Abstract
INTRODUCTION:
Microstructural alterations as assessed by diffusion tensor imaging (DTI) are key findings in both Alzheimer’s disease (AD) and small vessel disease (SVD). We determined the contribution of each of these conditions to diffusion alterations.
METHODS:
We studied six samples (N=365 participants) covering the spectrum of AD and SVD, including genetically-defined samples. We calculated diffusion measures from DTI and free water imaging. Simple linear, multivariable random forest, and voxel-based regressions were used to evaluate associations between AD biomarkers (amyloid-beta, tau), SVD imaging markers, and diffusion measures.
RESULTS:
SVD markers were strongly associated with diffusion measures and showed a higher contribution than AD biomarkers in multivariable analysis across all memory clinic samples. Voxel-wise analyses between tau and diffusion measures were not significant.
DISCUSSION:
In memory clinic patients, the effect of SVD on diffusion alterations largely exceeds the effect of AD, supporting the value of diffusion measures as markers of SVD.
Keywords: Alzheimer’s disease, cerebral small vessel disease, diffusion tensor imaging, free water imaging, white matter, biomarker
1. Introduction
Alzheimer’s disease (AD) and cerebral small vessel disease (SVD) are the two leading causes of cognitive decline and dementia.1 Altered white matter microstructure is considered a key finding in both conditions2,3 and has consistently been associated with cognitive deficits.4–6 The most commonly used method to study white matter microstructure in vivo is diffusion tensor imaging (DTI), which quantifies diffusion properties of water molecules in brain tissue.7,8 The typical finding described in both AD and SVD is an increase in the extent of water diffusion (mean diffusivity) and a decrease in diffusion directionality (fractional anisotropy), which can be detected both globally and regionally.4,5 Despite the wide use of diffusion alterations as efficient disease markers and their strong associations with clinical deficits, little is known about their underlying pathology.
In memory clinic patients, AD and SVD often co-exist.9 The extent to which each of these conditions contribute to diffusion MRI alterations is largely elusive. Free water imaging, an advanced diffusion model, improves the specificity of the DTI model and could therefore provide additional insight into the origin of diffusion MRI alterations.10 As such, free water imaging might be able to disentangle the effects of AD and SVD.11–14 Previous studies using DTI or free water imaging were limited by the lack of biomarker evidence of AD pathology or insufficient consideration of mixed pathology. Assessing the individual contributions of AD and SVD towards diffusion MRI alterations requires a systematic study covering the entire spectrum of “pure AD”, mixed disease, and “pure SVD”.
The uncertainty regarding the origin and interpretation of diffusion alterations in memory clinic patients impedes widespread implementation in research and clinical practice. Therefore, the aim of this study was to determine the effect of AD and SVD on diffusion MRI in a memory clinic setting. We examined associations between biomarkers of AD, MRI markers of SVD, and diffusion measures from both conventional DTI and free water imaging. Six study samples (N=365 participants) were included to systematically cover the entire spectrum of AD, mixed disease, and SVD, and to account for both cerebrospinal fluid (CSF) and positron emission tomography (PET) markers. In addition to the common memory clinic setting with predominantly mixed disease, our analysis also included patient samples with pure, genetically-defined AD or SVD. This enabled us to examine effects of both diseases on diffusion measures without confounding pathology. Analyses were performed separately within each sample in order to validate results and address generalizability using the six independently recruited samples.
2. Methods
2.1. Participants
We studied six independent samples (N=365 participants) covering the spectrum of AD, mixed disease, and SVD: four memory clinic samples with mixed disease with a recruitment focus on either AD or SVD, one sample each of genetically-defined AD and SVD. Memory clinic samples were drawn from single or multi-center studies, which were selected based on availability of (diffusion) MRI sequences and CSF or PET data. The compilation of samples, subject selection criteria, and exclusions are shown in Fig. 1, and further elaborated in 2.1.1–2.1.3. MRI, CSF, and PET data from subjects of the included samples were obtained within one year. Diagnostic criteria used in the AD and SVD focused memory clinic samples are summarized in Supplementary Table 1. All studies were approved by the ethics committees of the respective institutions and all subjects provided written informed consent.
Figure 1. Study concept and participant selection flowchart.
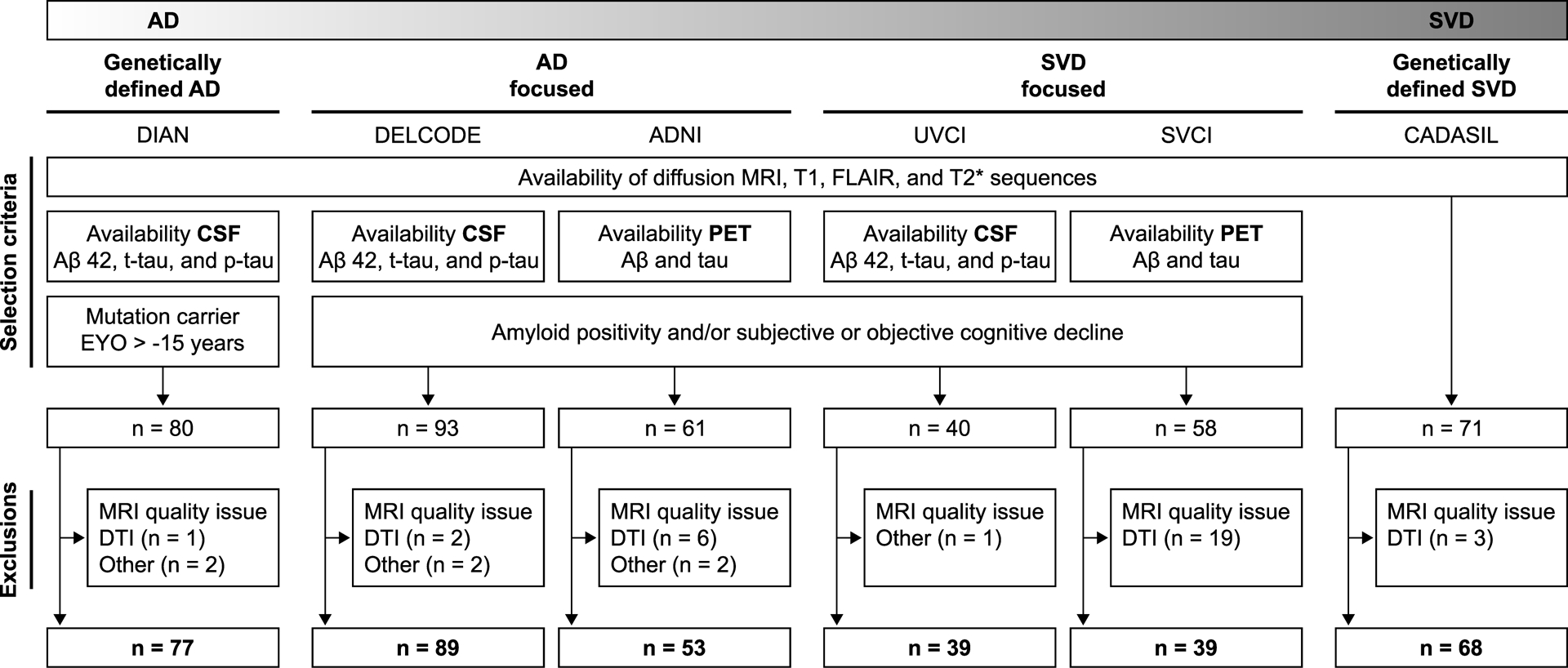
Samples cover the entire spectrum of AD, mixed disease, and SVD.
AD, Alzheimer’s disease; DTI, diffusion tensor imaging; EYO, estimated years from symptom onset; FLAIR, fluid-attenuated inversion recovery; p-tau, phosphorylated-tau181; SVD, small vessel disease; t-tau, total tau.
2.1.1. Alzheimer’s disease focused samples
We included 89 participants from the German multicentric DZNE-Longitudinal Cognitive Impairment and Dementia Study (DELCODE; downloaded in December 2018) with available CSF amyloid-beta1–40 (Aβ 40), amyloid-beta1–42 (Aβ 42), total-tau (t-tau), and phosphorylated-tau181 (p-tau) data. The sample consisted of Aβ 42-positive healthy controls (Aβ 42 cut-off see Supplementary Text 1) and patients with subjective cognitive decline, amnestic mild cognitive impairment, and mild dementia.15
We further included 53 participants from the multicentric Alzheimer’s disease Neuroimaging Initiative (ADNI, phase 3; downloaded in December 2018 at http://adni.loni.usc.edu) with available Aβ [18F]-florbetapir and tau [18F]AV-1451 flortaucipir (PET). The sample consisted of amyloid-positive (cut-off see Supplementary Text 1) healthy controls and patients with amnestic mild cognitive impairment and mild dementia (http://adni.loni.usc.edu).
2.1.2. Small vessel disease focused samples
We included 39 participants from the University Medical Center Utrecht, Netherlands (prospective Utrecht Vascular Cognitive Impairment study, UVCI) with available CSF data for Aβ 42, t-tau, and p-tau. The sample consisted of patients with subjective cognitive decline, mild cognitive impairment, and dementia and with no evidence of a primary etiology other than neurodegenerative disease or sporadic SVD and a high burden of SVD on MRI.16 We further included 39 participants from the Samsung Medical Center, Seoul, Republic of Korea (Seoul Vascular Cognitive Impairment study, SVCI) with available Aβ [18F]-florbetaben and tau [18F]AV-1451 flortaucipir (PET). The sample consisted of patients with objective cognitive impairment and a high burden of SVD on MRI.17,18
2.1.3. Genetically-defined samples
As a genetically-defined AD sample, we included 77 participants from the multicentric Dominantly Inherited Alzheimer Network (DIAN, data freeze 11; downloaded in August 2018).19 DIAN is a longitudinal cohort study of individuals at risk of developing autosomal dominant AD. Here we included PSEN1 (n=59), PSEN2 (n=5), and APP (n=13) mutation carriers with available Aβ 40, Aβ 42, t-tau, and p-tau CSF data. In our study, subjects had to be less than 15 years from estimated symptom onset in order to increase sensitivity to detect AD and SVD marker alterations in proximity to the onset of AD symptoms.5,20
As a genetically-defined SVD sample, we included 68 patients with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) recruited from a single-center study in Munich.4 Although CSF or PET data were not available in this dataset, we included CADASIL to judge the effect sizes of SVD markers in genetically-defined SVD.
2.2. MRI
All MRI data were obtained on 3 Tesla systems. All samples included diffusion MRI, T1-weighted, fluid-attenuated inversion recovery (T2-weighted), and gradient echo (T2*-weighted) sequences. While each study used a standardized protocol, acquisition parameters differed across studies. The MRI protocols have been published previously for DIAN,5 DELCODE,21 ADNI,22 UVCI,23 SVCI,17 and CADASIL.11 Diffusion MRI sequence parameters for all samples are summarized in Supplementary Table 2. All diffusion images were processed with the same pipeline as described in Supplementary Text 2. Global diffusion measures were calculated as mean of all voxels within a white matter skeleton. Regional analyses were based on voxel-wise diffusion measures.
2.3. Alzheimer’s disease markers
We used Aβ and tau (CSF or PET) as biomarkers of AD. Details on CSF assays, PET tracers, and calculations of PET standardized uptake value ratio (SUVR) scores have previously been published for DIAN,5 DELCODE,15 ADNI (http://adni.loni.usc.edu), UVCI,24 and SVCI.18 For the main analyses we used continuous CSF and PET measures. For a subgroup analysis in amyloid-positive individuals, we used study specific Aβ cut-off values. See Supplementary Text 1 for details.
2.4. Small vessel disease markers
We used an established total SVD score (ordinal variable)25 and white matter hyperintensity (WMH) volume (continuous variable) as MRI markers of SVD. The total SVD score summarizes the presence or severity of SVD lesions on an ordinal scale, i.e. WMH, lacunes, microbleeds, and enlarged perivascular spaces.25 Two trained raters (SF, NV) assessed these lesions according to the STRIVE consensus criteria:2 WMHs were rated using the Fazekas scale,26 the number of lacunes was determined on fluid-attenuated inversion recovery and T1-weighted images, the number of cerebral microbleeds on T2*-weighted gradient echo images, and the number of enlarged perivascular spaces in the basal ganglia on a single T1-weighted axial image slice with the highest number of perivascular spaces.27
WMH volume was calculated from a previously described semi-automated segmentation pipeline.4
2.5. Statistical analyses
All statistical analyses were performed in R (version 3.5.1).28 The statistical significance level was set at α < 0.05.
Associations between AD biomarkers, SVD markers, age, sex (independent variables), and global diffusion measures (dependent variables) were first assessed by simple linear regression analyses within each sample. Variables were power transformed in case of non-normal distribution (Shapiro-Wilk test).
To perform multivariable analysis in the presence of multicollinearity (i.e. intercorrelations among disease markers, Supplementary Fig. 1), we used random forest regressions (R package ‘party’; version 1.3–2).29 This method allows to assess the contribution of each AD biomarker, SVD marker, age, and sex to diffusion alterations, while accounting for all other variables. For each sample, we calculated 1501 conditional inference trees with unbiased variable selection and default parameters as previously described.11 We calculated conditional variable importance together with a 95% confidence interval from 100 repetitions.
An effect of Aβ on diffusion measures might be mediated by vascular pathology, in particular cerebral amyloid angiopathy, i.e. Aβ accumulation in perforating vessels.30 To address this possibility, we performed a post-hoc mediation analysis (R package ‘lavaan’; version 0.6–4)31 in samples where simple regression analysis showed an effect of Aβ on diffusion measures. Diffusion measures were entered as dependent variables, Aβ as independent variable, WMH volume as mediator, and age as covariate. Standard errors were based on bootstrapping (1000 iterations).
Because amyloid pathology has been shown to strengthen the association between tau accumulation and structural tract alterations as assessed by diffusion measures,32 we performed two additional analyses within each sample. First, we conducted a sensitivity analysis restricted to amyloid-positive individuals by repeating simple regression analyses. Second, we assessed the interaction effect of tau × Aβ on diffusion measures.
Finally, since tau is a localized pathology starting in the entorhinal cortex,33 we also performed regional analyses between voxel-wise diffusion measures and tau in the PET samples, i.e. ADNI and SVCI. We used permutation test theory with a standard general linear model as implemented in ‘randomise’ (FSL). We assessed associations between both global tau PET SUVR scores as well as regional tau PET SUVR scores in the entorhinal cortex and voxel-wise diffusion measures. The number of permutations was set at 5000. Significant voxels within the skeletonized diffusion measure maps were identified using threshold-free cluster enhancement with 2D optimization and P < 0.05, corrected for multiple comparisons.
3. Results
Sample characteristics are summarized in Table 1. As expected, patients with genetically-defined AD or SVD were considerably younger than memory clinic patients.
Table 1.
Sample characteristics
| Genetically defined AD | AD focused | SVD focused | Genetically defined SVD | |||
|---|---|---|---|---|---|---|
| DIAN (n=77) | DELCODE (n=89) | ADNI (n=53) | UVCI (n=39) | SVCI (n=39) | CADASIL (n=68) | |
| Age, years | 42 (14) | 72 (9) | 78 (13) | 74 (12) | 79 (10) | 55 (11) |
| Female, n (%) | 40 (52) | 36 (40) | 25 (47) | 13 (33) | 28 (72) | 44 (65) |
| Diagnosis, n (%) HC, SCD, MCI, dementia | na | 4 (4), 37 (42), 33 (37), 15 (17) | 22 (42), na, 23 (43), 8 (15) | 0 (0), 3 (8), 18 (46), 18 (46) | 0 (0), na, 22 (56), 17 (44) | na |
| CDR, n (%) 0, 0.5, 1, 2, 3 | 38 (49), 29 (38), 9 (12), 1 (1), 0 (0) | 29 (33), 52 (59), 7 (8), 0 (0), 0 (0)a | 22 (42), 23 (43), 6 (11), 2 (4), 0 (0) | 1 (3), 30 (77), 8 (20), 0 (0), 0 (0) | 0 (0), 26 (67), 7 (18), 6 (15), 0 (0) | 57 (84), 9 (13), 1 (1), 1 (1), 0 (0) |
| Aβ-positive, n (%) | 46 (60) | 44 (49) | 37 (70) | 22 (56) | 19 (49) | na |
| DTI | ||||||
| FAu, mm2/s | 0.45 (0.03) [0.38, 0.49] | 0.46 (0.03) [0.36, 0.52] | 0.45 (0.04) [0.38, 0.50] | 0.44 (0.04) [0.36, 0.48] | 0.42 (0.04) [0.35, 0.50] | 0.40 (0.06) [0.27, 0.49] |
| MDu, 10−4 mm2/s | 7.84 (0.64) [7.27, 9.31] | 7.68 (0.59) [6.71, 9.72] | 8.21 (0.63) [7.35, 9.77] | 8.05 (0.82) [7.23, 9.72] | 9.66 (0.76) [8.48, 11.0] | 9.40 (1.61) [7.79, 12.89] |
| FAt, mm2/s | 0.55 (0.02) [0.52, 0.58] | 0.56 (0.02) [0.52, 0.60] | 0.57 (0.02) [0.54, 0.60] | 0.56 (0.02) [0.52, 0.57] | 0.59 (0.01) [0.56, 0.63] | 0.55 (0.02) [0.50, 0.59] |
| MDt, 10−4mm2/s | 5.92 (0.07) [5.80, 6.01] | 5.97 (0.10) [5.51, 6.14] | 6.01 (0.63) [5.94, 6.09] | 5.82 (0.15) [5.63, 5.99] | 6.00 (0.04) [5.91, 6.12] | 5.97 (0.03) [5.89, 6.03] |
| FW, mm2/s | 0.18 (0.05) [0.14, 0.28] | 0.16 (0.04) [0.11, 0.29] | 0.20 (0.05) [0.13, 0.31] | 0.22 (0.06) [0.16, 0.35] | 0.25 (0.04) [0.17, 0.31] | 0.29 (0.11) [0.17, 0.51] |
| AD markers | ||||||
| CSF | ||||||
| Aβ 40, ng/L | 7634 (4516) [2215, 15622] | 7942 (3229) [3721, 13358] | - | na | - | - |
| Aβ 42, ng/L | 436 (332) [174, 1424] | 498 (380) [183, 1317] | - | 619 (279) [363, 1641] | - | - |
| T-tau, ng/L | 97 (132) [8, 563] | 425 (369) [98, 1477] | - | 524 (368) [140, 1274] | - | - |
| P-tau, ng/L | 56 (66) [14, 163] | 51 (39) [16, 192] | - | 67 (47) [19, 166] | - | - |
| PET | ||||||
| [18F]-florbetapir SUVR | - | - | 1.18 (0.36) [0.90, 1.70] | - | na | - |
| [18F]-florbetaben SUVR | - | - | na | - | 1.38 (0.49) [1.11, 2.17] | - |
| [18F]AV-1451 SUVR | - | - | 1.10 (0.13) [0.86, 1.67] | - | 1.11 (0.16) [0.89, 1.60] | - |
| SVD markers | ||||||
| WMHvol, ml | 2.22 (3.05) [0.00, 30.47] | 2.78 (5.36) [0.03, 34.50] | 3.35 (8.29) [0.00, 77.24] | 15.72 (1.85) [1.34, 67.27] | 32.19 (21.03) [10.48, 71.20] | 71.27 (73.74) [1.09, 257.74] |
| SVD score, n (%) 0, 1, 2, 3, 4 | 67 (87), 9 (12), 1 (1), 0 (0), 0 (0) | 23 (26), 33 (37), 28 (31), 3 (3), 2 (2) | 8 (15), 17 (32), 18 (34), 8 (15), 2 (4) | 4 (10), 15 (39), 11 (28), 6 (15), 3 (8) | 0 (0), 0 (0), 0 (0), 0 (0), 39 (100) | 0 (0), 16 (24), 19 (28), 17 (25), 16 (24) |
For numeric variables median (interquartile range) [min, max] is shown, except for age.
DELCODE: CDR of 1 subject missing;
AD, Alzheimer’s disease; CDR, clinical dementia rating; DTI, diffusion tensor imaging; FAu, uncorrected fractional anisotropy; FAt, free water corrected tissue compartment of fractional anisotropy; FW, free water content; HC, healthy control; MCI, mild cognitive impairment; MDu, uncorrected mean diffusivity; MDt, free water corrected tissue compartment of mean diffusivity; na, not available; p-tau, phosphorylated- tau181; SCD, subjective cognitive decline; SUVR, standardised uptake value ratio; SVD, small vessel disease; SVD score, total small vessel disease score; t-tau, total tau; WMHvol, white matter hyperintensity volume.
3.1. Small vessel disease shows stronger associations than Alzheimer’s disease with diffusion alterations in simple regression analyses
In simple regressions, both SVD markers, i.e. WMH volume and total SVD score, were consistently and strongly associated with conventional DTI measures (FAu, MDu; range of R2adj.. [0.08–0.79]) and FW (range of R2adj. [0.18–0.76]) across all six samples (Fig. 2, Supplementary Tables 3–5). In contrast, AD biomarkers, i.e. CSF and PET data, were not or only weakly associated with conventional DTI measures and FW (range of R2adj. [0.04–0.18]; Fig. 2, Supplementary Tables 3–5). Results were largely consistent across study samples, with a notable exception in the sample of genetically-defined AD (DIAN). Here, effect sizes for Aβ 42 (CSF) were similar to the effect sizes of WMH volume (Fig. 2, Supplementary Table 5). Associations between Aβ 42, WMH volume and diffusion measures in DIAN and DELCODE were further addressed in a post-hoc mediation analysis (see 3.3).
Figure 2. Simple regression analyses.
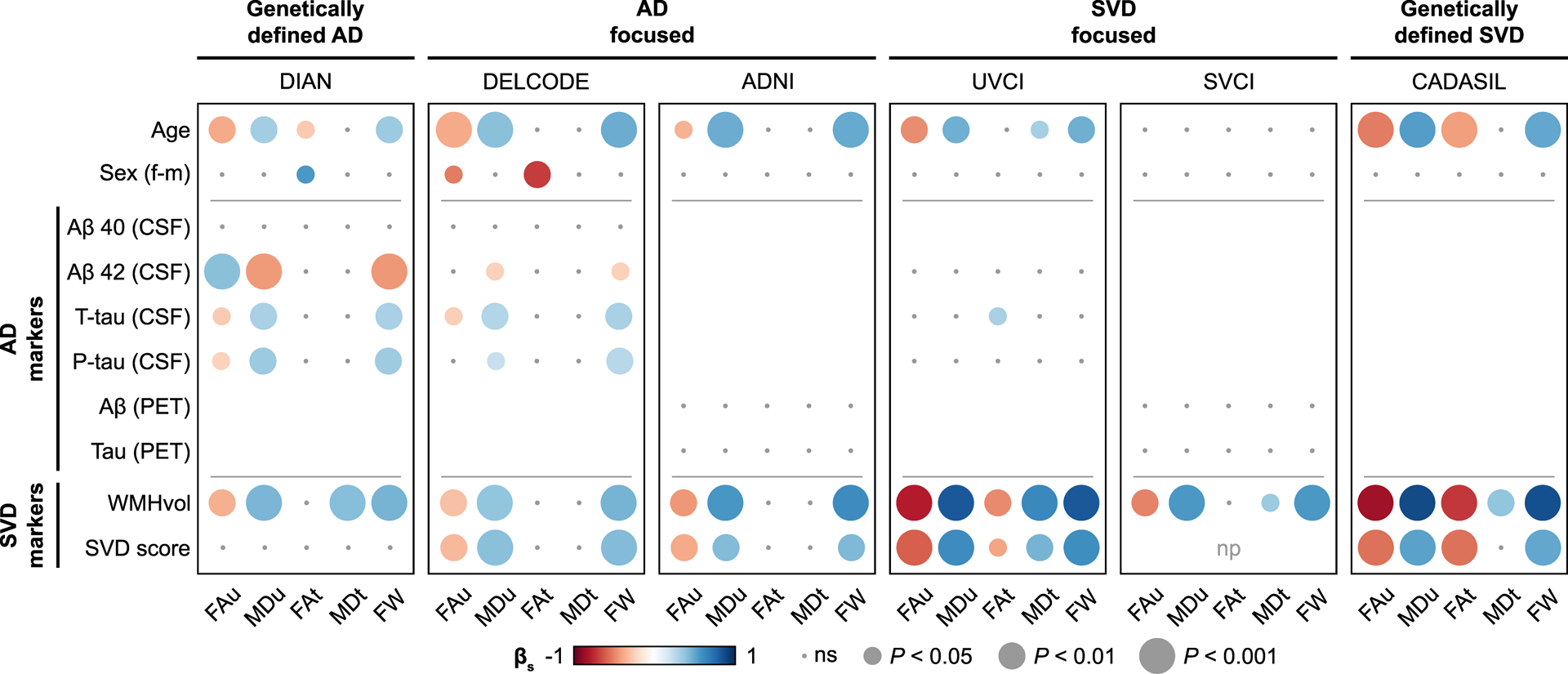
Simple linear regression analyses between diffusion measures and AD biomarkers or SVD markers. Standardized β is represented by color. AD, Alzheimer’s disease; βs, standardized beta; FAu, uncorrected fractional anisotropy; FAt, free water corrected tissue compartment of fractional anisotropy; FW, free water content; MDu, uncorrected mean diffusivity; MDt, free water corrected tissue compartment of mean diffusivity; np, not possible (all patients had the maximum score); ns, not significant; p-tau, phosphorylated- tau181; SVD, small vessel disease; SVD score, total small vessel disease score; t-tau, total tau; WMHvol, white matter hyperintensity volume.
3.2. Small vessel disease and age contribute most to diffusion alterations in multivariable analyses
Using random forest regression as a multivariable method, we assessed the contribution of each AD biomarker and SVD marker to diffusion measures, while accounting for multicollinearity. In all memory clinic samples, SVD markers showed higher variable importance than AD biomarkers for alterations of conventional DTI measures (FAu and MDu; Fig. 3) and FW (data not shown; nearly identical to MDu). The opposite was found only in DIAN, where AD biomarkers showed higher variable importance. For tissue measures (FAt and MDt), interpretation of random forest regressions was not feasible, because variable importances were zero or almost zero in all samples (data not shown).
Figure 3. Multivariable analyses.
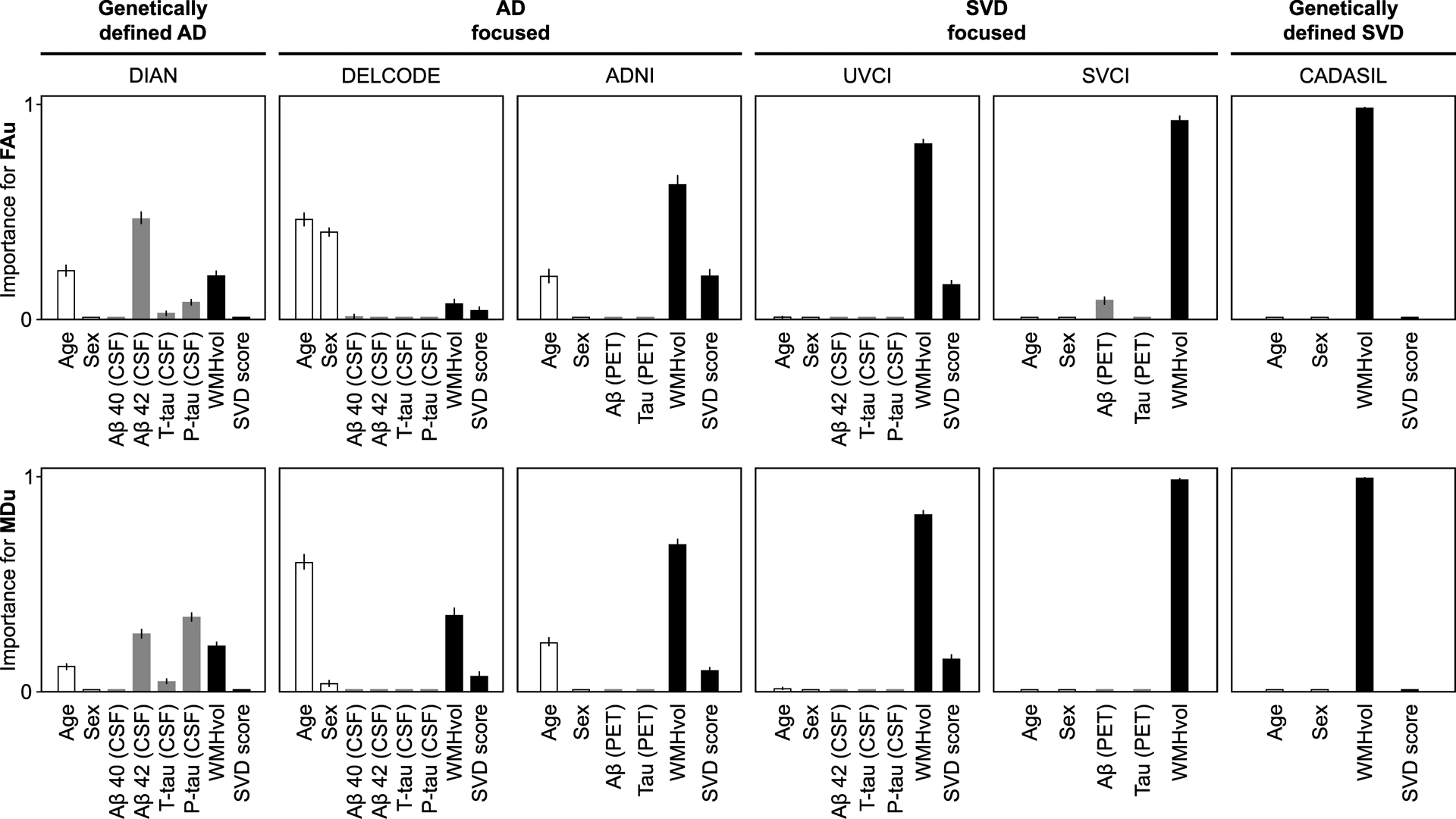
Random forest regression analyses for estimating the relative variable importance of AD biomarkers (grey bars), SVD markers (black bars), age and sex (white bars) with regard to conventional DTI measures (FAu, MDu) while accounting for all other variables (conditional importance). Lines indicate the 95% confidence interval for the conditional variable importance.
AD, Alzheimer’s disease; FAu, uncorrected fractional anisotropy; MDu, uncorrected mean diffusivity; p-tau, phosphorylated-tau181; SVD, small vessel disease; SVD score, total small vessel disease score; T-tau, total tau; WMHvol, white matter hyperintensity volume.
3.3. White matter hyperintensities partially mediate the effect of Aβ on diffusion alterations in genetically-defined Alzheimer’s disease
For diffusion measures significantly associated with Aβ 42 (CSF) in the simple regression analysis, i.e. in DIAN and DELCODE, we performed a post-hoc mediation analysis to explore whether these associations might be mediated by vascular pathology, such as cerebral amyloid angiopathy. In DIAN, the effect of Aβ 42 on MDu and FW was indeed partially mediated by WMH volume (MDu: βs=−0.06, SE=0.03, P=0.030; FW: βs=−0.06, SE=0.03, P=0.026). However, we also found a direct effect of Aβ 42 on MDu and FW (MDu: βs=−0.30, SE=0.12, P=0.005; FW: βs=−0.30, SE=0.11, P=0.005). For FAu, mediation analysis was not significant. As a further indication for the presence of cerebral amyloid angiopathy, most (8 out of 9) DIAN participants with cerebral microbleeds showed a strictly lobar distribution, and one participant had disseminated cortical superficial siderosis.
In DELCODE, where simple regression analysis showed only weak effects of Aβ 42, none of the mediation analyses were significant (all P > 0.136).
3.4. Tau is not associated with diffusion alterations in amyloid-positive individuals
It was recently reported that Aβ might strengthen the association between tau accumulation and diffusion alterations.32 We addressed this aspect in a sensitivity analysis restricted to amyloid-positive individuals (Supplementary Tables 6–8, Supplementary Fig. 2). Simple linear regressions between tau and diffusion measures in amyloid-positive individuals were not significant, except for DIAN (n=46; p-tau and MDu, βs=0.32, R2adj.=0.08, P=0.031; p-tau and FW, βs=0.31, R2adj.=0.07, P=0.038). In correspondence with the full DIAN sample, tau showed effect sizes comparable to those found for WMH volume (WMH volume and MDu, βs=0.35, R2adj.=0.10, P=0.017; WMH volume and FW, βs=0.37, R2adj.=0.12, P=0.011). None of the taú Aβ interaction models with diffusion measures as dependent variables were significant in any of the samples (all P > 0.051).
3.5. Regional tau is not associated with diffusion alterations
Tau is a localized pathology starting in the entorhinal cortex33 and previous literature suggests localized effects of tau on white matter microstructure.32,34,35 We therefore performed regional analyses in the PET samples, i.e. ADNI and SVCI, which allow to assess local tau load. Associations between regional tau PET SUVR scores in the entorhinal cortex or global tau PET SUVR scores and voxel-wise diffusion measures were not significant.
4. Discussion
We investigated the effect of AD and SVD on brain microstructure assessed by diffusion measures. As a unique feature, our study included six independently recruited samples covering the entire spectrum of AD, mixed disease, and SVD. The main finding is that in memory clinic patients, diffusion MRI alterations are largely determined by SVD. Results were consistent across all memory clinic samples, illustrating the robustness of our findings. Our study facilitates the interpretation of diffusion MRI alterations and the development towards clinical application.
The strong effect of SVD on diffusion measures was evident in all of the six study samples. In contrast, an association between AD and diffusion measures was only detectable in DELCODE and DIAN. While in DELCODE effect sizes of AD biomarkers were considerably smaller than those of SVD markers, effect sizes of Aβ 42 and WMH volume were similar in DIAN. Multivariable analyses using random forest regression showed a higher importance of SVD markers for diffusion alterations in all memory clinic samples. The only sample in which AD biomarkers had a higher variable importance was DIAN. As expected for a genetically-defined sample, these patients are considerably younger than typical memory clinic patients and less likely to show age-related comorbidities, such as SVD. Still, mediation analysis in DIAN suggested a vascular contribution to diffusion alterations also in this population, as the effect of Aβ on diffusion alterations was partly mediated by WMH volume. This might indicate a contribution of cerebral amyloid angiopathy, a specific subtype of SVD caused by deposition of Aβ in perforating vessels.30 Since the DIAN sample also included asymptomatic mutation carriers up to 15 years before estimated symptom onset, another explanation is that the association between Aβ and diffusion measures is strongest in early, preclinical AD. This view is supported by a recent study demonstrating an association between Aβ and diffusion measures over the adult lifespan in cognitively healthy participants.36 Overall, we conclude that while the effect of AD on diffusion measures is apparent in DIAN patients with pure and early AD, the presence of SVD in the memory clinic samples masks the effect of AD on diffusion measures.
Seemingly in contrast with our results, associations between AD biomarkers and alterations of white matter microstructure as assessed by DTI have been previously reported in memory clinic patients,13,14,32,34,37–39 although some studies found no association.40,41 Importantly, however, only one of these studies accounted for SVD. Hence, the effect of AD on diffusion alterations might have been overestimated. Only Strain and colleagues34 considered biomarkers of both diseases and found an association between tau PET (but not Aβ PET) in temporal regions and diffusion measures in temporal white matter projections, independently of WMHs. In line with our results, the effect size for WMH volume was larger than effect sizes of AD biomarkers. By considering both diseases, we conclude that SVD determines diffusion alterations to a much larger extent than AD, even in samples where AD was the clinically predominant disease. The strong effect of SVD has implications for future studies, which will need to take SVD into account as an important confounder, as well as for the interpretation of diffusion MRI alterations in clinical routine.
In the current study, neither the regional analysis nor the analysis in amyloid-positive individuals, where the effect of tau was expected to be stronger,32 indicated a significant association between tau and diffusion measures. In post-mortem studies, white matter alterations in AD patients have been attributed to axonal degeneration secondary to cortical deposition of hyperphosphorylated tau.42,43 Yet, post-mortem studies by design examine patients in very late stages of AD, while our memory clinic patients were mostly in earlier disease stages. Thus, it is conceivable that our patients have not yet reached the disease stage where associations between tau and axonal degeneration can be detected.
By design, our memory clinic samples were heterogeneous, which in our view accurately reflects a real-life memory clinic setting. To study pure forms of AD and SVD, we included genetically defined samples. Furthermore, the sensitivity analysis in subgroups with amyloid-positive individuals allowed to study memory clinic patients who met the biological definition of AD. Although statistical power was reduced, the strong effect of SVD on diffusion measures was also confirmed in these subgroups.
Our finding that diffusion alterations are predominantly driven by SVD is also supported by a genome-wide association study in the population-based UK Biobank. Polygenic risk scores for altered DTI measures were associated with SVD-related stroke and major depressive disorder, but not with AD.44 The study thus provided genetic evidence that mechanisms underlying diffusion alterations are shared with cerebrovascular disease.
Another aim of this study was to investigate whether free water imaging allows to disentangle the contribution of SVD and AD. The finding that SVD markers showed strongest associations with FW corroborates previous results indicating that diffusion alterations in SVD patients are predominantly driven by an increase in the free water content.11 However, our current analysis did not provide evidence that AD biomarkers are reflected in the tissue compartment. The latter result is in contrast to studies suggesting that AD-related neurodegeneration of the white matter might be specifically represented in free water corrected tissue measures: Tissue measures were associated with conversion from mild cognitive impairment to dementia in AD patients12 and showed Aβ-related longitudinal changes.14 It should be noted that the current study was cross-sectional and thus we cannot exclude that the tissue compartment holds valuable information for longitudinal studies.12,14 Furthermore, multi-shell diffusion data, which would be necessary for more complex parametrization of the fluid compartments,45–47 was not available in the study samples. This would have allowed to control for the effects of capillary blood flow (intravoxel incoherent motion) in the free water estimation.47
A limitation of our study is that elevated tau (especially in CSF) is not specific for AD as it could also indicate other tauopathies, such as Pick’s disease, corticobasal degeneration, or progressive supranuclear palsy. However, the tau PET tracer ([18F]AV-1451) employed mostly binds to tau deposits specific for AD.48 Also, the focus on recruitment of clinical AD, e.g. by including amnestic mild cognitive impairment in DELCODE and ADNI, clearly enriched for AD rather than other tauopathies. Another limitation is the lack of AD biomarkers in the CADASIL sample. Yet, the purpose of the CADASIL sample was to judge the effect sizes of SVD markers in genetically-defined disease, i.e. in young patients with pure SVD. Interestingly, we found similar effect sizes as in SVD focused samples with mixed pathology, in particular the UVCI sample. While we also included voxel-based analyses to identify regional associations, our study mostly focused on global, whole-brain averages of diffusion measures. Thus, we cannot exclude that analyses in specific subregions will yield different results. Because of limitations in the diffusion MRI acquisition protocols (no reversed phase-encoding, directions not sampled on entire sphere), we were not able to correct for susceptibility-induced distortions or to employ a more modern approach for correction of eddy current-induced distortions, motion, and outlier slices.49 Finally, the lack of pathological confirmation of the presence and extent of AD and SVD pathology originates from the paucity of autopsy studies with high quality, standardized antemortem diffusion MRI.
The main strength of our analysis is the inclusion of multiple samples from different countries and ethnicities, covering the entire spectrum of AD, mixed disease, and SVD. This has enabled us to independently validate results and to assess both CSF and PET biomarkers of AD in a robust manner. The differences in study protocols among the six samples, such as MRI acquisition, biomarker assessment techniques, and recruitment strategies indicate that our results might be generalizable to other populations along the spectrum of AD and SVD. We also included younger individuals with genetically-defined disease to minimize confounding by other age-related pathologies. Finally, the state-of-the art diffusion imaging analysis pipeline included modern pre-processing techniques and rigorous control for confounding by CSF partial volume effects, which is crucial in patients with atrophy and therefore enlarged CSF spaces.
In conclusion, we demonstrate that the effect of SVD on diffusion alterations largely exceeds the effect of AD. Our systematic analysis contributes to the interpretation of diffusion MRI in memory clinic patients and further advances its application in clinical practice. We validate diffusion measures as markers for SVD and as valuable tools to assess the vascular contribution to AD and dementia, which still needs to be adequately explored.50 Building upon our findings, future studies could assess if more advanced parameterization of diffusion processes, such as biophysical diffusion models, further increases the sensitivity in earlier or even asymptomatic stages.
Supplementary Material
Supplementary Table 1. Diagnostic criteria in memory clinic samples
Supplementary Table 2. Diffusion parameters
Supplementary Table 3. Simple regression models in Alzheimer’s disease focused samples
Supplementary Table 4. Simple regression models in small vessel disease focused samples
Supplementary Table 5. Simple regression models in genetically-defined samples
Supplementary Table 6. Simple regression models in Alzheimer’s disease focused samples in amyloid-positive individuals
Supplementary Table 7. Simple regression models in small vessel disease focused samples in amyloid-positive individuals
Supplementary Table 8. Simple regression models in genetically-defined samples in amyloid-positive individuals
Supplementary Table 9. DIAN consortium
Supplementary Table 10. DELCODE study group
Supplementary Figure 1. Correlation matrices
Supplementary Figure 2. Simple regression analyses in amyloid positive individuals.
Supplementary Text 1. CSF and PET markers
Supplementary Text 2. Processing of diffusion measures
Acknowledgements
We would like to thank all the researchers and the support staff from the DIAN (https://dian.wustl.edu/wp-content/uploads/2019/04/DIAN-TU-Publications_Acknowledgement_V14.pdf), DELCODE, ADNI, Utrecht VCI study group, Seoul VCI study group, and CADASIL study for their contributions to the present study. Investigators within DIAN, DELCODE, and ADNI contributed to the design and implementation of the respective studies and/or provided data but did not participate in analysis or writing of this report. A complete listing of the DIAN consortium and the DELCODE study group can be found in Supplementary Tables 9 and 10 and ADNI investigators at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. Members of the Utrecht VCI study group involved in the present study (in alphabetical order by department): University Medical Center Utrecht, the Netherlands, Department of Neurology: E. van den Berg, J.M. Biesbroek, M. Brundel, W.H. Bouvy, L.G. Exalto, C.J.M. Frijns, O. Groeneveld, S.M. Heringa, R. Heinen, N. Kalsbeek, L.J. Kappelle, J.H. Verwer; Department of Radiology/Image Sciences Institute: J. de Bresser, H.J. Kuijf, A. Leemans, P.R. Luijten, M.A. Viergever, K.L. Vincken, J.J.M. Zwanenburg; Department of Geriatrics: H.L. Koek; Hospital Diakonessenhuis Zeist, the Netherlands: M. Hamaker, R. Faaij, M. Pleizier, E. Vriens.
We acknowledge the altruism of the study participants and their families.
Funding
The study was funded by a cross-border grant from the Alzheimer Forschung Initiative e.V. (#16018CB)/Alzheimer Nederland AN WE.03-2016-1. BG and MDu were supported by the German Research Foundation (DU1626/1-1). The research of GJB is also supported by VICI grant 918.16.616 from NWO, the Netherlands Organization for Scientific Research.
DIAN: Data collection and sharing for this project was supported by The Dominantly Inherited Alzheimer’s Network (DIAN, U19AG032438) funded by the National Institute on Aging (NIA), the German Center for Neurodegenerative Diseases (DZNE), Raul Carrea Institute for Neurological Research (FLENI), Partial support by the Research and Development Grants for Dementia from Japan Agency for Medical Research and Development, AMED, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI). This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications.
DELCODE: The DELCODE study was funded by the German Center for Neurodegenerative Diseases (DZNE), Study-ID: BN012DZNE. We acknowledge support from the Max-Delbrück-Centrum für Molekulare Medizin in der Helmholtz-Gemeinschaft (MDC) and the Freie Universität Berlin Center for Cognitive Neuroscience Berlin (CCNB).
ADNI: Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
SVCI: This research was funded by Research of Korea Centers for Disease Control and Prevention (2018-ER6203-01).
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid-beta
- DTI
diffusion tensor imaging
- FAu
uncorrected fractional anisotropy
- FAt
free water corrected tissue compartment of fractional anisotropy
- FW
free water content
- MDu
uncorrected mean diffusivity
- MDt
free water corrected tissue compartment of mean diffusivity
- PET
positron emission tomography
- P-tau
phosphorylated- tau181
- SUVR
standardised uptake value ratio
- SVD
cerebral small vessel disease
- T-tau
total tau
- WMH
white matter hyperintensity
Footnotes
Declarations of Interest
None.
References
- 1.O’Brien JT, Thomas A. Vascular dementia. The Lancet. 2015;386(10004):1698–1706. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta neuropathologica communications. 2018;6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baykara E, Gesierich B, Adam R, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Annals of neurology. 2016;80(4):581–592. [DOI] [PubMed] [Google Scholar]
- 5.Araque Caballero MÁ, Suárez-Calvet M, Duering M, et al. White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer’s disease. Brain. 2018;141(10):3065–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mito R, Raffelt D, Dhollander T, et al. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain. 2018;141(3):888–902. [DOI] [PubMed] [Google Scholar]
- 7.Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience. 2014;276:206–215. [DOI] [PubMed] [Google Scholar]
- 8.Pasi M, van Uden IWM, Tuladhar AM, de Leeuw F-E, Pantoni L. White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: clinical consequences. Stroke. 2016;47(6):1679–1684. [DOI] [PubMed] [Google Scholar]
- 9.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta neuropathologica. 2017;134(2):171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2009;62(3):717–730. [DOI] [PubMed] [Google Scholar]
- 11.Duering M, Finsterwalder S, Baykara E, et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2018;14(6):764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier-Hein KH, Westin C-F, Shenton ME, et al. Widespread white matter degeneration preceding the onset of dementia. Alzheimer’s & Dementia. 2015;11(5):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoy AR, Ly M, Carlsson CM, et al. Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PloS one. 2017;12(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vipin A, Ng KK, Ji F, et al. Amyloid burden accelerates white matter degradation in cognitively normal elderly individuals. Human brain mapping. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen F, Spottke A, Boecker H, et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimer’s research & therapy. 2018;10(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aalten P, Ramakers IHGB, Biessels GJ, et al. The Dutch Parelsnoer Institute-Neurodegenerative diseases; methods, design and baseline results. BMC neurology. 2014;14(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Yang JJ, Kwon H, et al. Relative impact of amyloid-β, lacunes, and downstream imaging markers on cognitive trajectories. Brain. 2016;139(9):2516–2527. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Park S, Cho H, et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA neurology. 2018;75(8):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulder KL, Snider BJ, Mills SL, et al. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimer’s research & therapy. 2013;5(5):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA neurology. 2015;72(3):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franzmeier N, Ren J, Damm A, et al. The BDNF Val66Met SNP modulates the association between beta-amyloid and hippocampal disconnection in Alzheimer’s disease. Molecular psychiatry. 2019:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiaerken Y, Luo X, Yu X, et al. Microstructural and metabolic changes in the longitudinal progression of white matter hyperintensities. Journal of Cerebral Blood Flow & Metabolism. 2018:1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinen R, Vlegels N, de Bresser J, et al. The cumulative effect of small vessel disease lesions is reflected in structural brain networks of memory clinic patients. NeuroImage: Clinical. 2018;19:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wilde A, van Maurik IS, Kunneman M, et al. Alzheimer’s Biomarkers In Daily Practice (ABIDE) project: rationale and design. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2017;6:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staals J, Makin SDJ, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83(14):1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. American journal of roentgenology. 1987;149(2):351–356. [DOI] [PubMed] [Google Scholar]
- 27.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovascular diseases. 2015;39(3–4):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. [Google Scholar]
- 29.Strobl C, Boulesteix A-L, Zeileis A, Hothorn T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC bioinformatics. 2007;8(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charidimou A, Boulouis G, Gurol ME, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140(7):1829–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosseel Y Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). Journal of statistical software. 2012;48(2):1–36. [Google Scholar]
- 32.Jacobs HIL, Hedden T, Schultz AP, et al. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nature neuroscience. 2018;21(3):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho H, Choi JY, Hwang MS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Annals of neurology. 2016;80(2):247–258. [DOI] [PubMed] [Google Scholar]
- 34.Strain JF, Smith RX, Beaumont H, et al. Loss of white matter integrity reflects tau accumulation in Alzheimer disease defined regions. Neurology. 2018;91(4):e313–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantarci K, Murray ME, Schwarz CG, et al. White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiology of aging. 2017;56:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araque Caballero MÁ, Song Z, Rubinski A, et al. Age-dependent amyloid deposition is associated with white matter alterations in cognitively normal adults during the adult life span. Alzheimer’s & Dementia. 2020. [DOI] [PubMed] [Google Scholar]
- 37.Melah KE, Lu SY-F, Hoscheidt SM, et al. CSF markers of Alzheimer’s pathology and microglial activation are associated with altered white matter microstructure in asymptomatic adults at risk for Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2016;50(3):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racine AM, Merluzzi AP, Adluru N, et al. Association of longitudinal white matter degeneration and cerebrospinal fluid biomarkers of neurodegeneration, inflammation and Alzheimer’s disease in late-middle-aged adults. Brain imaging and behavior. 2019;13(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Racine AM, Adluru N, Alexander AL, et al. Associations between white matter microstructure and amyloid burden in preclinical Alzheimer’s disease: a multimodal imaging investigation. NeuroImage: Clinical. 2014;4:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantarci K, Schwarz CG, Reid RI, et al. White matter integrity determined with diffusion tensor imaging in older adults without dementia: influence of amyloid load and neurodegeneration. JAMA neurology. 2014;71(12):1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietroboni AM, Scarioni M, Carandini T, et al. CSF β-amyloid and white matter damage: a new perspective on Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2018;89(4):352–357. [DOI] [PubMed] [Google Scholar]
- 42.McAleese KE, Firbank M, Dey M, et al. Cortical tau load is associated with white matter hyperintensities. Acta neuropathologica communications. 2015;3(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAleese KE, Walker L, Graham S, et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta neuropathologica. 2017;134(3):459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutten-Jacobs LCA, Tozer DJ, Duering M, et al. Genetic study of white matter integrity in UK Biobank (N= 8448) and the overlap with stroke, depression, and dementia. Stroke. 2018;49(6):1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoy AR, Koay CG, Kecskemeti SR, Alexander AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. Neuroimage. 2014;103:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sepehrband F, Cabeen RP, Choupan J, et al. Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. NeuroImage. 2019;197:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rydhög AS, Szczepankiewicz F, Wirestam R, et al. Separating blood and water: perfusion and free water elimination from diffusion MRI in the human brain. Neuroimage. 2017;156:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta neuropathologica communications. 2016;4(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. November 1 2016;141:556–572. doi: 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- 50.Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction—The disregarded partner of Alzheimer’s disease. Alzheimer’s & Dementia. 2019;15(1):158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Diagnostic criteria in memory clinic samples
Supplementary Table 2. Diffusion parameters
Supplementary Table 3. Simple regression models in Alzheimer’s disease focused samples
Supplementary Table 4. Simple regression models in small vessel disease focused samples
Supplementary Table 5. Simple regression models in genetically-defined samples
Supplementary Table 6. Simple regression models in Alzheimer’s disease focused samples in amyloid-positive individuals
Supplementary Table 7. Simple regression models in small vessel disease focused samples in amyloid-positive individuals
Supplementary Table 8. Simple regression models in genetically-defined samples in amyloid-positive individuals
Supplementary Table 9. DIAN consortium
Supplementary Table 10. DELCODE study group
Supplementary Figure 1. Correlation matrices
Supplementary Figure 2. Simple regression analyses in amyloid positive individuals.
Supplementary Text 1. CSF and PET markers
Supplementary Text 2. Processing of diffusion measures


