Abstract
Rationale and Objective:
GFR estimation based on creatinine and cystatin C (eGFRcr-cys) is more accurate than eGFR based on either creatinine or cystatin C alone (eGFRcr or eGFRcys), but the inclusion of creatinine in eGFRcr-cys requires specification of a person’s race. Beta-2-microglobulin (B2M) and beta-trace protein (BTP) are alternative filtration markers that appear to be less influenced by race than creatinine.
Study Design:
Study of diagnostic test accuracy.
Setting and Participants:
Development in pooled population of seven studies with 5017 participants with and without chronic kidney disease. External validation in a pooled population of seven other studies with 2245 participants.
Tests compared:
Panel eGFR using B2M and BTP in addition to cystatin C (three-marker panel) or creatinine and cystatin C (four-marker panel) with and without age and sex or race.
Outcomes:
GFR measured as the urinary clearance of iothalamate, plasma clearance of iohexol, or plasma clearance of Cr-EDTA
Results:
Mean measured GFR was 58.1 and 83.2 ml/min/1.73m2 and the proportion of blacks was 38.6% and 24.0%, in the development and validation populations, respectively. In development, addition of age and sex improved the performance of all equations compared to equations without age and sex, but addition of race did not further improve the performance. In validation, the four-marker panels were more accurate than the three-marker panels (p<0.001). The three-marker panel without race was more accurate than eGFRcys [1- P30 of 15.6 vs 17.4% (p=0.014)], and the four-marker panel without race was as accurate as eGFRcr-cys [1- P30 of 8.6 vs 9.4% (p=0.17)]. Results were generally consistent across subgroups.
Limitations:
No representation of participants with severe comorbid illness and from geographic areas outside of North America and Europe.
Conclusions:
The four-marker panel eGFR is as accurate as eGFRcr-cys, without requiring specification of race. A more accurate race-free eGFR could be an important advance.
Keywords: Glomerular filtration rate, creatinine, cystatin C, beta trace protein, beta-2-microglobulin, estimating equations, race
Lay summary
Assessment of glomerular filtration rate (GFR) is critical for many aspects of medical practice. GFR estimation based on creatinine and cystatin C together (eGFRcr-cys) is more accurate than eGFR based on either creatinine or cystatin C alone, but the inclusion of creatinine in eGFRcr-cys requires specification of a person’s race. Beta-2-microglobulin (B2M) and beta-trace protein (BTP) are alternative filtration markers that appear to be less influenced by race than creatinine. In a pooled dataset of 7 studies (5017 participants), we developed new estimating equations based on the combinations of these markers, with and without age or sex and race. In a separate pooled dataset of 7 studies (2245 participants) we showed that the equation that used all four markers, age and sex, but not race, was as accurate as eGFRcr-cys. A more accurate race-free eGFR could be an important advance.
Introduction
Clinical assessment of kidney function is part of routine medical care for adults1. GFR estimates incorporate clinical and demographic factors (age, sex and race) that explain some of the variation of markers unrelated to GFR, and are more accurate and useful than serum concentrations of endogenous filtration markers alone in each demographic group. Most clinical laboratories report estimated glomerular filtration rate (eGFR) when serum creatinine is measured (eGFRcr)2. eGFR based on cystatin C (eGFRcys) or the combination of creatinine and cystatin C (eGFRcr-cys) are recommended as confirmatory tests for eGFRcr3, however, there are limitations of this approach. eGFRcys is not more accurate than eGFRcr, and although eGFRcr-cys is more accurate than either eGFRcr or eGFRcys, it is not independent of eGFRcr. Further, in some populations, neither marker provides accurate estimates because the demographic and clinical factors do not accurately account for the non-GFR determinants4,5.
There is increased scrutiny around use of race in GFR estimation, including current attention by the United States Congress to algorithms that include race 6–9. The use of Black race in the 2009 CKD-EPI creatinine equation leads to a 16% higher eGFRcr for the same level of creatinine compared to other people,10 which could worsen care for Blacks because of delayed referral for specialist care, dialysis and transplantation, and may represent an example of race-based medicine6,7. Conversely, omission of the Black race coefficient leads to lower eGFRcr compared to measured GFR and could worsen care because of contraindications to life-saving drugs and contrast-imaging procedures11,12. Thus, accurate GFR estimates matter in Black people; there is an urgent need to have more accurate GFR estimating equations that do not require a coefficient for race6,7,12,13.
A panel of endogenous filtration markers could improve the accuracy of GFR estimation by reducing the impact of the non-GFR determinants of each marker and by obviating the need for clinical and demographic factors, and in particular race14. Like cystatin C, beta-2-microglobulin (B2M) and beta-trace protein (BTP) are low molecular weight proteins that are filtered by the glomeruli and degraded by the tubules. 15,16 Like cystatin C, they have been shown to be useful in estimating GFR, are less influenced by age, sex and race than creatinine, and are more strongly associated with death and cardiovascular disease compared to creatinine or eGFRcr17–25. We previously reported that a four-marker panel eGFR including creatinine, cystatin C, B2M and BTP was not more accurate than eGFRcr-cys in a combined population of 3 US cohorts with CKD, but the panel was more accurate than eGFRcr-cys in two Chinese cohorts including participants with and without CKD where eGFRcr was less accurate than in the US cohorts26,27. We hypothesized that the advantage of a panel eGFR would be more apparent in diverse populations with and without CKD. The current analysis aimed to evaluate whether including B2M and BTP in a panel eGFR would enable performance comparable to or better than currently recommended equations without the need for creatinine or race.
Methods
Data Sources
Collaborators provided data from research studies and clinical populations (Tables S2a and S2b)10,26,28–46. GFR was measured using urinary or plasma clearance of exogenous filtration markers (Table S1). We allocated the datasets into development vs external validation such that each dataset representation of CKD and non-CKD studies and sufficient representation of Black people. We included 7 studies with a total of 5017 participants in the Development Population. We randomly divided this dataset into separate datasets for initial development (n=3,363) and internal validation (n=1,654) (Table S2a, Figure S1). We included 7 additional studies with a total of 2,245 participants in the External Validation Population (Table S2b). We calibrated all methods to urinary clearance of iothalamate (the reference method used for development of the reference equations10,47) by reducing the assigned value of other methods by 5%, based on a systematic comparison of all methods (Table S2a)48. The institutional review boards of all participating institutions approved each study or the current analysis. For GFR measurements done for research studies, informed consent was obtained by the participating studies at the time of the measurements.
Laboratory Methods
Table S1 describes the analytical methods used for all endogenous filtration markers. We calibrated serum creatinine assays or measured serum creatinine on the Roche enzymatic method (Roche-Hitachi P-Module instrument with Roche Creatininase Plus assay, Hoffman-La Roche, Ltd., Basel, Switzerland), traceable to National Institute Standardized Technology (NIST) creatinine standard reference material 96749. We calibrated serum cystatin C assays or measured serum cystatin C on the Siemens Dade Behring Nephelometer (Table S1), traceable to International Federation for Clinical Chemists (IFCC) Working Group for the Standardization of Serum Cystatin C and the Institute for Reference Materials and Measurements (IRMM) certified reference materials50,51. We measured B2M on the Siemens Prospec from 2011–2013, the Roche Mod P from 2013–2015, the Roche COBAS from 2015 to 2019. We measured BTP on the Siemens ProSpec from 2013 to 2019. Stability of the assays over time was evaluated using pooled QC material and calibration panels52.
Development and Validation of Equations
Our a priori hypothesis is that additional endogenous filtration markers can contribute to greater accuracy of GFR estimates because of diminished contribution from non GFR determinants of each marker, potentially eliminating the need for both creatinine and race coefficient. As such, we developed new equations using both B2M and BTP rather than either alone; with creatinine (hereafter referred to as four marker panels) and without creatinine (hereafter referred to as three marker panels); and tested with and without a race coefficient. We selected the 2009 CKD-EPI creatinine equation, 2012 CKD-EPI cystatin C equation and 2012 CKD-EPI creatinine cystatin C equation as reference equations since they are recommended by current guidelines3,10,47. Since all new and reference equations were developed by the CKD-EPI research group, we refer to reference equations only by the filtration marker and publication year.
As in previous work, we pre-specified a process for developing and validating equations26,47. In brief, we used least squares linear regression to relate log transformed measured GFR to log of the filtration markers, with or without age and sex or race communities. For each marker, we used nonparametric smoothing splines to characterize the shape of the relationship of log measured GFR with log filtration marker, and then approximated the smoothing splines by piecewise linear splines to represent observed non-linearity. We used the spline for creatinine and cystatin C that we had previously developed10,47. For comparison of the magnitude of the race coefficient across markers, we developed equations for each marker alone with and without use of age and sex or race.
In the initial development dataset we compared the new equations to the reference equations fit to this population (eGFRcys for three-marker panels, and eGFRcr-cys for four-marker panels. Equations that demonstrated improved performance, defined by 3% relative lower RMSE compared to the reference equation were brought into internal validation for verification of the statistical significance of demographic factors. Development and internal validation datasets were combined into one population (called the “Development Population” hereafter) to derive final coefficients.
In the external validation population (hereafter called the “Validation Population”), we compared the new equations to each other and the reference equations. For comparison of the magnitude of the coefficients for the filtration markers, we derived standardized coefficients by re-expressing the equations subtracting each participant’s value from the mean and dividing by the standard deviation, performed separately for each spline term. We compared performance of equations in the overall population and in subgroups, and final equations were selected based on ranking of RMSE overall and within subgroups and clinically significant differences.
Metrics for Equation Performance
We assessed bias as the median of the difference between measured and estimated GFR, and precision as the inter-quartile range (IQR) for the differences10,53. We assessed accuracy as root mean square error (RMSE) and as the percentage of estimates greater than 30% different from measured GFR (1- P30 respectively). Confidence intervals were calculated by bootstrap methods (2000 bootstraps)54. We focus our assessment of the significance of the differences among the new equations and the reference equations for accuracy (1-P30 using McNemar’s test and RMSE using the signed rank test) rather than bias, which may be more affected by differences in measurement methods and by regression to the mean. Accuracy metrics incorporate both bias and precision, and 1-P30 specifically reflects large errors, which are clinically relevant. We also assessed performance in subgroup of race communities (Black people vs others), eGFR (<30, 30-<60, 60-<89, and >90 ml/min/1.73 m2), age (< 40, 40–65 and > 65 years), sex, body mass index (BMI) (<20, 20-<25, 25-<30, and ≥30 kg/m2) and presence or absence of diabetes. Race was ascertained by the investigators or study participants at the time of data collection in each study.
Results
Clinical Characteristics
In the development population, mean (standard deviation) measured GFR was 58.1 (29.7) mL/min/1.73m2 (range 3.0 to 186.0 mL/min/1.73m2) (Table 1). The mean (standard deviation, range) age was 55.7 (15.9, 18–92) years, 43.8% female, and 38.6% were Black people. In the validation population, mean (standard deviation) measured GFR was 83.2 (27.4) mL/min/1.73m2 (range 8.0 to 184.0 mL/min/1.73m2), the mean (SD, range) age 52.8 (12.8, 18–91) years old and 29% were female. Black people were in 5 of the 7 development cohorts (> 5% in 3 of the 7 cohorts and 39% overall) and in all of validation cohorts (> 5% in 5 of the 7 cohorts and 24% overall) (Table 1). Clinical characteristics of the participants in each study are shown in Table S2.
Table 1.
Participant Characteristics in Study Populations
| Development (N=5017) N (%) or Mean (SD) | External Validation (N=2245) N (%) or Mean (SD) | |
|---|---|---|
| Age, years | 55.7 (15.9) | 52.8 (12.8) |
| <40 | 893 (17.8) | 331 (14.7) |
| 40–65 | 2689 (53.6) | 1570 (69.9) |
| >65 | 1435 (28.6) | 344 (15.3) |
| Female | 2198 (43.8) | 652 (29.0) |
| Black people | 1934 (38.6) | 539 (24.0) |
| BMI, kg/m2 | 29.0 (6.1) | 27.5 (5.4) |
| <20 | 131 (2.6) | 82 (3.7) |
| 20-<25 | 1212 (24.2) | 692 (30.9) |
| 25-<30 | 1870 (37.3) | 878 (39.2) |
| ≥30 | 1804 (36.0) | 588 (26.3) |
| Diabetes | 1296 (27.4) | 731 (34.7) |
| Measured GFR, ml/min/1.73m2 | 58.1 (29.7) | 83.2 (27.4) |
| <30 | 858 (17.1) | 52 (2.3) |
| 30-<60 | 2091 (41.7) | 414 (18.4) |
| 60-<90 | 1387 (27.7) | 846 (37.7) |
| ≥90 | 681 (13.6) | 933 (41.6) |
| Creatinine, mg/dL | 1.6 (0.9) | 1.1 (0.5) |
| Cystatin C, mg/L | 1.5 (0.6) | 1.2 (0.5) |
| B2M, mg/L | 3.8 (2.3) | 2.6 (1.5) |
| BTP, mg/L | 1.2 (0.8) | 0.8 (0.4) |
Development includes initial development and internal Validation (Figure S1). B2M, Beta 2-Microglobulin; BTP, Beta-Trace Protein.
Note: Values for categorical variables are given as number (percentage), for continuous variables, mean (standard deviation)
Development
As expected, all filtration markers were correlated negatively with measured GFR and positively with each other for cystatin C and B2M (Table S3). After adjusting for measured GFR, the correlations among filtration markers ranged from 0.508 (95% confidence intervals [CI] 0.487, 0.528) for creatinine and BTP to 0.774 (95% CI 0.763, 0.785) for cystatin C and B2M (Table S3).
We identified a spline for BTP, with a knot at 0.6 mg/L. In single-marker equations, race coefficients deviated further from 1.0 for equations with creatinine and BTP [1.160 (95% CI: 1.146, 1.174) and 0.861 (95% CI 0.848, 0.874, respectively] compared to those for cystatin C and B2M [0.991 (95% CI 0.979, 1.003) and 0.974 (95% CI 0.960, 0.987), respectively] (Table S4). The coefficient for race in the four-marker panel was significantly smaller than for the eGFRcr-cys [1.052 (95% CI: 1.040, 1.064) vs. 1.08 (95% CI 1.067, 1.093)].
In the overall population, regardless of the inclusion or exclusion of age and sex or race, four-marker panels were more accurate than the corresponding three-marker panels (Table S5). Addition of age and sex improved the performance of the three-marker and four-marker panels compared to panels without age and sex, but the addition of race did not further improve performance (Table S5). Results were generally similar in subgroups of people from Black vs other communities.
External Validation
Table 2 shows the equations for the three-marker and four-marker panels we are recommending (See Table S6 for additional formulas which might be of interest in research studies including equations which used either of the two novel markers). Variables in the three-marker panel include cystatin C, B2M, BTP, age and sex. Variables in the four-marker panel include creatinine, cystatin C, B2M, BTP, age and sex. Standardized coefficients for creatinine were less negative (weaker) for the four-marker panel compared to the eGFRcr and eGFRcr-cys [−0.208 (95% CI −0.219,−0.196) vs −0.558 (95% CI −0.558,−0.565) and −0.282 (95% CI −0.296, −0.268), respectively]. The new equations had less bias compared to 2015 B2M and BTP equations developed in CKD populations (Table S7).
Table 2 :
Variables and Coefficients in 2020 Equations in Development and Internal Validation Population
| Sex | Serum Creatinine mg/dl | Serum Cystatin C mg/L | Serum BTP mg/L | Equation for Estimating GFR |
|---|---|---|---|---|
| 2020 Cystatin C-B2M-BTP Equation | ||||
| Female | ≤0.8 | ≤0.6 | 110 × (Scys/0.8) −0.876 × B2M−0.205 × (SBTP/0.6) 0.038 × 0.999age | |
| >0.6 | 110 × (Scys/0.8) −0.876 × B2M−0.205 × (SBTP/0.6) −0.243 × 0.999age | |||
| >0.8 | ≤0.6 | 110 × (Scys/0.8) −0.697 × B2M−0.205 × (SBTP/0.6) 0.038 × 0.999age | ||
| >0.6 | 110 × (Scys/0.8) −0.697 × B2M−0.205 × (SBTP/0.6) −0.243 × 0.999age | |||
| Male | ≤0.8 | ≤0.6 | 120 × (Scys/0.8) −0.876 × B2M−0.205 × (SBTP/0.6) 0.038 × 0.999age | |
| >0.6 | 120 × (Scys/0.8) −0.876 × B2M−0.205 × (SBTP/0.6) −0.243 × 0.999age | |||
| >0.8 | ≤0.6 | 120 × (Scys/0.8) −0.697 × B2M−0.205 × (SBTP/0.6) 0.038 × 0.999age | ||
| >0.6 | 120 × (Scys/0.8) −0.697 × B2M−0.205 × (SBTP/0.6) −0.243 × 0.999age | |||
| 2020 Creatinine-Cystatin C-B2M-BTP Equation | ||||
| Female | ≤0.7 | ≤0.8 | ≤0.6 | 123 × (Scr/0.7) −0.243 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age |
| >0.6 | 123 × (Scr/0.7) −0.243 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.177 x0.996age | |||
| >0.8 | ≤0.6 | 123 × (Scr/0.7) −0.243 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age | ||
| >0.6 | 123 × (Scr/0.7) −0.243 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.177 × 0.996age | |||
| Female | >0.7 | ≤0.8 | ≤0.6 | 123 × (Scr/0.7) −0.471 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age |
| >0.6 | 123 × (Scr/0.7) −0.471 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.177 × 0.996age | |||
| >0.8 | ≤0.6 | 123 × (Scr/0.7) −0.471 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age | ||
| >0.6 | 123 × (Scr/0.7) −0.471 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.177 × 0.996age | |||
| Male | ≤0.9 | ≤0.8 | ≤0.6 | 131 × (Scr/0.9) −0.295 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age |
| >0.6 | 131 × (Scr/0.9) −0.295 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.177 × 0.996age | |||
| >0.8 | ≤0.6 | 131 × (Scr/0.9) −0.295 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age | ||
| >0.6 | 131 × (Scr/0.9) −0.295 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.177 × 0.996age | |||
| Male | >0.9 | ≤0.8 | ≤0.6 | 131 × (Scr/0.9) −0.471 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age |
| >0.6 | 131 × (Scr/0.9) −0.471 × (Scys/0.8) −0.519 × B2M−0.103 × (SBTP/0.6) −0.177 × 0.996age | |||
| >0.8 | ≤0.6 | 131 × (Scr/0.9) −0.471 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.004 × 0.996age | ||
| >0.6 | 131 × (Scr/0.9) −0.471 × (Scys/0.8) −0.423 × B2M−0.103 × (SBTP/0.6) −0.177 × 0.996age | |||
2020 Cystatin C- B2M-BPT equation can be expressed as a single equation: 120 X min(Scys/0.8,1) −0.876 X max(Scys/0.8,1) −0.697 X B2M−0.205 X min(SBTP/0.6,1) 0.038 X max(SBTP/0.6,1) −0.243 X 0.999age [X 0.922 if female], where Scys is serum cystatin C, B2M, Beta2-Microglobulin; BTP, Beta-Trace Protein
2020 Creatinine-Cystatin C-B2M-BTP Equation can be expressed as a single equation 131 X min(Scr/k,1)α X max(Scr/k,1) −0.471 X min(Scys/0.8,1) −0.519 X max(Scys/0.8,1) −0.423 X B2M−0.103 X min(SBTP/0.6,1) −0.004 X max(SBTP/0.6,1) −0.177 X 0.996age [X 0.937 if female] where Scr is serum creatinine Scys is serum cystatin C, SB2M is serum B2M, SBTP is serum BTP; B2M, Beta 2-Microglobulin; BTP, Beta-Trace Protein, k is 0.7 for females and 0.9 males, α is -0.243for females and -0.295for males, min indicates the minimum of Scr/k or 1, max indicates the maximum of Scr/k or 1
All equations were developed by the CKD-EPI research group.
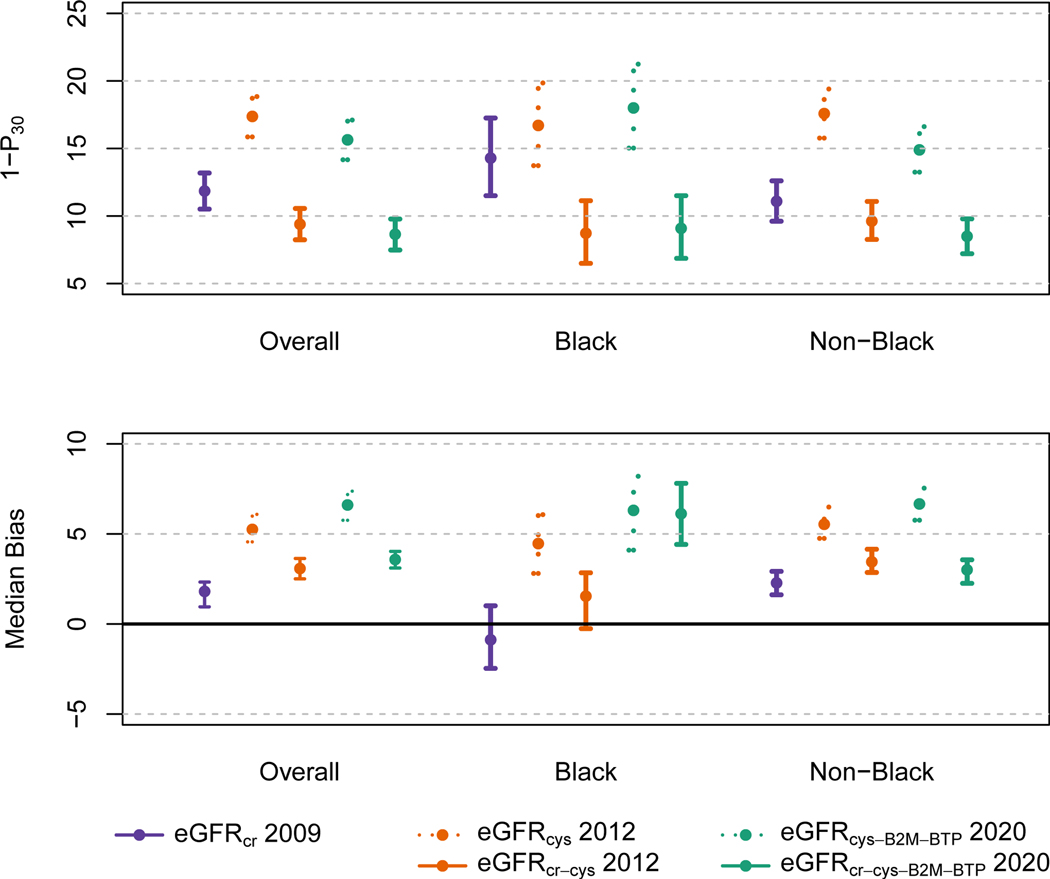
eGFRcr-cys (equation 5) was more accurate than both eGFRcr (equation 1) and eGFRcys (equation 2) (Tables 3 and S8). eGFRcr were more accurate than the eGFRcys equation and the four-marker panels (equations 6 and 7) were more accurate than the three-marker panels (equations 3 and 4) (p<0.001)]. The three-marker panel without race (equation 4) was more accurate than eGFRcys (equation 2) [1-P30 of 15.6 vs 17.4 (p=0.014)]. The four-marker panel without race (equation 7) was as accurate as eGFRcr-cys (equation 5) [1- P30 of 8.6 vs 9.4% (p=0.17)]. The addition of race to the three-marker (equation 3) and four-marker panel (equation 6) led to small further improvements in accuracy (1-P30 of 14.8 and 8.4%, respectively), and the four-marker panel with race (equation 6) was nominally significantly more accurate than eGFRcr-cys (equation 5) (p=0.048). Comparisons of RMSE were generally consistent. Results were generally consistent across subgroups in Black vs. other peoples (Figure 1 and Table S8), and across subgroups of eGFR, age, sex, diabetes and BMI (Figures S2 –S6). Results using non-calibrated measured GFR were generally more accurate than with calibrated measured GFR. Using non-calibrated measured GFR, the 4-marker panel without race was more accurate than eGFRcr-cys (Table S9).
Table 3:
Performance of Estimating Equations in the External Validation Dataset
| Equation Description |
Performance |
||||
|---|---|---|---|---|---|
| Group | Equation Number Year of Publication | Filtration markers | Demographics | Overall Population |
|
| 1-P30 | RMSE | ||||
| Equation with Creatinine only | 1. Ref (2009)10 | Creatinine | Age, sex, race | 11.8 (10.5, 13.2) | 0.199 (0.193, 0.206) |
| Equations with Cystatin C | 2. Ref (2012)47 | Cystatin C | Age, sex | 17.4 1 (15.9, 18.88) | 0.262 1 (0.250, 0.274) |
| 3. New (2020) | Cystatin C-B2M-BTP | Age, sex, race | 14.8 2 (13.4, 16.2) | 0.256 2 (0.243, 0.268) | |
| 4. New/Recommended (2020) | Cystatin C-B2M-BTP | Age, sex | 15.6 2 (14.2, 17.1) | 0.259 2 (0.247, 0.271) | |
| Equations with Creatinine and Cystatin C | 5. Ref (2012)47 | Creatinine -Cystatin C | Age, sex, race | 9.4 1 (8.2, 10.6) | 0.199 1 (0.191, 0.206) |
| 6. New (2020) | Creatinine - Cystatin C-B2M-BTP | Age, sex, race | 8.4 5, 3 (7.3, 9.5) | 0.195 5, 3 (0.187, 0.203) | |
| 7. New/Recommended (2020) | Creatinine -Cystatin C-B2M-BTP | Age, sex | 8.6 5,4 (7.5, 9.8) | 0.197 5, 4 (0.188, 0.205) | |
1-P30 and RMSE are measures of accuracy. 1-P30 is computed as 100 percent minus the percent of estimates greater than 30% of the measured GFR; RMSE, root mean square error; B2M, Beta2-Microglobulin; BTP, Beta-Trace Protein; A, age; S, sex; R, race;
Reference equations are the 2009 CKD-EPI creatinine equation (1), 2012 CKD-EPI cystatin C equation (2), and 2012 CKD-EPI creatinine-cystatin C equation (3). The superscript number indicates the comparator equation, and formatting of the superscript informs direction of the comparison. No underline marking indicates that the equation is better than the comparator equation for a p-value ≤ 0.05; an underline marking indicates that the equation is worse than the comparator equation for p-value ≤ 0.05. Double underline indicates that the equation is neither better nor worse than the comparator equation P >0.05.
Figure 1: Performance of Reference eGFR and Panel eGFR Equations in the External Validation Population and Overall and by Race.
Accuracy as measured by 1- P30 or the percentage of estimates greater than 30% of measured GFR. The vertical bars indicate 95% confidence intervals. Solid lines indicate equations that include creatinine. Purple indicates the 2009 CKD-EPI creatinine equation; Orange indicates the 2012 CKD-EPI cystatin C and creatinine-cystatin C equations; Green indicates the new 2020 CKD-EPI three and four marker panels.
eGFRcr-cys (equation 5) was unbiased, but eGFRcr (equation 1) overestimated and eGFRcys (equation 2) underestimated measured GFR. There was differential bias by race groups for eGFRcr, eGFRcys and eGFRcr-cys. The three-marker panels (equations 3–4) and four-marker panels (equations 6–7) underestimated measured GFR, but improved the differential bias between race groups (Figure 1 and Table S8).
Discussion
Accurate assessment of GFR is essential for detection, staging, and assessment of progression, management, prognostication, and drug dosage adjustment in chronic kidney disease. Estimated GFR using creatinine and cystatin C are widely used, but the inclusion of demographic variables in GFR estimating equations, particularly specification of race, has raised concerns about serious negative consequences for delivery of care and reinforcing implicit bias6,7,12,13. Availability of rigorously developed, more accurate GFR estimating equations that do not require specification of race could improve their utility and broad acceptance6,7. Our main findings are that the addition of BTP and B2M to cystatin C in a three marker panel without race improved the precision and accuracy compared to eGFRcys and the addition of BTP and B2M to creatinine and cystatin C in a four marker panel without race was more accurate than the three- marker panel and as accurate as eGFRcr-cys which includes race. More accurate equations that can be used as a confirmatory or alternative test to eGFRcr that do not require use of creatinine or race could be a major advance.
The serum concentrations of all endogenous filtration markers are influenced by their non-GFR determinants, including their generation, tubular reabsorption and secretion, and extra-renal elimination, all of which lead to error in GFR estimates1,55. Serum creatinine is affected by muscle mass and diet, and drugs that inhibit tubular secretion of creatinine or extra-renal elimination of creatinine. Demographic characteristics such as age, sex and race have been used as surrogates for some of the non-GFR determinants in GFR estimating equations, but they represent average values for the relationship between the marker and its non-GFR determinants and can lead to error in individuals, and bias and imprecision in populations with variation in non-GFR determinants of the marker that differ from the development population. Importantly, race is a social versus a biological construct. Prior studies have suggested that genetic measures of ancestry might be a better tool to account for the possible variation in creatinine generation by Black ancestry56. We do not advocate use of ancestry markers at this time as it would require their measurement for GFR estimation and would add complexity to the implementation of eGFR reporting. Moreover, it would not explain the observed geographical variation with the use of the current race coefficient between Black people in the United States and Europe vs Africa57–62. The panel eGFR equations reported here are a further advance as they do not require consideration of race or ancestry. Further work is required to determine if the new equations presented here are more robust across geographical regions.
Guidelines recommend use of confirmatory tests for eGFRcr in clinical scenarios where a more precise and accurate estimate of GFR is required3. Serum cystatin C is less affected by race than creatinine, but is affected by obesity, inflammation, smoking and alterations in thyroid and adrenal hormones63–69 and as such eGFRcys is not more accurate than eGFRcr47. We have previously shown that a panel of multiple non-correlated filtration markers can result in a more accurate estimate and minimize the requirement for demographic factors by diminishing the impact of the non GFR determinants of each marker on the resulting GFR estimate14. Here, we show that the addition of B2M and BTP to cystatin C in the three-marker panel eGFR provided greater accuracy than eGFRcys but not eGFRcr-cys, reflecting the important contribution of creatinine to GFR estimation in the populations included in this study. The addition of B2M and BTP to creatinine and cystatin C in the four-marker panel was more accurate than eGFRcr-cys, and allowed elimination of race with similar performance of eGFRcr-cys. Although the four-marker panel eGFR is also not independent of creatinine the magnitude of the creatinine coefficient is attenuated compared to the 2012 creatinine-cystatin C equation, thus reducing the contribution of creatinine to the four-marker panel eGFR. Overall, these findings are consistent with our hypothesis, and suggests a path forward to improved GFR estimation and without the need for specification of race.
Strengths of this study include its design, with separate large databases for development and validation of the new equations, a diverse development population including participants with and without CKD, higher measured GFR compared to our 2015 BTP and B2M equations, and a pre-specified rigorous statistical analytical plan for testing of all variables. The pooled development and validation databases in these diverse development populations allows for greater general applicability than our previous equations. Comparison of equations in a separate validation population overcomes limitations of differences among studies in patient characteristics and methods for measurement of GFR. We attempted to minimized differences by GFR measurement method by calibrating the measured GFR using a common method48.
The major limitations of these and existing GFR estimating equations is their development in ambulatory populations without serious comorbidity and lack of representation from geographically diverse groups. Specifically, our study population does not include participants with acute or serious chronic comorbidity that may cause malnutrition and muscle wasting, which may potentially affect creatinine more than cystatin C, B2M and BTP, such that eGFRcys or the three-marker panel without creatinine could be preferred as alternative tests initial tests for GFR evaluation. It is possible that in these settings, where creatinine is likely to perform poorly, the three-marker panel might provide greater accuracy, or conversely, the non GFR determinants might have a greater contribution to the overall eGFR and lead to decreased accuracy. Further evaluation in these populations is required to consider these possibilities.
There are other limitations. First, the mean GFR in the development population is higher than in the CKD populations used to develop the 2015 equations, it is lower than in the development population for the 2009 creatinine and 2012 cystatin C equations and in the External Validation population in this study, thus regression to the mean is a likely explanation for the underestimation of measured GFR in the validation population in the current study. However, performance was consistent across range of GFR suggesting that this may not decrease generalizability. Another limitation is possible variation in measurement methods for endogenous filtration markers over time, even though we used a single laboratory for calibration or measurement in all studies, and had calibration panels and quality control samples to evaluate stability over time52. In addition, GFR is known to be measured with error, which may account for some of the observed imprecision65.
Several steps would need to be done prior to implementation. First, clinical and laboratory practice guidelines should consider indications and preferred diagnostic strategies for laboratory testing and reporting panel eGFRs that include consideration of local public health priorities, clinical practice patterns, and cost/benefit analyses. Second, although our research laboratory has observed stability in filtration marker assays over a decade52, variation in these assays among laboratories could lead to errors, with potential for errors compounded with each additional analyte. Thus manufacturers and clinical chemists would need to develop standards as have been developed for creatinine and cystatin C.70 Finally, we suggest investigations into the cost effectiveness of these additional tests in clinical settings where GFR levels affect management decisions. Current attention by Congress suggests an avenue for advocacy for sensible cost structure for GFR confirmatory tests8,9
In conclusion, we present three-marker and four-marker panel eGFRs using B2M and BTP, which do not include race as confirmatory or alternative tests for eGFRcr. The four-marker panel eGFR is less dependent on creatinine and is as accurate as 2012 creatinine-cystatin C equation. An eGFR that does not require race and is less dependent on creatinine could provide more robust GFR estimates across a greater variety of populations. Further studies are required to understand how best to use these equations in clinical practice, especially in diverse clinical settings and geographical locations.
Supplementary Material
Figure S1. Flow chart showing the development of CKD-EPI pooled datasets
Figure S2: Performance of estimated of GFR equations in the overall population and by eGFR subgroups.
Figure S3: Performance of Estimated of GFR Equations by Age Subgroups.
Figure S4: Performance of Estimated of GFR Equations by BMI Subgroups.
Figure S5: Performance of Estimated of GFR Equations by Diabetes Subgroups.
Figure S6: Performance of Estimated of GFR Equations by Sex Subgroups.
Table S1: Assays and Quality Control for Creatinine, Cystatin C, B2M and BTP.
Table S2a Characteristics of the Development and Internal Validation Population by Study
Table S2b Characteristics of the External Validation Population by Study
Table S3. Correlations and Partial Correlations of Filtration Markers and measured GFR in Combined Development and Internal Validation and External Validation Populations
Table S4: Exponentiated Coefficients for the Race Coefficient in GFR Estimating Equations
Table S5. Panel eGFR Equation Performance in Development and Internal Validation Population With and Without including Age, Sex and Race
Table S6: Variables and Coefficients for Other New Equations that Might be Used in Research Developed in the Development and Internal Validation Population
Table S7. Performance of 2020 B2M and BTP Equations Compared to 2015 B2M and BTP Equations in External Validation Population
Table S8 New Panel eGFR Equation Performance Compared to Reference Equations in External Validation Overall and By Race
Table S9. New Panel eGFR Performance Compared to Reference Equations in External Validation Population using Non-calibrated measured GFR
Acknowledgements:
The authors thank Shiyuan Miao (Tufts Medical Center, Boston, MA), for assistance with creating the figures and Juhi Chaudhari (Tufts Medical Center, Boston, MA) with administrative aspects of the paper. The MESA authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Support: The measurements and analyses were supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK097020 “Estimating GFR from a Panel of Endogenous Filtration Markers” to Tufts Medical Center. Information on support for the pooled studies follows. AASK: NIDDK U01 DK045388 and the NCMHHD M01 RR00071. AGES-Kidney: NIDDK R01-DK082447 and supplement 01A1S1 to ASL. ALTOLD: funded by the National Institutes of Health (NIH) under the cooperative agreement U01 DK066013. The NIH participated in the interpretation of data, writing the report, and the decision to submit the report for publication. This study was also supported by the Minneapolis Medical Research Foundation, Minneapolis, MN, which did not participate in any aspect of the study. CCFP: NIDDK U01 DK053869. CRIC: cooperative agreements from NIDDK (U01 DK060990, U01 DK060984, U01 DK061022, U01 DK061021, U01 DK061028, U01 DK060980, U01 DK060963, and U01 DK060902), and supported in part by the following institutional Clinical Translational Science Awards (CTSA) and other NIH grants: University of Pennsylvania NIH/National Center for Advancing Translational Sciences (NCATS) UL1 TR000003, K01 DK092353, and K24 DK002651, Johns Hopkins University UL1 TR000424, University of Maryland General Clinical Research Center M01 RR16500, Clinical and Translational Science Collaborative of Cleveland, UL1 TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1 TR000433, University of Illinois at Chicago CTSA UL1 RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/National Center for Research Resources (NCRR) University of California San Francisco-Clinical and Translational Science Institute UL1 RR024131; HIV study: supported by Gilead Sciences, Inc. under an investigator-initiated protocol NCRR L1RR025752 and by the NIH/NCATS UL1 RR025752 (Tufts Medical Center), UL1 RR029887 (Mount Sinai School of Medicine) and UL1 RR025777 (University of Alabama at Birmingham). MACS: Baltimore CRS, U01-HL146201; Pittsburgh CRS, U01-HL146208; Chicago-Northwestern CRS, U01-HL146240; Los Angeles CRS, U01-HL146333; Data Analysis and Coordination Center U01-HL146193. MDRD: NIDDK U01 DK35073. MESA: Supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the NCATS. PERL: NIDDK (R03-DK-094484, R34-DK-097808, and UC4-DK-101108) and JDRF (17-2012-377). The iohexol used for the iGFR measurements was a generous gift of GE Healthcare. Research reported in this publication was supported by the NCATS, the NIDDK, and the National Institute on Aging (Claude Pepper Center grants) under award numbers P30-DK-036836,UL1-TR-002494, P30-DK-020572,UL1-TR-001422, UL1-TR-002556, UL1-TR-002319, UL1-TR-001105,UL1-TR-002319–02, P30-AG-08808,and P30-AG-02482. RASS: research grants from NIDDK (#DK51975); Merck & Co., USA; Merck Frosst, Canada; and Canadian Institutes of Health Research (CIHR) (#DCT 14281) Canada; supported in part by the University of Minnesota General Clinical Research Center (GCRC), M01-RR00400 NCRR, NIH. The remaining studies had no funding to report. Except as indicated, the funders had no a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Financial Disclosure: Dr Inker reports research funding to Tufts Medical Center from NIH, National Kidney Foundation, Retrophin, Omeros, Dialysis Clinic, Inc, and Reata Pharmaceuticals; and consulting fees to Tufts Medical Center from Tricida (to Tufts MC), Goldfinch Bio (Tufts MC), Diamtrix. Dr Abraham reports grant support from NIH. Dr Kasiske reports that all income is from the Hennepin Healthcare Research Institute, including funding from the NIH, HRSA and pharmaceutical companies (CSLB, Astellas). Dr Navis reports honoraria from Nutricia/Danone. Dr Rossing reports honoraria to institution from Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Merck, Mundipharma, Sanofi, Gilead, Vifor, Novo Nordisk. Dr Levey reports grant support from NIH, National Kidney Foundation, and Siemens. The remaining authors declare that they have no relevant financial interests.
Footnotes
Article Information
CKD-EPI GFR Collaborators: The Age, Gene/Environment Susceptibility Reykjavik study (AGES-RS): Margret B. Andresdottir, Hrefna Gudmundsdottir, Olafur S Indridason and Runolfur Palsson; Assessing Long Term Outcomes in Living Kidney Donors (ALTOLD): Paul Kimmel, Matt Weir, Roberto Kalil, Todd Pesavento; Chronic Renal Insufficiency Cohort (CRIC): Anna Porter, Jonathan Taliercio, Chi-yuan Hsu, Jing Chen; Groningen Renal Hemodynamic Cohort Study Group (GRECO): Steef Sinkeler; study of people with HIV: Christina Wyatt, Zipporah Krishnasami, James Hellinger; Multicenter AIDS Cohort Study (MACS), now the MACS/WIHS Combined Cohort Study (MWCCS): Joseph Margolick (Baltimore), Lawrence Kingsley (Pittsburgh), Mallory Witt (Los Angeles), Steven Wolinsky (Chicago Northwestern); Multi Ethnic Study of Atherosclerosis (MESA): Tariq Shafi, Wendy Post; Preventing Early Renal Loss in Diabetes (PERL): Alessandro Doria; Steno Diabetes Center study: Hans-Henrik Parving.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. The New England journal of medicine. June 08 2006;354(23):2473–2483. [DOI] [PubMed] [Google Scholar]
- 2.College of American Pathologists. Current Status Of Reporting Estimated Glomerular Filtration Rate (eGFR). 2012; http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt%7BactionForm.contentReference%7D=committees%2Fchemistry%2Fchemistry_resources.html&_state=maximized&_pageLabel=cntvwr. Accessed October 8, 2013.
- 3.Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150. [DOI] [PubMed] [Google Scholar]
- 4.Kervella D, Lemoine S, Sens F, et al. Cystatin C Versus Creatinine for GFR Estimation in CKD Due to Heart Failure. Am J Kidney Dis. February 2017;69(2):321–323. [DOI] [PubMed] [Google Scholar]
- 5.Torre A, Aguirre-Valadez JM, Arreola-Guerra JM, et al. Creatinine Versus Cystatin C for Estimating GFR in Patients With Liver Cirrhosis. Am J Kidney Dis. February 2016;67(2):342–344. [DOI] [PubMed] [Google Scholar]
- 6.Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight — Reconsidering the Use of Race Correction in Clinical Algorithms. New England Journal of Medicine. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA. July 9 2019;322(2):113–114. [DOI] [PubMed] [Google Scholar]
- 8.Warren E, Booker C, Wyden R, Lee B. Agency for Healthcare Research and Quality (AHRQ) requesting a review of the use of race-based clinical algorithms in standard medical practices. Washington DC: 2020. [Google Scholar]
- 9.Agarwal A. Response to Chairman Neal re Race and eGFR. Washington DC: American Society of Neprhology;2020. [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. May 5 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powe NR. Black Kidney Function Matters: Use or Misuse of Race? JAMA. August 25 2020;324(8):737–738. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney Disease, Race, and GFR Estimation. Clinical Journal of the American Society of Nephrology. 2020:CJN.12791019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Tighiouart H, Titan SM, Inker LA. Estimation of Glomerular Filtration Rate With vs Without Including Patient Race. JAMA internal medicine. March 16 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Levey AS, Coresh J. Estimated Glomerular Filtration Rate From a Panel of Filtration Markers-Hope for Increased Accuracy Beyond Measured Glomerular Filtration Rate? Advances in chronic kidney disease. January 2018;25(1):67–75. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann A, Nimtz M, Conradt HS. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology. June 1997;7(4):499–506. [DOI] [PubMed] [Google Scholar]
- 16.White CA, Ghazan-Shahi S, Adams MA. beta-Trace protein: a marker of GFR and other biological pathways. Am J Kidney Dis. January 2015;65(1):131–146. [DOI] [PubMed] [Google Scholar]
- 17.Astor BC, Shafi T, Hoogeveen RC, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. May 2012;59(5):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. February 2012;23(2):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavsar NA, Appel LJ, Kusek JW, et al. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis. December 2011;58(6):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster MC, Coresh J, Hsu CY, et al. Serum β-trace protein and β−2 microglobulin as Predictors End-stage Renal Disease in Adults with Chronic Kidney Disease [ASN Abstract SA-OR012]. J Am Soc Nephrol. 2014;25:83A. [Google Scholar]
- 21.Foster MC, Inker LA, Hsu CY, et al. Filtration Markers as Predictors of ESRD and Mortality in Southwestern American Indians With Type 2 Diabetes. Am J Kidney Dis. July 2015;66(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. February 2006;17(2):546–555. [DOI] [PubMed] [Google Scholar]
- 23.Spanaus KS, Kollerits B, Ritz E, Hersberger M, Kronenberg F, von Eckardstein A. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clinical chemistry. May 2010;56(5):740–749. [DOI] [PubMed] [Google Scholar]
- 24.Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney international. December 2012;82(12):1297–1303. [DOI] [PubMed] [Google Scholar]
- 25.Foster MC, Inker LA, Levey AS, et al. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis. July 2013;62(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inker LA, Tighiouart H, Coresh J, et al. GFR Estimation Using beta-Trace Protein and beta2-Microglobulin in CKD. Am J Kidney Dis. January 2016;67(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen N, Shi H, Zhang L, et al. GFR Estimation Using a Panel of Filtration Markers in Shanghai and Beijing. Kidney Med. Mar-Apr 2020;2(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modification of Diet in Renal Disease (MDRD) Study Group (prepared by Levey AS; Bosch JP; Lewis JB; Greene T; Rogers N; Roth D). A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. March 16 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 29.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. October 2001;38(4):744–753. [DOI] [PubMed] [Google Scholar]
- 30.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol. 2003;14(7):S148–153. [DOI] [PubMed] [Google Scholar]
- 31.Mauer M, Zinman B, Gardiner R, et al. ACE-I and ARBs in early diabetic nephropathy. Journal of the renin-angiotensin-aldosterone system : JRAAS. December 2002;3(4):262–269. [DOI] [PubMed] [Google Scholar]
- 32.Hansen HP, Tauber-Lassen E, Jensen BR, Parving HH. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney international. July 2002;62(1):220–228. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen P, Andersen S, Rossing K, Hansen BV, Parving HH. Dual blockade of the rennin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. June 2002;17(6):1019–1024. [DOI] [PubMed] [Google Scholar]
- 34.Harris TB, Ballard-Barbasch R, Madans J, Makuc DM, Feldman JJ. Overweight, weight loss, and risk of coronary heart disease in older women. The NHANES I Epidemiologic Follow-up Study. American journal of epidemiology. June 15 1993;137(12):1318–1327. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the rennin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney international. May 2003;63(5):1874–1880. [DOI] [PubMed] [Google Scholar]
- 36.Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. Brit Med J July 13 1991;303(6794):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarnow L, Rossing P, Jensen C, Hansen BV, Parving HH. Long-term renoprotective effect of nisoldipine and lisinopril in type 1 diabetic patients with diabetic nephropathy. Diabetes care. December 2000;23(12):1725–1730. [DOI] [PubMed] [Google Scholar]
- 38.Bosma RJ, Doorenbos CR, Stegeman CA, van der Heide JJ, Navis G. Predictive performance of renal function equations in renal transplant recipients: an analysis of patient factors in bias. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. September 2005;5(9):2193–2203. [DOI] [PubMed] [Google Scholar]
- 39.Rook M, Hofker HS, van Son WJ, van der Heide J, Ploeg R, Navis G. Predictive capacity of pre-donation GFR and renal reserve capacity for donor renal function after living kidney donation. Am J Trans. 2006;6:1653–1659. [DOI] [PubMed] [Google Scholar]
- 40.Inker LA, Wyatt C, Creamer R, et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. Journal of acquired immune deficiency syndromes (1999). November 1 2012;61(3):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okparavero AA, Tighiouart H, Krishnasami Z, et al. Use of glomerular filtration rate estimating equations for drug dosing in HIV-positive patients. Antiviral therapy. 2013;18(6):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr., The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. August 1987;126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 43.Maahs DM, Caramori L, Cherney DZI, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Current diabetes reports. 2013;13(4):550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. February 2005;16(2):459–466. [DOI] [PubMed] [Google Scholar]
- 45.Inker LA, Shafi T, Okparavero A, et al. Effects of Race and Sex on Measured GFR: The Multi-Ethnic Study of Atherosclerosis. Am J Kidney Dis. November 2016;68(5):743–751. [DOI] [PubMed] [Google Scholar]
- 46.Kasiske BL, Anderson-Haag T, Israni AK, et al. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis. July 2015;66(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. July 05 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soveri I, Berg UB, Bjork J, et al. Measuring GFR: a systematic review. Am J Kidney Dis. September 2014;64(3):411–424. [DOI] [PubMed] [Google Scholar]
- 49.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical chemistry. April 2007;53(4):766–772. [DOI] [PubMed] [Google Scholar]
- 50.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clinical chemistry and laboratory medicine. November 2010;48(11):1619–1621. [DOI] [PubMed] [Google Scholar]
- 51.Blirup-Jensen S, Grubb A, Lindstrom V, Schmidt C, Althaus H. Standardization of Cystatin C: development of primary and secondary reference preparations. Scandinavian journal of clinical and laboratory investigation. Supplementum. 2008;241:67–70. [DOI] [PubMed] [Google Scholar]
- 52.Karger A, Eckfeldt J, Rynders G, et al. Long-term longitudinal stability of kidney filtration marker measurements: implications for epidemiological studies and clinical care. Clinical Chemistry (In Press). 2020. [DOI] [PubMed] [Google Scholar]
- 53.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. Journal of nephrology. Nov-Dec 2008;21(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- 54.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 55.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. May 2014;63(5):820–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Udler MS, Nadkarni GN, Belbin G, et al. Effect of Genetic African Ancestry on eGFR and Kidney Disease. J Am Soc Nephrol. July 2015;26(7):1682–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Deventer HE, George JA, Paiker JE, Becker PJ, Katz IJ. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clinical chemistry. July 2008;54(7):1197–1202. [DOI] [PubMed] [Google Scholar]
- 58.Yayo E, Aye M, Yao C, et al. Measured (and estimated) glomerular filtration rate: reference values in West Africa. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. July 1 2018;33(7):1176–1180. [DOI] [PubMed] [Google Scholar]
- 59.Moodley N, Hariparshad S, Peer F, Gounden V. Evaluation of the CKD-EPI creatinine based glomerular filtration rate estimating equation in Black African and Indian adults in KwaZulu-Natal, South Africa. Clinical biochemistry. September 2018;59:43–49. [DOI] [PubMed] [Google Scholar]
- 60.Bukabau JB, Yayo E, Gnionsahé A, et al. Performance of creatinine- or cystatin C-based equations to estimate glomerular filtration rate in sub-Saharan African populations. Kidney international. 2019;95(5):1181–1189. [DOI] [PubMed] [Google Scholar]
- 61.Flamant M, Vidal-Petiot E, Metzger M, et al. Performance of GFR estimating equations in African Europeans: basis for a lower race-ethnicity factor than in African Americans. Am J Kidney Dis. July 2013;62(1):182–184. [DOI] [PubMed] [Google Scholar]
- 62.Fabian J, George JA, Etheredge HR, et al. Methods and reporting of kidney function: a systematic review of studies from sub-Saharan Africa. Clinical kidney journal. December 2019;12(6):778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inker LA, Huang N, Levey AS. Strategies for assessing GFR and albuminuria in the living kidney donor evaluation. Current transplantation reports. March 2017;4(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levey AS, Inker LA. GFR Evaluation in Living Kidney Donor Candidates. J Am Soc Nephrol. April 2017;28(4):1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nature reviews. Nephrology. January 2020;16(1):51–64. [DOI] [PubMed] [Google Scholar]
- 66.Schmid C, Ghirlanda C, Zwimpfer C, Tschopp O, Zuellig RA, Niessen M. Cystatin C in adipose tissue and stimulation of its production by growth hormone and triiodothyronine in 3T3-L1 cells. Mol Cell Endocrinol. February 15 2019;482:28–36. [DOI] [PubMed] [Google Scholar]
- 67.Naour N, Fellahi S, Renucci JF, et al. Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity (Silver Spring). December 2009;17(12):2121–2126. [DOI] [PubMed] [Google Scholar]
- 68.Risch L, Blumberg A, Huber AR. Assessment of renal function in renal transplant patients using cystatin C. A comparison to other renal function markers and estimates. Renal failure. May-Jul 2001;23(3–4):439–448. [DOI] [PubMed] [Google Scholar]
- 69.Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clinical chemistry. November 2001;47(11):2055–2059. [PubMed] [Google Scholar]
- 70.Myers G, Miller W, Coresh J, et al. Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clinical chemistry. January/01 2006;52:5–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart showing the development of CKD-EPI pooled datasets
Figure S2: Performance of estimated of GFR equations in the overall population and by eGFR subgroups.
Figure S3: Performance of Estimated of GFR Equations by Age Subgroups.
Figure S4: Performance of Estimated of GFR Equations by BMI Subgroups.
Figure S5: Performance of Estimated of GFR Equations by Diabetes Subgroups.
Figure S6: Performance of Estimated of GFR Equations by Sex Subgroups.
Table S1: Assays and Quality Control for Creatinine, Cystatin C, B2M and BTP.
Table S2a Characteristics of the Development and Internal Validation Population by Study
Table S2b Characteristics of the External Validation Population by Study
Table S3. Correlations and Partial Correlations of Filtration Markers and measured GFR in Combined Development and Internal Validation and External Validation Populations
Table S4: Exponentiated Coefficients for the Race Coefficient in GFR Estimating Equations
Table S5. Panel eGFR Equation Performance in Development and Internal Validation Population With and Without including Age, Sex and Race
Table S6: Variables and Coefficients for Other New Equations that Might be Used in Research Developed in the Development and Internal Validation Population
Table S7. Performance of 2020 B2M and BTP Equations Compared to 2015 B2M and BTP Equations in External Validation Population
Table S8 New Panel eGFR Equation Performance Compared to Reference Equations in External Validation Overall and By Race
Table S9. New Panel eGFR Performance Compared to Reference Equations in External Validation Population using Non-calibrated measured GFR