Abstract
Indoleamine 2–3 dioxygenase 1 (IDO1) expression may contribute to immunologic escape by melanoma metastases. However, a recent clinical trial failed to identify any clinical benefits of IDO1 inhibition in patients with unresectable metastatic melanoma, and prior characterizations of IDO1 expression have predominately studied primary lesions and local metastases, generating uncertainty regarding IDO1 expression in distant metastases. We hypothesized that IDO1 expression in such lesions would be low and correlated with decreased overall survival (OS).
Metastases from patients (n=96) with Stage IIIb-IV melanoma underwent tissue microarray construction and immunohistochemical staining for IDO1. Th-1 related gene expression was determined quantitatively. Associations between OS and IDO1 expression were assessed with multivariate models.
Of 96 metastatic lesions, 28% were IDOpos, and 85% exhibited IDO1 expression in <10% of tumor cells. IDOpos lesions were associated with improved OS (28.9 v. 10.5 months, p=0.02) and expression of Th1-related genes. OS was not associated with IDO1 expression in a multivariate analysis of all patients, however IDO1 expression (HR =0.25, p=0.01) and intratumoral CD8+ T-cell density (HR =0.99, p<0.01) were correlated with OS in patients who underwent metastasectomy with curative-intent. IDOpos metastases were less likely to recur after metastasectomy (54% vs. 16%, p= 0.01).
IDO1 expression was low in melanoma metastases and correlated with OS after metastasectomy with curative-intent. Intra-tumoral CD8+ T-cells and Th1-related genes were correlated with IDO1 expression, as was tumor recurrence. These suggest that IDO1 expression may be a marker of immunologic tumor control, and may inform participant selection in future trials of IDO1 inhibitors.
Keywords: melanoma, indoleamine 2–3 dioxygenase 1, IDO1, metastatic melanoma
Introduction
Immunotherapeutics have revolutionized the treatment of advanced melanoma, yet approximately 60% of patients fail to respond to current checkpoint blockade targeting PD-1 and CTLA-4. Consequently, there is a pressing need to investigate additional biochemical pathways which regulate anti-tumor immunity. One such pathway is mediated by indoleamine 2–3 dioxygenase 1 (IDO1), which catabolizes the conversion of tryptophan (Trp) into L-kynurenine (Kyn)(1). Depletion of Trp and accumulation of Kyn inhibits the immune response and can cause cell cycle arrest in T cells but not tumor cells(2–4).
Melanoma patients frequently exhibit abnormally high serum Kyn/Trp ratios compared to healthy controls(5). Small molecule inhibitors of IDO1 have shown promise in regulating this pathway and can normalize serum Kyn/Trp ratios(6). In a phase I clinical trial, IDO1 inhibition combined with checkpoint blockade resulted in objective responses in 55% of melanoma patients(7). However, a phase III trial (ECHO-301) randomized patients with unresectable melanoma to PD-1 blockade or PD-1 blockade combined with IDO1 inhibition (ECHO-301), and failed to detect differences in clinical outcomes(8). Several explanations could be offered for the negative findings of the ECHO-301 trial(9). One possibility is that metastatic melanoma lesions might exhibit low IDO1 expression. Prior enumerations of IDO1 expression by melanoma cells have focused on IDO1 expression in patients with Stage I-III disease, whose findings may not be generalizable to the Stage III-IV patients enrolled in ECHO-301. Furthermore, the prognostic significance of IDO1 expression by melanoma cells is controversial, and only a single prior study has reported a multivariate survival analyses controlling for clinical variables associated with IDO1 expression, and this report was heavily skewed towards patients with early (Stage I-II) disease (10–13).
We analyzed IDO1 expression in metastatic lesions from 96 patients with Stage IIIb-IV disease, whose disease state mimics the patient cohort enrolled in the ECHO-301 trial. Our primary hypothesis was that IDO1 expression would be limited to a small subset of melanoma metastases. We further hypothesized that IDO1 expression would be associated with induction of Th-1 associated cytokines. Finally, we hypothesized that IDO1 expression by melanoma cells or intra-tumoral endothelial cells, would be associated with shorter overall survival (OS), and that this finding would be significant in a multivariate model incorporating clinical and histologic data.
Methods
Patient Selection and Clinical
This study was conducted following institutional IRB approval (IRB-HSR #17816, #10598, #13310, #10803). Patients selected for inclusion had Stage IIIb-IV melanoma, appropriate clinical follow-up, and ample metastatic tumor tissue available. Data on the patients’ clinical histories, disease status, and outcomes had previously been collected. All patients were checkpoint-blockade therapy naïve, however some had been enrolled in clinical trials of melanoma peptide vaccines at our institution (Table 1). Given the advanced nature of our patient’s disease, some patients underwent palliative lesion resection for symptomatic metastases. Patients were considered to have undergone resection with palliative intent if any known metastatic lesions were left unresected after metastasectomy, while patients who underwent curative-intent resection underwent total metastasectomy of all known melanoma lesions at time of metastasectomy.
Table 1:
Patient Demographics
| Demographic | IDOneg | IDOpos | p-value |
|---|---|---|---|
| N | 69 | 27 | |
| Mean Age (SD) | 58.1 (15.7) | 56.4 (14.2) | 0.63* |
| Female Sex | 27 (39%) | 17 (65%) | 0.06 |
| Tissue Type | 0.49** | ||
| LN | 26 (38%) | 9 (33%) | |
| Skin | 38 (55%) | 16 (59%) | |
| Peritoneum | 0 (0%) | 1 (4%) | |
| Small Bowel | 5 (7%) | 1 (4%) | |
| Stage IV disease | 23 (33%) | 16 (59%) | 0.04 |
| Curative Intent Resection | 51 (75%) | 13 (50%) | 0.03 |
| BRAF mutant | 33 (48%) | 14 (52%) | 0.90 |
| Vaccination Status | 0.41 | ||
| Not vaccinated | 41 (59%) | 12 (44%) | |
| Surgery before vaccination | 11 (16%) | 6 (22%) | |
| Surgery after vaccination | 17 (25%) | 9 (33%) |
Student’s T-test.
Fisher’s Exact Test
Remainder of P values calculated with χ2 test.
SD = standard deviation.
Tumor microarray creation
Formalin-fixed paraffin-embedded (FFPE) blocks of melanoma metastases to skin, lymph nodes, small bowel, or peritoneum were obtained from the University of Virginia anatomic pathology archives. Tissue microarrays (TMAs) were constructed from 3–4 cores per tumor, each 1.0 mm in diameter, through regions of each selected FFPE block. Cores were taken from the tumor center in all cases (Supplemental Figure 1). Immune cells and PD-L1 expression in these tumors were enumerated through immunohistochemistry using standard protocols as has been previously reported(14). Histologic patterns of intra-tumoral immune cell infiltration had previously been determined(15), and tumors were consequently designated Immunotype A (no immune infiltrate), Immunotype B (immune infiltration adjacent to intratumoral blood vessels), or Immunotype C (diffuse immune infiltration).
IDO1 protein expression
IHC staining for IDO1 (IDO1 Antibody: Sigma Prestige, HPA 023072, 1:2,000 dilution) was performed on TMA slides using a Ventana Discovery Ultra platform. The HPA 023072 IDO1 antibody binds just its antigen on protein array, has been validated for IDO1 IHC by The Human Protein Atlas, and has been used extensively in prior publications(16–22). IDO1 staining was quantified by visual inspection by an experienced dermatopathologist (AG), and averaged across all cores for each patient. Tumors were classified as IDO1 positive (IDOpos) when definite cytoplasmic staining was present in >1% of tumor cells. Additionally, tumors were further classified into subgroups based on average percent of tumor cells expressing IDO1 (0–1%, 2–5%, 6–10% >10%). Tumor vasculature was also evaluated for IDO1 expression, and tumors were classified as positive (IDOendo+) if any intra-tumoral endothelial cells stained for IDO1.
Whole Tumor Section and IHC
To assess whether the average TMA IDO1 staining accurately represented whole tumor IDO1 staining, 25 tumors were submitted for whole tumor sectioning and IDO1 staining as above. As with TMA IDO1 staining, tumors were classified into subgroups based on average percent of tumor cells expressing IDO1 (0–1%, 2–5%, 6–10% >10%). Strength of association between TMA IDO1 staining and whole tumor IDO1 staining was assessed using Spearman’s rank order Correlation.
Quantitative Nuclease Protection Assay
The same FFPE tissue blocks sampled in TMAs, and analyzed by IHC, were also analyzed for expression of genes related to immune cell subtypes. Five micrometer sections of FFPE tumor specimens were submitted to HTG Molecular (Tucson, AZ) for this gene expression analysis using the HTG EdgeSeq Immuno-Oncology Assay, which included 558 probes with 15 housekeeper genes, 5 negative and 4 positive processor controls. For this analysis, functional DNA Nuclease Protection Probes (NPPs) are flanked by universal wing sequences that are hybridized to the target RNAs. S1 nuclease is added to digest excess non-hybridized RNA and DNA probes. This reaction then results in a stoichiometric quantity of NPPs:RNA hetero-duplexes of interest. Heat denaturization releases the protection probe allowing for enumeration by the Illumina NextSeq™ sequencing platform. Gene expression was standardized through a procedure that log transformed counts per million (cpm) and adjusted for total reads within a sample(23).
Statistics
The main endpoint for analysis was duration of overall survival (OS) as defined by the time from initial surgery to death. Patients who were alive at last follow-up were censored. The Kaplan-Meier product limit estimator was used to estimate survival distributions. A Kruskal-Wallis test was conducted to correlate percent tumor IDO1 staining with gene expression for seven genes of interest (IFNG, STAT1, TBX21, IL10, TGFB1, EOMES, CD8A) determined by quantitative nuclease protection assay. Tumors were dichotomized into those with intra-tumoral endothelial IDO1 expression and those without, and differences in mean mRNA expression of these same genes (IFNG, STAT1, TBX21, IL10, TGFB1, EOMES, CD8A) between these groups was assessed with Kruskal-Wallis tests. Multivariate Cox proportional hazards modeling was performed using R software version 3.5.3. Cox proportional hazards assumptions were tested using Schoenfield residual analysis using a level of significance of 0.01. Covariates of interest for multivariate Cox proportional hazards regression analysis included IDO staining, patient sex, Stage, intratumoral CD8 density, gene expression level (TBX21, EOMES, IFNG), mean PD-L1 expression, and whether the surgical resection was performed with palliative or curative intent.
Results
Detection of IDO1 by Immunohistochemistry
IHC staining and histologic evaluation were conducted on 153 lesions. Of these, 4 were excluded based on insufficient tumor sample, and 6 were primary tumors, leaving 143 melanoma metastases evaluable by IHC. mRNA expression had been previously determined for 96 of these 143 metastatic lesions, and consequently this group of 96 patients was ultimately the focus of our study. IDO1 was expressed by greater than 1% of tumor cells in 27 (28%) lesions, which were consequently classified as IDOpos (Figure 1). Of the IDOpos tumors, 18 (67%) exhibited IDO1 expression in 2–5% of tumor cells, while 5 (19%) had 6–10% IDO1 expression and 4 lesions (15%) had >10% IDO1 expression among tumor cells. Additionally, IDO1 expression was observed in intratumoral endothelial cells in 20 tumors (21%), which were classified as IDOendo+ (Figure 1c).
Figure 1: Examples of IHC staining of melanoma metastases for IDO1.
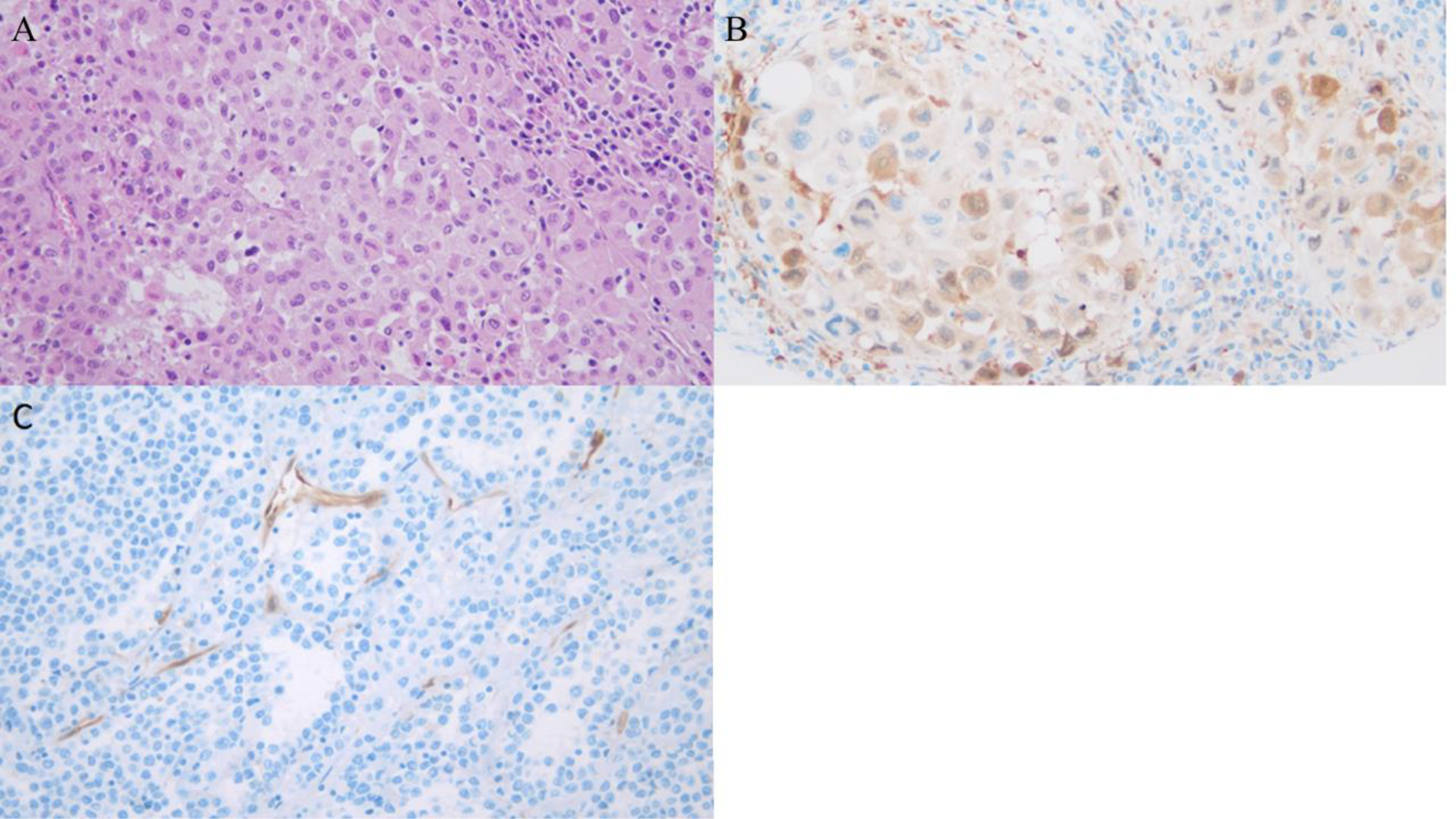
(A) H+E stain without IDO1 IHC, Immunotype-B Tumor (B) Lymph node metastasis showing a high level of IDO1 expression within tumor cells (Sigma Prestige, HPA 023072). (C) Metastatic melanoma showing no tumoral expression of IDO1, but a strong vascular expression pattern (Sigma Prestige, PHA 023072).
To test for associations between average IDO1 expression in TMA and IDO1 expression in a whole tumor section, 25 tumors were randomly selected for whole tumor sectioning and IDO1 staining. Percent of tumor cells expressing IDO1 in whole tumor sections was compared with the average percent of tumor cells expressing IDO1 in TMA for each patient. Percent of tumor cells in TMA expressing IDO1 and percent of tumor cells in whole tumor sections expressing IDO1 were highly correlated (Spearman’s Rho = 0.75, p-value <0.001, Supplemental Figure 2).
Patient Demographics
Patient demographics and clinical features were compared between IDOpos and IDOneg groups (Table 1). The IDOneg group contained a higher proportion of patients who underwent curative-intent resection and a lower proportion of patients with Stage IV disease. Additionally, there was a trend towards an increased proportion of females in the IDOpos group. There were no significant differences between the groups with respect to patient age, tissue origin, or BRAF mutation status. Given the advanced stage of our cohort’s disease, many had previously participated in experimental melanoma vaccine trials; however, the proportion of these patients did not differ significantly based on IDO expression.
Tumor Characteristics Related to IDO expression
Intra-tumoral immune cell infiltrates were characterized on the TMA for IDOpos and IDOneg tumors. As shown in Table 2, CD3+ and CD8+ cells more densely infiltrated IDOpos tumors (Supplemental Figure 3), which also expressed higher levels of PD-1 and PD-L1. There were also trends towards greater CD4+, CD20+, and FoxP3+ cell densities in IDOpos tumors which did not reach significance. Tumor Immunotype had been defined previously for these tumors(15) and did not differ significantly between IDOpos and IDOneg lesions, though Immunotype A tumors (which lacked immune infiltrates) represented 41% of IDOneg tumors and only 26% of IDOpos tumors. Likewise, CD163+ cell density was not different between IDOpos and IDOneg tumors.
Table 2:
Characterization of Tumor Immune Infiltrate and Gene Expression
| IDOneg | IDOpos | p-value | |
|---|---|---|---|
| Mean Intra-Tumoral Cell Density (cells/mm2) | |||
| CD20 (SD) | 29.1 (83.3) | 53.4 (128.1) | 0.28 |
| CD3 (SD) | 131.2 (194.3) | 282.7 (217.0) | <0.01 |
| CD4 (SD) | 46.1 (93.7) | 79.8 (100.0) | 0.12 |
| CD8 (SD) | 87.5 (109.7) | 210.0 (149.2) | <0.01 |
| PD-1 (SD) | 17.8 (32.1) | 41.4 (77.7) | 0.04 |
| PD-L1* (SD) | 9.0 (17.2) | 24.5 (26.2) | <0.01 |
| FoxP3 (SD) | 17.6 (29.6) | 31.4 (47.3) | 0.09 |
| CD163 (SD) | 60.4 (84.3) | 58.8 (63.5) | 0.93 |
| Immunotype (%) | 0.44** | ||
| A | 28 (41%) | 7 (26%) | |
| B | 37 (53%) | 18 (67%) | |
| C | 4 (6%) | 2 (7%) | |
| Gene Transcription*** | |||
| IDO1 | 9.69 | 10.46 | <0.01 |
| IDO2 | 4.46 | 5.64 | 0.04 |
| CD8A | 11.13 | 12.03 | 0.01 |
| IFNG | 10.91 | 11.20 | <0.01 |
| IL10 | 6.47 | 6.01 | 0.32 |
| TGFB1 | 10.95 | 10.87 | 0.53 |
| STAT1 | 14.17 | 14.61 | 0.09 |
| EOMES | 11.34 | 11.59 | 0.02 |
| TBX21 | 10.09 | 10.45 | <0.01 |
Differences between mean Intra-Tumoral Cell Density and Gene Transcription assessed with Kruskal-Wallis test.
PD-L1 expression by tumor cells
Fisher’s exact test
Log-transformed counts per million of RNA:NPP heteroduplexes.
SD = standard deviation.
Univariate Survival Analysis
Follow-up was determined from time of surgery and ranged from 1–104 months, with a median follow-up time of 14 months and 61 months for all patients and patients alive at time of last follow-up, respectively. Patients with IDOpos lesions had significantly longer OS than IDOneg patients in a univariate analysis (median OS 28.9 v. 10.5 months, log-rank p =0.02, Figure 2a). In contrast, intra-tumoral endothelial staining was not associated with differences in OS (log-rank p =0.29, Figure 2b). Having observed a correlation between tumor cell IDO1 expression and survival in a univariate analysis, we investigated whether IDO1 expression might be associated with other factors that could influence survival. Percent tumor IDO1 expression was determined along with mRNA expression of other genes associated with a Th1 predominant cytokine milieu, including the Th1 transcription factor t-bet (TBX21), CD8A, IFNG, STAT1, and EOMES, and also with genes for cytokines that regulate immune function (IL-10, TGFB1). As expected, IDOpos tumors exhibited significantly higher IDO1 mRNA expression than IDOneg tumors (Table 2). Additionally, another related gene, IDO2, was overexpressed in IDO1+ tumors. Expression of CD8A, IFNG, EOMES, and TBX21 was higher in IDOpos tumors, while there was a trend towards higher STAT1 transcription in IDOpos tumors that did not reach significance. In contrast, expression of IL10 and TGFB1, which are commonly associated with immune suppression, were not significantly different between the two groups.
Figure 2: Kaplan-Meier Survival Curves categorized by (a) Tumor Cell IDO1 expression and (b) Intra-tumoral Endothelial IDO1 expression.
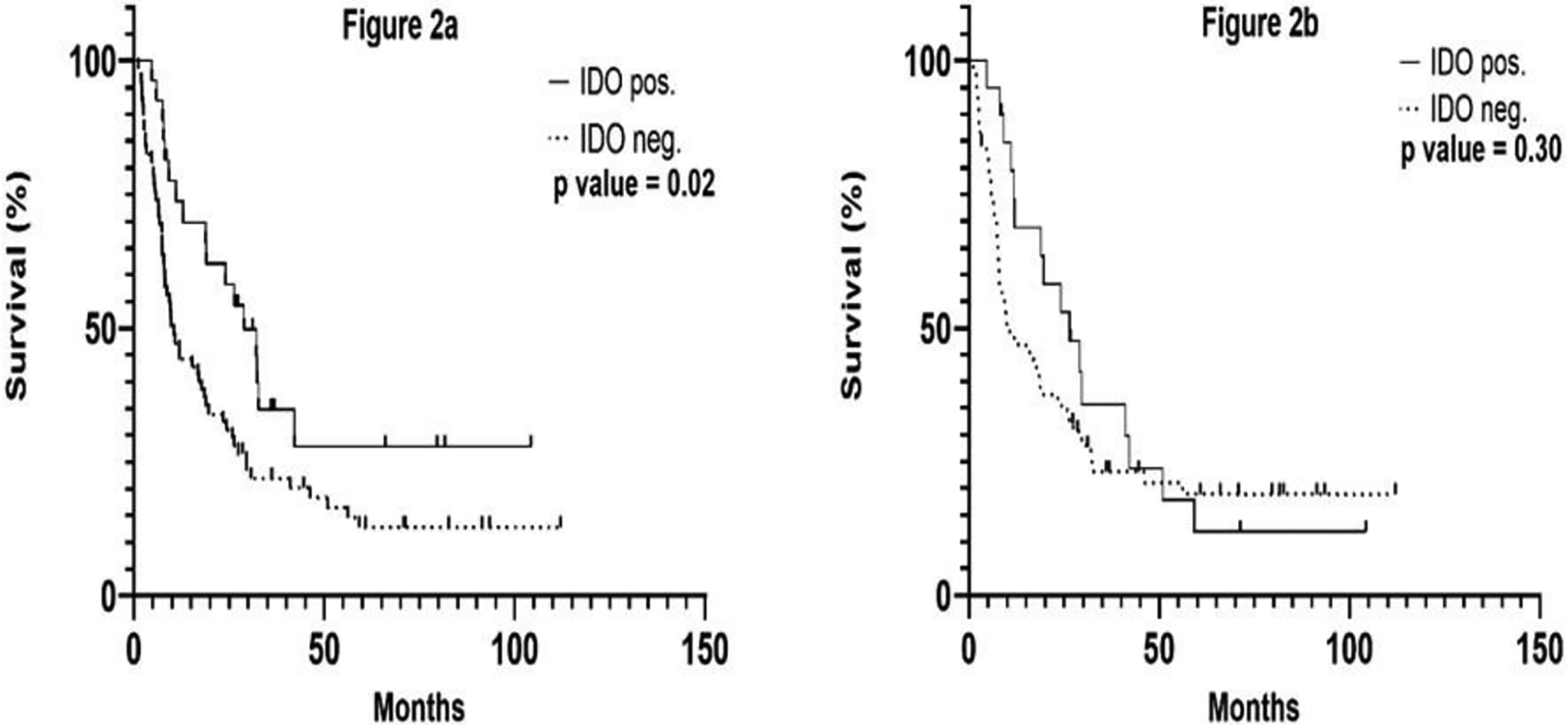
P value determined by log-rank test.
To further characterize the association between expression of these genes and tumor IDO1 staining, we correlated gene mRNA expression with the proportion of melanoma cells which stained for IDO1 by IHC. Percent IDO1 staining was directly correlated with increased CD8A, IFNG, TBX21, and STAT1 mRNA expression. In contrast, mRNA expression of EOMES, IL-10, and TGF-β1, and did not correlate with percent IDO1 staining. Finally, since IFNγ can increase with CD8+ T cell activation and, in turn, can enhance IDO1 expression, we compared percent IDO1 expression by tumor cells with intra-tumoral CD8+ T cell density. As expected, percent tumor IDO1 expression correlated strongly with intratumoral CD8+ T-cell density (Figure 3). Furthermore, on visual inspection tumor-cell IDO1 expression appeared to be upregulated at the interface of tumor and the immune-cell infiltration (Supplemental Figure 4).
Figure 3: Correlations between Gene Expression and Intra-Tumoral CD8+ T-cells with percent of tumor cells expressing IDO1.
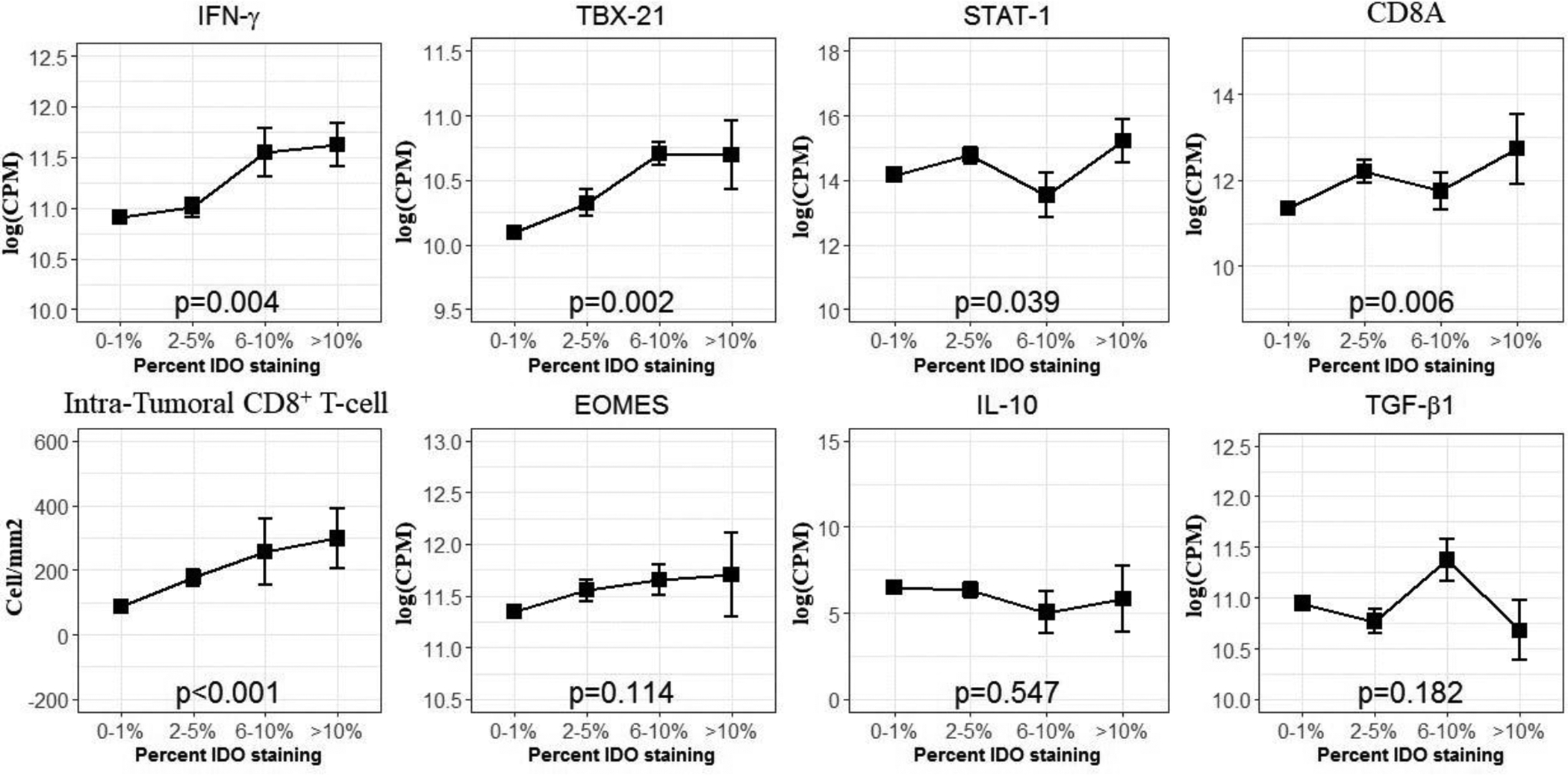
Associations between mRNA expression and Intra-Tumoral CD8+ T-cell density determined by Kruskal-Wallis test. Error bars correspond to the standard error of the mean.
To assess for changes in expression of CD8A, IFNG, TBX21, and STAT1 genes associated with endothelial IDO1 expression, mRNA levels of the same seven genes were compared between IDOendo+ tumors and tumors without endothelial IDO1 expression. As with IDOpos tumors, IDOendo+ tumors were associated with higher expression of CD8A, IFNG, TBX21, and STAT1 genes (p-value <0.01, 0.02, <0.01, 0.01, respectively, Kruskal-Wallis). As with IDOpos tumors, expression of TGFB1 and EOMES were not significantly elevated in IDOendo+ tumors; however, expression of IL10 was higher in IDOendo+ tumors relative to tumors lacking endothelial IDO1 expression (p-value = 0.05, Kruskal-Wallis) (Supplemental Figure 5).
Multivariate Survival Analysis
To determine whether the observed association between tumor IDO1 expression and OS might be related to additional clinical variables not evenly distributed between IDOpos and IDOneg tumors, we created a multivariate Cox-proportional hazards model which incorporated IDO1 expression, patient age, sex, disease Stage, tumor cell PD-L1 expression, intra-tumoral CD8+ T cell density, TBX21, EOMES and IFNG mRNA transcription, and whether surgical excision was intended to be palliative or curative. In this multivariate model, IDO expression was not associated with shorter or longer survival, but there was a weak trend toward longer OS for patients with IDO+ melanomas (HR: 0.65, p-value= 0.17); while palliative vs. curative intent at surgical resection was strongly associated with OS (Curative Intent: HR 0.35, p-value< 0.01, Table 3: Model 1).
Table 3:
Multivariate Cox Proportional Hazard Models
| Model 1: All Patients (n=96) | HR | 95% CI | p-value |
|---|---|---|---|
| IDOpos Tumor | 0.65 | 0.35 – 1.20 | 0.17 |
| IFNG | 0.99 | 0.47 – 2.11 | 0.99 |
| Stage 4 disease | 0.86 | 0.45 – 1.63 | 0.64 |
| Female sex | 0.71 | 0.43 – 1.16 | 0.17 |
| Curative-Intent Resection | 0.35 | 0.18 – 0.68 | <0.01 |
| Mean PD-L1 | 1.00 | 0.98 – 1.01 | 0.52 |
| Intratumoral CD8+ density | 1.00 | 1.00 – 1.00 | 0.10 |
| TBX21 | 0.88 | 0.43 – 1.82 | 0.74 |
| EOMES | 0.84 | 0.52 – 1.36 | 0.49 |
| Model 2: Curative-Intent Resection (n=64) | HR | 95% CI | p-value |
| IDOpos Tumor | 0.25 | 0.08 – 0.74 | 0.01 |
| IFNG | 1.49 | 0.54 – 4.10 | 0.44 |
| Stage 4 disease | 0.50 | 0.20 – 1.23 | 0.13 |
| Female sex | 0.56 | 0.29 – 1.06 | 0.07 |
| Intratumoral CD8+ density | 0.99 | 0.99 – 0.99 | <0.01 |
| TBX21 | 1.05 | 0.38 – 2.90 | 0.93 |
| EOMES | 1.43 | 0.79 – 2.61 | 0.24 |
Model assumptions confirmed with Schoenfeld residuals, significance level <0.01.
Sub-Group Analysis of Patients Undergoing Curative-Intent Resection
Because curative-intent at resection was a strong predictor of survival in our first multivariate model (Table 3: Model 1), as expected, we next performed a subgroup analysis of patients treated with curative-intent surgery, excluding patients who underwent palliative resection. This subgroup consisted of 64 patients, 13 (20%) of whose tumors were IDOpos. Of patients who underwent resection with curative intent, 49 (77%) had developed recurrent disease at time of last follow up, of whom 6 (12%) were IDOpos, and 43 (88%) were IDOneg. Patient demographics grouped by surgical intent are summarized in Supplemental Table 1. Interestingly, IDOneg tumors were associated with higher risk of disease recurrence at time of last follow up among patients who underwent resection with curative intent (84% vs. 46%, p-value = 0.01, χ2 test).
In a univariate analysis, IDOpos tumors were associated with significantly longer OS (Median OS: 23.4 vs. 11.9 months, Log-rank p <0.01). To determine if this association would persist after controlling for clinical variables known to the treating physician at time of surgery, and gene expression data in the tumor, we created a second multivariate Cox proportional hazards model incorporating IDO tumor status, tumor Stage, patient sex, and mRNA expression of TBX21, EOMES, IFNG. In this subgroup consisting of only patients who underwent curative-intent resection, tumor IDOpos expression and intratumoral CD8+ cell density were both associated with prolonged OS at the multivariate level (p=0.01, p<0.01, respectively; Table 3: Model 2).
Discussion
While prior studies have predominantly characterized IDO1 expression in patients with Stage I-III melanoma, we investigated IDO1 expression in a large (n=96) cohort of patients with Stage III-IV disease, who more closely match the cohort of patients treated in the ECHO-301 trial. In this cohort, only 28% of lesions were classified as IDOpos. Percent of tumor cells expressing IDOpos correlated with mRNA expression of IFNG, TBX21, and STAT1, and with intra-tumoral CD8+ T-cell penetration. Patients with IDOpos tumors appeared to have higher disease burden at baseline, as they were more likely to have Stage IV disease and more likely to undergo palliative intent resection (Table 1, Supplemental Table 1). Other authors have previously investigated the prognostic significance of IDO1 expression; however, these reports are contradictory, and multivariate survival analyses controlling for clinical factors that affect patient prognosis was conducted only in a single study whose sample was heavily skewed towards early melanoma (90/120 included patients had Stage I-II disease) (10–13). In this study, patients with IDOpos tumors experienced longer OS, but this association was not significant in a multivariate model which controlled for palliative-intent metastasectomy, which was more common in IDOpos tumors than IDOneg tumors. In a subgroup analysis including only patients undergoing curative-intent metastasectomy, IDOpos tumors were associated with lower risk of disease recurrence (54% vs. 16%, p-value=0.01), and with improved OS in a multivariate hazard model.
IDO1 expression occurs normally in a variety of tissue types associated with immune privilege or high antigen exposure (small intestine, epididymis, lung, female genital tract) (24,25). IDO1 expression can also be induced in most cell types by exposure to pro-inflammatory cytokines such as IFN-γ, IL-1, and TNF-α(26–28). Given IDO1’s immunosuppressive effects, its association with immune-privileged tissues, and its upregulation by Th1 cytokines, IDO1 expression is currently understood as a negative feedback mechanism protecting against auto-immune reactions(29,30). The potential for immunogenic tumors, such as melanomas, to co-opt the IDO1 pathway to evade immune regulation remains highly clinically relevant, particularly in the era of PD-1 blockade. Li and colleagues analyzed serum samples from CA209–038, a Phase 1 trial of melanoma patients treated with the anti-PD-1 antibody, nivolumab (Nivo). Of the 106 tested metabolites, Kyn, the byproduct of Trp degradation by IDO1, was the metabolite most elevated above pretreatment baseline at weeks 4 and 6 after starting Nivo. Furthermore, serum Kyn/Trp ratios that increased during treatment were associated with greater mortality risk (HR = 2.71, p<0.001) at a multivariate level, however pretreatment baseline Kyn/Trp ratios were not associated with overall survival(31), suggesting the possibility that induction of IDO1 expression during PD-1 antibody therapy may allow tumors to circumvent the effects of checkpoint blockade. Additionally, IDO1 expression may be associated with response to checkpoint blockade, as Helmick et al. recently reported that pretreatment IDO1 expression was enriched in tumors of melanoma patients who responded to checkpoint blockade compared to non-responders(32).
While promising, IDO1 inhibitors have thus far failed to demonstrate clinical benefit in combination with checkpoint blockade in phase III clinical trials in patients with Stage III-IV melanoma(8). The disconnect between the theoretical benefits of IDO1 inhibition and the lack of effects observed in recent clinical trials may be related to low IDO1 expression by metastatic tumors in contrast to primary tumors or resectable metastatic tumors, which comprised the majority of lesions included in prior analyses. In our cohort, which consisted of patients with Stage IIIB-IV melanoma, the observed fraction of patients with tumor-cell IDO1 expression was only 28%. This is comparable to that reported previously by Gide et al. (20%), but lower than that reported by Rubel et al. (65%)(10,11). Interestingly, Rubel’s study included only primary melanomas, and in that population, 44% percent of patients had IDO1 expression by >25% tumor of the tumor cells. In contrast, the large majority of IDOpos tumors in our study exhibited sparse IDO1 staining (only 4% of all lesions and 15% of all IDOpos tumors exhibited IDO1 expression in >10% of tumor cells). These data are consistent with the paradigm that IDO1 expression might be higher in primary tumors and locoregional metastases than in distant metastases. This possibility is supported by data reported by Gide et al., in which IDO1 was expressed by 24%, 31%, and 6% of primary tumors, locoregional metastases, and distant metastases, respectively. Given that all patients included in the ECHO-301 trial had unresectable Stage III-IV disease, these data raise the possibility that low IDO1 expression in advanced metastatic melanomas might explain the lack of survival benefit associated with IDO1 inhibition in combination with PD-1 blockade observed in this trial (8).
We initially hypothesized that IDO1 expression would correlate with worse OS. Contrary to our hypothesis, IDO1 expression by tumor cells correlated with a statistically significant and clinically relevant increase in OS using a univariate analysis (28.9 vs 10.5 months, p =0.02). There was no association between IDO1 expression by intratumoral endothelial cells and OS. Prior reports regarding the prognostic association between tumor-IDO1 expression and survival are conflicting, both in melanoma and in other tumors (1,33,34). In melanoma, higher IDO1 expression in lymph node metastases has been associated with decreased survival (13), and higher IDO1 expression in primary tumors has been correlated with lower median progression free survival (10). In contrast, in a prior study of 43 immunotherapy naïve patients with metastatic melanoma, a trend towards longer median melanoma specific survival was noted in patients with IDO1pos primary tumors or locoregional melanomas metastases (11). Of note, only Chevolet et al. conducted a multivariate survival analysis of IDO1 expression, and their sample overwhelmingly consisted of Stage I-II disease (90/120 patients), and no association was reported between OS and IDO1 expression by tumor cells (12). Similarly, in the present report of patients with more advance melanoma, IDO1 expression was not associated with OS in a multivariate analysis of all patients, however in a subgroup analysis including only patients who underwent metastasectomy with intention to cure, tumoral IDO1 expression and OS were significantly correlated after controlling for additional clinical variables and histologic features associated with patient prognosis. Density of CD8+ T-cell infiltrate was also a significant predictor of OS in this model, as has elsewhere been associated with improved prognosis in melanoma (15). Interestingly, among patients who underwent metastasectomy with curative intent, disease recurrence at time of last follow-up was more likely for IDOneg tumors than IDOpos tumors (84% vs. 46%, p-value=0.01), which may suggest that IDO positivity is a marker of microscopic metastatic burden at time of metastasectomy.
Others have suggested that the prognostic significance of IDO1 expression likely varies by the cell type expressing IDO1 (1,10,30); as it has been reported that IDO1 expression by melanoma cells in the primary tumor had no correlation with PFS or OS, but that IDO1 expression by peritumoral endothelial cells and high endothelial venules in sentinel lymph nodes correlated with OS in a multivariate model(12). Expression by melanoma cells may reflect IFNγ production by intra-tumoral lymphocytes (TIL), while expression by peritumoral dendritic cells (not included in our assessment) may reflect tumor antigen recognition and associated immune regulation. Melanoma cell IDO1 expression may, thus, represent a negative feedback mechanism initiated in response to a pro-inflammatory, Th1-dominant cytokine milieu. We found that IDO1 expression correlated with transcription of genes related to a Th1 predominant inflammatory response, including IFNG, STAT1 and TBX21. Additionally, IDOpos tumors tended to be immunotype B or C (74% of IDOpos vs. 59% of IDOneg tumors), and had a greater density of intra-tumoral cytotoxic T-cells and cells expressing PD-L1, which, like IDO1, can be upregulated by IFNγ(29). Tumor IDO1 expression was also noted to be upregulated at the tumor-TIL interface as shown in Supplemental Figure 4. These data are consistent with the hypothesis that melanoma cells may express IDO1 as a means of immunologic escape from a Th1 predominant cytokine milieu and anti-tumoral CD8+ activation. This paradigm, in which tumor cell IDO1 expression is a marker of CD8+ TIL activity in a Th1-dominant cytokine milieu, could explain the association between IDO1 expression and increased OS we observed in advanced melanoma patients, which, in patients who underwent curative intent resection, was significant in a multivariate Cox Hazard model controlling for clinical variables at time of resection.
Though not correlated with tumor cell IDO1 expression, IL-10 expression was upregulated in IDO1endo+ tumors. As IL-10 is associated with immune regulation, it is tempting to speculate that IDO1 expression by intra-tumoral endothelial cells may diminish the activity of adjacent TIL and contribute to worsened tumor control, which would be concordant with a report that endothelial IDO1 expression was associated with decreased OS in primary melanomas (13). In this study of metastatic melanomas, we observed as survival advantage associated with IDO1 expression by tumor cells, but not by intra-tumoral endothelial cells. Taken together, these data suggest that expression of IDO1 by intra-tumoral endothelial cells is regulated discordantly from expression by tumor cells, and that the prognostic significance of IDO1 expression is dependent on cell type.
To our knowledge, our report is the largest and most comprehensive exploration of IDO1 expression by melanoma metastases in patients with Stage III-IV disease, and the first report of a statistically significant survival improvement associated with IDO1 expression. IDO1 expression was low in melanoma metastases, with 72% of lesions expressing IDO1 in less than 1% of tumor cells. Additionally, even among IDOpos lesions, only 15% exhibited IDO1 expression in more than 10% of tumor cells. In contrast to a prior report, this association was significant in a multivariate hazard model controlling for clinical and histologic features associated with patient prognosis (12), however this was present only in patients who underwent resection with curative intent. Furthermore, tumor IDO1 expression was strongly correlated with IFNG mRNA expression and intra-tumoral CD8+ T-cell density, suggesting that tumor cell IDO1 expression might be driven by intra-tumoral CD8+ activity. Given the sparsity of metastatic lesions which were IDOpos, these data suggest that future trials of IDO1 inhibitors may benefit from patient selection or stratification based upon IDO1 expression.
A limitation of our study was that IDO1 expression was only characterized for melanoma cells and intratumoral endothelial cells. IDO1 expression by other cell types, such as macrophages and dendritic cells, may be associated with clinical outcome and may have an impact on tumor control, as discussed above. Additionally, our study was retrospective in nature, and unable to determine causation.
In summary, IDO1 expression is limited to a small subset (28%) of checkpoint blockade-naive melanoma patients with advanced (Stage III-IV) disease, and even among those with IDOpos lesions, the majority (85%) will express IDO1 in fewer than 10% of metastatic melanoma cells. The relative sparsity of IDO1 expression by melanoma cells in advanced metastatic lesions may have contributed to the negative outcome of the ECHO-301 trial. Future trials of IDO1 inhibitors may therefore benefit from more stringent patient selection criteria or stratification based on IDO1 expression. Additionally, IDO1 expression by tumor cells is correlated with improved survival in patients who underwent resection with curative intent and is likely reflective of an active CD8+ TIL population, which may protect against melanoma recurrence in patients with resectable metastases. Strong evidence persists that expression of IDO1 by tumor cells may be a means of immunologic escape from anti-tumoral immunity. IDO1 inhibitors may therefore offer additional therapeutic value for metastatic melanoma patients whose tumor cells express IDO1.
Supplementary Material
Acknowledgements:
Biorepository and Tissue Research Facility, University of Virginia, and HTG Molecular (Tusczon, AZ). Support provided by the United States Public Health Services Training Grants T32HL007849 (KL), T32CA009109-41 (MK) and T32CA163177 (MK, MM), as well as the Farrow Fellowship and Rebecca Clary Harris Memorial Fellowship, both from the University of Virginia (MK). Additional support was received from the Biostatistics Shared Resource, University of Virginia Cancer Center (NW) and the Cancer Center Support Grant P30CA044579 (CS).
Footnotes
Conflict of Interests: The authors declare no potential conflicts of interest
References
- 1.Zhai L, Ladomersky E, Lenzen A, Nguyen B, Patel R, Lauing KL, et al. IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol. 2018. May;15(5):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003. October;9(10):1269–74. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999. May 3;189(9):1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selvan SR, Dowling JP, Kelly WK, Lin J. Indoleamine 2,3-dioxygenase (IDO): Biology and Target in Cancer Immunotherapies. Curr Cancer Drug Targets. 2016;16(9):755–64. [DOI] [PubMed] [Google Scholar]
- 5.Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatol Basel Switz. 2007;214(1):8–14. [DOI] [PubMed] [Google Scholar]
- 6.Slingluff CL., Fling S, Mauldin I Ernstoff M, Hanks BA, Delman K, Lawson D, Gastman B, Kaiser J, Cheever M Pilot Trial of an Indoleamine 2,3-dioxygenase-1 (IDO1) inhibitor plus a multipeptide melanoma vaccine in patients with advanced melanoma. J Clin Oncol. 2018;36(15). [Google Scholar]
- 7.Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J Clin Oncol Off J Am Soc Clin Oncol. 2018. September 28;JCO2018789602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019. August;20(8):1083–97. [DOI] [PubMed] [Google Scholar]
- 9.Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41(1):41–8. [DOI] [PubMed] [Google Scholar]
- 10.Rubel F, Kern JS, Technau-Hafsi K, Uhrich S, Thoma K, Häcker G, et al. Indoleamine 2,3-Dioxygenase Expression in Primary Cutaneous Melanoma Correlates with Breslow Thickness and Is of Significant Prognostic Value for Progression-Free Survival. J Invest Dermatol. 2018;138(3):679–87. [DOI] [PubMed] [Google Scholar]
- 11.Gide TN, Allanson BM, Menzies AM, Ferguson PM, Madore J, Saw RPM, et al. Inter- and intrapatient heterogeneity of indoleamine 2,3-dioxygenase expression in primary and metastatic melanoma cells and the tumour microenvironment. Histopathology. 2019. May;74(6):817–28. [DOI] [PubMed] [Google Scholar]
- 12.Chevolet I, Speeckaert R, Haspeslagh M, Neyns B, Krüse V, Schreuer M, et al. Peritumoral indoleamine 2,3-dioxygenase expression in melanoma: an early marker of resistance to immune control? Br J Dermatol. 2014. November;171(5):987–95. [DOI] [PubMed] [Google Scholar]
- 13.Brody JR, Costantino CL, Berger AC, Sato T, Lisanti MP, Yeo CJ, et al. Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. Cell Cycle Georget Tex. 2009. June 15;8(12):1930–4. [DOI] [PubMed] [Google Scholar]
- 14.Obeid JM, Erdag G, Smolkin ME, Deacon DH, Patterson JW, Chen L, et al. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology [Internet]. 2016. September 20 [cited 2020 Apr 2];5(11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5139635/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012. March 1;72(5):1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills AM, Peres LC, Meiss A, Ring KL, Modesitt SC, Abbott SE, et al. Targetable Immune Regulatory Molecule Expression in High-Grade Serous Ovarian Carcinomas in African American Women: A Study of PD-L1 and IDO in 112 Cases From the African American Cancer Epidemiology Study (AACES). Int J Gynecol Pathol Off J Int Soc Gynecol Pathol. 2019. March;38(2):157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills A, Zadeh S, Sloan E, Chinn Z, Modesitt SC, Ring KL. Indoleamine 2,3-dioxygenase in endometrial cancer: a targetable mechanism of immune resistance in mismatch repair-deficient and intact endometrial carcinomas. Mod Pathol Off J U S Can Acad Pathol Inc. 2018;31(8):1282–90. [DOI] [PubMed] [Google Scholar]
- 18.Chinn Z, Stoler MH, Mills AM. PD-L1 and IDO expression in cervical and vulvar invasive and intraepithelial squamous neoplasias: implications for combination immunotherapy. Histopathology. 2019. January;74(2):256–68. [DOI] [PubMed] [Google Scholar]
- 19.Dill EA, Dillon PM, Bullock TN, Mills AM. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod Pathol Off J U S Can Acad Pathol Inc 2018;31(10):1513–22. [DOI] [PubMed] [Google Scholar]
- 20.Volaric A, Gentzler R, Hall R, Mehaffey JH, Stelow EB, Bullock TN, et al. Indoleamine-2,3-Dioxygenase in Non-Small Cell Lung Cancer: A Targetable Mechanism of Immune Resistance Frequently Coexpressed With PD-L1. Am J Surg Pathol. 2018;42(9):1216–23. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Zhao L, Liang Z, Liu S, Li Y, Liu S, et al. Indoleamine 2,3-dioxygenase 1 and Programmed Cell Death-ligand 1 Co-expression Predicts Poor Pathologic Response and Recurrence in Esophageal Squamous Cell Carcinoma after Neoadjuvant Chemoradiotherapy. Cancers. 2019. February 1;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sailer V, Sailer U, Bawden EG, Zarbl R, Wiek C, Vogt TJ, et al. DNA methylation of indoleamine 2,3-dioxygenase 1 (IDO1) in head and neck squamous cell carcinomas correlates with IDO1 expression, HPV status, patients’ survival, immune cell infiltrates, mutational load, and interferon γ signature. EBioMedicine. 2019. October;48:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law CW, Chen Y, Shi W, Smyth GK. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedlmayr P, Blaschitz A, Stocker R. The Role of Placental Tryptophan Catabolism. Front Immunol [Internet]. 2014. May 19 [cited 2020 Apr 2];5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4032907/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Routy J-P, Routy B, Graziani GM, Mehraj V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int J Tryptophan Res IJTR. 2016;9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, et al. Mesenchymal Stem Cells Employ IDO to Regulate Immunity in Tumor Microenvironment. Cancer Res. 2014. March 1;74(5):1576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medrano RFV, Hunger A, Mendonça SA, Barbuto JAM, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget. 2017. July 25;8(41):71249–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nirschl CJ, Suárez-Fariñas M, Izar B, Prakadan S, Dannenfelser R, Tirosh I, et al. IFNγ-Dependent Tissue-Immune Homeostasis Is Co-opted in the Tumor Microenvironment. Cell. 2017. June 29;170(1):127–141. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci Transl Med. 2013. August 28;5(200):200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv Exp Med Biol. 2017;1036:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Bullock K, Gurjao C, Braun D, Shukla SA, Bossé D, et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun [Internet]. 2019. September 25 [cited 2020 Apr 2];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6761178/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020. January;577(7791):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer Oxf Engl 1990. 2017;76:167–82. [DOI] [PubMed] [Google Scholar]
- 34.Löb S, Königsrainer A, Rammensee H-G, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009. June;9(6):445–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


