Abstract
Patients with obstructive sleep apnea (OSA) experience repetitive partial or complete airway collapse during sleep resulting in nocturnal hypoxia-normoxia cycling, and are at increased cardiovascular risk. The number of apneas and hypopneas indexed per hour of sleep (AHI) along with the associated intermittent hypoxia predict the increased cardiovascular risk; thus, their attenuation or prevention are objectives of OSA therapy. Continuous positive airway pressure (CPAP) is the gold-standard treatment for OSA and, when effective, mitigates both the AHI and hypoxemia. As such, it is reasonable to expect CPAP would decrease cardiovascular risk. However, three recent randomized clinical trials of CPAP versus usual care did not find any significant effects of CPAP in attenuating incident cardiovascular events in patients with OSA. In this review, we discuss these studies in addition to potential complementary therapeutic options to CPAP (e.g., neurostimulation) and conclude with suggested therapeutic targets for future interventional studies (e.g., the autonomic nervous system). While these areas of research are exciting, they have yet to be tested to any similar degree of rigor as CPAP.
Summary
Evidence implicates obstructive sleep apnea (OSA) as a mediator of increased cardiovascular risk. Pathologically, nocturnal hypoxia-normoxia cycling causes many of the deleterious effects of OSA via systemic inflammation, oxidative stress, and sympathetic activation. Recent data suggest continuous positive airway pressure (CPAP), the standard treatment for OSA, does not attenuate cardiovascular risk in these patients. We review potential reasons for these findings including efficacy of CPAP, highlight potential therapeutic targets for future interventional works, and discuss emerging treatments complementary to CPAP for OSA.
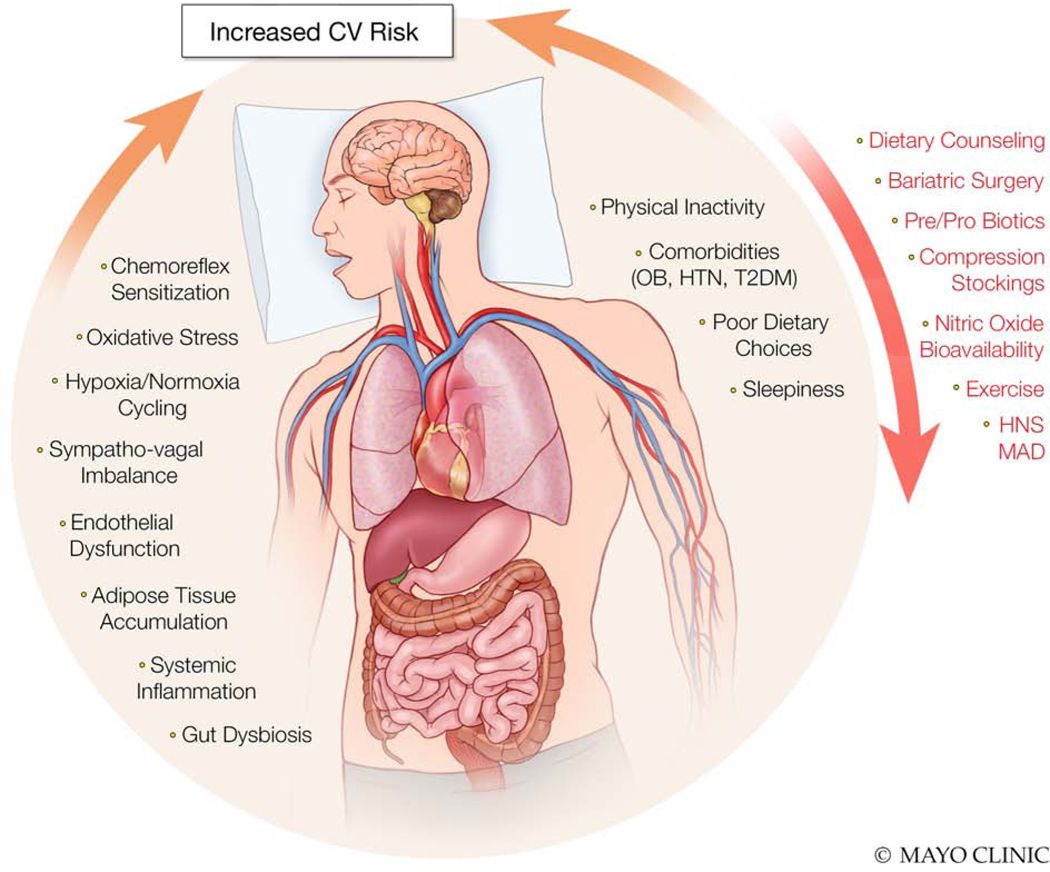
The prevalence of OSA has risen considerably over the past 30yrs1 with rates exceeding 40% in some populations,2 and significantly increases cardiovascular risk. Cardiovascular disease (CVD) has well-defined modifiable risk factors, such as hypertension,3 and is the leading cause of mortality in patients with OSA.4 One of the most robust studies linking sleep disorders to hypertension came from the Wisconsin Sleep Cohort Study 20yrs ago.5 Here, patients with OSA whose apnea-hypopnea index (AHI) was 5.0–14.9 were twice as likely to develop hypertension within four years whereas those with severe OSA (AHI>15) had a near three-fold increase in risk relative to individuals without OSA.5 Blood pressure regulation is a complex and integrative series of physiological processes;6 however, endothelial dysfunction7 and excessive visceral fat8 contribute to the hypertensive phenotype typified by OSA. Furthermore, elevated inflammatory cytokines9 and oxidative stress10 as well as deleterious alterations in the autonomic nervous system11 also play significant roles in blood pressure dysregulation commensurately increasing cardiovascular risk. When these factors are considered along with the frequent co-diagnosis of other pathologies (e.g., atrial fibrillation),12 it is evident comprehensive treatment strategies for OSA are required. The principle objective of this review is to bring to attention emerging alternative and potentially complementary treatments to continuous positive airway pressure (CPAP) for patients with OSA. We will also address pathologies and epiphenomena not exclusive to OSA, but which likely increase cardiovascular risk in these patients, as possible adjunct therapeutic targets for future interventional works (Figure 1).
Figure 1.
Overview of pathophysiologies and epiphenomema associated with obstructive sleep apnea which increase cardiovascular (CV) risk (dark text) along with potential adjunct treatments and therapeutic targets to reduce CV risk (red text). OB, obesity; HTN, hypertension; T2DM, type 2 diabetes mellitus; HNS; hypoglossal nerve stimulators; MAD, mandibular advancement devices.
Many deleterious effects of OSA are attributed to repetitive hypoxemia/reoxygenation cycles during sleep; an important differentiation from patients with compromised lung function (e.g., chronic obstructive lung disease) with tonic, low oxygen saturation. Pathophysiologically, nocturnal hypoxemia cycling contributes to the heightened sympathetic tone,11 poor endothelial health,7 blood pressure surges,13 and pro-oxidative state10 characteristic of patients with OSA as well as endothelin production14 and poor nitric oxide (NO) bioavailability.15 Not surprisingly, nocturnal hypoxemia was shown to predict major adverse cardiac events in post-infarct patients with OSA after adjusting for age, diabetes, AHI, and left ventricular function.16 Furthermore, patients with OSA are susceptible to developing atrial fibrillation12 and are at increased risk for sudden cardiac death17 with the magnitude of hypoxemia, rather than AHI, being independently associated with both. Given CPAP greatly improves hypoxemia in patients with OSA by maintaining airway patency, those with the most severe desaturation may consequently experience the greatest benefit from treatment. Additionally, excessive daytime sleepiness (EDS) is common with fragmented sleep which we18 and others19–22 have studied. Patients describe sleepiness as general lethargy, tiredness, or a lack of energy, and the Epworth Sleepiness Scale (ESS) is among the most common approaches to objectively quantify sleepiness.23 Alternative, more objective measures, include the Multiple Sleep Latency Test (MSLT) and Maintenance of Wakefulness Test (MWT); however, their application is limited due to the volume of resources they require and the paucity of studies validating their utility in patients with OSA. Nevertheless, post-infarction patients with OSA and EDS have a three-fold greater risk of major adverse cardiovascular events compared to those without EDS after adjusting for age and nadir oxygen saturation.18 Evidence has also linked EDS with all-cause (RR=1.23), CVD-related (RR=1.52), and stroke-related mortality (RR=1.52),20 increasing overall mortality risk 60%.19 Collectively, OSA commonly manifests as daytime somnolence and increases cardiovascular risk largely through elevations in blood pressure which exerts systemic deleterious effects.
Continuous Positive Airway Pressure
Continuous positive airway pressure (CPAP) therapy is the gold-standard treatment for OSA per the American Academy of Sleep Medicine.24 Mechanistically, nocturnal positive airway pressure prevents airway collapse as evidenced by significantly lower AHI and hypoxemia during use by patients with OSA; in this context, CPAP therapy can be thought of as a “stent” for the airway. Accordingly, literature has accumulated showing the beneficial-effects of CPAP treatment on a host of biomarkers including blood pressure,25 arterial stiffness,25 and endothelial function26 attributable to reduced inflammation,26 oxidative stress,26 and improved NO bioavailability.15, 27 However, more recent data suggest these surrogate outcomes may not translate to reduced cardiovascular risk. Specifically, intention to treat analyses of the SAVE (Sleep Apnea Cardiovascular Endpoints)28 and RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA)29 trials reported CPAP failed to attenuate the rate of future cardiovascular events in patients with OSA. Interestingly, the CPAP in Patients with Acute Coronary Syndrome and OSA (ISAACC) study found OSA in-and-of-itself was not associated with future cardiovascular events along with no added risk reduction in patients using CPAP.30
In 2016, McEvoy et al.28 reported data from the SAVE trial assessing the efficacy of CPAP to reduce cardiovascular events in patients with OSA and existing CVD. Over 2,700 patients from seven countries were randomized to CPAP and usual care or usual care alone and were then followed for nearly 4yrs. 17% of those assigned to CPAP had a myocardial infarction, stroke, or transient ischemic attack, were hospitalized for heart failure or acute coronary syndrome, or died from CVD whereas these endpoints were observed in 15% of controls. Moreover, no between-group differences were reported for newly diagnosed diabetes, atrial fibrillation, or all-cause mortality. That same year, Peker and colleagues29 published findings from a single-site randomized prospective study of 244 patients with OSA who also had coronary artery disease. Similar to the SAVE trial,28 myocardial infarction, stroke, and cardiovascular mortality were similar between CPAP and non-CPAP cohorts after a 4.75-year follow up in the RICCADSA trial (18 vs. 22%, respectively). Sánchez-de-la-Torre et al.30 reported on the ISAACC study which enrolled over 1,200 patients with OSA and acute coronary syndrome in 15 hospitals across Spain. These individuals were randomized to CPAP with usual care or usual care alone and studied, in parallel, over 3.35yrs (1.5–5.3yrs) with primary outcome measures being cardiovascular events (e.g., myocardial infarction) as well as admissions for heart failure or unstable angina. In accordance with the SAVE28 and RICCADSA29 trials, there was similar prevalence of cardiovascular events in CPAP (16%) and non-CPAP groups (17%). Interestingly, patients with OSA and acute coronary syndrome in the ISAACC study did not have more cardiovascular events than those with acute coronary syndrome alone. Collectively, these trials indicate CPAP may not be as beneficial as expected from the prior non-randomized observational data; however, experimental limitations from these studies are worth noting.
Chiefly, patients with EDS were excluded from the SAVE,28 RICCADSA,29 and ISAACC30 trials defined as an ESS score >15 (SAVE) and >10 (RICCADSA and ISAACC); thus, findings from these studies may not apply to all patients with OSA. Given patients with OSA and EDS have increased cardiovascular risk,22 excluding sleepy patients (those at greatest cardiovascular risk) may underestimate the benefits of CPAP. A second consideration for these trials is the exclusive investigation of CPAP on cardiovascular endpoints. While experimentally sound, this approach may not translate to clinical practice where patients with OSA frequently present with comorbidities and corresponding pharmacological interventions (e.g., ACE inhibitors). While medication usage was similar between CPAP and non-CPAP users in the SAVE,28 RICCADSA,29 and ISAACC30 studies, none reported sub-analyses for medication-specific effects on cardiovascular risk. Furthermore, adherence to non-CPAP treatments (e.g., prescription medications) was not reported in any of the aforementioned trials and should be discussed in future studies. That is, patients less adherent to CPAP could also be less adherent to other therapies potentially attenuating benefits in those assigned to CPAP therapy; an issue discussed in further detail below. An additional limitation to the SAVE study28 specifically, is the exclusion of patients with severe hypoxemia (10% of sleep <80%SpO2). With this in mind, conclusions drawn from the SAVE study,28 may also have underestimated the benefits of CPAP in patients with OSA.
Another important consideration when discussing CPAP is patient adherence. While use of CPAP throughout the night is ideal, “adherence” is frequently defined as ≥4hrs of usage per night; importantly, less than half of patients meet this criterion.26, 31 In the RICCADSA study,29 patients who met or exceeded the 4hr threshold were approximately 50% less likely to have an adverse cardiac event compared to non-adherent patients. Similarly, the SAVE trial reported CPAP-adherent patients had lower risk of stroke (HR=0.56, 95%CI 0.32–1.00) and other cerebrovascular events (HR=0.52, 95%CI 0.32–0.90) compared to non-adherent patients; however, these differences were no longer observed once corrected for multiple comparisons.28 Data from the ISAACC trial suggest CPAP adherence may not influence cardiovascular risk where 18% of adherent patients had adverse cardiovascular events compared to 15% in the non-adherent cohort and 17% in controls.30 Taken together, these data suggest average CPAP usage may not be the best metric when assessing cardiovascular risk in patients with OSA. Of consideration, OSA is more pronounced during rapid eye movement (REM) compared to non-REM sleep;32, 33 importantly, REM is more common during the second half.34 That is, if CPAP is primarily used during the initial hours of sleep, OSA would be largely untreated. Therefore, the apneic and hypoxemic burdens of OSA warrant consideration, along with duration of use, when identifying the presence or absence of benefit from CPAP. More directly, it is conceivable the absence of benefit on cardiovascular events previously reported28–30 may be attributable to sub-optimal usage and may not truly reflect the efficacy of CPAP. Nevertheless, as randomized clinical trials have shown CPAP does not attenuate cardiovascular risk in patients with OSA,28–30 exploration into potential complementary therapeutic targets is warranted.
Potential Therapeutic Targets beyond Airway Patency
As noted above, large-scale clinical trials report CPAP does not reduce cardiovascular risk in patients with OSA.28–30 Similarly, CPAP may not treat all consequences of OSA; for instance, periodic limb movements are associated with increased cardiovascular risk in patients with OSA and are largely untreated by CPAP.35 With this in mind, novel therapeutic targets which may compliment therapies which maintain airway patency in patients with OSA are worth discussing. It should be noted, each suggested target is supported by one or more of work from animal models, preliminary human findings, correlational evidence, or observational works as outlined in Table 1. To date, none have been tested with the scientific rigor reported in the aforementioned trials,28–30 if they have been tested at all; accordingly, they are discussed to stimulate future interventional studies rather than guide clinical practice.
Table 1.
Potential therapeutic targets beyond airway patency
| Target | Study | Findings |
|---|---|---|
| Gut Dysbiosis | Moreno-Indias et al.40 | IH promotes Firmicutes and reduces Bacteroidetes in mice |
| Ganesh et al.36 | Cbutyricum butyricum and Hylon supplementation prevented IH-induced HTN in mice | |
| Moreno-Indias et al.41 | Normoxia does not reverse IH-induced gut dysbiosis in mice | |
| Ko et al.38 | Attenuated short-chain fatty acid producing bacteria in OSA | |
| Poroyko et al.39 | Increased Lachnospiraceae and Ruminococcaceae with reduced Lactobacillaceae following sleep fragmentation in mice | |
| Durgan et al.37 | Intermittent tracheal balloon inflation reduced butyrate-producing bacteria in mice; fecal transplant from these mice induced HTN in controls | |
| Autonomic Nervous System | Santisteban et al.48 | Spontaneously hypertensive and chronic Ang-II infusion rats had heightened sympathetically-mediated HTN and increased gut epithelial permeability |
| Narkiewicz et al.47 | 100% FiO2 reduced MSNA and MAP in OSA but not controls | |
| Narkiewicz et al.49 | Greater ventilatory, cardiac, and MAP responses to hypoxia in OSA vs. controls | |
| Narkiewicz et al.50 | Impaired sympathetic baroreflex function in normotensive OSA | |
| Narkiewicz et al.52 | Increased blood pressure variability and MSNA burst frequency, decreased heart rate variability in OSA | |
| Body/Visceral Fat Mass | Covassin et al.58 | Visceral fat mass correlated with elevations in blood pressure in healthy individuals |
| Romero-Corral et al.59 | Association between visceral, but not subcutaneous, fat gain and endothelial function in healthy individuals | |
| Ng et al.61 | 3mo of CPAP did not reduce visceral fat, body mass, nor carotid intima-media thickness in OSA | |
| Chin et al.62 | >6mo of CPAP reduced visceral fat and LDL with increased HDL in OSA | |
| Dixon et al.112 | Surgical and non-surgical weight loss reduced AHI | |
| Peppard et al.113 | 10% weight gain increased AHI 32% and risk of sleep-disordered >breathing six-fold; 10% weight loss reduced AHI 26% | |
| Grassi et al.114 | Obesity propagates MSNA and plasma [norepinephrine] in OSA | |
| Systemic Inflammation | Horvath et al.68 | Associations between C3a and OSA severity/C-reactive protein |
| Murphy et al.69 | IH reduced insulin sensitivity and induced pro-inflammatory phenotype in visceral adipocytes of mice | |
| Braley et al.74 | 4mo dimethyl fumarate supplementation reduced NFκB signaling in OSA | |
| Shamsuzzaman et al.9 | Increased C-reactive protein in CPAP-naïve OSA relative to controls | |
| Nitric Oxide Bioavailability | Varadharaj et al.78 | eNOS-derived NO is reduced in adipose microvessels of OSA; reversed with BH4 |
| Kato et al.7 | Acetylcholine- and flow mediated vasodilation is reduced in OSA | |
| Jelic et al.26 | Phosphorylated eNOS is reduced with OSA and reversed with CPAP | |
| Ozkan et al.27 | Exhaled NO positively correlated with nadir SpO2 and inversely correlated with oxidative stress in patients with OSA | |
| Ip et al.15 | Plasma [nitrate] and [nitrite] are reduced in OSA and increase following CPAP | |
| Gerbe et al.86 | Flow-mediated dilation is reduced with OSA and increased with Vitamin C infusion | |
| Bock et al.87 | Acute inorganic nitrate supplementation blunted increases in blood pressure and peripheral chemoreflex sensitivity in OSA | |
IH, intermittent hypoxia; HTN, hypertension; OSA, patients with obstructive sleep apnea; MSNA, muscle sympathetic nerve activity, MAP, mean arterial pressure; CPAP, continuous positive airway pressure; AHI, apnea-hypopnea index; eNOS, endothelial nitric oxide (NO) synthase; BH4, tetrahydrobiopterin.
Recent studies suggest dysbiosis of the gut microbiome may be implicated in OSA-induced hypertension.36–38 Many of these studies have been conducted in rodents using disrupted sleep,39 tracheal balloons,37 or intermittent hypoxia to induce disturbances in the gut40 which may be ameliorated with pre- or probiotics.36 Surprisingly, exposing these rodents to normoxia (simulated CPAP) does not reverse the deleterious effects of simulated OSA.41 Although evidence shows gut dysbiosis in patients with OSA,38 interventional studies are lacking. Similar observations have been made in patients with heart failure (HF)42 which was later associated with elevated right atrial pressure and inflammation.43 Follow-up studies have shown dietary fiber, a prebiotic,44 prevents the manifestation of hypertension in a pre-clinical model of HF.45 While HF and OSA are vastly different pathologies, they are often present in the same patient46 and share pathophysiological components (e.g., elevated sympathetic nerve activity).47 Here, sympathetic activation may be a principal mechanism linking the gut to blood pressure.48 Given hypertension in OSA has been largely attributed to elevated sympathetic tone,11 improving gut microflora diversity may be a potential therapeutic target either alone, or to complement airway patency in future interventional studies.
We have previously reported patients with OSA have elevated basal sympathetic outflow which, acting on α-adrenergic receptors within the vasculature, promotes hypertension.11 Several lines of evidence suggest exaggerated peripheral (carotid) chemoreflex sensitivity is the cause of this heightened sympathetic state47, 49 as changes in muscle sympathetic nerve activity (MSNA) during hypercapnia (central chemoreflex) and the cold pressor test (total sympathoexcitatory arch) are similar between patients with OSA and controls.49, 50 In addition, alterations in baroreflex sensitivity have also been reported by Narkiewicz et al.50 who reported MSNA, heart rate, and blood pressure responses to intra-venous phenylephrine infusion (α1-agonist, simulates the baroreflex) were similar between normotensive patients with OSA and controls. However, a greater sympathetic response was observed in patients with OSA during sodium nitroprusside infusion (an NO pro-drug) to depress the baroreflex. Surprisingly, the heart rate and blood pressure responses to both infusions were comparable between groups. Alterations in vagal tone have been reviewed by Sequeira and colleagues51 reporting reduced high-frequency heart rate variability (low vagal tone) in patients with OSA. Similarly, increased blood pressure variability has been previously reported in patients with OSA.52 Clearly, autonomic regulation is impaired in patients with OSA and warrants further study.
Obesity, albeit not obligatory,53, 54 has long been associated with OSA,8 although the explicit signaling mechanisms linking the two remain incompletely defined. Importantly, an additive relationship has been reported as obese patients with OSA have increased CVD risk relative to their non-obese counterparts.55 Subcutaneous fat and obesity are commonly used interchangeably; however, visceral fat and ‘visceral obesity’ appears to increase CVD risk to a greater extent.56 Reports from our laboratory8 as well as others in the field55, 57 show patients with OSA have increased visceral fat which is linked to elevated ambulatory blood pressure,58 endothelial dysfunction,59 and insulin resistance.60 Some studies show promise for CPAP to reduce visceral fat deposition putatively via changes in lipid metabolism61, 62 although, as noted above, CPAP does not appear to reduce life-long cardiovascular risk.28–30 These contradictory studies clearly show more work on visceral fat and obesity in patients with OSA is warranted if they are to become adjunctive therapeutic targets to airway patency.
Recent evidence implicates adipose tissue as a source of the heightened inflammatory state characteristic of patients with OSA8, 55, 63 largely due to senescence.64–66 Chronic inflammation has been implicated in several pathophysiological consequences of OSA including endothelial dysfunction,67 hypertension,68 as well as insulin resistance.69 Given several studies report increased systemic inflammation in OSA,9, 68 and evidence implicating inflammation in cardiovascular risk,70, 71 whether anti-inflammatory interventions may mitigate cardiovascular risk in OSA is unknown. To this point, several interventional studies have shown CPAP may not reduce systemic inflammation in patients with OSA over a 4–12wk period.72, 73 However, a smaller clinical trial (n=50) by Braley et al.74 reported four months of supplementation with dimethyl fumarate, a potent anti-inflammatory ester,75 reduced indices of NFκB signaling in patients with OSA relative to a placebo. While provocative, these findings need to be replicated in larger clinical trials as their effects on cardiovascular risk were not studied and neither were the potential synergistic effects of concomitant CPAP therapy.
Endothelial dysfunction, or reduced endothelium-derived NO production, has been associated with increased CVD risk in a multitude of populations.76, 77 It is well known that patients with OSA have poor NO signaling7, 26, 78 as well as low circulating levels15, 27 which may promote CVD. Indeed, decades of work from the Schultz laboratory has shown NO produced by endothelial cells in the carotid body inhibit discharge frequency;79–81 albeit in a preclinical model of heart failure. As a ubiquitous gas molecule, NO can permeate various tissues such as the carotid body. Here, NO blunts carotid body discharge during hypoxia82 suggesting it could reduce the exaggerated peripheral chemoreflex sensitivity in patients with OSA.47, 49 A second mechanism linking NO to the peripheral chemoreflex is efferent inhibition where neuronal-derived NO from the glossopharyngeal neuron inhibits the carotid body.83 Additionally, evidence suggests attenuation of NO bioavailability facilitates cellular senescence84 which may be a consequence of increased mitochondrial-derived oxidative stress brought on by increased p66sch.85 Some evidence has indicated that augmenting NO bioavailability, through increased production,78 decreased scavenging,86 or via exogenous sources87 may reduce cardiovascular risk in patients with OSA. Collectively, these works suggest increasing NO bioavailability may be a viable therapeutic target for future interventional studies aiming to reduce cardiovascular risk in these individuals.
Emerging Treatment Avenues
As the exploration of these potential therapeutic targets will take a significant amount of time, funding, and resources to study thoroughly in humans, there is increasing attention to emerging novel therapeutic options either alone or as adjuncts to CPAP therapy (Table 2); for instance, renal denervation has been shown to reduce blood pressure in patients with OSA and resistant hypertension.88 Another promising treatment is hypoglossal nerve stimulation which was first introduced 20yrs ago.89 These devices stimulate the genioglossus muscle to maintain airway patency and prevent the tongue from breaching the airway. In 2014, Strollo et al.90 reported on the Stimulation Therapy for Apnea Reduction (STAR) trial which demonstrated 12mo of hypoglossal nerve stimulator usage reduced the AHI, ESS scores, and improved nocturnal oxygen saturation. More recently, a meta-analysis including 350 patients using hypoglossal nerve stimulators for one to five years, found reductions in AHI exceeding 15events/hour and a five-point lowering of ESS scores in concert with high implantation success rates with minimal long-term complications.91 Perhaps most importantly, these devices are largely exempt from adherence complications commonly observed with CPAP26, 31 and are consequently a promising therapeutic option for patients with OSA. However, caution should be exercised as hypoglossal nerve stimulators share a therapeutic target with CPAP (airway patency) and only surrogate outcomes have been evaluated so far; thus, there is no definitive evidence that they mitigate cardiovascular risk in patients with OSA.28–30
Table 2.
Emerging treatment avenues.
| Treatment | Study | Findings |
|---|---|---|
| Hypoglossal Nerve Stimulation | Schwartz et al.115 | Reduced AHI during REM and nREM as well as desaturation severity after 1mo, maintained after >6mo |
| Strollo et al.90 | AHI (68%) and ODI (70%) reduced after 12mo | |
| Eastwood et al.116 | Reductions in AHI and ESS after 6mo UAS | |
| Woodson et al.117 | STAR trial 3yr follow-up: ~22events/hr reduction in AHI and ~60% improvement in snoring | |
| Woodson et al.118 | STAR trial 5yr follow-up: 45% reduction in ESS and 52% increase in QOL | |
| Bariatric Surgery | Peromaa-Haavisto et al.119 | Roux-en-Y gastric bypass reduced AHI64% and cured OSA in 45% of patients |
| Tirado et al.96 | HO-1, insulin resistance, and inflammation decreased 12mo post-bariatric surgery | |
| Kardassis et al.97 | AHI, BMI, inflammation, and left ventricular mass were reduced 10yr post-bariatric surgery vs. obese cohort | |
| Auclair et al.98 | Bariatric surgery-induced changes in blood pressure and visceral fat are mediated via parasympathetic nerve activity but not inflammation or adipokines | |
| Compression Stockings | White et al.99 | AHI decreased following 2wks of daytime compression stocking use |
| Redolfi et al.102 | Correlation between AHI and neck circumference with overnight change in leg fluid volume | |
| Redolfi et al.103 | 1d of compression stocking use reduced AHI and nocturnal increased in neck circumference | |
| Redolfi et al.104 | Daytime compression stocking use attenuated AHI by 36% and nocturnal neck circumference increase 60% after 1wk | |
| Pharmacological Interventions | Blackman et al.106 | 32wks of ligutaride reduced AHI, body mass, and systolic blood pressure in CPAP-naïve OSA |
| Fiori et al.107 | 1wk of spironolactone and furosemide reduced AHI ~14% | |
| Carter et al.108 | 1mo zopiclone does not reduce AHI, sleepiness, or driving simulator performance in OSA | |
| Pépin et al.109 | Valsartan+CPAP reduced blood pressure to a greater extent than CPAP alone in hypertensive OSA | |
| Lifestyle Modifications | Fiori et al.107 | 1wk of diet counseling reduced AHI, sleepiness, and neck circumference |
| Phillips et al.92 | 6mo of sibutramine and 600kcal energy deficit reduced body mass, visceral fat, and time spent below 90%SpO2 in OSA | |
| Yee et al.93 | 6mo sibutramine and weight loss program reduced RDI and sleepiness in OSA | |
| Peppard et al.120 | Inverse relationship between exercise and AHI independent of body composition in OSA | |
| Kline et al.121 | 12wks of 150min/wk aerobic activity and 2 sessions/wk resistance training reduced AHI in sedentary OSA independent of body mass | |
AHI, apnea-hypopnea index; REM, rapid eye movement sleep; nREM, non-REM; ODI, oxygen desaturation index; ESS, Epworth Sleepiness Scale; UAS, upper airway stimulation; STAR, stimulation therapy for apnea reduction; QOL, quality of life; OSA, patients with obstructive sleep apnea; CPAP, continuous positive airway pressure.
Obesity is among the most prominent risk factors for development of OSA;63 however, this relationship is not always causative as patients with OSA of Asian descent have considerably less body mass than Americans.53, 54 Nevertheless, dietary interventions remain the mainstay for mild to moderately obese patients with weight loss of around 3–18% seen in clinical trials accompanied with improvements in AHI of 3–62%. Studies assessing the impact of weight loss have also shown significant improvements in AHI of 48–90% with weight reduction of around 12–37%.92, 93 Despite not being randomized and having a relatively small sample size, these studies promote relatively cost-effective approaches with other potential benefits such as improved insulin resistance. For reference, weight loss of 7–11% has been suggested as the level required to make significant, clinically-meaningful improvement in OSA status.94 The Swedish Obese Subjects (SOS) trial found a robust improvement in the symptoms of OSA following surgical weight loss. Improvements were also seen in OSA-related comorbidities such as diabetes, hyperlipidemia, and hypertension.95 Mechanistically, these beneficial-effects appear to be, in part, mediated by reduced systemic inflammation96, 97 although this is inconsistently reported.98 As such, bariatric surgery could be a viable complementary treatment to CPAP for obese patients with OSA which may lead to commensurate reduction in cardiovascular risk.
Over the past ten years, accumulating evidence supports the clinical utility of compression stockings in the treatment of OSA.99 Commonly used to treat lymphatic congestion100 and reduce the risk of post-surgical thromboembolism,101 compression stockings utilize external force to increase hydrostatic pressure in the legs shifting fluid centrally. During waking hours, this improves venous return and, when worn nocturnally (e.g., supine), prevents fluid accumulation in the neck which has been directly associated with AHI in patients with OSA.102 A small (n=6) proof-of-concept study by Redolfi et al.103 reported a 7.5events/hour decline in AHI which corresponded with a 40% reduction in leg fluid volume and 42% lowering of neck circumference in patients with OSA. Succeeding works from this group found similar results after one week of compression stocking use in combined OSA and chronic venous insufficiency.104 While these studies show promise for the integration of compression stockings into treatment paradigms for OSA, they will likely need to be combined with CPAP as, on their own, they do not ameliorate nocturnal apneas or hypopneas and were outperformed by CPAP during split-night studies.105 Nevertheless, compression stockings appear to provide cost-effective benefits to patients with OSA and follow up studies on the potential improvement in cardiovascular risk factors (e.g., endothelial function), particularly as adjunctive treatment to CPAP, are warranted.
Pharmacological options for the management of sleep apnea predominantly focus on addressing comorbid conditions. At the same time, few clinical trials have assessed the efficacy of pharmacological interventions for treatment of OSA. Liraglutide, an incretin mimetic used to treat type 2 diabetes mellitus, was found to reduce body weight (by 6%), BMI (by 10%), and AHI (by 12events/hour) in patients with moderate to severe OSA.106 Another study compared the efficacy of diuretic therapy (spironolactone and furosemide) versus nutritional consult with sodium restricted diet. After one week, the authors found a 14% reduction in AHI following the pharmacological arm and 22% after nutrition counseling.107 There has also been some recent interest in using central nervous system depressants to lower arousal thresholds of patients with OSA. One smaller study found a 25% reduction in AHI following one-month of zopiclone, a benzodiazepine, without impairing genioglossus activity.108 While these studies highlight emerging pharmacological approaches to treating OSA, none are regularly prescribed in clinical practice. Importantly, some evidence from Pépin et al.109 indicate CPAP may augment the beneficial-effects of specific medications in patients with OSA. That is, valsartan (an angiotensin 2 receptor antagonist) combined with CPAP led to a four-fold greater reduction in 24-hour blood pressure as compared to CPAP alone over 8wks.109 Indeed, studies on how intermittent hypoxemia influence pharmacokinetics/pharmacodynamics is seemingly non-existent and warrants exploration.
Beyond these specific examples, several other potential adjunctive or alternative treatments to CPAP have been studied with varying degrees of rigor. Most notably, mandibular advancement devices have been shown in several smaller clinical trials to improve cardiovascular health110 although large-scale randomized clinical trials are sparse. Alternatively, lifestyle modifications such as increasing physical activity and/or exercise improve AHI, sleepiness, and body mass in patients with OSA.111 While these preliminary data show promise and are indeed exciting, they require closer examination in addition to robust scientific studies similar to previous works investigating the effects of CPAP on cardiovascular risk.28–30
Conclusions & Perspectives
Continuous positive airway pressure has long been considered the gold-standard treatment for patients with OSA based upon the premise it reduced the risk of CVD by ameliorating apnea severity and nocturnal intermittent hypoxia. Recent evidence shows CPAP does not prevent incident cardiovascular events; thus, either CPAP is an ineffective therapy for cardioprotection, or intermittent airway collapse with hypoxemia is not the primary mechanism responsible for increasing CVD risk in these patients. In either case, other pathophysiologic mechanisms such as gut dysbiosis, alterations in the autonomic nervous system, and systemic inflammation should be investigated as potential targets for future therapies to complement CPAP. However, findings from studies discussed in the present text should be interpreted with caution as their ability to reduce cardiovascular risk has yet to be systematically investigated.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health HL007111 (J.M.B.), HL134885 (S.K. and V.K.S.), and HL065176 (V.K.S). S.V. is supported by funding from Sleep Number to Mayo Clinic.
Footnotes
Disclosures
J.M.B., S.V., and S.K. have no conflicts of interest to declare. V.K.S. has consulted for Baker Tilly, Respicardia, Jazz Pharmaceuticals, and is on the Scientific Advisory Board for Sleep Number.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- 3.Francula-Zaninovic S, Nola IA. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr Cardiol Rev. 2018;14:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 6.Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol. 2016;101:349–355. [DOI] [PubMed] [Google Scholar]
- 7.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. [DOI] [PubMed] [Google Scholar]
- 10.Svatikova A, Wolk R, Wang HH, et al. Circulating free nitrotyrosine in obstructive sleep apnea. Am J Physiol Regul Integr Comp Physiol. 2004;287:R284–287. [DOI] [PubMed] [Google Scholar]
- 11.Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens. 1997;15:1613–1619. [DOI] [PubMed] [Google Scholar]
- 12.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 13.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–515. [DOI] [PubMed] [Google Scholar]
- 15.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. [DOI] [PubMed] [Google Scholar]
- 16.Xie J, Sert Kuniyoshi FH, Covassin N, et al. Nocturnal Hypoxemia Due to Obstructive Sleep Apnea Is an Independent Predictor of Poor Prognosis After Myocardial Infarction. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. [DOI] [PubMed] [Google Scholar]
- 18.Xie J, Sert Kuniyoshi FH, Covassin N, et al. Excessive Daytime Sleepiness Independently Predicts Increased Cardiovascular Risk After Myocardial Infarction. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merlino G, Lorenzut S, Gigli GL, et al. Insomnia and daytime sleepiness predict 20-year mortality in older male adults: data from a population-based study. Sleep Med. 2020;73:202–207. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Liu Q, Heizhati M, Yao X, Luo Q, Li N. Association between Excessive Daytime Sleepiness and Risk of Cardiovascular Disease and All-Cause Mortality: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. J Am Med Dir Assoc. 2020. [DOI] [PubMed] [Google Scholar]
- 21.Endeshaw Y, Rice TB, Schwartz AV, et al. Snoring, daytime sleepiness, and incident cardiovascular disease in the health, aging, and body composition study. Sleep. 2013;36:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donadio V, Liguori R, Vetrugno R, et al. Daytime sympathetic hyperactivity in OSAS is related to excessive daytime sleepiness. J Sleep Res. 2007;16:327–332. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 24.Epstein LJ, Kristo D, Strollo PJ Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 25.Picard F, Panagiotidou P, Weinig L, Steffen M, Tammen AB, Klein RM. Effect of CPAP therapy on nocturnal blood pressure fluctuations, nocturnal blood pressure, and arterial stiffness in patients with coexisting cardiovascular diseases and obstructive sleep apnea. Sleep Breath. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozkan Y, Firat H, Simsek B, Torun M, Yardim-Akaydin S. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep Breath. 2008;12:149–154. [DOI] [PubMed] [Google Scholar]
- 28.McEvoy RD, Antic NA, Heeley E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016;375:919–931. [DOI] [PubMed] [Google Scholar]
- 29.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613–620. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8:359–367. [DOI] [PubMed] [Google Scholar]
- 31.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appleton SL, Vakulin A, Martin SA, et al. Hypertension Is Associated With Undiagnosed OSA During Rapid Eye Movement Sleep. Chest. 2016;150:495–505. [DOI] [PubMed] [Google Scholar]
- 34.Kryger M, Roth T, Dement W. Principles and practice of sleep medicine. 4th: Philadelphia: Saunders; 1985. [Google Scholar]
- 35.Schipper MH, Alvarez-Estevez D, Jellema K, Verbraecken J, Fulda S, Rijsman RM. Sleep-related leg movements in obstructive sleep apnea: definitions, determinants, and clinical consequences. Sleep Med. 2020;75:131–140. [DOI] [PubMed] [Google Scholar]
- 36.Ganesh BP, Nelson JW, Eskew JR, et al. Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertension. 2018;72:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durgan DJ, Ganesh BP, Cope JL, et al. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension. 2016;67:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko CY, Liu QQ, Su HZ, et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci (Lond). 2019;133:905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poroyko VA, Carreras A, Khalyfa A, et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci Rep. 2016;6:35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45:1055–1065. [DOI] [PubMed] [Google Scholar]
- 41.Moreno-Indias I, Torres M, Sanchez-Alcoholado L, et al. Normoxic Recovery Mimicking Treatment of Sleep Apnea Does Not Reverse Intermittent Hypoxia-Induced Bacterial Dysbiosis and Low-Grade Endotoxemia in Mice. Sleep. 2016;39:1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. [DOI] [PubMed] [Google Scholar]
- 43.Pasini E, Aquilani R, Testa C, et al. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016;4:220–227. [DOI] [PubMed] [Google Scholar]
- 44.Fiber Slavin J. and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marques FZ, Nelson E, Chu PY, et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–977. [DOI] [PubMed] [Google Scholar]
- 46.Khattak HK, Hayat F, Pamboukian SV, Hahn HS, Schwartz BP, Stein PK. Obstructive Sleep Apnea in Heart Failure: Review of Prevalence, Treatment with Continuous Positive Airway Pressure, and Prognosis. Tex Heart Inst J. 2018;45:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. [DOI] [PubMed] [Google Scholar]
- 48.Santisteban MM, Qi Y, Zubcevic J, et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res. 2017;120:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. [DOI] [PubMed] [Google Scholar]
- 50.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. [DOI] [PubMed] [Google Scholar]
- 51.Sequeira VCC, Bandeira PM, Azevedo JCM. Heart rate variability in adults with obstructive sleep apnea: a systematic review. Sleep Sci. 2019;12:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–1077. [DOI] [PubMed] [Google Scholar]
- 53.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110:1689–1693. [DOI] [PubMed] [Google Scholar]
- 54.Kohno T, Kimura T, Fukunaga K, et al. Prevalence and clinical characteristics of obstructive- and central-dominant sleep apnea in candidates of catheter ablation for atrial fibrillation in Japan. Int J Cardiol. 2018;260:99–102. [DOI] [PubMed] [Google Scholar]
- 55.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura T, Tokunaga K, Shimomura I, et al. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis. 1994;107:239–246. [DOI] [PubMed] [Google Scholar]
- 57.Toyama Y, Tanizawa K, Kubo T, et al. Impact of Obstructive Sleep Apnea on Liver Fat Accumulation According to Sex and Visceral Obesity. PLoS One. 2015;10:e0129513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Covassin N, Sert-Kuniyoshi FH, Singh P, et al. Experimental Weight Gain Increases Ambulatory Blood Pressure in Healthy Subjects: Implications of Visceral Fat Accumulation. Mayo Clin Proc. 2018;93:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, et al. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 2010;56:662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. [DOI] [PubMed] [Google Scholar]
- 61.Ng SS, Liu EK, Ma RC, et al. Effects of CPAP therapy on visceral fat thickness, carotid intima-media thickness and adipokines in patients with obstructive sleep apnoea. Respirology. 2017;22:786–792. [DOI] [PubMed] [Google Scholar]
- 62.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. [DOI] [PubMed] [Google Scholar]
- 63.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067–1074. [DOI] [PubMed] [Google Scholar]
- 64.Adams PD. Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell. 2009;36:2–14. [DOI] [PubMed] [Google Scholar]
- 65.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodier F, Coppé JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atkeson A, Jelic S. Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc Health Risk Manag. 2008;4:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horvath P, Tarnoki DL, Tarnoki AD, et al. Complement system activation in obstructive sleep apnea. J Sleep Res. 2018;27:e12674. [DOI] [PubMed] [Google Scholar]
- 69.Murphy AM, Thomas A, Crinion SJ, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J. 2017;49. [DOI] [PubMed] [Google Scholar]
- 70.Golia E, Limongelli G, Natale F, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16:435. [DOI] [PubMed] [Google Scholar]
- 71.Maffia P, Cirino G. Targeting inflammation to reduce cardiovascular disease risk. Br J Pharmacol. 2017;174:3895–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kohler M, Ayers L, Pepperell JC, et al. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax. 2009;64:67–73. [DOI] [PubMed] [Google Scholar]
- 74.Braley TJ, Huber AK, Segal BM, et al. A randomized, subject and rater-blinded, placebo-controlled trial of dimethyl fumarate for obstructive sleep apnea. Sleep. 2018;41. [DOI] [PubMed] [Google Scholar]
- 75.Stoof TJ, Flier J, Sampat S, Nieboer C, Tensen CP, Boorsma DM. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol. 2001;144:1114–1120. [DOI] [PubMed] [Google Scholar]
- 76.Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis. 2014;25:713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Konukoglu D, Uzun H. Endothelial Dysfunction and Hypertension. Adv Exp Med Biol. 2017;956:511–540. [DOI] [PubMed] [Google Scholar]
- 78.Varadharaj S, Porter K, Pleister A, et al. Endothelial nitric oxide synthase uncoupling: a novel pathway in OSA induced vascular endothelial dysfunction. Respir Physiol Neurobiol. 2015;207:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding Y, Li YL, Schultz HD. Downregulation of carbon monoxide as well as nitric oxide contributes to peripheral chemoreflex hypersensitivity in heart failure rabbits. J Appl Physiol (1985). 2008;105:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol (1985). 1999;86:1273–1282. [DOI] [PubMed] [Google Scholar]
- 81.Marcus NJ, Del Rio R, Ding Y, Schultz HD. KLF2 mediates enhanced chemoreflex sensitivity, disordered breathing and autonomic dysregulation in heart failure. J Physiol. 2018;596:3171–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iturriaga R, Mosqueira M, Villanueva S. Effects of nitric oxide gas on cat carotid body chemosensory response to hypoxia. Brain Res. 2000;855:282–286. [DOI] [PubMed] [Google Scholar]
- 83.Campanucci VA, Dookhoo L, Vollmer C, Nurse CA. Modulation of the carotid body sensory discharge by NO: an up-dated hypothesis. Respir Physiol Neurobiol. 2012;184:149–157. [DOI] [PubMed] [Google Scholar]
- 84.Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000;87:540–542. [DOI] [PubMed] [Google Scholar]
- 85.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. [DOI] [PubMed] [Google Scholar]
- 86.Grebe M, Eisele HJ, Weissmann N, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. [DOI] [PubMed] [Google Scholar]
- 87.Bock JM, Hanson BE, Asama TF, et al. Acute inorganic nitrate supplementation and the hypoxic ventilatory response in patients with obstructive sleep apnea. J Appl Physiol (1985). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warchol-Celinska E, Prejbisz A, Kadziela J, et al. Renal Denervation in Resistant Hypertension and Obstructive Sleep Apnea: Randomized Proof-of-Concept Phase II Trial. Hypertension. 2018;72:381–390. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Archives of Otolaryngology–Head & Neck Surgery. 2001;127:1216–1223. [DOI] [PubMed] [Google Scholar]
- 90.Strollo PJ Jr., Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. [DOI] [PubMed] [Google Scholar]
- 91.Costantino A, Rinaldi V, Moffa A, et al. Hypoglossal nerve stimulation long-term clinical outcomes: a systematic review and meta-analysis. Sleep Breath. 2020;24:399–411. [DOI] [PubMed] [Google Scholar]
- 92.Phillips CL, Yee BJ, Trenell MI, et al. Changes in regional adiposity and cardiometabolic function following a weight loss program with sibutramine in obese men with obstructive sleep apnea. J Clin Sleep Med. 2009;5:416–421. [PMC free article] [PubMed] [Google Scholar]
- 93.Yee BJ, Phillips CL, Banerjee D, Caterson I, Hedner JA, Grunstein RR. The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes (Lond). 2007;31:161–168. [DOI] [PubMed] [Google Scholar]
- 94.Garvey WT, Garber AJ, Mechanick JI, et al. American association of clinical endocrinologists and american college of endocrinology position statement on the 2014 advanced framework for a new diagnosis of obesity as a chronic disease. Endocr Pract. 2014;20:977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torgerson JS, Sjöström L. The Swedish Obese Subjects (SOS) study--rationale and results. Int J Obes Relat Metab Disord. 2001;25 Suppl 1:S2–4. [DOI] [PubMed] [Google Scholar]
- 96.Tirado R, Masdeu MJ, Vigil L, et al. Impact of Bariatric Surgery on Heme Oxygenase-1, Inflammation, and Insulin Resistance in Morbid Obesity with Obstructive Sleep Apnea. Obes Surg. 2017;27:2338–2346. [DOI] [PubMed] [Google Scholar]
- 97.Kardassis D, Grote L, Sjöström L, Hedner J, Karason K. Sleep apnea modifies the long-term impact of surgically induced weight loss on cardiac function and inflammation. Obesity (Silver Spring). 2013;21:698–704. [DOI] [PubMed] [Google Scholar]
- 98.Auclair A, Biertho L, Marceau S, et al. Bariatric Surgery-Induced Resolution of Hypertension and Obstructive Sleep Apnea: Impact of Modulation of Body Fat, Ectopic Fat, Autonomic Nervous Activity, Inflammatory and Adipokine Profiles. Obes Surg. 2017;27:3156–3164. [DOI] [PubMed] [Google Scholar]
- 99.White LH, Lyons OD, Yadollahi A, Ryan CM, Bradley TD. Effect of below-the-knee compression stockings on severity of obstructive sleep apnea. Sleep Med. 2015;16:258–264. [DOI] [PubMed] [Google Scholar]
- 100.Rabe E, Partsch H, Hafner J, et al. Indications for medical compression stockings in venous and lymphatic disorders: An evidence-based consensus statement. Phlebology. 2018;33:163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sachdeva A, Dalton M, Amaragiri SV, Lees T. Graduated compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2014:Cd001484. [DOI] [PubMed] [Google Scholar]
- 102.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–246. [DOI] [PubMed] [Google Scholar]
- 103.Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol. 2011;175:390–393. [DOI] [PubMed] [Google Scholar]
- 104.Redolfi S, Arnulf I, Pottier M, et al. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med. 2011;184:1062–1066. [DOI] [PubMed] [Google Scholar]
- 105.Silva BC, Santos RSS, Drager LF, Coelho FM, Elias RM. Impact of Compression Stockings vs. Continuous Positive Airway Pressure on Overnight Fluid Shift and Obstructive Sleep Apnea among Patients on Hemodialysis. Front Med (Lausanne). 2017;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fiori CZ, Martinez D, Montanari CC, et al. Diuretic or sodium-restricted diet for obstructive sleep apnea-a randomized trial. Sleep. 2018;41. [DOI] [PubMed] [Google Scholar]
- 108.Carter SG, Carberry JC, Cho G, et al. Effect of 1 month of zopiclone on obstructive sleep apnoea severity and symptoms: a randomised controlled trial. Eur Respir J. 2018;52. [DOI] [PubMed] [Google Scholar]
- 109.Pépin JL, Tamisier R, Barone-Rochette G, Launois SH, Lévy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182:954–960. [DOI] [PubMed] [Google Scholar]
- 110.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Jama. 2015;314:2280–2293. [DOI] [PubMed] [Google Scholar]
- 111.Aiello KD, Caughey WG, Nelluri B, Sharma A, Mookadam F, Mookadam M. Effect of exercise training on sleep apnea: A systematic review and meta-analysis. Respir Med. 2016;116:85–92. [DOI] [PubMed] [Google Scholar]
- 112.Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. Jama. 2012;308:1142–1149. [DOI] [PubMed] [Google Scholar]
- 113.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. Jama. 2000;284:3015–3021. [DOI] [PubMed] [Google Scholar]
- 114.Grassi G, Facchini A, Trevano FQ, et al. Obstructive sleep apnea-dependent and - independent adrenergic activation in obesity. Hypertension. 2005;46:321–325. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–1223. [DOI] [PubMed] [Google Scholar]
- 116.Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Woodson BT, Soose RJ, Gillespie MB, et al. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea: The STAR Trial. Otolaryngol Head Neck Surg. 2016;154:181–188. [DOI] [PubMed] [Google Scholar]
- 118.Woodson BT, Strohl KP, Soose RJ, et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol Head Neck Surg. 2018;159:194–202. [DOI] [PubMed] [Google Scholar]
- 119.Peromaa-Haavisto P, Tuomilehto H, Kössi J, et al. Obstructive sleep apnea: the effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med. 2017;35:85–90. [DOI] [PubMed] [Google Scholar]
- 120.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–484. [DOI] [PubMed] [Google Scholar]
- 121.Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.