Abstract
Isoquinoline alkaloids (IQs) from Macleaya cordata are promising natural products for enhancing the growth performance and overall health condition of farmed animals. The present study aimed to investigate the effects of two formulas of IQ extract, provided in either a powdered formula (IQ-E) or a water-soluble, granulated formula (IQ-WS) and containing the main active component sanguinarine at a concentration of 0.5% and 1%, respectively, on the growth, survival, immune response, and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei). In Experiment 1, the postlarvae were divided into five groups (four replicates/group and 100 shrimp/tank) and fed four times/day for 30 days with a control feed, IQ-E at 200 or 300 mg/kg of feed, or IQ-WS at 100 or 150 mg/kg of feed. In Experiment 2, the surviving shrimp from Experiment 1 were redistributed into six groups (four treatment groups as in Experiment 1 plus the positive and negative controls with four replicates/group and 30 shrimp/tank) and challenged with V. parahaemolyticus by immersion at a concentration of 103 colony-forming units (CFU)/mL and were fed with the same diets for another 14 days. The results revealed that all IQ-fed shrimp in Experiment 1 had significantly enhanced survival rates and immune parameters (total hemocyte count and phagocytic, phenoloxidase, and superoxide dismutase activities) compared to the control group, even though the growth performances were similar across all groups. In Experiment 2, all IQ-fed groups showed better growth performance and survival rates compared to the positive control. Other than in the positive control group, no histopathological lesions in the hepatopancreas and the intestine were found. In summary, the current study demonstrated the benefits of using IQs from M. cordata as feed additives for improving the growth performance, survival rate, immune responses, and resistance to vibriosis of Pacific white shrimp.
Introduction
Natural products from plants are becoming increasingly popularly utilized as feed additives in animal production for both terrestrial and aquatic species. Medicinal plants usually possess multiple biological activities such as antimicrobial, anti-inflammatory, antioxidant, immunostimulatory, and appetite-stimulating effects, which could potentially enhance the growth performance and health condition of animals [1–3]. Plant-derived isoquinoline alkaloids (IQs) are natural active components of various plants including Macleaya cordata or pink plume poppy, a herbaceous perennial plant in the family Papaveraceae which is widely distributed in China. M. cordata has long been used in traditional Chinese medicine as a topical agent for the treatment of inflammation and certain skin diseases [4–8].
The main bioactive isoquinoline alkaloids, namely sanguinarine and chelerythrine, are well known for their anti-inflammatory and antimicrobial properties [8,9]. It has generally been found that sanguinarine tends to be more active than chelerythrine with respect to these anti-inflammatory [10,11] and antimicrobial activities [7,12], although this result is not without exception [13]. Regarding the distribution of sanguinarine and chelerythrine within the plant, both alkaloids are found most abundantly in the capsules (32.08 and 7.36 mg/g dry weight, respectively) followed by the aerial part (4.51 and 2.88 mg/g dry weight, respectively), and at very low concentrations in the seeds (0.07 and 0.02 mg/g dry weight, respectively) [7]. Currently, IQs are considered promising feed additives to improve overall health condition and replace unnecessary antibiotic use in animal farming.
The potential health-promoting properties of IQ from M. cordata have been demonstrated in many animal species, including pigs [14], chickens [15–18], and fish [19–22]. These effects include growth performance enhancement, increased appetite, immunostimulation, alteration of the gut microbiome, and increased resistance to bacterial infection. For example, red tilapia fed with the IQ extracts at the rates of 25–100 mg/kg feed for 60 days had significantly higher final body weight (73.6–78.7 g), mean daily feed intake (1.19–1.25 g/fish/day), and leukocyte count (3.35–4.45 x 104 cell/μL) than the control fish which were 57.8 g, 1.03 g/fish/day, and 1.61 x 104 cell/μL, respectively [19]. Similarly, Caspian roach fed with the IQ extracts at the rates of 0.5–1.5 g/kg feed for 45 days had significantly higher final body weight (4.3–4.8 g) compared to the control (3.5 g) [20]. In addition, the IQ extracts (50–450 mg/kg feed) also showed anti-inflammatory and anti-oxidative effects, improved non-specific immunity, and enhanced resistance against Aeromonas hydrophila infection in koi carp [22]. Regarding the application of IQs in shrimp aquaculture, our previous study revealed that the IQ-fed Pacific white shrimp (100–200 mg/kg feed for 60 days and challenged with Vibrio harveyi) had a lower intestinal Vibrio spp. count compared to control shrimp, even though the effects on growth and survival were not significant [23]. We suspected that the limited effectiveness of the IQ extracts in promoting the growth and survival rate of the shrimp in our previous study might be related to the methodology of experimental diet preparation. Whereas our previous work utilized a simple top-coating approach which might result in the leaching of some IQ content from the pellet feed, the experimental feeds in the current study were made by mixing the IQ extracts with the other ingredients before pelleting to minimize the leaching problem of the IQs. Therefore, we expected that the health-promoting activity of the IQ extracts in the Pacific white shrimp in the present study would be more prominent.
The current study aimed to investigate under laboratory conditions the effects of two different formulations of IQ (a powdered formula (IQ-E; Sangrovit® Extra) and a water-soluble, granulated formula (IQ-WS; Sangrovit® WS), which contained the IQ sanguinarine as the main active compound at the concentrations of 0.5% and 1%, respectively) on the growth performance, survival rate, immune response, and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei). The study was divided into two experiments. In Experiment 1, the effects of IQs on growth, survival, and the immune system were investigated in healthy shrimp. In Experiment 2, the effects of IQs on shrimp growth, survival, and resistance to V. parahaemolyticus infection were evaluated after challenge with V. parahaemolyticus via immersion. The result of the present study provides useful information regarding the effects of IQ extracts as a shrimp feed additive for sustainable shrimp culture.
Materials and methods
Experiment 1: Effects of IQ on growth performance, survival, and immune response of healthy shrimp
Preparation of the experimental diets
IQs were obtained from cleaned, sieved and ground M. cordata plant material by solvent extraction with ethanol. Following extraction, the liquid extract was filtered and gently dried, milled and mixed with carrier materials. Five experimental diets were formulated: commercial pelleted feed without IQ supplementation (control diet), feed supplemented with a preparation of powdered IQ (IQ-E; Sangrovit® Extra) at 200 or 300 mg/kg of feed (providing 1 or 1.5 mg sanguinarine per kg of feed, respectively), and feed supplemented with a water-soluble, granulated preparation of IQ (IQ-WS; Sangrovit® WS) at 100 or 150 mg/kg of feed (providing 1 or 1.5 mg sanguinarine per kg of feed, respectively). Each experimental diet was prepared by mixing either of the two IQ preparations with a small amount of distilled water and mixed with the other ingredients by a pelleting machine to make the IQ-containing pellet feed. The ingredients and proximate composition of the basal diet was shown in Table 1.
Table 1. Ingredients and proximate composition (%) of the basal diet used in the current study.
| Ingredients | Percent |
|---|---|
| Fish meal | 15.00 |
| Soybean meal | 42.15 |
| Wheat bran | 1.25 |
| Corn protein concentrate | 2.90 |
| Wheat flour | 23.30 |
| Premix* | 7.05 |
| Binder | 0.10 |
| Squid liver powder | 2.50 |
| Fish soluble extract | 1.50 |
| Lecithin | 2.75 |
| Fish hydrolysate | 1.50 |
| Proximate composition | |
| Ash | 15.44 |
| Carbohydrate | 28.36 |
| Lipid | 8.00 |
| Moisture | 10.12 |
| Protein | 38.08 |
Note
*Premix composition (per kg) as follows: Vitamin A 6,700,000 IU, vitamin D 1,350,000 IU, vitamin E 67 g, vitamin K3 3.4 g, vitamin B1 6.7 g, vitamin B2 10 g, vitamin B6 8 g, vitamin B12 13.5 g, niacin 53 g, pantothenic 26.5 g, folic acid 3.3 g, and biotin 335 g.
Experimental animals
A total of 3000 healthy Pacific white shrimp (Litopenaeus vannamei) postlarvae 9 (PL-9) were obtained from a commercial shrimp hatchery in Chachoengsao Province, Thailand, and were transported to the Aquaculture Business Research Center (ABRC) laboratory at the Faculty of Fisheries, Kasetsart University, Thailand. Following 3 days of acclimation at 29°C in a 500 L fiberglass tank with 25 ppt salinity, a total of 2000 PL-12 shrimp (about 1.5 mg/PL) were randomly distributed into 20 fiberglass tanks (five groups with four replicates/treatment group and 100 shrimp/tank), resulting in approximately 150 shrimp/m2. Throughout the study period, the water temperature was maintained at 26–27°C using an aquarium heater, with a salinity of 25 ppt, pH of 7–8, dissolved oxygen above 6 mg/L, alkalinity above 120 mg/L as CaCO3, total ammonia below 0.3 mg/L, and nitrite below 0.2 mg/L. Water quality parameters were analyzed weekly. Temperature and dissolved oxygen were measured by YSI PRO 20 (YSI Inc./Xylem Inc., Yellow Springs, OH, USA). Salinity was measured by a salinometer. pH was measured by EcoScan pH 5 (Thermo Fisher Scientific Inc.). Alkalinity, total ammonia, and nitrite were analyzed according to American Public Health Association, American Water Works Association, and Water Environment Federation [24]. All water parameters were suitable for Pacific shrimp culture and were presented in Table 2.
Table 2. Water quality parameters throughout the 30 day-feeding trial.
| Water quality parameters | Treatment groups | ||||
|---|---|---|---|---|---|
| Control | IQ-E 200 mg/kg feed | IQ-E 300 mg/kg feed | IQ-WS 100 mg/kg feed | IQ-WS 150 mg/kg feed | |
| Temperature (°C) | 26.80 ± 0.17a | 26.69 ± 0.05a | 26.76 ± 0.06a | 26.78 ± 0.10a | 26.78 ± 0.06a |
| Dissolved oxygen (mg/L) | 6.64 ± 0.01a | 6.66 ± 0.18a | 6.72 ± 0.13a | 6.48 ± 0.09a | 6.57 ± 0.22a |
| pH | 7.65 ± 0.22a | 7.61 ± 0.18a | 7.64 ± 0.13a | 7.64 ± 0.09a | 7.62 ± 0.22a |
| Alkalinity (mg/L as CaCO3) | 143.75 ± 4.86a | 144.50 ± 6.76a | 149.25 ± 4.57a | 147.50 ± 5.00a | 147.50 ± 8.66a |
| Total ammonia (mg/L) | 0.25 ± 0.11a | 0.25 ± 0.07a | 0.23 ± 0.02a | 0.23 ± 0.11a | 0.16 ± 0.05a |
| Nitrite (mg/L) | 0.13 ± 0.01a | 0.14 ± 0.01a | 0.14 ± 0.01a | 0.12 ± 0.01a | 0.13 ± 0.01a |
The data was presented as mean ± SD. Means with different superscripts in a row are significantly different from each other (p < 0.05).
Growth and survival study
The shrimp in each tank were fed ad libitum with one of the five experimental diets four times per day (at 8:00 a.m., 11:00 a.m., 2:00 p.m., and 5:00 p.m.) for 30 days. On days 10, 20, and 30 of the feeding trial, 10 shrimp per tank were randomly selected and weighed. The shrimps were weighted individually using a balance with 2 decimal places. The survival rate in each tank was also determined at the same time.
Immunological study
On day 30, five shrimp from each group were randomly selected for the immunological study. A blood sample of 0.2 mL was withdrawn from the base of the third walking leg of each sample shrimp using a syringe containing 0.6 mL of anticoagulant (K-199 + 5% l-cysteine). The immune parameters evaluated in the current study were total hemocyte count, phagocytic activity, phenoloxidase activity, and superoxide dismutase (SOD) activity.
Total hemocyte count was determined from the blood (100 μL) using a hemocytometer under a light microscope and calculated as total hemocytes/mL of hemolymph.
Phagocytic activity was determined following the procedure reported by Itami et al. [25]. The collected shrimp hemocytes were rinsed with shrimp saline, and the viable cell number was adjusted to 1 × 106 cells/mL. The cell suspension (200 μL) was inoculated into a cover slip. After 20 min, the cell suspension was removed and rinsed three times with shrimp saline. Heat-killed yeast was added before incubation for 2 h. After incubation, the heat-killed yeast was removed, rinsed with shrimp saline five times to attain a concentration of 5 × 108 cells/mL, and fixed with 100% methanol. The cover slip was then stained with Giemsa stain and mounted with Permount Mounting Medium. A total of 200 hemocytes were counted. Phagocytic activity was expressed as the percentage of phagocytic hemocytes among the total hemocytes.
The method of phenoloxidase activity determination was modified from the procedure reported by Supamattaya et al. [26]. The hemolymph-anticoagulant mixture (200 μL) was washed three times with shrimp saline and centrifuged at 1000 rpm, 4°C, for 10 min. The hemocyte lysate (HLS) was prepared from hemocytes in a cacodylate buffer, pH 7.4, using a sonicator at 30 amplitude for 5 s. The suspension was then centrifuged at 10,000 rpm, 4°C for 20 min, and the supernatant was collected. Subsequently, 200 μL of 0.1% trypsin in cacodylate buffer was mixed with 200 mL of HLS, followed by the addition of 200 μL of 4 mg/mL l-dihydroxyphenylalanine (l-DOPA) as the substrate. Enzyme activity was measured as the absorbance of dopachrome at 490 nm. The protein content in the HLS was measured following the method of Lowry et al. [27]. The phenoloxidase activity was calculated as the increase in optimum density/min/mg of protein.
Superoxide dismutase (SOD) activity of the blood (20 μL) was measured using an SOD Assay Kit (Sigma-Aldrich) according to the manufacturer’s instructions.
Experiment 2: Effects of IQs on growth performance, survival, and resistance to V. parahaemolyticus infection of shrimp after immersion challenge
Experimental animals
After the completion of Experiment 1, the surviving shrimp (about 1.3 g) in the four IQ groups were transferred into new 16 fiberglass tanks (four replicates/treatment group and 30 shrimp/tank). Shrimp from the control group in Experiment 1 were randomly divided into two new groups: positive control (challenged with V. parahaemolyticus) and negative control (without V. parahaemolyticus inoculation). Thus, a total of 24 tanks (six groups with four replicates/treatment group) were used in Experiment 2. The animal husbandry and water quality parameters of Experiment 2 were similar to those of Experiment 1 throughout the study period.
Immersion Challenge with Vibrio parahaemolyticus
Vibrio parahaemolyticus (TISTR 1596) used in the current study was isolated from a diseased shrimp with vibriosis from a commercial farm in eastern Thailand. The bacteria were reisolated on tryptic soy agar (TSA) supplemented with 1.5% NaCl and incubated for 24 h at 35°C. The bacterial colonies were transferred into tryptic soy broth (TSB) supplemented with 1.5% NaCl and incubated for another 24 h at 35°C. The bacteria were then centrifuged at 1000 rpm for 15 min. The supernatant was discarded, and the bacterial pellet was resuspended in 1.5% NaCl. The bacterial suspension turbidity was adjusted to an optical density value of 0.02 at 540 nm (A540), equal to 105 CFU/mL. It was then inoculated into the water of all tanks (except for the negative control group) to produce a final concentration of 103 CFU/mL.
Growth and survival study
Three hours after the bacterial challenge, the shrimp in each tank were fed ad libitum with one of the five experimental diets four times/day (at 8:00 a.m., 11:00 a.m., 2:00 p.m., and 5:00 p.m.) as in Experiment 1 for 14 days. The shrimp body weight and the survival rates were determined at the end of the experiment.
Histopathological study
At the end of the experiment, five shrimp in each group were randomly selected and fixed in Davidson’s fixative for 48 h, and then transferred to 70% ethanol until they were processed for the histopathological study according to Bell and Lightner [28].
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test using IBM SPSS Statistics version 27 software (IBM Corporation, Armonk, NY, USA). Differences were considered statistically significant if p < 0.05.
Results
Experiment 1: Effects of IQs on growth performance, survival, and immune response of healthy shrimp
After feeding on the experimental diets for 30 days, the average shrimp body weight across all IQ-fed groups (i.e., IQ-E at 200 and 300 mg/kg of feed, and IQ-WS at 100 and 150 mg/kg of feed) and the control group was about 1.3 g (Table 3). No significant difference in shrimp body weight was observed between the five groups (p > 0.05). However, the average survival rates of all IQ-fed groups (ranging from 90.25–94.50%) were significantly greater (p < 0.05) than those of the control shrimp (83.00%) (Table 4).
Table 3. Effects of IQ extracts on growth performance of the healthy shrimp.
| Treatment group | Body weight (g) | ||
|---|---|---|---|
| Day 10 | Day 20 | Day 30 | |
| Control | 0.32 ± 0.01a | 0.75 ± 0.04a | 1.33 ± 0.02a |
| IQ-E 200 mg/kg feed | 0.34 ± 0.01a | 0.88 ± 0.02a | 1.35 ± 0.01a |
| IQ-E 300 mg/kg feed | 0.34 ± 0.02a | 0.86 ± 0.01a | 1.36 ± 0.02a |
| IQ-WS 100 mg/kg feed | 0.33 ± 0.01a | 0.84 ± 0.09a | 1.34 ± 0.02a |
| IQ-WS 150 mg/kg feed | 0.33 ± 0.03a | 0.88 ± 0.03a | 1.35 ± 0.03a |
The data was presented as mean ± SD. Means with different superscripts in a column are significantly different from each other (p < 0.05).
Table 4. Effects of IQ extracts on survival rate of the healthy shrimp.
| Treatment group | Survival rate (%) | ||
|---|---|---|---|
| Day 10 | Day 20 | Day 30 | |
| Control | 100.00 ± 0.00a | 94.50 ± 1.29a | 83.00 ± 2.94a |
| IQ-E 200 mg/kg feed | 100.00 ± 0.00a | 98.00 ± 1.50a | 94.50 ± 3.32b |
| IQ-E 300 mg/kg feed | 100.00 ± 0.00a | 95.00 ± 1.71a | 93.25 ± 2.06b |
| IQ-WS 100 mg/kg feed | 100.00 ± 0.00a | 97.00 ± 2.38a | 91.00 ± 2.16b |
| IQ-WS 150 mg/kg feed | 100.00 ± 0.00a | 95.00 ± 3.27a | 90.25 ± 1.89b |
The data was presented as mean ± SD. Means with different superscripts in a column are significantly different from each other (p < 0.05).
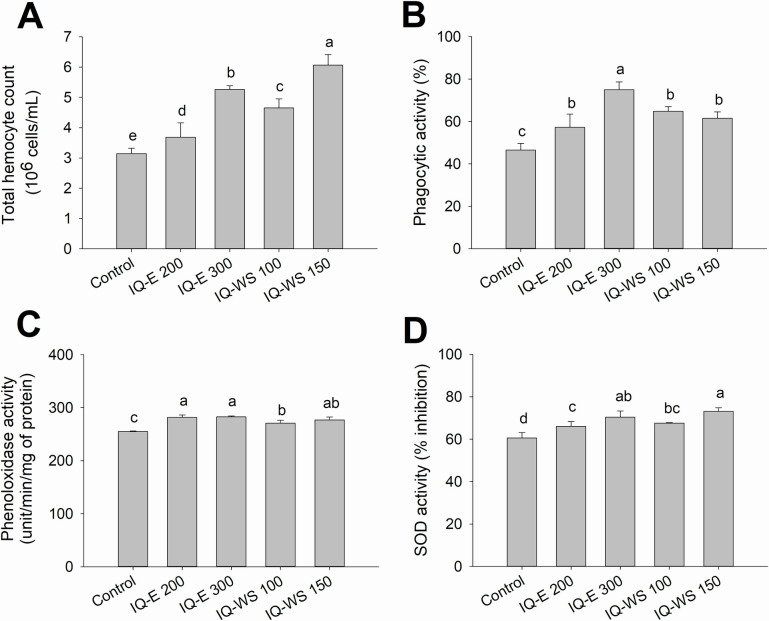
At the end of Experiment 1, all the IQ-supplemented groups showed a significant improvement (p < 0.05) in every immune parameter compared to the control shrimp. The total hemocyte counts of the control, IQ-E 200 and 300 mg/kg, and IQ-WS 100 and 150 mg/kg groups were 3.14, 3.68, 5.26, 4.56, and 4.65 × 106 cells/mL, respectively (Fig 1A). The phagocytic activities were 46.50%, 57.25%, 75.00%, 64.75%, and 61.50%, respectively (Fig 1B). The phenoloxidase activities were 255.09, 281.55, 282.59, 270.74, and 276.82 units/min/mg of protein, respectively (Fig 1C). The superoxide dismutase (SOD) activities were 60.62%, 66.01%, 70.40%, 67.52%, and 73.12%, respectively (Fig 1D).
Fig 1. Effects of IQ extracts on immune parameters of the healthy shrimp.
Total hemocyte count (106 cells/mL) (A), phagocytic activity (%) (B), phenoloxidase activity (unit/min/mg of protein) (C), and superoxide dismutase (SOD) activity (% inhibition) (D) of the shrimp (n = 5) fed the control diet, IQ-E at 200 or 300 mg/kg of feed, and IQ-WS at 100 or 150 mg/kg of feed on day 30. The data are presented as the mean ± standard deviation. Different letters above the bars indicate significant differences (p < 0.05).
Experiment 2: Effects of IQs on growth performance, survival, and resistance to V. parahaemolyticus infection of shrimp after immersion challenge
Following a further 14 days of the same experimental diets with the addition of V. parahaemolyticus immersion challenge at a concentration of 103 CFU/mL, the average body weights of the IQ-E 200 and 300 mg/kg and IQ-WS 100 and 150 mg/kg groups were 1.59, 1.71, 1.61, and 1.67 g (Table 5), respectively, which were significantly higher (p < 0.05) than both the negative control (without V. parahaemolyticus) (1.39 g) and the positive control shrimp (with V. parahaemolyticus) (1.35 g). The survival rates of the IQ-fed groups were 87.50%, 90.00%, 85.00%, and 87.50% (Table 5), which were similar to the negative control shrimp (91.67%) but significantly higher than the positive control (68.33%) (p < 0.05). No significant difference was observed between the four IQ groups and the negative control (p > 0.05).
Table 5. Effects of IQ extracts on growth performance and survival rate of the Vibrio parahaemolyticus-infected shrimp.
| Treatment group | Body weight (g) | Survival rate (%) |
|---|---|---|
| Negative control (without V. parahaemolyticus) | 1.39 ±0 .09b | 91.67 ± 1.92a |
| Positive control (with V. parahaemolyticus) | 1.35 ±. 017b | 68.33 ± 1.92b |
| IQ-E 200 mg/kg feed | 1.59 ± 0.10a | 87.50 ± 3.19a |
| IQ-E 300 mg/kg feed | 1.71 ± 0.12a | 90.00 ± 2.72a |
| IQ-WS 100 mg/kg feed | 1.61 ± 0.04a | 85.00 ± 3.33a |
| IQ-WS 150 mg/kg feed | 1.67 ± 0.10a | 87.50 ± 3.19a |
The data was presented as mean ± SD. Means with different superscripts in a column are significantly different from each other (p < 0.05).
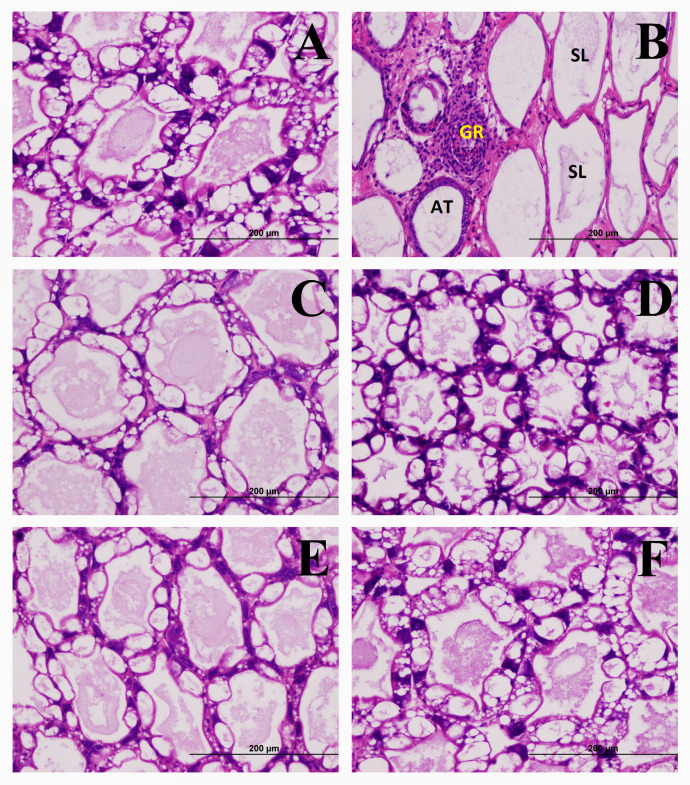
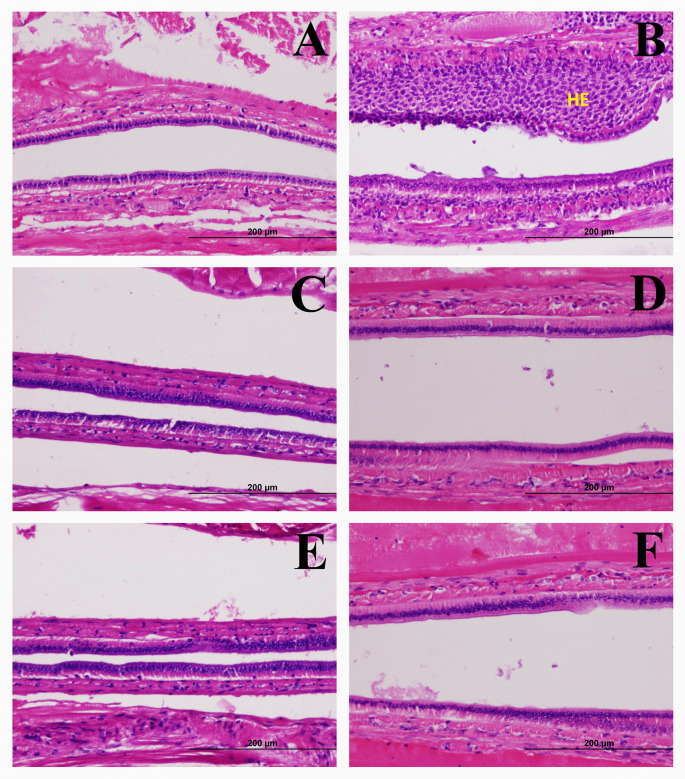
The hepatopancreas and intestine tissues of the negative control shrimp and all IQ groups had a normal appearance and no signs of bacterial infection (Figs 2 and 3). In contrast, the hepatopancreas tissues of the positive control shrimp showed signs of bacterial infection such as atrophy, sloughing of the hepatopancreas tubule epithelial cells, and granulomatous encapsulation. Unsurprisingly, the intestinal tissues of the positive control shrimp, but not of the other groups, showed signs of hemocytic infiltration in the submucosa due to bacterial infection. The finding that no histopathological change was observed in all IQ-fed groups was consistent with the higher survival rates of these groups. Taken together, they indicated better resistance to V. parahaemolyticus infection as a result of IQ extracts.
Fig 2. Effects of IQ extracts on histopathology of the hepatopancreas of the Vibrio parahaemolyticus-infected shrimp.
Hepatopancreas of the shrimp fed the negative control diet (A), positive control diet showing atrophy (AT), sloughing of the hepatopancreas tubule epithelial cells (SL), and granulomatous encapsulation (GR) (B), IQ-E 200 mg/kg of feed diet (C), IQ-E 300 mg/kg of feed diet (D), IQ-WS 100 mg/kg of feed diet (E), and IQ-WS 150 mg/kg of feed diet (F) on day 14 after immersion challenge with Vibrio parahaemolyticus (103 CFU/mL) (H&E stain).
Fig 3. Effects of IQ extracts on histopathology of the intestine of the Vibrio parahaemolyticus-infected shrimp.
Intestine of the shrimp fed the negative control diet (A), positive control diet showing hemocytic infiltration (HE) (B), IQ-E 200 mg/kg of feed diet (C), IQ-E 300 mg/kg of feed diet (D), IQ-WS 100 mg/kg of feed diet (E), and IQ-WS 150 mg/kg of feed diet (F) on day 14 after immersion challenge with Vibrio parahaemolyticus (103 CFU/mL) (H&E stain).
Discussion
The mechanisms of phytochemicals in improving overall animal health have yet to be firmly established, as they most likely involve many complex interactions between the plant compounds and the host factors. Nevertheless, it is arguable that plant secondary metabolites may exert health-improving effects in a similar fashion to antibiotic growth promoters (AGPs), to a certain degree. Currently, the main hypothesis for the growth-promoting effect of AGPs is the antimicrobial hypothesis, which postulates that the effect may be related to the inhibition of subclinical infection even though the concentration of active compounds may be lower than the minimum inhibitory concentration (MIC) of the bacteria [29–31]. In fact, drug concentrations at the sub-MIC level can still affect bacterial growth profile, virulence factor expression, and susceptibility to the host immune system [31]. Possibly the strongest evidence in support of the antimicrobial hypothesis is that AGPs cannot promote the growth of germ-free animals [32]. An alternative explanation is the anti-inflammatory hypothesis, which attributes the growth-promoting effect to the suppression of intestinal inflammation. Niewold [33] argued that the two commonly used groups of AGPs, namely tetracyclines and macrolides, also have significant anti-inflammatory properties. By suppressing the production of proinflammatory cytokines and tissue catabolism, more energy is conserved and available for growth. Given that these two hypotheses are not mutually exclusive, it could be speculated that both antimicrobial and anti-inflammatory mechanisms may be involved in the growth-enhancing properties of AGPs and phytochemicals alike.
The IQ formulations tested in the present study contained 0.5% and 1% sanguinarine, respectively. When incorporated into the pellet feeds, the shrimp diets contained sanguinarine at doses of 1 or 1.5 mg/kg of feed (see Materials and Methods). Since sanguinarine and other alkaloids found in M. cordata have antimicrobial and anti-inflammatory activities [8], it can potentially be used as a growth promoter. In fact, many previous studies have demonstrated the growth-stimulating effect of IQs in a variety of animal species including pigs [14], chickens [15–18], and fishes [19–21]. In line with these studies, our results revealed growth-enhancing effects of the four IQ-supplemented diets. It is worth mentioning that only the V. parahaemolyticus-infected shrimp fed IQs (in Experiment 2) showed a significant improvement in body weight compared to the control group, whereas the healthy shrimp (in Experiment 1) did not. These observations support the antimicrobial and anti-inflammatory hypotheses mentioned above as the main mechanisms for the growth-promoting effect of IQs [29–31].
In contrast to the present study, our previous research did not show the improved growth performance and survival rates of shrimp that were administered IQ-WS at 100 and 200 mg/kg of feed diets [23]. A possible explanation for the previous ineffective outcomes may be related to the different methods of experimental feed preparation. While the preparations were mixed with the feed ingredients prior to pelleting in the present study, the previous experiment employed a top-coating method without adding an oil binder which might result in significant leaching of IQs from the prepared diets, thereby rendering the administered doses of the IQ lower than expected. Therefore, to prevent leaching and obtain the best result, IQ preparations should be incorporated into shrimp feed mash before making the pellet feed instead of utilizing a simple top-dressing method.
Herbal extracts have long been studied as enhancers of the immune functions of aquatic animals with promising results, even though the exact molecular mechanisms are often unknown. The effects of plant immunostimulants usually include stimulation of phagocytosis, as well as increased nonspecific immunity mediators [34–36]. Our result demonstrated the immunostimulatory effect of IQs on total hemocyte count as well as phagocytic, phenoloxidase, and SOD activities of the shrimp hemocytes. Similarly, IQ-supplemented diets have been reported to increase total leukocyte count in red tilapia [19] and common carp [21], increase SOD, lysozyme, and complement activities in koi carp [22], and enhance antibody production in chickens [17] and pigs [14]. These improved immune responses may have partly contributed to the higher survival rates of the IQ-fed shrimp in Experiments 1 and 2.
In addition to enhanced immune function, the higher survival rates of the shrimp could be attributed to the antimicrobial activity of the active compound sanguinarine. Sanguinarine has been found to affect the bacterial cell membrane [37] and interfere bacterial cytokinesis [38]. The MICs of sanguinarine have been reported in the range of 12.5–50 μg/mL against fish pathogenic bacteria (Aeromonas hydrophila, A. salmonicida, Vibrio anguillarum, and V. harveyi) [39], 15.6–62.5 μg/mL against human pathogenic bacteria (Staphylococcus aureus, Escherichia coli, and Streptococcus agalactiae) [39], 3–50 μg/mL against Vibrio spp. (V. cholerae, V. parahaemolyticus, and non-agglutinable Vibrio spp.) [40], and 6.25–50 μg/mL against Helicobacter pylori [12]. Intraperitoneal administration of sanguinarine to A. hydrophila-infected common carp at doses of 10–20 mg/kg body weight resulted in lower tissue bacterial loads and higher survival rates of the carp [41]. In addition to the antibacterial property, sanguinarine and Macleaya extracts also possesses anti-parasitic activity against fish ectoparasites such as Ichthyophthirius multifiliis [42,43], Dactylogyrus intermedius [44], and Gyrodactylus kobayashii [45].
Although the direct evidence of antimicrobial activity of sanguinarine or IQ extracts against shrimp pathogens has yet to be reported so far, it is most likely that sanguinarine or IQ extracts may also exert such effect. Our previous work revealed a significant reduction of the intestinal Vibrio spp. count of the V. harveyi-infected shrimp that were fed IQ-WS at 100 and 200 mg/kg of feed, although the survival rates were not significantly improved [23]. However, the shrimp that were fed IQ-supplemented diets in the present study showed better resistance to V. parahaemolyticus infection, as suggested by the higher survival rates of all IQ-fed groups compared to the V. parahaemolyticus-infected control shrimp. The most likely explanation for the inconsistency between the two studies (in terms of survival rates) is associated with the different methods of experimental feed preparation, as mentioned above. The absence of histopathological lesions in the hepatopancreas and intestinal tissues of the IQ-fed shrimp but not the positive control shrimp also supports with the protective effect of IQs.
The bioavailability of sanguinarine and other IQs from M. cordata in shrimp and other aquatic animals has yet to be studied. However, it was reported that they were very poorly absorbed from the gastrointestinal (GI) tract of the pig [46] and rat [47] following oral administration. This characteristic is desirable for the prevention of digestive tract infection, but may not suitable for systemic infection. Given that the GI tract is one of the major routes of Vibrio spp. infection in the shrimp after immersion challenge [48], it is reasonable to assume that the oral administration of IQ extracts could prevent Vibrio spp. colonization in the shrimp’s GI tract, thereby suppressing the disease outbreak which was the case in the present study.
Conclusions
The two IQ formulations at the two sanguinarine concentrations (1 and 1.5 mg/kg of feed) supplemented into the shrimp diets showed equivalent efficacies in terms of improving growth performance, survival rate, immune response, and resistance to V. parahaemolyticus infection of Pacific white shrimp. Consequently, this natural product can be applied as a feed additive to support overall health condition for sustainable shrimp production.
Supporting information
The body weight (g) (n = 10) (A) and survival rates (%) (n = 4) (B) of the shrimp fed the control diet, powdered isoquinoline alkaloids (IQ-E) at 200 or 300 mg/kg of feed, and water-soluble, granulated isoquinoline alkaloids (IQ-WS) at 100 or 150 mg/kg of feed on days 10, 20, and 30. The data are presented as the mean ± standard deviation. Different letters above the bars indicate significant differences (p < 0.05).
(TIF)
The body weight (g) (n = 10) (A) and survival rates (%) (n = 4) (B) of the shrimp fed the control diet, IQ-E at 200 or 300 mg/kg of feed, and IQ-WS at 100 or 150 mg/kg of feed on day 14 after immersion challenge with Vibrio parahaemolyticus (103 colony-forming units (CFU)/mL). The data are presented as the mean ± standard deviation. Different letters above the bars indicate significant differences each other (p < 0.05).
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This paper was funded by grants from the Aquaculture Business Research Center (ABRC11082018), Kasetsart University, Thailand, to support graduate student PB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Phytobiotics (Thailand) Co., Ltd. provided support in the form of salary to WK. The specific roles of this author are articulated in the 'author contributions' section. Phytobiotics also provided material support in the form of the isoquinoline extracts for the study, but had no other role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hashemi SR, Davoodi H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet Res Commun. 2011;35(3):169–80. 10.1007/s11259-010-9458-2 [DOI] [PubMed] [Google Scholar]
- 2.Encarnação P. Functional feed additives in aquaculture feeds. In: Nates SF, editor. Aquafeed formulation. Amsterdam: Academic Press; 2016, pp. 217–237. [Google Scholar]
- 3.Valenzuela-Grijalva NV, Pinelli-Saavedra A, Muhlia-Almazan A, Domínguez-Díaz D, González-Ríos H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J Anim Sci Technol. 2017;59:8. 10.1186/s40781-017-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang KC. The pharmacology of Chinese herbs. 2nd ed. Boca Raton: CRC Press; 1999. [Google Scholar]
- 5.Yang X, Chen A, Ma Y, Gao Y, Gao Z, Fu B, et al. Encyclopedic reference of traditional Chinese medicine. Heidelberg: Springer-Verlag Berlin Heidelberg; 2003. [Google Scholar]
- 6.Zhou J, Xie G, Yan X. Encyclopedia of traditional Chinese medicines: Molecular structures, pharmacological activities, natural sources and applications. Vol. 5: Isolated compounds T-Z, references, TCM plants and congeners. Heidelberg: Springer-Verlag Berlin Heidelberg; 2011. [Google Scholar]
- 7.Kosina P, Gregorova J, Gruz J, Vacek J, Kolar M, Vogel M, et al. Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia. 2010;81(8):1006–12. 10.1016/j.fitote.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 8.Lin L, Liu YC, Huang JL, Liu XB, Qing ZX, Zeng JG, et al. Medicinal plants of the genus Macleaya (Macleaya cordata, Macleaya microcarpa): A review of their phytochemistry, pharmacology, and toxicology. Phytother Res. 2018;32(1):19–48. 10.1002/ptr.5952 [DOI] [PubMed] [Google Scholar]
- 9.Franz C, Bauer R, Carle R, Tedesco D, Tubaro A, Zitterl‐Eglseer K. Study on the assessment of plants/herbs, plant/herb extracts and their naturally or synthetically produced components as “additives” for use in animal production CFT/EFSA/FEEDAP/2005/01. EFSA Supporting Publications. 2007;4(4):070828. [Google Scholar]
- 10.Pěnčíková K, Kollár P, Závalová VM, Táborská E, Urbanová J, Hošek J. Investigation of sanguinarine and chelerythrine effects on LPS-induced inflammatory gene expression in THP-1 cell line. Phytomedicine. 2012;19(10):890–5. 10.1016/j.phymed.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 11.Hošek J, Šebrlová K, Kaucká P, Peš O, Táborská E. The capability of minor quaternary benzophenanthridine alkaloids to inhibit TNF-α secretion and cyclooxygenase activity. Acta Vet Brno. 2017;86(3):223–30. [Google Scholar]
- 12.Mahady GB, Pendland SL, Stoia A, Chadwick LR. In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phytother Res. 2003;17(3):217–21. 10.1002/ptr.1108 [DOI] [PubMed] [Google Scholar]
- 13.Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J Ethnopharmacol. 2002;79(1):57–67. 10.1016/s0378-8741(01)00350-6 [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Kang B, Yao K, Fu C, Zhao Y. Effects of dietary Macleaya cordata extract on growth performance, immune responses, antioxidant capacity, and intestinal development in weaned piglets. J Appl Anim Res. 2019;47(1):349–56. [Google Scholar]
- 15.Vieira SL, Berres J, Reis RN, Oyarzabal OA, Coneglian JLB, Freitas DM, et al. Studies with sanguinarine like alkaloids as feed additive in broiler diets. Rev Bras Cienc Avic 2008a;10(1):67–71. [Google Scholar]
- 16.Vieira SL, Oyarzabal OA, Freitas DM, Berres J, Pena JEM, Torres CA, et al. Performance of broilers fed diets supplemented with sanguinarine-like alkaloids and organic acids. J Appl Poult Res. 2008. b;17(1):128–33. [Google Scholar]
- 17.Karimi M, Foroudi F, Abedini MR. Effect of Sangrovit on performance and morphology of small intestine and immune response of broilers. Biosci Biotechnol Res Asia. 2014;11(2):855–61. [Google Scholar]
- 18.Lee K-W, Kim J-S, Oh S-T, Kang C-W, An B-K. Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J Poult Sci. 2015;52(1):15–22. [Google Scholar]
- 19.Rawling MD, Merrifield DL, Davies SJ. Preliminary assessment of dietary supplementation of Sangrovit® on red tilapia (Oreochromis niloticus) growth performance and health. Aquaculture. 2009;294(1–2):118–22. [Google Scholar]
- 20.Imanpoor MR, Roohi Z. Effects of Sangrovit‐supplemented diet on growth performance, blood biochemical parameters, survival and stress resistance to salinity in the Caspian roach (Rutilus rutilus) fry. Aquacult Res. 2016;47(9):2874–80. [Google Scholar]
- 21.Abdelnaby E, Mohamed MF, Gammaz HA-K. Pharmacological studies of feed additives (Sanguinarine and Saccharomyces cerevisiae) on growth performance, haematological and intestinal bacterial count with challenge test by Aeromonas hydrophila in Cyprinus carpio. Global Animal Science Journal. 2013;1(1):19–29. [Google Scholar]
- 22.Zhang R, Wang X-W, Zhu J-Y, Liu L-L, Liu Y-C, Zhu H. Dietary sanguinarine affected immune response, digestive enzyme activity and intestinal microbiota of Koi carp (Cryprinus carpiod). Aquaculture. 2019;502:72–9. [Google Scholar]
- 23.Rairat T, Chuchird N, Limsuwan C. Effect of Sangrovit WS on growth, survival and prevention of Vibrio harveyi in rearing of Pacific white shrimp (Litopenaeus vannamei). KU Fish Res Bull. 2013;37(1):19–29. [Google Scholar]
- 24.APHA, AWWA, WEF. Standard Methods for the Examination of Water and Wastewater. 23rd: American Public Health Association, American Water Works Association, and Water Environment Federation, Washington, DC; 2017. [Google Scholar]
- 25.Itami T, Takahashi Y, Tsuchihira E, Igusa H, Kondo M. Enhancement of disease resistance of kuruma prawn Penaeus japonicus and increase in phagocytic activity of prawn hemocytes after oral administration of β-1,3-glucan (Schizophyllan). Paper presented at: The Third Asian Fisheries Forum; 1992 Oct 26–30; Manila, Philippines.
- 26.Supamattaya K, Pongmaneerat J, Klowklieng T. Immunostimulant and vaccination in black tiger shrimp, Penaeus monodon Fabricius: III The effect of β-glucan (MacroGard®) on growth performance, immune response and disease resistance in black tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J Sci Technol. 2000;22:677–88. [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 28.Bell TA, Lightner DV. A handbook of normal penaeid shrimp histology: World Aquaculture Society, Baton Rouge, Louisiana; 1988. [Google Scholar]
- 29.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: History and mode of action. Poult Sci. 2005;84(4):634–43. 10.1093/ps/84.4.634 [DOI] [PubMed] [Google Scholar]
- 30.Modi CM, Mody SK, Patel HB, Dudhatra GB, Kumar A, Sheikh TJ. Growth promoting use of antimicrobial agents in animals. J Pharm Sci. 2011;1(08):33–6. [Google Scholar]
- 31.Broom LJ. The sub-inhibitory theory for antibiotic growth promoters. Poult Sci. 2017;96(9):3104–8. 10.3382/ps/pex114 [DOI] [PubMed] [Google Scholar]
- 32.Coates ME, Fuller R, Harrison GF, Lev M, Suffolk SF. A comparision of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. Br J Nutr. 1963;17(1):141–50. [DOI] [PubMed] [Google Scholar]
- 33.Niewold TA. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult Sci. 2007;86(4):605–9. 10.1093/ps/86.4.605 [DOI] [PubMed] [Google Scholar]
- 34.Harikrishnan R, Balasundaram C, Heo M-S. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture. 2011;317(1–4):1–15. [Google Scholar]
- 35.Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture. 2014;433:50–61. [Google Scholar]
- 36.Wang W, Sun J, Liu C, Xue Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquacult Res. 2017;48(1):1–23. [Google Scholar]
- 37.Obiang-Obounou BW, Kang O-H, Choi J-G, Keum J-H, Kim S-B, Mun S-H, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36(3):277–83. 10.2131/jts.36.277 [DOI] [PubMed] [Google Scholar]
- 38.Beuria TK, Santra MK, Panda D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry. 2005;44(50):16584–93. 10.1021/bi050767+ [DOI] [PubMed] [Google Scholar]
- 39.Kang YJ, Yi Yl, Zhang C, Wu SQ, Shi CB, Wang GX. Bioassay‐guided isolation and identification of active compounds from Macleaya microcarpa (Maxim) Fedde against fish pathogenic bacteria. Aquacult Res. 2013;44(8):1221–8. [Google Scholar]
- 40.Nandi R, Maiti M, Chaudhuri K, Mahato SB, Bairagi AK. Sensitivity of vibrios to sanguinarine. Experientia. 1983;39(5):524–5. 10.1007/BF01965187 [DOI] [PubMed] [Google Scholar]
- 41.Ling F, Wu ZQ, Jiang C, Liu L, Wang GX. Antibacterial efficacy and pharmacokinetic evaluation of sanguinarine in common carp (Cyprinus carpio) following a single intraperitoneal administration. J Fish Dis. 2016;39(8):993–1000. 10.1111/jfd.12433 [DOI] [PubMed] [Google Scholar]
- 42.Yao J-Y, Shen J-Y, Li X-L, Xu Y, Hao G-J, Pan X-Y, et al. Effect of sanguinarine from the leaves of Macleaya cordata against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idella). Parasitol Res. 2010;107(5):1035–42. 10.1007/s00436-010-1966-z [DOI] [PubMed] [Google Scholar]
- 43.Yao J-Y, Zhou Z-M, Li X-L, Yin W-L, Ru H-S, Pan X-Y, et al. Antiparasitic efficacy of dihydrosanguinarine and dihydrochelerythrine from Macleaya microcarpa against Ichthyophthirius multifiliis in richadsin (Squaliobarbus curriculus). Vet Parasitol. 2011;183(1–2):8–13. 10.1016/j.vetpar.2011.07.021 [DOI] [PubMed] [Google Scholar]
- 44.Wang G-X, Zhou Z, Jiang D-X, Han J, Wang J-F, Zhao L-W, et al. In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet Parasitol. 2010;171(3–4):305–13. 10.1016/j.vetpar.2010.03.032 [DOI] [PubMed] [Google Scholar]
- 45.Zhou S, Li WX, Wang YQ, Zou H, Wu SG, Wang GT. Anthelmintic efficacies of three common disinfectants and extracts of four traditional Chinese medicinal plants against Gyrodactylus kobayashii (Monogenea) in goldfish (Carassius auratus). Aquaculture. 2017;466:72–7. [Google Scholar]
- 46.Kosina P, Walterova D, Ulrichová J, Lichnovský V, Stiborová M, Rýdlová H, et al. Sanguinarine and chelerythrine: assessment of safety on pigs in ninety days feeding experiment. Food Chem Toxicol. 2004;42(1):85–91. 10.1016/j.fct.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 47.Psotova J, Vecera R, Zdarilova A, Anzenbacherova E, Kosina P, Svobodova A, et al. Safety assessment of sanguiritrin, alkaloid fraction of Macleaya cordata, in rats. Vet Med. 2006;51(4):145. [Google Scholar]
- 48.Aguirre‐Guzmán G, Sánchez‐Martínez JG, Pérez‐Castañeda R, Palacios‐Monzón A, Trujillo‐Rodríguez T, De La Cruz‐Hernández NI. Pathogenicity and infection route of Vibrio parahaemolyticus in American white shrimp, Litopenaeus vannamei. J World Aquacult Soc. 2010;41(3):464–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The body weight (g) (n = 10) (A) and survival rates (%) (n = 4) (B) of the shrimp fed the control diet, powdered isoquinoline alkaloids (IQ-E) at 200 or 300 mg/kg of feed, and water-soluble, granulated isoquinoline alkaloids (IQ-WS) at 100 or 150 mg/kg of feed on days 10, 20, and 30. The data are presented as the mean ± standard deviation. Different letters above the bars indicate significant differences (p < 0.05).
(TIF)
The body weight (g) (n = 10) (A) and survival rates (%) (n = 4) (B) of the shrimp fed the control diet, IQ-E at 200 or 300 mg/kg of feed, and IQ-WS at 100 or 150 mg/kg of feed on day 14 after immersion challenge with Vibrio parahaemolyticus (103 colony-forming units (CFU)/mL). The data are presented as the mean ± standard deviation. Different letters above the bars indicate significant differences each other (p < 0.05).
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.