Abstract
Background:
Lifestyle factors related to energy balance have been associated with ovarian cancer risk and influence the tumor immune microenvironment, including tumor-associated macrophages (TAMs). However, no studies have assessed whether these factors differentially impact ovarian cancer risk by TAM densities.
Methods:
We conducted a prospective analysis in the Nurses’ Health Studies to examine the associations of physical activity, sitting time, and a food-based empirical dietary inflammatory pattern (EDIP) score with invasive epithelial ovarian cancer risk by TAM density assessed by immunohistochemistry. We considered density of CD68 (marker of total TAMs) and CD163 (marker of pro-carcinogenic M2-type TAMs), and their ratios. We used multivariable Cox proportional hazards regression to calculate hazard ratios (HR) and 95% confidence intervals (CIs) of exposures with risk of ovarian tumors with high versus low TAMs, including analyses stratified by body mass index.
Results:
Analyses included 312 incident ovarian cancer cases with TAM measurements. Physical activity, sitting time, and EDIP score were not differentially associated with ovarian cancer risk by TAM densities (Pheterogeneity>0.05). Among overweight and obese women, higher EDIP score was associated with increased risk of CD163 low density tumors (HR comparing extreme tertiles=1.57, 95%CI=0.88–2.80; Ptrend=0.01), but not CD163 high density tumors (comparable HR=1.16, 95%CI=0.73–1.86; Ptrend=0.24), though this difference was not statistically significant (Pheterogeneity=0.22).
Conclusions:
We did not observe differential associations between lifestyle factors and ovarian cancer risk by TAM densities.
Impact:
Future investigations examining the interplay between other ovarian cancer risk factors and the tumor immune microenvironment may help provide insight into ovarian cancer etiology.
Keywords: ovarian cancer, physical activity, sedentary behavior, pro-inflammatory diet, tumor associated macrophages
Introduction
Ovarian cancer is the deadliest gynecologic cancer with 21,750 new cases expected in 2020 in the United States (US) (1). Local and systemic inflammation have been reported to influence ovarian cancer risk (2). Lifestyle factors related to energy balance including physical activity, sedentary behavior, and pro-inflammatory diet influence inflammation (3–5) and are associated with cancer risk such as breast and colorectal cancers (1). The literature on the associations between these lifestyle factors related to energy balance and ovarian cancer risk has been inconsistent (6–13), including the Nurses’ Health Studies reporting null associations between post-menopausal physical activity and pro-inflammatory diet and ovarian cancer risk (14, 15). While the inconsistent findings could be due to different methods and varying timing of assessment of lifestyle factors (16), it could also be due to these lifestyle factors being associated with specific tumor subtypes defined by tumor immune function. Studies report that risk factors for ovarian cancer differ by histotype, strongly supporting that ovarian cancer is a heterogeneous disease with differences in etiology by pathological features (17). Therefore, it is plausible that there may be heterogeneity in risk factors by tumor immune profiles.
We and others have reported chronic exposures being differentially associated with cancer risk by tumor immune profiles (18–24). Specifically, we have previously observed significant heterogeneity in the association between aspirin use and ovarian cancer risk by tumor infiltration of M2-type immunosuppressive macrophage density (18), supporting that chronic exposures may influence the immunomodulatory activity, impacting the development of tumor with specific immune profiles (25). Emerging evidence suggests that lifestyle factors influence the tumor immune microenvironment, including tumor associated macrophages (TAMs) in both animal models and humans with other cancer types (26–28). TAMs are prevalent in the ovarian tumor microenvironment, and are generally categorized by their dominant polarization as M1-type TAMs, which play a role in inhibiting tumor progression, and M2-type TAMs, which promote tumor progression via immune suppression (29). Several studies have observed associations of M2-type TAMs infiltration and low M1/M2 macrophage ratio with poor survival in ovarian cancer (29). Interestingly, one study reported that the distribution of TAMs differed by histotype, with TAMs most frequently infiltrating in serous histotypes (30). However, given there was no significant heterogeneity by histotype in the aspirin ovarian cancer association (31, 32), our observation of significant heterogeneity in the association between aspirin use and ovarian cancer risk by tumor infiltration of M2-type immunosuppressive macrophage density (18) supports that risk factors may vary by tumor subtypes defined by tumor immune profiles independent of histotype.
Given the roles of diet, activity, and sedentary behavior on inflammation and immunity, it is plausible that these factors impact ovarian cancer development by influencing tumor immune response. Thus, we conducted a prospective analysis to examine the association of physical activity, sitting time, and a food-based empirical dietary inflammatory pattern (EDIP) score with invasive epithelial ovarian cancer risk by TAM densities in two large cohorts of US women. Since obesity has been reported to influence polarization and infiltration of TAMs in the tumor microenvironment (33), we also conducted a stratified analysis to examine the potential differential associations between lifestyle factors related to energy balance and ovarian cancer risk by TAM densities stratified by body mass index (BMI).
Materials and Methods
Study population
The Nurses’ Health Study (NHS) is a prospective cohort study initiated in 1976 that enrolled 121,700 female registered nurses aged 30–55 years residing in 11 US states (34). The NHSII is a prospective cohort study initiated in 1989 that enrolled 116,429 female registered nurses aged 25–42 years residing in 14 US states (35). Participants completed a baseline questionnaire and have been followed biennially via questionnaires assessing updated information on various lifestyle and reproductive factors as well as incident diseases. The response rate ranged between 85–90% at each cycle in the NHS/NHSII cohorts. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. The IRB allowed participant’s return of self-administered questionnaire to be considered as implied informed consent.
Assessment of lifestyle exposures
Physical activity was assessed starting in 1986 in the NHS and 1989 in the NHSII and every 2–4 years thereafter using questionnaires that have been validated previously (36). A metabolic equivalent task (MET) score was assigned to the eight common leisure-time activities assessed (i.e. walking, jogging, running, bicycling, swimming, tennis, squash/racquetball and calisthenics/aerobics/rowing machine) to quantify its energy expenditure and MET-hours per week was calculated for each activity by multiplying the MET-score by the reported hours per week spent on that activity as described previously (37). MET-hours per week over all activities were summed to calculate total physical activity.
Sitting time was assessed using validated questionnaires in 1992, 2004, and every 4 years thereafter in NHS and in 1991 and every 4 years thereafter in NHSII (36). Participants were asked about their average hours per week spent sitting at work and time spent at home sitting watching television or other sitting (e.g. reading, meal times) (38). The average hours per week of these three sources of sitting time were summed to calculate total sitting time.
Dietary intake was assessed using a self-administered, semi-quantitative food frequency questionnaire (FFQ). The FFQ was administered in 1984, 1986, and every 4 years thereafter in NHS, and every 4 years since 1991 in NHSII (39, 40). Previous validation studies of the FFQ have demonstrated good correlations between foods and nutrients assessed by the FFQ compared with foods records (41). Pro-inflammatory diet was assessed using the EDIP score described previously (5). In brief, EDIP score is the weighted sum of 18 food groups, with higher scores indicating a more pro-inflammatory diet.
Ovarian cancer case ascertainment
Ovarian cancer cases were identified by self-report on biennial questionnaires or linkage with death certificates. Medical records or cancer registries were used to confirm the diagnosis and abstract tumor information such as histology, stage, and grade.
Tumor-associated macrophage density assessment
TAM density was measured using the NHS/NHSII ovarian tumor tissue microarrays as described previously (18). In brief, for each ovarian cancer paraffin-embedded tissue block containing representative tumor sample, three cores with 0.6mm diameter were embedded in a tissue microarray. CD68 (marker for total TAMs) and CD163 (marker for M2-type TAMs) were stained using immunohistochemistry (IHC) performed on the Leica Bond automated staining platform. Antibody CD68 from Dako catalogue # M0876 clone PG-M1 was run at 1:500 dilution using the Leica Biosystems Refine Detection Kit with CITRATE antigen retrieval. Antibody CD163 from Vector catalogue # VP-C374 clone 10D6 was run at 1:250 dilution using the Leica Biosystems Refine Detection Kit with EDTA antigen retrieval. CD68 and CD163 stains were evaluated in a semiquantitative manner by a gynecologic pathologist. CD68 and CD163 densities were scored separately for tumor stroma and epithelium as: none (1), low (2; <10% of cells, scattered), moderate (3; <10% of cells, with aggregation—at least three aggregates of three macrophages), and high (4; >10% of cells macrophages or an area of confluent macrophages). Tumors were classified as high density (i.e., CD68 high or CD163 high) when the sum of the epithelium and stromal scores were greater than 4 or low density (i.e. CD68 low or CD163 low) when the sum of scores was 4 or less.
Statistical analysis
Three analytic datasets were created to evaluate each exposure of interest: physical activity, sitting time, and diet as these three exposures were not always assessed at the same time. Person-years of follow-up were calculated from the return date of the first questionnaire that assessed the exposures (“baseline”, i.e., 1986 (NHS) or 1989 (NHSII) for physical activity; 1992 (NHS) or 1991 (NHSII) for sitting time; 1984 (NHS) or 1991 (NHSII) for diet) until the date of ovarian cancer diagnosis, death, bilateral oophorectomy, or the end of follow-up (May 31, 2016 for NHS and May 31, 2017 for NHSII), whichever came first. Women were excluded if they had a bilateral oophorectomy, pelvic irradiation, or a prior diagnosis of cancer (other than non-melanoma skin cancer) before baseline, or without data on the exposure of interest. Ovarian cancer cases with TAM data, which comprised 26% of the total incident ovarian cancer cases, contributed to the estimates of incidence and all other ovarian cancer cases were censored. As previously reported, reproductive/hormonal factors and tumor characteristics were similar between ovarian cancer cases eligible for block collection and cases with tissue data (42). The baseline cohort for analyses included 175,897 participants (NHS=64,503, NHSII=111,394) for physical activity, 152,022 participants (NHS=59,316, NHSII=92,706) for sitting time, and 155,461 participants (NHS=65,550, NHSII=89,911) for proinflammatory diet.
In primary analyses, we examined cumulative average (i.e., average of all previous measures since baseline) physical activity, sitting time, and EDIP score as this measure best represents long-term exposure status and minimizes measurement error due to within-person variation over time (43). In secondary analyses, we examined the most recently reported exposure prior to ovarian cancer diagnosis using questionnaire data one cycle prior to ovarian cancer diagnosis (i.e., 2–6 years prior to ovarian cancer diagnosis).
We used Cox proportional hazard regression models stratified by age, calendar year, and cohort to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs). Competing risk analyses were used to examine whether the associations between the three exposures of interest and ovarian cancer risk differed by the levels of immune markers (e.g., CD163 high, CD163 low) (44). All models were adjusted for established or putative ovarian cancer risk factors a priori including menopausal status (pre, post), parity (nulliparous, 1, 2, 3, >3 births), oral contraceptive use (never, <1, 1–4, 5–9, ≥10 years), hormone therapy (ever, never), tubal ligation (yes, no), hysterectomy (yes, no), family history of breast or ovarian cancer (yes, no), and BMI (<21, 21–24.9, 25–29.9, 30–34.9, ≥35 kg/m2). Physical activity and sitting time were modeled in three categories (<3, 3–17.9, ≥18 MET-hours/week; <21, 21–48.9, ≥49 hours/week, respectively). Further adjustment for aspirin use, smoking, and alcohol intake, which are associated with inflammation and thus may potentially influence the tumor microenvironment, as well as different types of hormone therapy use (i.e. estrogen only, estrogen and progestin, and other hormone therapy use) instead of the dichotomous hormone therapy use (i.e. ever, never) did not alter the risk estimates and were therefore not included in the final models. The EDIP score was categorized in tertiles based on the distributions of the NHS and NHSII combined with the lowest tertile as the reference group. Tests for linear trend were performed by entering the categorical variables as continuous parameters in the models. We assessed heterogeneity in HRs comparing a model constraining associations to be the same versus different for tumors with high versus low levels of immune markers using the likelihood ratio test. Since ovarian cancer is a heterogeneous disease comprising several histotypes with distinct epidemiologic, molecular, and clinical features, we conducted a sensitivity analysis restricting to high-grade serous and poorly differentiated ovarian cancers. We also conducted analyses of each exposure stratified by BMI (<25, ≥25 kg/m2) assessed at the same time as the relevant exposure assessment. We used SAS version 9.4 (SAS Institute, Cary, NC) for all analyses and considered a two-sided p-value of <0.05 to be statistically significant.
Results
These analyses included up to 175,897 participants with 273, 212, and 306 incident ovarian cancer cases (for a total of 312 ovarian cancer cases across all analyses) with TAM measurements included in the analyses for physical activity, sitting time, and EDIP score, respectively. When comparing the extreme categories of physical activity, sitting time, and pro-inflammatory diet at first assessment, the distribution of age-standardized characteristics was generally similar across each group (Table 1). Participants who reported lower cumulative average physical activity and lower cumulative average sitting time were more likely to be multiparous.
Table 1.
Age-standardized characteristics at the first assessment of physical activity, sedentary behavior, and EDIP score, NHS and NHSII
| Characteristic | Physical activity in 1986 (NHS)/1989 (NHSII), MET-hours/week | Sitting time in 1992 (NHS)/1991 (NHSII), hours/week | EDIP score in 1984 (NHS)/1991 (NHSII) | |||
|---|---|---|---|---|---|---|
| 0–2.9 | ≥18 | 0–21 | ≥49 | Tertile 1 | Tertile 3 | |
| n | 35,012 | 62,656 | 50,979 | 30,574 | 47,401 | 61,855 |
| Agea, years, median (IQR) | 42 (35,52) | 36 (32,42) | 39 (34,51) | 44 (36,58) | 43 (38,52) | 38 (34,43) |
| BMI, kg/m2, median (IQR) | 24 (22,28) | 23 (21,25) | 24 (21,27) | 25 (22,29) | 23 (21,26) | 24 (22,28) |
| Postmenopausal (%) | 18.9 | 18.6 | 28.1 | 28.2 | 18.1 | 17.8 |
| Parity (%) | ||||||
| Nulliparous | 15.9 | 24.2 | 15.2 | 22.0 | 16.8 | 17.3 |
| 1 child | 14.8 | 14.3 | 13.3 | 14.3 | 12.4 | 13.8 |
| 2 children | 33.0 | 29.6 | 33.5 | 30.6 | 32.7 | 32.1 |
| ≥3 children | 36.3 | 31.9 | 38.0 | 33.1 | 38.1 | 36.8 |
| Oral contraceptive use (%) | ||||||
| Never | 29.5 | 28.7 | 29.0 | 28.6 | 30.2 | 29.8 |
| Less than 1 year | 10.9 | 10.5 | 11.0 | 10.1 | 11.3 | 10.5 |
| 1-<5 years | 33.7 | 33.1 | 33.1 | 32.8 | 32.6 | 32.0 |
| 5-<10 years | 19.2 | 20.4 | 19.6 | 20.2 | 18.8 | 19.8 |
| ≥10 years | 6.8 | 7.3 | 7.3 | 8.3 | 7.1 | 7.8 |
| Ever postmenopausal hormone therapy use (%) | 7.4 | 8.6 | 15.4 | 16.0 | 7.4 | 6.9 |
| Tubal ligation (%) | 17.5 | 15.4 | 18.8 | 17.3 | 18.0 | 18.5 |
| Hysterectomy (%) | 9.3 | 9.1 | 11.0 | 10.4 | 9.2 | 11.0 |
| Family history of breast or ovarian cancer (%) | 8.2 | 8.4 | 10.0 | 10.1 | 8.8 | 8.5 |
Abbreviations: BMI, body mass index; EDIP, empirical dietary inflammatory pattern; MET, metabolic equivalent task; NHS, Nurses’ Health Study; IQR, interquartile range.
Percentages may not add up to 100% due to rounding.
Value is not age adjusted.
In our analytic dataset, we observed null associations between cumulative average physical activity, sitting time, and pro-inflammatory diet and overall ovarian cancer risk, which was consistent with our prior studies (14, 15). In the current study, these lifestyle factors were not differentially associated with ovarian cancer risk by TAM densities (Pheterogeneity≥0.54; Table 2). Furthermore, there were no significant associations when examining recent lifestyle exposures (Table S1) or when restricting to high-grade serous and poorly differentiated ovarian cancers (Table S2).
Table 2.
Associations between cumulative average physical activity, sitting time, EDIP score, and ovarian cancer risk by levels of CD163, CD68 and their ratio, NHS and NHSII1
| HR (95% CI) | Pheterogeneity2 | HR (95% CI) | Pheterogeneity2 | HR (95% CI) | Pheterogeneity2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| CD163 low | CD163 high | CD68 low | CD68 high | CD163/CD68<1 | CD163/CD68≥1 | ||||
| Total cumulative average physical activity, MET-hours/week | |||||||||
| n, case | 117 | 150 | 0.70 | 99 | 170 | 0.95 | 79 | 184 | 0.65 |
| <3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| 3–17.9 | 0.70 (0.44,1.11) | 0.99 (0.63,1.55) | 0.83 (0.50,1.40) | 0.86 (0.57,1.29) | 0.93 (0.50,1.72) | 0.79 (0.54,1.15) | |||
| ≥18 | 0.80 (0.40,1.61) | 0.83 (0.43,1.61) | 0.83 (0.37,1.84) | 0.84 (0.46,1.51) | 0.94 (0.39,2.26) | 0.77 (0.43,1.36) | |||
| Ptrend | 0.35 | 0.62 | 0.57 | 0.51 | 0.87 | 0.29 | |||
| Total cumulative average sitting time, hours/week | |||||||||
| n, case | 85 | 121 | 0.91 | 70 | 140 | 0.69 | 67 | 137 | 0.63 |
| <21 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| 21–48.9 | 1.49 (0.94,2.37) | 0.98 (0.67,1.44) | 1.31 (0.79,2.16) | 1.14 (0.79,1.63) | 1.06 (0.63,1.76) | 1.25 (0.87,1.80) | |||
| ≥49 | 0.41 (0.12,1.35) | 0.90 (0.47,1.72) | 0.44 (0.13,1.49) | 0.93 (0.50,1.73) | 0.66 (0.25,1.74) | 0.77 (0.39,1.54) | |||
| Ptrend | 0.94 | 0.78 | 0.72 | 0.87 | 0.61 | 0.92 | |||
| Total cumulative average EDIP score | |||||||||
| n, case | 135 | 164 | 0.54 | 116 | 186 | 0.68 | 86 | 209 | 0.74 |
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| T2 | 1.18 (0.95,1.47) | 1.08 (0.88,1.31) | 1.05 (0.83,1.33) | 1.12 (0.93,1.35) | 1.07 (0.81,1.42) | 1.14 (0.96,1.36) | |||
| T3 | 1.15 (0.81,1.64) | 1.24 (0.90,1.70) | 1.27 (0.87,1.84) | 1.19 (0.88,1.60) | 1.39 (0.91,2.14) | 1.16 (0.88,1.54) | |||
| Ptrend | 0.12 | 0.42 | 0.64 | 0.21 | 0.57 | 0.14 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; EDIP, empirical dietary inflammatory pattern; HR, hazard ratio; MET, metabolic equivalent task; NHS, Nurses’ Health Study; T, tertile.
HRs were calculated using Cox proportional hazards models stratified by age, calendar year, and cohort, and adjusted for menopausal status (pre, post), parity (nulliparous, 1, 2, 3, >3), oral contraceptive use (never, <1, 1–4, 5–9, ≥10 years), hormone therapy use (ever, never), tubal ligation (yes, no), hysterectomy (yes, no), family history of breast or ovarian cancer (yes, no), and BMI (<21, 21–24.9, 25–29.9, 30–34.9, and ≥35).
CD68 and CD163 density were scored separately for tumor stroma and epithelium as: none (1), low (2; <10% of cells, scattered), moderate (3; <10% of cells, with aggregation—at least three aggregates of three macrophages), high (4; >10% of cells macrophages or an area of confluent macrophages). Tumors were classified as high density (i.e., CD68 high or CD163 high) when the sum of the epithelium and stromal scores was greater than 4 out of 8, or low density when the sum of scores was less than 5.
Heterogeneity for associations of physical activity, sitting time, and EDIP score, with ovarian cancer risk by CD163, CD68, and their ratio.
In a stratified analysis by BMI, we observed that among women with BMI ≥25kg/m2, higher intake of a pro-inflammatory diet (EDIP score tertile 3) was suggestively associated with an increased risk of CD163 low density tumors (HR comparing extreme tertiles=1.57, 95%CI=0.88–2.80; Ptrend=0.01) but not CD163 high density tumors (comparable HR=1.16, 95%CI=0.73–1.86; Ptrend=0.24), though this difference was not statistically significant (Pheterogeneity=0.22) (Figure 1). We did not observe differential associations for physical activity or sitting time and TAM densities when stratified by BMI (Table S3).
Figure 1.
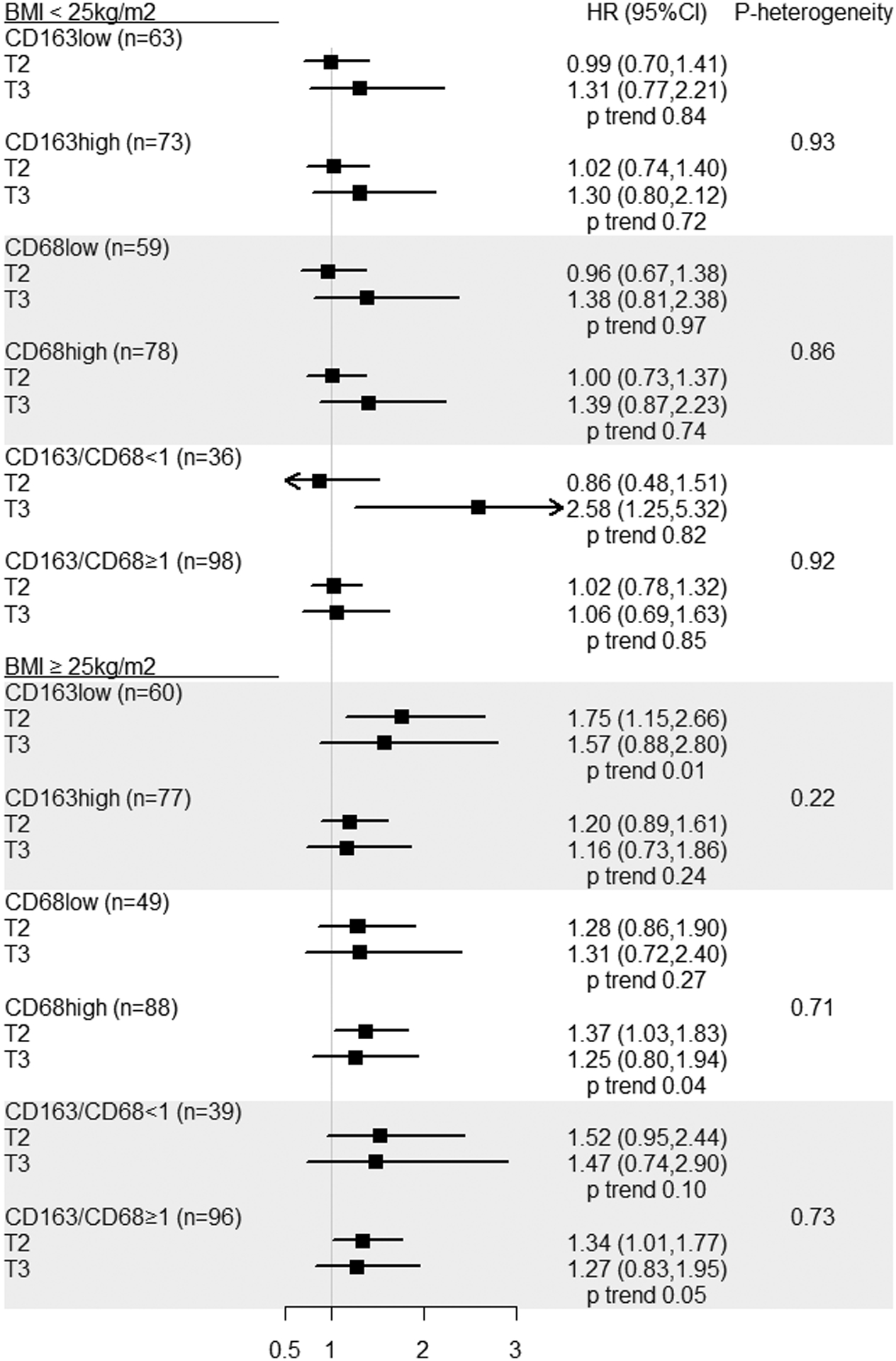
Association between cumulative average EDIP score and ovarian cancer risk by levels of CD163, CD68 and their ratio, stratified by BMI, NHS and NHSII.
Abbreviations: BMI, body mass index; CI, confidence interval; EDIP, empirical dietary inflammatory pattern; HR, hazard ratio; NHS, Nurses’ Health Study; T, tertile. Heterogeneity for associations of EDIP score with ovarian cancer risk by CD163, CD68, and their ratio.
Tertile 1 is the reference group for all models.
Discussion
In this pooled analysis of two large prospective cohort studies, we did not observe a differential association between physical activity, sitting time, and pro-inflammatory diet and ovarian cancer risk by TAM densities. To our knowledge, this is the first prospective study to assess lifestyle exposures related to energy balance in relation to ovarian cancer risk by TAM densities.
Many studies, including ours, have observed differential associations between chronic exposures (e.g. lifestyle factors) with cancer risk by tumor immune profiles (18–24). These results support the hypothesis that chronic exposures may impact anti- or pro-tumorigenic effect through immunomodulatory activities and thereby influence the development of tumors with specific immune profiles. Emerging evidence supports that some lifestyle factors have inflammatory and immuno-modulatory effects that operate systemically as well as on the tumor microenvironment (26–28). One study reported dietary modifications of protein or amino acid restricted diets altered macrophage polarization from a tumor-promoting (M2-type) to tumor-suppressive phenotype (M1-type) in animal models of prostate and renal cell carcinoma (26). Another study using mouse models reported links between exercise and reductions in tumor promoting (M2-type) macrophages and immunosuppressive myeloid-derived suppressor cells in breast tumor tissue (45). While most of the in vivo data report the interrelation between lifestyle factors and tumor immune profiles in the context of tumor progression, given that many factors have been reported to be associated with both risk and progression of ovarian cancer (18, 46, 47), it is plausible these lifestyle factors impact early tumor development through modulating the tumor immune microenvironment and have differential associations by subgroup defined by tumor immune profiles. This is supported by the studies in colorectal cancer observing the interrelation between lifestyle exposures and the colorectal tumor immune microenvironment, particularly T cells (19–24).
Obesity may influence polarization and infiltration of TAMs in the tumor microenvironment (33). Thus, we hypothesized that differential associations between lifestyle factors related to energy balance and ovarian cancer risk by TAM densities could differ by BMI. While we observed a suggestive association between a pro-inflammatory diet and CD163 low density ovarian cancer risk among those who were obese, there was no statistically significant heterogeneity by CD163 density levels. Higher BMI is associated with inflammation and increased risk of low-grade serous, invasive mucinous, endometrioid, and clear cell tumors (48–51). Therefore, exposure to a pro-inflammatory diet and having higher BMI may have synergistic effect in increasing ovarian cancer risk overall and also differentially impacting the polarization of TAMs in the tumor microenvironment. However, this result was based on limited sample size when stratified by BMI and therefore further examinations are needed in larger datasets.
The major strength of our study was the availability of physical activity, sitting time, and pro-inflammatory diet data assessed prospectively multiple times throughout follow-up, allowing us to examine the cumulative average of each exposure and thus minimize misclassification. Our study was limited by the number of ovarian cancer cases with tissue, which reduced power to identify significant associations. All exposures were measured by self-report which may increase the likelihood of misclassification. However, since these exposures were assessed years prior to ovarian cancer diagnosis, the misclassification is likely to be unrelated to the occurrence of the disease and therefore bias towards the null. We also acknowledge that our limited sample size may have resulted in inadequate power to detect significant heterogeneity by TAM density levels. We also have included a wide range of adjustment variables which will likely increase the standard errors. However, we decided to adjust for these established or putative ovarian cancer risk factors a priori to be consistent with prior literature to enhance comparison.
In summary, our results showed that physical activity, sitting time, and pro-inflammatory diet were not differentially associated with ovarian cancer risk by TAM densities. However, given emerging evidence of lifestyle factors impacting the tumor immune microenvironment, future investigations examining how other ovarian cancer risk factors may interplay with the tumor immune microenvironment may help provide insight into ovarian cancer etiology.
Supplementary Material
Acknowledgement:
We would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. We thank the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital as home of the Nurses’ Health Studies. The authors assume full responsibility for analyses and interpretation of these data.
Financial support: This work was supported in part by the National Institute of Health Award Numbers UM1 CA186107, P01 CA87969, U01 CA176726. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors have no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;9117:1459–67. 10.1093/jnci/91.17.1459 [DOI] [PubMed] [Google Scholar]
- 3.McTiernan A Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;83:205–11. 10.1038/nrc2325 [DOI] [PubMed] [Google Scholar]
- 4.Jochem C, Wallmann-Sperlich B, Leitzmann MF. The Influence of Sedentary Behavior on Cancer Risk: Epidemiologic Evidence and Potential Molecular Mechanisms. Curr Nutr Rep. 2019;83:167–74. 10.1007/s13668-019-0263-4 [DOI] [PubMed] [Google Scholar]
- 5.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and Validation of an Empirical Dietary Inflammatory Index. J Nutr. 2016;1468:1560–70. 10.3945/jn.115.228718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arthur R, Brasky TM, Crane TE, Felix AS, Kaunitz AM, Shadyab AH, et al. Associations of a Healthy Lifestyle Index With the Risks of Endometrial and Ovarian Cancer Among Women in the Women’s Health Initiative Study. Am J Epidemiol. 2019;1882:261–73. 10.1093/aje/kwy249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahmann PH, Friedenreich C, Schulz M, Cust AE, Lukanova A, Kaaks R, et al. Physical activity and ovarian cancer risk: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;181:351–4. 10.1158/1055-9965.Epi-08-0958 [DOI] [PubMed] [Google Scholar]
- 8.Merritt MA, Tzoulaki I, van den Brandt PA, Schouten LJ, Tsilidis KK, Weiderpass E, et al. Nutrient-wide association study of 57 foods/nutrients and epithelial ovarian cancer in the European Prospective Investigation into Cancer and Nutrition study and the Netherlands Cohort Study. Am J Clin Nutr. 2016;1031:161–7. 10.3945/ajcn.115.118588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz M, Lahmann PH, Boeing H, Hoffmann K, Allen N, Key TJ, et al. Fruit and vegetable consumption and risk of epithelial ovarian cancer: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2005;1411 Pt 1:2531–5. 10.1158/1055-9965.Epi-05-0159 [DOI] [PubMed] [Google Scholar]
- 10.Thomson CA, Neuhouser ML, Shikany JM, Caan BJ, Monk BJ, Mossavar-Rahmani Y, et al. The role of antioxidants and vitamin A in ovarian cancer: results from the Women’s Health Initiative. Nutr Cancer. 2008;606:710–9. 10.1080/01635580802233983 [DOI] [PubMed] [Google Scholar]
- 11.Thomson CA, Van Horn L, Caan BJ, Aragaki AK, Chlebowski RT, Manson JE, et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative dietary modification trial. Cancer Epidemiol Biomarkers Prev. 2014;2312:2924–35. 10.1158/1055-9965.Epi-14-0922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayedi A, Emadi A, Shab-Bidar S. Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv Nutr. 2018;94:388–403. 10.1093/advances/nmy015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J Physical activity, sitting time, and the risk of ovarian cancer: A brief research report employing a meta-analysis of existing. Health Care Women Int. 2019;404:433–58. 10.1080/07399332.2018.1505892 [DOI] [PubMed] [Google Scholar]
- 14.Huang T, Eliassen AH, Hankinson SE, Okereke OI, Kubzansky LD, Wang M, et al. A prospective study of leisure-time physical activity and risk of incident epithelial ovarian cancer: Impact by menopausal status. Int J Cancer. 2016;1384:843–52. 10.1002/ijc.29834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabung FK, Huang T, Giovannucci EL, Smith-Warner SA, Tworoger SS, Poole EM. The inflammatory potential of diet and ovarian cancer risk: results from two prospective cohort studies. Br J Cancer. 2017;1176:907–11. 10.1038/bjc.2017.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang T, Eliassen AH, Hankinson SE, Okereke OI, Kubzansky LD, Wang M, et al. A prospective study of leisure‐time physical activity and risk of incident epithelial ovarian cancer: Impact by menopausal status. International journal of cancer. 2016;1384:843–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;3424:2888–98. 10.1200/jco.2016.66.8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnard ME, Hecht JL, Rice MS, Gupta M, Harris HR, Eliassen AH, et al. Anti-Inflammatory Drug Use and Ovarian Cancer Risk by COX1/COX2 Expression and Infiltration of Tumor-Associated Macrophages. Cancer Epidemiol Biomarkers Prev. 2018;2712:1509–17. 10.1158/1055-9965.Epi-18-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song M, Nishihara R, Cao Y, Chun E, Qian ZR, Mima K, et al. Marine ω−3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA oncology. 2016;29:1197–206. 10.1001/jamaoncol.2016.0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y, Nishihara R, Qian ZR, Song M, Mima K, Inamura K, et al. Regular Aspirin Use Associates With Lower Risk of Colorectal Cancers With Low Numbers of Tumor-Infiltrating Lymphocytes. Gastroenterology. 2016;1515:879–92.e4. 10.1053/j.gastro.2016.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamada T, Nowak JA, Masugi Y, Drew DA, Song M, Cao Y, et al. Smoking and Risk of Colorectal Cancer Sub-Classified by Tumor-Infiltrating T Cells. J Natl Cancer Inst. 2019;1111:42–51. 10.1093/jnci/djy137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanyuda A, Ogino S, Qian ZR, Nishihara R, Song M, Mima K, et al. Body mass index and risk of colorectal cancer according to tumor lymphocytic infiltrate. Int J Cancer. 2016;1394:854–68. 10.1002/ijc.30122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Nishihara R, Qian ZR, Tabung FK, Nevo D, Zhang X, et al. Association Between Inflammatory Diet Pattern and Risk of Colorectal Carcinoma Subtypes Classified by Immune Responses to Tumor. Gastroenterology. 2017;1536:1517–30.e14. 10.1053/j.gastro.2017.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Liu L, Keum N, Qian ZR, Nowak JA, Hamada T, et al. Calcium Intake and Risk of Colorectal Cancer According to Tumor-infiltrating T Cells. Cancer Prev Res (Phila). 2019;125:283–94. 10.1158/1940-6207.Capr-18-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogino S, Nowak JA, Hamada T, Milner DA Jr., Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol. 2019;1483–103. 10.1146/annurev-pathmechdis-012418-012818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orillion A, Damayanti NP, Shen L, Adelaiye-Ogala R, Affronti H, Elbanna M, et al. Dietary Protein Restriction Reprograms Tumor-Associated Macrophages and Enhances Immunotherapy. Clin Cancer Res. 2018;2424:6383–95. 10.1158/1078-0432.Ccr-18-0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;1710:620–32. 10.1038/nrc.2017.78 [DOI] [PubMed] [Google Scholar]
- 28.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016;7614:4032–50. 10.1158/0008-5472.Can-16-0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol. 2017;1471:181–87. 10.1016/j.ygyno.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;719. 10.1186/1757-2215-7-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixon SC, Nagle CM, Wentzensen N, Trabert B, Beeghly-Fadiel A, Schildkraut JM, et al. Use of common analgesic medications and ovarian cancer survival: results from a pooled analysis in the Ovarian Cancer Association Consortium. Br J Cancer. 2017;1169:1223–28. 10.1038/bjc.2017.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trabert B, Poole EM, White E, Visvanathan K, Adami HO, Anderson GL, et al. Analgesic Use and Ovarian Cancer Risk: An Analysis in the Ovarian Cancer Cohort Consortium. J Natl Cancer Inst. 2019;1112:137–45. 10.1093/jnci/djy100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Incio J, Tam J, Rahbari NN, Suboj P, McManus DT, Chin SM, et al. PlGF/VEGFR-1 Signaling Promotes Macrophage Polarization and Accelerated Tumor Progression in Obesity. Clin Cancer Res. 2016;2212:2993–3004. 10.1158/1078-0432.Ccr-15-1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;55:388–96. 10.1038/nrc1608 [DOI] [PubMed] [Google Scholar]
- 35.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Spiegelman D, et al. Physical activity and breast cancer risk in a cohort of young women. J Natl Cancer Inst. 1998;9015:1155–60. 10.1093/jnci/90.15.1155 [DOI] [PubMed] [Google Scholar]
- 36.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;235:991–9. 10.1093/ije/23.5.991 [DOI] [PubMed] [Google Scholar]
- 37.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise. 1993;251:71–80. 10.1249/00005768-199301000-00011 [DOI] [PubMed] [Google Scholar]
- 38.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. Jama. 2003;28914:1785–91. 10.1001/jama.289.14.1785 [DOI] [PubMed] [Google Scholar]
- 39.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;1221:51–65. 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 40.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;937:790–6. 10.1016/0002-8223(93)91754-e [DOI] [PubMed] [Google Scholar]
- 41.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;1857:570–84. 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafrir AL, Rice MS, Gupta M, Terry KL, Rosner BA, Tamimi RM, et al. The association between reproductive and hormonal factors and ovarian cancer by estrogen-α and progesterone receptor status. Gynecol Oncol. 2016;1433:628–35. 10.1016/j.ygyno.2016.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;1496:531–40. 10.1093/oxfordjournals.aje.a009849 [DOI] [PubMed] [Google Scholar]
- 44.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;512:524–32 [PubMed] [Google Scholar]
- 45.Kim MK, Kim Y, Park S, Kim E, Kim Y, Kim Y, et al. Effects of Steady Low-Intensity Exercise on High-Fat Diet Stimulated Breast Cancer Progression Via the Alteration of Macrophage Polarization. Integr Cancer Ther. 2020;191534735420949678. 10.1177/1534735420949678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole EM, Konstantinopoulos PA, Terry KL. Prognostic implications of reproductive and lifestyle factors in ovarian cancer. Gynecol Oncol. 2016;1423:574–87. 10.1016/j.ygyno.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 47.Merritt MA, Rice MS, Barnard ME, Hankinson SE, Matulonis UA, Poole EM, et al. Pre-diagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): a cohort study. Lancet Oncol. 2018;198:1107–16. 10.1016/s1470-2045(18)30373-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;202:251–62. 10.1530/erc-12-0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;158:484–98. 10.1038/nrc3967 [DOI] [PubMed] [Google Scholar]
- 50.Schouten LJ, Rivera C, Hunter DJ, Spiegelman D, Adami HO, Arslan A, et al. Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2008;174:902–12. 10.1158/1055-9965.Epi-07-2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tworoger SS, Huang T. Obesity and Ovarian Cancer. Recent Results Cancer Res. 2016;208155–76. 10.1007/978-3-319-42542-9_9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


