Abstract
Background:
Previous studies have reported similarities in long-term outcomes following lung transplantation for connective tissue disease-associated interstitial lung disease (CTD-ILD) and idiopathic pulmonary fibrosis (IPF). However, it is unknown whether CTD-ILD patients are at increased risk of primary graft dysfunction (PGD), delays in extubation, or longer index hospitalizations following transplant compared to IPF patients.
Methods:
We performed a multicenter retrospective cohort study of CTD-ILD and IPF patients enrolled in the Lung Transplant Outcomes Group registry who underwent lung transplantation between 2012 and 2018. We utilized mixed effects logistic regression and stratified Cox proportional hazards regression to determine whether CTD-ILD was independently associated with increased risk for grade 3 PGD or delays in post-transplant extubation and hospital discharge compared to IPF.
Results:
32.7% (33/101) of patients with CTD-ILD and 28.9% (145/501) of patients with IPF developed grade 3 PGD 48-72 hours after transplant. There were no significant differences in odds of grade 3 PGD among patients with CTD-ILD compared to those with IPF (adjusted OR 1.12, 95% CI 0.64-1.97, p=0.69), nor was CTD-ILD independently associated with a longer post-transplant time to extubation (adjusted HR for first extubation 0.87, 95% CI 0.66-1.13, p=0.30). However, CTD-ILD was independently associated with a longer post-transplant hospital length of stay (median 23 days [IQR 14-35 days] vs.17 days [IQR 12-28 days], adjusted HR for hospital discharge 0.68, 95% CI 0.51-0.90, p=0.008).
Conclusion:
Patients with CTD-ILD experienced significantly longer post-operative hospitalizations compared to IPF patients without an increased risk of grade 3 PGD.
Keywords: Lung transplantation, primary graft dysfunction, connective tissue disease-associated interstitial lung disease
Background
Interstitial lung disease due to systemic autoimmune rheumatic diseases such as systemic sclerosis, rheumatoid arthritis, dermatomyositis, and polymyositis contributes significantly to morbidity and mortality.1 Lung transplantation is a potentially life-saving intervention for patients with connective tissue disease-associated interstitial lung disease (CTD-ILD). However, medical centers are often reluctant to offer transplant to patients with CTD-ILD due to concerns that extra-pulmonary manifestations of their underlying autoimmune disease may limit long-term survival.2 These concerns have been assuaged by several recent studies demonstrating comparable outcomes for patients with CTD-ILD and idiopathic pulmonary fibrosis (IPF), a widely accepted indication for lung transplantation.3-7 In addition, similar mortality rates after transplant have been observed between patients with systemic sclerosis-associated lung disease and those with pulmonary arterial hypertension, another widely accepted indication for transplant.7-9
Only a limited number of studies, most of which largely underrepresent CTD-ILD, have reported specifically on short-term in-hospital outcomes such as primary graft dysfunction (PGD) and hospital length of stay (LOS) following lung transplantation.10-14 PGD is a form of acute lung injury characterized by hypoxemia and alveolar infiltrates in the allograft(s) that occurs within 72 hours after transplant.15 The highest grade of PGD, grade 3, has been associated with a significantly longer duration of mechanical ventilation and post-transplant hospital LOS, as well as increased 90-day and one-year mortality compared to absent or lower grades of PGD.16-19
Despite a steady increase in lung transplantation for patients with CTD-ILD,20 little is understood about whether this unique population carries increased risk for the development of severe PGD and whether a higher incidence of PGD, along with other contributing factors, prolongs time to extubation or hospital LOS following transplant. The purpose of our study was to compare differences in incidence of grade 3 PGD, post-transplant time to extubation, and post-transplant hospital LOS between CTD-ILD and IPF patients. Given the critical role that systemic inflammation plays in the development of PGD,21 we hypothesized that CTD-ILD patients would be at higher risk of grade 3 PGD compared to IPF patients, which, in turn, would result in significant delays in extubation and increases in hospital LOS following transplant.
Methods
Study Design and Patient Population
We performed a retrospective cohort study of adults enrolled in the Lung Transplant Outcomes Group (LTOG) registry who underwent single or bilateral lung transplantation between July 2012 and August 2018. The LTOG registry is a multicenter prospective cohort study of patients aged 18-80 years who received a lung transplant at one of 10 participating U.S. medical centers.22-26 Subjects were included in the present study if they had a diagnosis of CTD-ILD or IPF. Of note, CTD patients with predominantly pulmonary arterial hypertension and minimal ILD were listed for transplant with a diagnosis of CTD-associated pulmonary arterial hypertension rather than CTD-ILD and were therefore not included in our study sample. Specific CTD diagnoses were extracted from the United Network for Organ Sharing (UNOS) database. Subjects were excluded if they received a combined heart-lung transplant or a bilateral lobar transplant. The institutional review boards at all participating centers approved the study, and all patients provided written informed consent.
Outcome Definitions
The primary outcome was grade 3 PGD at 48 or 72 hours after reperfusion, an outcome previously validated and utilized in earlier observational studies.16,18,24,27,28 Severity of PGD was assessed prospectively according to the 2017 International Society for Heart and Lung Transplantation (ISHLT) guidelines, which define grade 3 PGD as a partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2/FiO2) ratio <200 and the presence of infiltrates within the allograft(s) on chest radiograph imaging.15 Patients on extracorporeal life support who had supportive radiographic findings were classified as having grade 3 PGD. PGD was graded by two physicians blinded to participants’ clinical information who independently interpreted each subject’s chest radiographs with adjudication of conflicts by a third physician (kappa for grade 3 PGD classification was 0.95).18 Patients who died within 72 hours of transplant but who fulfilled criteria for grade 3 PGD were classified as having developed PGD. Dates of first extubation and index hospitalization discharge following transplant were used to quantify post-transplant time to extubation and post-transplant hospital LOS, respectively.
Statistical Analysis
Differences in baseline patient characteristics were analyzed using the Student’s t-test, Mann-Whitney U test, chi-squared test, or Fisher’s exact test, as appropriate. Individual data elements had varying degrees of missingness, ranging from 0-26%. To address any data missingness, we applied the method of chained equations by creating 10 imputed datasets using binomial, ordinal, and linear regression models for missing data.29-31 After the imputation process, out-of-range values were truncated to fall within an appropriate clinical range.
We performed univariate and multivariable mixed effects logistic regression to assess whether CTD-ILD patients were at higher risk of grade 3 PGD compared to IPF patients. A random effect for a patient’s transplant center was added to our multivariable model given the variability in incidence of PGD observed across participating centers and because prior research has shown differences in survival among lung transplant centers within the U.S.32 The following covariates were selected a priori for inclusion in our multivariable model based on preexisting mechanistic and biological knowledge: age, sex, race (Caucasian, Black, or other), body mass index (BMI), lung allocation score, intraoperative mean pulmonary arterial pressure (mPAP), transplant type (single or bilateral), use of intraoperative cardiopulmonary bypass or extracorporeal membrane oxygenation, and pre-transplant corticosteroid use.10,18,25,33-35 Corticosteroid use and non-corticosteroid immunosuppressant use were colinear, so only corticosteroid use was included in our multivariable model as it had stronger correlation with grade 3 PGD.
We generated Kaplan-Meier curves to graphically depict differences in post-transplant time to extubation and hospital LOS among survivors to first extubation and to hospital discharge, respectively. To examine the associations between listing diagnosis and both (1) post-transplant time to extubation and (2) post-transplant hospital LOS among survivors, we modeled listing diagnosis (CTD-ILD vs. IPF) as the independent binary variable of interest in Cox proportional hazards regression models where (1) time to extubation and (2) time to discharge were the dependent variables (i.e., first extubation and hospital discharge were the “events” in our analyses).36 For all of our analyses, a hazard ratio of less than 1 suggests a lower likelihood of achieving the event of interest (i.e., a lower likelihood of extubation and a lower likelihood of hospital discharge). We stratified our Cox models according to transplant center and adjusted for the same covariates that we did in our multivariable logistic regression model. The proportional hazards assumption was assessed via weighted versions of Kaplan-Meier curves using log-log plots and tests and graphical displays of Schoenfeld and scaled Schoenfeld residuals. We performed a sensitivity analysis in which in-hospital death after transplant was modeled as a competing risk, although death during the index hospitalization occurred in <5% of patients in our study population. Finally, we generated post-transplant hospital LOS models in which both grade 3 PGD and logarithmically-transformed time to extubation data were included as additional covariates.
According to our power calculations, an estimated 456 patients (380 with IPF and 76 with CTD-ILD) would be required to achieve 80% power at a two-sided alpha of 0.05 to detect a significant difference in risk for grade 3 PGD between patients with IPF and those with CTD-ILD, assuming a grade 3 PGD incidence of 15% among IPF patients18 and 30% among CTD-ILD patients.14 Statistical significance was defined as p<0.05. All analyses were performed using Stata/IC, version 15.1 (College Station, TX).
Results
During the study period, 872 study subjects with ILD underwent lung transplantation. After excluding those with an alternative ILD diagnosis or those who received a combined heart-lung transplant or a bilateral lobar transplant, 602 subjects remained (101 with CTD-ILD and 501 with IPF; Figure 1). Compared to those with IPF, patients with CTD-ILD were younger (median age 56 [IQR 47-64] years vs. 65 [IQR 59-69] years, p<0.001), a greater proportion were female (58.0% vs. 23.7%, p<0.001), and a smaller proportion were Caucasian (71.6% vs. 89.9%, p<0.001; Table 1). In addition, CTD-ILD patients had higher lung allocation scores (median score 47.2 [IQR 41.4-58.8] vs. 43.3 [38.0-54.5], p<0.001) and were more frequently prescribed pre-transplant immunosuppressive medications than those with IPF (corticosteroid use: 69.7% vs. 35.0%, p<0.001; non-corticosteroid immunosuppressant use: 61.8% vs. 15.1%, p<0.001). Finally, a greater proportion of patients with CTD-ILD underwent a bilateral lung transplant compared to those with IPF (83.3% vs. 61.6%, p<0.001). No differences were observed in BMI, intraoperative mPAP measurements, and intraoperative use of cardiopulmonary bypass or extracorporeal membrane oxygenation between the two groups.
Figure 1:
Flow diagram demonstrating how the study population was derived.
Table 1:
Baseline sociodemographic and clinical characteristics of study participants
| Total | CTD-ILD | IPF | P value | ||
|---|---|---|---|---|---|
| N | 602 | 101 | 501 | ||
| Age at transplant (years), median (IQR)* | 64 (58, 68) | 56 (47, 64) | 65 (59, 69) | <0.001 | |
| Female sex (%) | 173 (29.6) | 58 (58.0) | 115 (23.7) | <0.001 | |
| Race (%) | White/Caucasian | 504 (86.9) | 68 (71.6) | 436 (89.9) | <0.001 |
| Black/African American | 47 (8.1) | 21 (22.1) | 26 (5.4) | ||
| Other | 29 (5.0) | 6 (6.3) | 23 (4.7) | ||
| Ethnicity (%) | Hispanic/Latino | 29 (5.1) | 10 (10.4) | 19 (4.0) | 0.01 |
| Not Hispanic/Latino | 539 (94.9) | 86 (89.6) | 453 (96.0) | ||
| BMI (kg/m2), mean (SD) | 26.7 (3.6) | 26.1 (4.1) | 26.8 (3.5) | 0.10 | |
| Lung allocation score, median (IQR) | 43.8 (38.6, 56.4) | 47.2 (41.4, 58.8) | 43.3 (38.0, 54.5) | <0.001 | |
| Intraoperative mPAP (mmHg), median (IQR) | 28.0 (22.0, 36.3) | 29.3 (22.7, 38.3) | 28.0 (21.7, 36.0) | 0.21 | |
| Transplant type (%) | Single | 203 (34.8) | 16 (16.7) | 187 (38.4) | <0.001 |
| Bilateral | 380 (65.2) | 80 (83.3) | 300 (61.6) | ||
| Intraoperative cardiopulmonary bypass or ECMO (%) | 344 (62.4) | 68 (70.8) | 276 (60.7) | 0.06 | |
| Pre-transplant corticosteroid use (%) | 206 (40.2) | 53 (69.7) | 153 (35.0) | <0.001 | |
| Non-corticosteroid immunosuppressant use (%) | 113 (22.0) | 47 (61.8) | 66 (15.1) | <0.001 | |
Percent missing: age at transplant = 2.7; female sex = 2.8; race = 3.7; ethnicity = 5.7; BMI = 4.8; lung allocation score = 5.7; intraoperative mPAP = 26.1; transplant type = 3.2; intraoperative cardiopulmonary bypass or ECMO = 8.5; pre-transplant corticosteroid use = 14.8; non-corticosteroid immunosuppressant use = 14.6.
Abbreviations: CTD-ILD = connective tissue disease-associated interstitial lung disease; IPF = idiopathic pulmonary fibrosis; IQR = interquartile range; BMI = body mass index; SD = standard deviation; mPAP = mean pulmonary arterial pressure; ECMO = extracorporeal membrane oxygenation.
Among CTD-ILD patients, the most commonly represented autoimmune diseases were systemic sclerosis (n=42, 41.6%), rheumatoid arthritis (n=11, 11.9%), and mixed connective tissue disease (n=5, 5.0%). Sjogren’s syndrome, dermatomyositis and polymyositis, and systemic lupus erythematosus each comprised less than 5% of our CTD-ILD study population. Approximately one-third of patients (n=33, 32.7%) had a systemic autoimmune rheumatic disease that was not specified in the UNOS database (e-Supplemental Table 1).
Out of 602 participating subjects, 178 (30.0%) were diagnosed with grade 3 PGD 48-72 hours after reperfusion – 145 out of 501 (28.9%) patients with IPF and 33 out of 101 (32.7%) patients with CTD-ILD. Odds of grade 3 PGD were similar between subjects with CTD-ILD and IPF in both our univariate analysis (OR 1.19, 95% CI 0.75-1.88, p=0.45) and our multivariable-adjusted analysis (1.12, 95% CI 0.64-1.97, p=0.69; Table 2). Comparable multivariable-adjusted point estimates were observed using a complete case analysis approach in lieu of multiple imputation, in which only subjects with complete data were included (OR 1.20, 95% CI 0.62-2.34, p=0.59).
Table 2:
Association between interstitial lung disease diagnosis and incidence of grade 3 primary graft dysfunction
| Lung disease | No. at risk | No. with grade 3 PGD (%) |
Univariate | Multivariable* | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |||
| IPF | 501 | 145 (28.9) | Ref. | - | - | Ref. | - | - |
| CTD-ILD | 101 | 33 (32.7) | 1.19 | 0.75-1.88 | 0.45 | 1.12 | 0.64-1.97 | 0.69 |
Multivariable model is adjusted for age at transplant, sex, race (Caucasian, Black, or other), body mass index, lung allocation score, intraoperative mean pulmonary arterial pressure, type of transplant (single or bilateral), use of intraoperative cardiopulmonary bypass or extracorporeal membrane oxygenation, and pre-transplant corticosteroid use and includes transplant center as a random effect.
Abbreviations: PGD = primary graft dysfunction; OR = odds ratio; CI = confidence interval; IPF = idiopathic pulmonary fibrosis; CTD-ILD = connective tissue disease-associated interstitial lung disease.
Extubation data were available for 578 out of 602 subjects, 163 (28.2%) of whom were women. Twelve of the 24 subjects (50%) with missing extubation data were women (p=0.021). Baseline demographics otherwise did not statistically significantly differ between patients with and without extubation data. In addition, there were no differences noted in risk of death prior to or on the day of first extubation between CTD-ILD and IPF patients (n=0 [0.0%] for CTD-ILD vs. n=11 [2.3%] for IPF, p=0.14). Among survivors to first extubation (n=567), median post-transplant time to extubation was 2 days for both CTD-ILD and IPF with differences noted in interquartile ranges (CTD-ILD IQR 1-11 days, IPF IQR 1-5 days, p=0.02; Table 3). Unadjusted Kaplan-Meier estimates for time to extubation among survivors are shown in Figure 2A (log-rank test p=0.005). In our univariate analysis, subjects with CTD-ILD had a longer time to extubation (i.e., a lower “risk” of extubation) than those with IPF (HR 0.73, 95% CI 0.58-0.91, p=0.005; Table 3). However, after adjustment for prespecified clinical covariates, post-transplant time to extubation was similar between CTD-ILD and IPF patients (HR 0.87, 95% CI 0.66-1.13, p=0.30). A multivariable analysis in which death prior to or on the day of first extubation was modeled as a competing risk yielded a similar sub-hazard ratio (SHR 1.03, 95% CI 0.82-1.29, p=0.81; Table 3).
Table 3:
Associations between interstitial lung disease diagnosis and both time to extubation and hospital length of stay following lung transplantation
| Pre- transplant diagnosis |
No. at risk | Duration (days), median (IQR) |
Univariate Cox Model | Multivariable Cox Model* | Multivariable Competing Risk Model* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR† | 95% CI | P value | HR† | 95% CI | P value | SHR† | 95% CI | P value | |||
| Time to Extubation | |||||||||||
| IPF | 471 | 2 (1, 5) | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| CTD-ILD | 96 | 2 (1, 11)§ | 0.73 | 0.58-0.91 | 0.005 | 0.87 | 0.66-1.13 | 0.30 | 1.03 | 0.82-1.29 | 0.81 |
| Hospital Length of Stay | |||||||||||
| IPF | 465 | 17 (12, 28) | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| CTD-ILD | 92 | 23 (14, 35)§ | 0.74 | 0.59-0.93 | 0.01 | 0.68 | 0.51-0.90 | 0.008 | 0.76 | 0.57-1.01 | 0.06 |
Multivariable models are adjusted for age at transplant, sex, race (Caucasian, Black, or other), body mass index, lung allocation score, intraoperative mean pulmonary arterial pressure, type of transplant (single or bilateral), use of intraoperative cardiopulmonary bypass or extracorporeal membrane oxygenation, and pre-transplant corticosteroid use with stratification by transplant center.
A hazard or sub-hazard ratio less than 1 suggests a lower likelihood of achieving the event of interest (i.e., first extubation or hospital discharge).
P value for Mann-Whitney U test comparing CTD-ILD to IPF <0.05.
Abbreviations: IQR = interquartile range; HR = hazard ratio; CI = confidence interval; SHR = sub-hazard ratio; IPF = idiopathic pulmonary fibrosis; CTD-ILD = connective tissue disease-associated interstitial lung disease.
Figure 2:
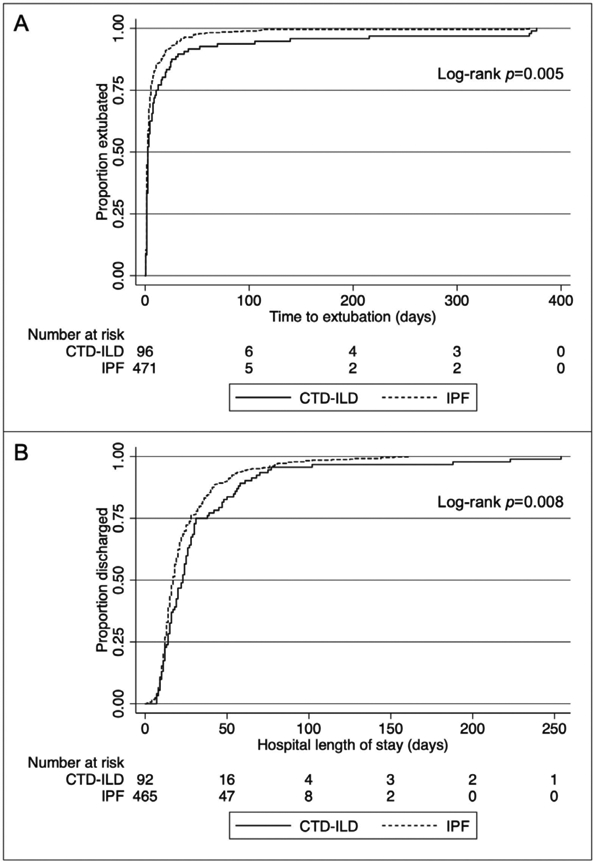
Unadjusted Kaplan-Meier estimates for A) post-transplant time to extubation among survivors to first extubation (n=567, log-rank test p=0.005) and B) post-transplant hospital length of stay among survivors to hospital discharge (n=557, log-rank test p=0.008).
Hospital discharge data were available for 577 out of 602 subjects, 162 (28.1%) of whom were female. Similar to the extubation data, 13 of the 25 subjects (52%) missing discharge data were women (p=0.01). Baseline demographics otherwise did not statistically significantly differ between patients with and without discharge data. In addition, there were no differences noted in risk of in-hospital death between CTD-ILD and IPF patients (n = 3 [3.2%] for CTD-ILD vs. n=17 [3.5%] for IPF, p=0.86). Among survivors to hospital discharge (n=557), median post-transplant hospital LOS was 23 days (IQR 14-35 days) for CTD-ILD and 17 days (IQR 12-28 days) for IPF patients (p=0.01; Table 3). Unadjusted Kaplan-Meier estimates for time to hospital discharge among survivors are shown in Figure 2B (log-rank test p=0.008). Among patients who survived to hospital discharge, subjects with CTD-ILD had a significantly longer post-transplant hospital LOS (i.e., a lower “risk” of hospital discharge) than those with IPF (HR 0.74, 95% CI 0.59-0.93, p=0.01; Table 3), a relationship that persisted after adjustment for prespecified clinical covariates (HR 0.68, 95% CI 0.51-0.90, p=0.008). A multivariable analysis in which in-hospital death was modeled as a competing risk yielded a similar sub-hazard ratio (SHR 0.76, 95% CI 0.57-1.01, p=0.06).
Finally, the relationship between diagnosis and post-transplant hospital LOS remained statistically significant with inclusion of grade 3 PGD and log-transformed time to extubation as additional covariates in a multivariable model, suggesting neither accounted for differences observed in post-transplant hospital LOS between CTD-ILD and IPF patients. In this model, both grade 3 PGD and log-transformed time to extubation were highly associated with post-transplant hospital LOS (p<0.001 for both).
Discussion
We did not detect an association between a diagnosis of CTD-ILD and PGD risk following lung transplantation relative to a diagnosis of IPF. CTD-ILD patients had significantly longer hospitalizations after transplant compared to IPF patients, but there was no significant difference between groups in time to extubation after transplant.
Our findings demonstrating a comparable risk for PGD are concordant with results from a recent single-center study of 15 CTD-ILD and 47 IPF patients.14 Several downstream consequences have been described as a result of PGD, including increased 90-day and one-year mortality rates.18 In addition, multiple studies have shown an association between PGD and risk for the subsequent development of chronic lung allograft dysfunction (CLAD).19,37-40 A large retrospective cohort study of non-systemic sclerosis CTD-ILD and IPF transplant recipients demonstrated similar risk for bronchiolitis obliterans syndrome, a subtype of CLAD.3 This observation may be explained in part by comparable rates of PGD, as demonstrated by our findings, although the authors did not specifically investigate this. In addition, a similar incidence of PGD may be one of many contributing factors leading to similarities in long-term outcomes among CTD-ILD and non-CTD-ILD transplant recipients, as described in previous studies.3-7,41
We anticipated delays in extubation among CTD-ILD patients relative to IPF patients, potentially driven by extra-pulmonary CTD manifestations (e.g., esophageal dysmotility in systemic sclerosis or muscle weakness in dermatomyositis or polymyositis); however, no significant differences in time to extubation were observed. Conversely, we did observe significant differences in post-transplant hospital LOS between these two groups. Specifically, median LOS was 6 days longer for CTD-ILD patients than for IPF patients, which is an increase of over 35%. This difference does not appear to have been driven by higher rates of PGD or delays in extubation, a finding supported by our sensitivity analysis in which PGD and log-transformed time to extubation were included as additional covariates in our multivariable model. Given the high proportion of systemic sclerosis patients present in our study (over 40% of the CTD-ILD patients had systemic sclerosis), one potential explanation for this difference in hospital LOS is the need to manage esophageal dysmotility prior to discharge,42,43 although these data are not specifically captured within the LTOG registry. Over 90% of systemic sclerosis patients have esophageal dysmotility,44 and many of these patients may have required placement of gastric or gastro-jejunal tubes for enteral nutrition prior to hospital discharge. Pre-transplant corticosteroid use likely does not account for the differences observed in post-transplant hospital LOS between CTD-ILD and IPF patients, as it was not significantly associated with post-transplant hospital LOS in our multivariable Cox model. In a previous study, patients who received pre-transplant corticosteroids did not have a longer overall hospital LOS or intensive care unit LOS following lung transplant than those who did not receive pre-transplant corticosteroids, although most patients carried a pre-transplant diagnosis of chronic obstructive pulmonary disease and not CTD-ILD.13
Our study has some limitations. Due to concerns for model overfitting, only a limited number of PGD risk factors could be included in our multivariable analyses, thus resulting in the potential for residual confounding. However, we intentionally selected covariates that have been found to be most significantly associated with PGD risk and omitted those that have been shown to be more weakly associated with PGD risk. There is a possibility that unmeasured confounding or bias due to missing data may have influenced our results. Although multiple imputation is a validated approach to address data missingness, some covariates had large percentages of missing data (e.g., 26% for intraoperative mPAP measurements and 15% for pre-transplant corticosteroid use), potentially leading to inflated variances caused by uncertainties of imputation. However, we observed similar multivariable-adjusted odds ratios in our PGD models when using imputed data or a complete case analysis approach. A limited number of subjects had missing extubation and hospital discharge data and thus were excluded from these analyses, potentially biasing our results. However, we compared baseline demographics between patients with and without extubation and discharge data and only noted statistically significant differences with regard to sex, suggesting these data were likely missing at random. Moreover, modeling in-hospital death as a competing risk in both the extubation and hospital discharge multivariable Cox proportional hazards regression models resulted in similar sub-hazard ratios compared to the original multivariable models. In addition, not all patients undergoing transplantation at an LTOG site were enrolled, thus potentially introducing selection bias. However, prior LTOG studies have demonstrated similar PGD risk profiles between enrolled and non-enrolled patients.18
Given the observational nature of our study, we were unable to definitively exclude other disease processes that could have resulted in similar radiographic findings compared to PGD (e.g., significant pulmonary contusion or multifocal pneumonia). However, our grade 3 PGD outcome definition has been validated and applied in numerous prior observational studies.16,18,24,27,28,45 For example, one prior multicenter prospective cohort study tested the discriminate validity of the ISHLT PGD grades with lung injury biomarker profiles and survival and showed that grade 3 PGD was associated with the most severely altered plasma biomarker profile and the worst outcomes, regardless of the time point of grading.16 In this study, PGD grade at 48 and 72 hours discriminated mortality better than PGD grade at 24 hours. More recently, a study utilizing the LTOG registry demonstrated that later time points for assessment of PGD grade (48-72 hours) had better discrimination for mortality and that all grading constructs based on P/F ratios performed well regardless of transplant type or mechanical ventilation status.45
Our study was inadequately powered to stratify analyses according to patients’ underlying autoimmune diseases, nor did we have the ability to study potential mechanisms driving differences in post-transplant LOS. Finally, we were unable to validate each subject’s diagnosis via chart review, although pulmonologists and rheumatologists at participating medical centers established diagnoses of IPF and CTD-ILD through multidisciplinary discussions and published classification criteria.46-50
In conclusion, CTD-ILD patients experienced significantly longer post-operative hospitalizations after lung transplantation compared to IPF patients. Additional research is needed to identify causal factors responsible for these differences. We did not, however, detect an increased risk of PGD in younger patients with CTD-ILD compared to those with IPF. Our findings add to a growing body of evidence that suggests carefully selected candidates with CTD-ILD have similar short- and long-term outcomes compared to patients with non-CTD-ILD and can safely undergo lung transplantation.
Supplementary Material
Acknowledgements and Funding
We gratefully acknowledge our research coordinators for their work on patient recruitment, data acquisition, and specimen storage and our patients for their participation in this study. We additionally acknowledge the methodological input of Michael O. Harhay, Ph.D. This work was supported by the National Heart, Lung, and Blood Institute, grant numbers T32HL007891 (JGN), K24HL103844 (SMK), K24HL15354 (JDC), U01HL145435 (JDC), and R01HL087115 (JDC), the National Institute of Arthritis and Musculoskeletal and Skin Diseases, grant number K23AR075112 (EJB), and the Rheumatology Research Foundation Scientist Development Award (EJB).
Footnotes
Disclosures
JGN, JMD, MKP, KMW, ABW, JBO, PDS, VNL, JFM, LDS, CAH, EC, MO, LK, JDC, and SMK have no relevant conflicts of interest. DJL is a full-time employee of Regeneron Pharmaceuticals, Inc. and owns Regeneron stock and stock options. JPS is a member of the Scientific Advisory Board of Altavant Sciences. LBW reports consulting fees from Merck, Bayer, Boehringer Ingelheim, CSL Behring, Quark, Foresee Pharmaceuticals, and Citius and research contracts from Genentech and CSL Behring, none of which are relevant to this manuscript. EJB reports consulting fees from Boehringer Ingelheim and Eicos Sciences, research grants from Boehringer Ingelheim and Pfizer, and clinical trial funding from Boehringer Ingelheim, Eicos Sciences, and Corbus Pharmaceuticals, all outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antin-Ozerkis D, Highland KB. Thoracic Manifestations of Rheumatic Disease. Clin Chest Med 2019;40:xiii. [DOI] [PubMed] [Google Scholar]
- 2.Lee JC, Ahya VN. Lung transplantation in autoimmune diseases. Clin Chest Med 2010;31:589–603. [DOI] [PubMed] [Google Scholar]
- 3.Courtwright AM, El-Chemaly S, Dellaripa PF, Goldberg HJ. Survival and outcomes after lung transplantation for non-scleroderma connective tissue-related interstitial lung disease. J Heart Lung Transplant 2017;36:763–9. [DOI] [PubMed] [Google Scholar]
- 4.Massad MG, Powell CR, Kpodonu J, Tshibaka C, Hanhan Z, Snow NJ, et al. Outcomes of lung transplantation in patients with scleroderma. World J Surg 2005;29:1510–5. [DOI] [PubMed] [Google Scholar]
- 5.Crespo MM, Bermudez CA, Dew MA, Johnson BA, George MP, Bhama J, et al. Lung transplant in patients with scleroderma compared with pulmonary fibrosis: short- and long-term outcomes. Ann Am Thorac Soc 2016;13:784–92. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705–11. [DOI] [PubMed] [Google Scholar]
- 7.Khan IY, Singer LG, de Perrot M, Granton JT, Keshavjee S, Chau C, et al. Survival after lung transplantation in systemic sclerosis. A systematic review. Respir Med 2013;107:2081–7. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein EJ, Bathon JM, Lederer DJ. Survival of adults with systemic autoimmune rheumatic diseases and pulmonary arterial hypertension after lung transplantation. Rheumatology (Oxford) 2018;57:831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein EJ, Peterson ER, Sell JL, D'Ovidio F, Arcasoy SM, Bathon JM, et al. Survival of adults with systemic sclerosis following lung transplantation: a nationwide cohort study. Arthritis Rheumatol 2015;67:1314–22. [DOI] [PubMed] [Google Scholar]
- 10.Banga A, Mohanka M, Mullins J, Bollineni S, Kaza V, Ring S, et al. Hospital length of stay after lung transplantation: Independent predictors and association with early and late survival. J Heart Lung Transplant 2017;36:289–96. [DOI] [PubMed] [Google Scholar]
- 11.Smith PJ, Rivelli SK, Waters AM, Hoyle A, Durheim MT, Reynolds JM, et al. Delirium affects length of hospital stay after lung transplantation. J Crit Care 2015;30:126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu HH, Chen JS, Ko WJ, Huang SC, Kuo SW, Huang PM, et al. Short-term outcomes of cadaveric lung transplantation in ventilator-dependent patients. Crit Care 2009;13:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SJ, Nguyen DQ, Savik K, Hertz MI, Bolman RM 3rd. Pre-transplant corticosteroid use and outcome in lung transplantation. J Heart Lung Transplant 2001;20:304–9. [DOI] [PubMed] [Google Scholar]
- 14.Park JE, Kim SY, Song JH, Kim YS, Chang J, Lee JG, et al. Comparison of short-term outcomes for connective tissue disease-related interstitial lung disease and idiopathic pulmonary fibrosis after lung transplantation. J Thorac Dis 2018;10:1538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1097–103. [DOI] [PubMed] [Google Scholar]
- 16.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2010;29:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest 2005;127:161–5. [DOI] [PubMed] [Google Scholar]
- 18.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porteous MK, Lee JC. Primary graft dysfunction after lung transplantation. Clin Chest Med 2017;38:641–54. [DOI] [PubMed] [Google Scholar]
- 20.Chambers DC, Cherikh WS, Harhay MO, Hayes D Jr., Hsich E, Khush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38:1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med 2002;165:211–5. [DOI] [PubMed] [Google Scholar]
- 22.Diamond JM, Akimova T, Kazi A, Shah RJ, Cantu E, Feng R, et al. Genetic variation in the prostaglandin E2 pathway is associated with primary graft dysfunction. Am J Respir Crit Care Med 2014;189:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond JM, Porteous MK, Roberts LJ 2nd, Wickersham N, Rushefski M, Kawut SM, et al. The relationship between plasma lipid peroxidation products and primary graft dysfunction after lung transplantation is modified by donor smoking and reperfusion hyperoxia. J Heart Lung Transplant 2016;35:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porteous MK, Lee JC, Lederer DJ, Palmer SM, Cantu E, Shah RJ, et al. Clinical risk factors and prognostic model for primary graft dysfunction after lung transplantation in patients with pulmonary hypertension. Ann Am Thorac Soc 2017;14:1514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med 2011;184:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawut SM, Okun J, Shimbo D, Lederer DJ, De Andrade J, Lama V, et al. Soluble p-selectin and the risk of primary graft dysfunction after lung transplantation. Chest 2009;136:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porteous MK, Ky B, Kirkpatrick JN, Shinohara R, Diamond JM, Shah RJ, et al. Diastolic dysfunction increases the risk of primary graft dysfunction after lung transplant. Am J Respir Crit Care Med 2016;193:1392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah RJ, Diamond JM, Cantu E, Flesch J, Lee JC, Lederer DJ, et al. Objective estimates improve risk stratification for primary graft dysfunction after lung transplantation. Am J Transplant 2015;15:2188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. [DOI] [PubMed] [Google Scholar]
- 30.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 32.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. Jama 2010;304:53–60. [DOI] [PubMed] [Google Scholar]
- 33.Whitson BA, Nath DS, Johnson AC, Walker AR, Prekker ME, Radosevich DM, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg 2006;131:73–80. [DOI] [PubMed] [Google Scholar]
- 34.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest 2003;124:1232–41. [DOI] [PubMed] [Google Scholar]
- 35.Kuntz CL, Hadjiliadis D, Ahya VN, Kotloff RM, Pochettino A, Lewis J, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant 2009;23:819–30. [DOI] [PubMed] [Google Scholar]
- 36.George B, Seals S, Aban I. Survival analysis and regression models. J Nucl Cardiol 2014;21:686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507–13. [DOI] [PubMed] [Google Scholar]
- 38.Balsara KR, Krupnick AS, Bell JM, Khiabani A, Scavuzzo M, Hachem R, et al. A single-center experience of 1500 lung transplant patients. J Thorac Cardiovasc Surg 2018;156:894–905.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher AJ, Wardle J, Dark JH, Corris PA. Non-immune acute graft injury after lung transplantation and the risk of subsequent bronchiolitis obliterans syndrome (BOS). J Heart Lung Transplant 2002;21:1206–12. [DOI] [PubMed] [Google Scholar]
- 40.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004–11. [DOI] [PubMed] [Google Scholar]
- 41.Pradère P, Tudorache I, Magnusson J, Savale L, Brugiere O, Douvry B, et al. Lung transplantation for scleroderma lung disease: an international, multicenter, observational cohort study. J Heart Lung Transplant 2018;37:903–11. [DOI] [PubMed] [Google Scholar]
- 42.Jablonski R, Dematte J, Bhorade S. Lung transplantation in scleroderma: recent advances and lessons. Curr Opin Rheumatol 2018;30:562–9. [DOI] [PubMed] [Google Scholar]
- 43.Miele CH, Schwab K, Saggar R, Duffy E, Elashoff D, Tseng CH, et al. Lung transplant outcomes in systemic sclerosis with significant esophageal dysfunction. A comprehensive single-center experience. Ann Am Thorac Soc 2016;13:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahcene M, Oumnia N, Matougui N, Boudjella M, Tebaibia A, Touchene B. Esophageal involvement in scleroderma: clinical, endoscopic, and manometric features. ISRN Rheumatol 2011;2011:325826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantu E, Diamond JM, Suzuki Y, Lasky J, Schaufler C, Lim B, et al. Quantitative Evidence for Revising the Definition of Primary Graft Dysfunction after Lung Transplant. Am J Respir Crit Care Med 2018;197:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Sadeleer LJ, Meert C, Yserbyt J, Slabbynck H, Verschakelen JA, Verbeken EK, et al. Diagnostic ability of a dynamic multidisciplinary discussion in interstitial lung diseases: a retrospective observational study of 938 cases. Chest 2018;153:1416–23. [DOI] [PubMed] [Google Scholar]
- 47.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–e68. [DOI] [PubMed] [Google Scholar]
- 48.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 49.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 50.Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


