.83 (2.56) | 170.46 (1.47) | 171.59 (2.14) | 168.68 (1.89) | 170.00 (2.88) | 166.03 (1.90) |
| BMI | 22.52 (.57) | 34.74 (1.08) | 25.04 (.61) | 40.27 (1.21) | 24.61 (.61) | 33.10 (1.35) |
| Waist Circumference (cm) | 79.79 (2.23) | 106.88 (2.66) | 91.57 (2.92) | 121.89 (2.11) | 89.87 (2.01) | 109.35 (2.79) |
| Systolic Blood Pressure | 119.52 (3.07) | 124.24 (2.69) | 124.93 (4.61) | 138.44 (2.66) | 145.45 (5.10) | 140.28 (3.86) |
| Diastolic Blood Pressure | 70.78 (2.33) | 75.01 (1.65) | 76.85 (2.83) | 83.85 (1.67) | 75.11 (2.50) | 72.33 (1.92) |
| Pulse Pressure | 48.74 (2.45) | 50.75 (1.80) | 48.08 (3.18) | 54.58 (1.92) | 70.34 (3.98) | 67.93 (4.07) |
| Stroke Risk (%) | 2.40 (.34) | 2.67 (.25) | 2.89 (.33) | 4.70 (.66) | 12.05 (1.66) | 14.87 (1.97) |
Note. MMSE = Mini-mental Status Examination; DRS = Dementia Rating Scale.
BMI = body mass index; lbs = pounds, cm = centimeters.
Procedures
Participants underwent two separate testing sessions, each session lasting approximately 2 hours. In the first session, participants were administered measures of general cognitive functioning and questionnaires regarding their metabolic status. During the second session, neuropsychological measures were administered.
Cognitive measures
The following tests were administered as part of a larger test battery.
Mini-Mental State Exam (MMSE)
The MMSE is a brief measure of cognition [48]. It is commonly administered to older adults as a screen for cognitive impairment and to track changes in cognition over time.
Dementia Rating Scale (DRS)
The DRS is a global measure of cognition that can be administered to older adults with known or suspected dementia [49]. The total scores from the MMSE and DRS were used to exclude those whose scores enter the clinically impaired range, defined as less than 24 for the MMSE and less than 130 for the DRS.
Subtests from the Delis-Kaplan Executive Function System (D-KEFS)
The D-KEFS is a comprehensive set of tests aimed at assessing higher-level cognitive functions [42]. The Verbal Fluency subtest measures verbal response generation and cognitive flexibility [42]. The Design Fluency subtest measures non-verbal response generation, inhibition, and cognitive flexibility [42]. The Color-Word Interference subtest assesses cognitive flexibility and inhibition [42]. It is based on the Stroop Color and Word Task [50] and consists of four conditions: Color Naming, Word Reading, Inhibition, and Inhibition Switching. Trails is designed to assess cognitive flexibility and executive functioning on a visual-motor task [42].
Conners’ Continuous Performance Test (CPT-2)
The CPT-2 assesses sustained attention, reaction time, and impulsivity [45].
California Verbal Learning Test- second edition (CVLT-II)
The CVLT-II is designed to measure verbal learning, short- and long-term memory, cued recall, and recognition [43].
Brief Visuospatial Memory Test-Revised (BVMT-R)
The BVMT-R assesses visuospatial learning, memory, and recognition [44].
Self-report questionnaires
Self-report questionnaires were administered to assess mood and impulsive personality traits. Specifically, the Beck Depression Inventory—Second Edition (BDI) [51] was used to assess depressive symptoms; the State Trait Anxiety Inventory (STAI) [52] was used to screen for anxiety symptoms/traits; The Three-Factor Eating Questionnaire (TFEQ) [53] was used to assess food intake-behavior, including disinhibition; and the Barratt Impulsiveness Scale (BIS) [54] was used to assess impulsiveness. The percentage of stroke risk was assessed using the Stroke Risk Assessment Test [55].
Statistical analyses
Self-report measures
Multivariate analyses of covariance (MANCOVAs) were performed to examine the potential associations of age group and metabolic status, with self-report measures, while controlling for gender and years of education, conducted in the following groupings: 1) BDI and STAI (state and trait indices); 2) TFEQ (cognitive restraint, disinhibition, hunger), and 3) BIS (first order factors: attention, motor, self-control, cognitive complexity, perseverance, cognitive instability).
Cognitive measures
Individual measures were analyzed using raw scores. MANCOVAs were conducted to examine potential associations of age group and metabolic status with cognitive functioning while accounting for gender and education level. The following indices were investigated in the following MANCOVA groupings: 1) DRS total score, MMSE total score, Digit span total score (due to significant correlations between these variables p < .01); 2) BVMT-R: total and delayed scores; 3) CVLT-II: total score of trials 1–5, short delay free and cued recall, and long delay free and cued recall; 4) CPT-2: clinical percentage, non-clinical percentage, omission, commission, variability, response style, and perseveratives; 5) D-KEFS Verbal Fluency: letter, category, category switching, switching accuracy, and set-loss errors; 6) D-KEFS Design Fluency: filled dots, empty dots, and switching;7) D-KEFS Trails: visual scanning, number sequencing, letter sequencing, number-letter switching, and motor speed; and 8) D-KEFS Color-Word Interference: color naming, word reading, inhibition, switching, and switching errors.
An alpha level of p = .05 was used for all analyses to achieve a balance between small sample size and Type I and Type II errors. Bonferroni post-hoc tests were used to probe the significant effects at an alpha of.05.
Exploratory analyses
As a follow-up to the main analysis, the raw data on cognitive measures were re-analyzed in MANCOVAs conducted to examine the role of weight in the context of metabolic status. As the middle-age group displayed higher mean weight than the other two age groups, we conducted MANCOVAs separated by age group to evaluate the relationship between metabolic status while controlling for weight, gender, and education level.
Results
Self-report measurements
MANCOVAs did not demonstrate significant interactions between age group and metabolic status on self-report measures while controlling for gender and education levels (Table 2); however, there were significant differences by age group and metabolic status for several measures. There was a main effect of age group on the BIS: Self-control ([F(2,113) = 4.05, p = .02]; Table 2); Bonferroni analyses revealed that middle-aged adults had significantly higher scores on this measure as compared to older adults (S1 Table). There was a main effect of metabolic status in which individuals with MetS had significantly higher scores on the BDI, TFEQ: disinhibition, and TFEQ: hunger as compared to controls (BDI [F(1,107) = 4.81, p = .04], TFEQ: disinhibition [F(1,118) = 29.46, p < .001], and TFEQ: hunger [F(1,118) = 14.87, p < .001], Table 2).
Table 2. Self-report measurements of participants.
| Age Group & Metabolic Status: Mean (Standard Error) | ||||||
|---|---|---|---|---|---|---|
| Variable | Young Control | Young Metabolic | Middle-age Control | Middle-age Metabolic | Older Control | Older Metabolic |
| BDI-II | 5.15 (.88) | 8.75* (2.21) | 7.06 (2.62) | 8.00* (1.72) | 4.42 (.85) | 9.30* (1.87) |
| STAI: State | 29.75 (1.58) | 31.17 (2.20) | 31.89 (2.92) | 31.00 (1.67) | 27.58 (1.76) | 32.83 (2.39) |
| STAI: Trait | 34.55 (1.67) | 37.00 (2.78) | 33.89 (2.72) | 32.35 (1.79) | 30.42 (1.70) | 35.65 (2.52) |
| TFEQ: Cognitive Restraint | 9.40 (1.00) | 9.59 (1.09) | 10.00 (1.18) | 8.04 (.90) | 10.62 (1.34) | 10.17 (.83) |
| TFEQ: Disinhibition | 5.05 (.69) | 7.41*** (.72) | 4.17 (1.05) | 9.52*** (.78) | 4.24 (.72) | 7.04*** (.72) |
| TFEQ: Hunger | 3.95 (.65) | 4.73*** (.62) | 3.61 (.69) | 7.09*** (.70) | 3.29 (.57) | 5.52*** (.67) |
| BIS: Attention | 9.90 (.57) | 9.85 (.51) | 9.39 (.37) | 9.55 (.51) | 9.57 (.54) | 10.35 (.60) |
| BIS: Motor | 14.10 (.58) | 13.65 (.79) | 14.11 (.84) | 15.40 (.67) | 13.81 (.46) | 13.43 (.54) |
| BIS: Self-control | 12.05 (.85) | 11.15 (.69) | 13.06* (.72) | 13.3* (.82) | 10.67 (.76) | 11.39 (.74) |
| BIS: Cognitive Complexity | 11.70 (.55) | 10.15 (.47) | 11.56 (.66) | 11.80 (.49) | 12.00 (.43) | 11.26 (.61) |
| BIS: Perseverative | 7.25 (.37) | 7.00 (.41) | 7.89 (.46) | 8.05 (.49) | 7.19 (.29) | 7.61 (.41) |
| BIS: Cognitive Instability | 5.70 (.411) | 6.40 (.43) | 5.67 (.26) | 5.10 (.37) | 5.24 (.34) | 5.96 (.43) |
| BIS: First order factors (total) | 60.65 (2.24) | 58.20 (2.21) | 61.67 (2.27) | 63.20 (2.55) | 58.48 (1.66) | 60.00 (2.09) |
Note. BDI = Beck Depression Inventory-II; STAI = State Trait Anxiety Inventory; TFEQ = Three-Factor Eating Questionnaire; BIS = Barratt Impulsiveness Scale.
Cognitive measures
Relationship between age group and cognitive functioning
MANCOVAs demonstrated significant main effects of age group on the MMSE, D-KEFS Verbal Fluency: category, switching, and switching accuracy; D-KEFS Design Fluency: filled dot, empty dot, and switching conditions; D-KEFS Trails: visual scanning, number sequencing, letter sequencing, number-letter switching, and motor speed; D-KEFS Color-word interference: color naming, word reading, inhibition, and inhibition switching; CVLT-II: total recall, short delay free and cued recall, and long delay free and cued recall; BVMT-R: total recall and long delay recall; digit span total; and CPT-2 clinical percentage and non-clinical percentage (Tables 3 and 4).
Table 3. Raw score means and standard error of cognitive performance for the main effect of age group.
| Age Group | ||||||
|---|---|---|---|---|---|---|
| Young | Middle-age | Older | ||||
| Raw Scores | Mean (Standard Error) | F | ||||
| MMSE | ||||||
| 29.54 (.14) | 29.07 (.22) | 28.48 (.22) | 5.05** | |||
| Verbal Fluency | ||||||
| Letter | 42.95 (1.89) | 41.91 (2.18) | 42.46 (1.97) | .07 | ||
| Category | 45.56 (1.19) | 43.60 (1.27) | 40.46 (1.33) | 4.87** | ||
| Switching | 15.18 (.45) | 15.51 (.42) | 13.14 (.47) | 8.99*** | ||
| Switching Accuracy | 14.28 (.50) | 14.69 (.51) | 12.14 (.53) | 8.12*** | ||
| Set loss | .54 (.15) | 1.14 (.27) | 1.79 (.32) | 5.75** | ||
| Design Fluency | ||||||
| Filled Dot | 11.15 (.56) | 10.94 (.65) | 8.98 (.61) | 3.69* | ||
| Empty Dot | 12.00 (.50) | 12.51 (.67) | 10.14 (.62) | 5.09** | ||
| Switching | 9.56 (.39) | 8.54 (.42) | 6.98 (.42) | 10.14*** | ||
| Set Loss | 1.90 (.44) | 2.20 (.44) | 3.19 (.50) | 1.45 | ||
| Trails | ||||||
| Visual Scanning | 18.77 (.91) | 19.31 (.70) | 24.67 (1.13) | 12.52*** | ||
| Number Sequencing | 25.36 (1.19) | 28.71 (1.37) | 41.26 (3.24) | 14.09*** | ||
| Letter Sequencing | 25.79 (1.36) | 30.34 (1.61) | 41.72 (2.67) | 16.72*** | ||
| Number-Letter Switching | 58.46 (2.87) | 75.17 (5.06) | 110.19 (8.32) | 20.36*** | ||
| Motor Speed | 22.15 (.98) | 25.69 (1.36) | 33.93 (2.46) | 11.89*** | ||
| Color-Word Interference | ||||||
| Color Naming | 28.46 (.89) | 30.80 (1.05) | 32.26 (1.14) | 3.79* | ||
| Word Reading | 21.21 (.73) | 22.74 (.79) | 24.43 (.97) | 4.22* | ||
| Inhibition | 47.56 (2.44) | 54.09 (2.12) | 68.02 (2.97) | 18.70*** | ||
| Inhibition Switching | 53.13 (1.47) | 60.11 (3.22) | 71.21 (4.26) | 8.20*** | ||
Note.
** = p < .01;
* = p < .05.
Table 4. Raw score means and standard error of cognitive performance for the main effect of age group.
| Age Group | ||||
|---|---|---|---|---|
| Young | Middle-age | Older | ||
| Raw Scores | Mean (Standard Error) | F | ||
| CVLT-II | ||||
| Total List Learning | 54.24 (1.31) | 53.71 (1.45) | 46.79 (1.90) | 7.74*** |
| Short Delay Free Recall | 11.87 (.42) | 11.80 (.51) | 9.33 (.56) | 9.30*** |
| Short Delay Cued Recall | 12.50 (.46) | 12.94 (.42) | 10.83 (.48) | 6.69** |
| Long Delay Free Recall | 12.39 (.46) | 12.66 (.50) | 9.71 (.55) | 11.50*** |
| Long Delay Cued Recall | 12.76 (.79) | 13.06 (.45) | 10.93 (.53) | 6.84** |
| BVMT-R | ||||
| Total Learning | 27.37 (.79) | 23.42 (1.16) | 20.46 (1.18) | 11.02*** |
| Delayed Recall | 10.32 (.25) | 9.39 (.44) | 8.51 (.47) | 5.26** |
| Digit Span | ||||
| Total | 20.23 (.69) | 18.20 (.64) | 16.60 (.58) | 8.17*** |
| CPT-2 | ||||
| Omission | 2.08 (.58) | 13.35 (5.01) | 7.54 (2.02) | 1.00 |
| Commission | 13.21 (1.40) | 17.86 (2.97) | 11.88 (1.04) | .38 |
| Response Time | 378.66 (10.40) | 338.06 (25.86) | 436.29 (12.40) | .56 |
| Variability | 8.15 (.75) | 16.92 (3.48) | 9.67 (1.10) | .12 |
| Perseveratives | .72 (.25) | 15.61 (7.22) | 1.20 (.52) | .10 |
| Clinical % | 41.21 (3.66) | 53.50 (3.03) | 63.14 (3.41) | 8.54*** |
| Non-Clinical % | 58.79 (3.66) | 46.33 (3.06) | 36.86 (3.41) | 8.51*** |
| Response Style | .60 (.11) | 10.68 (3.48) | 1.35 (.278) | 3.44* |
Note.
** = p < .01;
* = p < .05.
CVLT-II = California Verbal Learning Test-II, BVMT-R = Brief Visual Memory Test-Revised, and CPT-2 = Conner’s Continuous Performance Test-II.
Relationship between metabolic status and cognitive functioning
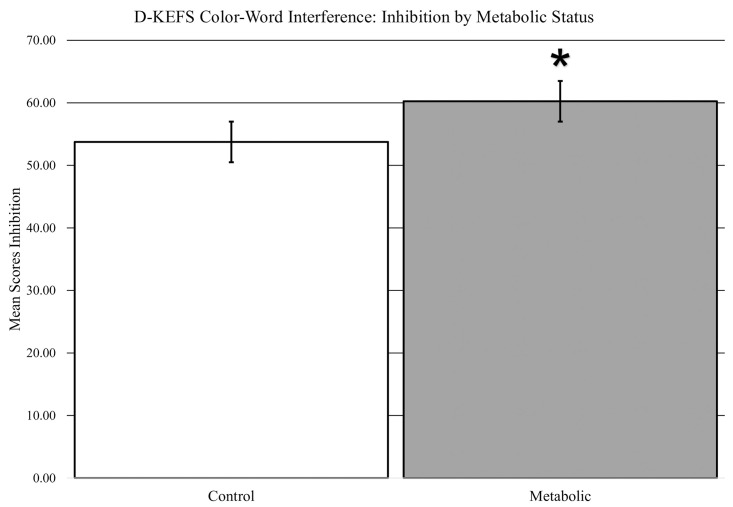
The MANCOVAs displayed a significant main effect of metabolic status on a single measure of executive functioning, Color-Word Interference Inhibition, in which participants with MetS took more time to complete the task as compared to controls (Fig 1, [F(1,107) = 6.14, p = .015]). There were no other significant main effects of metabolic status (Table 5).
Fig 1. Main effect of metabolic syndrome on cognitive inhibition.
* indicates p < .05.
Table 5. Status effects.
| Control | MetS | ||
|---|---|---|---|
| Mean (Standard Error) | F | ||
| TFEQ | |||
| Cognitive Restraint | 10.02 (.68) | 9.26 (.55) | .53 |
| Disinhibition | 4.49 (.47) | 8.00 (.44) | 26.46*** |
| Hunger | 3.61 (.36) | 5.79 (.40) | 14.87*** |
| BIS | |||
| Attention | 9.63 (.29) | 9.94 (.31) | .55 |
| Motor | 14.00 (.36) | 14.13 (.39) | 2.04 |
| Self-Control | 11.86 (.46) | 11.92 (.44) | .00 |
| Cognitive Complexity | 11.76 (.31) | 11.08 (.32) | 2.93 |
| Perseverative | 7.42 (.21) | 7.56 (.25) | .11 |
| Cognitive Instability | 5.53 (.20) | 5.83 (.24) | .79 |
| MMSE | |||
| Total | 29.16 (.17) | 28.86 (.17) | .93 |
| DRS | |||
| Total | 140.97 (.39) | 141.22 (.39) | 1.07 |
| Digit Span | |||
| Total | 18.47 (.62) | 18.10 (.48) | .05 |
| CPT-2 | |||
| Omission | 14.89 (4.25) | 17.60 (4.16) | .11 |
| Commission | 19.38 (2.81) | 19.28 (2.41) | .03 |
| Response Time | 328.42 (23.13) | 330.94 (22.56) | .01 |
| Variability | 17.87 (2.91) | 18.43 (3.04) | .03 |
| Perseveratives | 12.27 (3.36) | 15.07 (5.32) | .22 |
| Clinical % | 51.78 (3.39) | 53.56 (2.87) | .07 |
| Non-Clinical % | 48.10 (3.40) | 46.44 (2.87) | .08 |
| Response Style | 12.37 (3.29) | 12.76 (3.37) | .00 |
| CVLT-II | |||
| Total Trials 1–5 | 51.47 (1.47) | 51.23 (1.29) | .04 |
| SDFR | 11.07 (.44) | 10.77 (.44) | .37 |
| SDCR | 11.81 (.42) | 12.25 (.35) | .62 |
| LDFR | 11.47 (.46) | 11.52 (.44) | .00 |
| LDCR | 11.90 (.44) | 12.48 (.36) | 1.11 |
| BVMT-R | |||
| Total Trials 1–3 | 24.00 (.83) | 23.35 (1.05) | .27 |
| Delay | 9.70 (.28) | 9.05 (.39) | 1.89 |
| D-KEFS Verbal Fluency | |||
| Letter | 42.81 (1.60) | 42.10 (1.66) | .09 |
| Category | 42.39 (1.03) | 43.83 (1.11) | .74 |
| Switching | 14.78 (.38) | 14.28 (.41) | 1.25 |
| Switching accuracy | 13.92 (.40) | 13.31 (.48) | 1.44 |
| Set-loss errors | 1.03 (.21) | 1.33 (.24) | .91 |
| D-KEFS Design Fluency | |||
| Filled Dots | 10.85 (.52) | 9.72 (.49) | 2.33 |
| Empty Dots | 12.02 (.49) | 10.91 (.52) | 3.31 |
| Switching | 8.31 (.34) | 8.31 (.39) | .00 |
| Set-loss errors | 2.39 (.36) | 2.53 (.42) | .01 |
| D-KEFS Trails | |||
| Visual Scanning | 21.41 (.85) | 20.79 (.87) | .17 |
| Number Sequencing | 32.51 (2.21) | 31.90 (1.93) | .01 |
| Letter Sequencing | 32.85 (2.02) | 33.17 (1.77) | .06 |
| Switching | 82.25 (6.45) | 82.69 (5.00) | .05 |
| Motor Speed | 27.22 (1.83) | 27.86 (1.35) | .19 |
| Color-Word Interference | |||
| Color Naming | 29.75 (.83) | 31.37 (.91) | 2.22 |
| Word Reading | 22.15 (.69) | 23.54 (.72) | 2.55 |
| Inhibition | 53.75 (2.16) | 60.25 (2.56) | 6.14* |
| Inhibition Switching | 61.53 (2.65) | 62.05 (3.04) | .08 |
| Inhibition Switching errors | 1.64 (.31) | 1.60 (.26) | .09 |
Note.
*** = p < .001;
** = p < .01;
* = p < .05.
Relationship among age group, metabolic status, and cognitive functioning
There were significant interactions between age group, metabolic status, and cognitive functioning on the CPT-2: commission, response time, variability, perseveratives, and response styles sub-measures (Table 6). However, there were no additional significant interactions between age group and metabolic status when controlling for gender and education level (refer to Tables 7–9 for mean cognitive scores).
Table 6. Metabolic status by age group interactions for CPT-2.
| CPT-2 | F |
|---|---|
| Omission | 2.56 |
| Commission | 4.20* |
| Response Time | 3.64* |
| Variability | 4.28* |
| Perseveratives | 3.58* |
| Clinical % | 1.19 |
| Non-Clinical % | 1.23 |
| Response Style | 3.44* |
Note.
** = p < .01;
* = p < .05.
Table 7. Relationship between age, metabolic status, and cognitive functioning.
| Age Group & Metabolic Status: Mean (Standard Error) | ||||||
|---|---|---|---|---|---|---|
| Variable | Young Control | Young Metabolic | Middle-age Control | Middle-age Metabolic | Older Control | Older Metabolic |
| Digit Span | ||||||
| Total | 21.45** (1.10) | 18.95 (.74) | 17.82 (.92) | 18.56 (.90) | 16.27 (.83) | 16.95 (.81) |
| CPT-2 | ||||||
| Omission | 1.35 (.48) | 2.84 (1.07) | 7.46 (3.98) | 11.08 (4.57) | 6.00 (2.26) | 9.00 (3.34) |
| Commission | 17.33 (4.31) | 26.81 (5.42) | 14.69 (3.42) | 19.00 (4.64) | 28.20 (6.88) | 15.24 (2.72) |
| Response Time | 385.21 (15.57) | 371.77* (13.94) | 361.94 (31.04) | 330.08 (40.68) | 452.36 (18.97) | 420.98 (15.82) |
| Variability | 7.07 (.94) | 9.28 (1.13) | 13.95 (3.36) | 15.63 (4.66) | 10.80 (2.04) | 8.58 (.91) |
| Perseveratives | .60 (.28) | .84 (.44) | 5.93 (3.66) | 12.31 (5.45) | 2.05 (1.02) | .38* (.20) |
| Clinical Percentage | 37.10 (5.04) | 45.54 (5.28) | 56.49 (4.64) | 47.78 (2.88) | 68.31 (4.95) | 58.21 (4.55) |
| Non-Clinical Percentage | 62.90 (5.04) | 54.46 (5.28) | 43.16 (4.68) | 52.22 (2.88) | 31.69 (4.95) | 41.79 (4.55) |
| Response Style | .61 (.13) | .60 (.18) | 7.05 (4.40) | 11.75 (5.07) | 1.44 (.42) | 1.26 (.38) |
Note. CPT-2 = Conner’s Performance Test-II. Significant exploratory analyses are denoted here using *** = p < .001;
** = p < .01;
* = p < .05.
Table 9. Relationship between age, metabolic status, learning and memory.
| Age Group & Metabolic Status: Mean (Standard Error) | ||||||
|---|---|---|---|---|---|---|
| Variable | Young Control | Young Metabolic | Middle-age Control | Middle-age Metabolic | Older Control | Older Metabolic |
| CVLT-II | ||||||
| Trial 1 | 6.70 (.38) | 6.94 (.45) | 7.12 (.47) | 6.28 (.45) | 6.05 (.59) | 6.40 (.47) |
| Trial 2 | 10.05 (.54) | 9.67 (.40) | 10.24 (.53) | 9.61 (.51) | 8.45 (.60) | 8.45 (.53) |
| Trial 3 | 12.05 (.51) | 11.67 (.51) | 11.53 (.52) | 11.56 (.49) | 9.77 (.69) | 10.35 (.68) |
| Trial 4 | 12.90 (.48) | 12.44 (.52) | 12.82 (.52) | 12.50 (.49) | 10.86 (.65) | 10.95 (.57) |
| Trial 5 | 12.85 (.45) | 13.17 (.44) | 13.00 (.58) | 12.83 (.60) | 11.05 (.59) | 11.30 (.62) |
| Total Recall | 54.55 (1.91) | 53.89 (1.82) | 54.71 (2.15) | 52.78 (1.99) | 46.18 (2.86) | 47.45 (2.54) |
| Short Delay FR | 11.85 (.59) | 11.89 (.62) | 12.41 (.69) | 11.22* (.74) | 9.32 (.80) | 9.35 (.81) |
| Long Delay FR | 12.15 (.73) | 12.89 (.52) | 13.12 (.61) | 12.78 (.59) | 10.50 (.72) | 11.20 (.62) |
| Short Delay CR | 12.55 (.69) | 12.22 (.62) | 12.76 (.76) | 12.56 (.68) | 9.50 (.73) | 9.95 (.85) |
| Long Delay CR | 12.65 (.65) | 12.89 (.52) | 13.18 (.69) | 12.94 (.60) | 10.23 (.77) | 11.70 (.68) |
| BVMT-R | ||||||
| Total Recall | 27.50 (1.07) | 27.22 (1.20) | 24.13 (1.13) | 22.76 (2.01) | 20.57 (1.52) | 20.35 (1.85) |
| Long Delay Recall | 10.40 (.32) | 10.22 (.41) | 10.00 (.40) | 8.82 (.75) | 8.81 (.58) | 8.20 (.75) |
Note. CVLT-II = California Verbal Learning Test-II, FR = free recall, CR = cued recall, BVMT-R = Brief Visual Memory Test-Revised. Significant exploratory analyses are denoted here using *** = p < .001;
** = p < .01;
* = p < .05.
Exploratory analyses investigated the role of weight as a covariate when evaluating the possible influences of age group and metabolic status on cognitive functioning. Young adults demonstrated significant differences by metabolic status on Digit Span ([F(1,29) = 15.65, p < .01], Table 7) and CPT-2 Response time ([F(1,29) = 5.16, p = .03], Table 7) when controlling for weight in addition to gender and education level. In the middle-age group, these MANCOVAs revealed a significant relationship between metabolic status and performance on the D-KEFS Color-Word Inhibition ([F(1,34) = 6.00, p = .02], Table 8); CVLT-II Short Delay Free Recall ([F(1,34) = 5.49, p = .03], Table 9); D-KEFS Design Fluency: filled dots ([F(1,34) = 4.40, p = .04], Table 8), empty dots ([F(1,34) = 8.30, p < .01], Table 8), and switching ([F(1,34) = 5.10, p = .03], Table 8), when weight was added into the MANCOVA as a covariate. There were significant differences by metabolic status in the older adult group on the D-KEFS Color-Word Inhibition ([F(1,39) = 4.36, p = .04], Table 8) and CPT-2 Perseveratives ([F(1,33) = 5.03, p = .03], Table 7). There were not significant differences between metabolic status and cognitive performance when controlling for weight, gender, and education level for any other cognitive assessment in this study for young, middle-aged, or older adult age groups.
Table 8. Relationship between age, metabolic status, and cognitive functioning.
| Age Group & Metabolic Status: Mean (Standard Error) | ||||||
|---|---|---|---|---|---|---|
| Variable | Young Control (n = 20) | Young Metabolic (n = 22) | Middle-age Control (n = 18) | Middle-age Metabolic (n = 23) | Older Control (n = 22) | Older Metabolic (n = 23) |
| Color-Word Interference | ||||||
| Color Naming | 27.90 (1.05) | 29.05 (1.49) | 29.76 (1.56) | 31.78 (1.41) | 31.41 (1.56) | 33.20 (1.69) |
| Word Reading | 20.40 (.87) | 22.05 (1.19) | 22.82 (1.36) | 22.67 (.90) | 23.23 (1.27) | 25.75 (1.44) |
| Inhibition | 43.85 (1.32) | 51.47 (4.71) | 49.47 (2.98) | 58.44* (2.69) | 66.05 (3.98) | 70.20* (4.50) |
| Inhibition Switching | 50.65 (1.71) | 55.74 (2.30) | 59.35 (5.21) | 60.83 (4.00) | 73.09 (4.66) | 72.79 (6.85) |
| Trails | ||||||
| Visual Scanning | 20.20 (1.34) | 17.26 (1.16) | 18.82 (.79) | 19.78 (1.16) | 24.50 (1.66) | 24.86 (1.56) |
| Number | 27.00 (1.69) | 23.63 (1.63) | 27.82 (1.87) | 29.56 (2.02) | 41.14 (5.10) | 41.38 (4.06) |
| Letter | 25.45 (1.63) | 26.16 (2.25) | 28.00 (1.86) | 32.56 (2.53) | 43.32 (4.19) | 40.05 (3.33) |
| Number-Letter Switching | 60.95 (4.97) | 55.84 (2.72) | 65.82 (6.17) | 84.00 (7.50) | 114.32 (13.67) | 105.86 (9.51) |
| Motor Speed | 22.00 (1.71) | 22.32 (.94) | 26.18 (2.36) | 25.22 (1.48) | 32.77 (4.07) | 35.14 (2.78) |
| Design Fluency | ||||||
| Filled Dots | 10.65 (.69) | 11.68 (.90) | 12.06 (1.02) | 9.89* (.77) | 10.09 (.96) | 7.81 (.67) |
| Empty Dots | 11.80 (9.55) | 12.21 (.79) | 13.59 (.78) | 11.50**ss (1.04) | 11.00 (.96) | 9.24 (.76) |
| Switching | 9.55 (.44) | 9.58 (.67) | 8.71 (.57) | 8.39* (.63) | 6.86 (.59) | 7.10 (.61) |
| Verbal Fluency | ||||||
| Letter | 42.80 (2.79) | 43.11 (2.60) | 42.24 (3.07) | 41.61 (3.18) | 43.27 (2.67) | 41.62 (2.97) |
| Category | 44.95 (1.63) | 46.21 (1.77) | 43.47 (1.73) | 43.72 (1.90) | 39.22 (1.74) | 41.76 (2.04) |
| Switching | 15.55 (.56) | 14.79 (.72) | 15.82 (.61) | 15.22 (.59) | 13.27 (.63) | 13.00 (.72) |
Note. Significant exploratory analyses are denoted here using *** = p < .001;
** = p < .01;
* = p < .05.
Discussion
The primary aim of the current study was to investigate differences in cognitive functioning among young adults (classified as normal or at risk for MetS), middle-aged (classified as normal or MetS), and older adults (classified as normal or MetS).
Self report measures
Individuals with MetS rated themselves as less inhibited and more hungry than controls, regardless of age, on the TFEQ, a self-report measure of eating behavior (Table 2). Additionally, middle-aged adults reported significantly less self-control than young and older adults on the BIS, a self-report measure of impulsivity (S1 Table). Obese persons have been found to report significantly more disinhibited eating than their normal weight counterparts [56, 57]. Disinhibition increases likelihood of weight gain and has been associated with a sedentary lifestyle [58, 59], which contribute to the development of MetS [60].
Age group effects on cognitive performance
There were significant age group effects across neuropsychological assessments (Tables 3 and 4). Age-related cognitive decline has been consistently documented within the literature [61–68]. Of note, the present study showed age group effects across verbal and visual memory, executive functioning, and processing speed.
Metabolic status effects on cognitive performance
There was a main effect of metabolic status on cognitive performance in which participants with MetS were significantly slower on the Color-Word Interference Test: Inhibition condition (Table 8, Fig 1). The Inhibition condition from the D-KEFS requires intact processing speed and cognitive flexibility [42]. It is interesting that, in the present cohort, individuals with MetS rated themselves as more disinhibited with eating as compared to normal participants; however, there were no significant correlations between self-reported disinhibition (r = .137) or self-control (r = .034) and performance on the Color-Word Interference: Inhibition task, when controlling for age (r = .136). Given the task demands of the Inhibition condition, this finding may provide support for declines in executive functioning abilities for individuals with MetS. Studies examining larger cohorts that incorporate executive functioning measures provide additional support for the present results [17, 18, 36].
Clinically, changes in executive functioning are associated with declines in activities of daily living and medication adherence [69–72]. In addition, comorbidity of vascular risk factors is also associated with functional decline [73] and declines in one’s ability to manage vascular risk factors [74]. Thus, an individual with MetS may be at risk for poor medication compliance which could exacerbate MetS. As a result, poor medication compliance could ultimately increase the risk of developing dementia.
Metabolic status and age group effects on cognitive performance
Scores on measures of commission, response time, variability, perseveratives, and response style under the CPT-2 demonstrated significant interactions between age group and metabolic status. These interactions across the CPT-2 suggest that the young and middle-aged adults with MetS made more errors (commissions, perseveratives) and were more inconsistent in their responses as compared to controls. However, the older adults with MetS had fewer errors, better response times, and more consistency as compared to healthy older adults. Across age groups, participants with MetS also had faster response times (although this does not imply accuracy). These data support the notion of relative deficits related to attention in young and middle-aged adults with MetS. They also suggest the potential for a survivor effect or protective effect of MetS in older adults [35, 75, 76].
Due to the notable variability in weight in pounds for the middle-aged adults (Table 1), the extensive literature implicating obesity in cognitive dysfunction [25, 30, 77–84], and risk for dementia associated with weight in middle age [85–89], exploratory analyses were performed to assess the role of weight in cognition in the context of MetS. These analyses revealed significant differences on select executive functioning tasks (i.e., D-KEFS Color Word Interference: Inhibition and D-KEFS Design Fluency: Filled dots, Empty dots, and Switching subtests) between middle-aged adults with MetS versus control, with participant weight in pounds as a moderator. On each of these tasks, middle-aged control adults significantly outperformed those with MetS when weight in pounds was controlled for as an addition to gender and education level. These results are consistent with Yang and colleagues’ findings of deficits on executive functioning tasks for obese individuals and inhibition impairments for overweight individuals [75]. Of note, inherently poor executive functioning skills predicted obesity in longitudinal studies of children [30]. Based on these exploratory analyses, weight in pounds moderates the relationship between executive functioning on select tasks and MetS in middle-aged adults. This relationship likely highlights the compounding deleterious effects of excess weight and MetS on executive functioning in middle-aged adults in contrast with the purported protective effect of excess weight on cognition in older adults [35, 75]. However, this “protective effect” may potentially be partially accounted for by a survivor effect as MetS has been associated with increased mortality across the lifespan as compared to those without MetS [76].
Declines in executive functioning in MetS have been reported in the literature [17, 19, 31, 36, 90]. However, within these experiments executive functioning was assessed via screening measures or defined as a latent variable combining multiple processes such as novel problem solving, cognitive set-shifting, inhibition, and fluency. Based on the literature, it is difficult to determine which executive functioning processes are affected in MetS. In fact, primary criticisms in a review of the literature on cognitive performance in MetS were the “lack of consistency in the cognitive domains selected for assessment, differences in the quality of tests selected, and demographics of populations studied” [36]. Furthermore, a 2018 review examining the association between cognitive functioning and MetS concluded that the heterogeneity of results between studies was too great to infer MetS as a precursor to declines or changes in cognition [35]. As such, the present study adds to the literature through investigating the effects of MetS on individual tests of cognitive functioning.
There is a paucity of research regarding MetS in adolescents. One study found a significant negative impact of MetS on executive functioning and cognitive flexibility skills in Hispanic adolescents [91]. Additionally, adolescents with MetS demonstrated significantly poorer performances on measures of reading, attention, and working memory [92]. Obesity in adolescents has also been associated with executive dysfunction, decreased levels of inhibition and slower processing speed [26, 28, 93, 94]. When accounting for weight in this study, young adults at risk for MetS demonstrated significant differences on two tasks of attention (Digit Span and CPT-2 Response Time), in which those categorized as MetS performed significantly better than young controls. These results are inconsistent with the aforementioned literature and could be due to the small sample size. There were no effects of MetS on remaining measures of cognitive performance in the present study in this young adult sample (whether or not weight was accounted for).
Limitations
Strengths and limitations of this work should be recognized. The strengths of this work lie in the thorough neuropsychological evaluation and the life-span approach to assessing the effects of metabolic syndrome on cognitive function. There are also limitations. First, the study would have benefitted from larger sample sizes. The young adult metabolic cohort was comprised of obese individuals who are at risk for the development of MetS. The study is cross sectional and the duration during which an individual met criteria for MetS was not available, which could also underestimate the effect of MetS. Future studies will be needed to determine the effect of duration of MetS on lifespan cognitive function. Finally, the sample was largely Caucasian, middle class, and, on average, had some college education, suggesting the need for future studies in more diverse cohorts.
Conclusions
The current study investigated cognitive performance in a community sample of young, middle-aged and older adults with multiple risk factors for metabolic syndrome. Participants with MetS were significantly slower on the Color-Word Interference: Inhibition task as compared to controls. Middle-aged adults with MetS appeared to be more susceptible to executive functioning deficits with weight in pounds moderating this relationship. MetS in young and middle-aged adults may be associated with relative deficits in attention. Cognitive performance by older adults with MetS could suggest a survivor effect or protective effect of MetS. The results of the present study provide further evidence for age-related declines in cognitive functioning.
Innate executive dysfunction may be a causal factor in becoming obese [30]. While purely speculative, deficits in inhibition and executive function could potentially contribute to difficulties maintaining a healthy diet and adequate exercise, both of which contribute to the development and maintenance of MetS. Individuals with MetS self-report greater levels of disinhibited eating and hunger than controls, which may also have implications for the development and maintenance of MetS.
Given that individuals with MetS had significantly greater self-reported disinhibited eating and performed more poorly on a task of inhibition, future studies aimed at investigating potential causal relationships between MetS and disinhibited eating and executive dysfunction may provide insight into effective intervention targets to delay or prevent MetS.
Supporting information
(DOCX)
(XLSX)
Acknowledgments
We gratefully acknowledge the assistance of Aaron Jacobson and the members of the Lifespan Human Senses Laboratory.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
Supported by NIH grants R01AG004085-26 and R01AG062006-02 from the National Institute on Aging (CM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eckel R. H., Grundy S. M., & Zimmet P. Z. (2005). The metabolic syndrome. The Lancet, 365(9468) 1415–1428. 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 2.Grundy S. M., Cleeman J. I., Daniels S. R., Donato K. A., Eckel R. H., Franklin B. A., et al. (2005). Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 112(17), 2735–2752. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. (2006). Metabolic Syndrome. The IDF Consensus worldwide definition of the Metabolic Syndrome. Retrieved from http://www.idf.org/metabolic_syndrome.
- 4.Isomaa B., Almgren P., Tuomi T., Forsen B, Lahti K., Nissen M., et al. (2001). Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care, 24(4), 683–689. 10.2337/diacare.24.4.683 [DOI] [PubMed] [Google Scholar]
- 5.Lakka H. M., Laaksonen D. E., Lakka T. A., Niskanen L. K., Kumpusalo E., Tuomilehto J., et al. (2002). The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. The Journal of the American Medical Association, 288(21), 2709–2716. 10.1001/jama.288.21.2709 [DOI] [PubMed] [Google Scholar]
- 6.Kurl S., Laukkanen J. A., Niskanen L., Laaksonen D., Sivenius J., Nyyssonon K., et al. (2006). Metabolic syndrome and the risk of stroke in middle-aged men. Stroke, 37(3), 806–811. 10.1161/01.STR.0000204354.06965.44 [DOI] [PubMed] [Google Scholar]
- 7.Ninomiya J. K., Italien G., Criqui M.H., Whyte J. L., Ganst A., & Chen R. S. (2004). Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation, 109, 42–46. 10.1161/01.CIR.0000108926.04022.0C [DOI] [PubMed] [Google Scholar]
- 8.Aguilar M., Bhuket T., Torres S., Liu B., & Wong R.J. (2015). Prevalence of the Metabolic Syndrome in the United States, 2003–2012. JAMA 313(19), 1973–1974. 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 9.Ford E. S., Giles W. H., & Dietz W. H. (2002). Prevalence of the metabolic syndrome among US adults: findings from the third national health and nutrition examination survey. The Journal of the American Medical Association, 287(3), 356–359. 10.1001/jama.287.3.356 [DOI] [PubMed] [Google Scholar]
- 10.Loyd-Jones D., Adams R. J., Brown T. M., Carnethon M., Dai S, De Simone G., et al. (2010). Heart Disease and Stroke Statistics– 2010 Update. A report from the American Heart Association. Circulation, 121(7), e1–e170. 10.1161/CIRCULATIONAHA.109.192667 [DOI] [PubMed] [Google Scholar]
- 11.Gupta R., Misra A., Vikram N. K., Kondal D., Gupta S. S., Agrawal A., et al. (2009). Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovascular Disorders, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juonala M., Viikari J., Hutri-Kahonen N., Pietikainen M., Jokinen E., Taittonen L., et al. (2004). The 21-year follow-up of the Cardiovascular Risk in Young Finns Study: risk facto levels, secular trends and east-west difference. Journal of Internal Medicine, 255, 457–468. 10.1111/j.1365-2796.2004.01308.x [DOI] [PubMed] [Google Scholar]
- 13.Kivipelto M., Helkala E. L., Laakso M. P., Hanninen T., Hallikainen M., Alhainen K., et al. (2001). Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. British Medical Journal, 322(7300), 1447–1451. Retrieved from http://www.bmj.com/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kivipelto M., Ngandu T., Fratiglioni L., Vitanen M., Kareholt I., Winblad B., et al. (2005). Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology, 62(10), 1556–1560. Retrieved from http://archneur.ama-assn.org/. [DOI] [PubMed] [Google Scholar]
- 15.Kuusisto J., Koivisto K., Mykkanen L., Helkala E.L., Vanhanen M., Hanninen T., et al. (1997). Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: Cross sectional population based study. BMJ, 315(7115), 1045–1049. 10.1136/bmj.315.7115.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luchsinger J. A., & Mayeux R. (2004). Cardiovascular risk factors and Alzheimer’s disease. Current Atherosclerosis Reports, 6(4), 261–266. 10.1007/s11883-004-0056-z [DOI] [PubMed] [Google Scholar]
- 17.Bokura H., Yamaguchi S., Iijima K., Nagai A., & Oguro H. (2008). Metabolic syndrome is associated with silent ischemic brain lesions. Stroke, 39(5), 1607–1609. 10.1161/STROKEAHA.107.508630 [DOI] [PubMed] [Google Scholar]
- 18.Falkowski J., Atchison T., DeButte-Smith M., Weiner M., & O’Bryant S. (2014). Executive Functioning and the Metabolic Syndrome: A Project FRONTIER Study. Archives of Clinical Neuropsychology, 29(1), 47–53. 10.1093/arclin/act078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura B., Jurado M. A., Freixenet N., Albuin C., Muniesa J., & Junque C. (2009. a). Mental slowness and executive dysfunctions in patients with metabolic syndrome. Neuroscience Letters, 462, 49–53. 10.1016/j.neulet.2009.06.071 [DOI] [PubMed] [Google Scholar]
- 20.Chooi Y. C., Ding C., & Magkos F. (2019). The epidemiology of obesity. Metabolism, 92, 6–10. 10.1016/j.metabol.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 21.Grundy S. M. (2004). Obesity, metabolic syndrome, and cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism, 89(6), 2595–2600. [DOI] [PubMed] [Google Scholar]
- 22.Maayan L., Hoogendoorn C., Sweat V., & Convit A. (2011). Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity, 19(7), 1382–1387. 10.1038/oby.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yau P.L., Kang E.H., Javier D.C., & Convit A. (2014). Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Pediatric Obesity (22), 1885–1871. 10.1002/oby.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabia S., Kivimaki M., Shipley M. J., Marmot M. G., & Singh-Manous A. (2009). Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. The American Journal of Clinical Nutrition, 89, 601–607. 10.3945/ajcn.2008.26482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fergenbaum J. H., Bruce S., Lou W., Hanley A. J., Greenwood C., & Young T. K. (2009). Obesity and lowered cognitive performance in a Canadian first nations population. Obesity, 17(10), 1957–1963. 10.1038/oby.2009.161 [DOI] [PubMed] [Google Scholar]
- 26.Batterink L., Yokum S., & Stice E. (2010). Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. Neuroimage, 52(4), 1696–1703. 10.1016/j.neuroimage.2010.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cserjési R., Molnár D., Luminet O., & Lénárd L. (2007). Is there any relationship between obesity and mental flexibility in children?. Appetite, 49(3), 675–678. 10.1016/j.appet.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 28.Pauli-Pott U., Albayrak O., Hebebran J., & Pott W. (2010). Association between inhibitory control capacity and body weight in overweight and obese children and adolescents: dependence on age and inhibitory control component. Child Neuropsychology, 1, 1–12. 10.1080/09297049.2010.485980 [DOI] [PubMed] [Google Scholar]
- 29.Wirt T., Schreiber A., Kesztyüs D., & Steinacker J. M. (2015). Early life cognitive abilities and body weight: cross-sectional study of the association of inhibitory control, cognitive flexibility, and sustained attention with BMI percentiles in primary school children. Journal of obesity, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith E., Hay P., Campbell L., & Trollor J. N. (2011). A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obesity reviews, 12(9), 740–755. 10.1111/j.1467-789X.2011.00920.x [DOI] [PubMed] [Google Scholar]
- 31.Gatto N. M., Henderson V. W., St. John J. A., McCleary C., Hodis H. N., & Mack W. J. (2008). Metabolic syndrome and cognitive function in healthy middle-aged and older adults without diabetes. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 15(5), 627–641. 10.1080/13825580802036936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komulainen P., Lakka T. A., Kivipelto M., Hassinen M., Helkala E. L., Haapala I., et al. (2007). Metabolic syndrome and cognitive function: A population-based follow-up study in elderly women. Dementia and Geriatric Cognitive Disorders, 23, 29–34. 10.1159/000096636 [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K., Kanaya A., Lindquist K., Simonsick E. M., Harris T., Shorr R. I., et al. (2004). The metabolic syndrome, inflammation, and risk of cognitive decline. The Journal of the American Medical Association, 292(18), 2237–2242. 10.1001/jama.292.18.2237 [DOI] [PubMed] [Google Scholar]
- 34.Yaffe K., Weston A. L., Blackwell T., & Krueger K. A. (2009). The metabolic syndrome and development of cognitive impairment among older women. Archives of Neurology, 66(3), 324–328. Retrieved from http://archneur.ama-assn.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assuncao N., Sudo F. K., Drummond C., de Felice F. G., & Mattos P. (2018). Metabolic syndrome and cognitive decline in the elderly: a systematic review. PloS one, 13(3), e0194990. 10.1371/journal.pone.0194990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yates K.F., Sweat V.L., Po L.Y., Turchiano M.M., Convit A.M. (2012). Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(9), 2060–2067. 10.1161/ATVBAHA.112.252759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Berg E., Biessels G. J., de Craen A. M., Gussekloo J. J., & Westendorp R. J. (2007). The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology, 69(10), 979–985. 10.1212/01.wnl.0000271381.30143.75 [DOI] [PubMed] [Google Scholar]
- 38.Dik M. G., Jonker C., Comijs H. C., Deeg D. J., Kok A., Yaffe K., & Penninx B. W. (2007). Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care, 30(10), 2655–2660. 10.2337/dc06-1190 [DOI] [PubMed] [Google Scholar]
- 39.Crichton G. E., Elias M. F., Buckley J., Murphy K., & Bryan J., (2011). Metabolic syndrome, cognitive performance, and dementia. Journal of Alzheimer’s Disease, 28, 1–11. [DOI] [PubMed] [Google Scholar]
- 40.Akbaraly T., N., Kivimaki M., Shipley M. J., Tabak A. G., Jokela M., Virtanen M., et al. (2010). Metabolic syndrome over 10 years and cognitive functioning in late midlife: the Whitehall II study. Diabetes Care, 33, 84–89. 10.2337/dc09-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laudisio A., Marzetti E., Pagano F., Cocchi A., Franceshi C., Bernabei R., et al. (2008). Association of metabolic syndrome with cognitive function: The role of sex and age. Clinical Nutrition, 27(5), 747–754. 10.1016/j.clnu.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 42.Delis D. C., Kaplan E., & Kramer J. H. (2001). Delis-Kaplan Executive Function System (D-KEFS) examiner’s manual (pp. 79.–). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- 43.Delis D. C., Kramer J. H., Kaplan E., Ober B.A. (2000). California Verbal Learning Test—second edition. Adult Version. Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- 44.Benedict R. (1997). Brief Visuospatial Memory Test-Revised professional manual. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- 45.Conners C. K. (2000). Conners’ Continuous Performance Test (CPT-2) computer program for windows, technical guide, and software manual. Toronto, ON: Multi Health Systems Inc. [Google Scholar]
- 46.Alberti K.G., Eckel R.H., Grundy S.M. et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Associatin; World Heart Federation; International. Circulation, 120(18), 1640–1645. [DOI] [PubMed] [Google Scholar]
- 47.Andersen C. J., Murphy K. E., & Fernandez M. L. (2016). Impact of Obesity and Metabolic Syndrome on Immunity. Advances in nutrition (Bethesda, Md.), 7(1), 66–75. 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folstein M.F., Folstein S.E. & McHugh P.R. (1975). “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 49.Mattes S e(1975). Mental status examination for organic mental syndrome in the elderly patient. In: Bellad L. & Karash T.B. (Eds.), Geriatric Psychiatry. New York: Grune and Stratton. [Google Scholar]
- 50.Stroop J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology 18, 643–662. 10.1037/h0054651 [DOI] [Google Scholar]
- 51.Beck AT, Steer RA and Brown GK (1996) "Manual for the Beck Depression Inventory-II". San Antonio, TX: Psychological Corporation. [Google Scholar]
- 52.Spielberger C.D., Gorsuch R.L., Luchene R., Vagg P.R., & Jacobs G.A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- 53.Stunkard A.J. & Messick S. (1985). The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger, J. Psychosom Res, 29(1), 71–83. 10.1016/0022-3999(85)90010-8 [DOI] [PubMed] [Google Scholar]
- 54.Patton J. H., Stanford M. S., & Barratt E. S. (1995). Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology, 51(6), 768–774. [DOI] [PubMed] [Google Scholar]
- 55.D’Agostino R.B., Wolf P.A., Belanger A.J. & Kannel W.B. (1994). Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke, 25(1), 40–3. 10.1161/01.str.25.1.40 [DOI] [PubMed] [Google Scholar]
- 56.Harden C., Corte B. M., Richardson J. C., Dettmar P. W. & Paxman J. R. (2009). Body mass index and age effect three-factory eating questionnaires scores in male subjects. Nutrition Research, 29(6), 379–382. 10.1016/j.nutres.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 57.Mobbs O., Crepin C., Thiry C., Golay A., & Van der Liden M. (2010). Obesity and the four facets of impulsivity. Patient Education and Counseling, 79, 372–377. 10.1016/j.pec.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 58.Bryant E. J., Kiezebrink K., King N. A., & Blundell J. E. (2010). Interaction between disinhibition and restraint: implication for body weight and eating disturbance. Eating and Weight Disorders, 15(102), e43–51. [DOI] [PubMed] [Google Scholar]
- 59.Hays N. P., Bathalon G. P. McCory M. A., Roubenoff R., Lipman R., & Roberts S. B. (2002). Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. The American Journal of Clinical Nutrition, 75(3), 476–483. 10.1093/ajcn/75.3.476 [DOI] [PubMed] [Google Scholar]
- 60.Ho R. C., Niti M., Yap K. B., Kua E. H., & Ng T. P. (2008). Metabolic syndrome and cognitive decline in Chinese older adults: results from the Singapore longitudinal ageing studies. American Journal of Geriatric Psychiatry, 16(6), 519–522. 10.1097/JGP.0b013e31816b7841 [DOI] [PubMed] [Google Scholar]
- 61.Van der Elst W., Wan Boxtel M. P. Van Breukelen G. J., & Jolles J. (2006). The stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment, 13(1), 62–79. 10.1177/1073191105283427 [DOI] [PubMed] [Google Scholar]
- 62.Grady C.L., McIntosh A.R., & Craik F.I., (2005). Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 43(10), 1466–81. 10.1016/j.neuropsychologia.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 63.Hultsch D. F., MacDonald S. W., & Dixon R. A. (2002). Variability in reaction time performance of younger and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(2), P101–P115. 10.1093/geronb/57.2.p101 [DOI] [PubMed] [Google Scholar]
- 64.Finkel D., Reynolds C. A., McArdle J. J., Pedersen N. L. (2007). Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging, 22(3), 558–568. 10.1037/0882-7974.22.3.558 [DOI] [PubMed] [Google Scholar]
- 65.Schonknecht P., Pantel J., Kruse A., & Schroder J. (2005). Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. American Journal of Psychiatry, 162(11), 2071–2077. [DOI] [PubMed] [Google Scholar]
- 66.van Hooren S. A., Valentijn A. M., Bosma H., Ponds R. W. H., Boxtel M. P., & Jolles J. (2007). Cognitive functioning in healthy older adults aged 64–81: a cohort study into the effects of age, sex, and education. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology, and Cognition, 14, 40–54. [DOI] [PubMed] [Google Scholar]
- 67.Wilson R. S., Beckett L. A., Barnes L. L., Schneiger J. A., Bach J., Evans D. A., et al. (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology of Aging, 17(2), 179–193. [PubMed] [Google Scholar]
- 68.Drag L. L. & Bieliauskas L. A. (2010). Contemporary review 2010: Cognitive Aging. Journal of Geriatric Psychiatry Neurology, 23(2), 75–93. 10.1177/0891988709358590 [DOI] [PubMed] [Google Scholar]
- 69.Arlt S., Linder R., Rosler A., & von Renteln-Kruse W. (2008). Adherance to medication in patients with dementia: predictors and strategies for improvement. Drugs Aging, 25(12), 1033–1047. 10.2165/0002512-200825120-00005 [DOI] [PubMed] [Google Scholar]
- 70.Grigsby J., Kaye K., Baxter J., Shetterly S. M., & Hamman R. F. (1998). Executive cognitive abilities and functional status among community-dwelling older persons in the san luis valley health and aging study. Journal of the American Geriatrics Society, 46(5), 590–596. 10.1111/j.1532-5415.1998.tb01075.x [DOI] [PubMed] [Google Scholar]
- 71.Johnson J. K., Lui L., & Yaffe K. (2007). Executive function, more than global cognition, predicts functional decline and mortality in elderly women. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 62(10), 1134–1141. 10.1093/gerona/62.10.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoehr G., Lu S., Lavery L., Bilt J. V., Saxton J. A., Change C. H., et al. (2008). Factors associated with adherence to medication regimens by older primary care patients: the steel valley seniors survey. American Journal of Geriatric Pharmacotherapy, 6(5), 255–263. 10.1016/j.amjopharm.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stuck A. E., Walthert J. M., Nikolaus T., Bula C. J., Homann C., & Back J. C. (1999). Risk factors for functional status decline in community-living elderly people: a systematic literature review. Social Science and Medicine, 48(4), 445–469. 10.1016/s0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- 74.Munshi M., Grande L., Hayes M., Ayres D., Suhl E., Capelson R., et al. (2006). Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care, 29(8), 1794–1799. 10.2337/dc06-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi T. H., Wang B., & Natarajan S. (2020). The Influence of Metabolic Syndrome in Predicting Mortality Risk Among US Adults: Importance of Metabolic Syndrome Even in Adults With Normal Weight. Preventing chronic disease, 17, E36. 10.5888/pcd17.200020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y., Shields G.S., Guo C., Liu Y. (2018). Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neuroscience and Behavioral Reviews, 84, 225–244. 10.1016/j.neubiorev.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 77.Cheke L. G., Bonnici H. M., Clayton N. S., & Simons J. S. (2017). Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia, 96, 137–149. 10.1016/j.neuropsychologia.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conforto Richard Mark, and Gershman Louis. "Cognitive processing differences between obese and nonobese subjects." Addictive behaviors 10, no. 1 (1985): 83–85. 10.1016/0306-4603(85)90056-5 [DOI] [PubMed] [Google Scholar]
- 79.Coppin G., Nolan-Poupart S., Jones-Gotman M., & Small D. M. (2014). Working memory and reward association learning impairments in obesity. Neuropsychologia, 65, 146–155. 10.1016/j.neuropsychologia.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dye L., Boyle N. B., Champ C., & Lawton C. (2017). The relationship between obesity and cognitive health and decline. Proceedings of the Nutrition Society, 76(4), 443–454. 10.1017/S0029665117002014 [DOI] [PubMed] [Google Scholar]
- 81.Fagundo A. B., De la Torre R., Jiménez-Murcia S., Agüera Z., Granero R., Tárrega S., et al. (2012). Executive functions profile in extreme eating/weight conditions: from anorexia nervosa to obesity. PloS one, 7(8). 10.1371/journal.pone.0043382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzales M. M., Tarumi T., Miles S. C., Tanaka H., Shah F., & Haley A. P. (2010). Insulin sensitivity as a mediator of the relationship between BMI and working memory‐related brain activation. Obesity, 18(11), 2131–2137. 10.1038/oby.2010.183 [DOI] [PubMed] [Google Scholar]
- 83.Gunstad J., Paul R. H., Cohen R. A., Tate D. F., & Gordon E. (2006). Obesity is associated with memory deficits in young and middle-aged adults. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 11(1), e15–e19. [DOI] [PubMed] [Google Scholar]
- 84.Gunstad J., Paul R. H., Cohen R. A., Tate D. F., Spitznagel M. B., & Gordon E. (2007). Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive psychiatry, 48(1), 57–61. 10.1016/j.comppsych.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 85.Gustafson D. (2006). Adiposity indices and dementia. The Lancet Neurology, 5(8), 713–720. 10.1016/S1474-4422(06)70526-9 [DOI] [PubMed] [Google Scholar]
- 86.Prickett C., Brennan L., & Stolwyk R. (2015). Examining the relationship between obesity and cognitive function: a systematic literature review. Obesity research & clinical practice, 9(2), 93–113. 10.1016/j.orcp.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 87.Rosengren A., Skoog I., Gustafson D., & Wilhelmsen L. (2005). Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Archives of internal medicine, 165(3), 321–326. 10.1001/archinte.165.3.321 [DOI] [PubMed] [Google Scholar]
- 88.Whitmer R. A., Gunderson E. P., Barrett-Connor E., Quesenberry C. P., & Yaffe K. (2005). Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj, 330(7504), 1360. 10.1136/bmj.38446.466238.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whitmer R. A., Gunderson E. P., Quesenberry C. P., Zhou J., & Yaffe K. (2007). Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Current Alzheimer Research, 4(2), 103–109. 10.2174/156720507780362047 [DOI] [PubMed] [Google Scholar]
- 90.Cavalieri M., Ropele S., Petrovic K., Pluta-Fuerst A., Homayoon N., Enzinger C., et al. (2010). Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care, 33(12), 2489–2495. 10.2337/dc10-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mangone A., Yates K.F., Sweat V., Convit A.J., Convit A. (2018). Cognitive functions among predominantly minority urban adolescents with metabolic syndrome. Applied Neuropsychology: Child, 7(2), 157–163, 10.1080/21622965.2017.1284662 [DOI] [PubMed] [Google Scholar]
- 92.Rubens M., Ramamoorthy V., Saxena A., George F., Shehadeh N., Attonito J., et al. (2016). Relationship between metabolic syndrome and cognitive abilities in U.S. adolescents. Metabolic Syndrome and Related Disorders 14(8). 10.1089/met.2016.0015 [DOI] [PubMed] [Google Scholar]
- 93.Liang J., Matheson B.E., Kaye W.H & Boutelle K.N. (2014). Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International Journal of Obesity (Lon), 38(4), 494–506. 10.1038/ijo.2013.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sweat V., Yates K.F., Migliaccio R., Convit A. (2017). Obese Adolescents Show Reduced Cognitive Processing Speed Compared with Healthy Weight Peers. Childhood Obesity 13(3). 10.1089/chi.2016.0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.