Abstract
Purpose:
To characterize diphtheroid corneal infections in eyes in the chronic phase of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).
Methods:
Observational case series
Results:
Four eyes of three patients were included in this review. Each eye presented with persistent corneal epithelial defect with corneal thinning in the chronic phase of SJS/TEN. None of the epithelial defects were associated with stromal infiltration. The corneas were cultured at the time of workup of persistent epithelial defect (3 eyes) or at time of tectonic penetrating keratoplasty after perforation (1 eye). Cultures yielded abundant growth of Corynebacterium spp., including C. jeikeium (n=2), C. glucuronolyticum (n=1), and a multidrug-resistant C. striatum isolate (n=1). The ocular surface was stabilized with surgical intervention (1 eye), or with introduction of fortified topical antibiotic based on laboratory identification and susceptibility testing of the isolated organisms (3 eyes). Numerous risk factors for microbial keratitis were present in all four eyes.
Conclusion:
In eyes with a persistent corneal epithelial defect in the chronic phase of SJS/TEN, even in the absence of an infiltrate, corneal culture should be undertaken. Recognition and treatment of Corynebacterium spp. as opportunistic pathogens may lead to favorable outcomes in cases of clinically “sterile” ulceration during the chronic phase of SJS/TEN.
Keywords: microbial keratitis, Stevens-Johnson syndrome, Diphtheroid keratitis, PROSE lenses, scleral lenses
Introduction
Commensal bacteria exist in a symbiotic relationship with the ocular surface in physiologic conditions. When host mucosal immunity is intact,1 there is no inflammatory reaction to microbial presence.2 However, when ocular surface immunity is compromised by chronic Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), the fine balance between the conjunctival microbiota and the unique immune components of the ocular surface is disrupted, which may result in opportunistic infections caused by an otherwise harmless commensal organism.3 Corynebacterium spp., commonly referred to as diphtheroids, are one such group of bacteria that are common resident commensals of the normal conjunctiva and eyelids.4 However, there have been reports of diphtheroids causing ocular infection in the form of conjunctivitis and keratitis in eyes with a compromised ocular surface.5–7
SJS/TEN is a mucocutaneous disease that can affect the ocular surface. Ocular complications of SJS/TEN include eyelid malposition, posterior eyelid margin keratinization, and meibomian and lacrimal gland damage, which can lead to severe dry eye and persistent epithelial defect. Such a compromised ocular surface can promote infectious keratitis.8
In this case series, we report sight-threatening keratitis associated with Corynebacterium spp. infection in eyes in the chronic phase of SJS/TEN.
Methods
We reviewed the medical records of patients treated at Massachusetts Eye and Ear (MEE) and BostonSight (BoS) for persistent corneal epithelial defect (PED) during the chronic phase of SJS/TEN in whom corneal cultures yielded abundant growth of Corynebacterium spp. Demographic data, clinical history, clinical findings, culture results including antibiotic susceptibilities, and risk factors for microbial keratitis were recorded. Isolates were sent out (Quest Diagnostics) for species identification and antimicrobial susceptibility testing, which were performed by MALDI-TOF and broth microdilution, respectively. Species identification was confirmed molecularly for three available isolates (Cases 2, 3R and 3L) using their whole genomes. Draft genomes were sequenced as 2 × 250 bp reads on an Illumina HiSeq instrument (sequencing coverage ranging from approximately 105 – 912x). Trimmed reads were de novo assembled (CLC genomics workbench) and contigs were used to predict species identification and the pool of acquired antibiotic resistance genes using K-mer-based algorithms (KmerFinder 3.2 and KmerResistance, available at: http://genomicepidemiology.org/). This study was approved by the internal review boards at MEE and BoS. The study was conducted under Health Insurance Portability and Accountability Act (HIPAA) compliance and adhered to the tenets of the Declaration of Helsinki.
Results
Four instances of diphtheroid infection in four eyes of three patients were identified. The clinical data are reported in Table 1. All four eyes presented with PED during the chronic phase of SJS/TEN. All eyes had been treated for the PED with a combination of lubrication, bandage contact lens, amniotic membrane, and/or oral tetracyclines. All eyes were already on long-term topical antibiotic prophylaxis, topical corticosteroid, and daytime PROSE treatment prior to the development of the PED. Clinical exam in all cases was notable for lack of an infiltrate typically associated with infectious keratitis (Figure 1). In Case 3R, a plaque at the base of the epithelial defect was noted and attributed to chronic use of ciprofloxacin. Each of the four eyes was treated with continuous (i.e., full time) wear of customized prosthetic replacement of the ocular surface ecosystem (PROSE; BostonSight, Needham, MA) scleral lens devices for the purpose of healing the persistent epithelial defect. Corneal scrapings were obtained after failure to heal with continuous wear PROSE treatment in the setting of stromal thinning (Cases 2, 3R, 3L), or at the time of surgery for corneal perforation (Case 1). These were sent for Gram stain, and cultures in blood agar, chocolate agar, Sabouraud dextrose agar, and thioglycollate broth media.
Table 1.
Details of 3 patients (4 eyes) with culture positive microbial keratitis attributed to the Corynebacterium species in the chronic phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Case number | Age in years/Gender | Time interval between acute SJS/TEN and presentation | Clinical corneal examination findings | Species on culture | Susceptibility profile | Treatment | Outcome of treatment | Visual acuity |
|---|---|---|---|---|---|---|---|---|
| 1 | 59/Male | 5 years | PED + thinning with perforation, diffuse corneal haze, peripheral neovascularization | Corynebacterium glucuronolyticum | No sensitivities testing undertaken | Tectonic penetrating keratoplasty with amniotic membrane graft, tarsorrhaphy, and subconjunctival vancomycin (50 mg/ml), postoperative moxifloxacin four times a day | Stable ocular surface | Baseline after SJS: 20/200 —————— Upon presentation with epithelial defect: HM —————— Final follow up: LP |
| 2 | 70/Male | 6 months | PED + thinning, diffuse corneal haze, lid keratinization, peripheral neovascularization | Corynebacterium jeikeium | Sensitive to Vancomycin, Clindamycin, Tetracycline | Topical fortified vancomycin (20 mg/ml) every 1–2 hourly, subsequently delivered via continuous wear of a PROSE device | Re-epithelialization and arrest of thinning | Baseline after SJS: 20/30 —————— Upon presentation with epithelial defect: 20/100 —————— Final follow up: 20/40 |
| 3 R | 42/Female | >20 years | PED + focal plaque at base of epithelial defect, peripheral neovascularization and conjunctivalization of cornea | Corynebacterium striatum | Sensitive to Vancomycin | Topical fortified vancomycin (20 mg/ml) every 1–2 hourly, subsequently delivered via continuous wear of a PROSE device | Re-epithelialization arrest of thinning, and clearing of infiltrate over months | Baseline after SJS: 20/100 —————— Upon presentation with epithelial defect: 20/300 —————— Final follow up: 20/100 |
| 3 L | >21 years | PED + thinning with corneal melt + hypopyon, diffuse corneal haze, peripheral neovascularization, conjunctivalization, and keratinization | Corynebacterium jeikeium | Sensitive to Vancomycin, Clindamycin, Tetracycline, Penicillin | Topical fortified vancomycin (20 mg/ml) every 1–2 hourly, subsequently delivered via continuous wear of a PROSE device | Re-epithelialization and arrest of thinning | Baseline after SJS: HM —————— Upon presentation with epithelial defect: LP —————— Final follow up: HM |
SJS = Stevens-Johnson syndrome, TEN = toxic epidermal necrolysis, PED = persistent epithelial defect, PROSE = prosthetic replacement of the ocular surface ecosystem, HM = hand motion, LP = light perception
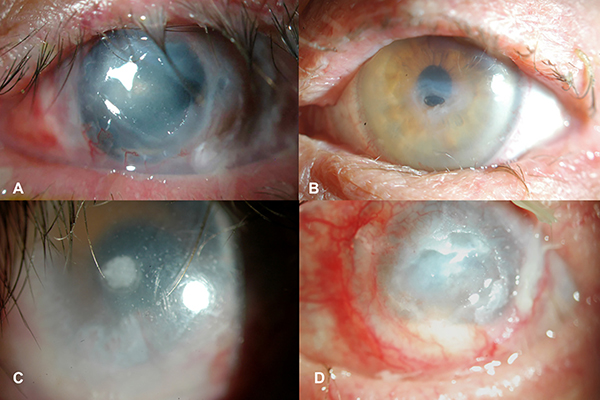
Figure 1. Clinical images of four cases of active diphtheroid keratitis.
A) Patient 1: Persistent epithelial defect (PED) of the central cornea with progressive thinning that led to corneal melt and perforation. B) Patient 2: PED of the central cornea with marked thinning. Patient is wearing a prosthetic replacement of the ocular surface ecosystem (PROSE) device. C) Patient 3 (right eye): PED with a focal plaque at base of the defect and only minimal corneal thinning. There is chronic peripheral corneal conjunctivalization. Patient is wearing a PROSE device. D) Patient 3 (left eye): PED and hypopyon, with peripheral corneal conjunctivalization and keratinization.
In each case, Gram positive bacilli were noted as the sole organism on Gram stain, with abundant diphtheroids reported within 1–2 days of culture of the corneal specimens. Susceptibilities were sought in all cases except in Case 1. The antibiotics that were processed for susceptibility testing were penicillin, erythromycin, vancomycin, clindamycin, and tetracycline. Speciation and susceptibility testing are reported in Table 1. Note that the two infections (Cases 3R and 3L) in the patient who suffered bilateral infection occurred eight months apart and yielded two different Corynebacterium spp. on culture (C. jeikeium and C. striatum). The left eye in this patient also grew moderate coagulase negative staphylococcus on culture. Corynebacterium jeikeium isolates from Cases 2 and 3L were commonly susceptible to the antibiotics tested and resistant only to erythromycin. Both isolates carried the erm(X) gene that confers resistance to macrolides, and did not carry any additional acquired resistance genes (data not shown). On the contrary, the C. striatum isolate (Case 3R) was phenotypically susceptible only to vancomycin and resistant to clindamycin, erythromycin, penicillin and tetracycline. This isolate carried a myriad of acquired resistance genes including three aminoglycosides phosphotransferases (aph(3’)-Ia, aph(3”)-Ib and aph(6)-Id), erm(X) and the chloramphenicol resistance-conferring cmx gene.
Topical fortified vancomycin (20 mg/ml) every 1–2 hours was initiated empirically at the time positive cultures for diphtheroids were reported. The frequency was reduced to four times daily after 48 hours of initiation to reduce toxicity. Prednisolone acetate 1% was continued in each case at either one or two times a day depending on level of inflammation. Susceptibility testing confirmed susceptibility to vancomycin. Continuous PROSE device wear was resumed to hasten the resolution of the PEDs in the three eyes that did not require tectonic grafting. PROSE wear was continuous with one drop of vancomycin added to the reservoir four times daily, then two times daily until the surface healed. See Figure 2 for healed ocular surface for Cases 2 and 3. Once healed, commercially available polymyxin/trimethoprim drops were applied to the eye two times a day, prior to insertion and after removal of the PROSE device, for ongoing prophylaxis against infection in these eyes with previous long-term fluoroquinolone use.9 The predisposing factors for microbial keratitis in these four cases are reported in Table 2.
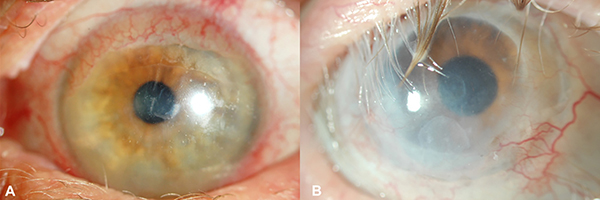
Figure 2. Clinical images of 2 of 4 eyes after healed diphtheroid keratitis.
A) Patient 2: Resolution of persistent epithelial defect (PED) of the cornea with presence of superior corneal conjunctivalization. Patient is wearing a prosthetic replacement of the ocular surface ecosystem (PROSE) device. B) Patient 3 (right eye): Resolution of PED and plaque previously seen at the base of the epithelial defect. Patient is wearing a PROSE device.
Table 2.
Potential pre-disposing factors in eyes with culture positive microbial keratitis attributed to the Corynebacterium species in the chronic phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Pre-disposing factors | Case 1 | Case 2 | Case 3R | Case 3L |
|---|---|---|---|---|
| Pre-treatment topical steroid use | Yes (dexamethasone 0.1%) | Yes (prednisolone acetate 1%) | Yes (prednisolone acetate 1%) | Yes (prednisolone acetate 1%) |
| Prophylactic antibiotic use/duration | Moxifloxacin, gatifloxacin, total of 2.5 years | Moxifloxacin, 4 weeks | Ciprofloxacin, 20+ years | Moxifloxacin, 20+ years |
| Keratinization of the eyelid margin | Yes | Yes | Yes | Yes |
| Cicatricial ectropion | Yes | Yes | No | No |
| Trichiasis and/or distichiasis | Yes | Yes | Yes | Yes |
| Limbal stem cell dysfunction | Yes | Yes | Yes | Yes |
| PROSE treatment with overnight wear (not FDA approved) | Yes | Yes | Yes | Yes |
| Therapeutic soft contact lens use | Yes | Yes | No | No |
| Systemic immune compromise | Type 2 diabetes mellitus | None | Chemotherapy and radiation therapy for treatment of secondary chondrosarcoma of the lung | |
PROSE = prosthetic replacement of the ocular surface ecosystem, FDA = Food and Drug Administration
Discussion
In the chronic phase of SJS/TEN, ocular sequelae such as eyelid margin keratinization and tarsal scarring cause blink-related microtrauma of the corneal epithelium resulting in limbal stem cell dysfunction, corneal scar, and corneal neovascularization.10 In the SJS/TEN eye where the ocular surface is commonly affected by aqueous and lipid tear deficiency dry eye, lagophthalmos and exposure from cicatricial ectropion, as well as trichiasis and distichiasis, are significant additional threats to the integrity of the ocular surface. When the epithelial barrier function is compromised, microbes can adhere easily to the unstable epithelium, causing an environment favorable for infection. In this way, eyes with eyelid-related keratopathy have an increased incidence of corneal complications including microbial keratitis.11
In addition, prophylactic topical antibiotics are often used. Maintenance medications may change the population of commensal flora of the ocular surface in these eyes, tipping the balance towards pathogens.1,2 Use of extended wear soft contact lenses, which is prevalent in this population as a bandage lens, may also alter the ocular surface flora in these eyes.12 Finally, some eyes within the chronic phase of SJS/TEN are maintained on low-dose topical steroid medication to suppress inflammation and thus may be predisposed to superinfection.
Corynebacterium spp., also known as diphtheroids, are ubiquitous Gram-positive bacilli typically thought of as contaminants when isolated in culture. Diphtheroids are often commensals of the skin and mucus membrane but can in some instances cause nosocomial infections. They can form biofilms on biotic surfaces and artificial medical devices, express multiple antibiotic-resistance phenotypes and cause difficult-to-treat infections.5 While Corynebacterium spp. are commonly found in the conjunctival flora of both normal and SJS/TEN eyes,5,13,14 the eyes in our series had resolution of epithelial defect only after treatment with vancomycin and PROSE. Corynebacterium spp. have also been identified as the causative agent of microbial keratitis in SJS/TEN eyes in previous studies.7,15 Das et al reported two eyes with chronic SJS/TEN with culture-positive diphtheroid keratitis. Both eyes presented with corneal infiltrates and corneal thinning and required cyanoacrylate glue application and bandage contact lens.7 One eye progressed to corneal perforation. Bagga et al. reported diphtheroids in 25.5% of bacterial isolates in eyes with SJS/TEN but it is unclear whether these were dominant organisms or part of a polymicrobial infection.16 In contrast with these two reports, however, the eyes in our series did not present with a corneal infiltrate and diphtheroids were the dominant organism growing on cultures.
Kang et al. studied 90 eyes with limbal stem cell dysfunction (LSCD) out of which 52 eyes had SJS/TEN, and noted infectious keratitis in 25 eyes overall, out of which six eyes had keratitis secondary to diphtheroids.15 All cases were vancomycin susceptible. The authors noted an increased incidence of infectious keratitis in eyes with SJS/TEN compared to other etiologies of LSCD secondary to its eyelid and corneal complications. Our series is notable in that all eyes had keratinization of the eyelid margin and a poor ocular surface, which may be predisposing factors to the emergence of diphtheroids as pathogens in eyes with an altered ocular surface microbiome and with prolonged use of topical steroid, contact lens, prophylactic antibiotic, and with an immunocompromised status.17 The LSCD in these eyes secondary to eyelid keratinization also likely contributed to the development of PED, allowing for Corynebacterium keratitis. Of note, none of the eyes had a known recent PED prior to the ones being described in this series. Although Cases 1 and 3 occurred before techniques such as mucous membrane grafting were widely known, Case 2 went on to receive bilateral upper and lower eyelid mucous membrane grafts a year and a half after resolution of PED.
This series also suggests that contact lens use, including soft and/or scleral, may be a risk factor in the development of diphtheroid keratitis. In addition to epithelial trauma, soft contact lenses may increase infectious risk by way of hypoxia.19 PROSE treatment involves the use of rigid gas permeable scleral lens prosthetic devices that are customized to vault the entire cornea and limbus. At the time of application, the reservoir is filled with non-preserved sterile saline. In a report of 86 patients with chronic SJS/TEN, PROSE offered sustained and significant improvement in visual acuity and visual function.18 PROSE lenses have been used for daytime and overnight (i.e., continuous) wear in eyes with chronic SJS/TEN with persistent corneal epithelial defects.20–22 Continuous wear of the PROSE device may hasten resolution of the epithelial defect in these eyes by providing a continuous mechanical barrier against blink-related microtrauma, by reducing evaporation of fluid from the ocular surface, and by supplying a continuous fluid reservoir with adequate oxygenation.20 PROSE treatment with continuous wear was being used in all four eyes in our series after failure to heal with other measures as described above (Table 3). In all cases fluoroquinolones were being used as prophylaxis in settings with multiple risk factors for infection.
Table 3.
Timeline and characteristics of PROSE use
| Case 1 | Case 2 | Case 3R | Case 3L | |
|---|---|---|---|---|
| Duration of daytime PROSE use prior to development of PED | 2.5 years | 0 | 20+ years | 20+ years |
| Duration of PED prior to continuous wear PROSE | 2 weeks | 6 weeks | 2 weeks | 2 weeks |
| Duration of continuous wear PROSE use prior to culture | 10 days | 3 weeks | Few weeks* | 1 week |
| Timepoint of sustained improvement of PED/Duration of continuous wear PROSE treatment until endpoint | NA/1 week (defect progressed to perforation) | 6 days/2 weeks (defect healed) | 1 week/12 weeks (defect healed) | 5 days/7 weeks (defect healed) |
exact amount of time is not known
PROSE = prosthetic replacement of the ocular surface ecosystem, PED = persistent epithelial defect
Culture was obtained during surgery for perforation for Case 1 and as workup for failure to heal in Cases 2 and 3. Case 1 was the outlier in management in this series as infection was not initially suspected in that case and therefore no cultures were done in the clinic setting. Only after the presentation of Case 2 was Case 1 deemed suspicious and retroactively reviewed. Though we don’t know what the outcome of Case 1 would have been had the same management strategy been followed, it may be that early culture and antibiotic treatment would have prevented progression to perforation. Case 1 did not have recurrence of disease after PKP and intraoperative subconjunctival antibiotics. The other three cases in this series also did not have recurrence of disease after the described treatment.
In all cases, Corynebacterium spp. were identified as the dominant organism in culture, and as the sole organism in three of four cases. Three available isolates were subjected to whole genome sequencing to confirm species identification and identify their pools of acquired antibiotic resistance genes. This analysis revealed that the most clinically relevant species causing opportunistic human infections, C. jeikeium (Cases 2 and 3L) and C. striatum (case 3R), were implicated in these cases. Of note, patient 3 presented with bilateral infections that occurred eight months apart and were caused by two different species, with the first presentation being associated with a highly multidrug-resistant C. striatum isolate. This patient was on ciprofloxacin 0.3% eye drops for more than 20 years as longstanding prophylaxis. In the setting of SJS/TEN with prolonged use of topical antibiotics, modifications in the ocular surface microbiome may occur that result in the selection and expansion of multidrug-resistant organisms.7 Keratoprosthesis literature also suggests that low-dose topical fluroquinolones increase antibiotic resistance.23,24
Case series which have so far been reported on infectious keratitis in SJS/TEN either do not report cases of diphtheroid keratitis or report diphtheroid cases as presenting with typical corneal ulceration and infiltrates.7,15,16,25 This is in contrast to our series, suggesting that corneal epithelial defects in the absence of infiltrates should also prompt corneal cultures as the absence of typical infectious signs may mislead or delay the clinician in diagnosis and effective management. Positive cultures prompted treatment with hourly topical, preservative-free vancomycin 20 mg/ml for at least 48h, before resumption of PROSE device wear.
Limitations of this series include that we cannot prove that in the absence of typical infiltrative signs that Corynebacteria, and not other bacteria that were not captured on culture, are solely responsible for infection. As infectious keratitis without infiltrate is uncommon and there were no controls with PED and lack of diphtheroids on culture, we cannot definitively conclude that diphtheroids are the pathogen. Cultures were not repeated, or done on the opposite eye, so it was not possible to compare Corynebacteria abundance before and after treatment or between eyes. additionally, vancomycin may be responsible for the resolution of the epithelial defect and associated disease through a mechanism other than eradication of Corynebacteria. Lastly, our pre-treatment interventions were not exhaustive in that therapies such as tarsorrhaphy were not considered and could have altered the clinical course. However, with abundant growth of diphtheroids on culture and the described response to the treatment regimen, particularly after the addition of vancomycin, this series would suggest that diphtheroids be considered as pathogens in these eyes. Interestingly, the presentation and predisposing factors in this case series are similar to those seen in methicillin-resistant S. aureus keratitis in a similar cohort of patients.26
The identification of Corynebacterium spp. as a dominant organism in these SJS/TEN patients with indolent but persistent epithelial defect and stromal thinning, and response to therapy with vancomycin warrants attention. In all cases there was gradual thinning, with minimal injection, discharge, opacity or infiltrate. Diphtheroids should be treated if they are identified as the dominant organism, with vancomycin chosen empirically. Speciation and susceptibility testing should be performed to guide therapy. Recognition and treatment of Corynebacterium spp. as opportunistic pathogens can lead to favorable outcomes in cases of seemingly sterile ulceration during the chronic phase of SJS/TEN.
Acknowledgments
Funding: National Eye Institute, award number K23EY028230
Footnotes
Conflicts of Interest: None
References
- 1.Ueta M, Kinoshita S. Ocular surface inflammation is regulated by innate immunity. Prog Retin Eye Res 2012;31:551–575. [DOI] [PubMed] [Google Scholar]
- 2.Miller D, Iovieno A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol 2009;9:466–470. [DOI] [PubMed] [Google Scholar]
- 3.White KD, Abe R, Ardern-Jones M, et al. SJS/TEN 2017: Building Multidisciplinary Networks to Drive Science and Translation. J Allergy Clin Immunol Pract. 2018;6:38–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res 2013;117:99–105. [DOI] [PubMed] [Google Scholar]
- 5.Lipsky BA, Goldberger AC, Tompkins LS, et al. Infections caused by nondiphtheria corynebacteria. Rev Infect Dis 1982;4:1220–1235. [DOI] [PubMed] [Google Scholar]
- 6.Rubinfeld RS, Cohen EJ, Arentsen JJ, et al. Diphtheroids as ocular pathogens. Am J Ophthalmol 1989;108:251–254. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Rao AS, Sahu SK, et al. Corynebacterium spp as causative agents of microbial keratitis. Br J Ophthalmol 2016;100:939–943. [DOI] [PubMed] [Google Scholar]
- 8.Kohanim S, Palioura S, Saeed HN, et al. Acute and Chronic Ophthalmic Involvement in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis - A Comprehensive Review and Guide to Therapy. II. Ophthalmic Disease. Ocul Surf 2016;14(2):168–88. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Bispo PJM, Tanner EEL, Mitragotri S, E Silva RN, Gipson I, Chodosh J, Behlau I, Paschalis EI, Gilmore MS, Dohlman CH The Search for Antifungal Prophylaxis After Artificial Corneal Surgery-An In Vitro Study. Cornea. 2020. August 4. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Di Pascuale MA, Espana EM, Liu DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology 2005;112:904–912. [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Shanbhag SS, Gokani A, et al. Chronic Ocular Sequelae of Stevens-Johnson Syndrome in Children: Long-term Impact of Appropriate Therapy on Natural History of Disease. Am J Ophthalmol 2018;189:17–28. [DOI] [PubMed] [Google Scholar]
- 12.Iskeleli G, Bahar H, Eroglu E, et al. Microbial changes in conjunctival flora with 30-day continuous-wear silicone hydrogel contact lenses. Eye Contact Lens 2005;31:124–126. [DOI] [PubMed] [Google Scholar]
- 13.Frizon L, Araujo MC, Andrade L, et al. Evaluation of conjunctival bacterial flora in patients with Stevens-Johnson Syndrome. Clinics (Sao Paulo) 2014;69:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venugopal R, Satpathy G, Sangwan S, et al. Conjunctival Microbial Flora in Ocular Stevens-Johnson Syndrome Sequelae Patients at a Tertiary Eye Care Center. Cornea 2016;35:1117–1121. [DOI] [PubMed] [Google Scholar]
- 15.Kang BS, Kim MK, Wee WR, et al. Infectious Keratitis in Limbal Stem Cell Deficiency: Stevens-Johnson Syndrome Versus Chemical Burn. Cornea 2016;35:51–55. [DOI] [PubMed] [Google Scholar]
- 16.Bagga B, Motukupally SR, Mohamed A. Microbial keratitis in StevensJohnson syndrome: clinical and microbiological profile. Ocul Surf 2018; 16:454–457. [DOI] [PubMed] [Google Scholar]
- 17.Shin H, Price K, Albert L, Dodick J, Park L, Dominguez-Bello MG. Changes in the eye microbiota associated with contact lens wearing. mBio 2016. March 22;7(2):e00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papakostas TD, Le HG, Chodosh J, et al. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology 2015;122:248–253. [DOI] [PubMed] [Google Scholar]
- 19.Weissman BA, Mondino BJ. Risk factors for contact lens associated microbial keratitis. Cont Lens Anterior Eye. 2002. March;25(1):3–9. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal P, Cotter JM, Baum J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am J Ophthalmol 2000;130:33–41. [DOI] [PubMed] [Google Scholar]
- 21.Lim P, Ridges R, Jacobs DS, et al. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol 2013;156:1095–1101. [DOI] [PubMed] [Google Scholar]
- 22.Ciralsky JB, Chapman KO, Rosenblatt MI, et al. Treatment of Refractory Persistent Corneal Epithelial Defects: A Standardized Approach Using Continuous Wear PROSE Therapy. Ocul Immunol Inflamm 2015;23:219–224. [DOI] [PubMed] [Google Scholar]
- 23.Torres-Netto EA, Silva LD, Bordon Riveros MA, Santos A, Sousa LB, Oliveira LA. Boston Type I Keratoprosthesis: Antibacterial Resistance and Microbiota Evaluation of Soft Contact Lenses Am J Ophthalmol. 2018. August;192:178–183. [DOI] [PubMed] [Google Scholar]
- 24.Robert MC, Eid EP, Saint-Antoine P, Harissi-Dagher M. Microbial colonization and antibacterial resistance patterns after Boston type 1 keratoprosthesis. Ophthalmology. 2013. August;120(8):1521–8. [DOI] [PubMed] [Google Scholar]
- 25.Sharma N, Venugopal R, Singhal D, et al. Microbial Keratitis in Stevens-Johnson Syndrome: A Prospective Study. Cornea 2019; 38:938–942. [DOI] [PubMed] [Google Scholar]
- 26.Sotozono C, Inagaki K, Fujita A, Koizumi N, Sano Y, Inatomi T, Kinoshita S. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002. October;21(7 Suppl):S94–101. [DOI] [PubMed] [Google Scholar]