Abstract
Purpose:
Performance status (PS) is one of the most common eligibility criteria. Many trials are limited to patients with high-functioning PS, resulting in important differences between trial participants and patient populations with the disease. Additionally, existing PS measures are subjective and susceptible to investigator bias.
Methods:
A multidisciplinary working group of the American Society of Clinical Oncology and Friends of Cancer Research evaluated how PS eligibility criteria could be more inclusive. The working group recommendations are based on a literature search, review of trials, simulation study, and multistakeholder consensus. The working group prioritized inclusiveness and access to investigational therapies, while balancing patient safety and study integrity.
Results:
Broadening PS eligibility criteria may increase the number of potentially eligible patients for a given clinical trial, thus shortening accrual time. It may also result in greater participant diversity, potentially reduce trial participant and patient disparities, and enable clinicians to more readily translate trial results to patients with low-functioning PS. Potential impact on outcomes was explored through a simulation trial demonstrating that when the number of Eastern Cooperative Oncology Group (ECOG) PS2 participants was relatively small, the effect on the estimated hazard ratio and power were modest, even when PS2 patients did not derive a treatment benefit.
Conclusion:
Expanding PS eligibility criteria to be more inclusive may be justified in many cases and could result in faster accrual rates and more representative trial populations.
INTRODUCTION
An important goal of the American Society of Clinical Oncology (ASCO), Friends of Cancer Research (Friends), and the oncology community at large is broadening clinical trial eligibility criteria to enhance trial access and accrual, and ensure trial populations better reflect patients with the disease.1 Performance status (PS) is one of the most common inclusion/exclusion criteria in oncology trials. Many trials are limited to high-functioning participants (i.e., “good” PS) and exclude low-functioning patients (i.e., “poor” PS).2
Two main PS scales are utilized in oncology clinical trials: Eastern Cooperative Oncology Group (ECOG) 3 and Karnofsky (KPS) scales.4 Multiple trials in various tumor types and settings have demonstrated that low-functioning PS (i.e., ECOG PS2–4 and KPS ≤70) is correlated with lower overall survival (OS) and progression-free survival (PFS) compared to high-functioning PS (ECOG 0–1 and KPS 80–100).5–13 Because of this, PS is included as a common eligibility criteria and stratification factor. However, this practice prevents trial enrollment for many patients and limits generalizability of trial results. Select trials that have focused exclusively on participants with low-functioning PS demonstrated patient and clinician interest and enrollment.14–17 The underlying etiology for low-functioning PS is also important; for patients whose low-functioning PS is due to disease burden, investigational treatment may result in improved PS with tumor control and symptom alleviation, especially with highly effective treatments. However current PS scales do not differentiate causes of low-functioning PS.
Additionally, there are limitations to PS assessments. PS is inherently subjective, which can affect inter-rater reliability18 and invite potential bias particularly for patients at the borderline between values. For example, studies demonstrate that clinicians assign patients aged >65 higher numeric PS scores than younger patients, despite no difference in objectively measured physical activity.19 Additionally, PS is less predictive of cancer-related outcomes for older adults.20,21
METHODS:
Because clinical trials frequently exclude PS2 patients, the working group chose to focus on this category. To understand the potential effect of including PS2 patients, the working group conducted a simulation study, where randomized trials of a hypothetical agent were simulated under various conditions. We also examined the literature to identify the potential risks and benefits of including PS2 patients on therapeutic clinical trials and evidence of the effectiveness of PS2 as a prognostic factor, reviewed past and current clinical trials to determine how often PS2 was included in inclusion/exclusion criteria, and developed consensus recommendations on how PS eligibility criteria could be revised while ensuring the safety of participants and integrity of the trial, and additional areas for research.
Benefits:
Increase Number of Patients Eligible and Shorten Enrollment Time:
Small, mainly single institution studies have demonstrated that of patients deemed ineligible for a clinical trial, exclusion was related to poor PS in a significant proportion of patients, with variability across disease type, investigational therapy, and therapy line.22,23 Even if other objective eligibility measures can be addressed, PS may remain a broad factor that excludes many patients. (Table 1)
Table 1:
Risks and benefits of expanding enrollment to patients with worse performance status.
| Patients/Prescribing Physicians | Sponsors/Investigators | |
|---|---|---|
| Benefits | • Earlier access to investigational agents for a larger population of patients • More complete safety and efficacy data to help inform standard of care decision-making in the “real world” once the agent is commercially available |
• Greater ability to generalize to “real world” populations • Larger population of potentially eligible patients may afford faster clinical trial accrual times • Efficacy/tolerability in an understudied population provides more informative drug labeling and may facilitate more use in these patients • Higher overall adverse events may make PS2 population more sensitive to demonstration of a potential comparative tolerability benefit • Where poor PS is due to advanced disease, benefits in a clinical outcome (survival, symptom or functional improvement) may be easier to demonstrate for a highly effective drug. |
| Risks | • Potentially higher rates of adverse events | • Potentially greater variability in outcomes if not stratified/balanced between treatment groups • Potentially higher rates of adverse events/more complicated attribution of adverse events – if PS balanced between treatment groups, should be able to account for this • Diminished treatment effect if PS2 patients do not have the same treatment benefit as patients with good PS |
Improve Assessment Accuracy, Particularly in Older Adults:
Most patients with cancer are aged ≥65, however, existing PS scales are inadequate in this population.20 Restrictive PS eligibility criteria contribute to the pervasive age disparity between trial participants and the overall cancer population, raising concerns about whether PS is unjustly limiting older populations’ ability to participate in trials.24–26 Multiple studies have demonstrated that other tools, such as the geriatric assessment (GA), are better than PS at evaluating older adults’ overall health status27 and at predicting chemotherapy toxicity.20 While a full GA may not be practical due to length, subcomponents may provide a better functional assessment, such as Instrumental Activities of Daily Living that measure functional independence.
Improve Generalizability:
Benefits for patients with high-functioning PS may not reflect outcomes for patients with low-functioning PS.28,29 Many eligibility restrictions from registration trials, such as line of therapy or cancer stage, are incorporated explicitly into the labeled indications with the exception of PS limitations. Therefore, therapies tested only in participants with high-functioning PS are administered to patients with lower functioning PS. This extrapolation may occur more readily with targeted and immunotherapies given greater efficacy.30 Therefore, evaluation of an investigational agent in participants reflective of the patient population is important. More inclusive PS eligibility will also likely increase enrollment of older adults24,31 and address the lack of evidence noted above.32,33
Risks:
Increased Adverse Events:
Rates of adverse events (AEs) may be greater in PS2 participants as compared to PS0 and PS1 participants, and this may influence patient’s outcomes and ability to comply with study procedures. As a result, investigators and sponsors may be reluctant to consider trial enrollment. PS2 patients risk AEs with standard therapy options as well, and thus participation on a trial may not necessarily pose a greater risk of AEs compared to standard therapy for a particular patient. Because targeted therapies often have higher response rates, PS2 patients may experience a greater therapeutic index in a targeted therapy trial than standard of care (e.g., cytotoxic chemotherapy), even if their absolute rate of AEs is higher than in patients with PS0 and PS1. Where the comparative tolerability between an investigational agent and standard therapy is less clear, including PS2 patients (who may be more sensitive to toxicity) may unmask subtle differences. Importantly, having a subset of PS2 patients will add important safety data to facilitate decision-making for patients in the post-approval setting. (Table 1) Determining appropriate timing for including PS2 participants is challenging. When possible, inclusion of a small number of PS2 participants in early phase trials is recommended to guide separate expansion cohorts for phase II or broader inclusion into registration trials.
Even when clinical trial eligibility allows PS2 patients to enroll, relatively few PS2 participants are actually enrolled.34,35 This may relate to clinicians’ lack of familiarity with the investigational agent and concerns about the tolerability and safety. Enhanced information about safety, tolerability, and efficacy from earlier phase trials with the agent may help to counteract this. Additionally, when clinically appropriate, allowing physician discretion in the treatment approach as a component of the clinical trial may help to mitigate this issue.36,37
Potential Impact on Trial Outcome Data:
In trials of novel therapies including PS2 participants, data suggests that outcomes may be inferior compared to participants with PS0–1, even though low proportions of PS2 participants were included.38–40 This information alone should not be used as a justification for excluding PS2 patients. Instead, similar to other high-risk prognostic markers identified in oncology, PS information could be considered as a stratification factor. When safe, inclusion of participants with low-functioning PS provides valuable evidence to guide clinical care for most patients. Outcomes in low-functioning PS participants can also better inform statistical considerations for future trials.
The risk of inferior outcomes from low-functioning PS participants is a potential concern to sponsors, especially if compared to historical cohorts including high-functioning PS. The FDA has addressed a similar concern in a March 2019 final guidance on enrollment of patients with brain metastases stating, “To mitigate uncertainties about including patients with brain metastases in clinical trials, consider enrolling these patients in a separate subgroup within the trial.”41 In addition, FDA commentary has further indicated a willingness to restrict primary efficacy analysis to the participant subset who meet more conventional eligibility criteria when a sponsor enrolls a broader range of participants.42 FDA also notes that including a broader group of participants could offer benefits, such as additional information in drug labeling and/or reduced post-marketing commitments.
Simulation Study Methods:
To explore the effects on inferences comparing trials that include vs. exclude participants with PS2, simulations were conducted under a variety of trial settings with three levels of PS: PS0, PS1, and PS2. Figure 1 presents results based on: (a) total sample size of 500 participants, (b) 1:1 randomization to two treatment groups, (c) accrual time of 24 months, (d) a time-to-event endpoint, and (e) follow-up until 283 events are observed, achieving power of 85% based on a hazard ratio (HR) of 0.70 vs. a null hypothesis of 1.0 and a two-sided alpha of 0.05. Participants were assumed to vary in their median survival: 12-, 9-, and 6-month median survival in PS0, PS1, and PS2 participants, respectively. Differences in drop-outs due to AEs or other factors varied: 5%, 10%, and 20% of PS0, PS1, and PS2, respectively, and AEs were assumed to have censored event times within the first 4 months. Simulations assumed 45% PS0, 45% PS1, and 10% PS2 participants, and the true HRs reflecting treatment benefit were varied across PS groups. Scenario 1 assumes all three PS groups have the same treatment effect: HR=0.7. Scenario 2 assumes PS 0 and PS1 participants derive benefit but PS2 participants do not (PS0 and PS1 HR=0.7 and PS2 HR=1.0). Scenario 3 assumes PS2 participants derive greater benefit compared to PS0 and PS1 participants (PS0 and PS1 HR=0.7 and PS2 HR=0.5). Outcome measures that were assessed to determine the differences in inferences due to the variability in HRs across the groups were (A) the estimated hazard ratio, (B) power, and (C) time to complete the study because fewer patients would be excluded (measured as the time from the first enrolled participant to the last event required for analysis). Inferences from simulated trials (10000 per scenario) were analyzed under two different approaches: (1) excluding PS2 participants (N=450 PS0 and PS1 patients included in analysis) and (2) including the PS2 participants (N=500 for analysis). When excluding PS2 participants, the analysis was undertaken when there were 283 events among the PS0 and PS1 participants.
Figure 1.
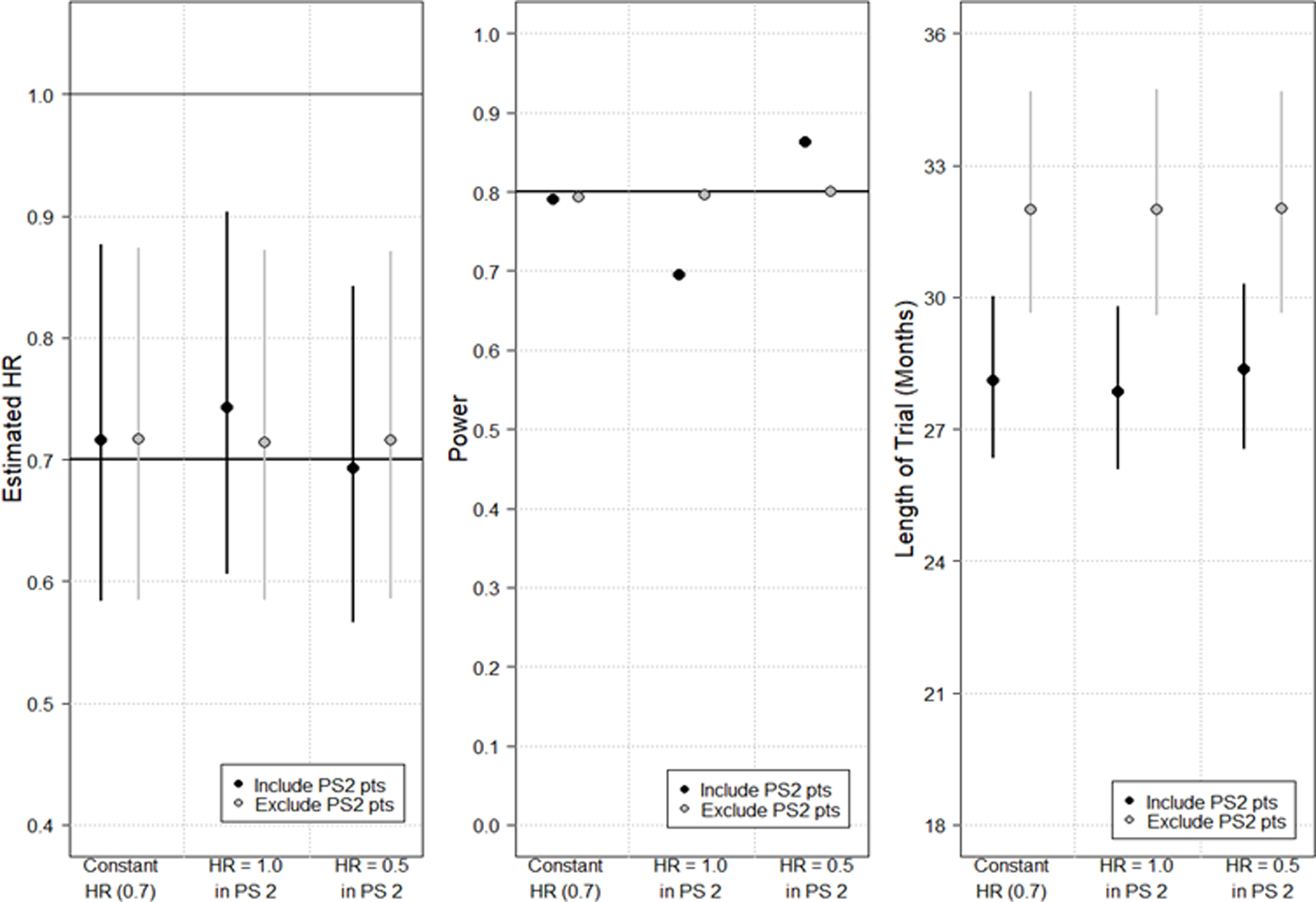
Simulation study results depicting changes in estimated hazard ratio (HR) (Panel A); power (Panel B); and length of trial from first accrual to last required event for analysis (Panel C). Within each panel, six analyses are depicted. In panels A and C, the median from the simulations is plotted as a circle with lines extending vertically to the 5th and 95th percentiles. For each analysis, the HR of PS0 and PS1 patients remains constant at 0.7 and the HR of PS2 patients is varied. Red points/lines depict results when PS2 patients are included in the final analysis (N=500); blue points/lines depict results when PS2 patients are excluded (N=450). Regardless of sample size, the trial end is assumed to be when the required number of events (283 events) have been accrued per the power calculation.
The simulation study demonstrated the following conclusions for including PS2 participants:
When the number of PS2 participants is relatively small (e.g., 10%), the effect on the estimated HR and power are relatively modest, even when the PS2 participants do not have a true treatment benefit (Figure 1, Panels A and B).
Including PS2 participants is likely to shorten duration of the trial by increasing the number of potentially eligible trial participants (Figure 1, Panel C) and due to the higher event rate in PS2 participants relative to PS0–1 participants.
These conclusions may not be generalized to all trial settings. Single-arm trials need attention given that previous trial results (to which the study results will be compared) may not have included PS2 participants. Similarly, trials with smaller (or larger) sample size may have more dramatic or muted effects depending on other trial parameters, such as the fraction of PS2 participants.
Mechanisms for Addressing Risks Associated with Expanding PS Eligibility Criteria:
Assessing safety concerns should take into account the potential increased risk in AE rates between standard of care and experimental intervention, rather than the absolute rate of expected AEs.
- Reassess and revise PS eligibility criteria at each phase of drug development, in accordance with growing knowledge about the investigational agent. Early phase data (AE rates, durable objective responses) for PS2 participants can decrease uncertainty of subsequent randomized trials. For example, trials could:
- Include an exploratory PS2 cohort in early phase trials to collect data without compromising internal validity and to inform inclusion in later phase trials, incorporating early stopping rules for unacceptable toxicity, or
- If tolerability/safety is acceptable during early phase for PS0–1 participants, expand to include PS2 participants in later phases.
- Consider alternate trial designs and settings. Examples may include:
- Trials specifically for PS2 participants and, where appropriate, PS3 participants. This may be most ideal for studies of modified (“de-intensified”) regimens where the overall goal is to develop a more tolerable therapy.
- Flexibility in the dosing schema, particularly for palliative trials. For example, enable investigator discretion to allow participants to initiate treatment at a reduced dosage with escalation to full dosage based on tolerability.37 This may be most appropriate for studies in advanced cancer where the goal of therapy is palliation.
- Consider expansion cohorts to enhance enrollment of PS2 patients. This may be the most effective strategy for therapies with novel mechanisms or less well defined AE profiles, whereby initial enrollment includes patients with high-functioning PS and once safety and tolerability are better understood, expansion to include PS2 patients occurs.
Discuss study design and statistical analysis approaches for broader eligibility and implications for post-marketing research with FDA during trial design, where appropriate. This may include performing simulations under a variety of assumptions regarding fraction of PS2 patients and heterogeneity of efficacy and safety across PS groups.
Recommendations for inclusion of PS2 participants are included in Table 2. Although discussion has focused on inclusion of PS2 participants, PS3 participants should also be considered. With targeted therapies for rare alterations, inclusion of PS3 participants may be considered to expand the eligible patient population, if the agent has demonstrated favorable toxicity and efficacy signals.
Table 2:
Recommendations
| Number | Recommendation |
|---|---|
| 1. | Patients with ECOG PS2 (or KPS 60–70) should be included unless there is a scientific and/or clinical rationale for exclusion justified by established safety considerations. a. PS eligibility criteria should be based on the patient population in which the intervention is expected to be applied in clinical practice. b. PS eligibility criteria should be continually re-evaluated and modified throughout the drug development process to reflect accumulated safety data of the investigational treatment. Decisions about PS eligibility criteria should be based on early clinical safety and efficacy data about the specific investigational agent or based on known data from other drugs in the same class with similar mechanism of action. Later phase trials (e.g. phase II/III) should generally mirror the intended use population and ECOG PS2 (or KPS 60–70) patients should be included, unless safety concerns have manifested in earlier phase trials. The rationale for exclusion should be justified and stated explicitly. c. Incorporating the rationale for inclusion of a broader population into the protocol could help encourage investigators to enroll these patients. d. Performance status data should still be collected for use as a stratification factor, regardless of how it is incorporated into eligibility criteria. |
| 2. | Consider alternative trial designs, such as pre-specified cohorts with lower-functioning PS that are exempt from the primary analysis, to encourage inclusion of these patients. These cohorts would generally be small in size and exploratory in nature and could be enrolled in an incremental way to enable an early stopping rule based upon safety data. Consideration of the data analysis approach for the broader eligibility cohort and subgroup analysis should be determined during the study design phase and its implications for marketing and post-marketing requirements discussed with FDA when appropriate. |
| 3. | Additional assessments of functional status should be considered to better characterize the functional status of ECOG PS2 patients and patients aged ≥65, such as Activities of Daily Living (ADLs) and Instrumental ADLs. |
AREAS OF NEED FOR FUTURE RESEARCH:
Methods to Incorporate Functional Status Assessment:
Alternate methods for assessing physical function exist, such as patient-reported outcome measures,45 objective performance measures (e.g., gait speed),46 and activity monitoring devices (e.g., wearable devices).47 Further research is needed to understand how to incorporate and use these alternative methods in oncology trials. Enhancing the objectivity of PS assessments may more accurately characterize functional capacity and improve trial suitability assessment, particularly if low-functioning PS is related to disease burden versus other factors around the time of diagnosis. Incorporating these methods may also reduce bias of PS assessments.
Associations Between PS and Safety/Toxicity in Targeted Therapies and Immunotherapy48
The majority of newly approved investigational agents have targeted mechanisms of action, however the safety and efficacy of many of these therapies remains unclear in the PS2 population given their underrepresentation on clinical trials leading to approval. Understanding safety and efficacy of novel therapies in PS2 patients, particularly for patients with low-functioning PS due to disease burden, is a critical area of need, as a targeted therapy or immunotherapy with a high objective response rate may afford improvement in PS by improving disease-related symptoms.
CONCLUSION:
Broadening PS eligibility criteria to be more inclusive can increase the number and diversity of trial participants. More effective biomarker-driven therapies warrant reconsideration of this traditional approach. Trial sponsors should justify exclusion of PS2 patients and limit exclusions to those affecting patient safety and trial integrity. Several strategies can encourage broader inclusion of PS2, and in select cases PS3, participants. Implementation of these recommendations will require cooperation of multiple stakeholders and can result in incentives following FDA approval.
Statement of Translational Relevance:
Performance status (PS) is one of the most common eligibility criteria, often resulting in exclusion of patients from trial participation and leading to clinical trial populations that are not reflective of populations afflicted with the disease. Existing PS tools are inherently subjective and invite bias. Additionally, PS is less predictive of outcomes for older adults. Broadening PS eligibility criteria to be more inclusive can increase the number and diversity of participants in clinical trials. Trial sponsors should justify any exclusion of low-functioning PS patients and limit exclusions to circumstances of participant safety and trial integrity. ASCO and Friends of Cancer Research outlined several strategies to encourage broader trial eligibility criteria. Implementation of these recommendations will require cooperation of multiple stakeholders and providing incentives for expanded PS eligibility may support this effort.
Acknowledgments:
This article was developed as a consensus document of the Performance Status Working Group as part of a collaboration between ASCO, Friends, the US Food and Drug Administration (FDA), and the National Cancer Institute (NCI). The contents of this document were presented on August 2, 2019 as part of an invitation-only, collaborative workshop. The authors would like to thank all the participants of the workshop, as well as the planning committee, ASCO, Friends, FDA, and NCI staff, for their expertise and thoughtful input on the project and article, and for logistical support.
Additional support: Dr. Magnuson is supported by a National Institute on Aging Beeson Career Development Award (NIA K76 AG064394). Dr. Lichtman is supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Disclosures:
Arnaldez: Dr. Arnaldez began this project as an NCI employee but is currently an employee of MacroGenics, Inc.
Klepin: Contributor to UpToDate
Melemed: Dr. Melemed began this project as an employee of Eli Lilly but is currently an employee of Chimerix.
Roy: Dr. Roy has received grant support from Merck and BMS Foundation to his organization, independent of the work presented in this manuscript.
Other authors report no disclosures.
Publisher's Disclaimer: DISCLAIMER:
The opinions expressed in this article are those of the authors and do not necessarily reflect the views or policies of the authors’ affiliated institutions.
REFERENCES:
- 1.Kim ES, Bruinooge SS, Roberts S, et al. Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(33):3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin S, Pazdur R, Sridhara R. Re-Evaluating Eligibility Criteria for Oncology Clinical Trials: Analysis of Investigational New Drug Applications in 2015. J Clin Oncol. 2017;35(33):3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 4.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of Chemotherapeutic Agents. Edited by: MacLeod CM. 1949. In: New York: Columbia University Press. [Google Scholar]
- 5.Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5(5):620–630. [DOI] [PubMed] [Google Scholar]
- 6.Carey MS, Bacon M, Tu D, Butler L, Bezjak A, Stuart GC. The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecol Oncol. 2008;108(1):100–105. [DOI] [PubMed] [Google Scholar]
- 7.Ishii H, Okada S, Nose H, Yoshimori M, Aoki K, Okusaka T. Prognostic factors in patients with advanced pancreatic cancer treated with systemic chemotherapy. Pancreas. 1996;12(3):267–271. [DOI] [PubMed] [Google Scholar]
- 8.Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer. 2010;46(1):72–83. [DOI] [PubMed] [Google Scholar]
- 9.Kodaira M, Takahashi S, Yamada S, et al. Bone metastasis and poor performance status are prognostic factors for survival of carcinoma of unknown primary site in patients treated with systematic chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(6):1163–1167. [DOI] [PubMed] [Google Scholar]
- 10.Tas F, Sen F, Odabas H, Kilic L, Keskin S, Yildiz I. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. 2013;18(5):839–846. [DOI] [PubMed] [Google Scholar]
- 11.Arboe B, Halgren Olsen M, Duun-Henriksen AK, et al. Prolonged hospitalization, primary refractory disease, performance status and age are prognostic factors for survival in patients with diffuse large B-cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation. Leuk Lymphoma. 2018;59(5):1153–1162. [DOI] [PubMed] [Google Scholar]
- 12.Song T, Wan Q, Yu W, et al. Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget. 2017;8(58):98974–98984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JR, Habbous S, Espin-Garcia O, et al. Comorbidity and performance status as independent prognostic factors in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(5):736–742. [DOI] [PubMed] [Google Scholar]
- 14.Langer C, Li S, Schiller J, et al. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(4):418–423. [DOI] [PubMed] [Google Scholar]
- 15.Lilenbaum R, Villaflor VM, Langer C, et al. Single-agent versus combination chemotherapy in patients with advanced non-small cell lung cancer and a performance status of 2: prognostic factors and treatment selection based on two large randomized clinical trials. J Thorac Oncol. 2009;4(7):869–874. [DOI] [PubMed] [Google Scholar]
- 16.Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(23):2849–2853. [DOI] [PubMed] [Google Scholar]
- 17.Spigel DR, Hainsworth JD, Joseph MJ, et al. Randomized phase 2 trial of pemetrexed, pemetrexed/bevacizumab, and pemetrexed/carboplatin/bevacizumab in patients with stage IIIB/IV non-small cell lung cancer and an Eastern Cooperative Oncology Group performance status of 2. Cancer. 2018;124(9):1982–1991. [DOI] [PubMed] [Google Scholar]
- 18.Chow R, Bruera E, Temel JS, Krishnan M, Im J, Lock M. Inter-rater reliability in performance status assessment among healthcare professionals: an updated systematic review and meta-analysis. Support Care Cancer. 2020. [DOI] [PubMed]
- 19.Broderick JM, Hussey J, Kennedy MJ, DM OD. Patients over 65 years are assigned lower ECOG PS scores than younger patients, although objectively measured physical activity is no different. Journal of geriatric oncology. 2014;5(1):49–56. [DOI] [PubMed] [Google Scholar]
- 20.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosn M, Ibrahim T, El Rassy E, Nassani N, Ghanem S, Assi T. Abridged geriatric assessment is a better predictor of overall survival than the Karnofsky Performance Scale and Physical Performance Test in elderly patients with cancer. J Geriatr Oncol. 2017;8(2):128–132. [DOI] [PubMed] [Google Scholar]
- 22.Network ACSCA. Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer: A Landscape Report. In:2018.
- 23.Lara PN Jr., Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. [DOI] [PubMed] [Google Scholar]
- 24.Ludmir EB, Mainwaring W, Lin TA, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed]
- 25.Canoui-Poitrine F, Lievre A, Dayde F, et al. Inclusion of Older Patients with Cancer in Clinical Trials: The SAGE Prospective Multicenter Cohort Survey. Oncologist. 2019;24(12):e1351–e1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biomedical USNCftPoHSo, Research B. The Belmont report: ethical principles and guidelines for the protection of human subjects of research. Vol 2: The Commission; 1978. [Google Scholar]
- 27.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(2):494–502. [DOI] [PubMed] [Google Scholar]
- 28.Azad AA, Eigl BJ, Leibowitz-Amit R, et al. Outcomes with abiraterone acetate in metastatic castration-resistant prostate cancer patients who have poor performance status. Eur Urol. 2015;67(3):441–447. [DOI] [PubMed] [Google Scholar]
- 29.Blackhall F, Ross Camidge D, Shaw AT, et al. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2(3):e000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh RB, Galsky MD, Gyawali B, et al. Trends in Checkpoint Inhibitor Therapy for Advanced Urothelial Cell Carcinoma at the End of Life: Insights from Real-World Practice. Oncologist. 2019. [DOI] [PMC free article] [PubMed]
- 31.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(22):4626–4631. [DOI] [PubMed] [Google Scholar]
- 32.Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015. [DOI] [PubMed]
- 33.Levit LA, Singh H, Klepin HD, Hurria A. Expanding the Evidence Base in Geriatric Oncology: Action Items From an FDA-ASCO Workshop. J Natl Cancer Inst. 2018;110(11):1163–1170. [DOI] [PubMed] [Google Scholar]
- 34.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. [DOI] [PubMed] [Google Scholar]
- 35.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Gruenigen VE, Huang HQ, Beumer JH, et al. Chemotherapy completion in elderly women with ovarian, primary peritoneal or fallopian tube cancer–An NRG oncology/Gynecologic Oncology Group study. Gynecologic oncology. 2017;144(3):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. [DOI] [PubMed] [Google Scholar]
- 39.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 40.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 41.Enhancing the Diversity of Clinical Trial Populations - Eligiblity Criteria, Enrollment Practices, and Trial Designs Guidance for Industry - Guidance Document. Published 2019. Accessed 2/3/2020.
- 42.Beaver JA, Ison G, Pazdur R. Reevaluating Eligibility Criteria - Balancing Patient Protection and Participation in Oncology Trials. N Engl J Med. 2017;376(16):1504–1505. [DOI] [PubMed] [Google Scholar]
- 43.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(24):2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nipp RD, Yao NA, Lowenstein LM, et al. Pragmatic study designs for older adults with cancer: Report from the U13 conference. Journal of geriatric oncology. 2016;7(4):234–241. [DOI] [PubMed] [Google Scholar]
- 45.Kluetz PG, Slagle A, Papadopoulos EJ, et al. Focusing on Core Patient-Reported Outcomes in Cancer Clinical Trials: Symptomatic Adverse Events, Physical Function, and Disease-Related Symptoms. Clin Cancer Res. 2016;22(7):1553–1558. [DOI] [PubMed] [Google Scholar]
- 46.Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. 2019;134(4):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J Oncol. 2016;2016:6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng S, Qureshi M, Pullenayegum E, Haynes A, Chan KK. Do patients with reduced or excellent performance status derive the same clinical benefit from novel systemic cancer therapies? A systematic review and meta-analysis. ESMO Open. 2017;2(4):e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]


