Abstract
Objectives
The FDA is considering the implementation of a national nicotine reduction policy for cigarettes, and such a policy may reduce the reinforcing efficacy of cigarettes and ultimately reduce tobacco dependence. However, it is not yet known how different levels of nicotine may affect the reinforcing efficacy of cigarettes in adolescents. We aimed to determine how reduced nicotine content may affect adolescents’ demand for cigarettes using the cigarette purchase task (CPT).
Methods
Adolescent daily smokers (ages 15–19, n = 50) completed a CPT for their usual brand cigarette and for each dose of SPECTRUM research cigarettes (15.8, 5.2, 1.3, 0.4 mg nicotine/g tobacco) during four laboratory sessions. We conducted repeated measures ANOVAs to evaluate the effect of nicotine dose on five demand indices derived from the CPT.
Results
Tests revealed significantly higher demand for usual brand than each research cigarette dose (all p’s < .01); dose did not significantly affect any measure when usual brand was excluded.
Conclusions
These results demonstrate the potential utility of the CPT for comparing the reinforcing efficacy of cigarettes varying in nicotine content in adolescents, and indicate a significantly reduced reinforcing efficacy of all research cigarettes relative to usual brand.
Keywords: adolescent, smoking, regulatory science, nicotine
INTRODUCTION
The passage of the Family Smoking Prevention and Tobacco Control Act (FSPTCA) gave the Food and Drug Administration (FDA) the authority to regulate the manufacture, distribution and marketing of tobacco products1. Recently, the FDA has announced its intention to move forward with a plan to reduce the nicotine content in cigarettes to decrease the addictiveness of these products and eventually lessen the public health toll of tobacco related disease2. Research studies investigating the potential impact of a mandated nicotine reduction policy on smoking, nicotine dependence and health have been reported3,4, with others currently underway. Of particular importance to the FDA is to investigate the implications of this potential widespread tobacco policy on those most vulnerable to tobacco use. As approximately 90% of cigarette smokers try their first cigarette before age 18, and each day in the U.S. over 1,000 adolescents become daily cigarette smokers, adolescents represent a population of concern to the FDA5.
Nicotine is the primary constituent in cigarettes responsible for addiction6. In 1994 it was proposed that mandating a reduction in the nicotine content of cigarettes below a reinforcing threshold that sustains addiction may reduce nicotine dependence and rates of addiction on a population scale7. The intent of the policy is to shift behavior away from combustible cigarette smoking and toward quitting or to the use of reduced harm products such as nicotine replacement therapies. However, the extent to which cigarette nicotine reduction may reduce the reinforcing efficacy of cigarettes in adolescents is unknown. Like adult smokers, adolescent smokers experience significant negative effects of smoking such as respiratory symptoms and withdrawal symptoms and craving during abstinence8–10. However, despite their shorter smoking histories, adolescents tend to be less responsive than adults to empirically-supported cessation interventions11. Furthermore, adolescent smoking is highly related to peer influence, marketing, and other non-nicotine factors that may influence cigarette reinforcing efficacy12,13; therefore, adolescents may not respond in the same way to reduced-nicotine content cigarettes as adults. Thus, studies assessing the reinforcing efficacy of reduced-nicotine content cigarettes are vital to understanding how adolescents may respond to a mandated nicotine reduction policy in order to make informed policy decisions. Furthermore, clinical trials using reduced nicotine cigarettes have provided these cigarettes to study participants free of charge; potentially introducing another variable that may influence smoking behavior. As adolescents are highly sensitive to changes in cigarette cost14, it is important to understand how their sensitivity to cost may be affected by nicotine reduction.
The concept of reinforcing efficacy is central to behavioral economics, a theoretical framework which applies consumer demand theory and economic concepts to understanding behavior15. A common behavioral economics task used to assess the reinforcing efficacy of cigarettes is the Cigarette Purchase Task (CPT16,17). The CPT is a questionnaire assessing cigarette demand in a hypothetical scenario, wherein participants are asked: “How many cigarettes would you smoke in a day if they cost $X?” From the resultant data, a demand curve can be constructed that relates price to consumption. Five demand indices can be derived from the data to provide an indication of how the cigarettes differ in reinforcing efficacy. The CPT has been validated in adolescent smokers with concordance between measures of demand and smoking rate, smoking biomarkers, and nicotine dependence18,19. The CPT provides an expedited and cost efficient method for measuring reinforcing efficacy of reduced and very low nicotine content (~0.4 mg nicotine /g tobacco, VLNC) cigarettes20.
A few recent studies have used the CPT to compare the relationships between the nicotine content of cigarettes, cigarette price, and cigarette demand in adults21,22. One large multi-site clinical trial randomly assigned adults to receive their usual brand cigarettes or a research cigarette with one of 5 nicotine contents (15.8, 5.2, 2.4, 1.3, or 0.4 mg nicotine/g tobacco)3,22. Following 6 weeks of exposure to their assigned study cigarette, participants completed the CPT. Responses to the purchase task correlated with actual smoking behavior during the study, and cigarette demand indices derived from the CPT showed that cigarette demand decreased systematically in response to the decreased nicotine content of the cigarettes. The second study, also a large multi-site trial, investigated the acute effects of cigarettes varying in nicotine content among several vulnerable adult populations (smokers with opioid use disorders, affective disorders, and socioeconomically-disadvantaged women)21. Participants completed the CPT after sampling four cigarettes varying in nicotine content (15.8, 5.2, 2.4, and 0.4 mg). Again, demand indices from the CPT varied dose-dependently and indicated a lower reinforcing efficacy for each of the lower-nicotine content cigarettes (5.2, 2.4, and 0.4 mg) relative to the normal nicotine content cigarette (15.8 mg/g) for three main indices from the CPT (Intensity, Break point and Maximum Output, described in further detail below).
To our knowledge, only one study has assessed the effect of VLNC cigarettes on withdrawal and subjective response in adolescents, and no studies have yet used the CPT to assess the relative reinforcing effects of reduced nicotine content cigarettes in adolescents. In the primary study from which these data came23, adolescent smokers smoked one cigarette of varying nicotine doses after overnight abstinence. While all doses of research cigarettes significantly reduced withdrawal and negative affect, there was not a significant effect across dose for these outcomes; however, there was a significant effect of dose such that higher nicotine content research cigarettes reduced craving and increased positive subjective evaluations to a greater extent than reduced nicotine content cigarettes. These data are generally consistent with findings from adults; however, reinforcing efficacy can provide a more robust measure of abuse liability than subjective evaluations as it measured demand across a series of increasing prices or constraints20. Thus, the current study aimed to test the relative reinforcing efficacy of nicotine in cigarettes following acute exposure in adolescents who had abstained from smoking overnight.
METHODS
Participants
50 adolescent (15–19 years old, inclusive) current smokers were recruited from the community and local high schools. Following a phone screen which queried cigarette use, alcohol and drug use, and quit intentions, participants who met initial eligibility criteria were invited to an in-person screening session. For participants under 18, parents were contacted via phone and asked to give verbal consent. Parents were then mailed a paper consent form which they were required to sign, and their child was required to bring with them to the session in order to participate. Minor participants also gave written assent. All procedures were approved by Brown University’s Institutional Review Board.
Inclusion Criteria
In addition to self-reporting daily smoking (≥ 28 of the last 30 days) and having been smoking daily for at least the past 6 months, participants had to submit a breath carbon monoxide (CO) sample measured using a Smokerlyzer ED50 meter (Bedfont Instruments) of > 6 ppm at their screening session. If they did not, they were required to submit a urine sample, which was tested for cotinine using NicAlert strips. A test result of 3 or higher (approximately equivalent to a Cotinine Concentration of 100–200 ng/mL) was required to verify smoking status. In addition, participants could not be suicidal, pregnant or breastfeeding, using alcohol or other drugs daily (except marijuana), or planning to quit smoking for good within the next month.
Experimental Procedures
At the baseline session, adolescents were screened in person for eligibility and also completed baseline measures. Following this session, if eligible, adolescents were given instructions about their next four sessions. They were instructed to abstain from smoking starting at 10 pm the night before their scheduled session and informed that their breath CO would be tested to determine any recent smoking. CO values had to be ≤6 ppm or a 50% decrease from their baseline CO measure, whichever was higher. Adolescents were also asked to sign an affidavit stating that they had not used tobacco since the night before. During the session, adolescents smoked a single research cigarette in the laboratory using a CReSS pocket device (CReSS Pocket, Borgwaldt KC, Richmond VA). Shortly after smoking the cigarette, adolescents answered questions about the cigarette they had just smoked. Doses of research cigarettes were presented in counterbalanced order across participants, and both participant and researchers were blind to the dose presented. Participants were paid $25 at screening, regardless of eligibility, and $35 for each experimental session completed; completing all four sessions resulted in a $100 bonus payment to incentivize study completion.
Products Tested
SPECTRUM brand research cigarettes were provided by the National Institute on Drug Abuse and produced by 22nd Century Group, Inc. The four doses tested in the current study had 15.8, 5.2, 1.3 and 0.4 mg/g of tobacco, and all tar yields were 9 ± 1.5 mg. 15.8 mg/g is roughly equivalent to nicotine content in a commercial cigarette. While not tested in the current study, we also collected data on participants’ usual brand cigarettes. These cigarettes, based on available FTC data, are generally above or in the range of the normal nicotine content cigarettes (15.8 mg/g condition).
Measures
Modified Fagerström Tolerance Questionnaire (mFTQ)
Adapted from the Fagerström Tolerance Questionnaire, this is a measure of nicotine dependence and has been validated for use in adolescents24,25.
Cigarette Purchase Task (CPT) - Usual Brand
In this questionnaire, adolescents were asked to imagine that the available cigarettes were their usual brand and they had no access to other sources of their usual brand cigarettes, and they were not to save or stockpile cigarettes for later. Given these instructions, they were to report how many of their usual brand cigarettes they would purchase in a 24-hour period at a series of increasing prices ($0.00, $0.02, $0.05, $0.10, $0.20, $0.30, $0.40, $0.50, $0.60, $0.70, $0.80, $0.90, $1.00, $2.00, $3.00, $4.00, $5.00). This task has been validated for use in adolescents17,19. This CPT was administered at baseline.
Cigarette Purchase Task (CPT) - Study Cigarettes
The questionnaire was identical to the Cigarette Purchase Task - Usual Brand, with the exception that in the instructional set participants were asked to imagine that the available cigarettes were their “study cigarette from today”22. These CPTs were administered in each study session immediately after smoking the research cigarette.
Data Analysis Plan
Cigarette Purchase Task Demand Indices
Several demand indices can be derived from the data that reflect multiple dimensions of the relative reinforcing efficacy of the drug to the individual across changes in response cost: Intensity, or the amount of cigarettes participants report they would consume when they are free; Breakpoint, the first price at which demand was suppressed to 0; Omax, the maximum amount that participants would spend on cigarettes across all prices; Pmax, the price at which Omax is reported and α, a measure of the ‘sensitivity of behavior to changes in price,’ which is derived from the following equation26:
where Q represents cigarettes consumed and C is cigarette price, k is set to a constant of 3 in the current analyses), and Q0 is the estimate of cigarette consumption at zero price.. The rate parameter alpha represents the rate of change in elasticity of demand across the demand function27 and is inversely related to reinforcing efficacy, such that lower alpha values indicate greater reinforcing efficacy. Thus, in the current analyses, all indices were empirically derived from observed data with the exception of alpha.
Prior to implementing statistical procedures, purchase task data were examined for orderliness according to the methods laid out by Stein et al. (2015). One participant’s data in dose 2 met criteria for an intensity value greater than 3 standard deviations above the mean; this value was recoded to 3 standard deviations above the mean. No data were excluded for inconsistent responding. Several participants’ data were excluded from analyses using the Exponentiated Demand Equation due to null demand; these data are explained further under the Null Demand section). For participants who did not suppress their responding to 0 (i.e., did not reach breakpoint), the breakpoint value was recoded as the highest tested price ($5).
Correlations
Correlations between demand indices and participant characteristics were examined to determine if relationships existed between reinforcing efficacy of usual brand cigarettes and gender, age, and cigarette dependence in this sample.
Dose Effects on Intensity, Breakpoint, Omax and Pmax
The effects of nicotine dose were examined using repeated measures ANOVAs in SPSS version 24 for Windows (IBM). We tested models including all four doses of research cigarettes and usual brand, as well as all four research doses without usual brand, in order to determine the effects of nicotine dose relative to usual brand and to determine any effects across dose within research cigarettes.
Comparison of α values across dose
The α values for each average curve at each dose were compared using an extra sum-of-squares F-test (Prism version 6 for Windows, GraphPad Software, San Diego California USA). This procedure tests the null hypothesis that one alpha parameter best fits the data; rejecting the null hypothesis indicates that the α parameter is not shared across data sets, indicating significantly different α values across dose.
Null Demand Analysis
Several of the participants noted that they would not purchase any study cigarettes, even if they were free. Though their data were included in the empirically derived parameters, fitted demand curves could be not be derived from these individuals’ data and they are therefore not reflected in the alpha value analysis. However, a lack of demand for these cigarettes is an important facet of abuse liability that we wished to capture. Thus, we expressed these data as the number (count) of participants who expressed null demand at price 0 as a function of nicotine dose.
RESULTS
Participant Characteristics
The average age of the participants was 17.7 (SD=1.0), and 56% were menthol smokers. There were equal numbers of females and males. Of the participants, 47.2% were White, 19.4% were Black, 13.9% were Asian and 14% reported Hispanic ethnicity. Participants smoked an average of 8.2 (SD=4.5) cigarettes per day and their average CO at baseline was 11.2 ppm (SD=7.2). The average dependence score was 4.2 (SD=1.5) indicating moderate dependence.
Correlations
Bivariate correlations are reported in Table 1. All demand indices with the exception of Pmax were significantly positively associated with dependence. No outcomes were significantly associated with age or gender. All demand indices were also significantly correlated with each other, with the exception of Pmax and intensity.
Table 1.
Correlations between baseline variables and usual brand demand indices derived from the CPT (N=50).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Gender | 1 | 0 | −.08 | .13 | .89 | .03 | .11 | −.04 |
| 2. Age | 1 | −.18 | −.03 | −.17 | −.20 | −.11 | .11 | |
| 3. Baseline Dependence (mFTQ score) | 1 | .39** | .28** | .12 | .40** | −.50** | ||
| 4. Intensity (Usual Brand) | 1 | .40** | .17 | .47** | −.49** | |||
| 5 Break Point (Usual Brand) | 1 | .81** | .57** | −.54** | ||||
| 6. Pmax (Usual Brand) | 1 | .67** | −.41** | |||||
| 7. Omax (Usual Brand) | 1 | −.50** | ||||||
| 8. Alpha (Usual Brand) | 1 |
Dose Effects on Intensity, Break point, Omax and Pmax
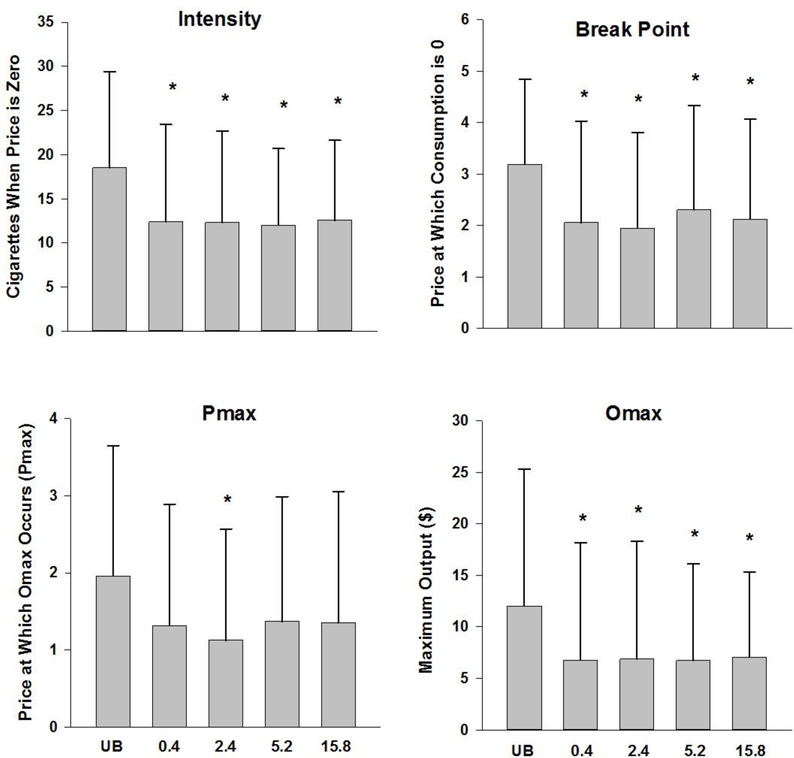
The raw values for Intensity, breakpoint, Omax, and Pmax across dose are shown in Figure 1. All demand indices (intensity, breakpoint, Omax, Pmax) were checked for normality; the distribution for Omax was non-normal and logarithmically transformed prior to running ANOVAs. All other variables were normal. When entering 5 doses (usual brand and four research cigarette doses), there was a main effect of cigarette type on intensity (F(2.915, 142.813)=9.62, p<.0001; Greenhouse-Geisser correction); Break point (F(2.694, 131.999)=11.743, p<.0001; Greenhouse-Geisser correction), log Omax (F(2.928, 143.489)=6.209, p<.001; Greenhouse-Geisser correction); and Pmax (F(3.03, 148.489)=4.801, p<.003; Greenhouse-Geisser correction). For intensity, break point, and Omax, post hoc comparisons indicated that usual brand was significantly different from all Spectrum doses (all p’s <.10), while no Spectrum doses were significantly different from one another. For Pmax, the only significant post hoc comparison was usual brand versus the 2.4 mg/g Spectrum dose. When usual brand was excluded from the analyses, dose was not significant for any outcome.
Figure 1.
Mean and standard deviation of intensity, breakpoint, Omax, and Pmax across dose.
Comparison of α values across dose
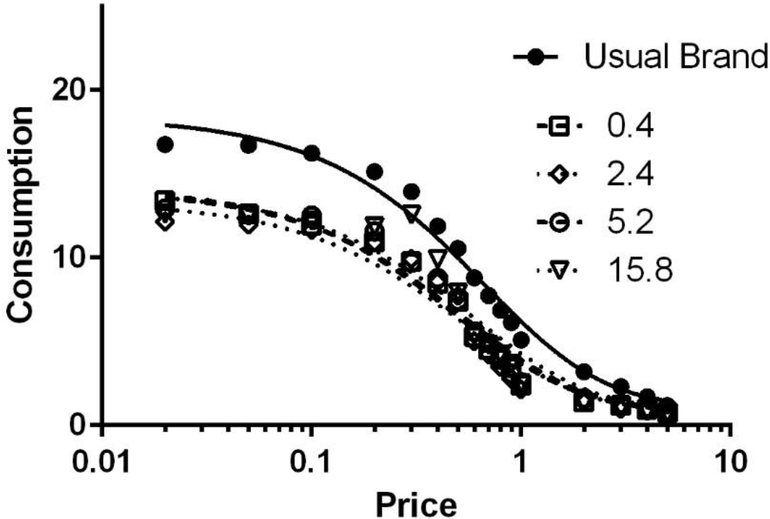
The global fits of Equation 1 to the data from each dose are shown in Figure 2. The average R2 values were greater than 0.85 across all doses (Usual brand, M=.88; 0.4 mg dose, M=.87; 1.2 mg dose, M=.88; 5.2 mg dose, M=.89; 15.8 mg, M=.86), indicating very good fit to the data. An extra sum-of-squares F Test indicated that α was not shared across cigarette type (F(4,4002)=11.90, p<.0001). In other words, consistent with the data from the other demand indices, one alpha value did not describe the data when including the usual brand condition.
Figure 2.
Mean fitted curves of demand for cigarettes across dose.
Null Demand Analysis
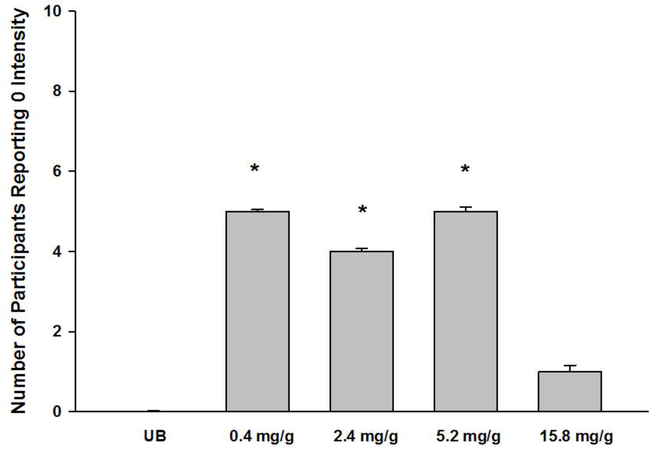
Figure 3 shows the count of participants who expressed zero demand for their usual brand cigarettes when they were free. A nonparametric test, the related-samples Cochran’s Q test, indicated that after re-coding the data sets for ‘zero’ or non-zero the distributions for the five doses tested were significantly different from each other (p=.016). Specifically, the distribution of participants who reported that they would not “buy” any study cigarettes if they were free of charge was significantly higher in the 0.4–5.2 mg/g Spectrum conditions (4–5 participants) relative to the usual brand condition (0 participants), and was significantly greater for the 0.4 mg/g and 5.2 mg/g dose relative to the 15.8 mg/g dose.
Figure 3.
Percent of participants reporting that they would smoke zero cigarettes if they were free at each dose. Asterisks denote a significant difference relative to usual brand.
DISCUSSION
As a nicotine reduction policy gains momentum, understanding how such a policy would reduce the reinforcing efficacy of cigarettes in vulnerable populations is an important goal. Furthermore, understanding how cigarette price may interact with cigarette nicotine reduction to affect smoking in adolescents is essential. To that end, we conducted the first, to our knowledge, laboratory examination of the effect of various nicotine doses on cigarette demand in adolescent smokers. This analysis complements and extends our previous report on the effects of reduced-nicotine cigarettes on cigarette craving, withdrawal symptoms, negative affect, and cigarette evaluations in this sample23. Our analysis showed that relative to usual brand, the reinforcing efficacy of all doses of research cigarettes was reduced; however, when compared to each other, no dose of research cigarettes differed significantly from each other. We did find that participants were more likely to report null demand (i.e, reported that they would not consume any cigarettes at any price) at the 0.4, 2.4, and 5.2 mg/g reduced nicotine doses, but not at the 15.8 mg/g research cigarette dose. The null demand results comport with data from the same study, which demonstrated that participants showed decreased subjective satisfaction from the research cigarettes as a function of dose.23 Intuitively, the lack of demand for any cigarettes, even when they are free, suggests that the null demand analysis may track more closely to subjective evaluation or ‘disliking’.
The current data differ from similar studies with adults. In contrast to the current data which did not show a significant difference across doses, Higgins et al.21 found a graded effect of nicotine dose on indices from the CPT in a similar acute model of exposure in adults. However, as noted above, in this same sample of adolescents we found a graded effect of dose on craving reduction and subjective responses23. This suggests that dose did influence some responses and underscores the idea that participants were able to discriminate nicotine dose. It is interesting to note that while our study did not show a dose-response effect, adolescent smokers had lower levels of demand for the SPECTRUM research cigarettes overall. For example, in adults, the reported intensity for the 15.8 mg/g cigarettes was around 18 cigarettes, while for adolescent participants it was 12.5 cigarettes. In contrast, intensity for usual brand cigarettes was similar to what is seen in adult smokers of similar cigarettes per day (e.g., around 19 cigarettes, see 28), suggesting that adolescents may be particularly sensitive to branding as all research cigarettes were of an unfamiliar brand regardless of dose. Similarly, while adults in the Higgins et al. (2017) trial described above gave 15.8 mg/g cigarette a smoking satisfaction score of 4.6 on a 1–5 scale, and VLNC cigarettes a 3.2, on average; adolescents in the current study reported an average smoking satisfaction score of just 3.2 for the 15.8 mg/g cigarette, and an average of 2.5 for the VLNC cigarette23. These results suggest that adolescents are relatively less subjectively satisfied by, and in turn do not show as strong demand for, research cigarettes when compared to adults.
While the extent to which these results may change after longer-term exposure is currently not known, some insights can be gleaned from data from a large trial in which adults were exposed to 6 weeks of research cigarettes at different doses. In that study, young adults (age 18–24) in the VLNC group (0.4 mg nicotine/g tobacco) showed less subjective reinforcement from these research cigarettes relative to older smokers (ages 25+) after two weeks of use29. Emerging from these results is an overall picture that points to reduced reinforcing efficacy for research cigarettes in younger smokers relative to older adults, which suggests that young people may be even more sensitive to the effects of nicotine reduction on cigarette reinforcement than older smokers. However, an important caveat remains that these data are all based on studies using an unfamiliar brand of research cigarettes; and given the apparent sensitivity to brand-switching in this population, it is difficult to determine if these results would be same with low nicotine cigarettes that retained the branding elements of participants’ usual brand cigarettes. In the current study, participants were exposed to each cigarette dose once in a blinded presentation. This may account for the lack of dose-response signal among the main demand indices; though such exposure did result in greater null demand at lower doses.
This study must be understood in the context of several limitations. The first is that while this study provides important information regarding the acute response to these cigarettes, it is not possible in the context of this study to determine how changes in reinforcing efficacy may impact quitting or switching to new products. In adults, Smith et al.22 found that not only was the reinforcing efficacy of VLNC cigarettes lower relative to higher dose cigarettes, extended exposure to VLNC cigarettes also reduced participants’ demand for their usual brand of cigarettes, indicating a potential mechanism by which such a policy may facilitate eventual abstinence from cigarettes. Second, due to ethical constraints related to administering cigarettes in the lab, our study population included only adolescent daily smokers. Many adolescents are nondaily smokers30, and thus our study may not generalize to these youth. At the same time, young daily smokers are at the highest risk of continuing to smoke as adults and are therefore a population of interest for regulatory effort, and these data also provide an important direct comparison with adult daily smokers. Third, participants completed the CPT for study cigarettes following only a single, blinded exposure following overnight abstinence. The usual brand CPT analyzed here was administered at baseline when participants were not in withdrawal. However, at each session, following the study CPT administration participants were also administered their usual brand CPT; the indices from the session CPTs did not differ from baseline, mitigating this concern. Finally, though extremely well-validated with real-world behavior, the CPT is a self-report measure of reinforcing efficacy, and it is likely that a behavioral choice task would delineate different aspects of dose differences in reinforcing efficacy. Future research should triangulate these results with behavioral self-administration tasks in adolescent tobacco users.
IMPLICATIONS FOR TOBACCO REGULATION
These results demonstrate the potential utility of the CPT for comparing the reinforcing efficacy of cigarettes varying in nicotine content in adolescents, and indicate a significantly reduced reinforcing efficacy of all research cigarettes relative to usual brand. This study, along with others, suggests overall that nicotine reduction would be an effective approach to reducing smoking reinforcement in adolescent daily smokers. As a comprehensive nicotine reduction plan moves forward, it is important to continue to specifically model the potential effects of this policy on adolescents and young adults. Indeed, recent models have suggested that if FDA enacts the policy by 2020, then by 2060 there will be a cumulative reduction in new smokers of ~16 million31. Such a policy has the potential to transform the future health of young people by reducing reinforcement from combustible cigarette use, and to reduce the public health toll of combustible cigarette use.
Acknowledgments
The study was funded by NCI grant 1K01CA189300 (RNC). Support for the preparation of this paper was also provided by NIDA grants U54DA031659 (JWT, RD-A and SMC) and P50DA036114 (JWT). Research reported in this publication was supported by NCI and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Research cigarettes were supplied by NIDA.
Footnotes
Conflict of Interest Disclosure Statement
The authors have no conflicts of interest to report.
Human Subjects Approval Statement
All procedures were approved by the Brown University Institutional Review Board.
References
- 1.Congress. Family Smoking Prevention and Tobacco Control Act (H.R. 1256). U.S.G.P.O., Washington; 2009. [Google Scholar]
- 2.Gottlieb S, Zeller M. A Nicotine-Focused Framework for Public Health. N Engl J Med. 2017;377(12). doi: 10.1056/NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- 3.Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15(6):1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.USDHHS. Preventing Tobacco Use Among Youth and Young Adults. 2016. https://www.surgeongeneral.gov/library/reports/preventing-youth-tobacco-use/factsheet.html. Accessed August 29, 2017.
- 6.USDHHS. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Rockville, MD: Public Health Service, Office on Public Health; 2014. [Google Scholar]
- 7.Benowitz NL, Henningfield JE. Establishing a Nicotine Threshold for Addiction -- The Implications for Tobacco Regulation. N Engl J Med. 1994;331(2):123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 8.Bidwell LC, Leventhal AM, Tidey JW, Brazil L, Niaura RS, Colby SM. Effects of abstinence in adolescent tobacco smokers: withdrawal symptoms, urge, affect, and cue reactivity. Nicotine Tob Res. 2013;15(2):457–464. doi: 10.1093/ntr/nts155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy RN, Roberts ME, Colby SM. Validation of a Respiratory Symptom Questionnaire in Adolescent Smokers. Tob Regul Sci. 2015;1(2):121–128. doi: 10.18001/TRS.1.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colby SM, Leventhal AM, Brazil L, et al. Smoking abstinence and reinstatement effects in adolescent cigarette smokers. Nicotine Tob Res. 2010;12(1):19–28. doi: 10.1093/ntr/ntp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colby SM, Gwaltney CJ. Pharmacotherapy for adolescent smoking cessation. JAMA. 2007;298(18):2182–2184. http://www.ncbi.nlm.nih.gov/pubmed/18027441. Accessed December 1, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman BR, Sussman S, Unger JB, Valente TW. Peer Influences on Adolescent Cigarette Smoking: A Theoretical Review of the Literature. Subst Use Misuse. 2006;41(1):103–155. doi: 10.1080/10826080500368892. [DOI] [PubMed] [Google Scholar]
- 13.Lovato C, Watts A, Stead LF. Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours. Cochrane Database Syst Rev. 2011;(10):CD003439. doi: 10.1002/14651858.CD003439.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavazos-Rehg PA, Krauss MJ, Sowles SJ, et al. Multiple Levels of Influence That Impact Youth Tobacco Use. Tob Regul Sci. 2016;2(2):106–122. doi: 10.18001/TRS.2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hursh SR. Behavioral economics. J Exp Anal Behav. 1984;42(3):435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999;7(4):412–426. http://www.ncbi.nlm.nih.gov/pubmed/10609976. Accessed June 26, 2015. [DOI] [PubMed] [Google Scholar]
- 17.MacKillop J, Murphy JG, Ray LA, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol. 2008;16(1):57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Bidwell LC, MacKillop J, Murphy JG, Tidey JW, Colby SM. Latent factor structure of a behavioral economic cigarette demand curve in adolescent smokers. Addict Behav. 2012;37(11):1257–1263. doi: 10.1016/j.addbeh.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 2011;113(2–3):207–214. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tidey JW, Cassidy RN, Miller ME, Smith TT. Behavioral Economic Laboratory Research in Tobacco Regulatory Science. Tob Regul Sci. 2016;2(4):440–451. doi: 10.18001/TRS.2.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins ST, Heil SH, Sigmon SC, et al. Addiction Potential of Cigarettes With Reduced Nicotine Content in Populations With Psychiatric Disorders and Other Vulnerabilities to Tobacco Addiction. JAMA Psychiatry. 2017;74(10):1056. doi: 10.1001/jamapsychiatry.2017.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith TTTT, Cassidy RNRN, Tidey JWJW, et al. Impact of smoking reduced nicotine content cigarettes on sensitivity to cigarette price: further results from a multi-site clinical trial. Addiction. 2017;112(2):349–359. doi: 10.1111/add.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassidy RN, Colby SM, Tidey JW, Jackson KJ, Cioe P, Krishnan-Sarin S, & Hatsukami D Adolescents’ Response to Nicotine Dose in Cigarettes: Evidence from a Within-Subjects Laboratory Study.
- 24.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. http://www.ncbi.nlm.nih.gov/pubmed/1932883. Accessed March 25, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Prokhorov A V, Pallonen UE, Fava JL, Ding L, Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav. 1996;21(1):117–127. http://www.ncbi.nlm.nih.gov/pubmed/8729713. Accessed December 1, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Koffarnus MN, Franck CT, Stein JS, Bickel WK. A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol. 2015;23(6):504–512. doi: 10.1037/pha0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- 28.Zhao T, Luo X, Chu H, Le CT, Epstein LH, Thomas JL. A two-part mixed effects model for cigarette purchase task data. J Exp Anal Behav. 2016;106(3):242–253. doi: 10.1002/jeab.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassidy RN, Tidey JW, Cao Q, Colby SM, McClernon FJ, Koopmeiners JS, Hatsukami D & Age D Moderates Smokers’ Subjective Response to Very Low Nicotine Content Cigarettes: Evidence from a Randomized Controlled Trial. 2018. [DOI] [PMC free article] [PubMed]
- 30.Rubinstein ML, Rait MA, Sen S, Shiffman S. Characteristics of adolescent intermittent and daily smokers. Addict Behav. 2014;39(9):1337–1341. doi: 10.1016/j.addbeh.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apelberg BJ, Feirman SP, Salazar E, et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med. March 2018:NEJMsr1714617. doi: 10.1056/NEJMsr1714617. [DOI] [PubMed] [Google Scholar]