Abstract
While there is significant investigation and investment in brain and neurodegenerative disease research, current understanding of the etiologies of illnesses like Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and brain cancer remains limited. Environmental exposure to the pollutant formaldehyde, an emerging neurotoxin widely used in industry, is suspected to play a critical role in mediating these disorders, although findings are limited and inconsistent. Focusing on highly exposed groups, we performed a meta-analysis of human epidemiological studies of formaldehyde and neurodegenerative disease (N = 19) or brain tumors (N = 12). To assess the biological plausibility of observed associations, we then conducted a bioinformatics analysis using WikiPathways and the Comparative Toxicogenomics Database and identified candidate genes and pathways that may be related to these interactions. We reported the meta-relative risk (meta-RR) of ALS following high exposures to formaldehyde was increased by 78% (meta-RR = 1.78, 95% confidence interval, CI 1.20–2.65). Similarly, the meta-RR for brain cancer was increased by 71% (meta-RR = 1.71; 95% CI 1.07–2.73) among highly exposed individuals. Multiple sensitivity analyses did not reveal sources of heterogeneity or bias. Our bioinformatics analysis revealed that the oxidative stress genes superoxide dismutase (SOD1, SOD2) and the pro-inflammatory marker tumor necrosis factor (TNF) were identified as the top relevant genes, and the folate metabolism, vitamin B12 metabolism, and the ALS pathways were highly affected by formaldehyde and related to the most brain diseases of interest. Further inquiry revealed the two metabolic pathways are also intimately tied with the formaldehyde cycle. Overall, our bioinformatics analysis supports the link of formaldehyde exposure to ALS or brain tumor reported from our meta-analysis. This new multifactorial approach enabled us to both interrogate the robustness of the epidemiological data and identify genes and pathways that may be involved in these interactions, ultimately lending strong evidence and potential biological plausibility for the association between formaldehyde exposure and brain disease.
Keywords: Neurodegenerative disease, Amyotrophic lateral sclerosis, Brain tumor, Human exposures, Comparative toxicogenomics database, WikiPathways
Background and Significance
Formaldehyde Exposure is Universal and Carcinogenic
Formaldehyde is ubiquitous in the environment and our bodies. In most organisms, including humans, formaldehyde is endogenously produced through amino acid and methanol metabolism, lipid peroxidation, demethylation of DNA, RNA, and histones (EFSA 2014; Swenberg et al. 2011) and is either consumed as an intermediate in the “one-carbon pool” (Neuberger 1981) or used for the biosynthesis of purines, thymidine, and some amino acids. It is reportedly kept at a concentration of approximately 80–100 μM in the blood (Casanova et al. 1988; Heck et al. 1985) with a delicate equilibrium maintained by endogenous metabolic pathways. Commercially, formaldehyde is indispensable for many products and processes that are important to the world’s economy (Tang et al. 2009). From its use in embalming to hair relaxation to the manufacture of plastics and composite wood products, the global formaldehyde production is rapidly growing and is projected to reach 36.6 million tons in 2026 (Bhisey 2026). Thus, chronic exogenous exposure to formaldehyde, a major public health concern, is known to cause nasopharyngeal cancer and myeloid leukemia in humans (IARC 2012). However, potential links between the exposure and brain cancers and other neural disorders are less understood.
Formaldehyde Has the Potential to Damage the Brain
Exposure to formaldehyde via inhalation is known to impair memory (Bach et al. 1990; Kilburn et al. 1987) and cognitive functions (Kilburn et al. 1985; Perna et al. 2001) in humans, to cause deficits in learning and memory (Qiang et al. 2014), neuronal damage (Sarsilmaz et al. 2007), and oxidative stress in the cerebellum (Songur et al. 2008) of experimental animals, and to induce misfolded neuronal tau and related proteins (Nie et al. 2007) in vitro. These formaldehyde-associated alterations and neurotoxicity have raised questions about its role in modulating diseases of the central nervous system (CNS), such as neurodegenerative disease (NDD) and brain tumor (BT).
Although the two are distinct pathological disorders, NDD and BT are suspected to share common mechanisms of genetic and molecular abnormalities (Du and Pertsemlidis 2011), indicating that the mechanisms of these seemingly dichotomous diseases may converge in the dysregulation of gene expression and at the post-translational level. For example, increased lipid peroxidation, a biomarker of oxidative stress, was found in animal models of Alzheimer’s disease (AD) (Pratico et al. 2001) and Huntington’s disease (Perez-De La Cruz et al. 2009), and in patients with Parkinson’s disease (PD) (Dexter et al. 1989) and brain cancer (Popov et al. 2003).
Formaldehyde Inhalation and Olfactory Function
A major route of exogenous exposure to formaldehyde in humans is via inhalation, where it could come in contact with and damage the olfactory bulb, an outgrowth of the forebrain specialized for the processing of signals that give rise to the sense of smell (Shepherd and Greer 1998). Repeated formaldehyde inhalation exposure impairs olfactory function in humans (Kilburn et al. 1985; Holmstrom and Wilhelmsson 1988; Hisamitsu et al. 2011; Edling et al. 1988), which has been linked to PD (Doty et al. 1988; Tissingh et al. 2001), amyotrophic lateral sclerosis (ALS) (Viguera et al. 2018), AD (McCaffrey et al. 2000; Morgan et al. 1995), and BT (Daniels et al. 2001). Given formaldehyde’s ability to damage the olfactory system that appears to be intimately tied to both NDD and brain cancer, here, we will focus on the effect of inhalation to formaldehyde on the brain in this study.
Formaldehyde Exposure and Brain Disorders
We previously reported the growing evidence that formaldehyde exposure may contribute to the risk of brain cancer (Zhang and Rana 2018a) and NDD (Zhang and Rana 2018b). A number of epidemiological studies conducted with professional workers, such as anatomists, pathologists, embalmers, and funeral home workers, and in industrial workers have analyzed the association between formaldehyde exposure and brain cancer, although the findings remain limited and inconsistent. In 2012, the International Agency for Research on Cancer (IARC) noted that despite several studies identifying statistically significant positive associations between exposure to formaldehyde and brain cancer, the results are inconsistent (IARC 2012). To the best of our knowledge, no authoritative entities have evaluated formaldehyde’s ability to cause NDD, though some studies have been suggestive.
Investigators in China have shown an association between mean endogenous levels of formaldehyde in normal, mild cognitive impairment, and AD patients (Tong et al. 2017). Other studies have reported the genesis of AD-like neuropathology in primates treated with methanol (Yang et al. 2014), a precursor of formaldehyde. The Western Pacific ALS-parkinsonism-dementia complex (ALS-PDC) disorder (Guam, Kii Peninsula, Honshu Island, Japan, and Papua, Indonesia on the island of New Guinea) is strongly associated with exposure to cycad seed genotoxins, namely methylazoxymethanol (the aglycone of cycasin) and beta-aminomethyl-L-alanine (L-BMAA), both of which are metabolized to formaldehyde (Spencer 2019; Spencer et al. 2012). Most recently, investigators in China reported that excess formaldehyde impaired memory in patients with mutations in formaldehyde metabolism enzyme ALDH2 and in the ALDH2 deficient mice (Ai et al. 2019).
To determine whether exogenous exposure to formaldehyde is associated with increased risk of NDD or brain cancer, we conducted a meta-analysis of all studies of formaldehyde-exposed workers. We then applied a bioinformatics analysis to identify candidate genes and pathways associated with both formaldehyde exposure and NDD/BT with the aim of investigating underlying mechanisms.
Meta-Analysis Approach to Evaluate the Human Evidence
Meta-analysis Objective
Given formaldehyde’s documented neurotoxicity in vivo (Sarsilmaz et al. 2007; Lu et al. 2008; Aslan et al. 2006; Tulpule and Dringen 2011) and in vitro (Nie et al. 2007; Tulpule et al. 2012; Song et al. 2010; Tang et al. 2013), we hypothesized formaldehyde plays a role in NDD and possibly brain cancer. We investigated this process by carefully reviewing the epidemiological evidence and performing a meta-analysis to examine the association between human exposure to high levels of formaldehyde and NDD and brain cancer. Meta-analysis provides a method for reviewing the current literature on a topic of interest, identifying heterogeneity in the literature and its possible sources, and evaluating major aspects of causal inference including the magnitude of the association, dose-response, and the possible presence and extent of bias and confounding.
Previously, two meta-analyses have reported mixed findings on formaldehyde exposure and brain cancer (Blair et al. 1990; Bosetti et al. 2008) and no meta-analysis has been conducted to date on formaldehyde-associated NDD. Our meta-analysis on brain tumors differs from previous reports by including a number of updated cohort studies (Beane Freeman et al. 2013; Coggon et al. 2014; Hauptmann et al. 2009; Meyers et al. 2013), multiple new analyses of heterogeneity and bias, and a focus on groups with higher formaldehyde exposure if available by employing an a priori hypothesis targeting exposure magnitude (A Priori Approach and Exposure Categories).
Searching and Identifying Relevant Human Studies
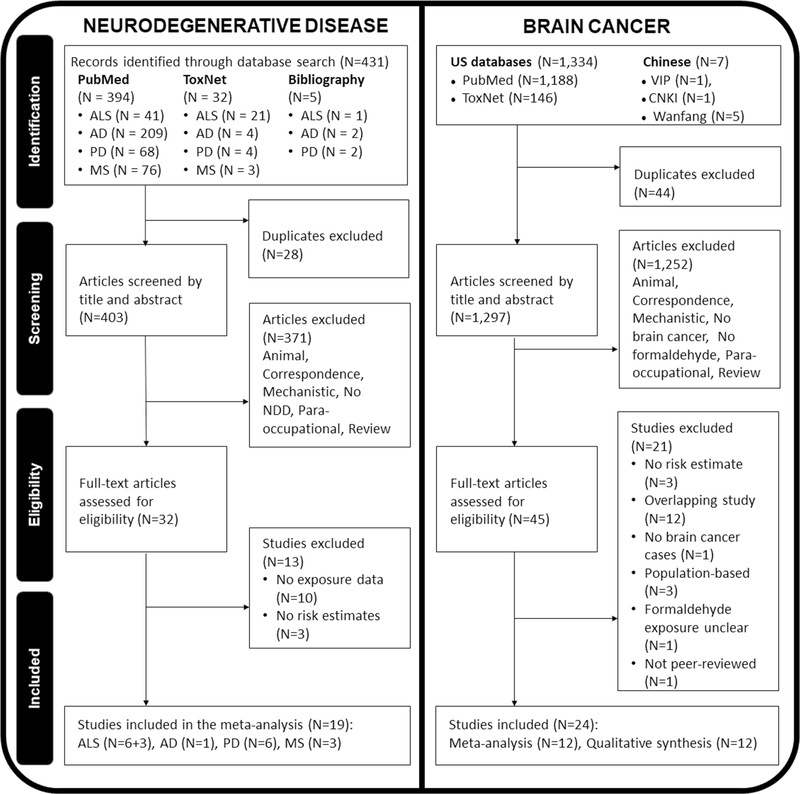
The literature search was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA, Moher D, Liberati A, Tetzlaff J, Altman DG, Group P 2009). The screening process and results are shown in Fig. 1. We conducted a systematic electronic literature review using PubMed in July 2018, which was updated in May 2019. We performed electronic searches of online databases (PubMed, Web of Science Core Collection, Biosis Previews, Embase, Google Scholar, ToxNet, Chinese National Knowledge Infrastructure, CNKI, Wanfang Data, and Chongqing VIP Information, CQVIP) using the search terms, inclusion, and exclusion criteria outlined in Supplementary Section A1.
Fig. 1.
Study selection process for meta-analyses using PRISMA guidelines
Selection of NDD Studies
We identified 394 studies from PubMed, 32 studies from ToxNet, and 5 studies from scanning bibliographies. After 28 duplicates were removed, 403 studies were primarily screened. Of these studies, 371 were excluded because they were reviews, correspondence, animal, mechanistic or para-occupational studies, or did not include the relevant exposure or outcome of interest (Fig. 1). When the final 32 studies were identified, 13 studies were further excluded for lacking clear formaldehyde exposure data or relative risk (RR) estimates. Finally, 19 NDD studies were selected, among which there were nine (6 + 3, detailed in Exposure Assessment and Estimate) studies on ALS, six on PD, one on AD, and three on multiple sclerosis (MS).
Exposure Assessment and Estimate
The NDD studies selected for the meta-analysis are described in Table 1. Six of the ALS studies conducted an exposure assessment specific to formaldehyde, defined as having a structured interview (Fang et al. 2009), job exposure matrix (Peters et al. 2017; Roberts et al. 2016; Seals et al. 2017), standardized questionnaire (Weisskopf et al. 2009), or studying a cohort of workers with known formaldehyde exposure (Pinkerton et al. 2013). An additional three studies of ALS lacked a formaldehyde exposure assessment (Chiò et al. 1991; Deapen and Henderson 1986; Gunnarsson et al. 1991), instead reporting only job title as "ever exposed" (Table 1).
Table 1.
Selected studies and their results used in our meta-analyses of neurodegenerative disease and brain tumors
| Study | Location | Population | Design | Type | RE | CIL | CIU | Cases | Exposure level | Weighta | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyotrophic lateral sclerosis (N = 9) | |||||||||||
| Fang et al. (2009) | USA | NEMC and BWH | CC | OR | 3.0 | 0.7 | 12.9 | 4 | > 60,000 h weighted | 6.04 | |
| Peters et al. (2017) | Sweden | National Patient Register | CC | OR | 1.22 | 0.95 | 1.57 | 123 | > 0.013 mg/m3 | 19.22 | |
| Pinkerton et al. (2013) | USA | Garment workers | Cohort | SMR | 0.94 | 0.19 | 2.75 | 3 | > 10-year duration | 6.94 | |
| Roberts et al. (2016) | USA | National Longitudinal Mortality Study | Cohort | HR | 4.43 | 1.16 | 16.85 | 2 | High intensity/probability | 6.93 | |
| Seals et al. (2017) | Denmark | Danish National Patient Register | CC | rr | 1.3 | 1.1 | 1.5 | 266 | > 0.28 ppm | 29.28 | |
| Weisskopf et al. (2009) | USA | ACSCPSII | Cohort | rr | 4.1 | 2.2 | 7.1 | 13 | > 10-year duration | 31.58 | |
| Chiò et al. (1991) b | Italy | Textile workers | CC | OR | 1.2 | 0.44c | 2.61c | 6 | Ever based on job title | N/Ab | |
| Deapen and Henderson (1986) b | USA | Plastics manufacturing | CC | OR | 3.7 | 1 | 20.5 | 11 | Ever based on job title | N/Ab | |
| Gunnarsson et al. (1991)b | Sweden | Male textile workers | CC | OR | 0.1 | 0.3 | 0.7 | 1 | Ever based on job title | N/Ab | |
| Male carpenter, woodworker | CC | OR | 1.0 | 0.6 | 1.5 | 55 | Ever based on job title | N/Ab | |||
| Female textile workers | CC | OR | 0.4 | 0.1 | 1.6 | 3 | Ever based on job title | N/Ab | |||
| Parkinson’s disease (N = 6) | |||||||||||
| Chaturvedi et al. (1995) | Canada | Hobby exposure plastic resins | CC | OR | 8.79 | 2.43 | 25.89 | 9 | Ever based on hobby | 0.9 | |
| Li et al. (2009) | Sweden | Male textile workers | Coh | SIR | 1.03 | 0.61 | 1.63 | 18 | Three censuses (job title)c | 5.1 | |
| Male woodworkers | Coh | SIR | 1.29 | 1.08 | 1.54 | 126 | Three censuses (job title)c | 39.5 | |||
| Female textile workers | Coh | SIR | 1.03 | 0.87 | 1.21 | 146 | Ever based on job title | 45.7 | |||
| Female woodworkers | Coh | SIR | 0.66 | 0.30 | 1.26 | 9 | Ever based on job title | 2.4 | |||
| Nee et al. (1991) | USA | Plastic monomers, additives worker | CC | OR | 5.25 | 2.79d | 8.98d | 13 | Ever based on job title | 3.6 | |
| Hertzman et al. (1990) | Canada | Planer mills workers | CC | OR | 4.11 | 0.91 | 18.5 | 8 | Ever based on job title | 0.5 | |
| Vanacore et al. (2005) | Europe | Wood and furniture worker | CC | OR | 0.43 | 0.04 | 5.17 | 1 | Ever based on job title | 0.7 | |
| Textile and clothing worker | CC | OR | 2.19 | 0.56 | 8.52 | 7 | Ever based on job title | N/A | |||
| Tanner et al. (1989) | China | O/R textile manufacture | CC | OR | 1.90 | 0.79 | 4.67 | unk | Ever based on O/R | 1.6 | |
| O/R lumber | CC | OR | 0.87 | 0.13 | 2.19 | unk | Ever based on O/R | N/Ab | |||
| O/R plastic | CC | OR | 1.62 | 0.61 | 4.31 | unk | Ever based on O/R | N/Ab | |||
| O/R paper mills | CC | OR | 1.08 | 0.17 | 6.76 | unk | Ever based on O/R | N/Ab | |||
| Multiple sclerosis (N = 3) | |||||||||||
| Li et al. (2008) | Sweden | Male textile workers | Coh | SIR | 0.31 | 0.00 | 1.80 | 1 | Three censuses | 56.0 | |
| Female textile workers | Coh | SIR | 1.34 | 0.75 | 2.21 | 15 | Three censuses | 16.6 | |||
| Male woodworkers | Coh | SIR | 0.88 | 0.51 | 1.41 | 17 | Three censuses | 18.7 | |||
| Dubrow and Gute (1988) | USA | Male textile workers | Coh | PMR | 1.83 | 0.67c | 3.98c | 6 | Ever based on job title | 6.1 | |
| Zorzon et al. (2003) | Italy | O/R formalin | CC | OR | 0.5 | 0.1 | 1.5 | 4 | Ever based on job title | 2.6 | |
| Woodworkers | CC | OR | 1.9 | 0.6 | 6.5 | 8 | Ever based on job title | N/Ab | |||
| Alzheimer’s disease (N = 1) | |||||||||||
| Tyas et al. (2001) | Canada | Liquid plastics and rubbers workers | Cohort | RR | 1.01 | 0.12 | 8.38 | 1 | Ever based on job title | N/Ab | |
| Glues, adhesives workers | Cohort | RR | 1.41 | 0.49 | 4.05 | 5 | Ever based on job title | N/Ab | |||
| Brain tumor: professional workers (N = 5) | |||||||||||
| Hall et al. (1991) | UK | Pathologists | Cohort | SMR | 2.18 | 0.80 | 4.75 | 6 | Ever | 10.87 | |
| Hauptmann et al. (2009) | USA | Embalmers | CC | OR | 1.7 | 0.5 | 5.3 | 12 | Peak ≥ 9.3 ppm | 8.38 | |
| Levine et al. (1984) | Canada | Undertakers | Cohort | SMR | 1.15 | 0.24 | 3.51 | 3 | Ever | 7.25 | |
| Stroup et al. (1986) | USA | Anatomists | Cohort | SMR | 3.9 | 1.0 | 9.9 | 9 | Gross anatomists | 8.64 | |
| Wang et al. (1989) | China | Anatomists | Cohort | SMR | 8.27 | 1.00 | 29.86 | 2 | Ever | 5.34 | |
| Brain tumor: industrial workers (N = 7) | |||||||||||
| Andjelkovich et al. (1995) | USA | Iron foundry workers | Cohort | SMR | 0.62 | 0.07 | 2.23 | 2 | Ever | 5.20 | |
| Beane Freeman et al. (2013) | USA | Industrial plant workers | Cohort | SMR | 0.87 | 0.49 | 1.56 | 20 | Peak ≥ 4.0 ppm | 14.05 | |
| Coggon et al. (2014) | UK | Chemical factory workers | CC | SMR | 0.56 | 0.24 | 1.11 | 8 | > 2.0 ppm | 12.10 | |
| Fondelli et al. (2007) | Italy | Library restoration workers | Cohort | SMR | 10.03 | 1.22 | 36.24 | 2 | Ever | 5.35 | |
| Jiang et al. (1990) | China | Resin factory workers | Cohort | SMR | 3.9 | 0.05 | 21.7 | 1 | Ever | 2.10 | |
| Meyers et al. (2013) | USA | Garment plant workers | Cohort | SMR | 1.72 | 0.86 | 3.08 | 11 | Duration 10+ years | 13.43 | |
| Wong and Gibson (1983) | USA | Formaldehyde plant workers | Cohort | SMR | 2.13 | 0.43 | 6.23 | 3 | Hired before 1961 | 7.28 | |
ACSCPII American Cancer Society Cancer Prevention Study II, BWH Brigham and Women’s Hospital, CC case-control, CIL lower 95% confidence interval, CIU upper 95% confidence interval, CS cross-sectional, hr hour, N/A not applicable, NEMC New England Medical Center, OR odds ratio, O/R occupational or residential exposure, RR relative risk, rr rate ratio, SIR standardized incidence ratios, SMR standardized mortality ratio, unk unknown.
Weight given to each study in the random effects model
Evaluated in sensitivity analyses only
Data for multiple censuses only reported for men
Calculated by us
Similarly, all epidemiological studies of PD, MS, and AD lacked a clear formaldehyde exposure assessment, relying on job titles, census data, hobbies, or occupational/residential exposure to activities with known formaldehyde exposure (Table 1). Given these individuals were likely exposed to formaldehyde and that the frequency and intensity of these exposures are unknown, we defined these groups as having “formaldehyde exposure by proxy.” The analogous group in studies with a formaldehyde exposure assessment would be “ever exposed,” defined as individuals with any type of exposure to formaldehyde at any level.
Due to the limited number of studies on NDD, we chose to include studies with estimated exposure assessment (proxy exposures). The studies that assessed formaldehyde exposure by proxy were used in (1) the ALS sensitivity analysis; (2) the analysis of PD and NDD, as no other studies of these outcomes; and (3) in an extended meta-analysis of all NDDs involving workers in occupations known to have formaldehyde exposure, namely studies of individuals with exposure to plastics (Deapen and Henderson 1986; Chaturvedi et al. 1995; Nee et al. 1991; Tanner et al. 1989; Tyas et al. 2001), woodwork (Gunnarsson et al. 1991; Tanner et al. 1989; Li et al. 2008, 2009; Vanacore et al. 2005; Zorzon et al. 2003; Hertzman et al. 1990), and textiles (Chiò et al. 1991; Gunnarsson et al. 1991; Tanner et al. 1989; Li et al. 2008, 2009; Vanacore et al. 2005; Dubrow and Gute 1988).
Regions of NDD Studies Selected
For ALS, five studies were conducted in the USA, two were conducted in Sweden, one was conducted in Italy, and one was conducted in Denmark. For PD, two studies were in Canada, one study was conducted in China, one was in Sweden, one was in the USA, and one was conducted across many countries in Europe. For MS, one study was in Sweden, one study was in the USA, and one study was in Italy. The only study of AD was in Canada.
Selection of Brain Tumor Studies
Following thorough searches from multiple resources and excluding 44 duplicates, we screened a total of 1,297 articles by title and abstract (Fig. 1). We excluded 1,252 articles that used animal or mechanistic study designs, lacked the exposure or outcome of interest, had para-occupational exposure, or were correspondence or a review, leaving 45 articles for full text review. Of these studies, 12 studies with 67,819 participants were eligible for inclusion in the meta-analysis. Table 1 also summarizes these selected brain cancer studies. Because brain cancer is a diverse cancer that may be defined differently among studies, the ICD codes from these studies have been reported in Supplementary Table 1.
There were five studies of professional workers and seven studies of industrial workers (Table 1). Two studies were case-control design (Coggon et al. 2014; Hauptmann et al. 2009), and ten were cohort studies. Six studies were conducted in the USA, one study was in Canada, two studies were in the UK, one study was in Italy, and two studies were in China.
Study Quality Evaluation
The methodological quality of the cohort and case-control studies included in the meta-analyses was assessed using the Newcastle Ottawa Scale (NOS) (Wells et al. 2009). Cohort studies (Supplementary Table 2) were evaluated based on the representativeness of the cohort, selection of the controls, ascertainment of exposure, outcome diagnosis at start of study, comparability of cohort on the basis of controlling for age, assessment of disease outcome, sufficiency of follow-up length, and response rate. Case-control studies (Supplementary Table 3) were evaluated on the validation of cases, representativeness of cases, selection of controls, absence of disease in the controls, whether the study controlled for age, exposure assessment, concordance of method among cases and controls, and similarity of response rate among both groups. A total of twelve points were available, and full results of the quality analysis are reported in Supplementary Section A2.
A Priori Approach and Exposure Categories
Our focus on groups with high formaldehyde exposure is based on the principle that, in general, if a true association exists, higher exposures are likely to yield higher relative risk estimates (Rom and Markowitz 2007). Including people with very low exposure in the exposed group can dilute relative risk estimates. All else being equal, higher relative risks are associated with greater statistical power and are less likely to be due to relatively minor biases or confounding (Hill 1965). Since our main goal is to evaluate the potential association of the exposure and risk of the disease outcomes and not to conduct a precise dose-response assessment or to evaluate risks in people with low exposures, our focus was on the groups with the highest available formaldehyde exposure. This a priori approach has been previously employed to estimate meta-risks for benzene (Steinmaus et al. 2008), formaldehyde (Zhang et al. 2009; Duong et al. 2011), and other agents (Welling et al. 2015; Zhang et al. 2019; Smith et al. 2017). Although the focus was on higher exposures (e.g., occupationally exposed cohorts), studies involving lower exposures (e.g., studies done in the general population) were also evaluated in sensitivity analyses.
Some studies reported multiple RRs or ORs for different exposure categories (high, “ever exposure,” or proxy exposures; see Exposure Assessment and Estimate). Based on our a priori hypothesis, when multiple RRs or ORs were given, we selected estimates in the following order: (1) peak exposure, (2) average exposure intensity, (3) cumulative exposure, (4) exposure duration, (5) earliest year of hire, and (6) ever exposure. We prioritized peak exposure because metrics like average intensity and cumulative exposure may be less accurate measures of true exposure if workers and/or professionals (such as embalmers) with periods of very high exposure also have intervening time periods with little or no exposure. We evaluated the impact of our a priori exposure selection criteria in sensitivity analyses. We conducted a separate meta-analysis of individuals with “ever exposure,” to assess the magnitude of potential bias caused by adding subjects with low exposures in the current literature. We also compared "ever exposure" with our a priori hypothesis to assess whether a representative dose-response relationship might be identifiable. Table 1 reports the results and weights of the studies used in the NDD and brain tumor meta-analyses.
Statistical Methods
We calculated overall summary estimates and weights of the studies using both the fixed effects inverse variance method (Greenland 1998) and the random effects method (DerSimonian and Laird 1986). Heterogeneity was evaluated using the general variance-based method (Petitti 1994). If heterogeneity was present, the random effects model was used. One benefit of the random effects model is the ability to incorporate between study variance into the summary variance estimate and confidence intervals (CI), which may help prevent artificially narrow CIs resulting from use of the fixed effects model in the presence of between-study heterogeneity (Petitti 1994). We evaluated publication bias through funnel plots, Egger’s test, and Begg’s test (Begg and Mazumdar 1994; Egger et al. 1997). All meta-analysis and statistical analysis were conducted with Stata IC 15.1 (StataCorp 2017) and Microsoft Excel 2013 (Corporation 2013).
Meta-analysis Findings
We first report our major findings for formaldehyde-associated ALS then the results of sensitivity analyses for ALS. Other subtypes of NDD, such as PD and MS, are also reported. Next, we report the findings of the meta-analysis on brain cancer with a detailed sensitivity analysis. A greater portion of this section is devoted to this discussion because we have identified more studies on brain tumor and there are previous meta-analyses present for comparison.
Formaldehyde Exposure Increases Risk of NDD
Major ALS Findings
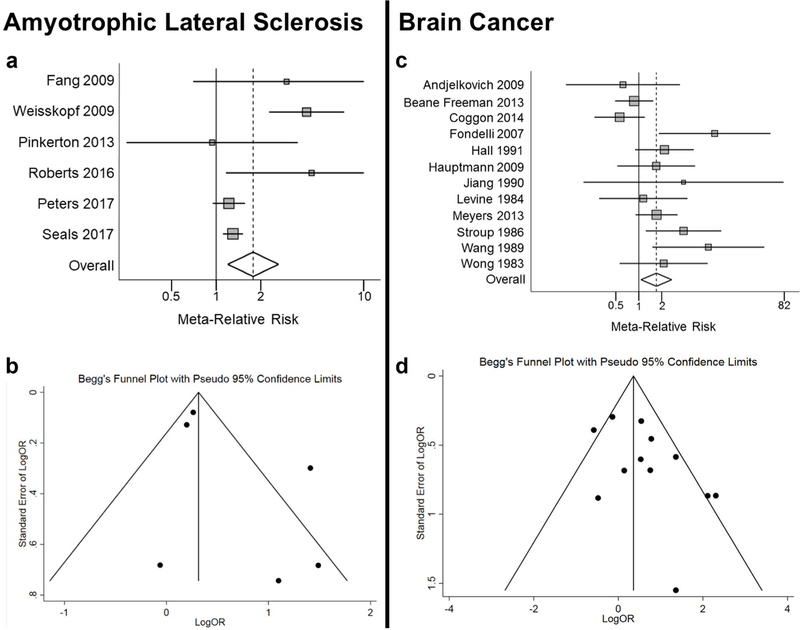
The findings of our meta-analysis of NDD are presented in Table 2. As discussed above in Exposure Assessment and Estimate, only six of nine studies of ALS had formaldehyde exposure assessments (Fang et al. 2009; Peters et al. 2017; Roberts et al. 2016; Seals et al. 2017; Weisskopf et al. 2009; Pinkerton et al. 2013). When examining the most highly exposed groups, we found a meta-relative risk (meta-RR) of 1.78 with a 95% CI of 1.20 to 2.65 (Fig. 2a). While there was somewhat high heterogeneity, we note that large majority of studies (5 of 6) have RRs that are greater than 1.0.
Table 2.
Meta-analysis results of amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases (NDD)
| Fixed effects model | Random effects modela | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Analysis | N | Meta-RR | CIL | CIU | Meta-RR | CIL | CIU | X2 | P |
| Amyotrophic lateral sclerosis (ALS) | |||||||||
| Main-highest peak exposure | 6 | 1.37 | 1.21 | 1.56 | 1.78 | 1.20 | 2.65 | 19.08 | < 0.01 |
| Ever exposureb | 6 | 1.25 | 1.17 | 1.33 | 1.17 | 0.93 | 1.47 | 20.59 | < 0.01 |
| Including studies with proxy exposurec | 11 | 1.10 | 0.98 | 1.23 | 1.18 | 0.65 | 2.17 | 158.33 | < 0.01 |
| Exclude one ALS studyd | |||||||||
| Fang et al. (2009) | 5 | 1.36 | 1.20 | 1.55 | 1.73 | 1.14 | 2.60 | 17.96 | < 0.01 |
| Weisskopf et al. (2009) | 5 | 1.30 | 1.14 | 1.48 | 1.31 | 1.08 | 1.59 | 4.96 | 0.29 |
| Pinkerton et al. (2013) | 5 | 1.37 | 1.21 | 1.56 | 1.89 | 1.24 | 2.88 | 18.77 | < 0.01 |
| Roberts et al. (2016) | 5 | 1.36 | 1.19 | 1.54 | 1.65 | 1.12 | 2.45 | 16.10 | < 0.01 |
| Peters et al. (2017) | 5 | 1.43 | 1.23 | 1.65 | 2.24 | 1.09 | 4.61 | 17.98 | < 0.01 |
| Seals et al. (2017) | 5 | 1.52 | 1.22 | 1.90 | 2.21 | 1.05 | 4.66 | 17.75 | < 0.01 |
| Other NDD subtypes | |||||||||
| Parkinson’s diseaseb | 9 | 1.23 | 1.10 | 1.38 | 1.70 | 1.17 | 2.48 | 46.49 | < 0.01 |
| Multiple sclerosisb | 5 | 0.54 | 0.43 | 0.68 | 0.80 | 0.37 | 1.73 | 35.33 | < 0.01 |
| Total NDD in EE studies | |||||||||
| Plastics | 5 | 4.06 | 2.63 | 6.25 | 3.64 | 1.84 | 7.19 | 7.44 | 0.11 |
| Wood workersb | 8 | 1.19 | 1.02 | 1.38 | 1.10 | 0.86 | 1.42 | 9.35 | 0.23 |
| Textile workersb | 12 | 0.73 | 0.65 | 0.83 | 0.77 | 0.44 | 1.34 | 158.22 | < 0.01 |
CI confidence interval, EE exposure estimated, NDD neurodegenerative disease, N number of studies, meta-RR meta-analysis relative risk
Random effects model was used when X2 heterogeneity statistic > degrees of freedom (number of studies minus 1)
Not reported in Roberts et al. 2016 and was calculated by the authors
Includes additional papers where formaldehyde exposure was not clearly defined but included professions with known exposure (plastics manufacturing, woodwork, and textile work)
One study excluded at a time.
Fig. 2.
Forest plot of ALS meta-analysis using random effects model a and funnel plot of ALS studies b. Forest plot of brain tumor meta-analysis using random effects model c and funnel plot of brain tumor d
The funnel plot revealed some evidence of asymmetry consistent with publication bias (Fig. 2b), although the number of studies is small and there was no evidence of bias in the in Egger’s (p = 0.204) or Begg’s tests (p = 0.188).
ALS Sensitivity Analysis
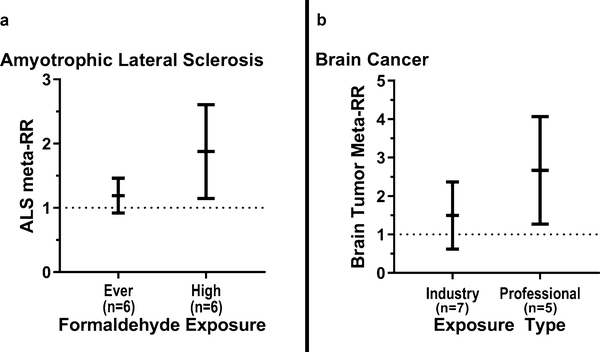
Examining “ever exposure” revealed a lower meta-RR of 1.17 (95% CI 0.93–1.47), in comparison with our a priori “high exposures” (meta-RR = 1.78, 95% CI 1.20–2.65; Table 2). Therefore, there was a 61% increase in meta-RR, suggesting the presence of an exposure-response relationship (Fig. 3a).
Fig. 3.
Meta-relative risk of ALS mortality a and brain cancer mortality b by level of formaldehyde exposure using random effects model. The relative risks are indicated by the black lines in the middle, and the 95% confidence intervals are indicated by the upper and lower vertical black lines
To determine the effects of adding the three ALS studies that assessed exposure by proxy, we included additional studies of individuals with known exposure to plastics, woodwork, and textiles (Chiò et al. 1991; Deapen and Henderson 1986; Gunnarsson et al. 1991) and found the risk of ALS was lower (meta-RR = 1.18, 95% CI 0.65–2.17; Table 2). When we excluded any one individual study included in our main analysis (meta-RR = 1.78), the meta-RRs of ALS in the sensitivity analysis fluctuated but did not change substantially (range 1.30 to 2.24).
Parkinson's Disease and Alzheimer's Disease
While the six ALS studies had adequate exposure assessments, all studies of PD and MS reported formaldehyde exposure by proxy (see Exposure Assessment and Estimate). PD appeared to be associated with jobs known to have formaldehyde exposure (meta-RR = 1.70, 95% CI 1.17–2.48). Although the meta-RR of PD is quite similar to the one from ALS (1.78), due to the lack of the appropriate formaldehyde exposure assessment, it remains uncertain whether the observed risk was truly attributable to formaldehyde and not to other confounding factors. Thus, one needs to interpret the PD results with caution. We could not perform a meta-analysis for AD because we only identified one AD study.
Multiple Sclerosis
We found no association for MS. It should be noted that MS, a chronic inflammatory demyelination disease of the CNS, is primarily considered an autoimmune disorder that can progress into a neurodegenerative disease (Schaeffer et al. 2015). MS was included in NDD in the meta-analysis for completeness but excluded from NDD in our mechanistic investigation with bioinformatics later (Integration of Gene-Association Data from the CTD).
Plastics Workers Have Increased Risk of NDD
When studies were stratified by type of industry, we observed a meta-RR for all NDD outcomes of interest (AD, ALS, MS, PD) of 3.64 (95% CI 1.84–7.19) for individuals exposed to plastics, a meta-RR of 1.10 (95% CI 0.86–1.42) for woodworkers, and no association for textile workers (Table 2). The lack of appropriate exposure assessments in these studies indicate these results must be interpreted with caution. Interestingly, these findings are consistent with formaldehyde exposure data in US industries, which reported average short-term formaldehyde exposures for miscellaneous plastic work, lumber, and textile industries to be 0.28, 0.27, and 0.20 mg/m3, respectively (Lavoue et al. 2008). Overall, they provide support for our hypothesis that higher formaldehyde exposure increases NDD risk.
Increased Meta-relative Risk of Brain Cancer
Next, we performed the meta-analysis and stratified sensitivity analyses on brain cancer; the results are reported in Table 3, and the forest plot is displayed in Fig. 2c. The meta-RR for all studies combined was 1.71 (95% CI 1.07–2.73). Figure 2 d shows the funnel plot for publication bias. Among all studies, there was no evidence of asymmetry consistent with obvious publication bias. Egger’s (p = 0.076) and Begg’s (p = 0.337) tests similarly did not show evidence of publication bias.
Table 3.
Major findings from brain cancer and formaldehyde exposure meta-analysis and sensitivity analysis
| Fixed effects model | Random effects modela | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Analysis | N | Meta-RR | CIL | CIU | Meta-RR | CIL | CIU | X2 | P |
| Exposure | |||||||||
| Main-highest peak exposure | 12 | 1.43 | 1.07 | 1.91 | 1.71 | 1.07 | 2.73 | 23.73 | 0.01 |
| Highest average intensity | 12 | 1.51 | 1.13 | 2.03 | 1.77 | 1.11 | 2.83 | 23.36 | 0.02 |
| Highest cumulative exposure | 12 | 1.51 | 1.13 | 2.03 | 1.72 | 1.09 | 2.70 | 21.46 | 0.03 |
| Longest duration | 12 | 1.42 | 1.06 | 1.90 | 1.75 | 1.07 | 2.84 | 24.29 | 0.01 |
| Ever exposure | 12 | 1.22 | 1.04 | 1.44 | 1.82 | 1.20 | 2.75 | 41.77 | <0.01 |
| Professionals vs. industry | |||||||||
| Professional workersb | 5 | 2.41 | 1.43 | 4.08 | 2.42 | 1.41 | 4.17 | 4.46 | 0.37 |
| Industrial workersc | 7 | 1.14 | 0.81 | 1.61 | 1.32 | 0.72 | 2.44 | 14.01 | 0.03 |
| Add population-based exposure/SPIR studies | |||||||||
| Add Dell and Teta 1995d | 14 | 1.29 | 0.99 | 1.69 | 1.47 | 0.96 | 2.25 | 27.55 | 0.01 |
| Add Lacourt et al. 2013e | 13 | 1.32 | 1.04 | 1.69 | 1.56 | 1.05 | 2.33 | 24.68 | 0.02 |
| Add Hansen and Olsen 1995f | 13 | 1.39 | 1.10 | 1.75 | 1.59 | 1.08 | 2.34 | 23.87 | 0.02 |
| Add all three | 16 | 1.25 | 1.03 | 1.53 | 1.35 | 0.99 | 1.85 | 27.96 | 0.02 |
| Hall 1991 [98] | |||||||||
| Only male subjects from Hall 1991g | 12 | 1.45 | 1.08 | 1.93 | 1.73 | 1.08 | 2.78 | 24.16 | 0.01 |
| Study location | |||||||||
| North America | 7 | 1.38 | 0.97 | 1.95 | 1.43 | 0.95 | 2.16 | 7.47 | 0.28 |
| Europe/Asia | 5 | 1.56 | 0.93 | 2.61 | 2.75 | 0.83 | 9.14 | 16.10 | <0.01 |
| Study design | |||||||||
| Cohort | 10 | 1.67 | 1.21 | 2.30 | 2.00 | 1.21 | 3.29 | 17.01 | 0.05 |
| Case-control | 2 | 0.78 | 0.41 | 1.48 | 0.89 | 0.30 | 2.59 | 2.39 | 0.12 |
CI confidence interval, N number of studies, meta-RR meta-analysis relative risk, SPIR standardized proportionate incidence ratio, USMR US Mortality Rate.
Random effects model was used when X2 heterogeneity statistic > degrees of freedom (number of studies minus 1)
Professionals are defined as anatomists, pathologists, embalmers, or funeral home directors
Industrial workers are defined as laborers in factories or restorative projects
Relative risk estimates presented in Dell and Teta 1995; since data are only reported for salaried workers and short-term workers separately, N = 2
Relative risk estimates presented in Lacourt et al. 2013
Relative risk estimates presented in Hansen and Olsen 1995
Cohort narrowed down to only male pathologists
We conducted a series of sensitivity analyses below to evaluate the impact of including or excluding each individual study or other possible studies. In general, results were similar across all analyses, demonstrating the robustness of our findings.
Alternative Exposure Criteria
We evaluated the impact of our a priori selection of peak exposure. When the relative risk for the highest average intensity was prioritized, the meta-RR changed nominally (meta-RR = 1.77, 95% CI 1.11–2.83). Similarly, when the relative risk for the highest cumulative exposure was selected first, the meta-RR was 1.72 (95% CI 1.09–2.70). When longest duration was used, the meta-RR remained relatively constant at 1.75 (95% CI: 1.07–2.84). When “ever exposure” was used, the meta-RR was elevated to 1.82 (95% CI 1.20–2.75; Table 3).
Professionals vs. Industrial Workers
Many of the brain tumor studies distinguished their study subjects either from professional or industrial working settings. Formaldehyde was associated with increased risk of brain cancer in professional workers (meta-RR = 2.42; 95% CI 1.41–4.17). Elevated risks of brain cancer among industrial workers were detected (meta-RR = 1.32; 95% CI 0.72–2.44), though not statistically significant. Overall, professionals had a 110% greater meta-relative risk of developing brain cancer than industrial workers, among the most highly exposed groups (Fig. 3b).
Similar to what we found earlier in formaldehyde-associated leukemia (Zhang et al. 2009), higher risk of brain cancer among professionals may be due to exposure level and pattern, as an embalmer or anatomist is more likely to experience high-probability, high-intensity exposure than an individual involved with garment work. Embalmers are exposed at 1.32–2.86 parts per million (ppm) (Hiipakka et al. 2001) and anatomists/pathologists are exposed at levels between 0.7–3.7 ppm (Dias-Teixeira et al. 2016). In addition to inhalation, professionals are more likely to absorb formaldehyde through their skin during embalming or specimen preparation, which may introduce another route of exposure (Boeniger and Stewart 1992).
Lower risks among industrial workers may be because these groups experience far lower exposures over longer periods of time; for example, garment workers are typically exposed between 0.09–0.20 ppm (Pinkerton et al. 2004). Further, industrial workers hold a wide variety of jobs which may result in a broader range of actual exposure levels, making it difficult to compare different cohorts of industry workers. The variance in exposure pattern and intensity may account for the differences in meta-relative risks.
Lastly, not only does our meta-analysis indicate that professionals are at the highest risk for brain tumor development, two previous meta-analyses (Blair et al. 1990;Bosetti et al. 2008) also consistently reported the similar findings (Table 4) that are detailed in Comparison with Previous Meta-analyses on Brain Tumors.
Table 4.
Comparison of current and previous meta-analyses on brain cancer
| Group |
Blair et al. 1990 |
Bosetti et al. (2008) |
Current meta-analysis (2020) |
|||
|---|---|---|---|---|---|---|
| N | Meta-RR (95% CI) | N | Meta-RR (95% CI) | N | Meta-RR (95% CI) | |
| Professionals | 9 | 1.5 (1.2–2.0)a,b | 7 | 1.56 (1.24–1.96) | 5 | 2.42 (1.41–4.17) |
| Industry workers | 3 | 0.9 (0.7–1.1)b | 4 | 0.92 (0.75–1.13) | 7 | 1.32 (0.72–2.44) |
| Overall | 12 | 1.29 (0.95–1.76)c | 11 | 1.26 (1.01–1.59)c,d | 12 | 1.71 (1.07–2.73) |
CI confidence interval, meta-RR meta-analysis relative risk
Reported white and nonwhite separately, so N = 2 Hayes et al. 1990
Confidence interval calculated by us
Meta-RR calculated by us using random-effects model because X2 heterogeneity statistic > degrees of freedom (number of studies minus 1)
Risk estimates used the numbers from Bosetti et al. 2008. When the numbers from original studies were used to calculate meta-RR, the result was slightly different as 1.24 (0.99–1.55)
Population-Based Exposure Studies and SPIR Studies Added
We analyzed studies with population-based exposure and studies that reported standardized proportionate incidence ratios (SPIR; Supplementary Section A1). As shown in Table 3, when population-based exposure studies (Dell and Teta 1995; Lacourt et al. 2013; Hansen and Olsen 1995) were added one at a time, the meta-RR decreased slightly. When we added the study that reported SPIR (Hansen and Olsen 1995), the meta-RR decreased to 1.59 (95% CI 1.08–2.34). Adding all three studies decreased the meta-RR to 1.35 (95% CI 0.99–1.85).
Study Exclusion
To ensure one study was not artificially inflating the risk estimate, we excluded all studies one at a time and found that they all nominally changed the meta-RR (Supplementary Fig. 1). Although Fondelli et al. (2007) hadthe highest RR, removal of this study did not significantly impact on the results (meta-RR = 1.51, 95% CI 0.98–2.34). Similarly, removal of any individual study included in our analysis (meta-RR = 1.71) of brain tumors did not significantly change our meta-RR (range 1.51 to 1.91).
Study Subpopulation in Males Only
In our meta-analysis, we used the relative risk from the Hall et al. (1991) study that included both males and females. Since all of the brain cancer cases in Hall et al. (1991) were male, the article also presented a relative risk for just male subjects. When this relative risk for male subjects only was used, the meta-RR remained unchanged at 1.73 (95% CI 1.08–2.78).
Study Location and Design
Studies from North America had a meta-RR of 1.43 (95% CI 0.95–2.16), whereas studies from Europe and Asia had a meta-RR of 2.75 (95% CI 0.83–9.14). This difference may have been driven by studies conducted in China before 1990, when the Chinese Ministry of Health’s maximum allowable concentration for formaldehyde was very high (3 mg/m3, MOH China).
Comparison with Previous Meta-analyses on Brain Tumors
While there are no previous meta-analyses of formaldehyde and NDD for comparison, two meta-analyses of formaldehyde and brain cancer have been published to date (Blair et al. 1990; Bosetti et al. 2008). Both studies conducted separate analyses for professional groups and for industrial workers but did not perform a meta-analysis of all studies together. Table 4 compares our present findings with the two previously published meta-analyses. The earliest meta-analysis by Blair et al. (1990) evaluated ten studies on formaldehyde exposure and found an increased RR of 1.5 (95% CI 1.2–2.0) in professionals and no excess mortality in three studies of industrial workers (RR = 0.9, 95% CI 0.7–1.1; Blair et al. 1990). Note that 95% confidence intervals were not reported but were calculated by us using the fixed effects model.
Similarly, the more recent meta-analysis of brain cancer among professionals (n = 7) performed by Bosetti et al. (2008) reported an overall statistically significant increased meta-RR of 1.56 (95% CI 1.24–1.96) in professionals, but not in industrial workers (RR = 0.92, 95% CI 0.75–1.13, n = 4, Bosetti et al. 2008). Both Blair et al. (1990) and Bosetti et al. ( 2008) didnot report an overall meta-RR including both groups, so we calculated it using the random effects model to be 1.29 (95% CI 0.95–1.76) and 1.26 (95% 1.01–1.59), respectively. A comparison of the individual studies and relative risks used in our meta-analysis with the two previous studies is presented in Supplementary Table 4.
Our new meta-analysis differs from the previous reports for two reasons: (1) we used four recently updated studies that were not available when the previous meta-analyses were conducted, and (2) we employed an a priori selection of the highest exposure groups. Consequently, we detected a 2.42 increased meta-relative risk among professional workers, and 1.32 increased meta-relative risk among industrial workers.
Strengths and Limitations of Meta-Analyses
Strengths of Meta-Analyses
The strengths of our meta-analyses are the inclusion of several updated cohort studies, use of multiple sensitivity analyses to evaluate heterogeneity and bias, and our novel a priori hypothesis. Selection of the highest exposure group in each study, when reported, appeared to improve our ability to detect the presence of an exposure-disease association particularly in the NDD analysis. Multiple sensitivity analyses conducted among occupational subgroups and high versus low exposure groups consistently revealed strong, positive associations, indicating formaldehyde may contribute to the risk of both brain tumor and ALS.
Another strength was the inclusion of multiple cohort studies, which are typically considered the gold standard in epidemiology (Rothman 2012; Rothman et al. 2008). The meta-RR of cohort studies (N = 10), was 2.00 (95% CI 1.21–3.29), which was notably higher than that of case–control studies (see Table 3).
Bias and Differential Risk
One potential source of bias is exposure misclassification, as many studies did not have complete information regarding the specific levels and duration of exposure, length of employment, and concomitant exposures. This misclassification was likely non-differential among cohort studies (exposure status equally misclassified among cases and controls) which tends to attenuate the risk (Pearce et al. 2007), and differential in case-control studies due to recall bias (exposures may have been remembered differently by cases or their proxies than controls).
Our brain tumor analysis was more likely to suffer from biases resulting from non-differential misclassification, as ten of the 12 studies were cohort design. Furthermore, the ascertainment of the brain tumor diagnosis (primary vs. metastatic origin) may have been questionable. While many of the tumors may have been primary malignancies, some death certificates may reflect misdiagnoses, as the brain is a common site for metastases of other primary cancers (Thomas and Waxweiler 1986). This non-differential misclassification of disease status would also bias results toward the null, thus indicating that our main meta-RR could potentially underestimate the risk for brain tumors.
Despite these limitations, our results suggest an increased risk of ALS and brain tumors among individuals highly exposed to formaldehyde.
Summary of Key Findings
Our meta-analyses have revealed for the first time that high exposure to formaldehyde increased ALS meta-relative risk by 78% (meta-RR = 1.78); individuals with higher exposures to formaldehyde had an increased meta-RR compared with those with low/"ever exposures" (Fig. 3a). This finding is also supported by elevated total NDD risk among plastics workers than other industrial workers (Table 2). Additionally, we reported high exposure to formaldehyde increased the relative risk of developing brain cancer (meta-RR = 1.71). Evidently, the increased risk was far greater among professionals (meta-RR = 2.42) compared with industrial workers (1.32) (Table 3 and Fig. 3b) likely due to their different exposure patterns and levels, which is comparable with and supported by previous meta-analyses (Table 4). In summary, inhaled formaldehyde can potentially damage the brain to cause ALS or tumors.
Potential Mechanisms Investigated with Bioinformatics
To examine our findings from human studies further, we investigated the biological plausibility of how inhaled formaldehyde could exert its toxic effects in the brain. Given these associations have historically been neglected, there is a paucity of studies investigating mechanisms underlying formaldehyde-mediated brain cancer and neurodegeneration. To address this limitation, we employed a bioinformatics approach to identify candidate genes and pathways that may be responsible for mediating these interactions.
Bioinformatics Approach
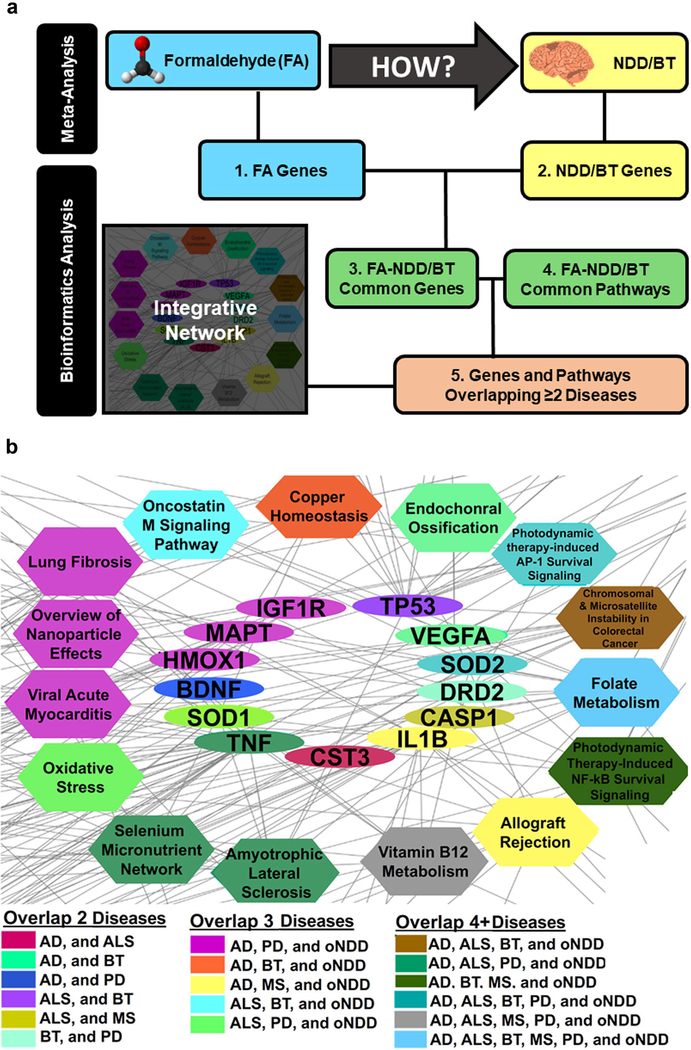
To deepen our understanding of the epidemiologic association between formaldehyde exposure and brain cancer and NDDs, we used gene-association data to identify (1) formaldehyde-associated genes, (2) the genes associated with the diseases of interest (ALS, AD, PD, MS, BT), (3) overlapped common genes from formaldehyde and NDD/BT, and (4) inferred formaldehyde–gene–NDD/BT associations and their associated pathways. Lastly, (5) we overlapped the genes and pathways identified from the inferred chemical–gene/pathway–disease associations to create an integrated network of candidate genes and pathways that could have played a role in mediating the associations observed in the human epidemiological studies (Fig. 4a).
Fig. 4.
Summary of bioinformatics analysis approach. a. Definitions of numbers are explained in “Bioinformatics Approach” in the text. Close-up of the integrative network depicted in Supplementary Fig. 2, which contains the genes and pathways that were overlapping between two or more NDDs of interest b. The color of the nodes depicts the respective combinations of the different disease types. The turquoise gene SOD2 (in the middle) and the gray pathway Vitamin B12 Metabolism (at the bottom), for example, are both observed to be affected in AD, ALS, brain tumor, PD, as well as in oNDD after formaldehyde exposure b
Integration of Gene-Association Data from the CTD
First, to investigate the relationship between formaldehyde exposure and NDD or BT, the Comparative Toxicogeno mics Database (CTD) (Davis et al. 2017) was queried to obtain appropriate gene association data. Details on the CTD and how the curated FA-genes and NDD/BT-genes were selected may be found in Supplementary Section A3.1.
Identifying Common Biomarker Genes
Second, to identify possible disease biomarkers following formaldehyde exposure, the obtained chemical–gene and gene–disease lists were compared using Venn Diagrams (Table 5). Details of how these resulting chemical-gene-disease associations were inferred may be found in Supplementary Section A3.2.
Table 5.
Chemical gene and pathway association data from the Comparative Toxicogenomics Database (CTD), related with formaldehyde exposure, were compared with curated gene-disease associations for AD, ALS, brain tumor, MS, PD, and oNDD
| Name | NDD |
MS | BT | Total |
|||
|---|---|---|---|---|---|---|---|
| oNDD | AD | ALS | PD | ||||
| Genes | |||||||
 Superoxide dismutase 2 (SOD2) Superoxide dismutase 2 (SOD2) |
✓ | ✓ | ✓ | ✓ | ✓ | 5 | |
 Tumor necrosis factor (TNF) Tumor necrosis factor (TNF) |
✓ | ✓ | ✓ | ✓ | 4 | ||
 Superoxide dismutase 1 (SOD1) Superoxide dismutase 1 (SOD1) |
✓ | ✓ | ✓ | 3 | |||
 Heme Oxygenase 1 (HMOX1) Heme Oxygenase 1 (HMOX1) |
✓ | ✓ | ✓ | 3 | |||
 Insulin Like Growth Factor 1 Receptor (IGF1R) Insulin Like Growth Factor 1 Receptor (IGF1R) |
✓ | ✓ | ✓ | 3 | |||
 Microtubule Associated Protein Tau (MAPT) Microtubule Associated Protein Tau (MAPT) |
✓ | ✓ | ✓ | 3 | |||
 Interleukin 1 Beta (IL1B) Interleukin 1 Beta (IL1B) |
✓ | ✓ | ✓ | 3 | |||
| Pathways | |||||||
 Folate Metabolism Folate Metabolism |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 |
 Photodynamic therapy-induced AP-1 survival signaling. Photodynamic therapy-induced AP-1 survival signaling. |
✓ | ✓ | ✓ | ✓ | ✓ | 5 | |
 Vitamin B12 Metabolism Vitamin B12 Metabolism |
✓ | ✓ | ✓ | ✓ | ✓ | 5 | |
 Photodynamic therapy-induced NF-kB survival signaling Photodynamic therapy-induced NF-kB survival signaling |
✓ | ✓ | ✓ | ✓ | 4 | ||
 Amyotrophic lateral sclerosis (ALS) Amyotrophic lateral sclerosis (ALS) |
✓ | ✓ | ✓ | ✓ | 4 | ||
 Selenium Micronutrient Network Selenium Micronutrient Network |
✓ | ✓ | ✓ | ✓ | 4 | ||
 Chromosomal and microsatellite instability in colorectal cancer Chromosomal and microsatellite instability in colorectal cancer |
✓ | ✓ | ✓ | ✓ | 4 | ||
 Oncostatin M Signaling Pathway Oncostatin M Signaling Pathway |
✓ | ✓ | ✓ | 3 | |||
 Copper homeostasis Copper homeostasis |
✓ | ✓ | ✓ | 3 | |||
 Oxidative Stress Oxidative Stress |
✓ | ✓ | ✓ | 3 | |||
 Viral Acute Myocarditis Viral Acute Myocarditis |
✓ | ✓ | ✓ | 3 | |||
 Lung fibrosis Lung fibrosis |
✓ | ✓ | ✓ | 3 | |||
 Overview of nanoparticle effects Overview of nanoparticle effects |
✓ | ✓ | ✓ | 3 | |||
 Allograft Rejection Allograft Rejection |
✓ | ✓ | ✓ | 3 | |||
Performing Gene Enrichment Analysis
Next, in order to gain more information into the biological processes that are enriched for the formaldehyde-induced disease genes, we performed gene set enrichment analysis (GSEA, detailed in Supplementary Section A3.3) using the Cytoscape (version 3.6.1), (Lotia et al. 2013) app ClueGO (version 2.5.2), (Bindea et al. 2009), a network visualization and analysis tool, in combination with the WikiPathways pathway repository containing 467 human pathways comprising 6235 human genes (version Oct 11, 2018) (Slenter et al. 2018).
Integrating Formaldehyde-Associated NDD/BT Genes and Pathways
Lastly, to understand what genes and which specific pathways are involved in the formaldehyde-associated NDD/BT, we created an integrative network of genes and pathways using Cytoscape (major findings reported in Fig. 4b). The full network, which is presented in Supplementary Fig. 2, allowed us to visualize similarities and differences between formaldehyde-related subtypes of NDD and brain tumors to further understand how these diseases are modulated.
Results of Bioinformatics Approach
Formaldehyde-Induced Brain Disorder Genes
When comparing gene lists for formaldehyde exposure versus the brain disorder of interest (Table 5), we identified the genes and pathways that overlapped the greatest number of diseases. The formaldehyde-associated genes that overlap three or more disease outcomes are reported in Table 5. Superoxide dismutase 2 (SOD2) was found for formaldehyde from 5 of 6 total brain-related disorders (other neurodegenerative disease [oNDD], AD, ALS, PD, and BT). Tumor necrosis factor (TNF) was found in formaldehyde from 4 NDDs (oNDD, AD, ALS, and PD). The complete formaldehyde-gene list all overlapping brain disorders can be found in Supplementary Table 5. As stated above in Multiple Sclerosis and Integration of Gene-Association Data from the CTD, because MS is a chronic inflammatory demyelinating disease that may progress into a NDD, it was analyzed separately.
Biological Pathways Affected in Formaldehyde-Induced Brain Disease
Using the respective formaldehyde-induced NDD gene lists, we conducted GSEA and identified significantly enriched pathways for formaldehyde and the NDD subgroups or brain tumor related genes (Supplementary Table 6). These unique pathways are affected by any of the NDD subgroups or brain cancer. The obtained lists of biological pathways were compared between individual NDD and with oNDD as a group. Fourteen pathways were found to be enriched by gene association data from three or more brain disorders (Table 5). The folate metabolism pathway was identified for all brain disorders of interest. Other pathways that were highly enriched included the photodynamic therapy-induced AP-1 survival signaling pathway, the vitamin B12 metabolism pathway, the ALS pathway, and the oxidative stress pathway.
An Integrative Network to Visualize Formaldehyde-Induced Effects on Brain Disorders
To summarize the results, an integrative network depicting the overlapping and unique genes and pathways was created in Cytoscape (Supplementary Fig. 2). The network is divided in two parts: (1) NDD and (2) brain tumors plus MS. The left panel of the figure depicts the unique genes and pathways that were found for PD, AD, ALS, and oNDDs (NDDs excluding AD, ALS, and PD). The upright panel of the network depicts the genes and pathways that were found for brain tumor and MS. The clusters of pathways and genes depicted next to each NDD are those overlapping with the oNDD group.
The most important results are depicted in Fig. 4b, which is the bottom right corner of Supplementary Fig. 2. This cluster of multicolor nodes represents the overlapping genes and pathways between two or more brain disorders (as were mentioned in Bioinformatics Approach and Results of Bioinformatics Approach). The genes presented in this network have been filtered because they could be involved in either of the enriched pathways (filtered list in comparison to Table 5). Figure 4b shows that the folate metabolism pathway overlapped all brain diseases of interest: PD, AD, ALS, MS, and brain tumors (5/5), as well as the oNDD group. Similarly, the vitamin B12 metabolism is enriched for gene association data related with AD, ALS, MS, and PD (4/5) plus the oNDD group.
Pathways Significantly Affected by Formaldehyde Exposure
The genes and pathways that were used to generate the network are reported in Supplementary Table 7. Using these data, we further analyzed the 14 pathways overlapping 3 + diseases to see which were significantly impacted by formaldehyde. To do so, we divided the number of genes affected by formaldehyde by the total number of genes involved in the pathway to calculate the percentage of the pathway affected (Supplementary Table 8). The ALS pathway had the greatest percent affected at 34%, lending support to our findings in the meta-analysis of human data. Other notable pathways included overview of nanoparticle effects, NF-kB survival signaling, folate metabolism, and oxidative stress, in which 32%, 31%, 23%, and 20% of genes were affected, respectively. A schematic of the formaldehyde genes affected in the ALS pathway is depicted in Supplementary Fig. 3. Another interesting pathway was the oncostatin M (OSM) signaling pathway, a homeostatic regulator in the central nervous system, in which 17% of genes were affected.
Genes and Pathways Involved in Formaldehyde-Induced Brain Disorders
We analyzed the results of the bioinformatics screen to determine concordance with known mechanisms of NDD/BT, including oxidative stress, abnormal protein aggregation, and inflammation, and to identify novel pathways that may be dysregulated.
Oxidative Stress and Lipid Peroxidation
One well-characterized feature of NDD and BT is oxidative stress. SOD2, depicted in the network to be affected within AD, ALS, brain tumor, and PD, plays a role in neutralizing oxidative stress. Additionally, some other genes listed in Table 5 and Supplementary Table 5, such as HMOX1 and GSTP1, can also be encoded to antioxidant enzymes to detoxify oxidative stress. Formaldehyde has been reported to induce oxidative stress not only in the brain but also in other tissues such as the bone marrow, spleen, liver, and testes of exposed mice (Ye et al. 2013).
Abnormal Protein Aggregation
Aggregation of proteins (such as amyloid-β and tau) and inclusion body formation is considered a hallmark of NDD (Ross and Poirier 2004). Cytostatin C (CST3), a formaldehyde-associated gene that was affected in ALS and AD, is a cysteine protease inhibitor that prevents amyloid-β deposition in mice (Mi et al. 2007). We also identified microtubule-associated protein tau (MAPT) in AD and PD; MAPT mutations can cause hyperphosphorylation and deposition of tau proteins into aggregates (Dujardin et al. 2018).
Mitochondrial Dysfunction and Vitamin B12 Metabolism
Mitochondrial dysfunction is another common mechanism in NDD pathology, manifesting as decreased energy production, excitotoxicity, impaired calcium buffering, and increased mitochondrial membrane permeability (Beal 1998). Deficiency in vitamin B12, a pathway overlapping ALS, AD, PD, and MS is known to cause mitochondrial toxicity as a result of citric acid cycle inhibition (Toyoshima et al. 1996) and predicts worsening mobility in PD patients (Christine et al. 2018).
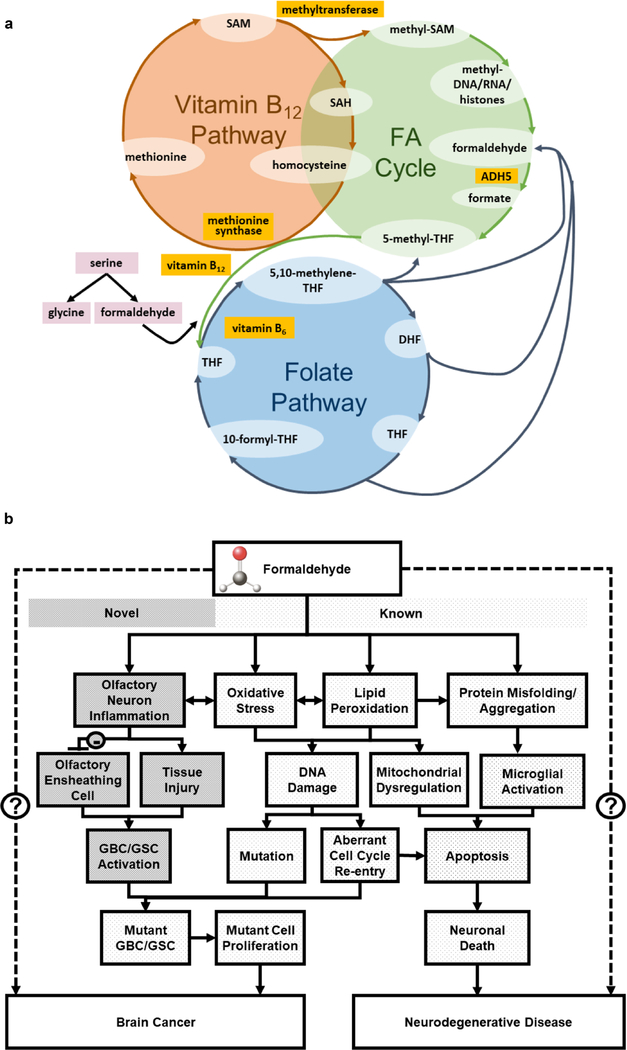
Low vitamin B12 levels inhibit the enzyme methionine synthase that catalyzes formation of methionine from homocysteine, leading to increased homocysteine (Fig. 5a). Hyperhomocystemia can cause the dysfunction or death of cells in the nervous system by impairing DNA repair mechanisms and inducing oxidative stress (Kruman et al. 2000, 2002; Obeid and Herrmann 2006). This mechanism was demonstrated in PC12 cells as a model for neuronal secretion and differentiation (Wagner et al. 1993), where formaldehyde exposure induced oxidative stress and inhibited hydrogen sulfide production, contributing to neurotoxicity induced by homocysteine (Tang et al. 2013). In human epidemiological studies, hyperhomocystemia has been strongly linked to development of dementia, AD (Seshadri et al. 2002), and cognitive decline in PD patients.
Fig. 5.
Intersection of folate driven one-carbon pool, formaldehyde cycle, and vitamin B12 pathway a. Potential mechanisms underlying formaldehyde-induced neurodegenerative disease and brain cancer b
Folate Metabolism
The only pathway identified through our bioinformatics analysis that overlapped every brain disorder of interest (ALS, BT, AD, PD, and MS) was the folate metabolism pathway, also known as the one-carbon cycle (Fig. 5a). Interestingly, the vitamin B12-dependent formation of methionine from homocysteine (discussed in detail in Mitochondrial Dysfunction and Vitamin B12 Metabolism) is also a major intersecting branch point in the one-carbon cycle, as it uses 5-methyltetrahydrofolate (5MTHF) from the folate metabolism pathway as a methyl donor.
The one-carbon metabolism is involved in many biochemical reactions such as the generation of DNA precursors, which may affect neuroprogenitor cell proliferation. Dysregulation of the one-carbon pool mediated by folate deficiency has been shown to inhibit adult hippocampal neuroprogenitor cell proliferation in vivo, a central function of neurogenesis in the olfactory bulb, subventricular zone of the lateral ventricle, and in the hippocampus (Kruman et al. 2005).
Formaldehyde Cycle Links Both Vitamin B12 and Folate Pathways
Metabolisms of vitamin B12 and folate (discussed in Mitochondrial Dysfunction and Vitamin B12 Metabolism and Folate Metabolism, respectively) are intimately linked with each other and connected with the recently discovered endogenous formaldehyde cycle (Burgos-Barragan et al. 2017) (Fig. 5a). In the folate metabolism pathway, it was previously known that formaldehyde generated from the enzymatic cleavage of serine serves as an intermediate in the cycle, where it rapidly reacts with tetrahydrofolate (THF) to form 5,10-methylene-THF. Formaldehyde has also been identified as a product of the one-carbon pool (Burgos-Barragan et al. 2017). This endogenously produced formaldehyde can react with glutathione and ultimately be hydrolyzed into formate, which is free to re-enter the canonical one-carbon metabolic pathway. Any excessive formaldehyde that is not recycled may induce DNA damage. Interestingly, our most current study using a CRISPR screen reported that genes encoding components of DNA damage response/repair and the formaldehyde-involved one-carbon metabolism were also identified as top candidate genes whose disruption altered FA cytotoxicity and conferred increased sensitivity to formaldehyde (Zhao et al. 2020a).
Peripheral and Neural Inflammation
Recent studies indicate peripheral inflammation in the body may be transmitted to the brain to induce neuroflammation (Cabrera-Pastor et al. 2019). We identified TNF, a gene overlapping AD, ALS, and PD, is a pro-inflammatory marker that has been shown to be increased significantly in mice after 15.5 mg/kg/day formaldehyde exposure (Liu et al. 2018). The IL1β gene encodes a cytokine protein that is an important mediator of the inflammatory response; increased production of IL1β causes a number of autoinflammatory syndromes (Masters et al. 2009; Bensi et al. 1987). Additionally, we identified photodynamic therapy-induced NF-kB and AP-1 signaling pathways (Table 5 and Supplementary Table 8) that involve with many other pro-inflammatory or inflammatory genes and intimately associated with formaldehyde-related NDDs and BT.
We summarize the mechanisms described in this section in Fig. 5b; many of which are well-known, and the novel pathway with olfactory neuron inflammation is discussed next in Potential Mechanisms: Neural Damage via Olfactory Bulb. It should be noted that the pathways denoted in the figure are interconnected and sometimes bidirectional; for example, both inflammation and oxidative stress are known to promote microglial activation which can then cause lipid peroxidation.
Potential Mechanisms: Neural Damage via Olfactory Bulb
As described above, we have established both an epidemiological association between formaldehyde exposure and brain cancer/NDD and described in detail the putative genes and pathways intersecting formaldehyde and multiple brain disorders of interest. Indeed, the question of how formaldehyde reaches these targets and modulates these different classes of disease remains; perhaps the common link is the nose, or more precisely, the olfactory bulb or olfactory neuron inflammation (Fig. 5b).
Formaldehyde and Olfactory Impairment
Although it is commonly postulated that most inhaled airborne formaldehyde is detoxified upon contact with the mucosal surfaces of the mouth and nose, formaldehyde encounters and could damage the olfactory bulb (Formaldehyde Inhalation and Olfactory Function). Repeated formaldehyde inhalation exposure impairs olfactory function in humans (Kilburn et al. 1985; Holmstrom and Wilhelmsson 1988; Hisamitsu et al. 2011; Edling et al. 1988) and in rats. (Zhang et al. 2014) Our recently published study shows that formaldehyde inhalation inhibits the growth of hematopoietic stem and progenitor cells in the olfactory mucosa of exposed mice in vivo and ex vivo (Zhao et al. 2020b). Exposure to formaldehyde also decreased glutamate, GABA, and nitric oxide synthase expression (Li et al. 2010) and reduced expression of synaptosomal-associated protein 25 (SNAP25) in the olfactory bulb, as well as mature and immature olfactory sensory neuron markers, olfactory marker protein (OMP), and Tuj-1 in rats (Zhang et al. 2014). Decreases in SNAP25 are predictive of neuron loss and decreased synaptogenesis (Washbourne et al. 2002).
Olfactory impairment has been linked to PD (Doty et al. 1988, 1995; Tissingh et al. 2001), ALS (Viguera et al. 2018), and AD (McCaffrey et al. 2000; Morgan et al. 1995). In particular, AD specific neuropathology has been detected in the olfactory epithelium (Yamagishi et al. 1994; Lee et al. 1993; Arnold et al. 1998), with phosphorylated tau and neurofilament proteins present in the axons and dendrites of olfactory neurons (Talamo et al. 1989; Reyes et al. 1993). AD patients also have altered olfactory evoked response potentials (Warner et al. 1986). Reduced olfactory performance has also been reported in patients with brain tumors (Daniels et al. 2001).
Interestingly, the mammalian olfactory epithelium has a unique capacity to replace olfactory receptor neurons throughout life (Graziadei and Graziadei 1979; Schwob 2002) using olfactory ensheathing cells (OECs) that wrap olfactory axons and support their continued regeneration (Su et al. 2013). When activated, these OECs have the ability to travel from the olfactory bulb in the peripheral nervous system (PNS) to primary tumor sites in the CNS and along the invasive tumor border where they can selectively target glioblastoma stem-like cells (GBC/GSC), which are thought to initiate glioblastomas (Carvalho et al. 2019).
Formaldehyde-Induced Toxicity in Olfactory Neurons and/or OECs
We hypothesize that formaldehyde could damage these OECs, which may in turn promote brain tumor proliferation. Olfactory neuronal inflammation may also promote oxidative stress, lipid peroxidation, and DNA damage acting along the apoptotic pathway, promoting neurodegenerative disease (Fig. 5b).
As reported above in Pathways Significantly Affected by Formaldehyde Exposure, the OSM signaling pathway was both affected by formaldehyde (17% of genes affected; Supplementary Table 8) and overlapped formaldehyde and both ALS and brain cancer (Table 5), the disorders for which we uncovered the strongest human epidemiological evidence. The OSM is a member of the interleukin-6 cytokine family. It modulates the homeostasis of neural precursor cells, which are responsible for the production of new neural cells in the olfactory bulb, among other brain regions (Houben et al. 2019). OSM has also been demonstrated to exhibit neuroprotective and anti-tumorigenic effects (Beatus et al. 2011), though its full role in the CNS is not completely understood.
Conclusion
In this systematic review and detailed meta-analysis, we describe the epidemiological evidence that appears to support an association between formaldehyde inhalation and the development of ALS and brain cancer. A similar association was also reported for PD, but the lack of rigorous exposure assessment in these studies indicates these results should be interpreted with caution. Indeed, the question of biological plausibility for the formaldehyde-associated brain damage (NDD and BT) remains.
Using multiple bioinformatics analyses, we reported that our new findings suggest formaldehyde may mediate these brain diseases through dysregulation of the ALS and folate metabolism pathways, among others. While cancer cells and neurons are distinct, as the former rapidly divide and the latter are non-replicating, there is evidence that supports common genetic mechanisms involved in brain cancer and NDD progression. Our findings highlight that formaldehyde exposure, particularly at high levels, may manifest in the initiation and progression of different neural diseases by mis-regulating common genes or pathways. We recommend conducting an animal exposure study employing CRISPR technology to knockdown critical genes identified from our bioinformatics analysis to further understand potential mechanisms supported by empirical data.
Supplementary Material
Acknowledgments
We are grateful to Profs. Marc Weisskopf from Harvard University, Christopher Chang, and Martyn Smith at UC Berkeley for their helpful discussions. Special thanks to Yun Zhao for creating Supplementary Fig. 3 depicting formaldehyde-genes in the ALS pathway, and for procurement and careful translation of the Chinese papers.
Funding This project was partially supported by the UC Berkeley Superfund Research Program (SRP, P42ES004705) to LZ and CS, who are project leaders. IR and LR are SRP trainees at UC Berkeley.
Abbreviations
- 5MTHF
5-Methyltetrahydrofolate
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- ALS-PDC
ALS-parkinsonism-dementia complex
- BT
Brain tumor
- CNS
Central nervous system
- CNKI
Chinese National Knowledge Infrastructure
- CQVIP
Chongqing VIP Information
- CTD
Comparative Toxicogenomics Database
- CI
Confidence interval
- CST3
Cytostatin
- GSEA
Gene set enrichment analysis
- GBC/GSC
Glioblastoma stem-like cells
- IARC
International Agency for Research on Cancer
- L-BMAA
Beta-aminomethyl-L-alanine
- Meta-RR
Meta-relative risk
- MAPT
Mictorubule associated protein tau
- MS
Multiple sclerosis
- NDD
Neurodegenerative disease
- NOS
Newcastle-Ottawa Scale
- OMP
Olfactory marker protein
- OSM
Oncostatin M
- oNDD
Other neurodegenerative disease
- PD
Parkinson’s disease
- ppm
Parts per million
- PNS
Peripheral nervous system
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RR
Relative risk
- SOD2
Superoxide dismutase 2
- SNAP25
Synaptosomal-associated protein 25
- THF
Tetrahydrofolate
- TNF
Tumor necrosis factor
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no competing interests.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s12640-020-00320-y.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- Ai L, Tan T, Tang Y, Yang J, Cui D, Wang R, Wang A, Fei X, Di Y, Wang X et al. (2019) Endogenous formaldehyde is a memory-related molecule in mice and humans. Commun Biol 2:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovich DA, Janszen DB, Brown MH, Richardson RB, Miller FJ (1995) Mortality of iron foundry workers: IV. Analysis of a subcohort exposed to formaldehyde. J Occup Environ Med 37(7):826–837 [DOI] [PubMed] [Google Scholar]
- Arnold SE, Smutzer GS, Trojanowski JQ, Moberg PJ (1998) Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer’s disease and schizophrenia. Ann N Y Acad Sci 855:762–775 [DOI] [PubMed] [Google Scholar]
- Aslan H, Songur A, Tunc AT, Ozen OA, Bas O, Yagmurca M, Turgut M, Sarsilmaz M, Kaplan S (2006) Effects of formaldehyde exposure on granule cell number and volume of dentate gyrus: a histopathological and stereological study. Brain Res 1122(1):191–200 [DOI] [PubMed] [Google Scholar]
- Bach B, Pedersen OF, Molhave L (1990) Human-performance during experimental formaldehyde exposure. Environ Int 16(2):105–113 [Google Scholar]
- Beal MF (1998) Mitochondrial dysfunction in neurodegenerative diseases. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1366(1–2):211–223 [DOI] [PubMed] [Google Scholar]
- Beane Freeman LE, Blair A, Lubin JH, Stewart PA, Hayes RB, Hoover RN, Hauptmann M (2013) Mortality from solid tumors among workers in formaldehyde industries: an update of the NCI cohort. Am J Ind Med 56(9):1015–1026 [DOI] [PubMed] [Google Scholar]
- Beatus P, Jhaveri DJ, Walker TL, Lucas PG, Rietze RL, Cooper HM, Morikawa Y, Bartlett PF (2011) Oncostatin M regulates neural precursor activity in the adult brain. Dev Neurobiol 71(7):619–633 [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101 [PubMed] [Google Scholar]
- Bensi G, Raugei G, Palla E, Carinci V, Buonamassa DT, Melli M (1987) Human interleukin-1 beta gene. Gene 52(1):95–101 [DOI] [PubMed] [Google Scholar]
- Bhisey R (2018) Global formaldehyde market is expected to reach 36.6 million tons towards the end of 2026. In: Transparency Market Research. Albany, New York: GlobeNewswire [Google Scholar]
- Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pages F, Trajanoski Z, Galon J (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25(8):1091–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A, Saracci R, Stewart PA, Hayes RB, Shy C (1990) Epidemiologic evidence on the relationship between formaldehyde exposure and cancer. Scand J Work Environ Health 16(6):381–393 [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Stewart P (1992) Biological markers for formaldehyde exposure in mortician students, Report II: Extent of exposure. Final Report, National Institute for Occupational Safety and Health Report Number IWSB
- Bosetti C, McLaughlin JK, Tarone RE, Pira E, La Vecchia C (2008) Formaldehyde and cancer risk: a quantitative review of cohort studies through 2006. Annals of oncology: official journal of the European Society for Medical Oncology 19(1):29–43 [DOI] [PubMed] [Google Scholar]
- Burgos-Barragan G, Wit N, Meiser J, Dingler FA, Pietzke M, Mulderrig L, Pontel LB, Rosado IV, Brewer TF, Cordell RL et al. (2017) Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature 548(7669):549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Pastor A, Llansola M, Montoliu C, Malaguarnera M, Balzano T, Taoro-Gonzalez L, García-García R, Mangas-Losada A, Izquierdo-Altarejos P, Arenas YM (2019) Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications. Acta Physiol 226(2):e13270. [DOI] [PubMed] [Google Scholar]
- Carvalho LA, Teng J, Fleming RL, Tabet EI, Zinter M, de Melo Reis RA, Tannous BA (2019) Olfactory ensheathing cells: a Trojan horse for glioma gene therapy. J Natl Cancer Inst 111(3):283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M, Heck HD, Everitt JI, Harrington WW Jr, Popp JA (1988) Formaldehyde concentrations in the blood of rhesus monkeys after inhalation exposure. Food Chem Toxicol 26(8):715–716 [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, Østbye T, Stoessl A, Merskey H, Hachinski V (1995) Environmental exposures in elderly Canadians with Parkinson’s disease. Can J Neurol Sci 22(3):232–234 [DOI] [PubMed] [Google Scholar]
- Chemical agents and related occupations: formaldehyde (2012) [http://monographs.iarc.fr/ENG/Monographs/vol100F/mono100F-29.pdf] [PMC free article] [PubMed]
- Chinese National Standard: Hygienic standards for the design of industrial enterprises (TJ36–79) (1979) [http://www.fm120.com/zt/law/laws/1/LDAQWSGL/LDAQWSGL1003.htm]
- Chiò A, Meineri P, Tribolo A, Schiffer D (1991) Risk factors in motor neuron disease: a case-control study. Neuroepidemiology 10(4):174–184 [DOI] [PubMed] [Google Scholar]
- Christine CW, Auinger P, Joslin A, Yelpaala Y, Green R, Investigators PSGD (2018) Vitamin B12 and homocysteine levels predict different outcomes in early Parkinson’s disease. Mov Disord 33(5):762–770 [DOI] [PubMed] [Google Scholar]
- Coggon D, Ntani G, Harris EC, Palmer KT (2014) Upper airway cancer, myeloid leukemia, and other cancers in a cohort of British chemical workers exposed to formaldehyde. Am J Epidemiol 179(11):1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corporation M (2013) Microsoft Excel Version 3. In. Redmond, Washington [Google Scholar]
- Daniels C, Gottwald B, Pause BM, Sojka B, Mehdorn HM, Ferstl R (2001) Olfactory event-related potentials in patients with brain tumors. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 112(8): 1523–1530 [DOI] [PubMed] [Google Scholar]
- Davis AP, Grondin CJ, Johnson RJ, Sciaky D, King BL, McMorran R, Wiegers J, Wiegers TC, Mattingly CJ (2017) The comparative toxicogenomics database: update 2017. Nucleic Acids Res 45(D1):D972–D978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deapen DM, Henderson BE (1986) A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol 123(5):790–799 [DOI] [PubMed] [Google Scholar]
- Dell L, Teta MJ (1995) Mortality among workers at a plastics manufacturing and research and development facility: 1946–1988. Am J Ind Med 28(3):373–384 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188 [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 52(2):381–389 [DOI] [PubMed] [Google Scholar]
- Dias-Teixeira M, Domingues V, Dias-Teixeira A, Teles T, Delerue-Matos C (2016) Risk of exposure to formaldehyde in pathological anatomy laboratories. In: Advances in Safety Management and Human Factors: Proceedings of the AHFE 2016 International Conference on Safety Management and Human Factors, July 27–31, 2016, Walt Disney World®, Florida, USA. Edited by Arezes P. Cham: Springer International Publishing; 379–385 [Google Scholar]
- Doty RL, Deems DA, Stellar S (1988) Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38(8):1237–1244 [DOI] [PubMed] [Google Scholar]
- Doty RL, Bromley SM, Stern MB (1995) Olfactory testing as an aid in the diagnosis of Parkinson’s disease: development of optimal discrimination criteria. Neurodegeneration: a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration 4(1):93–97 [DOI] [PubMed] [Google Scholar]
- Du L, Pertsemlidis A (2011) Cancer and neurodegenerative disorders: pathogenic convergence through microRNA regulation. J Mol Cell Biol 3(3):176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrow R, Gute DM (1988) Cause-specific mortality among male textile workers in Rhode Island. Am J Ind Med 13(4):439–454 [DOI] [PubMed] [Google Scholar]
- Dujardin S, Bégard S, Caillierez R, Lachaud C, Carrier S, Lieger S, Gonzalez JA, Deramecourt V, Déglon N, Maurage C-A (2018) Different tau species lead to heterogeneous tau pathology propagation and misfolding. Acta Neuropathol Commun 6(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong A, Steinmaus C, McHale CM, Vaughan CP, Zhang L (2011) Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat Res 728(3):118–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edling C, Hellquist H, Odkvist L (1988) Occupational exposure to formaldehyde and histopathological changes in the nasal mucosa. Br J Ind Med 45(11):761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2014) Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources. European Food Safety Authority Journal 12(2):3550 [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Quinlan P, Ye W, Barber MK, Umbach DM, Sandler DP, Kamel F (2009) Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect 117(9):1387–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondelli MC, Costantini AS, Ercolanelli M, Pizzo AM, Maltoni SA, Quinn MM (2007) Exposure to carcinogens and mortality in a cohort of restoration workers of water-damaged library materials following the River Arno flooding in Florence, 4 November 1966. La Medicina del lavoro 98(5):422–431 [PubMed] [Google Scholar]
- Graziadei PP, Graziadei GA (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 8(1):1–18 [DOI] [PubMed] [Google Scholar]
- Greenland S (1998) Meta-analysis. In: Modern Epidemiology. Edited by Rothman K, Greenland S, Second edn. Philadelphia: Lippincott Raven; 643–673 [Google Scholar]
- Gunnarsson LG, Lindberg G, Söderfeldt B, Axelson O (1991) Amyotrophic lateral sclerosis in Sweden in relation to occupation. Acta Neurol Scand 83(6):394–398 [DOI] [PubMed] [Google Scholar]
- Hall A, Harrington JM, Aw TC (1991) Mortality study of British pathologists. Am J Ind Med 20(1):83–89 [DOI] [PubMed] [Google Scholar]
- Hansen J, Olsen JH (1995) Formaldehyde and cancer morbidity among male employees in Denmark. Cancer Causes Control 6(4):354–360 [DOI] [PubMed] [Google Scholar]
- Hauptmann M, Stewart PA, Lubin JH, Beane Freeman LE, Hornung RW, Herrick RF, Hoover RN, Fraumeni JF Jr, Blair A, Hayes RB (2009) Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Natl Cancer Inst 101(24):1696–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RB, Blair A, Stewart PA, Herrick RF, Mahar H (1990) Mortality of U.S. embalmers and funeral directors. Am J Ind Med 18(6):641–652 [DOI] [PubMed] [Google Scholar]
- Heck HD, Casanova-Schmitz M, Dodd PB, Schachter EN, Witek TJ, Tosun T (1985) Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rats exposed to CH2O under controlled conditions. Am Ind Hyg Assoc J 46(1):1–3 [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Bowering D, Snow B, Calne D (1990) Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med 17(3):349–355 [DOI] [PubMed] [Google Scholar]
- Hiipakka DW, Dyrdahl KS, Garcia Cardenas M (2001) Successful reduction of morticians’ exposure to formaldehyde during embalming procedures. AIHAJ 62(6):689–696 [DOI] [PubMed] [Google Scholar]
- Hill AB (1965) The environment and disease: association or causation? In: Sage Publications [Google Scholar]
- Hisamitsu M, Okamoto Y, Chazono H, Yonekura S, Sakurai D, Horiguchi S, Hanazawa T, Terada N, Konno A, Matsuno Y et al. (2011) The influence of environmental exposure to formaldehyde in nasal mucosa of medical students during cadaver dissection. Allergology international: official journal of the Japanese Society of Allergology 60(3):373–379 [DOI] [PubMed] [Google Scholar]
- Holmstrom M, Wilhelmsson B (1988) Respiratory symptoms and pathophysiological effects of occupational exposure to formaldehyde and wood dust. Scand J Work Environ Health 14(5):306–311 [DOI] [PubMed] [Google Scholar]
- Houben E, Hellings N, Broux B (2019) Oncostatin M, an underestimated player in the central nervous system. Frontiers in immunology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhang R, Wang Y, Lu P, Gu X (1990) Study on the tumor mortality of a retrospective cohort of formaldehyde exposed workers in chemical industry. Chin J Ind Hyg Occup Dis 8(5):261–264 [Google Scholar]
- Kilburn KH, Seidman BC, Warshaw R (1985) Neurobehavioral and respiratory symptoms of formaldehyde and xylene exposure in histology technicians. Arch Environ Health 40(4):229–233 [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw R, Thornton JC (1987) Formaldehyde impairs memory, equilibrium, and dexterity in histology technicians: effects which persist for days after exposure. Arch Environ Health 42(2):117–120 [DOI] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP (2000) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20(18):6920–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Kumaravel T, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP (2002) Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci 22(5):1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Mouton PR, Emokpae R Jr, Cutler RG, Mattson MP (2005) Folate deficiency inhibits proliferation of adult hippocampal progenitors. NeuroReport 16(10):1055–1059 [DOI] [PubMed] [Google Scholar]
- Lacourt A, Cardis E, Pintos J, Richardson L, Kincl L, Benke G, Fleming S, Hours M, Krewski D, McLean D et al. (2013) INTEROCC case-control study: lack of association between glioma tumors and occupational exposure to selected combustion products, dusts and other chemical agents. BMC public health 13:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoue J, Vincent R, Gerin M (2008) Formaldehyde exposure in U.S. industries from OSHA air sampling data. J Occup Environ Hyg 5(9):575–587 [DOI] [PubMed] [Google Scholar]
- Lee JH, Goedert M, Hill WD, Lee VM, Trojanowski JQ (1993) Tau proteins are abnormally expressed in olfactory epithelium of Alzheimer patients and developmentally regulated in human fetal spinal cord. Exp Neurol 121(1):93–105 [DOI] [PubMed] [Google Scholar]
- Levine RJ, Andjelkovich DA, Shaw LK (1984) The mortality of Ontario undertakers and a review of formaldehyde-related mortality studies. J Occup Med 26(10):740–746 [DOI] [PubMed] [Google Scholar]
- Li X, Hemminki K, Sundquist K (2008) Regional, socioeconomic and occupational groups and risk of hospital admission for multiple sclerosis: a cohort study in Sweden. Mult Scler J 14(4):522–529 [DOI] [PubMed] [Google Scholar]
- Li X, Sundquist J, Sundquist K (2009) Socioeconomic and occupational groups and Parkinson’s disease: a nationwide study based on hospitalizations in Sweden. Int Arch Occup Environ Health 82(2):235–241 [DOI] [PubMed] [Google Scholar]
- Li YQ, Chen HH, Yin YF, Han F, Ye XS, Ling SC (2010) [Formaldehyde inhalation may damage olfactory bulb and hippocampus in rats]. Zhejiang da xue xue bao Yi xue ban = J Zheijang Univ Med Sci 39(3):272–277 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Wu R, Ye M, Zhao Y, Kang J, Ma P, Li J, Yang X (2018) Acute formaldehyde exposure induced early Alzheimer-like changes in mouse brain. Toxicol Mech Methods 28(2):95–104 [DOI] [PubMed] [Google Scholar]
- Lotia S, Montojo J, Dong Y, Bader GD, Pico AR (2013) Cytoscape App store. Bioinformatics 29(10):1350–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Li CM, Qiao Y, Yan Y, Yang X (2008) Effect of inhaled formaldehyde on learning and memory of mice. Indoor Air 18(2):77–83 [DOI] [PubMed] [Google Scholar]
- Masters SL, Simon A, Aksentijevich I, Kastner DL (2009) Horror auto-inflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol 27:621–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey RJ, Duff K, Solomon GS (2000) Olfactory dysfunction discriminates probable Alzheimer’s dementia from major depression: a cross-validation and extension. The Journal of neuropsychiatry and clinical neurosciences 12(1):29–33 [DOI] [PubMed] [Google Scholar]
- Meyers AR, Pinkerton LE, Hein MJ (2013) Cohort mortality study of garment industry workers exposed to formaldehyde: update and internal comparisons. Am J Ind Med 56(9):1027–1039 [DOI] [PubMed] [Google Scholar]
- Mi W, Pawlik M, Sastre M, Jung SS, Radvinsky DS, Klein AM, Sommer J, Schmidt SD, Nixon RA, Mathews PM (2007) Cystatin C inhibits amyloid-β deposition in Alzheimer’s disease mouse models. Nat Genet 39(12):1440. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CD, Nordin S, Murphy C (1995) Odor identification as an early marker for Alzheimer’s disease: impact of lexical functioning and detection sensitivity. J Clin Exp Neuropsychol 17(5):793–803 [DOI] [PubMed] [Google Scholar]
- Nee LE, Gomez MR, Dambrosia J, Bale S, Eldridge R, Polinsky RJ (1991) Environmental—occupational risk factors and familial associations in multiple system atrophy: a preliminary investigation. Clin Auton Res 1(1):9–13 [DOI] [PubMed] [Google Scholar]
- Neuberger A (1981) The metabolism of glycine and serine, vol 19a. Elsevier, Amsterdam [Google Scholar]
- Nie CL, Wang XS, Liu Y, Perrett S, He RQ (2007) Amyloid-like aggregates of neuronal tau induced by formaldehyde promote apoptosis of neuronal cells. BMC Neurosci 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580(13):2994–3005 [DOI] [PubMed] [Google Scholar]
- Pearce N, Checkoway H, Kriebel D (2007) Bias in occupational epidemiology studies. Occup Environ Med 64(8):562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-De La Cruz V, Elinos-Calderon D, Robledo-Arratia Y, Medina-Campos ON, Pedraza-Chaverri J, Ali SF, Santamaria A (2009) Targeting oxidative/nitrergic stress ameliorates motor impairment, and attenuates synaptic mitochondrial dysfunction and lipid peroxidation in two models of Huntington’s disease. Behav Brain Res 199(2):210–217 [DOI] [PubMed] [Google Scholar]
- Perna RB, Bordini EJ, Deinzer-Lifrak M (2001) A case of claimed persistent neuropsychological sequelae of chronic formaldehyde exposure: clinical, psychometric, and functional findings. Arch Clin Neuropsychol 16(1):33–44 [PubMed] [Google Scholar]
- Peters TL, Kamel F, Lundholm C, Feychting M, Weibull CE, Sandler DP, Wiebert P, Sparen P, Ye W, Fang F (2017) Occupational exposures and the risk of amyotrophic lateral sclerosis. Occup Environ Med 74(2):87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti D (1994) Statistical methods in meta-analysis. In: Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. New York, NY: Oxford University Press; 94–118 [Google Scholar]
- Pinkerton LE, Hein MJ, Stayner LT (2004) Mortality among a cohort of garment workers exposed to formaldehyde: an update. Occup Environ Med 61(3):193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton LE, Hein MJ, Meyers A, Kamel F (2013) Assessment of ALS mortality in a cohort of formaldehyde-exposed garment workers. Amyotroph Lateral Scler Frontotemporal Degener 14(5–6):353–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov B, Gadjeva V, Valkanov P, Popova S, Tolekova A (2003) Lipid peroxidation, superoxide dismutase and catalase activities in brain tumor tissues. Arch Physiol Biochem 111(5):455–459 [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM (2001) Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. The Journal of neuroscience: the official journal of the Society for Neuroscience 21(12):4183–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Xiao R, Su T, Wu BB, Tong ZQ, Liu Y, He RQ (2014) A novel mechanism for endogenous formaldehyde elevation in SAMP8 mouse. J Alzheimers Dis 40(4):1039–1053 [DOI] [PubMed] [Google Scholar]
- Reyes PF, Deems DA, Suarez MG (1993) Olfactory-related changes in Alzheimer’s disease: a quantitative neuropathologic study. Brain Res Bull 32(1):1–5 [DOI] [PubMed] [Google Scholar]
- Roberts AL, Johnson NJ, Cudkowicz ME, Eum KD, Weisskopf MG (2016) Job-related formaldehyde exposure and ALS mortality in the USA. J Neurol Neurosurg Psychiatry 87(7):786–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom WN, Markowitz S (2007) Environmental and occupational medicine, 4th edn. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(7s):S10. [DOI] [PubMed] [Google Scholar]
- Rothman KJ (2012) Epidemiology: an introduction: Oxford university press [Google Scholar]
- Rothman KJ, Greenland S, Lash TL (2008) Modern epidemiology, vol. 3: Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia [Google Scholar]
- Sarsilmaz M, Kaplan S, Songur A, Colakoglu S, Aslan H, Tunc AT, Ozen OA, Turgut M, Bas O (2007) Effects of postnatal formaldehyde exposure on pyramidal cell number, volume of cell layer in hippocampus and hemisphere in the rat: a stereological study. Brain Res 1145:157–167 [DOI] [PubMed] [Google Scholar]
- Schaeffer J, Cossetti C, Mallucci G, Pluchino S (2015) Chapter 30 -Multiple sclerosis. In: Zigmond MJ, Rowland LP, Coyle JT (eds) Neurobiology of brain disorders. Academic Press, San Diego, pp 497–520 [Google Scholar]
- Schwob JE (2002) Neural regeneration and the peripheral olfactory system. The Anatomical record 269(1):33–49 [DOI] [PubMed] [Google Scholar]
- Seals RM, Kioumourtzoglou MA, Gredal O, Hansen J, Weisskopf MG (2017) Occupational formaldehyde and amyotrophic lateral sclerosis. Eur J Epidemiol 32(10):893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346(7):476–483 [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Greer CA (1998) Olfactory bulb. In: The synaptic organization of the brain, 4th ed. New York, NY, US: Oxford University Press; 159–203 [Google Scholar]
- Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, Melius J, Cirillo E, Coort SL, Digles D et al. (2018) WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res 46(D1):D661–D667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Smith MT, La Merrill MA, Liaw J, Steinmaus C (2017) 2, 4-Dichlorophenoxyacetic acid (2, 4-D) and risk of non-Hodgkin lymphoma: a meta-analysis accounting for exposure levels. Ann Epidemiol 27(4):281–289. e284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Baker GB, Dursun SM, Todd KG (2010) The antidepressant phenelzine protects neurons and astrocytes against formaldehyde-induced toxicity. J Neurochem 114(5):1405–1413 [DOI] [PubMed] [Google Scholar]
- Songur A, Sarsilmaz M, Ozen O, Sahin S, Koken R, Zararsiz I, Ilhan N (2008) The effects of inhaled formaldehyde on oxidant and antioxidant systems of rat cerebellum during the postnatal development process. Toxicol Mech Methods 18(7):569–574 [DOI] [PubMed] [Google Scholar]
- Spencer PS (2019) Hypothesis: etiologic and molecular mechanistic leads for sporadic neurodegenerative diseases based on experience with Western Pacific ALS/PDC. Front Neurol 10:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Fry RC, Palmer VS, Kisby GE (2012) Western Pacific ALS-PDC: a prototypical neurodegenerative disorder linked to DNA damage and aberrant proteogenesis? Front Neurol 3:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp L (2017) Stata Statistical Software: Release 15. In. College Station, TX [Google Scholar]
- Steinmaus C, Smith AH, Jones RM, Smith MT (2008) Meta-analysis of benzene exposure and non-Hodgkin lymphoma: biases could mask an important association. Occup Environ Med 65(6):371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup NE, Blair A, Erikson GE (1986) Brain cancer and other causes of death in anatomists. J Natl Cancer Inst 77(6):1217–1224 [PubMed] [Google Scholar]
- Su Z, Chen J, Qiu Y, Yuan Y, Zhu F, Zhu Y, Liu X, Pu Y, He C (2013) Olfactory ensheathing cells: the primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia 61(4):490–503 [DOI] [PubMed] [Google Scholar]
- Swenberg JA, Lu K, Moeller BC, Gao L, Upton PB, Nakamura J, Starr TB (2011) Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and risk assessment. Toxicol Sci 120(Suppl 1):S130–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo BR, Rudel R, Kosik KS, Lee VM, Neff S, Adelman L, Kauer JS (1989) Pathological changes in olfactory neurons in patients with Alzheimer’s disease. Nature 337(6209):736–739 [DOI] [PubMed] [Google Scholar]
- Tang X, Bai Y, Duong A, Smith MT, Li L, Zhang L (2009) Formaldehyde in China: production, consumption, exposure levels, and health effects. Environ Int 35(8):1210–1224 [DOI] [PubMed] [Google Scholar]
- Tang XQ, Fang HR, Zhou CF, Zhuang YY, Zhang P, Gu HF, Hu B (2013) A novel mechanism of formaldehyde neurotoxicity: inhibition of hydrogen sulfide generation by promoting overproduction of nitric oxide. PLoS ONE 8(1):e54829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner C, Chen B, Wang W, Peng M, Liu Z, Liang X, Kao L, Gilley D, Goetz C, Schoenberg B (1989) Environmental factors and Parkinson’s disease: a case-control study in China. Neurology 39(5):660–660 [DOI] [PubMed] [Google Scholar]
- Thomas TL, Waxweiler RJ (1986) Brain-tumors and occupational risk-factors—a review. Scand J Work Environ Health 12(1):1–15 [DOI] [PubMed] [Google Scholar]
- Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC, Wolters EC (2001) Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Movement disorders: official journal of the Movement Disorder Society 16(1):41–46 [DOI] [PubMed] [Google Scholar]
- Tong Z, Wang W, Luo W, Lv J, Li H, Luo H, Jia J, He R (2017) Urine formaldehyde predicts cognitive impairment in post-stroke dementia and Alzheimer’s disease. J Alzheimers Dis 55(3):1031–1038 [DOI] [PubMed] [Google Scholar]
- Toyoshima S, Watanabe F, Saido H, Pezacka EH, Jacobsens DW, Miyatake K, Nakano Y (1996) Accumulation of methylmalonic acid caused by vitamin B 12-deficiency disrupts normal cellular metabolism in rat liver. Br J Nutr 75(6):929–938 [DOI] [PubMed] [Google Scholar]
- Tulpule K, Dringen R (2011) Formaldehyde stimulates Mrp1-mediated glutathione deprivation of cultured astrocytes. J Neurochem 116(4):626–635 [DOI] [PubMed] [Google Scholar]
- Tulpule K, Schmidt MM, Boecker K, Goldbaum O, Richter-Landsberg C, Dringen R (2012) Formaldehyde induces rapid glutathione export from viable oligodendroglial OLN-93 cells. Neurochem Int 61(8):1302–1313 [DOI] [PubMed] [Google Scholar]
- Tyas SL, Manfreda J, Strain LA, Montgomery PR (2001) Risk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol 30(3):590–597 [DOI] [PubMed] [Google Scholar]
- Vanacore N, Bonifati V, Fabbrini G, Colosimo C, De Michele G, Marconi R, Stocchi F, Nicholl D, Bonuccelli U, De Mari M (2005) Case—control study of multiple system atrophy. Movement disorders: official journal of the Movement Disorder Society 20(2):158–163 [DOI] [PubMed] [Google Scholar]
- Viguera C, Wang J, Mosmiller E, Cerezo A, Maragakis NJ (2018) Olfactory dysfunction in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 5(8):976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD, Vu N-D, Wu YN (1993) PC12 cells as a model for neuronal secretion. In: Botulinum and tetanus neurotoxins: neurotransmission and biomedical aspects. Edited by DasGupta BR. Boston, MA: Springer US; 105–115 [Google Scholar]
- Wang Y, Zhang RW, Jiang XZ (1989) Investigation of tumor-induced death in anatomical operators. J Labour Med 4:11–13 [Google Scholar]
- Warner MD, Peabody CA, Flattery JJ, Tinklenberg JR (1986) Olfactory deficits and Alzheimer’s disease. Biol Psychiat 21(1):116–118 [DOI] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Benditó G, Molnár Z, Becher MW, Valenzuela CF, Partridge LD (2002) Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci 5(1):19. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Morozova N, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, Ascherio A (2009) Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 80(5):558–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling R, Beaumont JJ, Petersen SJ, Alexeeff GV, Steinmaus C (2015) Chromium VI and stomach cancer: a meta-analysis of the current epidemiological evidence. Occup Environ Med 72(2):151–159 [DOI] [PubMed] [Google Scholar]
- Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2009) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute. Available in March 2016 [Google Scholar]
- Wong O (1983) An epidemiologic mortality study of a cohort of chemical workers potentially exposed to formaldehyde with a discussion on SMR and PMR. In: Gibson JE (ed) Formaldehyde toxicity. Hemisphere Pub. Corp, Washington, pp 256–272 [Google Scholar]
- Yamagishi M, Ishizuka Y, Seki K (1994) Pathology of olfactory mucosa in patients with Alzheimer’s disease. Ann Otol Rhinol Laryngol 103(6):421–427 [DOI] [PubMed] [Google Scholar]
- Yang M, Miao J, Rizak J, Zhai R, Wang Z, Huma T, Li T, Zheng N, Wu S, Zheng Y et al. (2014) Alzheimer’s disease and methanol toxicity (part 2): lessons from four rhesus macaques (Macaca mulatta) chronically fed methanol. J Alzheimers Dis 41(4):1131–1147 [DOI] [PubMed] [Google Scholar]
- Ye X, Ji Z, Wei C, McHale CM, Ding S, Thomas R, Yang X, Zhang L (2013) Inhaled formaldehyde induces DNA-protein crosslinks and oxidative stress in bone marrow and other distant organs of exposed mice. Environ Mol Mutagen 54(9):705–718 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Magana LC, Cui H, Huang J, McHale CM, Yang X, Looney MR, Li R, Zhang L (2020a) Formaldehyde-induced hematopoietic stem and progenitor cell toxicity in mouse lung and nose. Archives of toxicology [DOI] [PMC free article] [PubMed]
- Zhao Y, Wei L, Tagmount A, Loguinov A, Sobh A, Hubbard A, McHale CM, Chang CJ, Vulpe CD, Zhang L (2020b) Applying genome-wide CRISPR to identify known and novel genes and pathways that modulate formaldehyde toxicity. Chemosphere, 128701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rana I (2018a) Formaldehyde-associated brain tumors. In: Formaldehyde 192–210 [Google Scholar]
- Zhang L, Rana I (2018b) Formaldehyde-associated neurodegenerative diseases. In: Formaldehyde 211–240 [Google Scholar]
- Zhang L, Rana I, Taioli E, Shaffer RM, Sheppard L (2019) Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: a meta-analysis and supporting evidence. Mutation Research/Reviews in Mutation Research [DOI] [PMC free article] [PubMed]
- Zhang L, Steinmaus C, Eastmond DA, Xin XK, Smith MT (2009) Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat Res 681(2–3):150–168 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yan W, Bai Y, Zhu Y, Ma J (2014) Repeated formaldehyde inhalation impaired olfactory function and changed SNAP25 proteins in olfactory bulb. Int J Occup Environ Health 20(4):308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzon M, Zivadinov R, Nasuelli D, Dolfini P, Bosco A, Bratina A, Tommasi M, Locatelli L, Cazzato G (2003) Risk factors of multiple sclerosis: a case-control study. Neurol Sci 24(4):242–247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.