Abstract
Background:
Incidental or intentional durotomy in spine surgery is associated with a risk of cerebrospinal fluid (CSF) leakage and reoperation. Several strategies have been introduced but the incomplete closure is still relatively frequent and troublesome. In this study, we review current evidence on spinal dural repair strategies and evaluate their efficacy.
Methods:
PubMed, Web of Science and Scopus were used to search primary studies about the repair of the spinal dura with different techniques. Of 265 articles found, 11 studies, which specified repair techniques and post-operative outcomes, were included for qualitative and quantitative analysis. The primary outcomes were CSF leakage and post-operative infection.
Results:
The outcomes of different dural repair techniques were available in 776 cases. Pooled analysis of 11 studies demonstrated that the most commonly used technique was a combination of primary closure, patch or graft and sealant (22.7%, 176/776). A combination of primary closure and patch or graft resulted in the lowest rate of CSF leakage (5.5%, 7/128). In this study, sealants as an adjunct to primary closure (13.7%, 18/131) did not significantly reduce the rate of CSF leakage compared to primary closure alone (17.6%, 18/102). The rates of infection and postoperative neurological deficit were similar regardless of the repair techniques.
Conclusions:
Although the use of sealants has become prevalent, available sealants as an adjunct to primary closure did not reduce the rate of CSF leakage compared to primary closure. The combination of primary closure and patches or grafts could be effective in decreasing postoperative CSF leakage.
Keywords: dural repair, durotomy, primary closure, sealants, spine surgery, neurosurgery
Introduction
Dural tears are not uncommon complications in spine surgery, with an incidence varying from 1.6% to 10%1–6. Although primary repair of a dural tear is generally satisfactory, persistent CSF leakage resulting from incomplete closure can lead to complications such as postural headache, nausea, vomiting, dural cutaneous fistula formation, meningitis and even intracranial hemorrhage7–9. Moreover, treating these complications often requires prolonged bed rest, which is associated with subsequent complications including pneumonia, deep venous thrombosis and pulmonary embolism. Therefore, it is imperative to repair the durotomy during the initial surgery.
Many investigators have developed dural repair techniques to achieve watertight dural closure. The repair techniques have been described from simple sutures and sealants to different types of patches and grafts. Typically, sutures have been used to close simple dural tears. However, the application of sutures has limitations depending on the anatomical location and condition of the damaged dura. Over the past decades, various surgical sealants that address these limitations have been developed10–13 and have become an alternative solution for neurosurgeons. In addition, muscle, fascia, fat and synthetic materials have long been used to repair moderate-sized dural tears14, 15.
Although various strategies have been introduced for dural repair, studies that assess the efficacy of each strategy are scarce. We therefore aim to review current literature on the efficacy and safety of the available techniques. The primary outcome including post-operative CSF leakage and infection were examined. Also, adverse events resulting from the sealants, synthetic patches and biological grafts were discussed.
Methods
Search Strategy
A PubMed literature search was performed using the terms “(dural AND (repair OR closure) AND (spine OR spinal)) AND ((autologous OR allogenic OR synthetic) OR (seal OR glue) OR (suture OR clip)).” The search retrieved 265 studies from September 1976 to April 2020. Case reports, technical reports, cadaveric studies, animal studies, non-surgical studies and non-English articles were excluded. Also, studies with fewer than 15 subjects were excluded. Studies that stated the specific repair techniques and their corresponding CSF leakage rate were included. This literature review was designed and performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1)16. Ultimately, 11 studies met these exclusion and inclusion criteria for this systematic review. Then, a second literature search using Web of Science or Scopus was performed to include missing articles from the PubMed literature search. References of review articles were also examined for potential additional studies. Later, several case reports were included as examples for the discussion. Quality assessment of the included studies was conducted using the Levels of Evidence categorization system from Oxford Centre for Evidence-based Medicine. The scale ranges from 1 (highest level of evidence) to 5 (lowest level of evidence).
Figure 1.
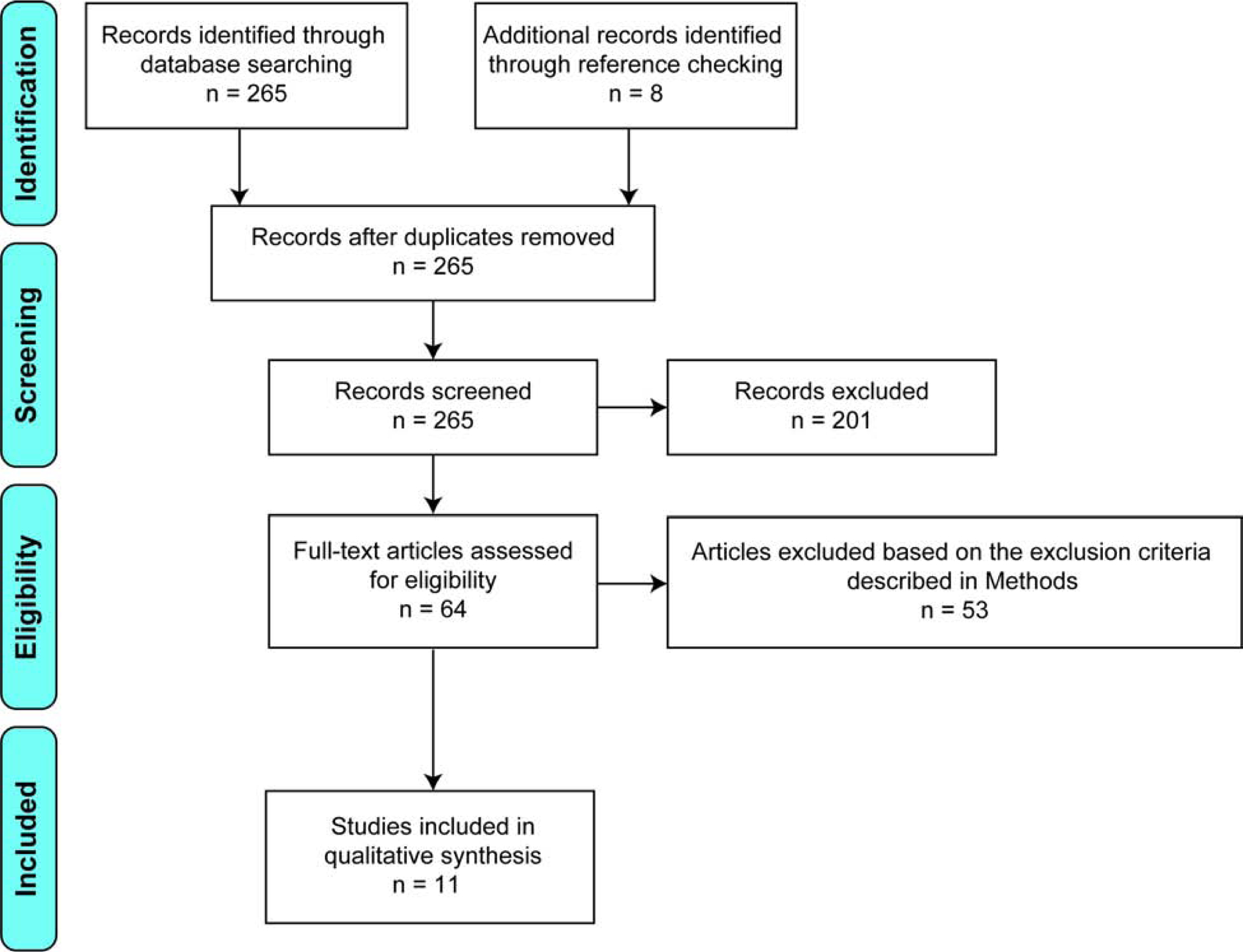
PRISMA diagram. Process of exclusion and inclusion of studies for the systematic review.
Extraction and Analysis of the Data
The following data were extracted from the included studies: year of publication, number of patients in each study, number of patients included for the analysis, mean age, sex, indication for surgery, involved location, use of drainage, repair technique, follow-up duration after dural repair, rate of post-operative CSF leakage, rate of post-operative surgical site infection and rate of post-operative neurological deficit. Dural repair technique was categorized as primary closure (suturing and/or clipping), sealant and patch or graft. Post-operative CSF leakage was defined as continuous CSF drainage through a conventional wound drain or direct wound leakage in two studies17, 18, whereas it was defined as dural leakage that requires revision surgery or conservative treatment in the remaining studies19–27. World Health Organization criteria for surgical-site infection was used to define surgical-site infection28. Surgical-site infection can be superficial infections involving the skin or more severe infections involving tissues under the skin, organs or implanted material28. Post-operative neurological deficit was defined as a reduction of two grade or more on manual muscle testing or post-operative sensory disturbance17. A direct comparison between studies was not possible due to heterogeneity. Thus, a pooled analysis was performed to measure rates of CSF leakage, infection or neurological deficit. The rates were calculated without adjustment for the surgeries, indication for surgeries or patient demographics.
Results
The initial search resulted in 265 studies. After the screening and assessment, a total of 11 studies fulfilled the inclusion criteria17–27. The type of study and level of evidence were summarized in Table 1. The level of evidence ranged from 2 to 4. Of the 11 studies, 6 studies were level of 4 evidence, 1 study was level of 3 evidence, and 4 studies were level of 2 evidence. A total of 776 cases (incidental durotomy: 628 cases, intentional durotomy: 148 cases) were included in this systematic review after excluding the cases that lost follow-up or did not receive dural repair (Table 1). The mean collective sample size study was 79. The mean collective age was 57.6 years. The collective ratio of male to female study participants was 1 to 1.1.
Table 1.
Characteristics of the included studies and patient demographics
| Study, year | Study type | Level of evidence | Number of total subjects (Incidental/Intentional) |
Number of subjects included in the systematic review (Incidental/Intentional) |
Mean age (years) |
Sex (M/F) |
Indication for surgery |
|---|---|---|---|---|---|---|---|
| Takenaka et al, 201917 | Retrospective cohort | 2 | 429 (429/0) |
354 (354/0) |
69.7 ± 12.7 | 206/223 | Disk herniation, Scoliosis, Spinal canal stenosis, Spondylolisthesis |
| Montano et al, 201818 | Prospective cohort | 2 | 35 (0/35) |
35 (0/35) |
58.1 ± 15.6 | 14/21 | Ependymoma, Ganglioglioma, Hemangioma, Hemangioblastoma Meningioma, Schwannoma |
| Arnautovic et al, 201619 | Case series | 4 | 40 (0/40) |
37 (0/37) |
57.4 ± 17.3 | 11/29 | Astrocytoma, Breast metastasis, Ependymoma, Hemangioblastoma, Meningioma, Myxopapillary, Schwannoma |
| Kamenova et al, 201620 | Retrospective cohort | 2 | 69 (69/0) |
69 (69/0) |
72.2 ± 12.8 | 35/34 | Disk herniation, Spinal canal stenosis |
| Masuda et al, 201621 | Case series | 4 | 75 (22/53) |
75 (22/53) |
57.1 | 34/41 | Epidural tumor, Intradural extramedullary tumor, Spinal canal stenosis, Metastatic spinal tumor, Ossification of ligamentum flavum, Spinal cord hernia, Spinal deformity, Spondylolisthesis, Spondylotic myelopathy, Subarachnoid cyst |
| Jo et al, 201522 | Case series | 4 | 15 (15/0) |
15 (15/0) |
42.2 ± 8.6 | 12/3 | Ankylosing spondylitis |
| Grannum et al, 201423 | Case control | 3 | 28 (28/0) |
13 (13/0) |
50.8 | 14/14 | Spinal canal stenosis |
| Tan et al, 201424 | Case series | 4 | 23 (0/23) |
23 (0/23) |
54.4 ±12.6 | 8/15 | Congenital fatty filum, Congenital meningocele, Ependymoma, Lymphoma, Meningioma, Schwannoma, Spinal dural arteriovenous fistula, Tarlov cyst |
| Anderson et al, 201325 | Case series | 4 | 50 (50/0) |
50 (50/0) |
58.9 | 28/22 | Adjacent segment degeneration, Cauda equina syndrome, Herniated disk, Scoliosis, Spinal canal stenosis, Spondylolisthesis |
| Sun et al, 201226 | Retrospective cohort | 2 | 85 (85/0) |
85 (85/0) |
54.3 | 44/41 | Ossification of ligamentum flavum |
| Hodges et al, 199927 | Case series | 4 | 20 (20/0) |
20 (20/0) |
58.1 ± 15.9 | 7/13 | NA |
| Total | 869 (718/151) |
776 (628/148) |
869 (413/456) |
NA: not available
The dural repair techniques were categorized as outlined in Table 2. Of the 776 cases, the most common technique was a combination of primary closure, patch or graft and sealant (22.7%, 176/776) followed by a combination of primary closure and sealant (16.9%, 131/776), a combination of primary closure and patch or graft (16.5%, 128/776), patch or graft (14.8%, 115/776), primary closure (13.1%, 102/776), sealant (9.9%, 77/776) and a combination of patch or graft and sealant (6.1%, 47/776). Primary closure was used as the basis for repairing all 148 intentional durotomies (Table 2). The studies involving these intentional durotomies were mostly metastatic and primary spinal tumor cases.
Table 2.
Characteristics of the repair techniques included in this study
| Repair technique | Number (%) | Incidental/Intentional |
|---|---|---|
| Primary closure (suturing and/or clipping) | 102 (13.1) | 102/0 |
| Sealant | 77 (9.9) | 77/0 |
| Primary closure + Sealant | 131 (16.9) | 108/23 |
| Patch or graft | 115 (14.8) | 115/0 |
| Primary closure + Patch or graft | 128 (16.5) | 93/35 |
| Patch or graft + Sealant | 47 (6.1) | 47/0 |
| Primary closure + Patch or graft + Sealant | 176 (22.7) | 86/90 |
| Total | 776 | 628/148 |
The involved location, use of drainage, follow-up duration after dural repair, repair technique and outcome were summarized in Table 3. Of the 11 studies, 10 studies reported the involved locations. The most commonly involved location was lumbar (77.8%, 548/704) followed by thoracic (16.8%, 118/704), cervical (2.3%, 16/704), lumbo-sacral (1.4%, 10/704), cervico-thoracic (0.7%, 5/704), thoraco-lumbar (0.7%, 5/704) and sacral (0.3%, 2/704) (Table 3).
Table 3.
Outcomes of the dural repairs in the included studies
| Study | Involved location | Drainage | Follow-up after dural repair (months) | Technique | CSF leakage | Post-operative infection | Post-operative neurological deficit | Revision technique for CSF leakage |
|---|---|---|---|---|---|---|---|---|
| Takenaka et al, 201917 | Lumbar (354/354) | Subfascial* | NA | Primary closure | 17/80 | 2/80 | 5/80 | NA |
| Sealant | 17/77 | 2/77 | 1/77 | |||||
| Primary closure + Sealant | 17/88 | 0/88 | 6/88 | |||||
| Patch + Sealant | 13/45 | 2/45 | 1/45 | |||||
| Primary closure + Patch + Sealant | 22/64 | 1/64 | 3/64 | |||||
| Montano et al, 201818 | Cervical (3/35) Cervico-thoracic (2/35) Thoracic (13/35) Thoraco-lumbar (3/35) Lumbar (14/35) |
NA | 23 | Primary closure + Patch | 1/35 | 0/35 | 0/35 | Needle aspiration + Bed rest |
| Arnautovic et al, 201619 | Cervical (11/40) Cervico-thoracic (3/40) Thoracic (11/40) Thoraco-lumbar (2/40) Lumbar (12/40) Sacral (1/40) |
NA | 45 | Primary closure + Patch + Sealant | 1/37 | 1/37 | 1/37 | Autologous fat + Lumbar drainage |
| Kamenova et al, 201620 | Lumbar (69/69) | Subarachnoid (2/69) Subfascial (60/69) Not available (7/69) |
NA | Primary closure | 1/13 | 0/13 | NA | NA |
| Patch | 4/22 | 4/22 | NA | |||||
| Primary closure + Patch | 3/34 | 0/34 | NA | |||||
| Masuda et al, 201621 | NA | Subarachnoid (75/75) | 28 | Primary closure + Patch + Sealant | 1/75 | 1/75 | 0/75 | Patch + Sealant |
| Jo et al, 201522 | Lumbar (15/15) | Subarachnoid (6/15) Subfascial (1/15) |
17 | Primary closure | 0/9 | 0/9 | 0/9 | No reoperation |
| Primary closure + Patch | 0/6 | 0/6 | 0/6 | |||||
| Grannum et al, 201423 | Lumbar (13/13) | NA | 60 | Patch | 0/13 | 0/13 | 0/13 | No reoperation |
| Tan et al, 201424 | Cervical (2/23) Thoracic (9/23) Lumbar (11/23) Sacral (1/23) |
Subarachnoid (0/23) Subfascial (0/23) |
20 | Primary closure + Sealant | 0/23 | NA | 0/23 | No reoperation |
| Anderson et al, 201325 | Lumbar (50/50) | Subfascial# | NA | Primary closure + Patch | 0/50 | 0/50 | 0/50 | No reoperation |
| Sun et al, 201226 | Thoracic (85/85) | Subfascial (84/85) Subfascial and subarachnoid (1/85) |
NA | Primary closure + Patch | 3/3 | 0/3 | 0/3 | NA |
| Patch | 60/80 | 1/80 | 0/80 | |||||
| Patch + Sealant | 2/2 | 1/2 | 0/2 | |||||
| Hodges et al, 199927 | Lumbar (10/20) Lumbo-Sacral (10/20) |
NA | >10 | Primary closure + Sealant | 1/20 | 0/20 | 0/20 | NA |
NA: not available;
exact number is not available but no patient underwent subarachnoid drainage;
only used for multilevel laminectomy or fusion cases
A direct comparison between studies was not possible due to heterogeneity. Thus, the rates of post-operative CSF leakage were combined based on the repair techniques (Table 4). The pooled rates of CSF leakage ranged from 5.5% (a combination of primary closure and patch or graft) to 55.7% (patch or graft) depending on the types of repair technique. A combination of primary closure and patch or graft resulted in the lowest rate of CSF leakage (5.5%, 7/128) followed by a combination of primary closure, patch or graft and sealant (13.6%, 24/176), a combination of primary closure and sealant (13.7%, 18/131), primary closure (17.6%, 18/102), sealant (22.1%, 17/77), a combination of patch or graft and sealant (31.9%, 15/47) and patch or graft (55.7%, 64/115). The pooled rates of post-operative infection and neurological deficit were analyzed in the same manner (Table 4). The pooled rates of infection ranged from 0.0% to 6.4% depending on the types of repair technique, but there was no significant difference between the groups. The pooled rates of neurological deficit ranged from 0.0% to 5.7% depending on the types of repair technique. In line with the pooled rates of infection, no significant difference in the pooled rates of neurological deficit between the groups was observed.
Table 4.
Pooled rates of CSF leakage, post-operative infection, and post-operative neurological deficit after dural repairs
| Repair technique | CSF leakage, number (%) | Post-operative infection, number (%) | Post-operative neurological deficit, number (%) |
|---|---|---|---|
| Primary closure | 18/102 (17.6) | 2/102 (2.0) | 5/ 89 (5.6) |
| Sealant | 17/77 (22.1) | 2/77 (2.6) | 1/77 (1.3) |
| Primary closure + Sealant | 18/131 (13.7) | 0/108 (0.0) | 6/131 (4.6) |
| Patch or graft | 64/115 (55.7) | 5/115 (4.3) | 0/93 (0.0) |
| Primary closure + Patch or graft | 7/128 (5.5) | 0/128 (0.0) | 0/94 (0.0) |
| Patch or graft + Sealant | 15/47 (31.9) | 3/47 (6.4) | 1/47 (2.1) |
| Primary closure + Patch or graft + Sealant | 24/176 (13.6) | 3/176 (1.7) | 4/176 (2.3) |
| Total | 163/776 (21.0) | 15/753 (2.0) | 17/707 (2.4) |
Discussion
Dural tears are common complications encountered by spine surgeons. Primary dural repair remains the treatment of choice, but recent literature has reported different repair techniques and adjuncts. This systematic review assesses the outcomes of spinal dural repairs with different repair techniques. We reviewed 776 cases from 11 studies for qualitative and quantitative analysis.
Dural Repair Techniques
Traditionally, sutures have been considered the gold standard for dural tear repair. Braided nylon suture, monofilament polypropylene suture and Gore-Tex suture have been routinely used and demonstrated their hydrostatic strengths29–31. Two different repair techniques including interrupted and running locked suture techniques have been commonly used10, 31. Two studies have reported no significant differences in CSF leakage between interrupted and running locked suture techniques10, 31. Although few studies suggested that dural tear repair could be achieved without sutures21, 24, 32, 33, this option is reasonable only in certain circumstances (e.g. the dural tear is located anteriorly or inaccessible). Indeed, only 1% and 5% of survey respondents preferred suturing to manage anterior dural tears and nerve root tears, respectively34. Suturing could also be challenging if the procedure is minimally invasive35, 36. As an alternative technique, nonpenetrating titanium clips have been used to achieve primary closure. The advantages of this technique include ease and speed of use, tighter closure and less need for extended dissection in confined spaces37, 38.
Various sealants have been introduced to augment sutures or clips. In general, there are two different types of sealants: the synthetic absorbable sealant containing PEG (polyethylene glycol) and the biologically absorbable sealant containing fibrinogen and thrombin. It has been known that these sealants polymerize on the dura and covers it. Although these sealants can improve the strength of sutured repair in calf spine model31, several lines of evidence have suggested that currently available sealants do not reduce the rate of CSF leakage in spine (sealant: 9.1% vs. control: 13.8%) and cranial surgery (sealant: 8.2% vs control: 8.4%)39, 40. These findings were consistent with other studies demonstrating fibrin (fibrin sealant: 8.3% vs. no fibrin sealant: 9.4%) and PEG (PEG sealant: 6.6% vs. control: 6.5%) do not significantly reduce the number of CSF leakage13, 41. In our study, a combination of primary closure and sealant did not significantly reduce the rate of CSF leakage compared to primary closure alone.
Closure of dural tears with grafts or patches has been another option. Autologous fat, muscle and fascia have been available options for the repair of dural tears. More recently, synthetic and absorbable patches including collagen matrix, gelatin sponge, polyglycolic acid sheet and collagen patch coated with fibrin have received Food and Drug Administration approval. These products have the advantage of ready availability and can be cut to shape. Also, the use of grafts or patches is advantageous when dural tears are relatively large23. Several lines of evidence suggested that collagen matrix can have a chemotactic interaction with dural fibroblasts and behave like a scaffold for the dural fibroblasts42, 43. On the other hand, grafts could release growth factors such as basic fibroblast growth factor, epidermal growth factor or transforming growth factor beta and promote the proliferation of dural fibroblasts, deposition of collagen and sutural fusion44–46. Although underlying molecular mechanisms enhancing dural repair are different, compelling evidence has demonstrated that grafts or patches could be an effective option. These data also partially explain that the combination of primary closure and patches or grafts could be effective in decreasing postoperative CSF leakage, as shown in our study.
Adverse Effects and Drawbacks of Dural Repair Techniques
Although primary durotomy repair is frequently implemented, it comes with the disadvantage that watertight closure is difficult to achieve in some circumstances29. Complications related to CSF leakage include pseudomeningoceles, wound infections, CSF fistulas and intracranial hypotension syndrome, which often require revision surgery47. A variety of suturing techniques can be implemented to help prevent CSF leakage, but the failure to form watertight closures has resulted in the development of nonpenetrating titanium clips37, 47. These clips may come with the advantages of reduced CSF leakage, dural exposure, scarring, and intradural adhesions as well as improved efficiency and ease of application as compared to traditional suturing29, 37. Although a concern with titanium clips has been the risk of causing metallic artifacts during post-operative imaging37, several studies have suggested that the clips are small enough that they do not have significant impact on the quality of post-operative imaging29, 37, 48, 49. Nonetheless, several disadvantages associated with non-penetrating clips have been reported: dural laceration, dislodgement, the inability to reposition or re-use clips once they have left the applier, greater cost, and even a high rate of CSF leaks as reported by Timothy and colleagues in 200749. Overall, nonpenetrating titanium clips appear to better reduce CSF leakage as compared to standard suturing, although further studies are needed to confirm the efficacy29, 37.
While fibrin sealant for dural repair may offer advantages as an adjunct to traditional suturing, including reduced CSF leakage, these biological systems can carry a risk of viral and prion infection as well as allergic responses50, 51. For these reasons, autologous fibrin tissue adhesives have been devised and have demonstrated efficacy in reducing CSF leakage in neurosurgical operations52, 53. However, they require a long production time (3 days) and are difficult to handle54. PEG has emerged as a hydrogel spinal sealant that may be superior to both traditional sutures and fibrin sealants in its ability to achieve watertight dural closure11. However, its negative effects are well-profiled, as it has demonstrated a tendency to swell postoperatively often leading to stenosis and nerve root compression55–59.
Furthermore, a collagen patch coated with fibrin has emerged as an alternative fibrin sealant that contains human blood components and may increase the risk of blood-borne disease transmission60. An additional logistical drawback is that this patch is not always large enough to completely cover dural injuries; as such, severable pieces may be required for dural reconstruction60. Finally, the high cost associated with this collagen patch may preclude its use at most centers60. Other grafts include autologous dural substitutes such as fascia lata, fat, muscle, skin, and pericranium61. Notably, autologous grafts do not come with a risk for infection or immunogenic reactions20. However, they can increase surgical time and risk for additional morbidity as a result of the intraoperative grafting process.
Limitations
The current systematic review has some limitations. First, more than half of the studies were retrospective case series. Thus, the average evidence level is relatively low. Moreover, it is common that a repair technique is determined by a surgeon in case series studies rather than predetermined by a protocol. Therefore, the selection bias of a repair technique is inevitable. Second, the included studies lack details about the size of dural tears and anatomical location. Although the included studies, except one study, reported the involved vertebral locations, they did not describe the exact location and size. Third, there were various types of patches or grafts, which were categorized to “patch or graft.” For example, patches could be subcategorized into collagen matrix, dural substitute, gelatin sponge, polyglycolic acid sheet and collagen patch coated with fibrin. Grafts could be subcategorized into autologous fat, muscle and fascia. The efficacy of each patch or graft was not evaluated in this study. Fourth, there was heterogeneity among the studies in terms of duration of bed rest and the use of subfascial or subarachnoid drainage. Seven studies described the use of drainage, whereas four studies did not. It is conceivable that the post-operative outcomes cannot be solely attributed to the specific repair technique. In addition, further information about the reoperation or treatment to manage CSF leakages following the initial repair would be interesting to note. However, only three studies reported the reoperation or treatment technique, and four studies reported that reoperation was not performed due to no CSF leakage. However, five studies did not mention about reoperation or treatment technique. Lastly, 45.6% (354/776 cases) of the data was extracted from one study17. Thus, the pooled analysis could be influenced by this study. These limitations should be considered when drawing conclusions from this systematic review.
Conclusion
In this systematic review, we analyzed the efficacy of different dural repair techniques in preventing post-operative CSF leakage, infection and neurological deficit. Primary closure resulted in a lower rate of CSF leakage than sealant, patch, or graft, suggesting that primary closure should be used as the basis for repairing durotomies if possible. A sealant as an adjunct to primary closure did not significantly reduce the rate of CSF leakage. Compared to other repair techniques, a combination of patch or graft and primary closure could be more effective for preventing post-operative CSF leakage. Regardless of the repair techniques, the rates of post-operative infection and neurological deficit were similar.
Dural tears are relatively common complications in spine surgery. However, further studies will be required to evaluate the efficacy of each repair method. Heterogeneity among the primary studies and various reporting methods preclude definitive message.
Conflict of interest and funding disclosure:
The authors report no conflicts of interest. Elliot H. Choi is supported by NIH MSTP funding (T32GM007250). Dr. Andrew K. Chan receives research support for a non-related study from Orthofix Medical, Inc.
Glossary
- CSF
Cerebrospinal fluid
- PEG
Polyethylene glycol
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams BJ, Sansur CA, Smith JS, et al. Incidence of unintended durotomy in spine surgery based on 108,478 cases. Neurosurgery. 2011;68(1): 117–123; discussion 123–114. 10.1227/NEU.0b013e3181fcf14e. [DOI] [PubMed] [Google Scholar]
- 2.Baker GA, Cizik AM, Bransford RJ, et al. Risk factors for unintended durotomy during spine surgery: a multivariate analysis. The spine journal : official journal of the North American Spine Society. 2012;12(2): 121–126. 10.1016/j.spinee.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahon P, Dididze M, Levi AD. Incidental durotomy after spinal surgery: a prospective study in an academic institution. Journal of neurosurgery Spine. 2012;17(1): 30–36. 10.3171/2012.3.Spine11939. [DOI] [PubMed] [Google Scholar]
- 4.Cammisa FP Jr., Girardi FP, Sangani PK, Parvataneni HK, Cadag S, Sandhu HS. Incidental durotomy in spine surgery. Spine. 2000;25(20): 2663–2667. 10.1097/00007632-200010150-00019. [DOI] [PubMed] [Google Scholar]
- 5.Kogias E, Klingler JH, Franco Jimenez P, et al. Incidental Durotomy in Open Versus Tubular Revision Microdiscectomy: A Retrospective Controlled Study on Incidence, Management, and Outcome. Clinical spine surgery. 2017;30(10): E1333–e1337. 10.1097/bsd.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 6.Murphy ME, Kerezoudis P, Alvi MA, et al. Risk factors for dural tears: a study of elective spine surgery(). Neurological research. 2017;39(2): 97–106. 10.1080/01616412.2016.1261236. [DOI] [PubMed] [Google Scholar]
- 7.deFreitas DJ, McCabe JP. Acinetobacter baumanii meningitis: a rare complication of incidental durotomy. Journal of spinal disorders & techniques. 2004;17(2): 115–116. 10.1097/00024720-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman RM, Kebaish KM. Intracranial hemorrhage following incidental durotomy during spinal surgery. A report of four patients. The Journal of bone and joint surgery American volume. 2007;89(10): 2275–2279. 10.2106/jbjs.F.01550. [DOI] [PubMed] [Google Scholar]
- 9.Khong P, Jerry Day M. Spontaneous cerebellar haemorrhage following lumbar fusion. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2009;16(12): 1673–1675. 10.1016/j.jocn.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Cain JE Jr., Dryer RF, Barton BR. Evaluation of dural closure techniques. Suture methods, fibrin adhesive sealant, and cyanoacrylate polymer. Spine. 1988;13(7): 720–725. [PubMed] [Google Scholar]
- 11.Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine. 2011;36(23): 1906–1912. 10.1097/BRS.0b013e3181fdb4db. [DOI] [PubMed] [Google Scholar]
- 12.Wright NM, Park J, Tew JM, et al. Spinal sealant system provides better intraoperative watertight closure than standard of care during spinal surgery: a prospective, multicenter, randomized controlled study. Spine. 2015;40(8): 505–513. 10.1097/brs.0000000000000810. [DOI] [PubMed] [Google Scholar]
- 13.Kim KD, Ramanathan D, Highsmith J, et al. DuraSeal Exact Is a Safe Adjunctive Treatment for Durotomy in Spine: Postapproval Study. Global spine journal. 2019;9(3): 272–278. 10.1177/2192568218791150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Lage JF, Perez-Espejo MA, Palazon JH, Lopez Hernandez F, Puerta P. Autologous tissues for dural grafting in children: a report of 56 cases. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2006;22(2): 139–144. 10.1007/s00381-005-1232-3. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Li S, Song B, et al. The pathological changes in the spinal cord after dural tear with and without autologous fascia repair. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014;23(7): 1531–1540. 10.1007/s00586-014-3326-7. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339: b2700. 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takenaka S, Makino T, Sakai Y, et al. Prognostic impact of intra- and postoperative management of dural tear on postoperative complications in primary degenerative lumbar diseases. The bone & joint journal. 2019;101-b(9): 1115–1121. 10.1302/0301-620x.101b9.Bjj-2019-0381.R1. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Sun C, Liu X, et al. The frequency and treatment of dural tears and cerebrospinal fluid leakage in 266 patients with thoracic myelopathy caused by ossification of the ligamentum flavum. Spine. 2012;37(12): E702–707. 10.1097/BRS.0b013e31824586a8. [DOI] [PubMed] [Google Scholar]
- 19.Montano N, Pignotti F, Auricchio AM, Fernandez E, Olivi A, Papacci F. Results of TachoSil(R) associated with fibrin glue as dural sealant in a series of patients with spinal intradural tumors surgery. Technical note with a review of the literature. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2019;61: 88–92. 10.1016/j.jocn.2018.10.138. [DOI] [PubMed] [Google Scholar]
- 20.Arnautovic KI, Kovacevic M. CSF-Related Complications After Intradural Spinal Tumor Surgery: Utility of an Autologous Fat Graft. Medical archives (Sarajevo, Bosnia and Herzegovina). 2016;70(6): 460–465. 10.5455/medarh.2016.70.460-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamenova M, Leu S, Mariani L, Schaeren S, Soleman J. Management of Incidental Dural Tear During Lumbar Spine Surgery. To Suture or Not to Suture? World neurosurgery. 2016;87: 455–462. 10.1016/j.wneu.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 22.Masuda S, Fujibayashi S, Otsuki B, Kimura H, Neo M, Matsuda S. The dural repair using the combination of polyglycolic acid mesh and fibrin glue and postoperative management in spine surgery. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2016;21(5): 586–590. 10.1016/j.jos.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Jo DJ, Kim KT, Lee SH, Cho MG, Seo EM. The Incidence and Management of Dural Tears and Cerebrospinal Fluid Leakage during Corrective Osteotomy for Ankylosing Spondylitis with Kyphotic Deformity. Journal of Korean Neurosurgical Society. 2015;58(1): 60–64. 10.3340/jkns.2015.58.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grannum S, Patel MS, Attar F, Newey M. Dural tears in primary decompressive lumbar surgery. Is primary repair necessary for a good outcome? European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014;23(4): 904–908. 10.1007/s00586-013-3159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan LA, Takagi I, Straus D, O’Toole JE. Management of intended durotomy in minimally invasive intradural spine surgery: clinical article. Journal of neurosurgery Spine. 2014;21(2): 279–285. 10.3171/2014.3.Spine13719. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DG, Popov V. Repair of lumbar dural tears with a suture patch: retrospective single-surgeon case series. American journal of orthopedics (Belle Mead, NJ). 2013;42(9): E72–75. [PubMed] [Google Scholar]
- 27.Hodges SD, Humphreys SC, Eck JC, Covington LA. Management of incidental durotomy without mandatory bed rest. A retrospective review of 20 cases. Spine. 1999;24(19): 2062–2064. 10.1097/00007632-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 28.Organization WH. Global guidelines for the prevention of surgical site infection: World Health Organization; 2016. [PubMed] [Google Scholar]
- 29.Faulkner ND, Finn MA, Anderson PA. Hydrostatic comparison of nonpenetrating titanium clips versus conventional suture for repair of spinal durotomies. Spine. 2012;37(9): E535–539. 10.1097/BRS.0b013e31824cf756. [DOI] [PubMed] [Google Scholar]
- 30.Ghobrial GM, Maulucci CM, Viereck MJ, et al. Suture Choice in Lumbar Dural Closure Contributes to Variation in Leak Pressures: Experimental Model. Clinical spine surgery. 2017;30(6): 272–275. 10.1097/bsd.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 31.Dafford EE, Anderson PA. Comparison of dural repair techniques. The spine journal : official journal of the North American Spine Society. 2015;15(5): 1099–1105. 10.1016/j.spinee.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Narotam PK, Jose S, Nathoo N, Taylon C, Vora Y. Collagen matrix (DuraGen) in dural repair: analysis of a new modified technique. Spine. 2004;29(24): 2861–2867; discussion 2868–2869. 10.1097/01.brs.0000148049.69541.ad. [DOI] [PubMed] [Google Scholar]
- 33.Black P Cerebrospinal fluid leaks following spinal surgery: use of fat grafts for prevention and repair. Technical note. Journal of neurosurgery. 2002;96(2 Suppl): 250–252. 10.3171/spi.2002.96.2.0250. [DOI] [PubMed] [Google Scholar]
- 34.Oitment C, Aref M, Almenawar S, Reddy K. Spinal Dural Repair: A Canadian Questionnaire. Global spine journal. 2018;8(4): 359–364. 10.1177/2192568217724132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller SJ, Burkhardt BW, Oertel JM. Management of Dural Tears in Endoscopic Lumbar Spinal Surgery: A Review of the Literature. World neurosurgery. 2018;119: 494–499. 10.1016/j.wneu.2018.05.251. [DOI] [PubMed] [Google Scholar]
- 36.Wolff S, Kheirredine W, Riouallon G. Surgical dural tears: prevalence and updated management protocol based on 1359 lumbar vertebra interventions. Orthop Traumatol Surg Res. 2012;98(8): 879–886. 10.1016/j.otsr.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman BA, Matthews AE, Zwienenberg-Lee M, Lew SM. Spinal dural closure with nonpenetrating titanium clips in pediatric neurosurgery. Journal of neurosurgery Pediatrics. 2010;6(4): 359–363. 10.3171/2010.7.Peds09545. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Aoyama T, Horiuchi T, Hongo K. Utility of nonpenetrating titanium clips for dural closure during spinal surgery to prevent postoperative cerebrospinal fluid leakage. Journal of neurosurgery Spine. 2015;23(6): 812–819. 10.3171/2015.3.Spine141215. [DOI] [PubMed] [Google Scholar]
- 39.Kinaci A, Moayeri N, van der Zwan A, van Doormaal TPC. Effectiveness of Sealants in Prevention of Cerebrospinal Fluid Leakage after Spine Surgery: A Systematic Review. World neurosurgery. 2019;127: 567–575.e561. 10.1016/j.wneu.2019.02.236. [DOI] [PubMed] [Google Scholar]
- 40.Kinaci A, Algra A, Heuts S, O’Donnell D, van der Zwan A, van Doormaal T. Effectiveness of Dural Sealants in Prevention of Cerebrospinal Fluid Leakage After Craniotomy: A Systematic Review. World neurosurgery. 2018;118: 368–376.e361. 10.1016/j.wneu.2018.06.196. [DOI] [PubMed] [Google Scholar]
- 41.Jankowitz BT, Atteberry DS, Gerszten PC, et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2009;18(8): 1169–1174. 10.1007/s00586-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narotam PK, Van Dellen JR, Bhoola K, Raidoo D. Experimental evaluation of collagen sponge as a dural graft. British journal of neurosurgery. 1993;7(6): 635–641. 10.3109/02688699308995092. [DOI] [PubMed] [Google Scholar]
- 43.Narotam PK, van Dellen JR, Bhoola KD. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. Journal of neurosurgery. 1995;82(3): 406–412. 10.3171/jns.1995.82.3.0406. [DOI] [PubMed] [Google Scholar]
- 44.Mehrara BJ, Mackool RJ, McCarthy JG, Gittes GK, Longaker MT. Immunolocalization of basic fibroblast growth factor and fibroblast growth factor receptor-1 and receptor-2 in rat cranial sutures. Plastic and reconstructive surgery. 1998;102(6): 1805–1817; discussion 1818–1820. 10.1097/00006534-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Tachibana E, Saito K, Fukuta K, Yoshida J. Evaluation of the healing process after dural reconstruction achieved using a free fascial graft. Journal of neurosurgery. 2002;96(2): 280–286. 10.3171/jns.2002.96.2.0280. [DOI] [PubMed] [Google Scholar]
- 46.Nurata H, Cemil B, Kurt G, Uçankuş NL, Dogulu F, Omeroğlu S. The role of fibroblast growth factor-2 in healing the dura mater after inducing cerebrospinal fluid leakage in rats. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2009;16(4): 542–544. 10.1016/j.jocn.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Suter A, Spirig JM, Fornaciari P, et al. Watertightness of wound closure in lumbar spine-a comparison of different techniques. Journal of spine surgery (Hong Kong). 2019;5(3): 358–364. 10.21037/jss.2019.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito K, Seguchi T, Nakamura T, et al. Evaluation of Metallic Artifacts Caused by Nonpenetrating Titanium Clips in Postoperative Neuroimaging. World neurosurgery. 2016;96: 16–22. 10.1016/j.wneu.2016.08.086. [DOI] [PubMed] [Google Scholar]
- 49.Timothy J, Hanna SJ, Furtado N, Shanmuganathan M, Tyagi A. The use of titanium non-penetrating clips to close the spinal dura. British journal of neurosurgery. 2007;21(3): 268–271. 10.1080/02688690701246210. [DOI] [PubMed] [Google Scholar]
- 50.Buchta C, Hedrich HC, Macher M, Hocker P, Redl H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal and Vivostat in comparison to the homologous fibrin sealant product Tissucol/Tisseel. Biomaterials. 2005;26(31): 6233–6241. 10.1016/j.biomaterials.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Jackson MR. Fibrin sealants in surgical practice: An overview. American journal of surgery. 2001;182(2 Suppl): 1s–7s. 10.1016/s0002-9610(01)00770-x. [DOI] [PubMed] [Google Scholar]
- 52.Stechison MT. Rapid polymerizing fibrin glue from autologous or single-donor blood: preparation and indications. Journal of neurosurgery. 1992;76(4): 626–628. 10.3171/jns.1992.76.4.0626. [DOI] [PubMed] [Google Scholar]
- 53.Yuyama R, Mishima K, Fujimaki T, et al. [Clinical experience of autologous blood transfusion and fibrin glue in neurosurgery]. No shinkei geka Neurological surgery. 1998;26(8): 685–690. [PubMed] [Google Scholar]
- 54.Nakamura H, Matsuyama Y, Yoshihara H, et al. The effect of autologous fibrin tissue adhesive on postoperative cerebrospinal fluid leak in spinal cord surgery: a randomized controlled trial. Spine. 2005;30(13): E347–351. 10.1097/01.brs.0000167820.54413.8e. [DOI] [PubMed] [Google Scholar]
- 55.Neuman BJ, Radcliff K, Rihn J. Cauda equina syndrome after a TLIF resulting from postoperative expansion of a hydrogel dural sealant. Clinical orthopaedics and related research. 2012;470(6): 1640–1645. 10.1007/s11999-011-2071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulder M, Crosier J, Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: case report. Spine. 2009;34(4): E144–148. 10.1097/BRS.0b013e31818d5427. [DOI] [PubMed] [Google Scholar]
- 57.Lee G, Lee CK, Bynevelt M. DuraSeal-hematoma: concealed hematoma causing spinal cord compression. Spine. 2010;35(25): E1522–1524. 10.1097/BRS.0b013e3181edfe2c. [DOI] [PubMed] [Google Scholar]
- 58.Blackburn SL, Smyth MD. Hydrogel-induced cervicomedullary compression after posterior fossa decompression for Chiari malformation. Case report. Journal of neurosurgery. 2007;106(4 Suppl): 302–304. 10.3171/ped.2007.106.4.302. [DOI] [PubMed] [Google Scholar]
- 59.Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): a possible complication. Spine. 2010;35(1): E25–26. 10.1097/BRS.0b013e3181b9fc45. [DOI] [PubMed] [Google Scholar]
- 60.Kivelev J, Gohre F, Niemela M, Hernesniemi J. Experiences with TachoSil(R) in microneurosurgery. Acta neurochirurgica. 2015;157(8): 1353–1357; discussion 1357. 10.1007/s00701-015-2473-x. [DOI] [PubMed] [Google Scholar]
- 61.Di Vitantonio H, De Paulis D, Del Maestro M, et al. Dural repair using autologous fat: Our experience and review of the literature. Surgical neurology international. 2016;7(Suppl 16): S463–468. 10.4103/2152-7806.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]


