Abstract
Purpose of review
Remote monitoring (RM) of cardiac implantable electronic devices (CIEDs) is recommended as part of the individualized multidisciplinary follow-up of heart failure (HF) patients. Aim of this article is to critically review recent findings on RM, highlighting potential benefits and barriers to its implementation.
Recent findings
Device-based RM is useful in the early detection of CIEDs technical issues and cardiac arrhythmias. Moreover, RM allows the continuous monitoring of several patients' clinical parameters associated with impending HF decompensation, but there is still uncertainty regarding its effectiveness in reducing mortality and hospitalizations.
Summary
Implementation of RM strategies, together with a proactive physicians' attitude towards clinical actions in response to RM data reception, will make RM a more valuable tool, potentially leading to better outcomes.
Keywords: Remote monitoring, Telemedicine, Heart failure, pacemaker, Implantable cardioverter defibrillator, Cardiac resynchronization device
Introduction
Heart failure (HF) is a highly prevalent cardiovascular (CV) disease affecting approximately 1-2% of the adult population in developed countries [1–3]. Due to a high rate of morbidity and mortality, it imposes a remarkable economic burden on healthcare systems. A growing number of cardiac implantable electronic devices (CIEDs) are used to treat bradyarrhythmias and tachyarrhythmias in HF patients and in a subset of appropriately selected patients to correct electrical and mechanical dyssynchrony through biventricular pacing [4, 5••, 6–8]. Many modern CIEDs harbor remote monitoring (RM) systems which can gather, store, and transmit to hospitals/clinicians data regarding the status of the device itself and a multitude of clinical parameters while the patient is at home [9]. These include early recognition of device-related malfunctions, detection of arrhythmias, heart and respiratory rate statistics and, in some cases, heart souds, intrathoracic impedance, and early sign and symptoms of HF [10, 11], potentially leading to a timely clinical action. Thus, RM has joined in-person evaluation in the follow-up of HF patients. Nevertheless, despite the undoubted potential benefits, robust data showing an improvement of outcomes in patients followed-up with RM as compared with in-office only evaluations are scant.
Aim of this article is to critically review recent data on RM of CIEDs in HF patients, highlighting potential benefits and barriers to its implementation. The evaluation of the effects of other types of RM, namely, structured telephone support, telemedicine, and remote monitoring with implanted monitoring-only devices, is outside the object of this review.
Detection of CIEDs-related complications
Device-based RM has a well-established role beside in-person office visits in the early detection of CIEDs technical issues [12–16]. Moreover, it is useful to reduce the incidence of inappropriate ICD shocks. The number of system-related complications per year (including lead complications and generator malfunctions) is not negligible [17–20] and their prompt identification can improve patients' management. In the TRUST trial, 1339 ICD patients were randomized in a 2:1 fashion to RM with daily transmissions or to conventional care with office visits only. During the 15th month follow-up, RM detected generator and lead problems earlier than conventional care (median of 1 vs. 5 days respectively; p = 0.05) [13]. RM proved also safe and useful in reducing total in-hospital device evaluations [11] and demonstrated robust transmission reliability (91%) without reducing battery longevity [21]. Recently, Watanabe et al. [22•] studied 1274 consecutive patients implanted with a PM randomized to RM only or in-office follow-up (2 visits per year). After 24 months, RM only follow-up did not increase the occurrence of death, stroke, or cardiovascular events requiring surgery (10.9% vs. 11.8%, respectively, p < 0.01 for noninferiority) suggesting that RM is safe and able to reduce resource consumption. Device and lead advisories represent a major concern for the physician and for the patient as well. Despite rare [23], device malfunctions can be life-threatening and, on the other hand, replacement of the generator/leads before an overt malfunction may expose the patient to unnecessary risks [24, 25] as well as an organizational burden and costs for hospitals and the health care system [26]. Guédon-Moreau et al. [14] reported a 7.5% lead dysfunction rate in 40 recipients of a high-voltage lead prone to fracture, remotely followed for 22 ± 4 months. In a retrospective cohort of patients with ICD lead fractures, RM sent alert messages in 91% of all lead-related ICD complications [27]. In this setting, RM offers a double benefit: (1) provides an immediate detection of abnormal device behavior through a continuous surveillance of several parameters such as lead impedance and sensing and (2) avoids too early device replacements.
Detection and management of cardiac arrhythmias
CIEDs can record, analyze, and store different types of atrial and ventricular arrhythmias through one or more intracavitary catheters. The continuous monitoring of atrial activity can identify arrhythmic episodes characterized by high atrial rate (AHREs) in asymptomatic patients with no history of clinical atrial fibrillation (AF). These episodes, common in patients necessitating CIEDs, include different forms of atrial tachyarrhythmias such as atrial tachycardias, atrial flutter and AF [28]. AHREs are associated with a considerable risk of adverse clinical events including death [29], hospitalizations [30, 31], stroke/systemic thromboembolism [32–34], occurrence of heart failure [35], and progression to clinical AF [36]. In 2012, 2580 patients with no history of AF were enrolled in the prospective ASSERT [32] trial and were followed for a mean of 2.5 years. AHREs were associated with a 2.5-fold (95% CI 1.28-4.89) higher risk of stroke or systemic embolism at the multivariate analysis. These findings were later confirmed in large observational studies [37–39] and meta-analysis [40]. It also emerged that the higher the burden of AHREs, the higher the risk of future thromboembolic events [41]. RM proved successful in the early identification of AHREs and may reduce the time to potentially meaningful clinical decision such as the institution of an oral anticoagulant therapy, which offers huge and well-established benefits in patients with clinical AF and, presumably, also in selected patients with AHREs [11, 41–46]. Ricci et al. [47] conducted a Monte Carlo simulation showing that in patients with AHREs daily RM may reduce the stroke risk with respect to standard in-person visits scheduled every 6 to 12 months, but ad hoc studies are needed to demonstrate the possible clinical benefits of RM in this setting. In a subanalysis of the ASSERT trial [31], AHREs progression to episodes lasting more than 24 h or to clinical AF was independently associated with HF hospitalization (HR 4.58; 95% CI 1.6-12.8). Therefore, a timely identification of AHREs and of their progression to a higher AF burden or to clinical AF has the potential to improve the outcome of HF patients [36, 48]. Finally, ICDs have a well-recognized life-saving role [49–52], but inappropriate ICD shocks are fearful and common events associated with increased mortality [53]. In the THORN registry [54] (a large RM database of 1882 ICD patients), a 9% prevalence of inappropriate ventricular arrhythmia detection and a 3% prevalence of inappropriate shocks over 13.7 ± 3.4 months of follow-up was reported. In a substudy of the ECOST trial [55], during 27 months follow-up, 5% of patients in the RM group received 1 or more inappropriate shocks versus 10.4% in the control group, suggesting that RM can be effective in the prevention of inappropriate ICD shocks.
Heart failure: a major public health threat
The prevalence of chronic HF (1-2% of the adult population in developed countries) is expected to increase with ageing population [1–3]. Over the last decades, new treatments improved patients outcomes, but morbidity, mortality, and hospitalization rates remain still high [56]. Acute exacerbations of HF often require prolonged in-hospital treatments and also contribute to disease progression and adverse prognosis. Thirty days all-cause readmission rate reaches up to 20% [57] and 10-year mortality approaches 99% [58]. Hospitalizations are at the center of the high cost of HF care accounting for approximately 70% of the global costs [59]. Therefore, huge efforts should address this unmet need. The vast majority of HF readmissions are due to fluid overload [60] and the process of decompensation starts weeks before the acute event through subtle hemodynamic changes which can be detected by some RM systems [61]. A persistent increase in filling pressures in response to small augmentation in intravascular volume is the first measurable event that can be observed. Shortly after, autonomic adaptation through sympathetic activation and vagal withdrawal intervene to increase cardiac output. Heart rate variability is a physiologic parameter that can be measured by CIEDs and directly relates to the autonomic control of the heart: the lower the heart rate variability, the higher the sympathetic tone. One study found that heart rate variability was lower in unstable patients at risk for hospitalization and changes could have been seen 16 to 20 days before symptoms of worsening heart failure with a 70% sensitivity [62]. The next pathophysiologic step is progression to pulmonary circulation congestion, which can be detected by changes in intrathoracic impedance about 2 weeks before hospitalization [63]. Weight changes (>2 pounds in 24-36 h) occur approximately 7 days before hospitalization but, although specific (97%), this is not a sensitive (9%) nor an early marker [64]. Several studies failed to demonstrate that weight gain alone is valuable for HF management [65, 66]. Finally, symptoms develop in the last phase of this process, just before the hospitalization [61].
Remote monitoring of heart failure patients
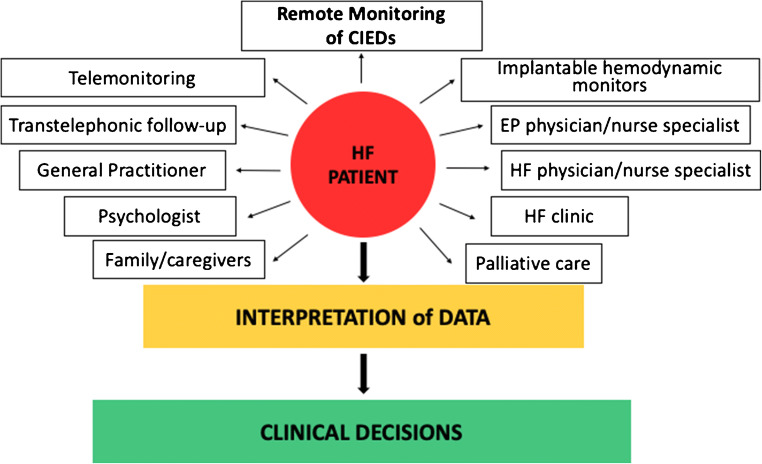
RM aims to respond to the unmet need of HF hospitalizations and deaths prevention and it is recommended as part of a multidisciplinary approach to the management of HF patients [5, 67] (Fig. 1). Among the multiple parameters that can be continuously or frequently assessed, many commercially available CIEDs allow also the measurement of intrathoracic impedance, which is inversely correlated with pulmonary capillary wedge pressure and fluid balance. A decrease in intrathoracic impedance precedes and predicts patient symptoms and hospital admissions [63, 68–71]. In 2011, van Veldhuisen et al. [72] randomized 335 chronic HF patients implanted with an ICD/CRTD featuring a monitoring tool capable of tracking changes in intrathoracic impedance in two groups. In the access arm physicians received RM information in case of preset threshold crossings, while in the control arm they did not. During 14.9 ± 5.4 months of follow-up, 29% of patients in the access arm and 20% of patients in the control arm reached the composite endpoint of all-cause mortality and HF hospitalizations (HR 1.52; 95% CI 0.97-2.37), showing that the use of the monitoring tool was not beneficial. Additional algorithms incorporating multiple HF related indexes such as thoracic impedance, heart sounds (S1, S3), respiratory rate and relative tidal volume, activity response and heart rate have been developed to overcome the limited efficacy of single parameters [73]. In the study by Boehmer et al. [10], the device-based diagnostic algorithm combining these indexes showed 70% sensitivity in predicting impending HF decompensation. The reported 34 days median time between the alert and the HF events is potentially valuable to establish an early therapy and the 1.47 per patient-year unexplained alert rate is acceptable. Clinical usefulness of this algorithm will be clarified in upcoming clinical trials (MANAGE-HF, NCT03237858 and PREEMPT-HF, NCT 03579641) targeted to assess if decision making based on the information provided by these algorithms may result in significant changes in hospitalization burden and cardiovascular mortality as compared to standard clinical judgment. More recently, in a cohort of 918 ICD/CRTD patients, D'Onofrio et al. combined the Seattle Heart Failure Score with the temporal trends of specific individual device-based variables to test an index capable of predicting the first HF hospitalization post-implant. Preliminary data show a 73.3% sensitivity, with low false alert rate [74]. Similarly, in 2010, the TRUST randomized controlled trial (RCT) showed that RM was safe and allowed an early detection of actionable events (defined as an event that prompted initiation/up-titration of antiarrhythmic medications or significant ICD reprogramming/system revision) compared with standard care [11], but this advantage failed to translate into a clinical benefit in most of the following RCTs (Table 1). In the MORE-CARE prospective, multicenter, randomized controlled trial, 865 CRTD patients were randomized to RM checks alternating with in-office follow-up or in-office follow-up only. No significant difference was found in the primary endpoint (a composite of death and cardiovascular and device-related hospitalization) between the 2 groups (HR 1.02; 95% CI 0.80-1.30). However, the authors found a significant 38% reduction in the use of healthcare resources (i.e., 2-year rates of CV hospitalizations, CV emergency department admissions, CV in-office follow-up) in favor of the RM group, mainly as a result of a decrease in in-office visits [84]. A total of 1650 HF patients implanted with a CIED (ICD, CRTD or CRTP) were randomly assigned to active RM or to usual care in the REM-HF randomized controlled trial. RM consisted of weekly transmissions in the active arm and also transmissions every 6 months in the usual care arm, but in the latter group, they were not used to manage HF in any form. After a median of 2.8 years follow-up, no significant differences were observed between the 2 arms in the composite endpoint of all-cause death or CV hospitalizations (HR 1.01; 95% CI 0.87-1.18) or in its individual components. The authors concluded that RM strategy provided no benefit over usual care for patients with HF [85•]. A considerable proportion of patient (38% at 24 months) transmitted data for <75% of the weeks. Beside this gap in achiveing a comprehensive monitoring, centers were overloaded by unfiltered data (a total of 79325 downloads with 10-15 transmission/day per site) rarely leading to a significant clinical action. Less than 1.2% of the transmissions lead to an advise to medical attention and less than 0.3% lead to a medicantion change. These data highlight the need for collecting the right data and that the benefit on outcomes depend on prompt reactions to a critical interpretation of data and not by informations themselves.
Fig. 1.
Remote monitoring as part the multidisciplinary approach to the treatment of heart failure patients. CIEDs cardiac implantable electronic devices, EP electrophysiology, HF heart failure
Table 1.
Randomized clinical trials (RCTs) comparing remote monitoring (RM) versus in-office only follow-up
| Study | RM system | Sample size (n) | Average follow-upa(months) | Device type | Primary endpoint | Results |
|---|---|---|---|---|---|---|
| PREFER, 2009 [75] | CLN |
897 (14% HF) |
12 | PM | • Mean time to first diagnosis of clinically actionable events | • 5.7 (RM) vs. 7.7 (CG) months (p < 0.01) |
| Al-Khatib et al., 2010 [12] | CLN | 151 | 12 | ICD, CRTD | • Composite of CV hospitalization, emergency room visit for cardiac cause and unscheduled visit to the electrophysiology clinic for a device-related issue | • 32% (RM) vs. 34% (CG) (p = 0.8) |
| ECOST substudy, 2010b [14] | HM | 40 | 22 | ICD | • Monitoring of device status and leads function in recipients of high-voltage ICD leads under advisory | • 3/18 (RM) vs. 0/18 (CG) lead fracture detection |
| TRUST, 2010b [11, 13] | HM | 1339 | 12 | ICD |
• Number of total in-hospital device evaluations • Adverse event (deaths, stroke, surgical intervention) rate • Time from arrhythmic event to physician evaluation • Detection of device-related complications |
• 2.1 (RM) vs. 3.8 ppy (p < 0.01) • 10.4% in both groups (p = 0.01 for noninferiority) • 1 (RM) vs. 36 (CG) days (p < 0.01) • 4.4% (RM) vs. 1.4% (CG) (p < 0.01) |
| CONNECT, 2011 [42] | HM | 1997 | 15 | ICD, CRTD |
• Time from clinical event (arrhythmias, CV disease progression and device issues) to clinical decision • Mean length of CV hospitalization |
• 4.6 (RM) vs. 22 (CG) days (p < 0.01) • 3.3 (RM) vs. 4 (CG) days (p < 0.01) |
| DOT-HF, 2011 [72] | OV (CLN) | 335 | 14.9 | ICD, CRTD | • Composite of all-cause mortality and HF hospitalizations | • 29% (RM) vs. 20% (CG) (p = 0.06), HR 1.52 (95% CI 0.97-2.37) |
| COMPAS, 2012b [43] | HM | 538 | 18.3 | PM | • Composite of all-cause death and hospitalizations for device-related or CV adverse events | • 17.3% (RM) vs. 19.1% (CG) (P < 0.01 for noninferiority) |
| EVATEL, 2012 [76] | NA | 1501 | 12 | ICD | • Composite of death, CV hospitalization, and ineffective or inappropriate device therapy | • 30.1% (RM) vs. 28.5% (CG) (p = NS) |
| EVOLVO, 2012 [77] | CLN | 200 | 16 | ICD, CRTD | • Rate of emergency department or urgent in-office visits for HF, arrhythmias, or ICD-related events | • 75 vs. 117 visits, 35% reduction (p < 0.01) |
| ECOST, 2013b [78] | HM | 433 | 24.2 | ICD | • Proportion of patients with ≥ 1 MAE (deaths and CV/procedure/device-related MAE) | • 38.5% (RM) vs. 41.5% (CG) (p < 0.05 for noninferiority), HR 0.91 (95% CI 0.68-1.23) |
| SAVE-HM, 2013 [79] | HM |
115 PM 36 ICD |
17.1 26.3 |
PM, ICD |
• Number of outpatient follow-ups • Number of adverse events |
• 57.8% reduction in RM group • No difference between groups |
| IN-TIME, 2014b [80] | HM | 664 | 12 | ICD, CRTD | • Worsened composite score of all-cause death, hospital admission for HF, change in NYHA class and in patient global self-assessment | • 18.9% (RM) vs. 27.2% (CG) (p = 0.01), OR 0.63 (95% CI 0.43-0.9) |
| EuroEco, 2015b [81] | HM | 303 | 24 | ICD | • Total follow-up-related cost for providers | • 204€ (RM) vs. 213€ (CG) (p = NS) |
| IMPACT, 2015b [44] | HM | 2718 | 24 | ICD, CRTD | • Composite of stroke, systemic embolism and major bleeding | • 2.4 (RM) vs. 2.3 (CG) p100-py, HR 1.06 (95% CI 0.75-1.51) |
| LIMIT-CHF, 2015 [82] | CLN, MER | 80 | 12 | ICD, CRTD | • Number of hospital readmission per patient | • 0.3 (RM) vs. 0.2 (CG) (p = 0.95) |
| OptiLink HF, 2016 [83] | CLN | 1002 | 23 | ICD, CRTD | • Composite of death and CV hospitalization | • 45% (RM) vs. 48.1% (CG), HR 0.87 (95% CI 0.72-1.04) |
| MORE-CARE, 2017 [84] | CLN | 865 | 24 | CRTD | • Composite of death, CV hospitalization and device related hospitalization | • 29.7 (RM) vs. 28.7 (CG), HR: 1.02 (95% CI 0.80-1.30) |
| REM-HF, 2017 [85•] | CLN, LAT, MER | 1650 | 33.6 | ICD, CRTD/P | • Composite of death and CV hospitalization | • 42.4 (RM) vs. 40.8 (CG), HR 1.01 (95% CI 0.87-1.18) |
| REMOTE-CIED, 2019 [86] | LAT | 595 | 24 | ICD |
• Effects of RM on health status • Effects of RM ICD acceptance |
• No effect on KCCQ total score • No effect on FPAS total score |
| At-Home, 2020b [22•] | HM | 1274 (25% HF) | 24 | PM | • Composite of death, stroke, or cardiovascular events requiring surgery | • 10.9% (RM) vs. 11.8% (CG) (p < 0.01 for noninferiority) |
Table 1 shows pivotal RCTs comparing RM vs. in-office only follow-up in heart failure patients implanted with CIEDs in terms of mortality, hospitalizations, and other potential RM benefits
CI confidence interval, CLN CareLink Network (Medtronic Inc.; Minneapolis and Tempe, USA); CG control group, CRT-D cardiac resynchronization therapy defibrillator, CRT-P cardiac resynchronization therapy pacing (no defibrillator), CV cardiovascular, FPAS Florida Patient Acceptance Survey, HF heart failure, HM Home Monitoring (Biotronik SE & Co. KG; Berlin, Germany), HR hazard ratio, ICD implantable cardioverter-defibrillator, KCCQ Kansas City Cardiomyopathy Questionnaire, LAT Latitude Patient Management System (Boston Scientific; St Paul, USA), MAE major adverse event, MER Merlin.net (St. Jude Medical; Sylmar, USA), NA not available, NYHA New York Heart Association class, NS nonsignificant, OR odds ratio, OV OptiVol (pulmonary congestion) algorithm, PM pacemaker, ppy per patient-year, RM remote monitoring, RR relative risk
aMean or median, whatever provided in the original publication.
bDaily RM transmissions.
Parthiban et al. [87] meta-analyzed data extracted from 9 RCTs comparing RM versus conventional in-office follow-up. All-cause mortality and hospitalizations data were available for 7 RCTs, including 4932 and 5372 patients, respectively. No significant difference between RM and conventional care groups was observed for neither outcome (odds ratio (OR) 0.83; 95% CI 0.58-1.17 and OR 0.83; 95% CI 0.63-1.10, respectively). The meta-analysis by Klersy et al. [88••] included 11 RCTs for a total of 5702 patients followed for 12-36 months. Consistently with the previous meta-analysis, rates of cardiac hospitalizations (RR 0.96; 95% CI 0.82-1.12) and the composite of emergency room, unplanned hospital visits or hospitalizations (RR 0.99; 95% CI 0.68-1.43) were similar between the RM and the conventional care groups (Table 2). A recent meta-analysis showed no differences in all-cause mortality and HF-related hospitalizations in patients with RM compared with standard care [89]. However, this meta-analysis included also invasive hemodynamic monitoring systems and not only CIEDs-based RM. Finally, Versteeg et al. [86] tried to evaluate the effects of RM on patient-reported outcomes in a cohort of 595 HF patients in the first 2 years after ICD implant. The authors found no difference in terms of patients' health status (assessed by the Kansas City Cardiomyopathy Questionnaire) and ICD acceptance (assessed by Florida Patient Acceptance Survey) between the group of patients randomized to RM and the group randomized to in-office visits only.
Table 2.
Meta-analysis of randomized clinical trials on remote monitoring of cardiac implantable electronic devices from various device manufacturers: effects on mortality, hospitalizations, and visits
| Meta-analysis | RM system | Sample size (n) | Average follow-upa (Months) |
No of studies included | Primary endpoint | Results |
|---|---|---|---|---|---|---|
| Parthiban et al. (2015) [87] | HM, CLN |
4932 5372 |
14.4 NA |
7 7 |
• All-cause mortality (RM vs. CG) • Hospitalizations (RM vs. CG) |
• OR 0.83 (95% CI 0.58-1.17) • OR 0.83 (95% CI 0.63-1.10) |
| Klersy et al., (2016) [88••] | HM, CLN | 5702 | 12-36 | 11 |
• Reduction in total number of visits (RM vs. CG) • Cardiac hospitalizations (RM vs. CG) • Composite of emergency room, unplanned hospital visits, or hospitalizations (RM vs. CG) |
• RR 0.56 (95% CI 0.43-0.73) • RR 0.96 (95% CI 0.82-1.12) • RR 0.99 (95% CI 0.68-1.43) |
CI confidence interval, CLN CareLink Network (Medtronic Inc.; Minneapolis and Tempe, USA), CG control group, HM Home Monitoring (Biotronik SE & Co. KG; Berlin, Germany), No number, NA not available, OR odds ratio, RM, remote monitoring, RR, relative risk
aMean or median, whatever provided in the original publication
IN-TIME is the only RCT up to now that showed a significant mortality benefit of automatic, daily, multiparameter telemonitoring as compared with usual care alone (3% of patients in the RM arm vs. 8.2% in the control arm, p = 0.004) in HF patients implanted with ICD or CRTD, albeit hospitalizations for worsening HF did not differ between the two groups. Mean follow-up duration was ≈1 year and mortality was not the primary end point. A considerable deployment of resources has to be acknowledged in this trial. In the RM group (333 patients), the investigators contacted patients on the basis of telemonitored data, starting a standardized telephone interview to establish whether the patient's overall condition or symptoms had worsened or not, whether the patient was regularly taking prescribed drugs, whether there was a sudden increase in body weight or whether and additional clinic follow-up or a visit to the family doctor was scheduled. On the other hand, in the control group (331 patients), telemonitoring data were not accessible until study completion [80]. Large-scale nonrandomized studies point in a similar direction, showing a survival advantage for patients undergoing RM as compared with those receiving in-person only follow-up [90–92]. However, given the nonrandomized design of these studies, several biases may have affected the results and their generalizability, thus requiring caution in data interpretation.
In recent years, the possibility that daily RM transmissions may increase data processing capacity leading to higher sensitivity and specificity as compared with weekly transmissions has been investigated. Hindricks et al. [93] performed a pooled patient-level meta-analysis of 3 RCTs (TRUST, ECOST, IN-TIME) using the Home Monitoring system that is based on daily verification of transmissions. The authors reported a 1.9% (p = 0.037) reduction in the absolute risk of all-cause death at 1 year in the RM group and a 5.6% (p = 0.007) reduction in the composite endpoint of all-cause mortality or hospitalization for worsening HF. The latter analysis was conducted including only 2 trials (ECOST, IN-TIME). Daily transmission of data is an alternative approach as compared to RM systems transmitting preset alerts activated at specific predefined thresholds. In the absence of direct comparisons between these 2 approaches, the superiority of daily RM remains speculative and should be tested towards clinical outcomes at long-term in dedicated randomized trials.
Altogether, these data indicate that RM of CIEDs represents a valuable tool in the early diagnosis of HF decompensation, but its effectiveness in reducing mortality and hospitalizations is still uncertain. They anyway suggest that implementation of RM can be a worth doing strategy, especially in consideration of the impact of COVID-19 pandemic [94, 95] with need for more accurate analysis in the next future.
Progresses and pitfalls in everyday implementation of remote monitoring
The use of RM has markedly increased in recent years, as shown by the comparison of two Italian surveys conducted in 2012 and 2017 [96]. The global COVID-19 pandemic is further boosting the RM implementation in order to keep social-distancing to the utmost [95]. However, RM is still largely underused in clinical practice [92]. Barriers to its implementation are mainly the lack of reimbursement, need for significant changes in hospitals' workflows, data overload, and increased workload for health-care providers [97–101]. The growing bunch of clinical evidence on the safety and usefulness of RM, combined with the overcoming of the reimbursement issue, will probably lead to a wider overall adoption of this valuable tool, which will obviously will markedly benefit from active involvement of general practitioners, caregivers, and empowered patients [102].
Conclusions
RM is recommended for the early detection of CIEDs technical issues and early diagnosis and management of cardiac arrhythmias [5, 67]. In recent years, multiparameter RM has gained relevance in the individualized management of HF patients implanted with a CIED. Despite good sensitivity in predicting worsening HF, the role of RM in improving patients' outcome is still matter of debate. Factors that may lead to a more profitable use of RM include a better selection of parameters to monitor and patients to candidate to RM and a more proactive attitude towards disease management of HF, with an appropriate organization of care strictly linking hospital care to home care. A paradigm shift from remote patient monitoring to remote patient management is warranted, translating data into prompt clinical actions.
Compliance with ethical standards
Conflict of interest
Dr. Boriani has received small speaker's fees from Medtronic, Boston, Biotronik, Boehringer, and Bayer outside of the submitted work. The other authors report no conflict of interest.
Consent for publication
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Myocardial Disease
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jacopo Francesco Imberti, Email: jacopo.imberti@hotmail.it.
Alberto Tosetti, Email: albertotosetti1@gmail.com.
Davide Antonio Mei, Email: davide.mei93@gmail.com.
Anna Maisano, Email: annamaisano1991@gmail.com.
Giuseppe Boriani, Email: giuseppe.boriani@unimore.it.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, et al. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail. 2002;4(4):531–539. doi: 10.1016/S1388-9842(02)00034-X. [DOI] [PubMed] [Google Scholar]
- 2.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25(18):1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34(8):1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 6.Boriani G, Berti E, Belotti LM, Biffi M, De Palma R, Malavasi VL, et al. Cardiac device therapy in patients with left ventricular dysfunction and heart failure: 'real-world' data on long-term outcomes (mortality, hospitalizations, days alive and out of hospital) Eur J Heart Fail. 2016;18(6):693–702. doi: 10.1002/ejhf.509. [DOI] [PubMed] [Google Scholar]
- 7.Boriani G, Ziacchi M, Nesti M, Battista A, Placentino F, Malavasi VL, et al. Cardiac resynchronization therapy: How did consensus guidelines from Europe and the United States evolve in the last 15 years? Int J Cardiol. 2018;261:119–129. doi: 10.1016/j.ijcard.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Mullens W, Auricchio A, Martens P, Witte K, Cowie MR, Delgado V, et al. Optimized Implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care: A joint position statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. Eur J Heart Fail. 2020;22(12):2349–69. 10.1002/ejhf.2046. [DOI] [PubMed]
- 9.Boriani G, Diemberger I, Martignani C, Biffi M, Valzania C, Bertini M, et al. Telecardiology and remote monitoring of implanted electrical devices: the potential for fresh clinical care perspectives. J Gen Intern Med. 2008;23(Suppl 1):73–77. doi: 10.1007/s11606-007-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results From the MultiSENSE Study. JACC Heart Fail. 2017;5(3):216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C, Investigators T. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122(4):325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 12.Al-Khatib SM, Piccini JP, Knight D, Stewart M, Clapp-Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol. 2010;21(5):545–550. doi: 10.1111/j.1540-8167.2009.01659.x. [DOI] [PubMed] [Google Scholar]
- 13.Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: the Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial. Circ Arrhythm Electrophysiol. 2010;3(5):428–436. doi: 10.1161/CIRCEP.110.951962. [DOI] [PubMed] [Google Scholar]
- 14.Guédon-Moreau L, Chevalier P, Marquié C, Kouakam C, Klug D, Lacroix D, et al. Contributions of remote monitoring to the follow-up of implantable cardioverter-defibrillator leads under advisory. Eur Heart J. 2010;31(18):2246–2252. doi: 10.1093/eurheartj/ehq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen JC, Kottkamp H, Zabel M, Aliot E, Kreutzer U, Bauer A, et al. Automatic home monitoring of implantable cardioverter defibrillators. Europace. 2008;10(6):729–735. doi: 10.1093/europace/eun099. [DOI] [PubMed] [Google Scholar]
- 16.Folino AF, Chiusso F, Zanotto G, Vaccari D, Gasparini G, Megna A, et al. Management of alert messages in the remote monitoring of implantable cardioverter defibrillators and pacemakers: an Italian single-region study. Europace. 2011;13(9):1281–1291. doi: 10.1093/europace/eur154. [DOI] [PubMed] [Google Scholar]
- 17.Ranasinghe I, Parzynski CS, Freeman JV, Dreyer RP, Ross JS, Akar JG, et al. Long-Term Risk for Device-Related Complications and Reoperations After Implantable Cardioverter-Defibrillator Implantation: An Observational Cohort Study. Ann Intern Med. 2016;165(1):20–29. doi: 10.7326/M15-2732. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins NM, Grubisic M, Andrade JG, Huang F, Ding L, Gao M, et al. Long-term complications, reoperations and survival following cardioverter-defibrillator implant. Heart. 2018;104(3):237–243. doi: 10.1136/heartjnl-2017-311638. [DOI] [PubMed] [Google Scholar]
- 19.Eckstein J, Koller MT, Zabel M, Kalusche D, Schaer BA, Osswald S, et al. Necessity for surgical revision of defibrillator leads implanted long-term: causes and management. Circulation. 2008;117(21):2727–2733. doi: 10.1161/CIRCULATIONAHA.107.740670. [DOI] [PubMed] [Google Scholar]
- 20.van Rees JB, van Welsenes GH, Borleffs CJ, Thijssen J, van der Velde ET, van der Wall EE, et al. Update on small-diameter implantable cardioverter-defibrillator leads performance. Pacing Clin Electrophysiol. 2012;35(6):652–658. doi: 10.1111/j.1540-8159.2011.03338.x. [DOI] [PubMed] [Google Scholar]
- 21.Varma N, Love CJ, Schweikert R, Moll P, Michalski J, Epstein AE, et al. Automatic remote monitoring utilizing daily transmissions: transmission reliability and implantable cardioverter defibrillator battery longevity in the TRUST trial. Europace. 2018;20(4):622–628. doi: 10.1093/europace/eux059. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe E, Yamazaki F, Goto T, Asai T, Yamamoto T, Hirooka K, et al. Remote Management of Pacemaker Patients With Biennial In-Clinic Evaluation: Continuous Home Monitoring in the Japanese At-Home Study: A Randomized Clinical Trial. Circ Arrhythm Electrophysiol. 2020;13(5):e007734. doi: 10.1161/CIRCEP.119.007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrotta L, Pieragnoli P, Ricciardi G, Sacchi S, Mascia G, Padeletti M, et al. Multicenter experience with implantable defibrillators subject to recall. Pacing Clin Electrophysiol. 2011;34(8):998–1002. doi: 10.1111/j.1540-8159.2011.03083.x. [DOI] [PubMed] [Google Scholar]
- 24.Gould PA, Krahn AD, Advisories CHRSWGoD. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295(16):1907-1911. [DOI] [PubMed]
- 25.Boriani G, Merino J, Wright DJ, Gadler F, Schaer B, Landolina M. Battery longevity of implantable cardioverter-defibrillators and cardiac resynchronization therapy defibrillators: technical, clinical and economic aspects. An expert review paper from EHRA. Europace. 2018;20(12):1882–1897. doi: 10.1093/europace/euy066. [DOI] [PubMed] [Google Scholar]
- 26.Palmisano P, Ziacchi M, Belotti G, Rapacciuolo A, Santini L, Stabile G, et al. Clinical and organizational management of cardiac implantable electronic device replacements: an Italian Survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing) J Cardiovasc Med (Hagerstown) 2019;20(8):531–541. doi: 10.2459/JCM.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 27.Spencker S, Coban N, Koch L, Schirdewan A, Müller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11(4):483–488. doi: 10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 28.Gorenek B, Bax J, Boriani G, Chen SA, Dagres N, Glotzer TV, et al. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE) Europace. 2017;19(9):1556–1578. doi: 10.1093/europace/eux163. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JE, et al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014;11(12):2214–2221. doi: 10.1016/j.hrthm.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Pastori D, Miyazawa K, Li Y, Székely O, Shahid F, Farcomeni A, et al. Atrial high-rate episodes and risk of major adverse cardiovascular events in patients with cardiac implantable electronic devices. Clin Res Cardiol. 2020;109(1):96–102. doi: 10.1007/s00392-019-01493-z. [DOI] [PubMed] [Google Scholar]
- 31.Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J, et al. Progression of Device-Detected Subclinical Atrial Fibrillation and the Risk of Heart Failure. J Am Coll Cardiol. 2018;71(23):2603–2611. doi: 10.1016/j.jacc.2018.03.519. [DOI] [PubMed] [Google Scholar]
- 32.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 33.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 34.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST) Circulation. 2003;107(12):1614–1619. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 35.Khan AA, Boriani G, Lip GYH. Are atrial high rate episodes (AHREs) a precursor to atrial fibrillation? Clin Res Cardiol. 2020;109(4):409–416. doi: 10.1007/s00392-019-01545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boriani G, Glotzer TV, Ziegler PD, De Melis M, Mangoni di S Stefano L, Sepsi M, et al. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm. 2018;15(3):376–383. doi: 10.1016/j.hrthm.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices) Eur Heart J. 2014;35(8):508–516. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, et al. Atrial Fibrillation Burden and Short-Term Risk of Stroke: Case-Crossover Analysis of Continuously Recorded Heart Rhythm From Cardiac Electronic Implanted Devices. Circ Arrhythm Electrophysiol. 2015;8(5):1040–1047. doi: 10.1161/CIRCEP.114.003057. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke Risk as a Function of Atrial Fibrillation Duration and CHA. Circulation. 2019;140(20):1639–1646. doi: 10.1161/CIRCULATIONAHA.119.041303. [DOI] [PubMed] [Google Scholar]
- 40.Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39(16):1407–1415. doi: 10.1093/eurheartj/ehx731. [DOI] [PubMed] [Google Scholar]
- 41.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020:ehaa612.
- 42.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH, Investigators C. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57(10):1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, et al. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial) Eur Heart J. 2012;33(9):1105–1111. doi: 10.1093/eurheartj/ehr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36(26):1660–1668. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 45.Ricci RP, Vaccari D, Morichelli L, Zanotto G, Calò L, D'Onofrio A, et al. Stroke incidence in patients with cardiac implantable electronic devices remotely controlled with automatic alerts of atrial fibrillation. A sub-analysis of the HomeGuide study. Int J Cardiol. 2016;219:251–256. doi: 10.1016/j.ijcard.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Boriani G, Healey JS, Schnabel RB, Lopes RD, Calkins H, Camm JA, et al. Oral anticoagulation for subclinical atrial tachyarrhythmias detected by implantable cardiac devices: an international survey of the AF-SCREEN Group. Int J Cardiol. 2019;296:65–70. doi: 10.1016/j.ijcard.2019.07.039. [DOI] [PubMed] [Google Scholar]
- 47.Ricci RP, Morichelli L, Gargaro A, Laudadio MT, Santini M. Home monitoring in patients with implantable cardiac devices: is there a potential reduction of stroke risk? Results from a computer model tested through monte carlo simulations. J Cardiovasc Electrophysiol. 2009;20(11):1244–1251. doi: 10.1111/j.1540-8167.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- 48.Boriani G, Vitolo M. Atrial fibrillation in patients with cardiac implantable electronic devices: new perspectives with important clinical implications. Kardiol Pol. 2019;77(12):1119–1120. doi: 10.33963/KP.15110. [DOI] [PubMed] [Google Scholar]
- 49.Investigators AIDA. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337(22):1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 50.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 51.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 52.Boriani G, Malavasi VL. Extending survival by reducing sudden death with implantable cardioverter-defibrillators: a challenging clinical issue in non-ischaemic and ischaemic cardiomyopathies. Eur J Heart Fail. 2018;20(3):420–426. doi: 10.1002/ejhf.1080. [DOI] [PubMed] [Google Scholar]
- 53.Proietti R, Labos C, Davis M, Thanassoulis G, Santangeli P, Russo V, et al. A systematic review and meta-analysis of the association between implantable cardioverter-defibrillator shocks and long-term mortality. Can J Cardiol. 2015;31(3):270–277. doi: 10.1016/j.cjca.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 54.Perrin T, Boveda S, Defaye P, Rosier A, Sadoul N, Bordachar P, et al. Role of medical reaction in management of inappropriate ventricular arrhythmia diagnosis: the inappropriate Therapy and HOme monitoRiNg (THORN) registry. Europace. 2019;21(4):607–615. doi: 10.1093/europace/euy284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guédon-Moreau L, Kouakam C, Klug D, Marquié C, Brigadeau F, Boulé S, et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial. J Cardiovasc Electrophysiol. 2014;25(7):763–770. doi: 10.1111/jce.12405. [DOI] [PubMed] [Google Scholar]
- 56.Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2013;15(7):808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 57.Bergethon KE, Ju C, DeVore AD, Hardy NC, Fonarow GC, Yancy CW, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the get with the guidelines-heart failure registry. Circ Heart Fail. 2016;9(6). [DOI] [PMC free article] [PubMed]
- 58.Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5(4):414–421. doi: 10.1161/CIRCHEARTFAILURE.111.964791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 60.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168(8):847–854. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 61.Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009;6(4):287–292. doi: 10.1007/s11897-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 62.Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation. 2003;108(3):266–269. doi: 10.1161/01.CIR.0000083368.75831.7A. [DOI] [PubMed] [Google Scholar]
- 63.Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 64.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116(14):1549–1554. doi: 10.1161/CIRCULATIONAHA.107.690768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Goode KM, Cuddihy PE, Cleland JG, Investigators T-H. Predicting hospitalization due to worsening heart failure using daily weight measurement: analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur J Heart Fail. 2009;11(4):420–427. doi: 10.1093/eurjhf/hfp033. [DOI] [PubMed] [Google Scholar]
- 66.Lyngå P, Persson H, Hägg-Martinell A, Hägglund E, Hagerman I, Langius-Eklöf A, et al. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur J Heart Fail. 2012;14(4):438–444. doi: 10.1093/eurjhf/hfs023. [DOI] [PubMed] [Google Scholar]
- 67.Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12(7):e69–100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Small RS, Wickemeyer W, Germany R, Hoppe B, Andrulli J, Brady PA, et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15(6):475–481. doi: 10.1016/j.cardfail.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 69.Vanderheyden M, Houben R, Verstreken S, Ståhlberg M, Reiters P, Kessels R, et al. Continuous monitoring of intrathoracic impedance and right ventricular pressures in patients with heart failure. Circ Heart Fail. 2010;3(3):370–377. doi: 10.1161/CIRCHEARTFAILURE.109.867549. [DOI] [PubMed] [Google Scholar]
- 70.Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55(17):1803–1810. doi: 10.1016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 71.Cowie MR, Sarkar S, Koehler J, Whellan DJ, Crossley GH, Tang WH, et al. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J. 2013;34(31):2472–2480. doi: 10.1093/eurheartj/eht083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124(16):1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 73.Kotalczyk A, Kalarus Z, Wright DJ, Boriani G, Lip GYH. Cardiac Electronic Devices: Future Directions and Challenges. Med Devices (Auckl) 2020;13:325–338. doi: 10.2147/MDER.S245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.D'Onofrio A, Solimene F, Calò L, Calvi V. Combining home monitoring temporal trends and baseline patient risk profile for predicting impending heart failure hospitalizations. Results from the SELENE HF (BIO.Detect HF IV) study. Eur Heart J. 2020:1551. [DOI] [PMC free article] [PubMed]
- 75.Crossley GH, Chen J, Choucair W, Cohen TJ, Gohn DC, Johnson WB, et al. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol. 2009;54(22):2012–2019. doi: 10.1016/j.jacc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Mabo P, Defaye P, Sadoul N. Remote follow-up of patients implanted with an ICD: the prospective randomized Evatel study (abstr). Heart Rhythm. 2012:S226–S7.
- 77.Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation. 2012;125(24):2985–2992. doi: 10.1161/CIRCULATIONAHA.111.088971. [DOI] [PubMed] [Google Scholar]
- 78.Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida JS, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34(8):605–614. doi: 10.1093/eurheartj/ehs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perl S, Stiegler P, Rotman B, Prenner G, Lercher P, Anelli-Monti M, et al. Socio-economic effects and cost saving potential of remote patient monitoring (SAVE-HM trial) Int J Cardiol. 2013;169(6):402–407. doi: 10.1016/j.ijcard.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 80.Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384(9943):583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 81.Heidbuchel H, Hindricks G, Broadhurst P, Van Erven L, Fernandez-Lozano I, Rivero-Ayerza M, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): a provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J. 2015;36(3):158–169. doi: 10.1093/eurheartj/ehu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Domenichini G, Rahneva T, Diab IG, Dhillon OS, Campbell NG, Finlay MC, et al. The lung impedance monitoring in treatment of chronic heart failure (the LIMIT-CHF study) Europace. 2016;18(3):428–435. doi: 10.1093/europace/euv293. [DOI] [PubMed] [Google Scholar]
- 83.Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J. 2016;37(41):3154–3163. doi: 10.1093/eurheartj/ehw099. [DOI] [PubMed] [Google Scholar]
- 84.Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017;19(3):416–425. doi: 10.1002/ejhf.626. [DOI] [PubMed] [Google Scholar]
- 85.Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38(30):2352–2360. doi: 10.1093/eurheartj/ehx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Versteeg H, Timmermans I, Widdershoven J, Kimman GJ, Prevot S, Rauwolf T, et al. Effect of remote monitoring on patient-reported outcomes in European heart failure patients with an implantable cardioverter-defibrillator: primary results of the REMOTE-CIED randomized trial. Europace. 2019;21(9):1360–1368. doi: 10.1093/europace/euz140. [DOI] [PubMed] [Google Scholar]
- 87.Parthiban N, Esterman A, Mahajan R, Twomey DJ, Pathak RK, Lau DH, et al. Remote Monitoring of Implantable Cardioverter-Defibrillators: A Systematic Review and Meta-Analysis of Clinical Outcomes. J Am Coll Cardiol. 2015;65(24):2591–2600. doi: 10.1016/j.jacc.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 88.Klersy C, Boriani G, De Silvestri A, Mairesse GH, Braunschweig F, Scotti V, et al. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta-analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail. 2016;18(2):195–204. doi: 10.1002/ejhf.470. [DOI] [PubMed] [Google Scholar]
- 89.Alotaibi S, Hernandez-Montfort J, Ali OE, El-Chilali K, Perez BA. Remote monitoring of implantable cardiac devices in heart failure patients: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev. 2020;25(3):469–479. doi: 10.1007/s10741-020-09923-1. [DOI] [PubMed] [Google Scholar]
- 90.Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122(23):2359–2367. doi: 10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]
- 91.Akar JG, Bao H, Jones PW, Wang Y, Varosy PD, Masoudi FA, et al. Use of remote monitoring is associated with lower risk of adverse outcomes among patients with implanted cardiac defibrillators. Circ Arrhythm Electrophysiol. 2015;8(5):1173–1180. doi: 10.1161/CIRCEP.114.003030. [DOI] [PubMed] [Google Scholar]
- 92.Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65(24):2601–2610. doi: 10.1016/j.jacc.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 93.Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon-Moreau L, et al. Daily remote monitoring of implantable cardioverter-defibrillators: insights from the pooled patient-level data from three randomized controlled trials (IN-TIME, ECOST, TRUST) Eur Heart J. 2017;38(22):1749–1755. doi: 10.1093/eurheartj/ehx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mattioli AV, Cossarizza A, Boriani G. COVID-19 pandemic: usefulness of telemedicine in management of arrhythmias in elderly people. J Geriatr Cardiol. 2020;17(9):593–596. doi: 10.11909/j.issn.1671-5411.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boriani G, Palmisano P, Guerra F, Bertini M, Zanotto G, Lavalle C, et al. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing) Intern Emerg Med. 2020;15(8):1445–1456. doi: 10.1007/s11739-020-02487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palmisano P, Melissano D, Zanotto G, Perego GB, Toselli T, Landolina M, et al. Change in the use of remote monitoring of cardiac implantable electronic devices in Italian clinical practice over a 5-year period: results of two surveys promoted by the AIAC (Italian Association of Arrhythmology and Cardiac Pacing) J Cardiovasc Med (Hagerstown) 2020;21(4):305–314. doi: 10.2459/JCM.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 97.Braunschweig F, Anker SD, Proff J, Varma N. Remote monitoring of implantable cardioverter-defibrillators and resynchronization devices to improve patient outcomes: dead end or way ahead? Europace. 2019;21(6):846–855. doi: 10.1093/europace/euz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zanotto G, Melissano D, Baccillieri S, Campana A, Caravati F, Maines M, et al. Intrahospital organizational model of remote monitoring data sharing, for a global management of patients with cardiac implantable electronic devices: a document of the Italian Association of Arrhythmology and Cardiac Pacing. J Cardiovasc Med (Hagerstown) 2020;21(3):171–181. doi: 10.2459/JCM.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 99.Boriani G, Imberti JF, Vitolo M. Atrial fibrillation and remote monitoring through cardiac implantable electronic devices in heart failure patients. Eur J Heart Fail. 2020;22(3):554–556. doi: 10.1002/ejhf.1745. [DOI] [PubMed] [Google Scholar]
- 100.Maines M, Tomasi G, Moggio P, Peruzza F, Catanzariti D, Angheben C, et al. Implementation of remote follow-up of cardiac implantable electronic devices in clinical practice: organizational implications and resource consumption. J Cardiovasc Med (Hagerstown) 2020;21(9):648–653. doi: 10.2459/JCM.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 101.Boriani G. Remote monitoring of cardiac implantable electrical devices in Europe: quo vadis? Europace. 2015;17(5):674–676. doi: 10.1093/europace/euv031. [DOI] [PubMed] [Google Scholar]
- 102.Padula MS, D'Ambrosio GG, Tocci M, D'Amico R, Banchelli F, Angeli L, et al. Home care for heart failure: can caregiver education prevent hospital admissions? A randomized trial in primary care. J Cardiovasc Med (Hagerstown) 2019;20(1):30–38. doi: 10.2459/JCM.0000000000000722. [DOI] [PubMed] [Google Scholar]