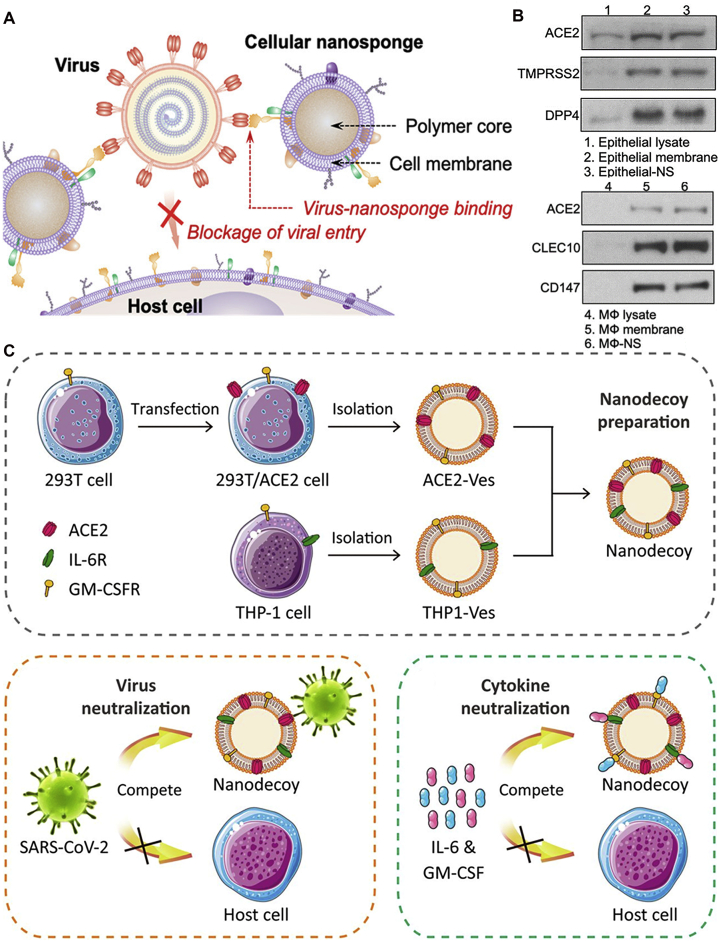
Abstract
Acute respiratory distress syndrome (ARDS) is characterized by the severe inflammation and destruction of the lung air–blood barrier, leading to irreversible and substantial respiratory function damage. Patients with coronavirus disease 2019 (COVID-19) have been encountered with a high risk of ARDS, underscoring the urgency for exploiting effective therapy. However, proper medications for ARDS are still lacking due to poor pharmacokinetics, non-specific side effects, inability to surmount pulmonary barrier, and inadequate management of heterogeneity. The increased lung permeability in the pathological environment of ARDS may contribute to nanoparticle-mediated passive targeting delivery. Nanomedicine has demonstrated unique advantages in solving the dilemma of ARDS drug therapy, which can address the shortcomings and limitations of traditional anti-inflammatory or antioxidant drug treatment. Through passive, active, or physicochemical targeting, nanocarriers can interact with lung epithelium/endothelium and inflammatory cells to reverse abnormal changes and restore homeostasis of the pulmonary environment, thereby showing good therapeutic activity and reduced toxicity. This article reviews the latest applications of nanomedicine in pre-clinical ARDS therapy, highlights the strategies for targeted treatment of lung inflammation, presents the innovative drug delivery systems, and provides inspiration for strengthening the therapeutic effect of nanomedicine-based treatment.
KEY WORDS: Acute respiratory distress syndrome, Nanomedicine, Anti-inflammatory therapy, Drug delivery, Targeting strategy, Acute lung injury, COVID-19, Pathophysiologic feature
Abbreviations: ACE2, angiotensin-converting enzyme 2; AEC II, alveolar type II epithelial cells; AM, alveolar macrophages; ARDS, acute respiratory distress syndrome; BALF, bronchoalveolar lavage fluid; BSA, bovine serum albumin; CD, cyclodextrin; CLP, cecal ligation and perforation; COVID-19, coronavirus disease 2019; cRGD, cyclic arginine glycine-d-aspartic acid; DOPE, phosphatidylethanolamine; DOTAP, 1-diolefin-3-trimethylaminopropane; DOX, doxorubicin; DPPC, dipalmitoylphosphatidylcholine; ECM, extracellular matrix; ELVIS, extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration; EPCs, endothelial progenitor cells; EphA2, ephrin type-A receptor 2; EPR, enhanced permeability and retention; Esbp, E-selectin-binding peptide; EVs, extracellular vesicles; FcgR, Fcγ receptor; GNP, peptide-gold nanoparticle; H2O2, hydrogen peroxide; HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule-1; IKK, IκB kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MERS, Middle East respiratory syndrome; MPMVECs, mouse pulmonary microvascular endothelial cells; MPO, myeloperoxidase; MSC, mesenchymal stem cells; NAC, N-acetylcysteine; NE, neutrophil elastase; NETs, neutrophil extracellular traps; NF-κB, nuclear factor-κB; PC, phosphatidylcholine; PCB, poly(carboxybetaine); PDA, polydopamine; PDE4, phosphodiesterase 4; PECAM-1, platelet-endothelial cell adhesion molecule; PEG, poly(ethylene glycol); PEI, polyetherimide; PEVs, platelet-derived extracellular vesicles; PLGA, poly(lactic-co-glycolic acid); PS-PEG, poly(styrene-b-ethylene glycol); RBC, red blood cells; RBD, receptor-binding domains; ROS, reactive oxygen species; rSPANb, anti-rat SP-A nanobody; S1PLyase, sphingosine-1-phosphate lyase; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; scFv, single chain variable fragments; SDC1, syndecan-1; Se, selenium; Siglec, sialic acid-binding immunoglobulin-like lectin; SORT, selective organ targeting; SP, surfactant protein; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; TPP, triphenylphosphonium cation; YSA, YSAYPDSVPMMS
Graphical abstract
Nanomedicine has demonstrated great potential in achieving specific targeting and amplifying therapeutic efficiency. This review focuses on the recent breakthroughs and targeting strategies to provide insights into nanomedicine for acute respiratory distress syndrome treatment.
1. Introduction
Acute respiratory distress syndrome (ARDS) is a common critical illness that seriously threatens the respiratory system and places a tremendous burden on the healthcare system worldwide1,2. The most common inducers for ARDS are pneumonia and sepsis3. Many respiratory viruses, such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and global pandemic coronavirus disease 2019 (COVID-19), can also result in ARDS4, 5, 6. Cumulatively, ∼122 million people have been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and as many as 2.69 million people have died since the outbreak7. Patients with COVID-19 and ARDS share similar clinical manifestations and face a high incidence of deadly complications4,5. About 20% of patients developed ARDS, and around 90% of non-survivors suffered from ARDS6,8, 9, 10, 11. The COVID-19 pandemic has sparked new interest in understanding the intricate pathologies of ARDS12. The overwhelming inflammation and destruction of lung barrier are the fundamental pathophysiology involved in the development of ARDS3. According to the Berlin Definition, ARDS is classified as mild, moderate, or severe type according to the degree of hypoxemia13. ARDS has long been recognized as a heterogeneous disease3. It not only varies among patients due to distinct pathogenic factor, but also displays spatial–temporal differences in lung injury characterized as regional heterogeneous damage and time-dependent rapid progression. The complicated heterogeneity makes ARDS challenging to be cured, with a high mortality rate of 30%–40%14.
Current treatments for ARDS include respiratory support and medication1. However, improper use of ventilators may cause a second hit that aggravates lung injury3,15, and ventilator equipment often faces severe shortage when lethal epidemic rages out. Before the emergence of specific antiviral drugs or effective vaccines, inchoate management for ARDS is a priority. Early intervention may help alleviate inflammation and respiratory symptom, prevent irreversible damage to lung, decrease the possibility of requiring mechanical ventilation, and reduce severe ARDS incidence16,17. The main drugs used to treat ARDS emphasize on the pathophysiological process, including suppressing inflammation (corticosteroids, statin, IFN-β, and sivelestat), reducing pulmonary edema (lung surfactant, β2-agonist), promoting selective vasodilation (nitric oxide), and facilitating the repair of alveolar epithelial and vascular endothelial cells (keratinocyte growth factor)18. Drug candidates for ARDS are emerging continuously, however, none has been proved beneficial in terms of reducing mortality in clinical trials14. On account of the unique pulmonary physiological barrier, drug delivery efficiency to the lungs is relatively low, resulting in a considerable reduction in drug efficacy and many undesired side effects19. Moreover, the heterogeneity of ARDS may primarily impede the therapeutic effectiveness.
Nanomedicine-based delivery systems have emerged to improve biopharmacokinetic property and therapeutic outcome of drugs20. With adjustable size distribution, tunable surface properties, and unique modifiability for targeting, nanocarriers have shown great potential in drug delivery for various disease treatment21, 22, 23, 24. Specifically, nanocarriers can accumulate in inflammatory lung sites through passive, active, or physicochemical targeting strategies, thereby boosting drug potency and significantly reducing side effects25, 26, 27, 28. Besides, nanocarriers are capable of loading multiple drugs29, 30, 31. Thus, the synergy can be achieved through pleiotropic pharmacological mechanisms30, 31, 32. Based on the pathophysiology of ARDS and delivery strategies, this review introduces the features and advantages of nanocarriers, then discusses the factors that affecting delivery and therapeutic efficiency, underlines the targeting delivery strategies and novel delivery systems applied for ARDS, and finally provides insights into the rational design of nanocarriers and precise management of ARDS.
2. ARDS pathophysiology and model establishment
2.1. Pathophysiologic features
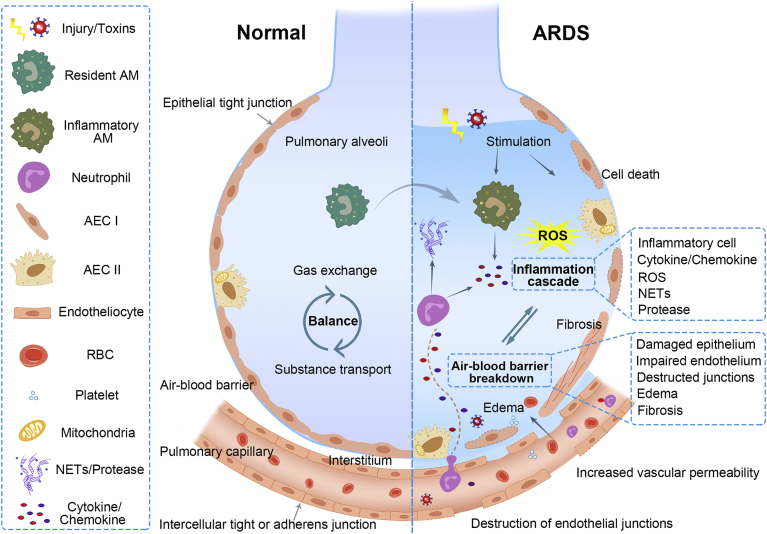
As illustrated in Fig. 1, the dominating pathophysiology of ARDS can be classified into the cascade of inflammation and the destruction of the air–blood barrier3,14,33. The etiologies of ARDS, particularly infections, such as SARS-CoV-2 invasion, can bring about cytokine storms in the pulmonary environment and systemic circulation, severely damaging lung tissues, and account for high mortality34,35. Both direct and indirect injuries, such as inhalation of harmful substances, trauma, infection, and shock, can cause pulmonary inflammation, activate resident alveolar macrophages (AM) and alveolar type II epithelial cells (AEC II), and trigger cytokines/chemokines secretion, recruiting circulating immune cells to the lungs14. Specifically speaking, stimulated AEC and monocytes/macrophages can lead to the high infiltration of pro-inflammatory cytokines and chemokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, and CCL2 in ARDS patients36, 37, 38. Of note, a large number of neutrophils are recruited and assembled in the lungs, which are closely associated with the protein-rich edema fluid in ARDS39. The activated neutrophils can produce noxious reactive oxygen species (ROS), neutrophil extracellular traps (NETs), as well as proteases such as neutrophil elastase (NE), myeloperoxidase (MPO), and matrix metalloproteinases, complicating the inflammatory microenvironment3,39.
Figure 1.
Pathophysiology of ARDS. Healthy lungs maintain a balance of gas change and substance transport, ascribing to the integrated structure and function of the air–blood barrier (the left sections). The air–blood barrier is mainly consisting of epithelium and endothelium barrier, where cells are lined up continuously and connected through intercellular junctions. Once injury or toxins stimulate, AM and AEC cells are initiated, secreting inflammatory cytokines and chemokines, and recruiting neutrophils to the inflamed lungs (the right sections). The activated neutrophils can release NETs and multiple proteases in response, while excessive productions can be harmful and induce lung edema. Various inflammatory cells may overproduce pro-inflammatory cytokines, ROS, and other inflammatory mediators, resulting in cytokine storms and severe lung injury. The inflammatory environment can damage the lung cells, dissolve the intercellular junctions, increase epithelium and vascular permeability, leading to the breakdown of epithelium and endothelium barrier. The impairment of lung barrier can prompt more extravasation of inflammatory cells into alveoli, which can exacerbate the inflammatory state and cause tissue damage, forming a vicious circle between inflammation cascade and air–blood barrier breakdown. Adapted with permission from Ref. 3. Copyright © 2017, Massachusetts Medical Society.
The lung air–blood barrier, including alveolar epithelial and vascular endothelial barrier, performs vital functions in guaranteeing the intact alveolar structure and maintaining the balance of alveolar fluid clearance and substance exchange40. The alveolar epithelial barrier is primarily composed of two alveolar epithelial cells (types I and II) that line continuously in a monolayer and link through tight junctions14. The pulmonary endothelial barrier comprises endothelial cells connected by intercellular tight or adhesive junctions, regulating the entry of fluid and inflammatory entities into the interstitium41. However, the hyperinflammatory environment during the acute stage of ARDS, including infiltrated inflammatory cells in the alveoli, along with the simultaneously released pro-inflammatory cytokines, chemokines, and other inflammatory mediators, can damage the pulmonary epithelium and endothelium, causing apoptosis/necrosis of cells and dissolution of the intercellular junctions3,42. It is documented that the activation, migration, and degranulation of neutrophils are responsible for the increased vascular and paracellular epithelial permeability, as well as the function loss of normal endothelial/epithelial barrier39. Besides, red blood cells (RBC) and activated platelets can also infiltrate into alveolar space, resulting in lung endothelial injury39,43. Consequently, the increased permeability of pulmonary barrier may lead to alveolar edema, which in return promotes the migration of leukocytes, forms a vicious circle of tissue injury and pulmonary inflammation, and breaks the balance of substance transport and gas exchange. Histological and pathological injuries of lung tissues, such as diffuse alveolar damage, alveolar hemorrhage and collapse, pulmonary fibrosis, etc., were found in ARDS patients, which seriously led to the dysfunction of the respiratory system4,13,37.
Moreover, ARDS patients display spatio-temporal heterogeneity due to the distinct etiology and severity of injury. Pathological changes in lung tissues can be diverse44. As shown by X-rays or computed tomography images, pulmonary infiltrates are often heterogeneously and patchy distributed3. Taking virus-induced ARDS as an example, respiratory virus primarily infects lower airways through binding with specific receptors on pneumocytes5. SARS, MERS, and COVID-19-related ARDS demonstrate similar pathological features and hallmarks, such as diffuse alveolar damage, pneumocyte injury, endothelium damage, mononuclear inflammatory infiltration, and cytokine storms35. However, there are some differences. For instance, both SARS-CoV and MERS-CoV can induce ARDS, while their patients show distinct injury sites5. MERS-CoV infection often causes lesions that are located in lower lobes instead of upper lobes, and progresses more rapidly compared with SARS-CoV infection. For COVID-19-related ARDS, it was found that damage of alveolar epithelial cells was the leading cause, while endothelial cells were less injured accompanied with less exudation4. Besides, the onset time of ARDS can be different, and the duration of respiratory failure may last long or brief, causing different degrees of lung damage in various stages45.
2.2. Model establishment
Animal model is of great importance for investigating etiology and pathological mechanisms of ARDS and exploiting available treatments. It can be established by activating lung inflammation directly or indirectly to mimic human lung environment. Intratracheal/intranasal instillation or inhalation of lipopolysaccharide (LPS) is regarded as the practical method to establish an ARDS model in mice or rats46. Except for LPS or other pathogens stimulation, clinically-related diseases such as sepsis, acute pancreatitis and transfusion injury, as well as chemical factors like bleomycin and hydrochloric acid (HCl) can also trigger ARDS, and the corresponding models have been established47. Besides, human models of ARDS including low-dose of LPS inhalation and one-lung ventilation are also performed48. The ex vivo lung perfusion (EVLP) model has been utilized to resemble the pulmonary ventilation and circulation in ARDS49.
Plenty of drugs have demonstrated certain degree of therapeutic effects in animal models. Unfortunately, among thousands of drugs tested in clinical trials, none has succeeded for ARDS therapy. Therefore, effective pharmacologic treatments and proper animal models remain challenging. It is conducive to provide precise management on lung injury through more comprehensive investigation into the underlying pathogenesis of ARDS, more reliable models to mimic the complicated pathological process, and deeper exploitation on pharmaceutic means.
3. Characteristics, advantages and disadvantages of nanomedicine for ARDS treatment
3.1. Characteristics of nanomedicine
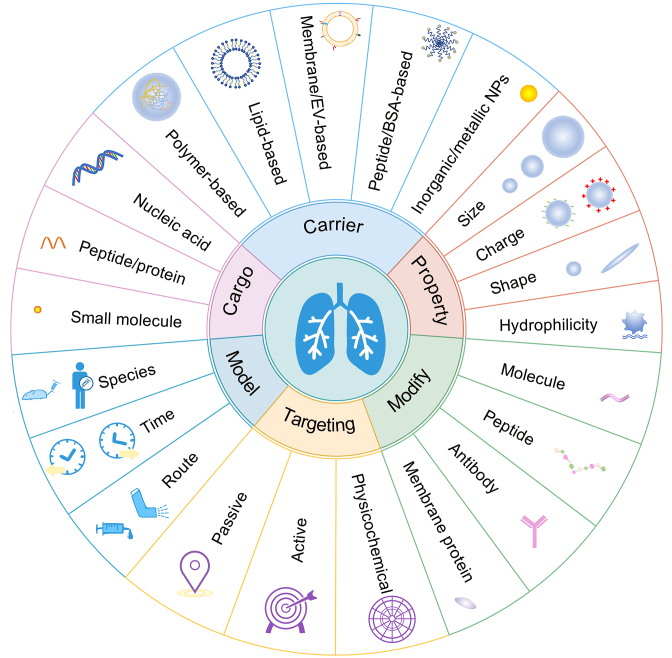
Nanomedicine refers to a new type of drug delivery system made of drugs and carrier materials with particle size of 10–1000 nm. There are many types of nanomedicine, such as liposomes, polymer micelles, dendrimers, inorganic nanocarriers, extracellular vesicles, etc.50 Nanomedicine has shown the characteristics of improving drug stability, reducing side effects, targeting to specific sites or even specific cells that can be hardly reached by general drugs, achieving precise drug release, etc., and its function can be designed according to requirements51. Owing to the unique characters, more and more nanocarriers with excellent properties are discovered and synthesized. Nanomedicine has been widely used in the delivery of various agents with different structure, physicochemical property, and pharmacological activity for ARDS treatment. Supporting Information Table S125,27, 28, 29,52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 summarized the basic properties of drugs in nanocarriers for ARDS treatment.
The principal reason of employing nanomedicine for ARDS is to improve the therapeutic index of active agent. Free drugs may fail to reach the desired site of effect with required dose, and the drugs in non-target organs may be associated with undesired side reactions76. Wrapped with nanocarriers, free drugs can be protected from degradation in the blood circulation, thus improving the pharmacokinetics and pharmacodynamics. Besides, further modification of targeting ligands on nanocarriers can endow the delivery systems with targeting capacity. Therefore, nanomedicine may provide a promising paradigm by preferentially boosting drug concentration, enhancing lung targeting, and thus limiting the toxicity and side effects. Compared with traditional formulations, nanomedicine can improve the bioactivity and provide versatile means to control the release behavior of agents. Importantly, ARDS patients are more prone to suffer from the dysfunction of multiple organs, and nanomedicine can reduce side effects by improving the targeting efficiency.
3.2. Advantages and disadvantages of nanomedicine in ARDS treatment
Nanocarriers have distinctive advantages in solving the defects of conventional drug or gene therapy for ARDS. They have revealed great potentiality for critical illness management, with manifold benefits such as improving the biopharmacokinetics of drugs, achieving targeted delivery to increase efficiency while reducing toxicity, and realizing co-delivery of agents for early diagnosis and synergetic treatment21. Moreover, some materials with inherent anti-inflammatory or antioxidant activity have been proposed as favorable carriers in ARDS treatment77, 78, 79. Additionally, several kinds of materials demonstrate potential antiviral activity, which could help to fight against virus-induced ARDS80,81.
3.2.1. Improve biopharmacokinetic properties of therapeutic agents
A variety of nanocarriers display desirable features in drug delivery, especially for improving solubility, stability, or activity of hydrophobic agents, nucleic acids, enzymes, and peptides70, 71, 72,82, 83, 84, 85. Inhibitors or antagonists of key inflammatory pathways that act on cells or mediators have been extensively inspected for ARDS treatment, such as phosphodiesterase 4 (PDE4) inhibitor86,87, neutrophil elastase inhibitor16, IKK-2 inhibitor27, and chemokines receptor 2 antagonist88. Some of them encounter unsatisfied characteristics such as inadequate solubility, instability, and short half-life in vivo, severely impeding the therapeutic efficacy and leading to unsatisfactory clinical outcomes83. For instance, the Src tyrosine kinase inhibitor (PP2) can be applied to treat acute lung inflammation, while its low solubility restricts its use. To minimize the component of solvent dimethyl sulfoxide (a toxic organic solvent to increase the solubility for injection), a nanoformulation was developed to improve the solubility, employing self-assembled peptides (EAK16-II) together with amino acids83. Therefore, the biocompatibility was significantly enhanced, displaying reduced infiltration of inflammatory cells and secretion of TNF-α in pretreatment for ARDS. Exogenous NE inhibitor, sivelestat, has shown benefits in ARDS mouse model, but it is still controversial in clinical trials89,90. It may be due to the inefficiency of drug administration, as sivelestat is a small molecular selective inhibitor with poor pharmacokinetics and requires multiple doses91. To improve the efficacy, lipid-based nanocarriers were fabricated for sivelestat delivery, which executed effective inhibition on neutrophils and provided better therapeutic effects over free sivelestat70. Endogenous NE inhibitors, such as secretory leukocyte protease inhibitor, failed to tackle NE activity92. In another study, recombinant secretory leukocyte protease inhibitor was encapsulated in liposomes with further micronization into liposomal dry powders92. The liposomal dry powders improved structural integrity and the size stability during storage, maintained the activity of anti-NE and protected it from degradation by cathepsin. Enzymes like DNase-I and catalase are used for suppressing inflammation, but the instability can discount their effect. Various nanocarriers have been developed to achieve effective delivery by keeping the enzyme activity and extending action time71,72,84.
Importantly, nanocarriers can greatly alter the drug biodistribution in pre-clinical studies. Accumulated evidences indicated that nanoparticles may undergo distinct biodistribution behavior between healthy and ARDS animals, regardless of targeting modification56,64,78,93,94. It was found that cationic liposomes demonstrated up to 1.54-fold accumulation in inflamed lungs compared to healthy ones93. Similarly, β-cyclodextrin nanoparticles reached 1.3-fold accumulation in inflamed lungs78. Besides, it is documented that cationic liposomes consisting of 50% (mol/mol) 1-diolefin-3-trimethylaminopropane (DOTAP) generated up to double accumulation in the inflamed lung tissues compared with healthy lungs in rats after intravenous (i.v.) injection93. The apparent difference in biodistribution of nanocarriers between normal and ARDS animals reflects the leakage of vascular system during the pathophysiological process, which may promote the passive targeting of nanocarriers, thus improving therapeutic efficiency. Furthermore, active targeting modification on nanocarriers can be realized by taking advantage of specific and highly expressed molecules in the inflammatory microenvironment, to accomplish more precise distribution57.
3.2.2. Enhance therapeutic efficiency and reduce toxicity
For the fact that ARDS patients are often vulnerable to multiple organ dysfunction, non-target side effects of drugs may aggravate the progress of the disease95. Therefore, there is tremendous clinical significance to realize targeted delivery for increasing efficiency while reducing toxicity.
Both passive and active targeting strategies can motivate lung distribution and cellular uptake, ultimately promoting the therapeutic effects while avoiding adverse reactions. For instance, glucocorticoids are regarded as potent anti-inflammatory drugs, while clinicians have to weigh the benefits and detriments. In this regard, multiple nanocarriers have been utilized to improve the therapeutic efficiency and decline side effects, including polymeric nanoparticles96, liposomes28,58,61, nanostructured lipid carriers56, polymer micelles55, nanogels59, bovine serum albumin (BSA) nanoparticles62, and monocyte membrane-derived vesicles60. For another example, PDE4 inhibitors can be detrimental due to unintended brain distribution. Encapsulation into lipid-based nanocarriers such as nanovesicles and nanoemulsions can reduce their biodistribution in the brain considerably86,87. Statins possess anti-inflammation and antioxidant properties, while the potential hepatotoxicity discourages their clinical application97. Thereby, nanostructured lipid carriers with intercellular adhesion molecule-1 (ICAM-1) antibody modification were adapted for the targeting delivery to provide effective therapy with negligible side effects65,66.
3.2.3. Realize co-delivery of agents for diagnosis and treatment
Combination therapy has been proposed for effective ARDS treatment30,52,66,96,98,99. Currently, combination strategies have mainly focused on gene therapy with anti-inflammatory or antioxidant agents, such as dexamethasone96, resveratrol99, curcumin29, and LPS binding peptide30. Polymer or lipid-based nanocarriers and peptides can be used to deliver various nucleic acids, including cDNA, plasmid DNA, siRNA, and miRNA29,30,66. Multiple drugs can be incorporated into nanocarriers to accomplish the co-delivery for improving delivery efficiency, diminishing off-target side effects, and exerting coordinated therapeutic functions29,30,96,99. Polymers can serve as an ideal gene carrier for ARDS therapy73,100. For example, dexamethasone-conjugated polyamidoamine achieved higher efficiency in delivering the adiponectin gene with reduced pro-inflammatory cytokines96. Cholesterol conjugated polyamidoamine was utilized for heme oxygenase-1 (HO-1) gene delivery99. Meanwhile, the hydrophobic drug resveratrol was loaded into the hydrophobic core of polymer micelles for pulmonary inhalation. Consequently, the polymer micelles exerted more effective transfection capacity and significant inhibition on transcription factor nuclear factor-κB (NF-κB) through the combined delivery. Besides, polyetherimide (PEI) with deoxycholic acid conjugated was employed as a carrier for the co-delivery of HO-1 plasmid and LPS binding peptide30. This ternary complex exerted higher transfection efficiency, thus effectively reducing the expression of HO-1 as well as the level of pro-inflammatory cytokines in the lungs. Peptides could also be consumed as versatile gene carriers for co-delivery. For instance, R7L10 peptide was employed for the co-delivery of curcumin and plasmid DNA to the lungs29. Despite the lower transfection efficiency in vitro, R7L10-curcumin demonstrated lower cytotoxicity than PEI (25 kDa) and indicated an opposite trend of transfection efficiencies in vivo. With the help of R7L10 peptide, the curcumin delivery efficiency was also improved by increasing solubility and enhancing uptake. Besides, complex formation with plasmid DNA can also function as a structural element to enhance the delivery of hydrophobic agents. R3V6 peptide, with the composing of both positively charged and hydrophobic regions, can form micelles and complexes with siRNA through charge interaction31. Thus, it was utilized to form nanoparticles with sphingosine-1-phosphate lyase (S1PLyase) siRNA and recombinant high mobility box-1 box A peptide to realize the co-delivery for treating ARDS. The complex demonstrated a higher uptake and delivery efficiency of siRNA than lipofectamine and PEI and exerted a synergistic effect in reducing the inflammatory response, which may be attributed to the combinational therapy.
Additionally, nanocarriers have been applied for other collaborative strategies. Cationic liposomes encapsulated with various antioxidants [N-acetylcysteine (NAC), vitamins C and E] manifested a remarkable decrease in oxidative damage, which contributed to the restoration of redox balance in cecal ligation and perforation (CLP)-induced lung injury52. Moreover, nanoparticles can provide a platform for ARDS diagnosis and treatment55. For instance, a diagnosis-therapy material was constructed by the conjugation of prednisolone with a two-photon fluorophore55. This nanoplatform not only achieved the effective control of acute inflammation but also realized the dimensional diagnosis of injured lungs, thus holding great potential for ARDS theranostics.
Nanomedicine has displayed many benefits and demonstrated potential in ARDS therapy. However, there also exist some disadvantages which have hindered their clinical applications. For example, nanoformulations usually integrate multiple drugs to exert synergistic effect, which adds the complexity with regard to scale-up, manufacturing and quality control. Besides, some nanomaterials possess the potential of activating inflammatory pathways which may aggravate inflammation. Importantly, although nanomedicine can enhance drug delivery efficiency to some extent, it remains unsatisfied. It is reported that merely a small portion (∼1%) of nanoparticles are able to reach the lesion sites of tumor after systemic administration, which indicates that off-target effects are existing101. Hence, it is still of great interest to optimize more specific targeting strategies to enhance drug delivery.
4. Parameters that affecting delivery and therapeutic efficiency of nanomedicine against ARDS
The factors that dominate the efficiency of drug delivery and therapeutic effect for ARDS are outlined in Fig. 2. Numerous studies have been conducted to tune the properties for optimal nanocarriers in ARDS treatment. Nanoparticle fate may be quite different for inflamed lungs due to permeability edema and complicated inflammatory environment caused by disordered inflammatory cells and overproduction of cytokines or enzymes102,103. Except for the fact that inflammatory conditions may alter the fate of nanoparticles, therapeutic scheme (intervention time and administration route), and physicochemical properties (size, shape, surface charge, hydrophilicity, and modification) can have a specific influence on the pharmacokinetics and cellular uptake behavior, which ultimately determine the therapeutic effect79,104.
Figure 2.
Parameters that affecting delivery and therapeutic efficiency of nanomedicine in ARDS. Various drugs of distinct properties are applied for ARDS treatment. Both organic and inorganic/metallic carriers have been employed for drug delivery. Physicochemical properties can be manipulated for optimal drug delivery, including particle size, charge, shape, and hydrophilicity. Through engineering approaches such as modification or conjugation with specific molecules, peptides antibody, or membrane proteins on the surface, nanocarriers can accomplish passive, active, and physicochemical targeting. The therapeutic effect of drug delivery systems was mainly conducted in animals with few studies on human lung sections. Therapeutic regimens including administration time and route can also influence drug efficiency.
4.1. Therapeutic schedule
In pre-clinical studies, therapeutic schedule can be diverse, depending on drug property, acting site, and targeting mechanism. Both systemic and pulmonary administration routes are adopted in ARDS treatment. The administration time and routes may impact the delivery and therapeutic efficiency to some extent79. As an example, the therapeutic outcome of polydopamine (PDA) nanoparticles was evaluated among different intervention timepoint post-LPS challenge via different administration pathway (i.v. or intranasally administration)79. It was reported that the earlier PDA nanoparticles were administrated, the better therapeutic outcome could be achieved in both injection ways. Given the rapid development of this disease, the time window for practical application of nanomedicine may be very narrow. In clinical, it was evidenced that the first week after ARDS onset represented a critical period for potential therapeutics105. It was also reported that the intranasal administration route achieved a better therapeutic outcome as indicated by a ∼1.3-fold decrease in both total protein content and neutrophil counts in bronchoalveolar lavage fluid (BALF)79.
The pulmonary administration route provides a non-invasive method for drug delivery to the lungs19. Various nanocarriers have been evaluated for pulmonary delivery, as listed in Supporting Information Table S2 29, 30, 31,52, 53, 54,61,73, 74, 75,79,83,96,99,100,106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116. Importantly, gene therapies usually adopt pulmonary administration to take immediate effect for ARDS treatment29, 30, 31,73, 74, 75,96,99,100. In addition, some antioxidant and anti-inflammatory molecules delivered by nanocarriers also conducted pulmonary administration52,53,61,83,112. Nanoparticles administrated through pulmonary route need to surmount multiple structured layers or biological barriers, including mucus, epithelium, endothelium, interstitial space, pulmonary surfactant, and innate immune system117. Physicochemical properties, including particle size, surface charge, and hydrophilicity, could impact the clearance and translocation of nanoparticles in lung tissues118. As physicochemical properties that affect the fate of nanoparticles after lung deposition have been excellently reviewed118, we mainly focus on factors influencing non-pulmonary drug delivery for ARDS treatment and provide general discussions in the following parts.
4.2. Size
Particle size has a significant influence on the fate of nanoparticles, which can alter physical properties of nanoparticles, resulting in the differences in drug release, cellular uptake, and pharmacokinetic behavior in vivo119. For example, larger-sized nanoemulsions showed lower lipophilicity than smaller-sized nanoemulsions67. Thus, the drug release rate was much slower than smaller-sized formulations.
In general, enhanced lung accumulation and uptake by phagocytic cells is correlated to the increase of particle size67,120. Gentile et al.121 reported that larger particles were more likely to attach firmly to blood vessels' walls in the case of flow than smaller ones. For instance, microspheres of different sizes (250, 423, and 851 nm) exhibited various biodistributions and cell uptake activities, among which the largest one exerted the best lung targeting property and cellular internalization64. Likewise, simvastatin-loaded nanostructured lipid carriers of distinct sizes (from 143.7 to 337.8 nm) were also examined65. It was revealed that simvastatin-loaded nanostructured lipid carriers with the largest size exhibited the best distribution in vivo. In Yu et al.’s work, oleic acid-based nanoparticles of 105, 153, and 225 nm were fabricated to examine the size effect on inflammation inhibition87. According to reports, these nanosystems displayed a trend that the smallest size nanoparticles displayed the most ingestion by isolated human neutrophils. Nevertheless, the larger nanoparticles demonstrated a greater extent of pulmonary accumulation and presented a higher significant reduction in neutrophil recruitment and inflammatory mediators, including TNF-α, IL-6, and MPO.
However, there is still controversy as to which size of nanoparticles can achieve maximum lung aggregation in inflamed lungs. A study demonstrated that the smallest nanoemulsions displayed the most vigorous-intensity compared to the middle and larger sized nanoemulsions in ex vivo bioimaging of lungs67. The leaky endothelium may account for this opposite trend67. Small droplets can readily penetrate the endothelial wall. In contrast, the larger droplets showed better retention but poor permeability across the endothelium. Moreover, biodistribution detected by drug concentration also indicated that the smallest formulation held the highest ratios of lung/liver and lung/plasma.
4.3. Surface charge
The surface characteristic is also vital for lung accumulation and cellular uptake119. Both negatively charged and positively charged nanocarriers are investigated in ARDS treatment. It seems that positively charged nanocarriers can attain better accumulation and cellular uptake. Some cationic materials have been harnessed for enhancing lung accumulation via passive targeting66,93,122,123. For example, positively charged gene-loaded ICAM-nanostructured lipid carriers exerted ∼1.26-fold of lung accumulation than that of negatively charged ones66. Recent research reported a generally applicable strategy, namely selective organ targeting (SORT), in which lipid nanoparticles were designed to accurately edit extrahepatic tissues with the supplement of a SORT molecule122. For lung-targeted distribution in normal mice, with the increasing molar percentage of cationic DOTAP, luciferase expression can be transferred from liver to lungs after i.v. injection significantly and exerted selectively editing of lung epithelial and endothelial cells. DOTAP demonstrated satisfactory potential in aiding lipid nanocarriers for pulmonary targeting and the development of gene therapeutics in ARDS. However, the underlying mechanism for this passive targeting has not been fully understood. The possible reason may be ascribed to that cationic lipid-based nanocarriers could interact with angiogenic endothelial cells of negatively charged cell surfaces93.
However, there are also pieces of evidence showing that surface charge would not affect lung accumulation obviously or even on the contrary with the results described previously86. Anionic and cationic nanovesicles with a PDE4 inhibitor loaded were examined for the comparison of anti-inflammation effects86. The study exhibited that both cationic and anionic nanovesicles enhanced the distribution of lungs, while no significant difference was observed between them. However, the cationic nanovesicles showed better inhibition on neutrophilic inflammation, thus dramatically reversing alveolar wall damage and reducing the pro-inflammatory cytokines. Another study showed that anionic nanostructured lipid carriers were more likely to distribute in inflammatory site than the cationic carriers in ARDS mice56. And it also enhanced cellular uptake of activated endothelial cells in vitro and exhibited a more robust distribution of 1.25-fold than cationic ones in inflamed lungs in vivo.
4.4. Shape
Although the morphology of nanoparticles has proved to be associated with cellular internalization, shape effects have not yet been completely elucidated in ARDS treatment124. The extensively studied particles in vivo are spherical. The trend of cell uptake may vary from cell to cell125,126. ICAM or platelet-endothelial cell adhesion molecule (PECAM-1) antibody-coated spheres were internalized more efficiently by endothelial cells than polymorphous conjugates, which could further benefit the endothelial delivery of superoxide dismutase and exert their protection against ROS125. For macrophages, particle shape is central for the phagocytosis process, and elongated particles are known to undergo reduced uptake126. In contrast, both in vitro and in vivo studies have exhibited opposite trends that polymeric rod particles were preferentially phagocytosed by neutrophils compared with sphere-shaped particles, which could offer an opportunity for selective targeting in acute inflammations featured with excessive neutrophils126. Further understanding of how physical parameters of particles influence their specific interaction within various immune cells will inspire us with more specific and feasible designs for drug delivery in ARDS treatment.
4.5. Hydrophilicity
Hydrophobic nanoparticles have long been regarded to boost immune activation, while hydrophilic nanoparticles can modulate the immune system in the opposite direction106,127. The underlying mechanism may be ascribed to that hydrophobic portions are essential parts of endogenous and exogenous immune stimulators, which may initiate innate immune responses106. The role of hydrophilic polymer nanoparticles in immune regulation was studied by exploring poly(ethylene glycol) (PEG), poly(carboxybetaine) (PCB), and poly(sulfobetaine) hydrogels106. In vitro and in vivo findings revealed that hydrophilic nanogels could weaken the immune response promoted by LPS and rebuild the balance of the immune system. Among them, PCB nanogels possessed the highest degree of hydrophilicity and showed the most significant relief of immune response, as indicated by a reduced number of infiltrating cells as well as levels of TNF-α and IL-6, and further observation of decreased phagocyte activation. Thus, the immune-regulation ability of nanoparticles may be associated with their hydrophilicity, which helps to resist non-specific binding. Since surface hydrophilicity is closely related to the immunomodulatory properties, modifying these factors can reduce possible pro-inflammatory properties of delivery systems and make them suitable drug carriers128.
4.6. Surface modification
To achieve better accumulation and retention in the lungs, surface modification on nanoparticles has been widespread74,129, 130, 131, 132. For pulmonary delivery, surface modification can help to penetrate mucus barrier. Mucus is responsible for nanoparticle clearance as it exerts functions to trap and remove pollutants and pathogens intrinsically117. Hence, the rational design of nanoparticles that could penetrate mucus barriers while keeping their therapeutic properties is of great significance for powerful pulmonary delivery. PEGylation and fluorinated functional decoration have been reported to facilitate the transport of nanoparticles across mucosal barrier74,130. For non-pulmonary delivery, PEGylation has also be hired for extending the blood circulation71,72. Importantly, small molecules, antibodies, peptides, and membrane proteins can be harnessed as targeting ligands to facilitate the delivery to desired areas, thus improving the therapeutic index. Surface modification can achieve the targeting for specific cells, including vascular endothelium cells and immune cells, which will be introduced in detail in the following parts.
The fate and impact of nanoparticle delivery in ARDS situations have not been investigated or recorded in detail. Deeper understanding of the influencing factors in the nanocarriers-based delivery system is of great importance to improve lung biodistribution and evade the possible but unwanted clearance or side effects in vivo, ensuring subsequent good therapeutic effect. More detailed surveys on the progression of barrier disruptions, corresponding vascular permeability, and other characteristics of inflammatory environment may provide directions on the property optimization of nanocarriers and delivery strategies for ARDS therapies14.
5. Strategies for targeting delivery in ARDS treatment
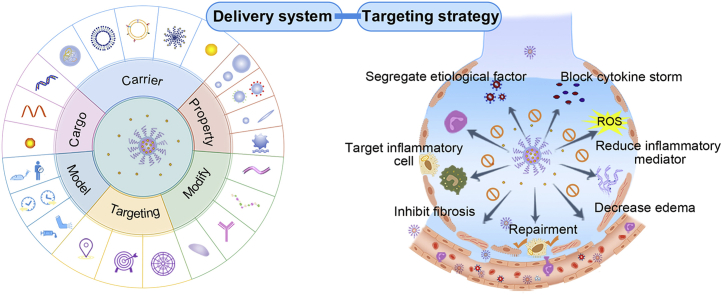
Targeted delivery strategies have been generally applied in ARDS treatment, especially for systemic drug delivery, which remains a common choice for nanodrug therapy. It was observed that leaky vasculature could form due to the destruction of pulmonary interstitial and alveolar structure39,133. Taking advantage of pathological features, nanocarriers can exert their specific strengths through passive targeting in circulation25. Active targeting drug delivery systems via specific modification of ligands can be designed to boost the therapeutic efficacy. Stimulus responsive materials based on ROS, MPO, NE, or other excessive pro-inflammatory mediators, can be devised for inflammatory microenvironment targeting. Besides, pulmonary delivery of nanocarriers have also been exploited in ARDS treatment by leveraging the direct interaction with lung environment and abnormal pathologic conditions. Taken together, nanocarrier-mediated drug delivery built on various targeting strategies have been investigated, as highlighted in Fig. 3.
Figure 3.
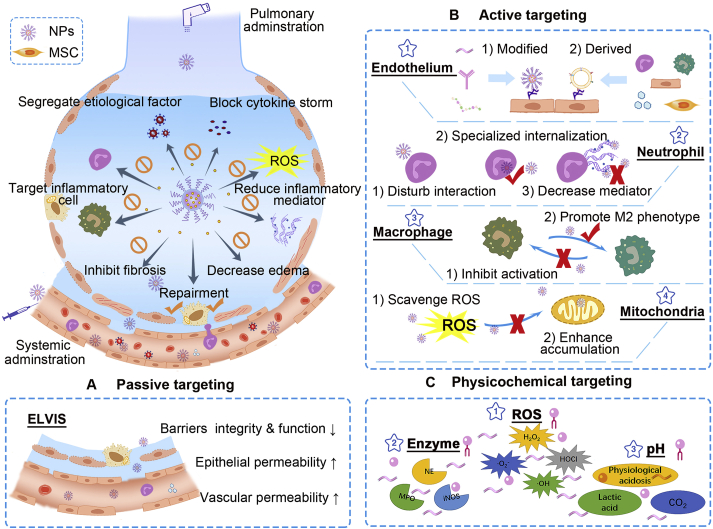
Nanocarriers-mediated drug delivery for ARDS therapy. Current targeting strategies have been focused on handling overwhelming inflammations and restoring pulmonary functions by inhibiting inflammatory cells, capturing toxins and cytokines, decreasing inflammatory mediators, and recovering the air–blood barrier. Passive, active, and physicochemical targeting tactics were applied. (A) The passive targeting delivery has primarily relied on ELVIS (extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration) effect. (B) Active targeting has been concentrated on inflamed endothelium, inflammatory neutrophils and macrophages, and impaired mitochondria. (i) targeting endothelium: nanocarriers with modification of particular molecules, antibodies, and peptides can be applied for targeting inflamed endothelium, where specific markers are highly expressed; biomimetic carriers derived from various functional cells (neutrophils, macrophages/monocytes, endothelial cells) can inherit good tropism to inflammatory endothelium; (ii) targeting neutrophils: particular nanocarriers can interfere with neutrophils to disturb their migrations to the lungs; some nanocarriers can be specifically internalized by activated neutrophils and hitchhiked to inflammatory site subsequently; nanocarriers can be employed for decreasing inflammatory mediators released by neutrophils; (iii) targeting macrophages: inhibiting pro-inflammatory M1 and promoting polarization to M2 phenotype; (iv) targeting mitochondria: scavenge ROS to protect mitochondria from damage; employing mitochondria-targeted materials for enhancing intracellular drug accumulation. (C) Physicochemical targeting: utilizing the aberrant inflammatory state such as excessive ROS, overproduced enzyme, and low pH to achieve site-specific drug delivery and stimuli-responsive release.
5.1. Passive targeting delivery
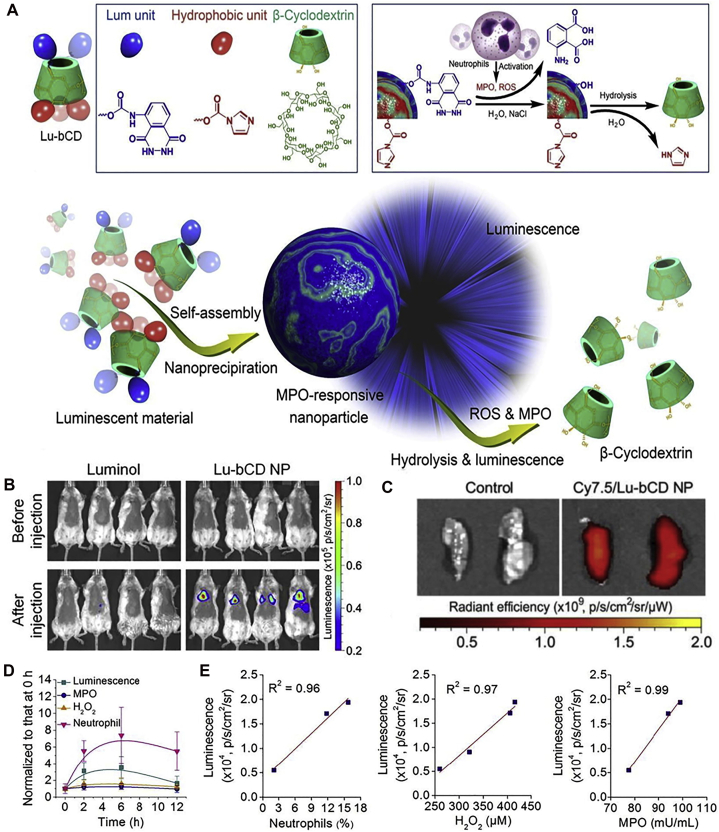
The lung is regarded as the most important organ for gas change, where the pulmonary vasculature occupies 25%–30% of whole endothelial surface in vascular networks134. Nanocarriers can passively accumulate in lung injury sites after systemic administration, which may be attributed to the increased permeability of pathological vasculature in inflammatory areas. The applications of nanomedicine for passive targeting delivery are summarized in Table 125,55,67,70,77,78,86,87,93,135, 136, 137.
Table 1.
Passive targeted delivery of nanomedicine in ARDS.
| Platform | Drug | Size (nm) | Charge (mV) | Animal model | Therapeutic schedule | Note | Ref. |
|---|---|---|---|---|---|---|---|
| PEI NPs | β2-Adrenergic receptor DNA | ∼60 | +30 | i.t., LPS, mice | i.v., 1 or 24 h after injury | Increased alveolar fluid clearance to reduce lung edema | 135 |
| Cyclodextrin NPs | – | 238 | −31 | i.t., LPS, mice | i.v., 1 h after injury | Internalized by inflammatory cells and inhibited their migration | 77 |
| – | 109 | −16 | i.t., LPS, mice | i.v., 1 h after injury | ROS-scavenging and anti-inflammatory properties; lung/liver: ∼0.4 | 78 | |
| Luminol | 228 | – | i.t., LPS, mice | i.v., not mentioned | MPO-responsive nanoplatform | 136 | |
| PMPC-PMEMA polymer micelles | Prednisolone | 57.5 | – | i.t., LPS, mice | i.v., 8 h after injury | High-resolution for pulmonary inflammation diagnosis | 55 |
| Liposomes | – | 100–200 | – | i.t., LPS, rat | i.v., 4 h after injury | Accumulated in the acutely inflamed sites selectively | 93 |
| Clodronate | – | – | i.t., LPS, mice | i.v., 2 d before injury | Depleted circulating monocytes and reduced neutrophil infiltration | 137 | |
| Nanovesicles | Rolipram | 154 | −34 | i.t., LPS, mice | i.v., 1 h before injury | Increased lung uptake, accumulation and drug biocompatibility; lung/liver: ∼0.9 | 25 |
| Cilomilast | 100.29 | +32.43 | i.t., LPS, mice | i.v., 30 min before injury | Inhibition on neutrophilic inflammation; lung/liver: ∼0.7 | 86 | |
| Oleic acid-based NPs | – | 105–225 | ∼−46 | i.t., LPS, mice | i.v., 30 min before injury | Reduced pulmonary neutrophil recruitment and inflammatory mediator | 87 |
| Interbilayer-crosslinked multilamellar vesicles | Sivelestat | 266 | −41.8 | i.p., LPS, mice | i.v., 1 h after injury | Inhibited the formation of NETs and decreased neutrophil elastase; lung/liver: ∼0.1 | 70 |
| Nanoemulsions | Rolipram | 68–188 | ∼−47.7 | i.t., LPS, mice | i.v., 30 min before injury | Internalized by neutrophils and reduced the distribution in the brain | 67 |
‒, not applicable; i.p., intraperitoneal; i.t., intratracheal; i.v., intravenous; LPS, lipopolysaccharide; MPO, myeloperoxidase; NETs, neutrophil extracellular traps; NPs, nanoparticles; PEI, polyetherimide; ROS, reactive oxygen species.
Under pathophysiological conditions of ARDS, the complicated inflammatory microenvironment and impaired lung air-blood barrier may hinder drug entry and penetration into disease lesions, affecting therapeutic efficacy138. On the other hand, nanocarriers can be designed, making use of those aberrant changes. EPR (enhanced permeability and retention) effect is accredited in tumor microenvironment, and on this basis, numerous nanoparticles have been devised to improve anti-tumor outcome139. Specially, a similar effect described as ELVIS (extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration) was proposed, which represents the abnormal neoangiogenesis and enhanced vascular permeability that occurs in inflammatory diseases140,141. The loss of endothelium/epithelium integrity and overproduced inflammatory mediators promoting vascular/epithelium permeability may contribute to ELVIS in ARDS. The alveolar injury of ARDS can be divided into three stages: exudation, proliferative, and fibrotic phase, indicating that vascular permeability changes dynamically with the disease progression93. In this regard, the leakage condition (leaky area and degree) may transform over time. In ARDS animal models, it was displayed that the increased lung endothelial permeability could last quite long duration, up to several weeks142. Due to nanoscale dimensions, nanocarriers can achieve passive targeting exploiting the leaky vascular system at injured tissues25,143. It was observed that with the extension of time, nanoparticles accumulated rapidly within 12 h, which may be attributed to the raised permeability caused by the injury of pulmonary endothelial and epithelial barrier55.
The biodistribution investigation revealed that most nanocarriers relying on passive targeting only increased lung accumulation to a limited extent and displayed a massive distribution in liver with a low lung-to-liver ratio25,67,70,144. For example, it was reported that sivelestat-loaded interbilayer-crosslinked multilamellar vesicles demonstrated a relatively low lung-to-liver ratio of ∼0.170. It suggests that although these nanocarriers have improved the efficacy over free sivelestat, the drug accumulation in lungs is still lacking. Cilomilast encapsulated nanovesicles and rolipram loaded phosphatiosomes amplified delivery to the lung via passive targeting, but neither of them showed superior accumulation in lung over liver, with a lung-to-liver ratio of ∼0.7 and 0.9, respectively25,67,86. Besides, a study using cyclodextrin-derived nanoparticles for passive targeting demonstrated that the fluorescence signal observed in the lungs was less than half of that in the liver78.
As concluded from these studies, most nanocarriers adopted for passive targeting have not performed outstanding targeting efficiency25,67,70,144. Overall, though passive targeting strategies have been commonly employed, their facility to enhance lung accumulation is insufficient, thereby more precise targeting is on demand for ARDS treatment. Based on ELVIS and other inflammatory features in ARDS situation, nanomedicine can be further exploited to achieve active, physicochemical, or combined targeting.
5.2. Active targeting delivery
Compared with passive targeting, active targeting strategy may provide more feasibility on engineering nanoparticles to target interesting cells using specific ligands, aiming for the optimal and accurate drug delivery138. In some cases, the decoration of functional molecules can not only endow the nanocarriers with targeting properties, but also participate in the regulation of inflammatory145. A variety of cells including immune cells, vascular cells, and alveolar cells, are intimately involved in ARDS development, thus targeting these cells may represent efficient ways. As listed in Table 226, 27, 28,56, 57, 58, 59, 60,62, 63, 64, 65, 66,68,69,71,72,94,145, 146, 147, 148, 149, 150, 151, 152, 153, active targeting therapies have mainly aimed at vascular endothelial cells, alveolar epithelial cells, neutrophils, macrophages, and organelle mitochondria in pre-clinical studies. Endothelial and epithelial cells are principal cells that are often injured in ARDS conditions. Neutrophils and macrophages are the two foremost immune cells that participate in lung inflammation42,154,155. With the understanding of pathophysiological mechanism, more and more functional components have been sought and exploited for targeted delivery and therapy28,145,156. Regarding the organelle level, mitochondrial targeting has also been developed to realize better antioxidant and anti-inflammatory effects64,157. With active targeting modification, the lung-to-liver ratio of particular nanocarriers could reach 3–7, which was much higher than that of conventional passive targeting, displaying superior lung accumulation28,65,146.
Table 2.
Active targeted delivery systems in ARDS.
| Targeting | Platform | Modification | Drug | Size (nm) | Charge (mV) | Animal model | Therapeutic schedule | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Endothelial | Liposomes | PECAM antibody | EUK-134 | 197.8 | −4.78 | i.t., LPS, mice | i.v., 15 min before injury | Decreased lung edema and inflammation; lung/liver: ∼4 or ∼7 (with two different antibody conjugation) | 146 |
| NLC | ICAM-1 antibody | Simvastatin | 354.7 | −32.1 | i.t., LPS, mice | i.v., 6 h after injury | Reduced side effects, decreased the infiltration of inflammatory cells and cytokines | 65 | |
| NLC | ICAM-1 antibody | Dexamethasone | 249.9 | −30.3 | i.t., LPS, mice | i.v., 6 h after injury | Targeting ability and enhanced internalization in activated endothelial cells | 56 | |
| NLC | ICAM-1 antibody | Simvastatin, protamine and angiopoietin 1 gene | 258–352 | 12.5–20.4 | LPS, mice | i.v., not mentioned | Up-regulated the expression of angiopoietin 1 protein, improved vascular permeability and reduced endothelial inflammation; lung/liver: ∼1.2 | 66 | |
| Polymer micelles | ICAM-1 antibody | TPCA-1 | 100 | – | Nebulization, LPS, mice | i.v., 4 h after injury | pH-Responsive, enhanced pulmonary deposition | 27 | |
| Lysozyme dextran nanogel | ICAM-1 antibody | Dexamethasone | 160 | – | i.v., LPS, mice | i.v., 15 min before injury | Reduced the off-target effect and blocked the expression of ICAM; lung/liver: ∼3 | 59 | |
| PLGA NPs | γ3 peptide | Sparfloxacin and tacrolimus | 183.7 | −40 | i.t., P. aeruginosa, mice | i.v., 4 h after injury | Targeted delivery to inflammation sites; lung/liver: ∼5 | 26 | |
| PLGA NPs | YSA peptide | Lipophilic fluorescent dye | 219.4 | −32.76 | i.t., bleomycin, mice | i.v., 4 d after injury | Improved lung accumulation; lung/liver: ∼0.2 | 94 | |
| Liposome-like nanovesicles | Macrophage membrane protein | – | 94 | −27 | i.p., LPS, mice | i.v., 30 min after injury | Interacted with macrophages as decoy and escaped from mononuclear phagocytic system | 147 | |
| Monocyte membrane derived vesicles | – | Dexamethasone | ∼130 | ∼−40 | i.p., E. coli OMVs, mice | i.v., 1 h before, 1, 3, and 6 h after injury | Inhibited the pro-inflammatory responses and reduced the adverse reactions | 60 | |
| Macrophage membrane camouflaged nano-iron oxide clusters | – | – | – | −19.5 | i.v., LPS, mice | i.v., immediately after injury | Adsorbed and separated LPS, neutralized pro-inflammatory cytokines; lung/liver: ∼1.2 | 148 | |
| Neutrophils-derived vesicles | – | Piceatannol | ∼180 | ∼−18 | i.p., LPS, mice | i.v., 2 h after injury | Reduced neutrophil infiltration and reversed pulmonary edema | 69 | |
| Platelet-derived EVs | – | TPCA-1 | 100–150 | −30 | i.t., LPS, mice | i.v., 4 h after injury | Inhibition on pulmonary inflammatory cells and blocked cytokine storms; lung/liver: ∼2.6 | 63 | |
| MPMVECs derived EVs | – | Syndecan-1 | 100 | – | i.t., LPS, mice | i.v., 2 and 12 h after injury | Improved the function of pulmonary microvascular barrier | 149 | |
| PEG-PLGA microspheres | Sialic acid | Curcumin | 852 | −24.2 | i.t., LPS, mice | i.v., 4 h after injury | Dual-targeting for inflamed lungs and mitochondria | 64 | |
| Neutrophils | BSA NPs | – | Piceatannol | 100 | – | i.p., LPS, mice | i.v., 2 h after injury | Denatured albumin nanoparticles for targeting activated neutrophils | 68 |
| BSA NPs | – | TPCA-1 | ∼130 | ∼−35 | Nebulization, LPS, mice | i.v., 4 or 12 h after injury | Transported across the vascular barrier and increased accumulation in lung tissues | 62 | |
| BSA NPs | Esbp peptide | Dexamethasone | 251.7 | −20.03 | i.t., LPS, mice | i.v., 24 h after injury | Improved the biocompatibility and avoided potential side effects; lung/liver: ∼0.25 | 57 | |
| MNSs | PDA | DNase-I | 170 | −10.9 | CLP, mice | i.v., 12 or 24 h after injury | Alleviated NETosis dysregulation and prolonged DNase-I circulation | 72 | |
| PLGA NPs | PDA | DNase-I | 217 ± 1.63 | −12.0 | CLP, mice | i.v., 12 or 24 h after injury | Decreased NETosis | 71 | |
| Macrophages | PLGA NPs | Siglec-E | – | – | +0.3 | i.t., LPS, mice | i.p., 2 h after injury | Regulated neutrophil infiltration and inhibited TLR-mediated inflammation | 145 |
| Au NPs | – | – | 24 | −32 | i.p., LPS, mice | i.p., 12 h after injury daily up to the seventh day | Reduced peritoneal leukocyte and regulated activity of oxidants | 150 | |
| Alveolar epithelial | Liposomes | SP-A antibody | Dexamethasone | 136 | – | i.t., bleomycin, rat | i.v., once daily for 1–2 weeks | Increased the concentration of dexamethasone and reduced adverse effects; lung/liver: ∼2.5 | 58 |
| Liposomes | SP-A nanobody | Methylprednisolone | 106 | – | i.t., bleomycin, rat | i.v., once daily for 2 weeks | Reduced the adverse effect and decreased the inflammatory cytokines; lung/liver: ∼3.9 | 28 | |
| Mitochondria | Porous Se@SiO2 nanospheres | – | – | ∼55 | – | Intragastric administration, paraquat, rat | i.p., every 24 h, for 3 days | Inhibition of ROS and reduction of NF-κB pathways | 151 |
| Cerium oxide NPs | – | – | 10–30 | – | Irradiation, rat | i.p., twice a week for 2 weeks | Served as radioprotector | 152 | |
| Cerium oxide NPs | – | – | 38.11 | +19.1 | Irradiation, mice | i.p., twice weekly for 4 weeks | Improved pulmonary function | 153 |
‒, not applicable; BSA, bovine serum albumin; CLP, cecal ligation and perforation; Esbp, E-selectin-binding peptide; EVs, extracellular vesicles; i.p., intraperitoneal; i.t., intratracheal; i.v., intravenous; ICAM-1, intercellular adhesion molecule-1; LPS, lipopolysaccharide; MNSs, melanin-like nanospheres; MPMVECs, mouse pulmonary microvascular endothelial cells; NETs, neutrophil extracellular traps; NF-κB, transcription factor nuclear factor-κB; NLC, nanostructured lipid carriers; NPs, nanoparticles; OMVs, outer membrane vesicles; PDA, polydopamine; PECAM, platelet-endothelial cell adhesion molecule; PECAM, platelet-endothelial cell adhesion molecule; PLGA, poly(lactic-co-glycolic acid); ROS, reactive oxygen species; Siglec-E, sialic acid-binding immunoglobulin-like lectin-E; SP, surfactant protein; TLR, Toll-like receptor; YSA, YSAYPDSVPMMS.
5.2.1. Endothelium-targeting therapies
The pulmonary endothelium performs as a critical regulatory interface between blood and alveoli, playing a pivotal role in the occurrence and further expansion of ARDS inflammation33. Endothelium damage has been confirmed in COVID-19 hospitalized patients as one of the essential features related to severe illness and death158,159. It is proposed that strategies to reduce endothelium damage may improve the prognosis of COVID-19. Endothelium-targeted drug therapies have been extensively employed in treating common acute pathological conditions138,160. Activated endothelium has offered targeting sites in the inflamed tissue for therapeutic nanoparticles138. The highly expressed surface markers on inflammatory endothelial cells prompt researchers to explore targeting modification160.
Multiple tactics have been engaged in endothelial targeting through ligand conjugation on the surface of nanocarriers160,161. Antibody and their derivatives, such as single chain variable fragments (scFv), peptides, and receptor ligands, are commonly used targeting ligands160,161. The targeting efficiency differs according to the type and affinity of ligands, as well as the size, shape, and surface charge of the whole system125,162. More advanced means inspiring by nature have been investigated using cell-derived biomimetic materials. Overall, the endothelium-targeted therapies can be recapitulated as follows, from the straightforward modification of antibody and scFv of adhesion molecules, to the conjugation of functional peptide/molecule, and eventually decoration of membrane protein on nanoparticles.
5.2.1.1. Modification of monoclonal antibody and scFv
Pulmonary endothelial cells can be activated with upregulated expression of cell adhesion molecules to platelets and leukocytes, such as PECAM-1 and ICAM, in response to ARDS163,164. Thus, antibodies, such as anti-PECAM-1 and anti-ICAM-1, could be modified on liposomes, nanostructured lipid carriers, polymer micelles, or nanogels to achieve the endothelial targeted delivery27,56,59,65,146. It is worth noting that the delivery of antioxidants usually accompanies endothelial targeted strategies, as antioxidants need to be delivered to lung inflammation areas for decreasing oxidative stress, and it is hard to reach effective concentrations when administrated as free forms138. Besides, the encapsulated antioxidants or anti-inflammatory agents can also exert their functions to decrease adhesion molecule expression, such as ICAM, which can serve as a pro-inflammatory factor and thus enhance therapeutic outcomes59,146.
Lipid-based nanocarriers such as liposomes and nanostructured lipid carriers have been extensively utilized for their capacity of encapsulating antioxidants or anti-inflammatory agents while providing extra targeting ability by anchoring antibodies146. Loaded with a potent antioxidant EUK-134 (EUK), the PECAM-1 antibody-coated liposomes (Ab/EUK/liposomes) were developed to target the vascular endothelium146. The Ab/EUK/liposomes obtained ∼15-fold of pulmonary uptake compared with IgG/liposomes (the negative control), thus remarkably reducing their non-specific uptake by liver and spleen. The formulation of Ab/EUK/liposomes exerted conductive protection against pulmonary inflammation and edema, attributed to the effective delivery and preferable accumulation in the lungs. Nanostructured lipid carriers have been popular in drug delivery for treating various lung diseases, attributing to its excellent biocompatibility and encapsulation properties165. Another anti-ICAM-1 antibody modified nanostructured lipid carriers were suggested for dexamethasone delivery56. Owing to the satisfactory targeting ability and enhanced internalization, this system significantly reduced the infiltration of pulmonary inflammatory cells and the production of pro-inflammatory cytokines in ARDS mice. Analogously, with the conjugation of ICAM-1 antibody, the formulated simvastatin-loaded nanostructured lipid carriers showed ideal lung targeting characteristics and reduced the infiltration of inflammatory cells as well as cytokines (TNF-α, IL-6) effectively, compared with free drugs or non-targeted carriers65.
In addition, polymeric micelles and nanogels can also be utilized for endothelial targeting delivery in ARDS treatment27,59. Polymeric micelles modified with anti-ICAM-1 antibodies were constructed27. The decoration of anti-ICAM antibody enhanced the lung deposition remarkably, thus achieving the goal of improving drug delivery and reducing inflammation and injury effectively. For another example, ICAM-1 targeted lysozyme dextran nanogels accomplished targeting delivery of dexamethasone to the lungs, as in vivo biodistribution showed of ∼12 times increase in pulmonary vasculature than that of non-specific nanogel59. As a consequence, the off-target side effects were reduced, and the expression of ICAM was blocked.
Moreover, endothelial targeting can also be applied in the diagnosis and imaging of ARDS166. Radiolabeled liposomes with PECAM-1 or ICAM-1 targeting were devised through copper-free click chemistry functionalization to obtain effective conjugation with antibodies or scFv166. With surface chelation with 111Indium, the radiolabeled liposomes acted as a direct and quantitative tracing tool for molecular imaging and visualized analysis. According to microSPECT/CT imaging, scFv decorated liposomes exhibited higher immune specificity than antibody-modified liposomes, especially for PECAM-1 targeting scFv-liposomes where 10-fold of accumulation was observed in the pulmonary vasculature. Thereby, this platform showed excellent potential in screening and evaluating active drugs for acute injury treatment.
5.2.1.2. Conjugation of functional peptide/molecule
Apart from cell adhesion molecules that can be changed in ARDS situations, other inflammatory markers, such as E-selectin, αvβ5 integrin, and ephrin type-A receptor 2 (EphA2), are also elevated26,64,94,167. Functional peptides and molecules that are able to bind with these highly expressed markers can also be anchored on the surface of nanocarriers to achieve targeted delivery. The roster of current functional peptides and molecules that are applied in ARDS treatment includes γ3 peptide26, YSA (YSAYPDSVPMMS) peptide94, E-selectin-binding peptide (Esbp)57, cyclic arginine glycine-d-aspartic acid (cRGD) peptide167, and sialic acid64.
Based on the characteristic that γ3 peptide can specifically bind to ICAM-1, γ3 peptide modified PLGA nanoparticles were constructed to obtain targeting delivery toward inflammation lesions26. Compared to blank PLGA nanoparticles, about 5-fold higher fluorescent intensity in the lung was observed with γ3-PLGA nanoparticles after i.v. injection. Besides, the lung/liver ratio can reach ∼5 according to ex vivo organ fluorescence signals at 16 h. Another peptide, YSA, can also serve as a targeting ligand94. It can mimic EphA2, a transmembrane receptor that is overexpressed in injured lungs. With high affinity to EphA2 receptors on cell surface, the YSA-functionalized PLGA nanoparticles enhanced the delivery to vascular endothelial cells and improved the lung distribution with a 1.3-fold increase compared with non-functionalized nanoparticles. Besides, cRGD-peptide could target lung αvβ5 integrin receptors. cRGD-peptide modified liposomes achieved the detection and visualization of lung inflammation sites with fluorescence dye DiR18 incorporated, providing non-invasive detection for ARDS167. For another example, with a high-affinity for E-selectin binding, Esbp modified BSA nanoparticles can increase the uptake by activated human umbilical vein endothelial cells in vitro57. Also, the Esbp modified BSA nanoparticles enhanced the lung accumulation, showing 1.5-fold higher than that of free dexamethasone, thus, improving the biocompatibility and circumventing potential side effects of glucocorticoid. Besides, sialic acid can also bind to E-selectins which are expressed in inflamed sites effectively64. Thus, sialic acid modified PEG-PLGA microspheres accomplished the targeted delivery to inflamed lungs.
Essentially, endothelial-targeted therapies achieved by modification of specific markers’ ligand can partially mimic the interactions of inflammatory cells with activated endothelial cells. These methods cannot mediate the strongest targeting as they are designed only based on single or several biological signals among multiple inflammatory markers. As an emerging technology, cell-membrane or extracellular vesicles-based nanocarriers have exerted inherent virtue for endothelial targeting, which will be introduced in a separate part (seen in Section 6.2).
5.2.2. Neutrophils-targeting therapies
As the most abundant and essential white blood cells in the innate immune system, neutrophils play a vital role in manipulating acute inflammation42. When ARDS occurs, neutrophils are firstly recruited to the lungs to resist foreign pathogenic microorganisms14,168. However, the accumulation of excessive neutrophils in the pulmonary microcirculation, interstitium, and alveolar spaces, can trigger uncontrolled inflammatory response, further leading to the damage of pulmonary microvascular and endothelial, and tissue injury154. Treatment strategy based on neutrophils has attracted much attention. It is reported that reducing the number of neutrophils alone may lead to a neutropenia-related infection154,169. Therefore, it is necessary to design more intelligent drug delivery system to effectively reverse the overactivation of neutrophils while keeping the normal function of neutrophils. Therapeutic strategies for targeting neutrophils involve disturbing neutrophils recruitment and migration, increasing internalization by activated neutrophils, and inhibiting neutrophil-derived inflammatory mediators.
5.2.2.1. Disturb neutrophils recruitment and migration
It is well-documented that neutrophils can leave the circulation and migrate to inflamed sites through complicated sequence of recruitment processes in response to environmental stimuli169. In this regard, various intercellular adhesions and migration gradient get involved in the interactions between neutrophils and vascular systems, which may finally cause immoderate neutrophils recruitment and migration170. Thus, nanoparticles that can segregate circulating neutrophils, or interrupt the interaction with recruitment mediators, may reduce the migration and infiltration of neutrophils towards lung tissues.
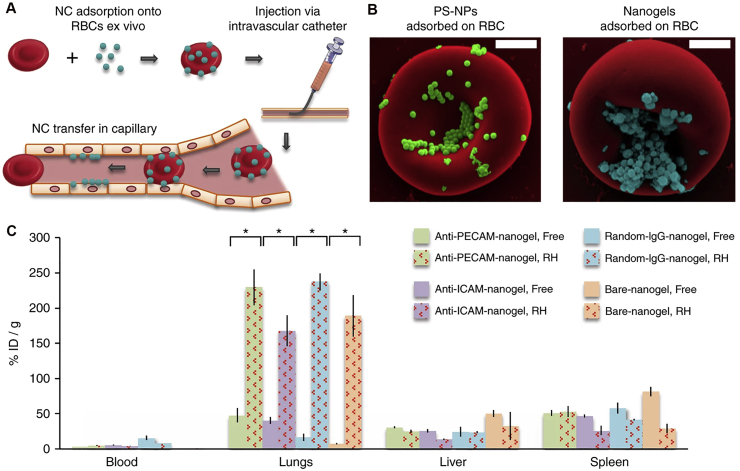
Polystyrene carboxylate-modified particles with size of 0.5 or 2 μm could interfere with neutrophils adhesion and migration within vasculature171. As observed in ARDS model, these particles displayed the capability of rapidly binding and sequestering with circulating blood neutrophils after i.v. injection, and decreased airway neutrophil accumulation as much as ∼95% versus particle-free BALB/c mice. Such findings may inspire researchers to search for novel designs toward trapping inflammatory cells such as neutrophils, monocytes/macrophages, and platelets, which could induce inflammation and injury during ARDS.
Inhibiting the interactions of neutrophils with endothelium is another approach to decrease the recruitment and migration of neutrophils61. The RGD-domain of extracellular matrix (ECM) ligands can interact with integrin receptors of endotheliocyte, contributing to the infiltration of neutrophils. Thus RGD-peptide was employed for blocking the interactions of neutrophil-ECM. Methylprednisolone loaded cRGD-peptide grafted liposomes (MPS-LcRGD) were manufactured for the spatial–temporal delivery61. The nebulized liposomes could deposit in the alveolar region to achieve high drug concentration in the lungs, demonstrating significant suppression of neutrophils infiltration and the release of pro-inflammatory mediators.
5.2.2.2. Increase neutrophils internalization and inactivate neutrophils
As critical immune cells, neutrophils can express a variety of surface receptors to trigger signal transduction and mediate effector functions169. Particular kinds of nanocarriers can interfere with activating cell surface receptors on neutrophils and be specifically hijacked by activated neutrophils in vivo68,172,173. Thus, taking those advantages to increase internalization by neutrophils and further release the encapsulated agents could inactivate the pro-inflammatory function of activated neutrophils.
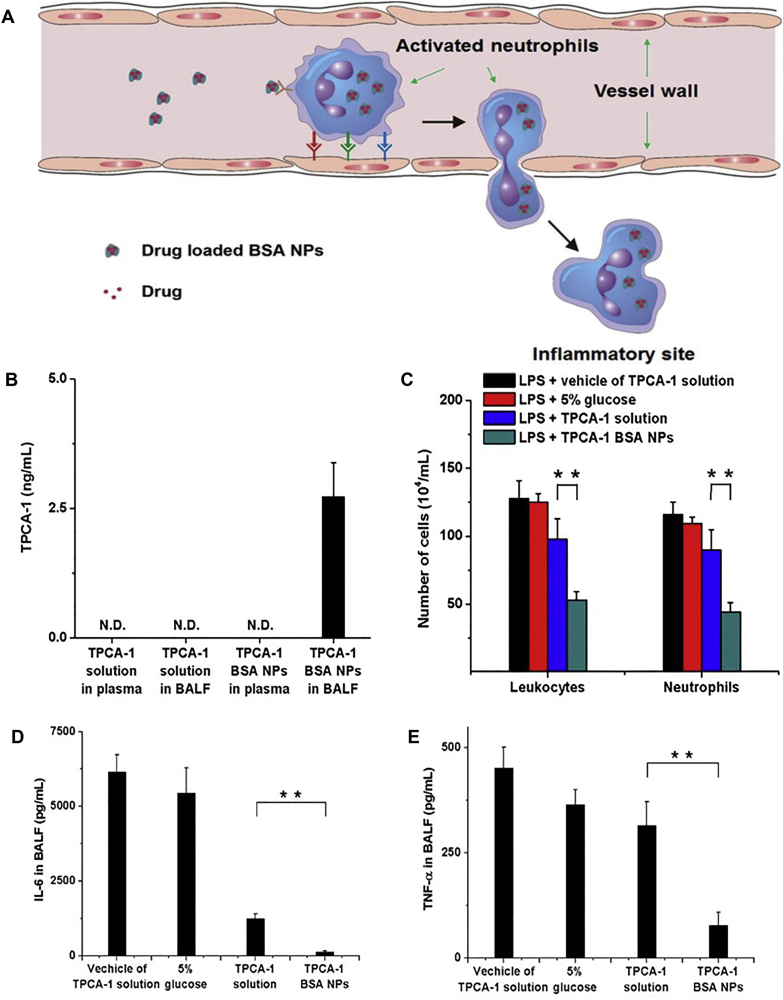
Under inflammation circumstance, a multitude of activated neutrophils with highly expressed Fcγ receptor (FcgR) adheres to the vascular wall174. Studies have evinced that denatured albumin nanoparticles can target these neutrophils and be specifically internalized, partly mediated by FcgR signaling68. Therefore, BSA nanoparticles loaded with anti-inflammatory drugs can be hijacked by in situ activated neutrophils, hitch a ride to the inflammatory tissue, and then be released to block downstream inflammatory pathways62,68,172. Given as an example, BSA nanoparticles with piceatannol loaded could block the β2 integrin signal transduction of neutrophils and reduce the adhesion and migration of neutrophils towards the endothelium significantly68. As the uptake of those nanoparticles depends on the vascular endothelial adhesion of activated neutrophils, they could target inflammatory neutrophils and avoid affecting the anti-bactericidal function of normal neutrophils in the circulatory system. Analogously, BSA nanoparticles with TPCA-1 loaded could be transported across the vascular barrier (Fig. 4A)62, achieving intensive drug accumulation in lungs (Fig. 4B), alleviating pulmonary inflammation (Fig. 4C−E), and reducing lung permeability effectively. Besides, antibiotic cephalosporin could also be encapsulated into albumin nanoparticles to decrease bacterial proliferation and treat acute pneumonia.
Figure 4.
Targeting neutrophils by drug-loaded BSA nanoparticles. (A) The schematic of neutrophils-mediated delivery of BSA nanoparticles to reach the inflammatory site. (B) TPCA-1 concentration in plasma and BALF after TPCA-1 or TPCA-1 BSA nanoparticles injection. (C) Cell count of leukocytes and neutrophils in BALF. (D) IL-6 and (E) TNF-α concentration in BALF after drug administration (vehicle of TPCA-1 solution, 5% glucose, TPCA-1 solution, or TPCA-1 BSA nanoparticles). All data represent mean ± SD (n = 3–4, per group). Statistics were performed by a two-sample Student's t test (∗∗P < 0.01). Reprinted with the permission from Ref. 62. Copyright © 2015, American Chemical Society.
Recently, another research found that liposomes composed of inverse phosphocholine lipids could rapidly enrich complement fragment iC3b and be hijacked by activated neutrophils via the interaction with cell-bound complement receptor CR3173. Then, the neutrophils containing liposomes could migrate across the alveolar-capillary barrier, accumulate in the inflamed lungs in a few hours, and release drugs to achieve remarkable therapeutic efficacy.
5.2.2.3. Inhibit neutrophil-derived mediators
Neutrophils can undergo a typical process, namely NETosis, to generate and release reticular NETs (consisting of DNA and proteins), thus performing their anti-infective activity175. However, it was conveyed that overproduced NETs may also cause tissue damage and be associated with the onset and activation of ARDS176. Thus, targeting NET-mediated pathology by blocking neutrophil-derived mediators provides new insight for ARDS.
NE is an essential inflammatory mediator formed by neutrophils, which can promote NETs formation. The inhibition of NE can suppress NETs induced tissue injury70. As an exogenous NE inhibitor, sivelestat has displayed outstanding performance in ARDS medication. Free sivelestat did not show efficacy in mouse suffered endotoxic shock, while sivelestat-loaded vesicles could promote drug uptake by neutrophils and remarkably improve the effectiveness in disturbing NET formation and reducing lung injury70.
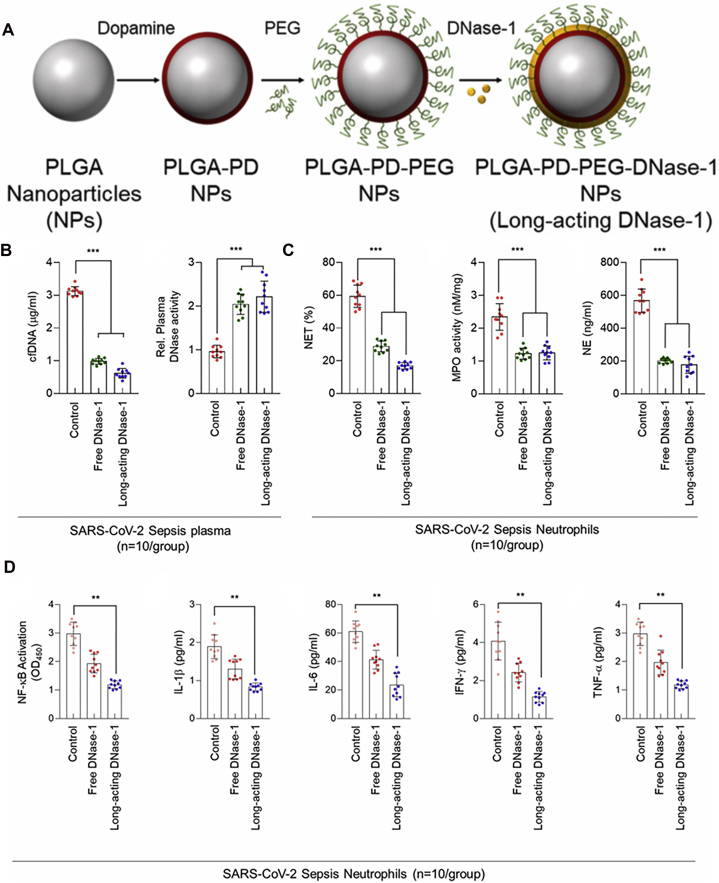
Besides, DNase-I, a kind of DNA degrading enzyme, is found to combat inflammation by inhibiting NETosis in experimental animals71,72. However, its short half-life has restricted the activity. PDA nanoparticles with this enzyme immobilized on the surface were developed to improve the stability, extend lung accumulation, and achieve long-acting in circulation (Fig. 5A)71. These nanoparticles maintained their activity and resolved sepsis-associated NETosis, as evidenced by the decline of neutrophil numbers and inflammatory markers in lung tissues compared with free DNase-I (Fig. 5B‒D). Likewise, another research employed PDA to settle DNase-I on the surface of melanin-like nanospheres72. This system also reduced the inflammatory response and alleviated lung damage in the septic ARDS model.
Figure 5.
Long-acting DNase-I nanoparticles for COVID-19 treatment. (A) Fabrication of long-acting DNase-I nanoparticle. (B) Quantitative analysis of plasma cell-free DNA (cfDNA) level and DNase-I activity from patient with SARS-CoV-2 sepsis after free DNase-I or long-acting DNase-I treatment (n = 10). (C) NET ratio, MPO, and NE concentration after free drug or long-acting nanoparticles therapy in SARS-CoV-2 Sepsis patient’ PBMCs. (D) NF-κB p65 binding activity and plasma cytokine levels with free DNase-I or long-acting DNase-I treatment in PBMCs from SARS-CoV-2 patients. The experiment was repeated at least three times. Statistics were analyzed using a two-tailed unpaired t-test. Data are displayed as mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001. Reprinted with the permission from Ref. 71. Copyright © 2020, Elsevier Inc.
5.2.3. Macrophage-targeting therapies
During the ARDS inflammatory activation process, the polarization of AM exists a dynamic conversion between pro-inflammatory M1 and anti-inflammatory M2 phenotype177. Once inflammation is triggered, circulating monocytes migrate to the lung, and resident AM undertakes a phenotypic transition from M2 to M1. M1 macrophages can clear pathogens through phagocytosis, and release pro-inflammatory cytokines/chemokines155. However, in the recovery phase, the M1 phenotype polarized into the M2 phenotype, and aging neutrophils can be swallowed, encouraging inflammation regression178. Therefore, targeting macrophages is of great value for hindering pulmonary inflammatory pathologies. It was reported that some nanoformulations could interact with macrophages to enhance the intracellular delivery, and further exert their functions such as regulating negative inflammatory receptors or pro-inflammatory signaling pathways of acute responses, and manipulating macrophage polarization for ARDS therapy179,180.
The surface markers on macrophages are commonly utilized for macrophage-targeted drug delivery. As negative inflammation regulatory receptors, sialic acid-binding immunoglobulin-like lectin (Siglec)-E receptors are expressed on the surface of macrophages, and they can abrogate toll-like receptor (TLR)-mediated responses181. In this regard, PLGA nanoparticles modified with natural Siglec ligands were exploited145. The constructed nanoparticles induced the oligomerization of Siglec-E receptors, thus promoting the secretion of anti-inflammatory cytokine IL-10, which in return enhancing the expression of Siglec-E and subsequently uptake of nanoparticles. Besides, anti-inflammatory effects were observed in both primary human monocytes and monocyte-derived macrophages.
Small-sized gold nanoparticles with peptide modification have been surveyed for their anti-inflammatory properties in adjusting macrophage phenotype and function112,113,115,182. A specific peptide-gold nanoparticle (GNP) hybrid, P12, consisting of hydrophobicity and aromatic amino acids, was proved with immunomodulatory activity182. P12 was verified effective in inhibiting TLR4-triggered activation of NF-κB and IRF3 and decreasing pro-inflammatory cytokines. Further studies have clarified the mechanism. It was discovered that GNP could inhibit TLR signaling pathways by targeting macrophages113. Besides, P12 could increase M2 alveolar macrophages while reducing M1 type in BALF as well as pulmonary alveolar and interstitial112. Moreover, active components of cigarette smoke extract were proved to inhibit the inflammatory response of TLR4. On this basis, cigarette smoke extract could adsorb onto the P12 hybrid to program the surface chemistry, thus significantly enhancing cellular uptake in macrophages115.
Besides, many drugs are reported to regulate the polarization of macrophages. For instance, spironolactone, a glucocorticoid receptor antagonist, participates in the regulation of inflammatory monocytes/macrophages. Spironolactone-loaded liposomes increased the bioavailability of drugs, inhibited Ly6Chi-mediated M2 polarization of alveolar macrophages, and thus significantly improved the pathological injury of lung tissue183.
5.2.4. Mitochondria-targeting therapies
Mitochondria can be attacked by excessive ROS and is often severely damaged in ARDS64,116. Antioxidants can diminish oxidative stress, and thus can be used to mitigate mitochondria-related damage. Antioxidants, such as NAC, curcumin, selenium (Se), and cerium oxide, have been broadly examined, whereas they may fail to exert satisfactory therapeutic effects for their non-specific distribution and low concentration at inflamed sites53,116. Therefore, nanocarriers were employed for antioxidants delivery into mitochondria to improve pharmacokinetics and protect antioxidative activity. NAC-loaded PLGA nanoparticles demonstrated superior properties in reducing protein and inflammatory cells, as well as decreasing levels of MPO and malondialdehyde, thus reducing lung inflammation and edema and preserving pulmonary architecture53. The improved outcomes can be attributed to enhancing NAC delivery to the lung with higher pulmonary concentrations and retention. Triphenylphosphonium cation (TPP) has been widely used as mitochondrial targeting ligands due to its unique structure with three benzene rings and delocalized positive charges, which can penetrate the double-layered hydrophobic mitochondria membrane184. TPP-modified curcumin can be loaded into PEG-PLGA microspheres to achieve mitochondria targeting64. In addition, the targeting strategy at the organ or organelle level can be combined in an effort to implement more efficient drug delivery. Thus, with further SA decorated, this dual-targeting system not only realized the inflammation targeted delivery, but also enhanced antioxidant effects. Other materials can also exert mitochondrial targeting facility. For example, curcumin could be encapsulated into amphipathic dequalinium formed vesicles (DQAsomes)157. In vitro studies verified that curcumin-loaded DQAsomes could achieve mitochondrial-specific accumulation, hence showing mitochondrial targeting ability and improving antioxidant activity markedly.
Inorganic nanoparticles with intrinsic antioxidant properties are also exploited116,151,152. Among which, nano-selenium is widely employed. As an ingredient of GSH peroxidase, Se can serve as a scavenger for intracellular free radicals. Nano-selenium has demonstrated favorable properties over other forms of Se, such as low toxicity, good bioavailability, and elevated competence in clearing free radicals185. For example, Se@SiO2 nanospheres were developed to minimize the side effects that Se may also cause damage to cellular components due to the catalyzing of the oxidation and generating of superoxide151. It could alleviate oxidative stress and inflammatory response through the inhibition of ROS and reduction of NF-κB, p-NF-κB, and inflammatory cytokines. Further investigation revealed that nanoparticles could target mitochondria to avoid mitochondrial dysfunction, thus providing cytoprotection for airway epithelial cells against oxidative injury116. Besides, cerium oxide nanoparticles could function as a radioprotector for normal cells, benefiting from their antioxidant properties152.
5.3. Physicochemical targeting delivery
Physicochemical targeting delivery has also been broadly used in ARDS treatment based on the inflammatory microenvironment characterized as extensive infiltration of inflammatory cells and overproduction of inflammatory mediators such as ROS, MPO, NE, and inducible nitric oxide synthase (iNOS). Based on this theory, a variety of bioactive or responsive nanocarriers have been created to realize targeted drug release and therapy for ARDS.
5.3.1. ROS scavenging or responsive
ROS participates in acute inflammation in response to pathogens and foreigners eliminating, while its overproduction can result in tissue injury186. Thus, ROS-scavenging has been a promising strategy to relieve oxidative stress for ARDS. Moreover, ROS-responsive materials have been devised to achieve the intelligent release of drugs. Besides, the ROS-responsive approach can also combine with other modifications for targeted delivery to lung inflammatory tissues187. Aside from antioxidative treatment, ROS-responsive materials also possess the ability for diagnosis55.
Various materials and agents exert ROS-scavenging properties79,84. With enriched phenol groups, PDA is recognized as a free radical scavenger79. PDA nanoparticles displayed a remarkable anti-inflammatory effect to ameliorate lung injury by eliminating ROS through direct reaction with ROS and decomposition of hydrogen peroxide (H2O2). Meanwhile, PDA nanoparticles exhibited abilities in suppressing neutrophils, which consequently diminished neutrophils infiltration and pro-inflammatory cytokines. Besides, with functionalized modifications for ROS-scavenging, a series of cyclodextrin (CD)-based nanoparticles were constructed77,78. Luminol-conjugated β-CD materials could form nanoparticles through nanoprecipitation and be degraded under oxidative conditions77. These intrinsically bioactive nanoparticles could obstruct inflammatory macrophage recruitment and prevent downstream pro-inflammatory proceedings such as production of H2O2 and inflammatory cytokines. On account of that one single antioxidant may not be enough to exert ROS-eliminating capability, another study designed functional β-CD based nanocarriers with two kinds of ROS-scavenging materials (tempol and phenylboronic acid pinacol ester) simultaneously linked78. The constructed nanosystem was endowed with broad-spectrum ROS-scavenging capability, showing efficient elimination of multiple ROS, such as radical, H2O2, O2·-, and ClO–. Besides, it could reduce oxidative stress-induced apoptosis in vitro and protect mice from ARDS in vivo. Catalase has strong ability to breakdown H2O2 molecules, while it exhibits poor stability and quick clean-off in vivo. Thus, nanocapsules with catalase embedded were proposed84. Through encapsulating catalase with a thin polymer shell via in situ polymerization, the fabricated nanocapsules demonstrated improved enzyme stability and high activity. Thus, the system showed superior properties on lung tissue protection against oxidative injury.
ROS-responsive materials have been broadly used for precise treatment through the introduction of stimuli-responsive bonds55. For example, a multifunctional nanocarrier with serial ROS responsiveness was constructed, which could accomplish the accurate delivery of prednisolone as well as the dimensional diagnosis of pulmonary inflammation55. A two-photon fluorophore and prednisolone were conjugated via ROS sensitive bond and encapsulated into amphipathic polymer PMPC-PMEMA to fabricate micelles. These micelles could accumulate in the inflammatory sites and respond to the overexpressed ROS. After interrupted by the ROS, the ROS-sensitive bond was broken, followed by the release of prednisolone to exert efficient anti-inflammatory activity. Furthermore, this system revealed high-resolution on pulmonary inflammation diagnosis through distinct two-photon imaging on account of the aggregation-induced emission, thereby displaying great potential in ARDS theranostics.
5.3.2. Enzyme responsive
In ARDS, many inflammatory cells can release proteases3. In this regard, various enzyme-responsive materials have been utilized to improve drug delivery efficiency136. For example, MPO is abnormally produced in neutrophils under inflammatory conditions. Based on this theory, biodegradable nanovesicles were fabricated by self-assembly of CD-derived materials with luminescence (Lu-bCD) for effective tracking, imaging, and quantification of activated neutrophils (Fig. 6A)136. This MPO-responsive nanoprobe showed specific and sustainable luminescence signals after triggering even in deep tissues of acute lung injury, implying a promising strategy for precise delivery of therapeutic drugs (Fig. 6B‒E).
Figure 6.
MPO and ROS dual-responsive nanoparticles for real-time imaging of inflammatory site. (A) The fabrication of luminescent materials with dual-responsive properties. (B) In vivo luminescence images of luminol or Lu-bCD nanoparticles before and after i.v. injection in ARDS mice. (C) Ex vivo fluorescence imaging of lung tissue after Lu-bCD nanoparticles treatment. (D) The luminescent intensities, MPO, and H2O2 levels, and neutrophil amounts in the lungs at different time points after LPS challenge. (E) Linear correlation analysis between luminescent intensity and neutrophil count, H2O2, and MPO level. Error bars, mean ± SD (n = 4, B and C; n = 6, D and E). Reprinted with the permission from Ref. 136. Copyright © 2017, Elsevier Inc.
NE-responsive materials were also exploited156. Taking advantage of the excessive neutrophil elastase released from the pulmonary inflammatory microenvironment, NE-sensitive peptides could be employed to achieve specific protease-triggered rapid degradation of microgels, leading to the release of encapsulated Nexinhib20 (a potent neutrophil degranulation inhibitor), for neutrophil inhibition156.
Based on the overproduced nitric oxide, polyphosphoester-based cationic nanoparticles were fabricated, which could undergo hydrolytic or enzymatic degradation into various fragments after entering cells. The degradation products mediated inhibition on iNOS expression, thus eventually suppressed nitric oxide overproduction188. Besides, multifunctional biosynthetic hybrid nanostructures were prepared to selectively bind and inhibit iNOS mRNA sequence that is overexpressed in the inflammatory site of ARDS189.
5.3.3. pH-Triggered release
Except for the elevated ROS and enzyme levels, lower pH than normal tissue was also reported27,190,191. Although the exact acidity and pH value in ARDS lungs has not been detected, several pH-responsive materials have been designed. In sepsis or trauma-induced ARDS conditions, severe metabolic acidosis usually occurs, which may induce respiratory acidosis, releasing lactic acid and CO2, and damaging normal cellular function192. Some medications have been proposed for correcting the acidosis. Besides, it is known that low pH values exist in subcellular organelles such as endosomes, lysosomes, and Golgi apparatus, showing different inner acidic microenvironment, ranged from 4.5 to 6.7191. Thus, pH-sensitive materials can be devised for responsiveness to physiological or pathological acidosis27,172,190. For example, pH-responsive polymeric micelles were designed for ARDS therapy27. This novel system was capable of encapsulating hydrophobic TPCA-1 and releasing drugs responsively under acidic environment. Besides, pH-sensitive BSA nanoparticles conjugated with doxorubicin (DOX) via hydrazine bonds were designed to target activated neutrophils specifically172. The internalized DOX-BSA nanoparticles could degrade in the acidic intracellular environment, release DOX to promote neutrophil apoptosis, thus protecting mice from sepsis-related lung injury significantly. In contrast, free DOX or BSA nanoparticles without pH-responsive properties failed to show substantial efficiency. Moreover, dual responsive of pH and ROS may provide more precise delivery to target inflammatory conditions190. ROS-responsive phenylboronic acid and acid-sensitive acetalated-dextran-based polymer were blended to obtain dual-responsive nanoparticles with high specificity to the inflammatory environment. With naproxen loaded, these nanoparticles could effectively reduce the level of pro-inflammatory cytokines, including IL-6 and TNF-α, to much lower degree190.
Taken together, endogenous triggers have been exploited currently based on the inflammatory situations to reach the site-selective delivery with stimuli-responsive and controlled release of encapsulated agents.
6. Novel drug delivery systems in ARDS treatment
Apart from the universal design of nanoparticles for drug delivery, other novel strategies have been emerging, including cell hitchhiking technology, bioinspired engineering, pulmonary surfactant-based strategy, and particle allosteric strategy, and nanovaccines.
6.1. Cell hitchhiking-based drug delivery system
Multiple circulatory cells including RBCs, neutrophils, monocytes/macrophages, and platelets, can migrate to lung tissues during the physiological or pathological process, such as blood circulation and inflammation homing193. Therefore, many innovative drug delivery systems have been widely developed by utilizing these features193, aiming to solve the limit of nanocarriers that most drugs will be eliminated in circulation and may not be able to reach the affected area directly194.
Cell hitchhiking technology has been engaged in drug delivery for ARDS therapy195, 196, 197. With the excellent flexibility and fluidity in the vascular circulation, RBC can serve as a versatile platform for hitchhiking nanoparticles to the targeted organ134,195,198. For example, nanoparticles could be adsorbed on RBC surface through non-covalent adsorption or electrostatic interactions, taking a hitchhike after intravascular injection to reach the lungs, and transferring to pulmonary endothelial cells through mechanical extrusion in narrow pulmonary capillaries, thus increasing the concentration of nanocarriers in lungs while reducing potential side effects (Fig. 7A and B)195. RBC-hitchhiking liposomes could dramatically increase the accumulation of liposomes in lungs by ∼40-fold after i.v. injection, compared with that of free liposomes. Furthermore, with the modification of active targeting ligand on RBC-hitchhiking carriers, their lung distributions were prominently elevated (Fig. 7C). It was evaluated that RBC-hitchhiking nanocarriers could be internalized by intravascular resident leukocytes in ARDS mice. Besides, in human ARDS lungs ex vivo, it was found that the deposition of hitchhiking carriers was 3.7-fold higher, compared with that of free carriers. Another study exploited RBC-hitchhiking strategies in treating lung cancers, where the delivery of non-targeted particles to lungs was significantly enhanced by ∼120-fold, in contrast to the conventional one194.
Figure 7.
RBC-hitchhiking for lung targeted delivery. (A) Scheme of RBC-hitchhiking: nanocarriers (NCs) were attached to RBCs, followed by the injection via an intravascular catheter, then the NCs transferred to the first downstream capillary. (B) Representative scanning electron micrographs of nanoparticles or nanogels absorbed on RBC (scale bars, 1 μm). (C) i.v. injection of RBC-hitchhiking NCs enhanced lung delivery. Mice were injected with nanogels that were uncoated (bare) or different antibodies (anti-PECAM, anti-ICAM, or IgG) coated, with or without RBC-hitchhiking. Data are plotted as % of the injected dose (%ID) per organ. Each data point represents mean ± SEM (n = 3). ∗P < 0.05, non-paired, two-tailed t-test. Reprinted with the permission from Ref. 195. Copyright © 2018, Nature Publishing Group.
Neutrophil or macrophage-based hitchhiking strategies have been adopted for acute and chronic inflammation targeting in treating various diseases such as skeletal muscle inflammation, myocardial ischemia reperfusion injury, neuroinflammation, and postoperative malignant glioma recurrence199, 200, 201, 202. Of note, nanoparticles by activated neutrophil hitchhiking in situ have been emerged, showing fantastic delivery properties to lung inflamed sites. For example, BSA nanoparticles and inverse phosphocholine lipids-decorated liposomes could be specially internalized by activated neutrophils in vivo, thereby accomplishing neutrophil-mediated delivery to inflammatory sites62,68,172,173. Thus, nanocarriers hitchhiked by these circulatory cells have provided promising way for optimizing pharmacokinetics and targeting delivery to inflamed lungs for ARDS treatment195.
Additionally, another approach hired Sertoli cells to carry curcumin-loaded chitosan nanoparticles (CUR-CS) for targeted delivery to deep lungs196. After i.v. administration, a high density of around 92% of CUR-CS distributed throughout the lungs, implying the potential in inhibiting deep lung inflammation of ARDS. Through cell hitchhiking, nanoparticles could also be used as a tool for detecting delivery in pulmonary197. Dual-polymer-coated UCNP-PEG-PEI nanoparticles were labeled on human amniotic fluid stem cells to obtain upconversion luminescence imaging in vivo. Through the imaging, it was found that human amniotic fluid stem cells performed much better than mouse bone marrow mesenchymal stem cells in terms of lung tissue repair, highlighting a promising technology for imaging-guided therapy in ARDS197.
6.2. Biomimetic drug delivery system
Cell membrane/EV-based delivery strategies hold great potential for effective countermeasures against overwhelming inflammations with multiple benefits in improving biocompatibility, exerting targeting properties, neutralizing endotoxin/virus or inflammatory derivatives, and modulating immune systems69,203,204.
6.2.1. Membrane-based nanoformulations
In essence, both modifications of monoclonal antibodies/single chain variable fragments and conjugation of functional peptides/molecules are designed to partly imitate the binding process of biological reaction, using the vital or representative bits to achieve the targeting delivery. More innovatively, instead of extra decoration of targeting agents, cell membrane-based biomimetic strategy has emerged in recent years, exhibiting great promise in combating diverse diseases203,205, 206, 207, 208. Taking advantage of their parent cells, the inherited biologic characteristics can endow the systems with fantastic biocompatibility and targeting properties203.
Macrophage membrane-based biomimetic strategies have been appealing for anti-inflammatory treatment60,147,148. Macrophage membrane camouflaged nano-iron oxide clusters could retain the TLR4 and CD14 receptors inherited from the parent membrane and possess the ability to capture and inactivate LPS that could otherwise trigger inflammation148. Meanwhile, the magnetic iron oxide core with positively charged could stabilize the membrane coating structure, adsorb and separate the negatively charged LPS, as well as neutralize different pro-inflammatory cytokines. Therefore, this biomimetic nanoparticle evidently improved lung morphology, including pulmonary edema and alveolar hemorrhage, reversed inflammatory infiltration, and reduced mortality.
A recent study has employed cellular nanosponges that demonstrated the power of inhibiting SARS-CoV-2 infectivity significantly (Fig. 8A)204. Human lung AEC II and macrophages membrane coated PLGA nanoparticles inherited similar protein receptors from parent cells, such as angiotensin-converting enzyme 2 (ACE2) and CD147, which were required by SARS-CoV-2 for cellular entry (Fig. 8B). This cellular nanosponge provided a broad-acting platform for neutralizing viruses and inflammatory mediators, which could benefit the inhibition of pulmonary inflammation and tissue destructions. Another finding employed decoy nanovesicles, prepared by fusing cellular membrane derived from human THP-1 and genetically engineered 293T cells expressing ACE2 (Fig. 8C)209. These nanodecoys efficaciously protected the lung from the infection of SARS-CoV-2 in vitro and exerted suppression on acute pneumonia in vivo, attributing to their cytokine neutralization and virus binding ability inherited from parent cells. As pneumonia and sepsis are listed as the most common etiology for ARDS3, it is also vital to cut the source, such as sweeping away toxins or pathogens. Besides, other nanomaterials that can trap or suppress inflammatory stimulations are in exploration210, 211, 212, 213.
Figure 8.
Biomimetic drug delivery systems for virus and cytokine neutralization. (A) The scheme of cellular nanosponges preventing viruses from entering host cells through surface antigen. (B) Western blotting analysis of cell lysate, cell membrane, and cellular nanosponges obtained from epithelial cells and macrophages. Data are presented as mean ± SD. n = 3. (C) The illustration of nanodecoy preparation. The nanodecoy was derived from cell membrane vesicles of human THP-1 cells and genetically engineered 293T/ACE2. Reprinted with the permission from Ref. 204. Copyright © 2020, American Chemical Society and Ref. 209. Copyright © 2020, the Author(s).
In the ARDS course, vascular endothelial cells are activated, and neutrophils migrate and aggregate to the lungs through the interaction between β2 integrin on neutrophils and ICAM-1 on endothelial cells. Based on the inflammatory tendency of neutrophils, neutrophils membrane can also be used to realize the targeted delivery to inflammatory sites168. With the aid of the inherent adhesion function of neutrophils, neutrophils-derived vesicles can target the inflammatory endothelium69. Prepared by nitrogen cavitation, the membrane protein composition of vesicles was similar to that of their parents32,69. Besides, pH gradient method was employed to encapsulate weakly acidic anti-inflammatory drugs to improve the loading rate. Hence, this biomimetic carrier effectively improved the potency of piceatannol, reduced neutrophil infiltration in lung tissue and reversed pulmonary inflammation and edema. Besides, human neutrophil membrane-derived nanovesicles co-loaded with ceftazidime and resolvin D1 were engineered, demonstrating superior properties in targeting pathogens and inhibiting inflammation pathways synergistically32. The nanovesicles showed much more accumulation in the inflamed lung after systemic administration, compared with RBC derived nanovesicles, owing to the interaction between integrin β2 on nanovesicles and the ICAM-1 on inflamed endothelium.
To combine the advantages of liposomes and membrane-based nanocarriers, liposome-like nanovesicles such as leukosomes were formulated by the fusion of macrophage membrane proteins and synthetic lipids147. The incorporated biomimetic nanovesicles were endowed with biological functions that could escape the capture of the mononuclear phagocytic system and target inflammatory endothelium. Leukosomes demonstrated the ability to interact with macrophages to modulate the expression of pro-inflammatory and anti-inflammatory genes. Besides, the leukosomes could regulate endothelial cells to reduce expression levels of representative cell adhesion molecules and chemokines147.
6.2.2. EVs-based nanoformulations
Extracellular vesicles (EVs) are lipid membrane-enclosed nano-sized vesicles, secreted by various cells that can mediate intercellular communication through the delivery of biological components such as lipids, proteins, mRNA, and miRNA214,215. EVs participate in numerous critical physiological and pathological processes by modulating immune systems and facilitating tissue regeneration216. Several types of EVs have been utilized as bioactive drug delivery vehicles for ARDS treatment, attributing to the specific targeting and transfer of diverse biologic cargos217.
6.2.2.1. Mesenchymal stem cells derived EVs
Mesenchymal stem cells (MSC) are recognized as a versatile cell in regenerative medicine and have demonstrated great potential in dealing with tissue injury such as COVID-19 related ARDS treatment218, 219, 220. With the favorable immune-modulating ability, MSC can target inflammatory sites and interact with other immune cells and lung endothelial or epithelial cells to exert multiple functions. The communication can be mainly achieved by EVs. Due to their non-cell structure with smaller size and inherited functions, MSC-derived EVs (MSC-EVs) have exhibited advantageous properties over MSC in several aspects such as decreasing the possibility of iatrogenic tumor formation, retaining activity while storage, and improving pharmacokinetics in circulation221. We note that superb reviews have summarized the applications of MSC-EVs for ARDS therapy. Thus, it will only be discussed briefly as follow221,222.
MSC-EVs can be derived from diverse stem cells of murine or human tissues such as bone marrow, adipose tissues, and umbilical cord blood, and their therapeutic effects have been explored in pre-clinical and clinical researches for ARDS223, 224, 225, 226. Many studies have revealed that MSC-EVs could transfer various substances such as miRNA, mRNA, proteins, and mitochondrion to realize the amelioration for ARDS217. MSC-EVs can not only target immune cells to exert immune regulating effects but also communicate with various pulmonary cells, including lung epithelial or endothelial cells, to adjust the lung permeability and repair damaged tissues221. A variety of methods have been arisen to potentiate the therapeutic efficiency of MSC-EVs, including pretreatment MSC with poly(I:C)227, interferon γ228, or IL-1β228,229. Besides, pretreated MSC through genetically engineering modification was also investigated to fabricate EVs with overexpressed functional elements such as ACE2230, IL-10231, and HO-1232. Furthermore, EVs can be adapted through various physicochemical methods to enhance the targeting ability of inflammatory nidus and carry therapeutic agents for combinational therapy, and thus, MSC-EVs have attracted more and more attention to ARDS treatment.
6.2.2.2. Platelet-EVs
Despite the fact that activated platelets may serve as pro-inflammatory cells to exacerbate the lung inflammation and injury in ARDS progression, it was reported that platelet-derived extracellular vesicles (PEVs) did not aggravate inflammation after reaching the inflamed regions63. Taking advantage of the intrinsic affinity of platelets to inflammation sites, the engineered PEVs improved the lung delivery of TPCA-1, exerted significant inhibition on the infiltration of pulmonary inflammatory cells, and blocked cytokine storm (Fig. 9A)63. The PEVs demonstrated 3.6-fold of enhanced lung accumulation in ARDS mice compared to normal mice, possibly due to their selective binding to activated/inflamed sites via receptor patterns (Fig. 9B and C), such as CD40L, glycoproteins, and P-selectin63,233.
Figure 9.
Platelet-derived extracellular vesicles for targeting inflammatory lungs. (A) Schematic of platelet-derived EVs with TPCA-1 loaded for pneumonia therapy. The inflammatory cytokine storm was remarkably reduced, as indicated by TNF-α, IL-6, and IL-1β. (B) Ex vivo imaging and corresponding fluorescence level of main organs after i.v. injection of DiD-labeled PEVs. Data are presented as mean ± SEM (n = 3–5). Statistical significance is calculated by one-way ANOVA using Tukey's post-test. ∗∗P < 0.01; ∗∗∗P < 0.005. Reprinted with the permission from Ref. 63. Copyright ©2020, Elsevier Inc.
6.2.2.3. Endothelial exosomes
Endothelial cells derived exosomes can deliver biomacromolecules such as proteins or nucleic acids111,149. Through lentivirus transfection on mouse pulmonary microvascular endothelial cells (MPMVECs), exosomes with up-regulated syndecan-1 (SDC1) were isolated149. This SDC1-high exosome could enter into endothelium and preserve the endothelial glycocalyx thickness, thus improving the function of pulmonary microvascular barrier. For another instance, exosomes derived from endothelial progenitor cells (EPCs) demonstrated therapeutic efficiency for acute lung injury by delivering miRNA-126, which can reduce alveolar and interstitial neutrophil infiltration, reduce MPO activity, restore the integrity of epithelial barrier system and reverse pulmonary edema111.
6.3. Pulmonary surfactant-related drug delivery system
When delivering drugs to deep lungs, it is hard to hurdle the biological barrier that consists of pulmonary surfactants, a thin film covering the respiratory surface of the lungs234. Pulmonary surfactants can be secreted by AEC, and their dysfunction was identified in ARDS235. Supplement of exogenous surfactants has been explored for a long time in the treatment of ARDS236. For example, natural surfactants prepared from porcine lungs (Curosurf®), constituting of phospholipids with a small amount of surfactant protein (SP)-B and SP-C, have improved the outcome of neonatal respiratory distress syndrome in clinical236. Besides, pharmaceutical liposomal products such as the synthetic lung surfactant Alveofact® for pulmonary instillation have entered the market for neonatal respiratory distress syndrome treatment237. However, no apparent therapeutic effect has been evidenced in adult ARDS for dose and technical problems. Specifically speaking, there is a dynamic adsorption–desorption process of pulmonary surfactant during the compression-expansion of the lung, and excessive pulmonary surfactant may be required to make it saturated for this function, and it is hard to dope out the proper dosage108. Notably, the inflammatory environment may inactivate the surfactant. Additionally, direct intrapulmonary administration has encountered technical problems as pulmonary surfactant is prone to foaming during the compression procedure when a large dose is required. Thus, efforts have been made to settle these problems based on nanotechnology by incorporating pulmonary surfactant lipids/proteins or searching for synthetic materials.
6.3.1. Phospholipids-based nanocarriers
Phospholipids are the main components of pulmonary surfactants, in which phosphatidylcholine (PC) is regarded as the predominant lipid that can be used to facilitate nanoparticle delivery to the alveoli238. The PEGylated PC-rich nanovesicles could target the lungs and maintain in circulation for a long time239. Rolipram, a PDE4 inhibitor that can suppress active neutrophils, was encapsulated in phosphatiosomes (novel nanovesicles containing PC)25. The phosphatiosomes attained the increased lung accumulation and good biocompatibility, which can be ascribed to the existence of phospholipids that probably interact with alveolar lipoproteins and generate the aggregates in the lungs. Besides, a decrease in brain permeability was exhibited, showing that these nanovesicles could diminish the toxicity to the nervous system. Analogously, another PDE4 inhibitor, cilomilast, was encapsulated into phosphatiosomes86. These phosphatiosomes also enhanced pulmonary targeting, thus increasing the therapeutic index of cilomilast. The targeting mechanism may be due to the abundance of PC components, which could interact strongly with dipalmitoylphosphatidylcholine (DPPC), the prime component of pulmonary surfactant, through hydrogen bond and hydrophilic bond240.
Nanovesicles with abundant soy phosphatidylcholine can also interact with pulmonary surfactants, which are beneficial for lung targeting239,241. Using soy phosphatidylcholine, Poloxamer 188, and mineral oil as carrier materials, highly lipophilic oleic acid could be encapsulated into lipid-based nanocarriers87. However, the pulmonary biodistribution was not as satisfactory because these nanocarriers demonstrated broad distribution in peripheral organs and exceptionally high in the gastrointestinal tract. Similarly, with the incorporation of SPC and Poloxamer 188, the constructed nanoemulsions were beneficial for pulmonary delivery241. With rolipram entrapped, these nanoemulsions could be internalized by neutrophils to exert anti-inflammatory effects in inflamed lungs67. The nanoemulsions significantly reduced rolipram concentration in plasma and promoted the distribution to peripheral organs. Importantly, a notable decline in brain distribution was detected, which helps to minimize side effects. However, the nanoemulsions still demonstrated more accumulation in the gastrointestinal tract and liver than other organs by ∼45% and 40%, respectively.
Nanovesicles of nonlamellar lipids using DPPC and phosphatidylethanolamine (DOPE) were developed as pulmonary surfactant aerosols110. The DPPC-DOPE nanovesicle aerosols improved the resistance of pulmonary surfactants to inactivation from inflammatory mediators and were expected to be utilized as a non-invasive aerosol therapy in ARDS.
6.3.2. Surfactant protein-based nanocarriers
As surfactant proteins are critical ingredients of lung surfactant, thus they can be utilized for alveolar epithelial targeting modification28,58,235. SP-A is highly and specifically expressed in AEC II. Thereby, SP-A antibody functionalized immunoliposome can be employed for lung-specific targeting58. Consequently, the concentration and residence time of dexamethasone in the lung tissue remarkably increased. However, the complete antibody may lead to rapid clearance from the circulation. Thus, anti-rat SP-A nanobody (rSPANb) with low molecular weight and low immunogenicity was further studied. Methylprednisolone was loaded into nano-sterically stabilized unilamellar liposomes with rSPANb functionalized28. Compared with the SP-A polyclonal antibody, these rSPANb conjugated nanoparticles exerted high affinity and stable structure, showing more senior safety and more specific lung targeting properties. For another example, lipoplexes with SP-C antibody conjugated could be a popular platform for microRNA delivery and treatment for pulmonary disease, attributing to targeting lung AEC II with specificity as much as 70%109. In addition, with a pulmonary surfactant layer coating, the reconstructed siRNA-loaded nanogels improved the stability of particles and enhanced the intracellular siRNA delivery observably75. With fusogenic properties, SP-B was identified to be the determining factor that enhanced cytosolic siRNA delivery. Besides, these hybrid nanogels could be efficiently internalized by resident alveolar macrophages and demonstrated low toxicity.
Instead of active ingredients such as lipids, proteins, and peptides, synthetic polymers can be exploited as promising candidates for pulmonary surfactants108. Poly(styrene-b-ethylene glycol) (PS-PEG) polymer nanomicelles displayed a strong affinity to the air–water interface, thus producing extraordinarily low surface tension under high compression108.
6.4. Particle allosteric strategy
For the pulmonary delivery of nanoparticles, an aerodynamic particle size of 1–5 μm is requested for inhalation to deliver the active ingredient to lower respiratory systems242, whereas particles with this geometric diameter are readily phagocytosed by macrophages and rapidly cleared. Most nanoparticles are out of this size range and might result in aggregation into larger particles or get exhaled243. However, smaller particles may achieve more effective intracellular delivery244. Those paradoxical constraints on particle size may obstruct the effective therapy for lung disease, and particle size allosteric strategy is promising to satisfy the specific needs for pulmonary delivery156. For instance, neutrophil elastase-sensitive nanoparticles-in-microgels (N-in-M) were designed (Fig. 10A)156. With a size under 200 nm, Nexinhib20-loaded PLGA nanoparticles could facilitate the intracellular delivery. PLGA nanoparticles were embedded into degradable microgels that were cross-linked by NE-sensitive peptides. With a diameter of 3.9 μm, the microgels achieved an appropriate aerodynamic size for deep lung deposition. The microgels were degraded triggered by neutrophil elastase, after which PLGA nanoparticles accomplished the delivery of Nexinhib20 to the airway and internalized by polymorphonuclear neutrophils, leading to substantial reduction of systemic and lung inflammation signaling (Fig. 10B‒D).
Figure 10.
Neutrophil elastase-sensitive nanoparticles-in-microgels for particle allosteric strategy. (A) The preparation of neutrophil elastase-sensitive nanoparticles-in-microgels (N-in-M) microgels and illustration of the mechanism of NE-responsive drug release. (B) MPO activity detected by fluorescence image of lung tissue. (C) Neutrophil numbers and (D) neutrophil percentage in BALF after treatment. Statistical tests: Shapiro-Wilk followed by Kruskal-Wallis (C) and 1-way ANOVA (D), with ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 between groups denoted. n = 5 each group. Reprinted with the permission from Ref. 156. Copyright © 2019, The American Society for Clinical Investigation.
6.5. Nanovaccines
Currently, many repurposed and antiviral drugs that aim to suppress ARDS symptoms have been approved to manage COVID-19245. As for direct prevention of viral infections, vaccines can serve as effective means to exert protective effect and fight against virus-induced ARDS246. Recently, nanovaccines have attracted many attentions. Multiple nanoformulations such as liposomes247, lipid nanoparticles248, protein nanoparticles249, and virus-like particles can be employed for nanovaccines. With high surface energy and sharing similar size distribution like virus, nanoparticles can imitate the virus to enter targeted cells and elicit anti-viral immune reactions245. Nanoformulations can provide advantages for vaccines, such as enhancing antigen stability, allowing sustained antigen release, and achieving targeting delivery via surface engineering245. Moreover, nanovaccines can achieve the codelivery of multiple antigens and adjuvant, improve the targeting efficacy on antigen presenting cells, thereby facilitating the activation of innate and adaptive immune system, and exerting effective vaccine responses249,250. For example, Ma et al.250 constructed ferritin nanoparticle vaccines with conjugation of both receptor-binding domains (RBDs) and heptad repeat antigens. Compared with monomers, these vaccines promoted more effective immune responses. Besides, they can produce cross-reactive immune responses, thus protecting against other coronaviruses. Walls et al.249 developed protein nanovaccines displaying sixty SARS-CoV-2 Spike RBD, which can induce robust neutralizing antibody responses to target multiple distinct epitopes. Thus, nanovaccines can be of great potential in the therapy for virus-induced ARDS.
7. Conclusions and perspectives
Based on the comprehension of pathogenesis, nanoparticles for early diagnosis, drug delivery, and gene therapy of ARDS have achieved considerable progress in the past decade. The major applications based on nanomaterials for ARDS can be summarized into four aspects: (i) employing as a carrier for small molecules, peptides, nucleic acids, etc., to improve drug solubility, stability, and bioavailability, or realize co-delivery; (ii) utilizing inherent antioxidant and immunomodulatory effects of materials; (iii) exploiting distinct nanoparticles which are prone to be internalized by targeted cells to provoke effective cellular delivery; (iv) overcoming multiple pulmonary barriers to enhance drug accumulation at inflammatory sites through passive or active targeting effect, or developing bioactive or responsive nanomaterials to obtain site-specific or stimuli-responsive drug release.
To date, precise nano-therapy for ARDS has primarily concentrated on the endothelium-targeted delivery, inhibiting pathological-related inflammatory cells by modification of targeting ligands and encapsulation of anti-inflammatory/antioxidant drugs, and promoting the drug accumulation in inflamed lungs and improving drug efficiency. However, it remains in the early stage, none of nanoformulations has entered the clinical trial phase, and the effective therapeutic strategy of ARDS is still facing great challenges:
Accurate model: Although distinct experimental models caused by various pathogenic factors have been established, none could fully reflect the heterogeneity, inflammatory exudation and resolution of human ARDS251, 252, 253. High-risk ARDS patients in clinical usually accompany by other complications and need respiratory support, where a higher mortality rate lies in elderly patients163. In this regard, the animal model, which is more in line with the clinical characteristics, such as using aged animals with decreased adaptive immunity, employing humanized mice to better replicate ARDS pathophysiology, or utilizing modified EVLP model, may predict the actual biodistribution and therapeutic effect of nanodrugs49.
Mechanism investigation: The excessive inflammation and impairment of air–blood barrier has been illuminated to explain ARDS complexity. For combating inflammation, studies have primarily centered on neutrophils and macrophages, whereas further investigation on the role of other immune cells, such as dendritic cells, B cells, T cells, etc., which may provide us with a brand-new direction to improve the effectiveness of targeting delivery and therapy. Besides, researches have been focused on the exudation stage of ARDS, there are relatively few studies about promoting the regression of inflammation. Many survivors suffered from pulmonary fibrosis in the later stage, thus preventing or inhibiting lung fibrosis is also essential. Expoiting the role of immune cells and understanding the dynamic change of ARDS pathophysiology may be helpful for novel therapeutic strategies.
Therapeutic direction: Current treatment emphasizes pulmonary inflammation management, while in many cases, ARDS could develop acute systemic inflammation and result in multiple organ failure. Thus, treatment of their complications should be considered. Given the complexity of ARDS disease, single-drug therapy is not enough to block the progression of the illness. Coordinated therapy of multiple targets, that is, targeting multiple inflammatory/damaged cells and pathways simultaneously, will significantly optimize the treatment of ARDS and frustrate the progression of the disease. In addition, more specific targets regarding the recruitment and activation of neutrophils may provide suitable therapeutic strategy. For example, it was reported that the recruitment of neutrophils in lungs would not follow the classic mechanism. Dipeptidase-1 on the surface of pulmonary vascular endothelial cells is the main receptor for neutrophil adhesion and recruitment, mediating about 50% of neutrophil recruitment to lungs254. Therefore, it is necessary to improve our understanding of pathophysiological mechanisms and multiple heterogeneity of ARDS, detect new receptors and biomarkers on objective inflamed/impaired cells, and discover new therapeutic drugs in future researches.
Drug delivery: Nanocarriers have shown great feasibility and specialty in increasing lung distribution and cellular uptake while limiting toxicity and side effects. Still, there are issues to be settled. For example, the lung targeting efficiency is still unqualified. Optimizing drug delivery systems to circumvent the biologic obstacles, including clearance, degradation, and lung barriers, is of great importance. Cell membrane-based biomimetic delivery systems and hitchhiking strategies that taking advantages of the over-recruitment of inflammatory cells, hold great potential in targeted delivery to inflamed lungs. Moreover, nanocarriers may encounter the aggregation of non-target organs and other potentially undiscovered problems255. Ensuring the safety of nanocarriers is essential for clinical translation. Although some are non-inflammatory with certain safety or even display internal anti-inflammatory or antioxidant activity, it is still important to clarify their potential toxicity, especially for pulmonary delivery. A comprehensive understanding of the interaction of nanoparticles with immune systems and lung cells is conducive to accomplish safer and more efficient nano-therapy for ARDS.
In summary, nanomedicine-based drug delivery systems for the prevention, diagnosis, and treatment of ARDS have excellent clinical application prospects, which are expected to provide a new paradigm for ARDS drug therapy. More reliable models and more in-depth understanding of underlying mechanisms and interactions will encourage more rational designs of novel drug delivery systems with precise targeting for ARDS therapy, which could fill the existing gap in clinical translation. Considering numerous links between ARDS and COVID-19, it is promising that ARDS relevant investigations will contribute to promoting potential therapies for COVID-19.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81872810), Program for HUST Academic Frontier Youth Team (2018QYTD13, China), and the Fundamental Research Funds for the Central Universities (2018KFYYXJJ019 and 2019KFYRCPY049, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.04.023.
Contributor Information
Conglian Yang, Email: conglianyang@hust.edu.cn.
Zhiping Zhang, Email: zhipingzhang@mail.hust.edu.cn.
Author contributions
Zhiping Zhang and Conglian Yang conceived and designed this review. Qi Qiao investigated references and wrote the manuscript with assistance of Xiong Liu, Ting Yang, and Kexin Cui. Zhiping Zhang, Conglian Yang, and Li Kong revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. J Am Med Assoc. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 Countries. J Am Med Assoc. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care. 2020;24:198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan C., Li C.Y., Ma H., Li Y.S., Zhang H.L. Immunopathogenesis of coronavirus-induced acute respiratory distress syndrome (ARDS): potential infection-associated hemophagocytic lymphohistiocytosis. Clin Microbiol Rev. 2021;34 doi: 10.1128/CMR.00074-20. e00074−20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R.H., Fan G.H., Liu Y., Liu Z.B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO COVID-19 global situation. 2021. https://covid19.who.int/ Available form:
- 8.Li L., Huang Q.H., Wang D.C., Ingbar D.H., Wang X.D. Acute lung injury in patients with COVID-19 infection. Clin Transl Med. 2020;10:20–27. doi: 10.1002/ctm2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X.B., Yu Y., Xu J.Q., Shu H.Q., Xia J.A., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100:1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin definition. J Am Med Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beitler J.R. Lung protection in acute respiratory distress syndrome: what should we target? Curr Opin Crit Care. 2020;26:26–34. doi: 10.1097/MCC.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kido T., Muramatsu K., Yatera K., Asakawa T., Otsubo H., Kubo T. Efficacy of early sivelestat administration on acute lung injury and acute respiratory distress syndrome. Respirology. 2017;22:708–713. doi: 10.1111/resp.12969. [DOI] [PubMed] [Google Scholar]
- 17.Hayashida K., Fujishima S., Sasao K., Orita T., Toyoda Y., Kitano M. Early administration of sivelestat, the neutrophil elastase inhibitor, in adults for acute lung injury following gastric aspiration. Shock. 2011;36:223–227. doi: 10.1097/SHK.0b013e318225acc3. [DOI] [PubMed] [Google Scholar]
- 18.Silva P.L., Pelosi P., Rocco P.R.M. Personalized pharmacological therapy for ARDS: a light at the end of the tunnel. Expet Opin Invest Drugs. 2020;29:49–61. doi: 10.1080/13543784.2020.1699531. [DOI] [PubMed] [Google Scholar]
- 19.Murgia X., de Souza Carvalho C., Lehr C.M. Overcoming the pulmonary barrier: new insights to improve the efficiency of inhaled therapeutics. Eur J Nanomed. 2014;6:157–169. [Google Scholar]
- 20.Li C., Wang J., Wang Y., Gao H., Wei G., Huang Y. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadikot R.T. The potential role of nano- and micro-technology in the management of critical illnesses. Adv Drug Deliv Rev. 2014;77:27–31. doi: 10.1016/j.addr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Dong X.Y., Zhang C.Y., Gao J., Wang Z.J. Targeting of nanotherapeutics to infection sites for antimicrobial therapy. Adv Ther. 2019;2:1900095. doi: 10.1002/adtp.201900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J., Burgess D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm Sin B. 2020;10:2110–2124. doi: 10.1016/j.apsb.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao H.L. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang C.L., Wen C.J., Aljuffali I.A., Sung C.T., Huang C.L., Fang J.Y. Passive targeting of phosphatiosomes increases rolipram delivery to the lungs for treatment of acute lung injury: an animal study. J Control Release. 2015;213:69–78. doi: 10.1016/j.jconrel.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y., Ding Y., Fan B., Wang Y., Mao Z., Wang W. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J Control Release. 2020;321:463–474. doi: 10.1016/j.jconrel.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C.Y., Lin W., Gao J., Shi X., Davaritouchaee M., Nielsen A.E. pH-responsive nanoparticles targeted to lungs for improved therapy of acute lung inflammation/injury. ACS Appl Mater Interfaces. 2019;11:16380–16390. doi: 10.1021/acsami.9b04051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N., Weng D., Wang S.M., Zhang Y., Chen S.S., Yin Z.F. Surfactant protein-A nanobody-conjugated liposomes loaded with methylprednisolone increase lung-targeting specificity and therapeutic effect for acute lung injury. Drug Deliv. 2017;24:1770–1781. doi: 10.1080/10717544.2017.1402217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J.H., Kim H.A., Park J.H., Lee M. Amphiphilic peptide carrier for the combined delivery of curcumin and plasmid DNA into the lungs. Biomaterials. 2012;33:6542–6550. doi: 10.1016/j.biomaterials.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.Y., Piao C., Kim G., Lee S., Lee M.S., Jeong J.H. Combined delivery of a lipopolysaccharide-bonding peptide and the heme oxygenase-1 gene using deoxycholic acid-conjugated polyethylenimine for the treatment of acute lung injury. Macromol Biosci. 2017;17 doi: 10.1002/mabi.201600490. [DOI] [PubMed] [Google Scholar]
- 31.Oh B., Lee M. Combined delivery of HMGB-1 box A peptide and S1PLyase siRNA in animal models of acute lung injury. J Control Release. 2014;175:25–35. doi: 10.1016/j.jconrel.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Gao J., Wang S., Dong X., Leanse L.G., Dai T., Wang Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun Biol. 2020;3:680. doi: 10.1038/s42003-020-01410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniatis N.A., Orfanos S.E. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 34.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 37.Butt Y., Kurdowska A., Allen T.C. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 38.Pelaia C., Tinello C., Vatrella A., De Sarro G., Pelaia G. Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther Adv Respir Dis. 2020;14:1–9. doi: 10.1177/1753466620933508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthay M.A., Zemans R.L. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Physiol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuchiya T., Doi R., Obata T., Hatachi G., Nagayasu T. Lung microvascular niche, repair, and engineering. Front Bioeng Biotechnol. 2020;8:105. doi: 10.3389/fbioe.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhattacharya J., Matthay M.A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 42.Grommes J., Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Middleton E.A., Weyrich A.S., Zimmerman G.A. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev. 2016;96:1211–1259. doi: 10.1152/physrev.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han J., Li Y., Li Y. Strategies to enhance mesenchymal stem cell-based therapies for acute respiratory distress syndrome. Stem Cell Int. 2019;2019:5432134. doi: 10.1155/2019/5432134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson J.G., Calfee C.S. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24:102. doi: 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Domscheit H., Hegeman M.A., Carvalho N., Spieth P.M. Molecular dynamics of lipopolysaccharide-induced lung injury in rodents. Front Physiol. 2020;11:36. doi: 10.3389/fphys.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rawal G., Yadav S., Kumar R. Acute respiratory distress syndrome: an update and review. J Transl Int Med. 2018;6:74–77. doi: 10.1515/jtim-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proudfoot A.G., McAuley D.F., Griffiths M.J.D., Hind M. Human models of acute lung injury. Dis Model Mech. 2011;4:145–153. doi: 10.1242/dmm.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussain M., Xu C., Ahmad M., Majeed A., Lu M., Wu X. Acute respiratory distress syndrome: bench-to-bedside approaches to improve drug development. Clin Pharmacol Ther. 2018;104:484–494. doi: 10.1002/cpt.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557–4588. doi: 10.7150/thno.38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torchilin V.P. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2012;64:302–315. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Galvao A.M., Galvao J.S., Pereira M.A., Cadena P.G., Magalhaes N.S.S., Fink J.B. Cationic liposomes containing antioxidants reduces pulmonary injury in experimental model of sepsis liposomes antioxidants reduces pulmonary damage. Respir Physiol Neurobiol. 2016;231:55–62. doi: 10.1016/j.resp.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Zarchi A.A.K., Faramarzi M.A., Gilani K., Ghazi-Khansari M., Ghamami G., Amani A. N-acetylcysteine-loaded PLGA nanoparticles outperform conventional N-acetylcysteine in acute lung injuries in vivo. Int J Polym Mater Polym Biomater. 2017;66:443–454. [Google Scholar]
- 54.Yu Z., Liu X., Chen H., Zhu L. Naringenin-loaded dipalmitoylphosphatidylcholine phytosome dry powders for inhaled treatment of acute lung injury. J Aerosol Med Pulm Drug Deliv. 2020;33:194–204. doi: 10.1089/jamp.2019.1569. [DOI] [PubMed] [Google Scholar]
- 55.Ma B., Xu H., Zhuang W., Wang Y., Li G., Wang Y. Reactive oxygen species responsive theranostic nanoplatform for two-photon aggregation-induced emission imaging and therapy of acute and chronic inflammation. ACS Nano. 2020;14:5862–5873. doi: 10.1021/acsnano.0c01012. [DOI] [PubMed] [Google Scholar]
- 56.Li S.J., Chen L., Wang G.K., Xu L.X., Hou S.S., Chen Z.W. Anti-ICAM-1 antibody-modified nanostructured lipid carriers: a pulmonary vascular endothelium-targeted device for acute lung injury therapy. J Nanobiotechnol. 2018;16:105. doi: 10.1186/s12951-018-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y., Yang B., Zhao X., Xi M., Yin Z. E-selectin-binding peptide-modified bovine serum albumin nanoparticles for the treatment of acute lung injury. AAPS PharmSciTech. 2019;20:270. doi: 10.1208/s12249-019-1403-2. [DOI] [PubMed] [Google Scholar]
- 58.Chen X.Y., Wang S.M., Li N., Hu Y., Zhang Y., Xu J.F. Creation of lung-targeted dexamethasone immunoliposome and its therapeutic effect on bleomycin-induced lung injury in rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrer M.C.C., Shuvaev V.V., Zern B.J., Composto R.J., Muzykantov V.R., Eckmann D.M. ICAM-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Go G., Lee J., Choi D.S., Kim S.S., Gho Y.S. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Adv Healthc Mater. 2019;8 doi: 10.1002/adhm.201801082. [DOI] [PubMed] [Google Scholar]
- 61.Desu H.R., Thoma L.A., Wood G.C. Nebulization of cyclic arginine-glycine-(d)-aspartic acid-peptide grafted and drug encapsulated liposomes for inhibition of acute lung injury. Pharm Res (N Y) 2018;35:94. doi: 10.1007/s11095-018-2366-9. [DOI] [PubMed] [Google Scholar]
- 62.Chu D.F., Gao J., Wang Z.J. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano. 2015;9:11800–11811. doi: 10.1021/acsnano.5b05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Q.L., Fan Q., Xu J.L., Bai J.Y., Han X., Dong Z.L. Calming cytokine storm in pneumonia by targeted delivery of TPCA-1 using platelet-derived extracellular vesicles. Matter. 2020;3:287–301. doi: 10.1016/j.matt.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin F., Liu D., Yu H., Qi J., You Y., Xu X. Sialic acid-functionalized PEG-PLGA microspheres loading mitochondrial-targeting-modified curcumin for acute lung Injury therapy. Mol Pharm. 2019;16:71–85. doi: 10.1021/acs.molpharmaceut.8b00861. [DOI] [PubMed] [Google Scholar]
- 65.Li S.J., Wang X.J., Hu J.B., Kang X.Q., Chen L., Xu X.L. Targeting delivery of simvastatin using ICAM-1 antibody-conjugated nanostructured lipid carriers for acute lung injury therapy. Drug Deliv. 2017;24:402–413. doi: 10.1080/10717544.2016.1259369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang S., Li S., Hu J., Xu X., Wang X., Kang X. Combined delivery of angiopoietin-1 gene and simvastatin mediated by anti-intercellular adhesion molecule-1 antibody-conjugated ternary nanoparticles for acute lung injury therapy. Nanomedicine. 2019;15:25–36. doi: 10.1016/j.nano.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Yu H.P., Liu F.C., Lin C.Y., Umoro A., Trousil J., Hwang T.L. Suppression of neutrophilic inflammation can be modulated by the droplet size of anti-inflammatory nanoemulsions. Nanomedicine. 2020;15:773–791. doi: 10.2217/nnm-2019-0407. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z., Li J., Cho J., Malik A.B. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9:204–210. doi: 10.1038/nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J., Wang S., Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okeke E.B., Louttit C., Fry C., Najafabadi A.H., Han K., Nemzek J. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials. 2020;238:119836. doi: 10.1016/j.biomaterials.2020.119836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee Y.Y., Park H.H., Park W., Kim H., Jang J.G., Hong K.S. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials. 2020;267:120389. doi: 10.1016/j.biomaterials.2020.120389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park H.H., Park W.S., Lee Y.Y., Kim H., Seo H.S., Choi D.W. Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation. Adv Sci. 2020;7:2001940. doi: 10.1002/advs.202001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bohr A., Tsapis N., Andreana I., Chamarat A., Foged C., Delomenie C. Anti-inflammatory effect of anti-TNF-α siRNA cationic phosphorus dendrimer nanocomplexes administered intranasally in a murine acute lung injury model. Biomacromolecules. 2017;18:2379–2388. doi: 10.1021/acs.biomac.7b00572. [DOI] [PubMed] [Google Scholar]
- 74.Ge C.L., Yang J.D., Duan S.Z., Liu Y., Meng F.H., Yin L.C. Fluorinated α-helical polypeptides synchronize mucus permeation and cell penetration toward highly efficient pulmonary siRNA delivery against acute lung injury. Nano Lett. 2020;20:1738–1746. doi: 10.1021/acs.nanolett.9b04957. [DOI] [PubMed] [Google Scholar]
- 75.Merckx P., De Backer L., Van Hoecke L., Guagliardo R., Echaide M., Baatsen P. Surfactant protein B (SP-B) enhances the cellular siRNA delivery of proteolipid coated nanogels for inhalation therapy. Acta Biomater. 2018;78:236–246. doi: 10.1016/j.actbio.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 76.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo J., Li D., Tao H., Li G., Liu R., Dou Y. Cyclodextrin-derived intrinsically bioactive nanoparticles for treatment of acute and chronic inflammatory diseases. Adv Mater. 2019;31 doi: 10.1002/adma.201904607. [DOI] [PubMed] [Google Scholar]
- 78.Li L., Guo J., Wang Y., Xiong X., Tao H., Li J. A broad-spectrum ROS-eliminating material for prevention of inflammation and drug-induced organ toxicity. Adv Sci. 2018;5:1800781. doi: 10.1002/advs.201800781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao H., Zeng Z., Liu L., Chen J., Zhou H., Huang L. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale. 2018;10:6981–6991. doi: 10.1039/c8nr00838h. [DOI] [PubMed] [Google Scholar]
- 80.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl Mater Interfaces. 2018;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 81.Cojocaru F.D., Botezat D., Gardikiotis I., Uritu C.M., Dodi G., Trandafir L. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12:171. doi: 10.3390/pharmaceutics12020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koo O.M., Rubinstein I., Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Fung S.Y., Oyaizu T., Yang H., Yuan Y.F., Han B., Keshavjee S. The potential of nanoscale combinations of self-assembling peptides and amino acids of the Src tyrosine kinase inhibitor in acute lung injury therapy. Biomaterials. 2011;32:4000–4008. doi: 10.1016/j.biomaterials.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Qin M., Cao Z., Wen J., Yu Q., Liu C., Wang F. An antioxidant enzyme therapeutic for COVID-19. Adv Mater. 2020;32 doi: 10.1002/adma.202004901. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Y., Niu B., Wu B., Luo S., Fu J., Zhao Y. A homogenous nanoporous pulmonary drug delivery system based on metal-organic frameworks with fine aerosolization performance and good compatibility. Acta Pharm Sin B. 2020;10:2404–2416. doi: 10.1016/j.apsb.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu F.C., Yu H.P., Lin C.Y., Elzoghby A.O., Hwang T.L., Fang J.Y. Use of cilomilast-loaded phosphatiosomes to suppress neutrophilic inflammation for attenuating acute lung injury: the effect of nanovesicular surface charge. J Nanobiotechnol. 2018;16:35. doi: 10.1186/s12951-018-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu H.P., Liu F.C., Umoro A., Lin Z.C., Elzoghby A.O., Hwang T.L. Oleic acid-based nanosystems for mitigating acute respiratory distress syndrome in mice through neutrophil suppression: how the particulate size affects therapeutic efficiency. J Nanobiotechnol. 2020;18:25. doi: 10.1186/s12951-020-0583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao Z., Liu J.L., Wu S., Wang Q. Mechanism of MCP-1 in acute lung injury and advanced therapy by drug-loaded dextrin nanoparticle. Int J Polym Sci. 2018;2018:1–7. [Google Scholar]
- 89.Zeiher B.G., Artigas A., Vincent J.L., Dmitrienko A., Jackson K., Thompson T. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32:1695–1702. doi: 10.1097/01.ccm.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]
- 90.Tamakuma S., Ogawa M., Aikawa N., Kubota T., Hirasawa H., Ishizaka A. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Therapeut. 2004;17:271–279. doi: 10.1016/j.pupt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Aikawa N., Ishizaka A., Hirasawa H., Shimazaki S., Yamamoto Y., Sugimoto H. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm Pharmacol Therapeut. 2011;24:549–554. doi: 10.1016/j.pupt.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Gibbons A., McElvaney N.G., Cryan S.A. A dry powder formulation of liposome-encapsulated recombinant secretory leukocyte protease inhibitor (rSLPI) for inhalation: preparation and characterisation. AAPS PharmSciTech. 2010;11:1411–1421. doi: 10.1208/s12249-010-9500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herber-Jonat S., Mittal R., Gsinn S., Bohnenkamp H., Guenzi E., Schulze A. Comparison of lung accumulation of cationic liposomes in normal rats and LPS-treated rats. Inflamm Res. 2011;60:245–253. doi: 10.1007/s00011-010-0260-y. [DOI] [PubMed] [Google Scholar]
- 94.Patil M.A., Upadhyay A.K., Hernandez-Lagunas L., Good R., Carpenter T.C., Sucharov C.C. Targeted delivery of YSA-functionalized and non-functionalized polymeric nanoparticles to injured pulmonary vasculature. Artif Cells Nanomed Biotechnol. 2018;46 doi: 10.1080/21691401.2018.1528984. S1059−S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brenner J.S. Nanomedicine for the treatment of acute respiratory distress syndrome the 2016 ATS bear cage award-winning proposal. Ann Am Thorac Soc. 2017;14:561–564. doi: 10.1513/AnnalsATS.201701-090PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piao C., Park J.H., Lee M. Anti-Inflammatory therapeutic effect of adiponectin gene delivery using a polymeric carrier in an acute lung injury model. Pharm Res (N Y) 2017;34:1517–1526. doi: 10.1007/s11095-017-2175-6. [DOI] [PubMed] [Google Scholar]
- 97.Cornier M.A., Eckel R.H. Non-traditional dosing of statins in statin-intolerant patients-is it worth a try?. Curr Atherosclerosis Rep. 2015;17:475. doi: 10.1007/s11883-014-0475-4. [DOI] [PubMed] [Google Scholar]
- 98.Sadikot R.T., Rubinstein I. Long-acting, multi-targeted nanomedicine: addressing unmet medical need in acute lung injury. J Biomed Nanotechnol. 2009;5:614–619. doi: 10.1166/jbn.2009.1078. [DOI] [PubMed] [Google Scholar]
- 99.Kim G., Piao C., Oh J., Lee M. Self-assembled polymeric micelles for combined delivery of anti-inflammatory gene and drug to the lungs by inhalation. Nanoscale. 2018;10:8503–8514. doi: 10.1039/c8nr00427g. [DOI] [PubMed] [Google Scholar]
- 100.Ravikumar P., Menon J.U., Punnakitikashem P., Gyawali D., Togao O., Takahashi M. Nanoparticle facilitated inhalational delivery of erythropoietin receptor cDNA protects against hyperoxic lung injury. Nanomedicine. 2016;12:811–821. doi: 10.1016/j.nano.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1 [Google Scholar]
- 102.Hussain S., Vanoirbeek J.A.J., Haenen S., Haufroid V., Boland S., Marano F. Prior lung inflammation impacts on body distribution of gold nanoparticles. BioMed Res Int. 2013;2013:923475. doi: 10.1155/2013/923475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y.B., Watts A.B., Peters J.I., Williams R.O. The impact of pulmonary diseases on the fate of inhaled medicines—a review. Int J Pharm. 2014;461:112–128. doi: 10.1016/j.ijpharm.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 104.Li S.D., Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 105.Thille A.W., Esteban A., Fernandez-Segoviano P., Rodriguez J.M., Aramburu J.A., Vargas-Errazuriz P. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med. 2013;1:395–401. doi: 10.1016/S2213-2600(13)70053-5. [DOI] [PubMed] [Google Scholar]
- 106.Li B., Xie J., Yuan Z., Jain P., Lin X., Wu K. Mitigation of inflammatory immune responses with hydrophilic nanoparticles. Angew Chem Int Ed Engl. 2018;57:4527–4531. doi: 10.1002/anie.201710068. [DOI] [PubMed] [Google Scholar]
- 107.Zhu L., Li M., Dong J., Jin Y. Dimethyl silicone dry nanoemulsion inhalations: formulation study and anti-acute lung injury effect. Int J Pharm. 2015;491:292–298. doi: 10.1016/j.ijpharm.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 108.Kim H.C., Suresh M.V., Singh V.V., Arick D.Q., Machado-Aranda D.A., Raghavendran K. Polymer lung surfactants. ACS Appl Bio Mater. 2018;1:581–592. doi: 10.1021/acsabm.8b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu Y., Ma J.Y., Woods P.S., Chesarino N.M., Liu C., Lee L.J. Selective targeting of alveolar type Ⅱ respiratory epithelial cells by anti-surfactant protein-C antibody-conjugated lipoplexes. J Control Release. 2015;203:140–149. doi: 10.1016/j.jconrel.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaviratna A.S., Banerjee R. Nanovesicle aerosols as surfactant therapy in lung injury. Nanomedicine. 2012;8:665–672. doi: 10.1016/j.nano.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Y., Li P.F., Goodwin A.J., Cook J.A., Halushka P.V., Chang E. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care. 2019;23:44. doi: 10.1186/s13054-019-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L., Zhang H.S., Sun L.Y., Gao W., Xiong Y., Ma A. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J Nanobiotechnol. 2020;18:38. doi: 10.1186/s12951-020-00593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiong Y., Gao W., Xia F., Sun Y., Sun L., Wang L. Peptide-gold nanoparticle hybrids as promising anti-inflammatory nanotherapeutics for acute lung injury: in vivo efficacy, biodistribution, and clearance. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800510. [DOI] [PubMed] [Google Scholar]
- 114.Gao W., Wang Y., Xiong Y., Sun L., Wang L., Wang K. Size-dependent anti-inflammatory activity of a peptide-gold nanoparticle hybrid in vitro and in a mouse model of acute lung injury. Acta Biomater. 2019;85:203–217. doi: 10.1016/j.actbio.2018.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao W., Wang L., Wang K., Sun L., Rao Y., Ma A. Enhanced anti-inflammatory activity of peptide-gold nanoparticle hybrids upon cigarette smoke extract modification through TLR inhibition and autophagy induction. ACS Appl Mater Interfaces. 2019;11:32706–32719. doi: 10.1021/acsami.9b10536. [DOI] [PubMed] [Google Scholar]
- 116.Wang M.Y., Wang K., Deng G.Y., Liu X.J., Wu X.D., Hu H.Y. Mitochondria-modulating porous Se@SiO2 nanoparticles provide resistance to oxidative injury in airway epithelial cells: implications for acute lung injury. Int J Nanomed. 2020;15:2287–2302. doi: 10.2147/IJN.S240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Souza Carvalho C., Daum N., Lehr C.M. Carrier interactions with the biological barriers of the lung: advanced in vitro models and challenges for pulmonary drug delivery. Adv Drug Deliv Rev. 2014;75:129–140. doi: 10.1016/j.addr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 118.Liu Q., Guan J., Qin L., Zhang X., Mao S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov Today. 2020;25:150–159. doi: 10.1016/j.drudis.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 119.Sadat S.M.A., Jahan S.T., Haddadi A. Effects of size and surface charge of polymeric nanoparticles on in vitro and in vivo applications. J Biomaterials Nanobiotechnol. 2016:91–108. [Google Scholar]
- 120.Hoshyar N., Gray S., Han H., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11:673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gentile F., Curcio A., Indolfi C., Ferrari M., Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J Nanobiotechnol. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan W., Chen W., Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol Immunol. 2007;44:3672–3681. doi: 10.1016/j.molimm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 124.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 125.Shuvaev V.V., Muro S., Arguiri E., Khoshnejad M., Tliba S., Christofidou-Solomidou M. Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates. J Control Release. 2016;234:115–123. doi: 10.1016/j.jconrel.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Safari H., Kelley W.J., Saito E., Kaczorowski N., Carethers L., Shea L.D. Neutrophils preferentially phagocytose elongated particles—an opportunity for selective targeting in acute inflammatory diseases. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seong S.Y., Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 128.Dobrovolskaia M.A., Mcneil S.E. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 129.Lai S.K., Wang Y.Y., Hida K., Cone R., Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ibricevic A., Guntsen S.P., Zhang K., Shrestha R., Liu Y.J., Sun J.Y. PEGylation of cationic, shell-crosslinked-knedel-like nanoparticles modulates inflammation and enhances cellular uptake in the lung. Nanomedicine. 2013;9:912–922. doi: 10.1016/j.nano.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khutoryanskiy V.V. Beyond PEGylation: alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv Drug Deliv Rev. 2018;124:140–149. doi: 10.1016/j.addr.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 132.Mahri S., Hardy E., Wilms T., De Keersmaecker H., Braeckmans K., De Smedt S. PEGylation of recombinant human deoxyribonuclease I decreases its transport across lung epithelial cells and uptake by macrophages. Int J Pharm. 2020;593:120107. doi: 10.1016/j.ijpharm.2020.120107. [DOI] [PubMed] [Google Scholar]
- 133.Wilhelm D.L. Mechanisms responsible for increased vascular permeability in acute inflammation. Agents Actions. 1973;3:297–306. doi: 10.1007/BF01986484. [DOI] [PubMed] [Google Scholar]
- 134.Anselmo A.C., Gupta V., Zern B.J., Pan D., Zakrewsky M., Muzykantov V. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7:11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin E.H., Chang H.Y., Yeh S.D., Yang K.Y., Hu H.S., Wu C.W. Polyethyleneimine and DNA nanoparticles-based gene therapy for acute lung injury. Nanomedicine. 2013;9:1293–1303. doi: 10.1016/j.nano.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Guo J.W., Tao H., Dou Y., Li L.L., Xu X.Q., Zhang Q.X. A myeloperoxidase-responsive and biodegradable luminescent material for real-time imaging of inflammatory diseases. Mater Today. 2017;20:493–500. [Google Scholar]
- 137.Jiang Z., Zhou Q., Gu C., Li D., Zhu L. Depletion of circulating monocytes suppresses IL-17 and HMGB1 expression in mice with LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312:L231−L42. doi: 10.1152/ajplung.00389.2016. [DOI] [PubMed] [Google Scholar]
- 138.Brenner J.S., Greineder C., Shuvaev V., Muzykantov V. Endothelial nanomedicine for the treatment of pulmonary disease. Expet Opin Drug Deliv. 2015;12:239–261. doi: 10.1517/17425247.2015.961418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fang J., Islam W., Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020;157:142–160. doi: 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 140.Brusini R., Varna M., Couvreur P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv Drug Deliv Rev. 2020;157:161–178. doi: 10.1016/j.addr.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yuan F., Quan L.D., Cui L., Goldring S.R., Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Adv Drug Deliv Rev. 2012;64:1205–1219. doi: 10.1016/j.addr.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gotts J.E., Abbott J., Matthay M.A. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol. 2014;307:L395−L406. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y.J., Sun D.D., Fan Q., Ma Q.L., Dong Z.L., Tao W.W. The enhanced permeability and retention effect based nanomedicine at the site of injury. Nano Res. 2020;29:564–569. [Google Scholar]
- 144.Johnson E.R., Matthay M.A. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spence S., Greene M.K., Fay F., Hams E., Saunders S.P., Hamid U. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med. 2015;7:303ra140. doi: 10.1126/scitranslmed.aab3459. [DOI] [PubMed] [Google Scholar]
- 146.Howard M.D., Greineder C.F., Hood E.D., Muzykantov V.R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J Control Release. 2014;177:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Molinaro R., Pasto A., Corbo C., Taraballi F., Giordano F., Martinez J.O. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11:13576–13586. doi: 10.1039/c9nr04253a. [DOI] [PubMed] [Google Scholar]
- 148.Shen S., Han F., Yuan A., Wu L., Cao J., Qian J. Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials. 2019;189:60–68. doi: 10.1016/j.biomaterials.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 149.Zhang C.K., Guo F., Chang M.L., Zhou Z.D., Yi L., Gao C.J. Exosome-delivered syndecan-1 rescues acute lung injury via a FAK/p190RhoGAP/RhoA/ROCK/NF-kappa B signaling axis and glycocalyx enhancement. Exp Cell Res. 2019;384:111596. doi: 10.1016/j.yexcr.2019.111596. [DOI] [PubMed] [Google Scholar]
- 150.Dos Santos Haupenthal D.P., Mendes C., de Bem Silveira G., Zaccaron R.P., Correa M., Nesi R.T. Effects of treatment with gold nanoparticles in a model of acute pulmonary inflammation induced by lipopolysaccharide. J Biomed Mater Res. 2020;108:103–115. doi: 10.1002/jbm.a.36796. [DOI] [PubMed] [Google Scholar]
- 151.Zhu Y., Deng G.Y., Ji A.Q., Yao J.Y., Meng X.X., Wang J.F. Porous Se@SiO2 nanospheres treated paraquat-induced acute lung injury by resisting oxidative stress. Int J Nanomed. 2017;12:7143–7152. doi: 10.2147/IJN.S143192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kadivar F., Haddadi G., Mosleh-Shirazi M.A., Khajeh F., Tavasoli A. Protection effect of cerium oxide nanoparticles against radiation-induced acute lung injuries in rats. Rep Practical Oncol Radiother. 2020;25:206–211. doi: 10.1016/j.rpor.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu P.T., Maidment B.W., Antonic V., Jackson I.L., Das S., Zodda A. Cerium oxide nanoparticles: a potential medical countermeasure to mitigate radiation-induced lung injury in CBA/J mice. Radiat Res. 2016;185:516–526. doi: 10.1667/RR14261.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vassallo A., Wood A.J., Subburayalu J., Summers C., Chilvers E.R. The counter-intuitive role of the neutrophil in the acute respiratory distress syndrome. Br Med Bull. 2019;131:43–55. doi: 10.1093/bmb/ldz024. [DOI] [PubMed] [Google Scholar]
- 155.Huang X., Xiu H., Zhang S., Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediat Inflamm. 2018;2018:1264913. doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mejias J.C., Forrest O.A., Margaroli C., Frey Rubio D.A., Viera L., Li J. Neutrophil-targeted, protease-activated pulmonary drug delivery blocks airway and systemic inflammation. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Š Zupančič, Kocbek P., Zariwala M.G., Renshaw D., Gul M.O., Elsaid Z. Design and development of novel mitochondrial targeted nanocarriers, DQAsomes for curcumin inhalation. Mol Pharm. 2014;11:2334–2345. doi: 10.1021/mp500003q. [DOI] [PubMed] [Google Scholar]
- 158.Vassiliou A.G., Kotanidou A., Dimopoulou I., Orfanos S.E. Endothelial damage in acute respiratory distress syndrome. Int J Mol Sci. 2020;21:8793. doi: 10.3390/ijms21228793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H.M., Bahel P. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7 doi: 10.1016/S2352-3026(20)30216-7. e575−e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shuvaev V.V., Brenner J.S., Muzykantov V.R. Targeted endothelial nanomedicine for common acute pathological conditions. J Control Release. 2015;219:576–595. doi: 10.1016/j.jconrel.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kiseleva R.Y., Glassman P.M., Greineder C.F., Hood E.D., Shuvaev V.V., Muzykantov V.R. Targeting therapeutics to endothelium: are we there yet?. Drug Deliv Transl Res. 2018;8:883–902. doi: 10.1007/s13346-017-0464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Greineder C.F., Chacko A.M., Zaytsev S., Zern B.J., Carnemolla R., Hood E.D. Vascular immunotargeting to endothelial determinant ICAM-1 enables optimal partnering of recombinant scFv-thrombomodulin fusion with endogenous cofactor. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maniatis N.A., Kotanidou A., Catravas J.D., Orfanos S.E. Endothelial pathomechanisms in acute lung injury. Vasc Pharmacol. 2008;49:119–133. doi: 10.1016/j.vph.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shuvaev V.V., Han J.Y., Yu K.J., Huang S.H., Hawkins B.J., Madesh M. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25:348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Patil T.S., Deshpande A.S. Nanostructured lipid carriers-based drug delivery for treating various lung diseases: a state-of-the-art review. Int J Pharm. 2018;547:209–225. doi: 10.1016/j.ijpharm.2018.05.070. [DOI] [PubMed] [Google Scholar]
- 166.Hood E.D., Greineder C.F., Shuvaeva T., Walsh L., Villa C.H., Muzykantov V.R. Vascular targeting of radiolabeled liposomes with bio-orthogonally conjugated ligands: single chain fragments provide higher specificity than antibodies. Bioconjugate Chem. 2018;29:3626–3637. doi: 10.1021/acs.bioconjchem.8b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Desu H.R., Wood G.C., Thoma L.A. Non-invasive detection of lung inflammation by near-infrared fluorescence imaging using bimodal liposomes. J Fluoresc. 2016;26:241–253. doi: 10.1007/s10895-015-1706-y. [DOI] [PubMed] [Google Scholar]
- 168.Chu D., Dong X., Shi X., Zhang C., Wang Z. Neutrophil-based drug delivery systems. Adv Mater. 2018;30 doi: 10.1002/adma.201706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nemeth T., Sperandio M., Mocsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19:253–275. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- 170.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 171.Fromen C.A., Kelley W.J., Fish M.B., Adili R., Noble J., Hoenerhoff M.J. Neutrophil-particle interactions in blood circulation drive particle clearance and alter neutrophil responses in acute inflammation. ACS Nano. 2017;11:10797–10807. doi: 10.1021/acsnano.7b03190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang C.Y., Dong X., Gao J., Lin W., Liu Z., Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li S., Li M., Huo S., Wang Q., Chen J., Ding S. Voluntary-opsonization-enabled precision nanomedicines for inflammation treatment. Adv Mater. 2020 doi: 10.1002/adma.202006160. [DOI] [PubMed] [Google Scholar]
- 174.Nakatani K., Takeshita S., Tsujimoto H., Kawamura Y., Kawase H., Sekine I. Regulation of the expression of Fcg receptor on circulating neutrophils and monocytes in Kawasaki disease. Clin Exp Immunol. 1999;117:418–422. doi: 10.1046/j.1365-2249.1999.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Daniel C., Leppkes M., Munoz L.E., Schley G., Schett G., Herrmann M. Extracellular DNA traps in inflammation, injury and healing. Nat Rev Nephrol. 2019;15:559–575. doi: 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- 176.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2017;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 177.Chen X., Tang J., Shuai W., Meng J., Feng J., Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm Res. 2020;69:883–895. doi: 10.1007/s00011-020-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnston L.K., Rims C.R., Gill S.E., McGuire J.K., Manicone A.M. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim H., Wang S.Y., Kwak G., Yang Y., Kwon I.C., Kim S.H. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6:1900513. doi: 10.1002/advs.201900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.He W., Kapate N., Shields CWt, Mitragotri S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv Drug Deliv Rev. 2019;165–166:15–40. doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 181.Ando M., Tu W., Nishijima K., Iijima S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem Biophys Res Commun. 2008;369:878–883. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- 182.Yang H., Fung S.Y., Xu S., Sutherland D.P., Kollmann T.R., Liu M. Amino acid-dependent attenuation of Toll-like receptor signaling by peptide-gold nanoparticle hybrids. ACS Nano. 2015;9:6774–6784. doi: 10.1021/nn505634h. [DOI] [PubMed] [Google Scholar]
- 183.Ji W.J., Ma Y.Q., Zhang X., Zhang L., Zhang Y.D., Su C.C. Inflammatory monocyte/macrophage modulation by liposome-entrapped spironolactone ameliorates acute lung injury in mice. Nanomedicine. 2016;11:1393–1406. doi: 10.2217/nnm-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Paleos C.M., Tsiourvas D., Sideratou Z. Triphenylphosphonium decorated liposomes and dendritic polymers: prospective second generation drug delivery systems for targeting mitochondria. Mol Pharm. 2016;13:2233–2241. doi: 10.1021/acs.molpharmaceut.6b00237. [DOI] [PubMed] [Google Scholar]
- 185.He L., Zhao J., Wang L., Liu Q., Fan Y., Li B. Using nano-selenium to combat coronavirus disease 2019 (COVID-19)?. Nano Today. 2021;36:101037. doi: 10.1016/j.nantod.2020.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee I.T., Yang C.M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol. 2012;84:581–590. doi: 10.1016/j.bcp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 187.Hou C.Y., Bai H., Wang Z.J., Qiu Y.H., Kong L.L., Sun F.F. A hyaluronan-based nanosystem enables combined anti-inflammation of mTOR gene silencing and pharmacotherapy. Carbohydr Polym. 2018;195:339–348. doi: 10.1016/j.carbpol.2018.04.113. [DOI] [PubMed] [Google Scholar]
- 188.Shen Y.F., Zhang S.Y., Zhang F.W., Loftis A., Pavia-Sanders A., Zou J. Polyphosphoester-based cationic nanoparticles serendipitously release integral biologically-active components to serve as novel degradable inducible nitric oxide synthase inhibitors. Adv Mater. 2013;25:5609–5614. doi: 10.1002/adma.201302842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shrestha R., Shen Y.F., Pollack K.A., Taylor J.S.A., Wooley K.L. Dual peptide nucleic acid- and peptide-functionalized shell cross-linked nanoparticles designed to target mRNA toward the diagnosis and treatment of acute lung injury. Bioconjugate Chem. 2012;23:574–585. doi: 10.1021/bc200629f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee S., Stubelius A., Hamelmann N., Tran V., Almutairi A. Inflammation-responsive drug-conjugated dextran nanoparticles enhance anti-inflammatory drug efficacy. ACS Appl Mater Interfaces. 2018;10:40378–40387. doi: 10.1021/acsami.8b08254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dou Y., Li C., Li L., Guo J., Zhang J. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J Control Release. 2020;327:641–666. doi: 10.1016/j.jconrel.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kallet R.H., Jasmer R.M., Luce J.M., Lin L.H., Marks J.D. The treatment of acidosis in acute lung injury with tris-hydroxymethyl aminomethane (THAM) Am J Respir Crit Care Med. 2000;161:1149–1153. doi: 10.1164/ajrccm.161.4.9906031. [DOI] [PubMed] [Google Scholar]
- 193.Anselmo A.C., Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zelepukin I.V., Yaremenko A.V., Shipunova V.O., Babenyshev A.V., Balalaeva I.V., Nikitin P.I. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale. 2019;11:1636–1646. doi: 10.1039/c8nr07730d. [DOI] [PubMed] [Google Scholar]
- 195.Brenner J.S., Pan D.C., Myerson J.W., Marcos-Contreras O.A., Villa C.H., Patel P. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun. 2018;9:2684. doi: 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kumar A., Glam M., El-Badri N., Mohapatra S., Haller E., Park S. Initial observations of cell-mediated drug delivery to the deep lung. Cell Transplant. 2011;20:609–618. doi: 10.3727/096368910X536491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu Y.Y., Xiang J., Zhao H., Liang H.S., Huang J., Li Y. Human amniotic fluid stem cells labeled with up-conversion nanoparticles for imaging-monitored repairing of acute lung injury. Biomaterials. 2016;100:91–100. doi: 10.1016/j.biomaterials.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 198.Zhao Z.M., Ukidve A., Gao Y.S., Kim J., Mitragotri S. Erythrocyte leveraged chemotherapy (ELeCt): nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Che J., Najer A., Blakney A.K., McKay P.F., Bellahcene M., Winter C.W. Neutrophils enable local and non-invasive liposome delivery to inflamed skeletal muscle and ischemic heart. Adv Mater. 2020;32 doi: 10.1002/adma.202003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang C., Zhang L., Wu W., Gao F., Li R.Q., Song W. Artificial super neutrophils for inflammation targeting and HClO generation against tumors and infections. Adv Mater. 2019;31 doi: 10.1002/adma.201901179. [DOI] [PubMed] [Google Scholar]
- 201.Xue J., Zhao Z., Zhang L., Xue L., Shen S., Wen Y. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 202.Brynskikh A.M., Zhao Y., Mosley R.L., Li S., Boska M.D., Klyachko N.L. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson's disease. Nanomedicine. 2010;5:379–396. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yan H., Shao D., Lao Y.H., Li M., Hu H., Leong K.W. Engineering cell membrane-based nanotherapeutics to target inflammation. Adv Sci. 2019;6:1900605. doi: 10.1002/advs.201900605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rao L., Tian R., Chen X. Cell-membrane-mimicking nanodecoys against infectious diseases. ACS Nano. 2020;14:2569–2574. doi: 10.1021/acsnano.0c01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tan S., Wu T., Zhang D., Zhang Z. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5:863–881. doi: 10.7150/thno.11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guo Y., Wang D., Song Q., Wu T., Zhuang X., Bao Y. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for Induction of antitumor immunity against melanoma. ACS Nano. 2015;9:6918–6933. doi: 10.1021/acsnano.5b01042. [DOI] [PubMed] [Google Scholar]
- 208.Zhang D., Qin X., Wu T., Qiao Q., Song Q., Zhang Z. Extracellular vesicles based self-grown gold nanopopcorn for combinatorial chemo-photothermal therapy. Biomaterials. 2019;197:220–228. doi: 10.1016/j.biomaterials.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 209.Rao L., Xia S., Xu W., Tian R., Yu G.C., Gu C.J. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci U S A. 2020;117:27141–27147. doi: 10.1073/pnas.2014352117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14:6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 211.Cagno V., Andreozzi P., D'Alicarnasso M., Silva P.J., Mueller M., Galloux M. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater. 2018;17:195–2203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 212.Jones S.T., Cagno V., Janecek M., Ortiz D., Gasilova N., Piret J. Modified cyclodextrins as broad-spectrum antivirals. Sci Adv. 2020;6 doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Al-Lawati H., Aliabadi H.M., Makhmalzadeh B.S., Lavasanifar A. Nanomedicine for immunosuppressive therapy: achievements in pre-clinical and clinical research. Expet Opin Drug Deliv. 2018;15:397–418. doi: 10.1080/17425247.2018.1420053. [DOI] [PubMed] [Google Scholar]
- 214.Wiklander O.P.B., Brennan M.A., Lotvall J., Breakefield X.O., El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11:eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang D., Wu T., Qin X., Qiao Q., Shang L., Song Q. Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Lett. 2019;19:6635–6646. doi: 10.1021/acs.nanolett.9b02903. [DOI] [PubMed] [Google Scholar]
- 216.Wu T., Qi Y., Zhang D., Song Q.L., Yang C.L., Hu X.M. Bone marrow dendritic cells derived microvesicles for combinational immunochemotherapy against tumor. Adv Funct Mater. 2017;27:1703191. [Google Scholar]
- 217.Mahida R.Y., Matsumoto S., Matthay M.A. Extracellular vesicles: a new frontier for research in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2020;63:15–24. doi: 10.1165/rcmb.2019-0447TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Behnke J., Kremer S., Shahzad T., Chao C.M., Böttcher-Friebertshäuser E., Morty R.E. MSC based therapies-new perspectives for the injured lung. J Clin Med. 2020;9:682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Qu W., Wang Z., Hare J.M., Bu G., Mallea J.M., Pascual J.M. Cell-based therapy to reduce mortality from COVID-19: systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:1007–1022. doi: 10.1002/sctm.20-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Du J., Li H., Lian J., Zhu X., Qiao L., Lin J. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res Ther. 2020;11:192. doi: 10.1186/s13287-020-01699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Abraham A., Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shah T.G., Predescu D., Predescu S. Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clin Transl Med. 2019;8:25. doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Khatri M., Richardson L.A., Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Monsel A., Zhu Y.G., Gudapati V., Lim H., Lee J.W. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expet Opin Biol Ther. 2016;16:859–871. doi: 10.1517/14712598.2016.1170804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ahn S.Y., Park W.S., Kim Y.E., Sung D.K., Sung S.I., Ahn J.Y. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Monsel A., Zhu Y.G., Gennai S., Hao Q., Hu S., Rouby J.J. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Varkouhi A.K., Jerkic M., Ormesher L., Gagnon S., Goyal S., Rabani R. Extracellular vesicles from interferon-γ-primed human umbilical cord mesenchymal stromal cells reduce Escherichia coli-induced acute lung injury in rats. Anesthesiology. 2019;130:778–790. doi: 10.1097/ALN.0000000000002655. [DOI] [PubMed] [Google Scholar]
- 229.Song Y., Dou H., Li X., Zhao X., Li Y., Liu D. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cell. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 230.He H., Liu L., Chen Q., Liu A., Cai S., Yang Y. Mesenchymal stem cells overexpressing angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced lung injury. Cell Transplant. 2015;24:1699–1715. doi: 10.3727/096368914X685087. [DOI] [PubMed] [Google Scholar]
- 231.Wang C.F., Lv D., Zhang X.B., Ni Z.A., Sun X.F., Zhu C.Q. Interleukin-10-overexpressing mesenchymal stromal cells induce a series of regulatory effects in the inflammatory system and promote the survival of endotoxin-induced acute lung injury in mice model. DNA Cell Biol. 2018;37:53–61. doi: 10.1089/dna.2017.3735. [DOI] [PubMed] [Google Scholar]
- 232.Chen X.X., Wu S.S., Tang L., Ma L., Wang F., Feng H.S. Mesenchymal stem cells overexpressing heme oxygenase-1 ameliorate lipopolysaccharide-induced acute lung injury in rats. J Cell Physiol. 2019;234:7301–7319. doi: 10.1002/jcp.27488. [DOI] [PubMed] [Google Scholar]
- 233.Zaldivia M.T.K., McFadyen J.D., Lim B., Wang X., Peter K. Platelet-derived microvesicles in cardiovascular diseases. Front Cardiovasc Med. 2017;4:74. doi: 10.3389/fcvm.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hidalgo A., Cruz A., Perez-Gil J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur J Pharm Biopharm. 2015;95:117–127. doi: 10.1016/j.ejpb.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 235.Guagliardo R., Perez-Gil J., De Smedt S., Raemdonck K. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J Control Release. 2018;291:116–126. doi: 10.1016/j.jconrel.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 236.Speer C.P., Gefeller O., Groneck P., Laufkötter E., Roll C., Hanssler L. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 1995;72:F8−F13. doi: 10.1136/fn.72.1.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Muller R.H., Mader K., Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 238.De Backer L., Cerrada A., Perez-Gil J., De Smedt S.C., Raemdonck K. Bio-inspired materials in drug delivery: exploring the role of pulmonary surfactant in siRNA inhalation therapy. J Control Release. 2015;220:642–650. doi: 10.1016/j.jconrel.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 239.Hsu C.Y., Sung C.T., Aljuffali I.A., Chen C.H., Hu K.Y., Fang J.Y. Intravenous anti-MRSA phosphatiosomes mediate enhanced affinity to pulmonary surfactants for effective treatment of infectious pneumonia. Nanomedicine. 2018;14:215–225. doi: 10.1016/j.nano.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 240.Tavano L., Pinazo A., Abo-Riya M., Infante M.R., Manresa M.A., Muzzalupo R. Cationic vesicles based on biocompatible diacyl glycerol-arginine surfactants: physicochemical properties, antimicrobial activity, encapsulation efficiency and drug release. Colloids Surf B Biointerfaces. 2014;120:160–167. doi: 10.1016/j.colsurfb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 241.Hsieh P.W., Wen C.J., Yu H.P., Aljuffali I.A., Huang Y.H., Fang J.Y. Nanostructured lipid carriers containing a high percentage of a pluronic copolymer increase the biodistribution of novel PDE4 inhibitors for the treatment of traumatic hemorrhage. J Biomed Nanotechnol. 2014;10:1520–1535. doi: 10.1166/jbn.2014.1858. [DOI] [PubMed] [Google Scholar]
- 242.Hickey A.J. Controlled delivery of inhaled therapeutic agents. J Control Release. 2014;190:182–188. doi: 10.1016/j.jconrel.2014.05.058. [DOI] [PubMed] [Google Scholar]
- 243.Scherliess R. Future of nanomedicines for treating respiratory diseases. Expet Opin Drug Deliv. 2019;16:59–68. doi: 10.1080/17425247.2019.1553955. [DOI] [PubMed] [Google Scholar]
- 244.Zhang S.L., Gao H.J., Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9:8655–8671. doi: 10.1021/acsnano.5b03184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Machhi J., Shahjin F., Das S., Patel M., Abdelmoaty M.M., Cohen J.D. Nanocarrier vaccines for SARS-CoV-2. Adv Drug Deliv Rev. 2021;171:215–239. doi: 10.1016/j.addr.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.He C., Qin M., Sun X. Highly pathogenic coronaviruses: thrusting vaccine development in the spotlight. Acta Pharm Sin B. 2020;10:1175–1191. doi: 10.1016/j.apsb.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang J., Li P., Yu Y., Fu Y., Jiang H., Lu M. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science. 2020;367 doi: 10.1126/science.aau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 249.Walls A.C., Fiala B., Schafer A., Wrenn S., Pham M.N., Murphy M. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell. 2020;183:1367–1382. doi: 10.1016/j.cell.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ma X., Zou F., Yu F., Li R., Yuan Y., Zhang Y. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity. 2020;53:1315–1330. doi: 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Stormann P., Becker N., Kunnemeyer L., Wutzler S., Vollrath J.T., Lustenberger T. Contributing factors in the development of acute lung injury in a murine double hit model. Eur J Trauma Emerg Surg. 2020;46:21–30. doi: 10.1007/s00068-019-01121-5. [DOI] [PubMed] [Google Scholar]
- 252.Katalan S., Falach R., Rosner A., Goldvaser M., Brosh-Nissimov T., Dvir A. A novel swine model of ricin-induced acute respiratory distress syndrome. Dis Model Mech. 2017;10:173–183. doi: 10.1242/dmm.027847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sun J., Zhuang Z., Zheng J., Li K., Wong R.L., Liu D. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell. 2020;182:734–743. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Choudhury S.R., Babes L., Rahn J.J., Ahn B.Y., Goring K.R., King J.C. Dipeptidase-1 is an adhesion receptor for neutrophil recruitment in lungs and liver. Cell. 2019;178:1205–1221. doi: 10.1016/j.cell.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 255.Fang G., Zhang Q., Pang Y., Thu H.E., Hussain Z. Nanomedicines for improved targetability to inflamed synovium for treatment of rheumatoid arthritis: multi-functionalization as an emerging strategy to optimize therapeutic efficacy. J Control Release. 2019;303:181–208. doi: 10.1016/j.jconrel.2019.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.