Abstract
Effective treatments for patients with mCRPC following disease progression on enzalutamide is currently an unmet clinical need. Simultaneous inhibition of the HIF-1α and AR pathways has been shown to overcome enzalutamide resistance in vitro. Combination treatment with NLG207, a nanoparticle-drug conjugate of camptothecin and inhibitor of HIF-1α, and enzalutamide was evaluated in preclinical prostate cancer models of enzalutamide resistance. The effect of NLG207 and enzalutamide on average tumor volume and tumor re-growth after 3 weeks of treatment was evaluated in vivo using the subcutaneous 22Rv1 xenograft and castrated subcutaneous VCaP xenograft models. Correlative assessments of anti-tumor activity were evaluated in vitro using cell proliferation and qPCR assays. NLG207 8 mg/kg alone and in combination with enzalutamide reduced average tumor volume by 93% after 3 weeks of treatment (p<0.05) in comparison to vehicle control in the subcutaneous 22Rv1 xenograft model. Notably, the addition of NLG207 also enhanced the efficacy of enzalutamide alone in the castrated subcutaneous VCaP xenograft model, decreasing the median rate of tumor growth by 51% (p=0.0001) in comparison to enzalutamide alone. In vitro assessments of cell proliferation and gene expression further demonstrated anti-tumor activity via AR-HIF-1α crosstalk inhibition. Combination treatment with NLG207 and enzalutamide was shown to be effective in preclinical prostate cancer models of enzalutamide resistance. Clinical investigation of this treatment combination is ongoing (NCT03531827).
INTRODUCTION
Enzalutamide, an androgen receptor antagonist (ARA), and abiraterone acetate, a CYP17 inhibitor, are current primary standard of care treatment options for patients with metastatic castration-resistant prostate cancer (mCRPC).1 Acquired resistance to these agents is inevitable, ultimately resulting in clinical disease progression and initiation of additional lines of therapy.2 Mechanisms of acquired resistance include alterations of DNA damage repair mechanisms, activation of alternative cell signaling pathways (e.g. PI3K, Wnt/β-catenin), and aberrations in androgen receptor (AR) activity.3 Notably, AR-full length (AR-FL) overexpression and expression of AR splice variants (e.g. AR-V7) are commonly associated with disease progression following enzalutamide monotherapy.4–7 Recent clinical investigations often focus on combinatorial treatment approaches aimed to target one or multiple pathways of acquired resistance.
Intratumoral hypoxia serves an important role in prostate cancer aggressiveness and metastatic potential, as cells adapt to hypoxic environments via co-opting blood vessel formation and migrating towards vessels.8–11 Hypoxia-Inducible Factor (HIF)-1α, a transcription factor upregulated in response to hypoxia, is responsible for promoting tumor angiogenesis, anaerobic metabolism, immunity, adaptation, and invasion.11–13 Androgen deprivation can also contribute to prostate cancer cell adaptation to hypoxic environments, upregulating transcriptional activity of the androgen receptor.14,15 Crosstalk between the HIF-1α and AR pathways has been suggested via a ternary complex comprising AR, HIF-1α and β-catenin on androgen response elements of AR target genes.16–18 Thus, targeting HIF-1α was hypothesized to down-regulate AR-mediated gene expression and reduce prostate cancer cell proliferation. Our laboratory previously investigated the dual targeting of both axes via combination treatment of enzalutamide with HIF-1α inhibition in prostate cancer cells to define the molecular mechanisms by which HIF-1α inhibition potentiates anti-AR therapy in CRPC. HIF-1α inhibition, achieved via chetomin (disruptor of HIF-1α-p300 interactions) or siRNA silencing of HIF-1α, in combination with enzalutamide synergistically reduced AR-regulated and HIF-1α-mediated transcription, reduced VEGF protein expression, and inhibited cell growth.18 In 22Rv1 cells, a cell line with enzalutamide resistance mediated via androgen independence and significant AR splice variant expression,19–21 enzalutamide activity was significantly enhanced following HIF-1α inhibition.18
In the current study, we further investigate this therapeutic approach by evaluating the combination treatment of enzalutamide with NLG207, formerly known as CRLX101. NLG207 is a nanoparticle-drug conjugate of camptothecin designed to overcome the poor physicochemical properties associated with small molecule camptothecin, while also utilizing the enhanced permeation and retention (EPR) effect to more optimally facilitate drug delivery to tumors.22,23 Camptothecins, potent inhibitors of topoisomerase I, have previously been shown to block the accumulation of HIF-1α and subsequently the expression of VEGF.24,25 Studies with NLG207 have also demonstrated effective targeting of HIF-1α and inhibition of angiogenesis in models of ovarian cancer, breast cancer and glioblastoma, either as monotherapy or in combination with bevacizumab.26–29 Thus, we assessed the anti-tumor activity of NLG207 in combination with enzalutamide in subcutaneous xenograft models of prostate cancer with clinically relevant mechanisms of acquired resistance to enzalutamide.
MATERIALS AND METHODS
Cell Culture
22Rv1 cells were maintained in phenol red-free RPMI 1640, and VCaP cells were maintained in DMEM, both supplemented with 10% fetal bovine serum (FBS), 50 U/mL Penicillin, and 50 mg/mL Streptomycin. Both 22Rv1 and VCaP cells were purchased from American Type Culture Collection (ATCC; Manassas, VA). ATCC uses Short Tandem Repeat (STR) profiling for testing and authentication of cell lines. Cells were thawed and sub-cultured per ATCC recommendations, with cells designated for in vitro experiments cultured for fewer than 3 months and routinely screened for mycoplasma. For in vivo experiments, cells were thawed directly from original ATCC vials and expanded until the sufficient amount of cells needed for inoculation to generate subcutaneous xenografts (4–7 passages).
Reagents
R1881 and S-(+)-Camptothecin were purchased from Millipore Sigma (Rockville, MD), enzalutamide was purchased from Selleck Chemicals (Houston, TX), and NLG207 was provided by NewLink Genetics (Ames, IA). All doses of NLG207 (mg) were measured using camptothecin (CPT) equivalents, or the mass of CPT contained within the nanoparticle formulation.
Cell Proliferation Assays
22Rv1 and VCaP cells were seeded in 96-well plates in 100 μL 10% Charcoal-Dextran Stripped (CDS) FBS supplemented phenol-red free RPMI 1640 and DMEM (supplemented with 4 mM L-gluatmine) medium, respectively. Cells were seeded at densities of 5,000 and 30,000 cells per well for 22Rv1 and VCaP, respectively. Following 24–48 hour incubation, cells were treated with the corresponding 10% CDS-FBS supplemented media containing 0.1 nM R1881 and either DMSO control, CPT, NLG207, enzalutamide (ENZ), CPT + ENZ, or NLG207 + ENZ (day 0). Cells were re-dosed on day 4 post-treatment. Cell viability was measured on days 0, 3, 5, and 7 using the Cell Counting Kit-8 cell proliferation/cytotoxicity assay according to the manufacturer’s instructions (Dojindo, Rockville, MD), and absorbance was read at 450 nm using a SpectraMax iD3 fluorescence plate reader (Molecular Devices, Sunnyvale, CA).
Semiquantitative Real-Time Polymerase Chain Reaction
VCaP cells were plated at a density of 800,000 cells per well in a 6-well dish in phenol red-free DMEM media supplemented with 10% CDS-FBS for 48 hours. Cells were then treated with 10% CDS-FBS supplemented media with or without 0.1 nM R1881 and combinations of DMSO control, 500 nM CPT, 500 nM NLG207, and/or 500 nM ENZ for 24 hours. Total RNA was extracted using the RNAeasy mini kit (Qiagen, Germantown, MD) per manufacturer’s protocol. Purified RNA (~0.24 μg) was reverse transcribed via 30 μL cDNA synthesis reaction using the SuperScript III First Strand Synthesis System (Invitrogen, Carlsbad, CA) per manufacturer’s protocol.
cDNA synthesis products were amplified in triplicate with forward and reverse primers: KLK3 (Hs02576345_m1, Applied Biosystems, Foster City, CA), ERG (Hs01554629_m1, Applied Biosystems), VEGFA (Hs00900055_m1, Applied Biosystems), LDHA (Hs00855332_g1, Applied Biosystems), and ACTB (Hs99999903_m1, Applied Biosystems). Custom primers (Applied Biosystems) for AR-FL and AR-V7, previously described by Markowski et al., were also used to amplify cDNA.30,31 The specificity of these primers were evaluated in PC3, 22Rv1, and VCaP cells, and compared to LNCaP95 cells. All other primers used were commercially available and previously validated.18,32
Two microliters of cDNA per sample was mixed with 1 μL forward and reverse primers, 7 μL water, and 10 μL Taqman Gene Expression Master Mix (Applied Biosystems) for a total of 20 μL. Semiquantitative real-time polymerase chain reaction (qPCR) was performed using an Applied Biosystems StepOnePlus Real-Time PCR system with StepOne Software. All qPCR reactions were run in triplicate using the standard Taqman protocol for 40 cycles, with use of β-actin (ACTB) as the reference housekeeping gene. Fold-change in RNA levels was calculated using the ΔΔCt method. For AR-FL and AR-V7 primer validation, the same qPCR reaction conditions were used for 30 cycles. PCR products were mixed with DNA gel loading dye (6x; Thermo Fisher Scientific, Waltham, MA), applied to 4–20% TBE gels (Invitrogen) and stained with SYBR Green (Invitrogen). The GeneRuler 50 bp DNA Ladder (Thermo Fisher Scientific) was used as a size standard, and PCR fragments were visualized using an Odyssey Fc Imager (LI-COR Biosciences, Lincoln, NE) (Fig. S1).
Animal Care
All animals were housed in a pathogen-free facility of the National Cancer Institute, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and follows the Public Health Service (PHS) Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the NCI Animal Care and Use Committee (ACUC).
22Rv1 Subcutaneous Xenografts
Approximately 5×106 22Rv1 cells (suspended in DPBS) were subcutaneously injected into the rear flank of six-week old, male, severe combined immunodeficiency (SCID) mice. Tumors were grown to a volume greater than 50 mm3 before stratification into treatment groups (Study 1: n = 4–5; Study 2: n =9–10): vehicle control, ENZ only (25 mg/kg in 50:50 PEG-400:Tween 80 via daily oral gavage), NLG207 4 or 8 mg/kg (in DPBS via weekly i.p. injection) ± ENZ. Mice were treated for 3 weeks, with weight measurements daily and tumor volume measurements three times weekly, using the formula V=(L*W2)/2 (length corresponding to longer dimension of tumor). Following 3 weeks of treatment in the first study, the mice were euthanized and tumors were harvested to obtain final weight and volume (using L*W*H*(π/6)) measurements. In a second study, mice were followed after treatment course completion three times weekly until tumors ulcerated or reached >2 cm in any dimension per NCI ACUC guidance. Mice with >20% reduction in body weight were removed and censored.
Castrated VCaP Subcutaneous Xenograft
Approximately 2.5×106 VCaP cells (suspended in 50:50 DPBS:Matrigel) were injected into the rear flank of six-week old, male, SCID mice. When the average tumor volume reached ~200 mm3 (measured via V = (L*W2)/2), the mice were castrated (removal of the testes facilitated via scrotal incision and vaginal tunic access) under isoflurane anesthesia. The mice were followed up post-surgically for 10 days, and tumors were allowed to regrow to an average volume of ~200 mm3. The mice were then stratified on the basis of tumor volume into 4 treatment groups (n=9–10): vehicle control, ENZ 25 mg/kg daily, NLG207 8 mg/kg once weekly, and combination. Mice were treated for 3 weeks, with tumor measurements obtained 3 times weekly and body weight measurements collected daily. Following 3 weeks of treatment, animals were monitored for tumor size and body weight three times weekly. Mice were removed from study if tumors reached >2cm in any dimension per NCI ACUC guidance; mice with >20% reduction in body weight were removed from study and censored. Following 6 weeks post treatment, all remaining animals were euthanized.
Statistical Analyses
Unpaired t tests were used for between group comparisons of cell proliferation. Tumor growth curves were reported as mean tumor volumes ± SEM and mean percentage change in tumor volume (start of treatment as baseline) ± SEM for the 22Rv1 and VCaP xenograft models, respectively. Body weight curves were reported as mean body weight ± SEM. Comparisons of average tumor volumes, average tumor volume change, average tumor weights, and fold change in gene expression at specified timepoints were made using Mann-Whitney tests. Kaplan-Meier method was used to assess median survival (i.e. time to tumor >2 cm in one dimension) and progression-free survival (i.e. tumor doubling in size from baseline) for the 22Rv1 and VCaP xenograft models, respectively. Log-rank (Mantel-Cox) tests were used to determine statistical significance of survival differences between treatment groups. Statistical analyses were performed using GraphPad Prism 8 (p<0.05 was used as the threshold for statistical significance).
RESULTS
Enhanced Activity of Enzalutamide in Combination with NLG207 in 22Rv1 Cells
First, the effect of NLG207 and enzalutamide on the growth of 22Rv1 cells was evaluated in vitro. The approximate IC50 values for NLG207 and CPT in 22Rv1 cells were 10 nM and 5 nM, respectively (Fig. S2A); CPT was included to confirm activity was associated with the CPT component of NLG207. The IC50 value for enzalutamide in 22Rv1 cells was 1 μM, consistent with previous literature.21 ENZ, NLG207 and CPT each significantly down-regulated cell proliferation in the presence of 0.1 nM R1881 by 5 days of treatment (Figure 1A and 1B). Treatment with NLG207 or CPT in combination with ENZ in 22Rv1 cells enhanced the effect of ENZ alone by 58.3% and 59.9% by day 7, respectively, (p<0.0001) post-treatment initiation. The enhancement of enzalutamide anti-tumor activity via NLG207 co-treatment mirrored previous data with siHIF + ENZ in 22Rv1 cells.18
Figure 1: Effect of NLG207, camptothecin, and enzalutamide on cell proliferation of 22Rv1 cells.
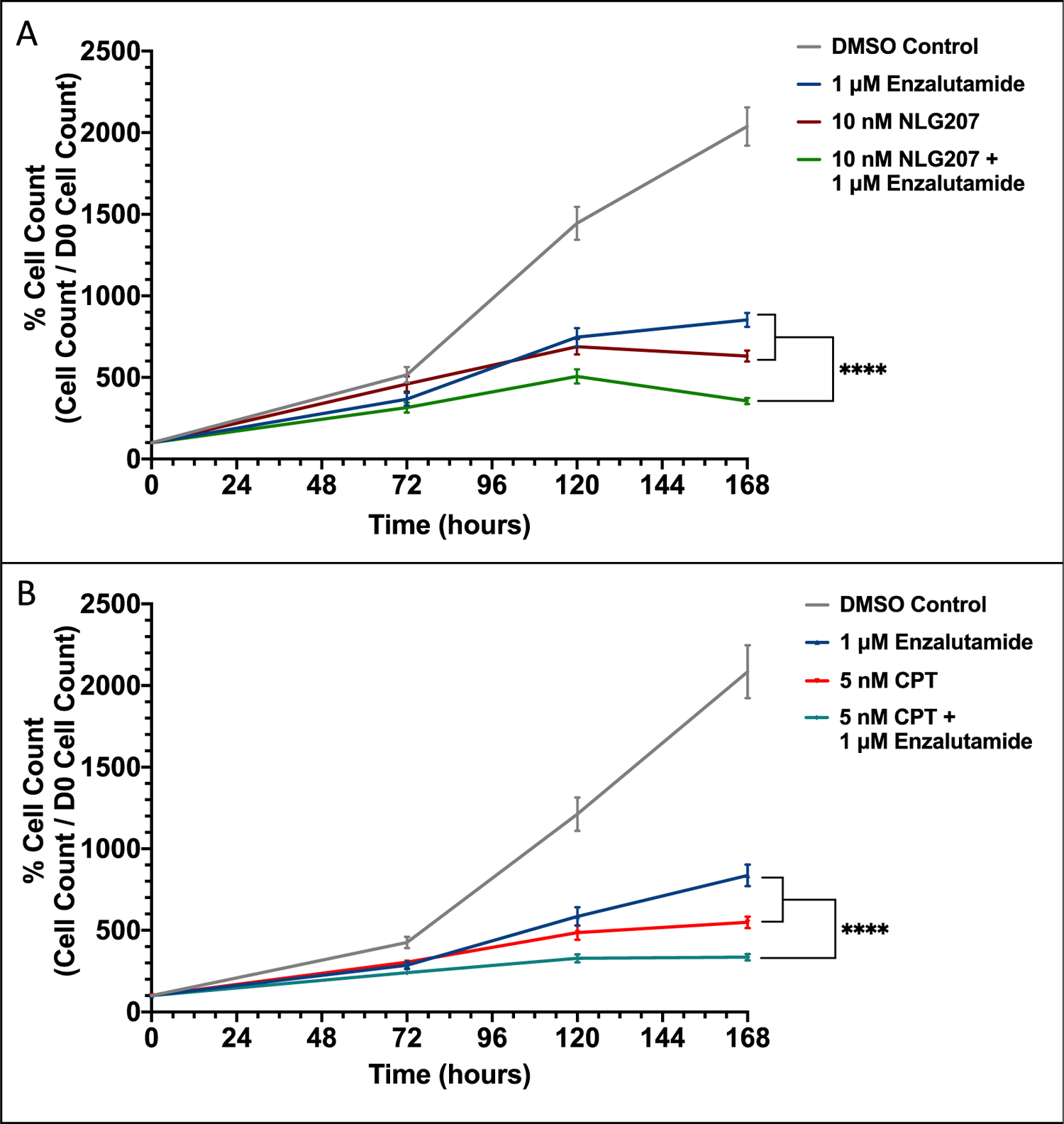
Growth of 22Rv1 cells in media containing 0.1 nM R1881 with or without 1 μM enzalutamide was evaluated over 168 hours following treatment with 10 nM NLG207 (A) or 5 nM camptothecin (B). Cell viability was determined using the Cell Counting Kit-8 cell proliferation/cytotoxicity assay at the indicated time points. The result is representative of three independent experiments. Statistical significance between groups by day 7 is denoted: **** - p<0.0001.
Efficacy of NLG207 and Enzalutamide in 22Rv1 Xenograft Model
The efficacy of NLG207 and ENZ was first evaluated via analysis of tumor volume reduction following 3 weeks of treatment using the subcutaneous 22Rv1 xenograft model, a model previously used by Liu et al..33,34 The 4 mg/kg and 8 mg/kg doses of NLG207 were derived from prior xenograft studies by Pham et al.28,29 Comparisons of daily tumor measurements collected on study showed NLG207 8 mg/kg ± ENZ and NLG207 4 mg/kg ± ENZ significantly reduced average tumor volume compared to vehicle control by day 7 and day 11 post-treatment, respectively (p<0.05) (Figure 2A). All groups treated with NLG207 had significant reductions in tumor volume compared to ENZ alone by day 14 (p<0.05). In harvested tumors, average volume and weight comparisons between vehicle control and ENZ alone were consistent with enzalutamide resistance (p>0.05; Figure 2B, 2C, and 2D). All NLG207 treated groups had significantly reduced average harvested tumor weights compared to either vehicle control or ENZ alone (p<0.05). Relative to vehicle control, treatment with NLG207 8 mg/kg + ENZ reduced harvested tumor volume by 93%, and NLG207 4 mg/kg + ENZ, by comparison, only showed an 81% reduction (p<0.05). Based on this data, we used the 8 mg/kg dose of NLG207 for future xenograft studies. An additional xenograft study assessing tumor re-growth (an endpoint commonly used to evaluate NLG207 efficacy)35 following 3 weeks of treatment with 8 mg/kg NLG207 and ENZ confirmed these findings while demonstrating significantly increased survival in mice treated with NLG207 (Fig. S3).
Figure 2: Efficacy of NLG207 and enzalutamide in subcutaneous 22Rv1 xenografts after 3 weeks of treatment.
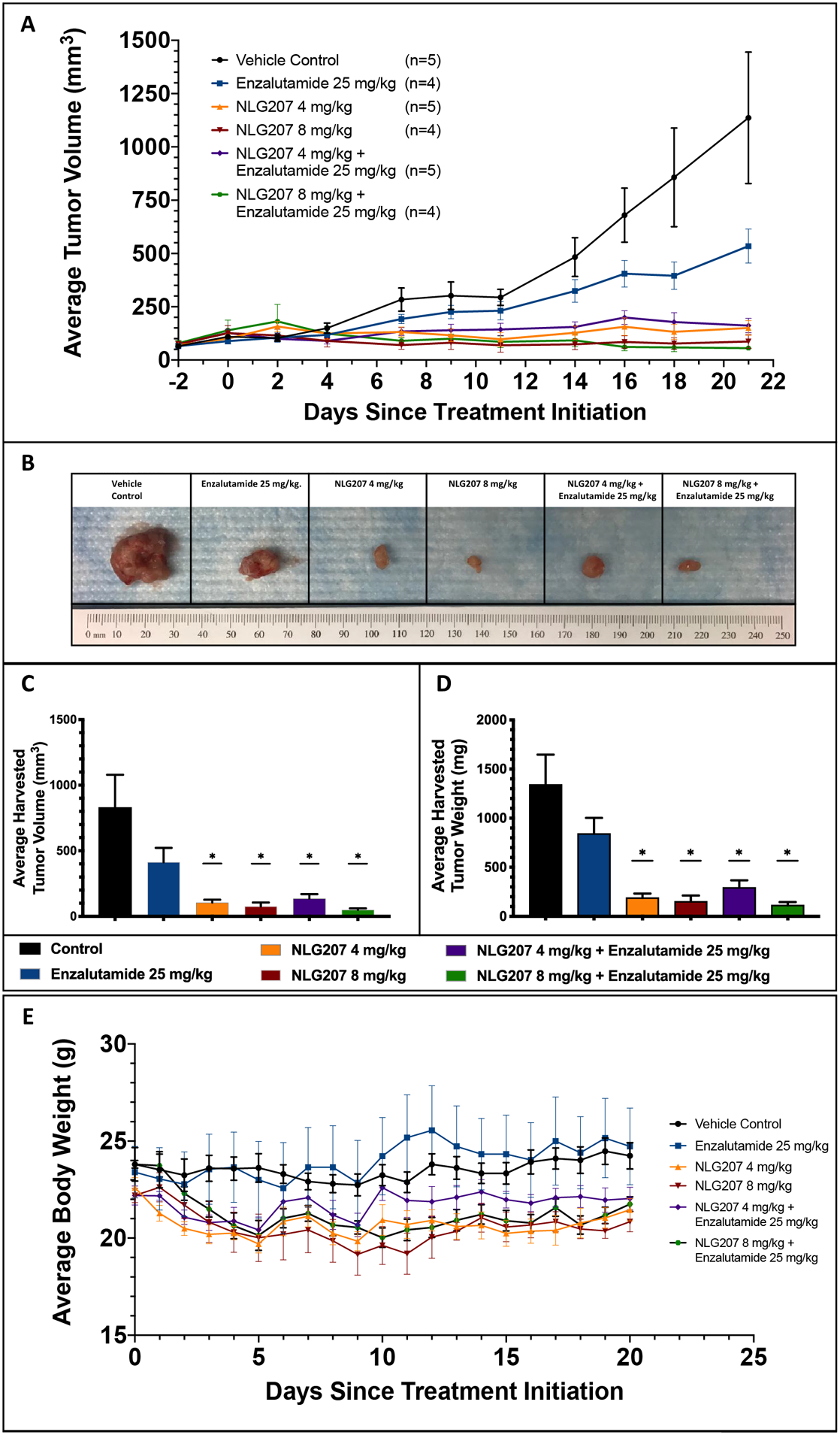
Mice with subcutaneous 22Rv1 xenografts were treated for 3 weeks with the specified doses of vehicle controls, enzalutamide and/or NLG207, and euthanized at the completion of treatment. Average tumor volume measurements collected are plotted during the course of the study (A). Tumors, harvested following 3 weeks of treatment, were compared, as shown via photograph with one tumor per group (B), average tumor volume (C) and average tumor weight (D) at time of harvest; statistical comparisons to vehicle control above each treatment group are denoted: * - p<0.05. Daily average body weight measurements collected are summarized over the course of the study (E).
Toxicity was evaluated via average body weight measurements, taken daily during treatment and three times weekly post-treatment (Figure 2E). Two mice were removed from both studies due to >20% reduction in body weight: one mouse receiving NLG207 8 mg/kg and one mouse receiving NLG207 8 mg/kg + ENZ. Trends of decreased average body weight were seen in groups receiving NLG207 compared to either vehicle control or ENZ alone.
Effect of NLG207 on Cell Proliferation and Gene Expression in VCaP Cells
The anti-tumor activity of NLG207 + ENZ was further evaluated in the VCaP cell line. Unlike 22Rv1 cells, which express AR-V7 and other splice variants via AR intragenic rearrangement, VCaP cells express AR-V7 via significantly increased AR gene transcript generation.19,20,33,36 VCaP cells also overexpress AR-FL and harbor the TMPRSS2-ERG fusion, relevant characteristics in advanced mCRPC that are not present in 22Rv1 cells.36 The approximate in vitro IC50 values were 500 nM for NLG207 and 500 nM for CPT in VCaP cells (Fig. S2B), a 50-fold and 100-fold decrease in potency, respectively, compared to 22Rv1 cells. In VCaP cells, the IC50 value for ENZ was 500 nM, a two-fold increase in potency compared to 22Rv1 and similar to the previously reported value.37 ENZ, NLG207 and CPT each significantly down-regulated cell proliferation in the presence of 0.1 nM R1881 by 3 days post-treatment (Figure 3A and 3B). Treatment of NLG207 or CPT in combination with ENZ enhanced cell growth inhibition of ENZ alone by 60% and 61.5%, respectively, by day 7 (p<0.0001).
Figure 3: Effect of NLG207, camptothecin, and enzalutamide on cell proliferation of VCaP cells.
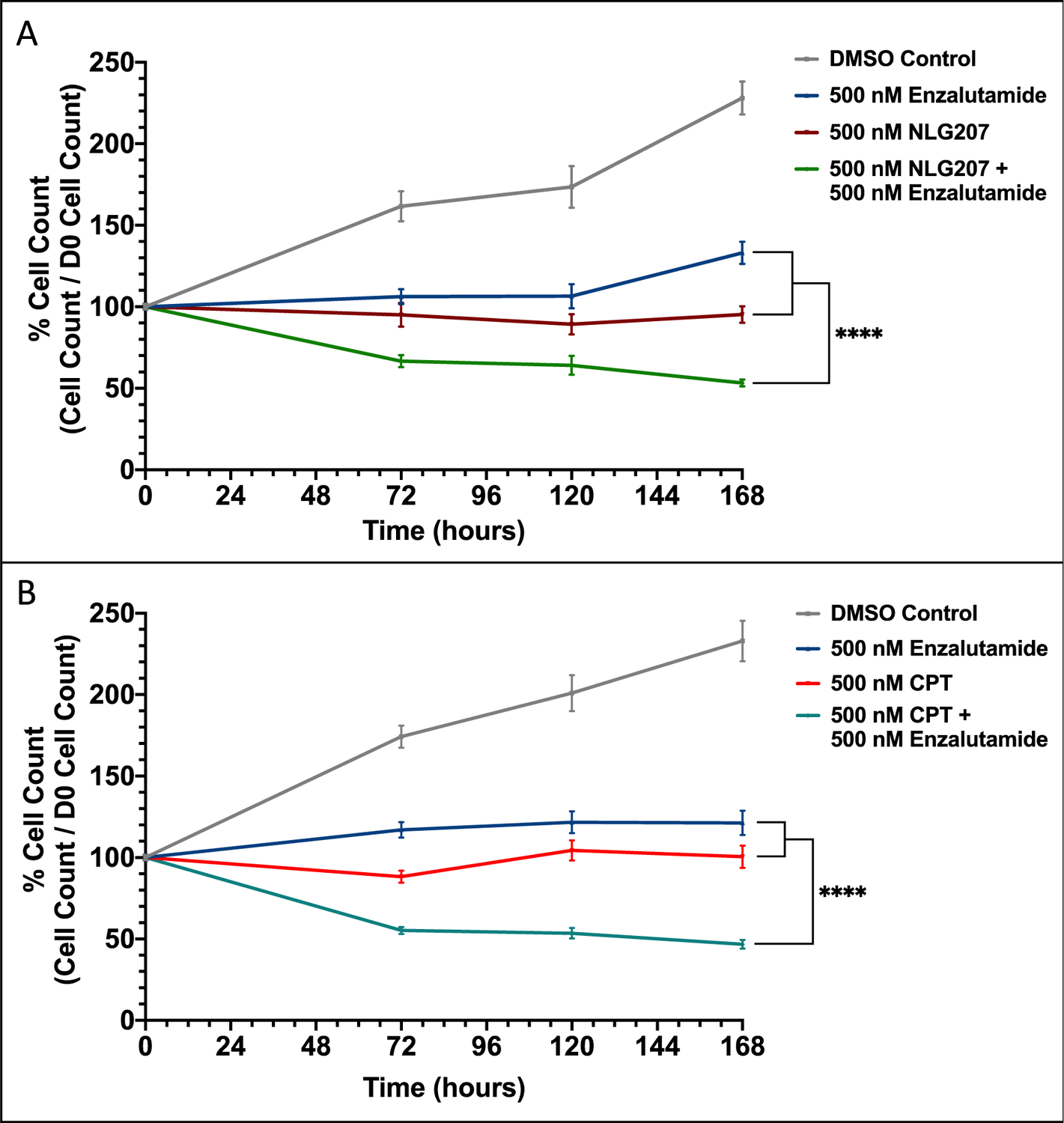
Growth of VCaP cells in media containing 0.1 nM R1881 with or without 500 nM enzalutamide was evaluated over 168 hours following treatment with 500 nM NLG207 (A) or 500 nM camptothecin (B). Cell viability was determined using the Cell Counting Kit-8 cell proliferation/cytotoxicity assay at the indicated time points. The result is representative of three independent experiments. Statistical significance between groups by day 7 is denoted: **** - p<0.0001.
We next examined the effect of drug treatments on changes in mRNA expression of AR pathway genes in VCaP cells treated with 0.1 nM R1881 in vitro. Trends in AR-associated gene expression in response to androgen stimulation with or without enzalutamide (Figure 4) was consistent with prior literature.36,38–40 Androgen stimulation or NLG207 alone preferentially suppressed AR-V7 vs. AR-FL mRNA expression while this effect is blunted following ENZ treatment (Figure 4A & 4B). Treatment with 500 nM NLG207 alone had a more robust effect on AR-FL/AR-V7 compared to androgen stimulation. The addition of NLG207 to ENZ treatment more effectively attenuated AR-V7 expression (2.9-fold compared to ENZ alone, p<0.0001). Downstream AR target genes, KLK3 and ERG, were significantly downregulated following combination treatment, with 2.8-fold and 4.6-fold reductions observed compared to ENZ alone, respectively (Figure 4C & 4D; p<0.0001). Treatment with 500 nM CPT instead of NLG207 resulted in similar but more robust changes in gene expression, likely explained via nanoparticle release kinetics (Fig. S4). VEGFA and LDHA, HIF-1α downstream target genes, were also down-regulated following treatment with NLG207 or CPT (Fig. S5); combination CPT + ENZ treatment resulted in 1.7-fold and 2.5-fold reductions in VEGFA and LDHA, respectively, when compared to ENZ alone (p<0.0001). Similar findings were noted in our prior study examining the effect of combination chetomin and enzalutamide on target gene expression.18
Figure 4: Effect of NLG207 and enzalutamide on mRNA expression of relevant AR pathway associated genes in VCaP cells.
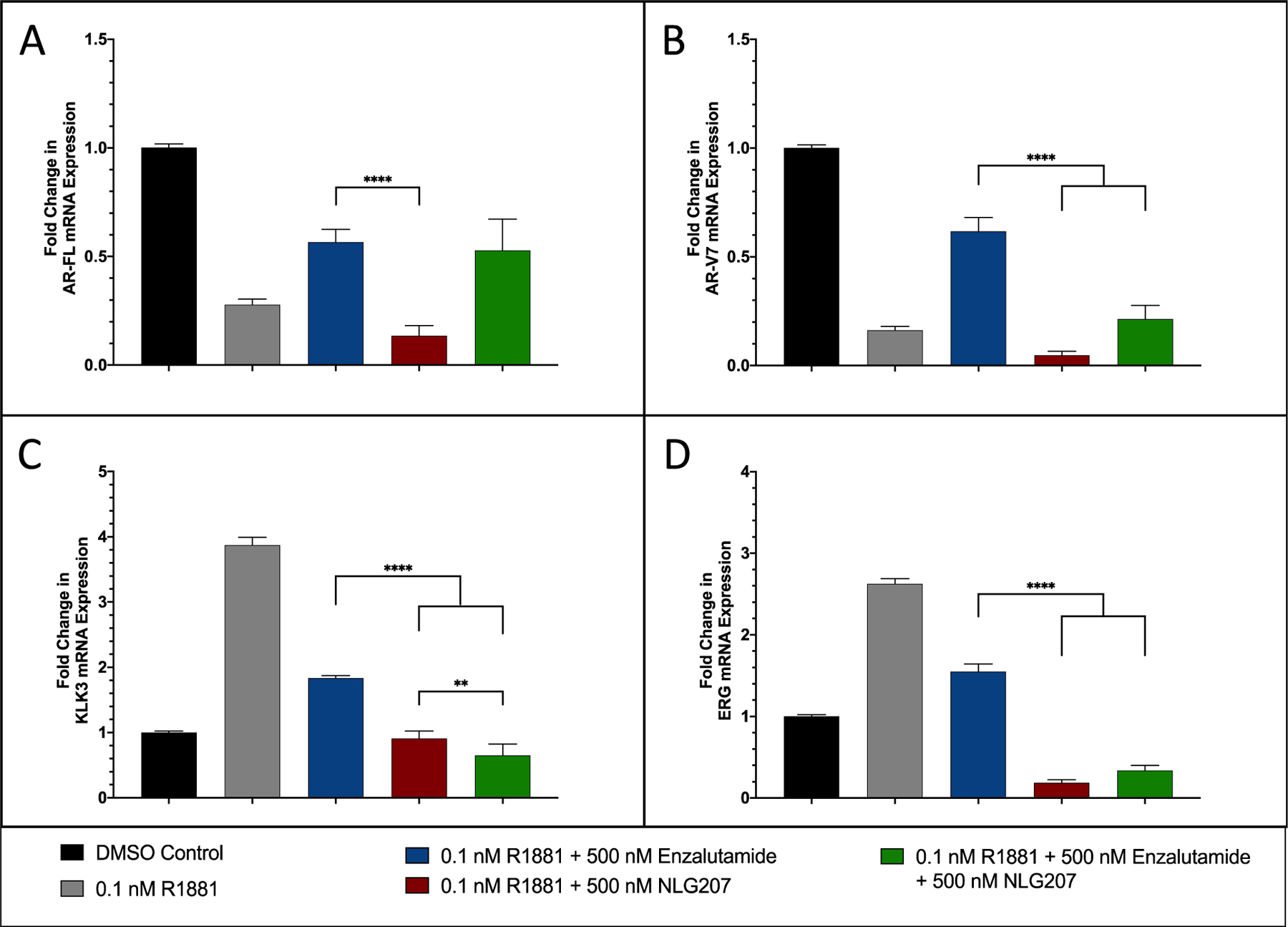
The fold change in mRNA expression of AR-FL (A), AR-V7 (B), KLK3 (C), ERG (D) following 24 hours of treatment with combinations of R1881, enzalutamide and NLG207 compared to cells in 10% CDS-FBS supplemented media. Results are representative of three independent experiments. Significance of indicated comparisons are denoted: ** - p < 0.01, **** - p < 0.0001.
NLG207 Enhances Enzalutamide Activity in Castrated VCaP Xenografted Mice
Finally, we investigated the efficacy of NLG207 8 mg/kg + ENZ 25 mg/kg in the castrated VCaP subcutaneous xenograft model. Cai et al. first described the effects of castration on pre-established VCaP xenografted tumors, demonstrating that AR activity and ERG expression are restored following castration, with marked increases in AR mRNA expression.41 Evaluation of NLG207 and enzalutamide anti-tumor activity in the castrated VCaP subcutaneous xenograft model was implemented similarly to recent preclinical evaluations of second-generation AR antagonist-based treatments.37,42 The average percent change in tumor volume from baseline per treatment group is summarized in Figure 5A. The NLG207 treated groups had significant reductions in tumor volume compared to vehicle control or ENZ by day 10 post-treatment initiation (p<0.05). NLG207 + ENZ was significantly better than NLG207 alone (p≤0.05) throughout several timepoints post-treatment initiation, including day 8 (post-second NLG207 injection), day 22 (end of treatment [EOT]), and day 36 (two weeks post-EOT). While the effect of NLG207 with enzalutamide provided a persistent reduction in tumor growth in comparison to NLG207 alone well after treatment cessation, the effect of enzalutamide in comparison to vehicle control dissipated prior to the end of treatment. The median progression-free survival (i.e tumor doubling time) was different between the vehicle control and ENZ alone groups (Figure 5B), both reached prior to EOT (13 and 20 days, respectively; p<0.05); the anti-tumor effect of ENZ alone was consistent with prior studies describing minimal sensitivity to the agent in the castrated VCaP xenograft model.37,42 The median progression-free survival of the NLG207 8 mg/kg + ENZ treatment group was significantly improved in comparison to NLG207 8 mg/kg alone (31 and 41 days, respectively, p<0.05). Importantly, the addition of NLG207 to ENZ treatment reduced the median rate of tumor growth (assessed via progression-free survival) by 51% in comparison to ENZ alone (p = 0.0001).
Figure 5: Efficacy of NLG207 and enzalutamide in castrated subcutaneous VCaP xenografted mice.
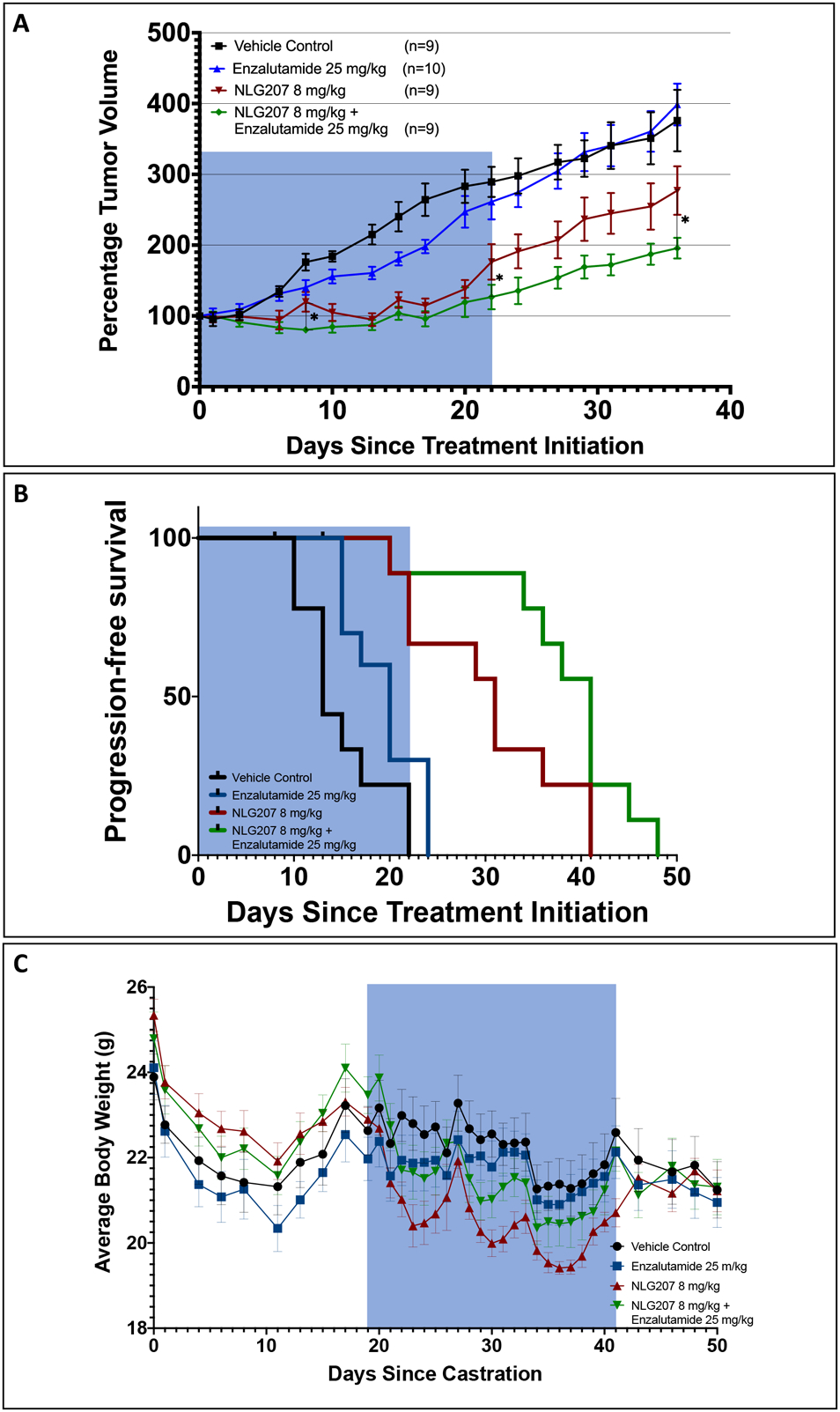
Castrated mice with subcutaneous VCaP xenografts were treated for 3 weeks with specified doses of vehicle control, enzalutamide, and/or NLG207, then monitored for tumor re-growth. Average percent change in tumor volume measurements (baseline at the start of treatment) through day 36 post-treatment initiation are summarized, with statistical significance of comparisons at specified time points denoted: * - p≤ 0.05 (A). Kaplan-Meier survival analysis progression-free survival (progression indicated when a tumor doubled in size) for each treatment group (B). Mice were censored if treatment resulted in >20% reduction in body weight. Average body weight measurements per treatment group collected following castration through 10 days post-drug treatment (C). The blue shading indicates the time frame for on-study drug treatment.
Castration and NLG207 treatment were associated with declines in average body weight measurements (Figure 5C). A brief reduction in average body weight was observed during post-castration follow-up, with weight stabilizing close to baseline prior to treatment initiation. NLG207 alone was the least well tolerated, with average body weight nadirs occurring 3 days post-i.p. injection; ENZ appeared to negate this effect, as NLG207 + ENZ average body weight nadirs were smaller by comparison. Three mice were euthanized following >20% body weight reduction: one during post-castration follow-up, one from the NLG207 alone group (day 7 of treatment) and one from the NLG207 + ENZ group (day 10 of treatment).
DISCUSSION
The use of NLG207 in combination with enzalutamide presented a reasonable approach to down-regulate AR-HIF crosstalk, as suggested by prior study in enzalutamide-resistant 22Rv1 cells.18 First, NLG207 was shown to be a potent inhibitor of tumor growth, both as monotherapy and in combination with ENZ, in the 22Rv1 xenograft model. The addition of NLG207 was then shown to potentiate the anti-AR effects of ENZ in the castrated VCaP xenograft model, which exhibits AR amplification and splice variant expression, two relevant mechanisms of AR-mediated acquired resistance to ENZ. The combination treatment demonstrated robust activity in both the 22Rv1 and castrated VCaP xenograft models, with evidence supporting the downregulation of AR pathway-gene expression in VCaP cells, including AR-FL, AR-V7 and downstream targets.
Camptothecin, the “payload” of the β-cyclodextrin-polyethylene glycol co-polymer NLG207 formulation and a potent topoisomerase I (TOP1) inhibitor,22 has been previously shown to exhibit indirect effects on HIF-1α and AR activity, both dependent on anti-TOP1 activity. Rapisarda et al. first demonstrated the effect of topotecan (TPT), a CPT derivative, on HIF-1α accumulation if given at low, persistent doses, which was dependent upon TOP1 inhibition.43 TOP1 was previously shown to co-occupy enhancer binding sites with NKX3.1 to regulate AR transcription, with the catalytic activity of TOP1 to nick DNA important for AR-regulated enhancer activation.44 A similar β-cyclodextrin nanosponge formulation of CPT (CN-CPT) was previously shown to exert activity against TOP1 via the up-regulation of γ-H2AX, a marker of DNA damage, in AR-null PC3 and DU-145 cells; with increased doses, similar effects on γ-H2AX were seen in the AR T878A variant expressing LNCaP cell line, while also accompanied by depletion of the AR.45 Extension of these studies with CN-CPT in DU-145 and PC-3 cells showed inhibition of adhesion and migration of tumor cells while decreasing STAT3 phosphorylation, supporting beneficial anti-angiogenic activity when coupled with in vivo anti-tumor activity in PC3 xenografted mice.46 NLG207 was previously shown to induce γ-H2AX formation and decrease HIF-1α levels in rectal cancer cells, and down-regulate HIF-1α in breast cancer tumor xenografts.27,47 Evaluation of clinical tumor specimens via immunohistochemistry following NLG207 treatment demonstrated both activity against Ki-67 and downstream targets of HIF-1α (CAIX, VEGFA), while decreasing TOP1 expression.48 Lastly, TOP1 was shown reside on HIF-1α, AR-FL, and AR-V7 mRNA; co-treatment with TPT and ENZ in hypoxic 22Rv1 cells resulted in HIF-1α knockdown, reduced AR-V7 nuclear localization, and synergistic dose response.49 The summation of these data highlight the multi-faceted nature of CPT anti-tumor activity stemming from potent TOP1 inhibition.
Using validated AR species specific primers,30,31 our in vitro analyses demonstrated decreases in AR-FL and notably AR-V7 mRNA expression in VCaP cells following treatment with NLG207. Further, NLG207 enhanced enzalutamide’s ability to down-regulate the mRNA expression of KLK3 and ERG, both downstream AR targets, mirroring previous data shown with chetomin and enzalutamide treatment.18 Downstream targets of HIF-1α signaling, VEGFA and LDHA, were also down-regulated in response to NLG207 and CPT under normoxia. The impact of treatment VEGFA and LDHA mRNA expression was not evaluated in the context of hypoxia and subsequent HIF-1α accumulation, a limitation of the present study.18 Additionally, we lacked sufficient tumor tissue to compare in vivo gene and protein expression of relevant pharmacodynamic biomarkers post-treatment due to our xenograft study endpoint selection.33,35 Given the robust suppression of NLG207 on AR-FL/AR-V7, whether NLG207 has an effect on the heterodimerization of AR-FL with AR-V7 remains to be determined; however, preliminary evidence has been suggested by TPT in 22Rv1 cells.50
NLG207 was highly potent in both the 22Rv1 and castrated VCaP subcutaneous xenograft models. Prior subcutaneous xenograft models, including colorectal models evaluating combination NLG207 treatment with 5-FU and oxaliplatin, have similarly shown significant reductions in tumor volume followed by decreased rates of tumor re-growth;47 subcutaneous xenograft studies evaluating NLG207 have limited dosing frequency and have established efficacy on the basis of re-growth rates.35 Unsurprisingly, 4 mg/kg and 8 mg/kg NLG207 with or without ENZ significantly decreased tumor growth in the 22Rv1 model following just 3 weeks of treatment, although a dose-dependent effect was not observed in this prostate cancer model because of the sensitivity of 22Rv1 cells to NLG207. In the castrated VCaP xenograft model, similar reductions in tumor growth were observed after 3 weeks of treatment with NLG207 8 mg/kg, with enhanced activity of the combination in comparison to both NLG207 or ENZ alone at the end of treatment and 2 weeks post-end of treatment. Though the VCaP model does display some ENZ sensitivity from days 13 through 17, the effect dissipates prior to treatment cessation to indicate ENZ resistance, a finding similar to previous literature.51 With the addition of NLG207, ENZ has sustained anti-tumor effect long after treatment cessation, increasing the tumor doubling time by 20 days. These findings when coupled with in vitro data point towards both the potency of NLG207 to TOP1 inhibition and sustained reduction of tumor growth driven by indirect activity of CPT on the AR pathway.
Our in vitro analysis also demonstrated a 50-fold increase in the potency of NLG207 in 22Rv1 cells when compared to VCaP cells. The absence of a measurable effect of combination treatment versus NLG207 alone in 22Rv1 xenografts as opposed to VCaP xenografts further demonstrated this significant potency difference. Upregulation of ERG expression, a transcription factor of the ETS family promoted via the TMPRSS2-ERG fusion, is present in VCaP cells but not 22Rv1 cells.52 In addition to its effects on tumor cell invasiveness and proliferation,53 ERG has been implicated to disrupt the interaction of topoisomerase I and DNA-PKcs, which regulates the cellular response of CPT independently of DNA repair.54 A comparison of CPT sensitivity between VCaP and DU-145, the latter cell line with similar levels of ERG expression to that of 22Rv1,52 demonstrated a >100-fold difference in potency to CPT,54 a near identical finding to our analysis of IC50 concentrations of CPT in VCaP and 22Rv1 cells. These data suggest the role of reduced ERG expression to significantly enhance the potency of NLG207. Interestingly, our findings show CPT can decrease ERG mRNA expression in VCaP cells and another study implicates enhanced responses to ENZ associated with ERG expression in VCaP cells;55 despite the latter finding, recent data has suggested TMPRSS2-ERG to not be a predictive biomarker of enzalutamide efficacy in chemo-naïve patients with mCRPC in the first-line setting.56 Future investigations of ERG expression are necessary to better understand CPT sensitivity in prostate cancer cells and the role of TMPRSS2-ERG as a predictive biomarker of clinical outcomes following enzalutamide treatment.
In addition to ERG expression, the expression profile of the AR appears to be important to explain the sustained enhancement of ENZ activity when combined with NLG207 treatment in VCaP xenografts. CPT significantly reduced both AR-FL and AR-V7 mRNA expression in the present study, as well as AR mRNA expression in prior studies with LNCaP cells.57,58 Selective AR-variant knockdown has been previously shown to restore enzalutamide activity via AR-FL, or androgen-dependent, signaling in 22Rv1 cells;21 both the enhanced potency of NLG207 and potentially complete pan-AR variant inhibition limited our ability to ascertain a true effect of ENZ in 22Rv1 xenografts. However, AR-FL overexpression found in VCaP cells coupled with decreased potency of NLG207 may indicate incomplete suppression of AR-FL with NLG207 alone, enabling the re-sensitization to ENZ with lower intracellular concentrations of AR-FL. In a similar study, JQ1, a BET bromodomain inhibitor targeting AR activity, also significantly down-regulated AR-FL and AR-V7 transcription and enhanced the tumor growth inhibition of enzalutamide in the castrated VCaP xenograft model;42 combination NLG207 and enzalutamide reduced tumor volume below baseline measurement, an effect not seen with JQ1 and enzalutamide.42 Additionally, when compared to a previous study that assessed niclosamide, an agent that specifically promotes AR-V7 degradation, in the subcutaneous 22Rv1 xenograft model,33 the anti-tumor effect of NLG207 appears more potent. The inhibition of AR-FL and AR-V7 from the treatment combination will be further addressed in future biomarker directed studies derived from the ongoing clinical investigation in patients with advanced mCRPC (NCT03531827).
Inhibition of AR-V7 expression appears to impact body weight for both xenograft models. Weight loss has been associated with NLG207 in prior reports in the literature.28 Interestingly, NLG207 and ENZ combination therapy appeared to have a weight-sparing effect, most notably in the castrated VCaP xenograft model. In the 22Rv1 xenografted mice, a similar weight-sparing effect was also implicated with 4 mg/kg doses of NLG207, but not the 8 mg/kg dose. The expression of AR-V7 has been previously shown to restore AR-mediated lipid biosynthesis via study of 22Rv1 and VCaP cells in vitro and in vivo.59 Increased AR-V7 mRNA expression following combination treatment in comparison to NLG207 alone, as shown via our in vitro data, may explain the weight-sparing effect observed in these models.
In conclusion, NLG207 in combination with enzalutamide had significant anti-tumor activity in two different preclinical prostate cancer models harboring clinically relevant mechanisms of enzalutamide resistance. AR-V7 expression, a potentially attractive predictive biomarker,4 is ultimately a result of two diverse oncogenic mechanisms driving enzalutamide resistance. AR amplification mediated via AR copy number gain, modeled using VCaP cells,36,41 has been well-characterized in the context of disease progression on enzalutamide in numerous clinical studies.5–7 Constitutively active splice variant generation via AR intragenic gene rearrangement, modeled using 22Rv1 cells,19,21 has recently been characterized in clinical CRPC tumors following enzalutamide treatment; intriguingly, AR gene rearrangements were not only shown to generate diverse AR-V species, but to also correlate with AR overexpression in the context of AR amplification.20 Our data indicates relevant downregulation of AR-FL and AR-V7 expression via a TOP1 inhibition dependent mechanism facilitated by NLG207, enhancing the efficacy of ENZ. Anti-tumor activity of NLG207 and enzalutamide was demonstrated in both prostate cancer models of AR amplification and AR intragenic rearrangement, suggesting the potential of the treatment combination to effectively target tumors with heterogeneous mechanisms of enzalutamide resistance. Additionally, the treatment combination was effective in the presence of the TMPRSS2-ERG fusion, a tumor characteristic present in nearly half of advanced prostate cancer cases in North America.60 Clinical investigation of this treatment combination to confirm anti-tumor activity in patients with mCRPC following disease progression on enzalutamide is currently ongoing (NCT03531827).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (ZIA BC 010547).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTERESTS
None.
REFERENCES
- 1.Teo MY, Rathkopf DE, Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med 2019;70:479–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakazawa M, Paller C, Kyprianou N. Mechanisms of Therapeutic Resistance in Prostate Cancer. Curr Oncol Rep 2017;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annala M, Vandekerkhove G, Khalaf D, et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov 2018;8:444–57. [DOI] [PubMed] [Google Scholar]
- 6.Azad AA, Volik SV, Wyatt AW, et al. Androgen Receptor Gene Aberrations in Circulating Cell-Free DNA: Biomarkers of Therapeutic Resistance in Castration-Resistant Prostate Cancer. Clin Cancer Res 2015;21:2315–24. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt AW, Azad AA, Volik SV, et al. Genomic Alterations in Cell-Free DNA and Enzalutamide Resistance in Castration-Resistant Prostate Cancer. JAMA Oncol 2016;2:1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharti SK, Kakkad S, Danhier P, et al. Hypoxia Patterns in Primary and Metastatic Prostate Cancer Environments. Neoplasia 2019;21:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraga A, Ribeiro R, Principe P, Lopes C, Medeiros R. Hypoxia and Prostate Cancer Aggressiveness: A Tale With Many Endings. Clin Genitourin Cancer 2015;13:295–301. [DOI] [PubMed] [Google Scholar]
- 10.Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int 2010;105:8–13. [DOI] [PubMed] [Google Scholar]
- 11.Schito L, Semenza GL. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016;2:758–70. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010;29:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J 2012;31:2448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shabsigh A, Ghafar MA, de la Taille A, et al. Biomarker analysis demonstrates a hypoxic environment in the castrated rat ventral prostate gland. J Cell Biochem 2001;81:437–44. [PubMed] [Google Scholar]
- 15.Halin S, Hammarsten P, Wikstrom P, Bergh A. Androgen-insensitive prostate cancer cells transiently respond to castration treatment when growing in an androgen-dependent prostate environment. Prostate 2007;67:370–7. [DOI] [PubMed] [Google Scholar]
- 16.Park C, Kim Y, Shim M, Lee Y. Hypoxia enhances ligand-occupied androgen receptor activity. Biochem Biophys Res Commun 2012;418:319–23. [DOI] [PubMed] [Google Scholar]
- 17.Mitani T, Harada N, Nakano Y, Inui H, Yamaji R. Coordinated action of hypoxia-inducible factor-1alpha and beta-catenin in androgen receptor signaling. J Biol Chem 2012;287:33594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez EV, Reece KM, Ley AM, et al. Dual targeting of the androgen receptor and hypoxia-inducible factor 1alpha pathways synergistically inhibits castration-resistant prostate cancer cells. Mol Pharmacol 2015;87:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Alsagabi M, Fan D, Bova GS, Tewfik AH, Dehm SM. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res 2011;71:2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Yang R, Henzler CM, et al. Diverse AR Gene Rearrangements Mediate Resistance to Androgen Receptor Inhibitors in Metastatic Prostate Cancer. Clin Cancer Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013;73:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young C, Schluep T, Hwang J, Eliasof S. CRLX101 (formerly IT-101)-A Novel Nanopharmaceutical of Camptothecin in Clinical Development. Curr Bioact Compd 2011;7:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark AJ, Wiley DT, Zuckerman JE, et al. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc Natl Acad Sci U S A 2016;113:3850–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beppu K, Nakamura K, Linehan WM, Rapisarda A, Thiele CJ. Topotecan blocks hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression induced by insulin-like growth factor-I in neuroblastoma cells. Cancer Res 2005;65:4775–81. [DOI] [PubMed] [Google Scholar]
- 25.Rapisarda A, Zalek J, Hollingshead M, et al. Schedule-dependent inhibition of hypoxia-inducible factor-1alpha protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res 2004;64:6845–8. [DOI] [PubMed] [Google Scholar]
- 26.Lin CJ, Lin YL, Luh F, Yen Y, Chen RM. Preclinical effects of CRLX101, an investigational camptothecin-containing nanoparticle drug conjugate, on treating glioblastoma multiforme via apoptosis and antiangiogenesis. Oncotarget 2016;7:42408–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conley SJ, Baker TL, Burnett JP, et al. CRLX101, an investigational camptothecin-containing nanoparticle-drug conjugate, targets cancer stem cells and impedes resistance to antiangiogenic therapy in mouse models of breast cancer. Breast Cancer Res Treat 2015;150:559–67. [DOI] [PubMed] [Google Scholar]
- 28.Pham E, Birrer MJ, Eliasof S, et al. Translational impact of nanoparticle-drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin Cancer Res 2015;21:808–18. [DOI] [PubMed] [Google Scholar]
- 29.Pham E, Yin M, Peters CG, et al. Preclinical Efficacy of Bevacizumab with CRLX101, an Investigational Nanoparticle-Drug Conjugate, in Treatment of Metastatic Triple-Negative Breast Cancer. Cancer Res 2016;76:4493–503. [DOI] [PubMed] [Google Scholar]
- 30.Markowski MC, Silberstein JL, Eshleman JR, Eisenberger MA, Luo J, Antonarakis ES. Clinical Utility of CLIA-Grade AR-V7 Testing in Patients With Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lokhandwala PM, Riel SL, Haley L, et al. Analytical Validation of Androgen Receptor Splice Variant 7 Detection in a Clinical Laboratory Improvement Amendments (CLIA) Laboratory Setting. J Mol Diagn 2017;19:115–25. [DOI] [PubMed] [Google Scholar]
- 32.Font-Tello A, Juanpere N, de Muga S, et al. Association of ERG and TMPRSS2-ERG with grade, stage, and prognosis of prostate cancer is dependent on their expression levels. Prostate 2015;75:1216–26. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Lou W, Zhu Y, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res 2014;20:3198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, Lou W, Zhu Y, et al. Intracrine Androgens and AKR1C3 Activation Confer Resistance to Enzalutamide in Prostate Cancer. Cancer Res 2015;75:1413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schluep T, Hwang J, Cheng J, et al. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin Cancer Res 2006;12:1606–14. [DOI] [PubMed] [Google Scholar]
- 36.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene 2014;33:3140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep 2015;5:12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo J, Li Y, Zheng W, et al. Characterization of a Prostate- and Prostate Cancer-Specific Circular RNA Encoded by the Androgen Receptor Gene. Mol Ther Nucleic Acids 2019;18:916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kregel S, Chen JL, Tom W, et al. Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget 2016;7:26259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai C, He HH, Chen S, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011;20:457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai C, Wang H, Xu Y, Chen S, Balk SP. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res 2009;69:6027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asangani IA, Wilder-Romans K, Dommeti VL, et al. BET Bromodomain Inhibitors Enhance Efficacy and Disrupt Resistance to AR Antagonists in the Treatment of Prostate Cancer. Mol Cancer Res 2016;14:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res 2004;64:1475–82. [DOI] [PubMed] [Google Scholar]
- 44.Puc J, Kozbial P, Li W, et al. Ligand-dependent enhancer activation regulated by topoisomerase-I activity. Cell 2015;160:367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minelli R, Cavalli R, Ellis L, et al. Nanosponge-encapsulated camptothecin exerts anti-tumor activity in human prostate cancer cells. Eur J Pharm Sci 2012;47:686–94. [DOI] [PubMed] [Google Scholar]
- 46.Gigliotti CL, Minelli R, Cavalli R, et al. In Vitro and In Vivo Therapeutic Evaluation of Camptothecin-Encapsulated beta-Cyclodextrin Nanosponges in Prostate Cancer. J Biomed Nanotechnol 2016;12:114–27. [DOI] [PubMed] [Google Scholar]
- 47.Tian X, Nguyen M, Foote HP, et al. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1alpha. Cancer Res 2017;77:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaur S, Wang Y, Kretzner L, et al. Pharmacodynamic and pharmacogenomic study of the nanoparticle conjugate of camptothecin CRLX101 for the treatment of cancer. Nanomedicine 2014;10:1477–86. [DOI] [PubMed] [Google Scholar]
- 49.Fruehauf JP, Gomez DC, Spithalny L, Kim JH. Topotecan-mediated inhibition of HIF1α/ARV7 heterodimers in 22RV1 prostate cancer cells prevents ARV7 nuclear entry, reversing enzalutamide resistance. Cancer Res 2020;80 (16 Suppl):Abstrat nr 4098. [Google Scholar]
- 50.Fruehauf JP, Farrokhian N, Sarkissian S, Kim JH. Blockade of ARV7: HIF1alpha heterodimers after topotecan reverses enzalutamide resistance in 22Rv1 cells. J Clin Oncol 2016;34(15_suppl):e16594. [Google Scholar]
- 51.Li Q, Deng Q, Chao HP, et al. Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses. Nat Commun 2018;9:3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mertz KD, Setlur SR, Dhanasekaran SM, et al. Molecular characterization of TMPRSS2-ERG gene fusion in the NCI-H660 prostate cancer cell line: a new perspective for an old model. Neoplasia 2007;9:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St John J, Powell K, Conley-Lacomb MK, Chinni SR. TMPRSS2-ERG Fusion Gene Expression in Prostate Tumor Cells and Its Clinical and Biological Significance in Prostate Cancer Progression. J Cancer Sci Ther 2012;4:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roche E, Montaudon D, Kayali S, Houede N, Pourquier P. Role of the ERG transcription factor in the resistance of prostate cancer cells to the topoisomerase I inhibitor camptothecin. Cancer Res 2014;74(19 Suppl):Abstract nr 3813. [Google Scholar]
- 55.Semaan L, Mander N, Cher ML, Chinni SR. TMPRSS2-ERG fusions confer efficacy of enzalutamide in an in vivo bone tumor growth model. BMC Cancer 2019;19:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grande E, Fernandez Perez MP, Wetterskog D, et al. A phase II multicenter biomarker trial to study the predictive value of TMPRSS2-ERG before enzalutamide treatment in chemo-naïve metastatic castration-resistant prostate cancer. J Clin Oncol 2019;37, no. 15_suppl:5040. [Google Scholar]
- 57.Chiang KC, Tsui KH, Chung LC, et al. Topoisomerase inhibitors modulate gene expression of B-cell translocation gene 2 and prostate specific antigen in prostate carcinoma cells. PLoS One 2014;9:e89117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S, Yuan Y, Okumura Y, Shinkai N, Yamauchi H. Camptothecin disrupts androgen receptor signaling and suppresses prostate cancer cell growth. Biochem Biophys Res Commun 2010;394:297–302. [DOI] [PubMed] [Google Scholar]
- 59.Han W, Gao S, Barrett D, et al. Reactivation of androgen receptor-regulated lipid biosynthesis drives the progression of castration-resistant prostate cancer. Oncogene 2018;37:710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou CK, Young D, Yeboah ED, et al. TMPRSS2:ERG Gene Fusions in Prostate Cancer of West African Men and a Meta-Analysis of Racial Differences. Am J Epidemiol 2017;186:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


