Abstract
Background:
Fine particulate matter (PM2.5) is associated with chronic kidney disease progression and may impact the health of patients living with kidney failure. While older (aged≥65) adults are most vulnerable to the impact of PM2.5, it is unclear whether older patients on dialysis are at elevated risk of mortality when exposed to fine particulate matter.
Methods:
Older adults initiating dialysis (2010–2016) were identified from US Renal Data System. PM2.5 concentrations were obtained from NASA’s SEDAC Global Annual PM2.5 Grids. We investigated the association between PM2.5 and all-cause mortality using Cox proportional hazard models with linear splines [knot at the current Environmental Protection Agency (EPA) National Ambient Air Quality Standard for PM2.5 of 12 μg/m3] and robust variance.
Results:
For older dialysis patients who resided in areas with high PM2.5, a 10 μg/m3 increase in PM2.5 was associated with 1.16-fold (95%CI:1.08–1.25) increased risk of mortality; furthermore, those who were female (aHR=1.26, 95%CI:1.13–1.42), Black (aHR=1.31, 95%CI:1.09–1.59) or had diabetes as primary cause of kidney failure (aHR=1.25, 95%CI:1.13–1.38) were most vulnerable to high PM2.5. While the mortality risk associated with PM2.5 was stronger at higher levels (aHR=1.19, 95%CI:1.08–1.32), even at lower levels (≤12 μg/m3), PM2.5 was significantly associated with mortality risk (aHR=1.04, 95%CI:1.00–1.07) among patients aged≥75 (Pslope difference=0.006).
Conclusions:
Older adults initiating dialysis who resided in ZIP codes with PM2.5 levels>12 μg/m3 are at increased risk of mortality. Those aged >75 were at elevated risk even at levels below the EPA Standard for PM2.5.
Keywords: Air Pollution, kidney failure, Mortality
INTRODUCTION
Elevated levels of fine particulate matter (particulate matter with diameter<2.5μM [PM2.5]) are a well-recognized risk factor for cardiovascular mortality,[1] and are associated more broadly with an increased risk of death as well as a reduced life expectancy.[2–5] Older adults are one of the most vulnerable groups to the harmful effect of air pollution.[1, 6, 7] It was estimated that more than 2 million deaths among older adults were attributed to ambient particulate matter globally in 2015.[8]
Furthermore, there is a high global burden of kidney disease that is attributable to ambient air pollution.[9, 10] Experimental studies showed that pollutants in the air such as diesel exhaust and heavy metal could promote oxidative stress, inflammation, and DNA damages in renal tissues.[11, 12] In the United States (US), elevated levels of fine particulate matter, are associated with incident chronic kidney disease, decline in estimated glomerular filtration rate (eGFR), and progression to kidney failure.[13, 14] In fact, proximity to major roads and increased levels of PM2.5 leads to reduced eGFR.[15]
Previous studies also suggested that those with diabetes or preexisting coronary disease were susceptible to the harmful effect of PM2.5.[1, 16, 17] Therefore, older patients undergoing dialysis were extremely vulnerable to air pollution given their advanced age and high burden of comorbidity.[18] However, it remains unclear whether older dialysis patients who live in areas with elevated levels of fine particulate matter are at increased risk of mortality and whether the current US Environmental Protection Agency (EPA) National Ambient Air Quality Standard for annual PM2.5 (≤12μg/m3)[19] is sufficient to protect this vulnerable population.
Our a priori hypothesis was that older dialysis patients were at elevated risk of mortality when living in a ZIP code with high ambient air pollution exposure. Understanding the role of air pollution on mortality among older patients undergoing dialysis will help inform policy discussions and clinical practice in the US. Therefore, we estimated the risk of mortality by level of fine particulate matter among adults aged≥65 years who initiated dialysis in the US.
MATERIALS AND METHODS
Study population and data source
Older kidney failure patients aged≥65 years at dialysis initiation, who started maintenance dialysis (either hemodialysis or peritoneal dialysis) between 1/1/2010–12/31/2016 without a previous solid organ transplant were identified from US Renal Data System (USRDS). USRDS is a national data system that collects, analyzes and distributes information about chronic kidney disease and kidney failure in the US. The database includes kidney failure patient demographic and diagnosis data, biochemical values, dialysis claims and information on treatment history, hospitalization events and physician/supplier services. The data in USRDS originated from The Centers for Medicare & Medicaid Service, the Organ Procurement and Transplantation Network, the Centers for Disease Control, the ESRD Networks, the US Census and select data from past and current USRDS Special Studies.[20] This study was reviewed by the Johns Hopkins School of Medicine Institutional Review Board and was determined to be exempt.
Only older patients who were diagnosed with renal failure and received their first chronic dialysis treatment were included in our analysis. Among 396,259 older patients who initiated dialysis between 2010–2016, we excluded those who had missing or unidentified ZIP codes or lived in ZIP codes without available air pollution data (N=9,183). We also excluded older patients who lived in ZIP code areas without available socioeconomic status (SES) data (N=1,333) and older patients with missing age, sex, race/ethnicity, body mass index (BMI), cause of kidney failure, smoking status, nephrology care, and comorbidities (N=1,467). Our analytic sample included 384,276 (97% of original population) older patients.
Ambient air pollution exposure
The primary exposure of our analysis was annual PM2.5 concentration at the ZIP code level. Ambient air pollution data gridded at 0.01 degrees between 2010–2016 was obtained from NASA’s Socioeconomic Data and Application Center (SEDAC) Global Annual PM2.5 Grids from NASA Moderate Resolution Imaging Spectroradiometer, Multi-angle Imaging Spectroradiometer, and the Sea-Viewing Wide Field-of-View Sensor Aerosol Optical Depth with Geographically Weighted Regression.[21, 22] PM2.5 data was then aggregated to ZIP code level by year. We linked older kidney failure patients and the PM2.5 data through their ZIP code of residence and the year of first dialysis. In this analysis, we defined a concentration ≤12μg/m3 as lower level and >12μg/m3 as higher level of PM2.5, as is suggested by the US EPA National Ambient Air Quality Standard for annual PM2.5.
Mortality
The outcome of interest in the study was all-cause mortality. USRDS obtained death dates of the patients from multiple sources including CMS Medicare Enrollment Database, CMS forms 2746 and 2728 (1995–2005), Organ Procurement and Transplantation Network transplant follow-up form, CROWNWeb database and Inpatient Claims.[20] Older patients were followed from the date of their first dialysis through date of death, date of transplant, or administrative censoring (12/31/2017), whichever came first. We also explored the association between PM2.5 and cause-specific mortality. Given the large number of missingness in the data for cause of deaths, we only classified cause-specific mortality by three categories: CVD, other, and unknown.
Patient and geographic factors
Individual-level factors were obtained from USRDS database (patient profile and CMS-2728 Form), including demographics (age, sex, race/ethnicity) and health-related factors (cause of kidney failure, smoking status, BMI and nephrology care status). Considering comorbidities are potential mediators in the association between PM2.5 and mortality, we did not include them in the models of our main analysis.
ZIP code-level SES factors included percent below 200% of the federal poverty line, mean years of education, median household income, median housing cost per month, percent non-Hispanic Black, percent Hispanics, population density and urbanicity. Population density was obtained from SEDAC Gridded Population of the World 2010.[23] Urbanicity of the area in the year of 2013 was retrieve from the Census Bureau TIGER/Line shapefiles, and was categorized into urban, urban clusters (incorporating places that contained at least 2,500 but less than 50,000 people within its boundaries) and rural.[24] Other ZIP code-level characteristics were abstracted from 2014 American Community Survey.[25]
Statistical analysis
Baseline characteristics were summarized by levels of ZIP code-level PM2.5 exposure (≤12μg/m3, >12μg/m3). Continuous variables were presented as median (IQR) while categorical variables were presented as frequency (%).
Cox proportional hazard models with robust variance accounting for cluster effect of ZIP codes were used to investigate the unadjusted and adjusted continuous association between PM2.5 and all-cause and cause-specific mortality among the study population. Considering the variation of air pollution by different time periods and that more than 50% of the older patients with PM2.5 exposure level higher than 12μg/m3 came from California, we allowed different baseline hazard functions by the year of dialysis initiation and whether the older patients lived in California. We accounted for demographics, health-related factors and ZIP code-level factors in our adjusted models. We fit linear spline models with a knot at 12μg/m3 to allow for different dose-response associations below and above the current EPA National Ambient Air Quality Standard. To explore other potential non-linear exposure-outcome associations, we additionally fit restricted cubic spline models.
To investigate whether the association between ZIP code-level PM2.5 concentration and all-cause mortality among older dialysis population varied by age (<75 and ≥75 years), sex (male, female), race/ethnicity (non-Hispanic white, non-Hispanic Black, and Hispanic/Latino, other), history of smoking (current and non-current), and cause of kidney failure (diabetes, hypertension, glomerular nephritis and other), we fit linear spline models within different subgroups.
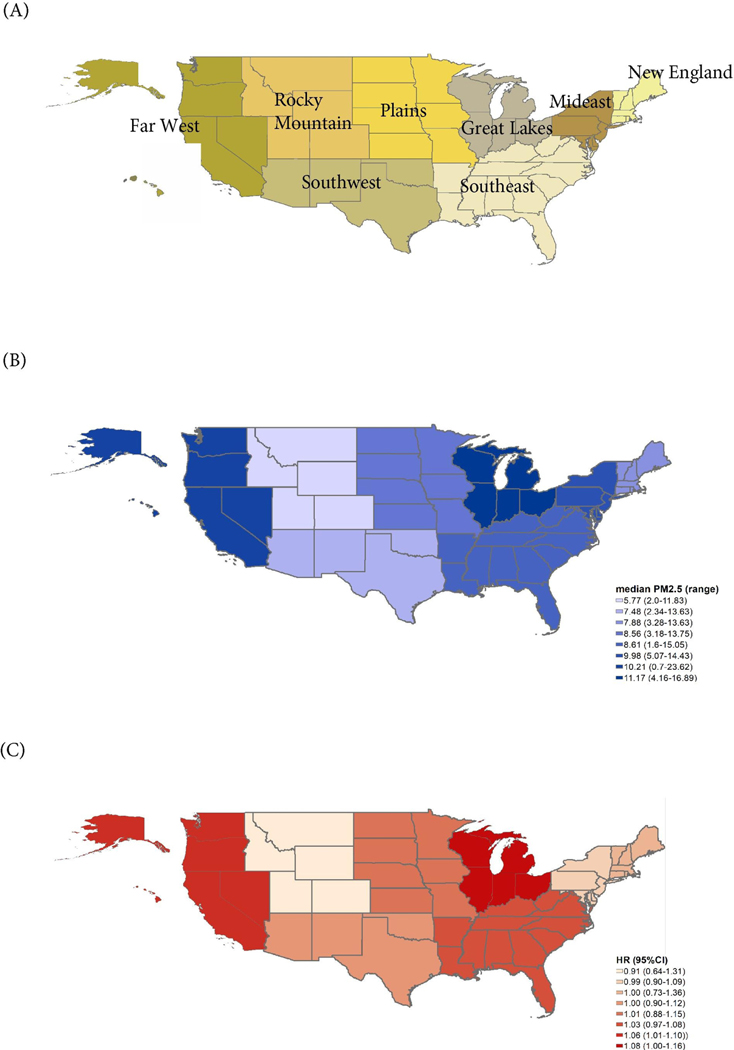
Considering the levels and composition of PM2.5 can vary greatly across the US, we further investigated the association within different regions of the country. We grouped our study population by their states of residence into eight regions [Figure 1]. Adjusted Cox proportional hazard models were used to obtain the linear association between continuous PM2.5 and all-cause mortality in different regions, separately.
Figure 1.
PM2.5 level and PM2.5-mortality association in eight regions of the US
(A) Eight regions in the Unites States; (B) PM2.5 level by regions; (C) PM2.5-mortality association in eight regions; HR: Hazard Ratio for mortality per 10 μg/m3 increase in PM2.5
In our sensitivity analysis, we further adjusted for comorbidities (cardiovascular disease, chronic obstructive pulmonary disease and cancer) in our linear spline models as additional potential confounders. The first six months after dialysis initiation was a complicated period with high mortality, we did additional analysis among those who survived the first three and six months of maintenance dialysis. Considering a large proportion of the older patients resided in areas with higher level of PM2.5 concentration were from California, we also conducted analyses separately for older patients who came from California and those who were from other states to see if the association was consistent between the two groups.
Statistical analyses were conducted using Stata SE, version 15 (Stata Corp, College Station, TX) and R 3.6.1,[26],[27] and a p-value of less than 0.05 was considered statistically significant.
RESULTS
Older adults initiating dialysis
There were 384,276 older patients (across 25,100 ZIP codes) who initiated dialysis between 2010 and 2016 and were followed for a median of 1.84 (IQR:0.77–3.25) years. Overall, 14% were female, 63% were non-Hispanic white, 20% were non-Hispanic Black, and 11% were Hispanic/Latino. The median age at dialysis initiation was 74 years (IQR:69–80).
Ambient air pollution (PM2.5)
The median ZIP code-level PM2.5 concentration for older patients was 9.17μg/m3 (IQR:7.63–10.78, range 0.70–23.62 μg/m3). Among our study population, 48,885 (13%) had ZIP-code level PM2.5 concentration >12μg/m3. Furthermore, the distribution of ZIP code-level PM2.5 varied across the US with the highest ZIP code-level air pollution occurring in Far west region.
When compared to those residing in areas with lower PM2.5 concentrations (≤12μg/m3), older patients in areas with higher level of concentration (PM2.5 >12μg/m3) were less likely to be non-Hispanic white (49% vs 65%) and more likely to be Hispanic/Latino (20% vs 10%). Only 23% of the older patients living in areas with higher level of PM2.5 had been on nephrology care for more than 12 months at the time of dialysis initiation, compared to 32% in the older patients with lower exposure [Table 1].
Table 1.
Baseline characteristics of older (aged≥65) patients initiating dialysis by PM2.5 levels (N=384,276).
| Factor | PM2.5≤12μg/m3 | PM2.5>12μg/m3 | |
|---|---|---|---|
| N | 335,391 | 48,885 | |
| Individual level characteristics | |||
| Age at dialysis initiation (years) | 74 (69, 80) | 75 (69, 81) | |
| Female | 148,233 (44.2%) | 22,369 (45.8%) | |
| Race/ethnicity | White | 218,650 (65.2%) | 24,170 (49.4%) |
| Black | 68,831 (20.5%) | 10,048 (20.6%) | |
| Hispanic | 32,161 (9.6%) | 9,606 (19.7%) | |
| Other | 15,749 (4.7%) | 5,061 (10.4%) | |
| BMIa (kg/m2) | 27.4 (23.5, 32.3) | 26.6 (23.1, 31.5) | |
| Primary cause of kidney failure | Diabetes | 148,217 (44.2%) | 23,503 (48.1%) |
| Hypertension | 117,073 (34.9%) | 16,882 (34.5%) | |
| Glomerulonephritis | 16,817 (5.0%) | 1,903 (3.9%) | |
| Other | 53,284 (15.9%) | 6,597 (13.5%) | |
| Current smoker | 13,565 (4.0%) | 1,399 (2.9%) | |
| Nephrology care at dialysis initiation | No care | 74,798 (22.3%) | 10,921 (22.3%) |
| Unknown care | 43,655 (13.0%) | 10,589 (21.7%) | |
| <6 months of care | 44,806 (13.4%) | 7,949 (16.3%) | |
| 6–12 months | 63,555 (19.0%) | 8,204 (16.8%) | |
| >12 months | 108,566 (32.4%) | 11,221 (23.0%) | |
| ZIP code-level characteristics | |||
| Percent Black | 6.2 (1.7, 21.1) | 5.2 (2.0, 17.6) | |
| Percent Hispanic | 6.9 (2.7, 18.4) | 19.0 (4.6, 50.0) | |
| Population density | 393 (71, 1,275) | 1,974 (873, 3,761) | |
| Mean education year | 13.2 (12.6, 14.0) | 13.0 (12.2, 13.8) | |
| Median income | 48,520 (38,583, 63,677) | 50,083 (39,222, 64,211) | |
| Monthly housing cost | 928 (721,1245) | 1,145 (871, 1437) | |
| Percent below 200% FPL | 36.0 (24.5, 47.3) | 39.9 (27.2, 52.1) | |
| Urbanicity | Urban | 243,681 (72.7%) | 45,017 (92.1%) |
| Urban cluster | 62,219 (18.6%) | 2,989 (6.1%) | |
| Rural | 29,491 (8.8%) | 879 (1.8%) |
Continuous variables were presented as [median (IQR)], categorical variables were presented as [frequency (%)]
BMI: Body Mass Index; FPL: Federal poverty line
The older patients in ZIP codes with higher levels of PM2.5 concentrations were more likely to live in ZIP codes with a higher proportion of Hispanic residents (median 19.0% vs 6.9%), population density (median 1,974/KM2 vs 393/KM2), and housing cost (median $1,145 vs $928 USD/month). Furthermore, they were more likely to be in urban areas; 93.0% of the older patients in high PM2.5 concentration ZIP codes lived in urban areas compared to 73.3% in the lower group [Table 1].
Ambient air pollution and mortality among older dialysis patients
Overall, 117,091 patients residing in areas with low PM2.5 level and 29,995 patients in areas with high PM2.5 died during the follow-up period, among which 84,109 (34%) died from CVD, 94,923 (38%) died from other causes, and 69,093 (28%) had unknown cause of death.
Restricted cubic spline model suggested that there was non-linear association between PM2.5 and mortality risk and shown that the dose-response association changed when PM2.5 level reached ~12μg/m3 [Figure 2].
Figure 2.
Concentration-response function of PM2.5 and mortality among older (aged≥65) patients initiating dialysis:
Dose-response function was estimated using restrict-cubic spline model. Association adjusted for demographics (age, sex, race), ZIP-code level characteristics (percent below 200% of the federal poverty line, mean years of education, median household income, median housing cost per month, percent Black, percent Hispanics, population density and urbanicity), health related factors (cause of kidney failure, smoking status, BMI and nephrology care status)
In the unadjusted linear spline model, a 10μg/m3 increase in PM2.5 was associated with a 1.03-fold (95%CI:1.01–1.06) increased risk of mortality when PM2.5 was at lower level; a 10μg/m3 increase in PM2.5 was associated with a 1.21-fold (95%CI:1.11–1.33) increased risk of mortality when PM2.5 was at higher level. After accounting for demographics, health-related factors and ZIP code-level characteristics, no association was found between PM2.5 and mortality risk when the PM2.5 level was below 12μg/m3 (HR=1.01 per 10μg/m3 increase, 95%CI:0.99–1.03). However, when PM2.5 was above 12 μg/m3, 10μg/m3 increase was associated with 1.16-fold (95%CI:1.08–1.25) increase in mortality risk among older dialysis patients [Table 2].
Table 2.
Associations between PM2.5 and all-cause mortality among older (aged≥65) patients initiating dialysis (N=384,276).
| HR (95%CI) | p for slope difference | ||
|---|---|---|---|
| PM2.5≤12μg/m3 | PM2.5>12μg/m3 | ||
| Unadjusted | 1.03 (1.01–1.06) | 1.21 (1.11–1.33) | 0.002 |
| Model1a: Demographic adjusted | 1.03 (1.01–1.05) | 1.26 (1.17–1.36) | <0.001 |
| Model2b: Model1+ ZIP code factors | 1.05 (1.02–1.07) | 1.23 (1.14–1.32) | <0.001 |
| Model3c: Model2+ health-related factors | 1.01 (0.99–1.03) | 1.16 (1.08–1.25) | 0.001 |
HR: Hazard Ratio for mortality per 10 μg/m3 increase in PM2.5; p for slope difference indicated the p effect difference of per 10 μg/m3 increase in PM2.5 between areas with PM2.5≤12 and areas with PM2.5>12
Model1 adjusted for demographics (age, sex, race)
Model2 adjusted for demographics + ZIP-code level characteristics (percent below 200% of the federal poverty line, mean years of education, median household income, median housing cost per month, percent Black, percent Hispanics, population density and urbanicity)
Model3 adjusted for demographics + ZIP-code level characteristics + health-related factors (cause of kidney failure, smoking status, BMI and nephrology care status)
At higher levels, increased PM2.5 was associated with an increased risk of CVD mortality (HR=1.38; 95%CI 1.21–1.58) and unknown causes of mortality (HR=1.33; 95%CI 0.1.14–1.55), but not death from other cause [Table S1].
Ambient air pollution and mortality among subgroups of older patients initiating dialysis
The association between PM2.5 concentration and mortality varied across different subgroups of older patients initiating dialysis [Figure 3]. At higher levels, we found that older patients who were female (HR=1.26 per 10μg/m3 increase, 95%CI:1.13–1.42), Black (HR=1.31 per 10μg/m3 increase, 95%CI:1.09–1.59) or had diabetes (HR=1.25 per 10μg/m3 increase, 95%CI:1.13–1.38) as the primary cause of kidney failure had higher PM2.5 associated mortality risk than the overall study population.
Figure 3.
PM2.5 and all-cause morality among subgroups of older patients on dialysis in the US (N=384,276)
HR: Hazard ratio for mortality per 10 μg/m3 increase in PM2.5; p for slope difference indicated the p effect difference of per 10 μg/m3 increase in PM2.5 between areas with PM2.5≤12 and areas with PM2.5>12; Association adjusted for adjusted for demographics (age, sex, race), ZIP-code level characteristics (percent below 200% of the federal poverty line, mean years of education, median household income, median housing cost per month, percent Black, percent Hispanics, population density and urbanicity), health related factors (cause of kidney failure, smoking status, BMI and nephrology care status)
Although the risk of mortality was stronger when PM2.5 was above 12 μg/m3 (HR=1.19 per 10 μg/m3 increase, 95%CI:1.08–1.32), even at lower levels, PM2.5 was significantly associated with increased mortality risk among older patients who were aged>75 years (HR=1.04 per 10 μg/m3 increase, 95%CI:1.00–1.07).
Ambient air pollution and mortality by geographic region
Across eight geographic regions, the Great Lakes region had the highest median ZIP code-level PM2.5 concentration (median=11.17μg/m3), while the Rocky Mountains had the lowest (median=5.77μg/m3) [Figure 1].
Significant associations between PM2.5 and mortality risk were only found in the Far West region (Median PM2.5=10.22μg/m3), where per 10 μg/m3 increase in PM2.5 was associated with 1.06-fold (95%CI:1.01–1.11) increase in mortality risk. We also found marginally significant exposure-outcome association in the Great Lakes region (HR=1.08 per 10 μg/m3 increase, 95%CI:1.00–1.16). No association was observed in other regions with lower PM2.5 level [Figure 1].
Sensitivity analysis
Further adjustment for comorbidities did not significantly change the inferences from the main analyses; for every 10μg/m3 increase, PM2.5 was associated with 1.14-fold (95%CI:1.07–1.22) increase in mortality risk for higher levels, and no association was observed at lower air pollution levels. After excluding patients who died within first three and six months of maintenance dialysis, the result remained consistent. The exposure-outcome association was similar between older patients from California and other states [Table S1]. Mortality rates among our study population between 2010–2016 are shown in table S2.
DISCUSSION
In this study of 384,276 older patients initiating dialysis between 2010–2016 in the US, the median ZIP code-level PM2.5 concentration was 9.17μg/m3, and 13% lived in ZIP code with PM2.5 level >12μg/m3. A 10 μg/m3 increase in PM2.5 was associated with 1.23-fold (95%CI:1.14–1.32) increase in mortality among older patients initiating dialysis when at higher level of PM2.5 and 1.05-fold (95%CI:1.02–1.07) increase when at lower level after adjusting for demographics and ZIP code-level socioeconomic status. When further adjusted for health-related factors, the association was attenuated such that PM2.5 was associated with mortality only at levels above 12 μg/m3 (HR=1.15, 95%CI:1.07–1.23). We also found that PM2.5 was associated with elevated mortality risk among older patients who were aged>75 years at dialysis initiation even at lower levels of PM2.5. However, older patients who were female, Black or had diabetes as primary cause of kidney failure were most vulnerable when exposed to higher levels of PM2.5 (>12 μg/m3).
To our knowledge, while many cohort studies have evaluated the association between long-term exposure of air pollution and mortality risk in the general population[3, 28–30], this is the first study to examine this relationship with a focus on older patients with kidney failure in the US. We observed a positive association between long-term exposure to PM2.5 and all-cause and CVD mortality among older patients initiating dialysis, which is consistent with a recent study in Hong Kong (median PM2.5 concentration=38.9μg/m3). Ran et.al[31] found an interquartile change (4 μg/m3) in PM2.5 was associated with 14% (95%CI:1.01–1.30) increase in mortality, which could be translated into more than 35% increase in mortality every 10 μg/m3 increase in PM2.5, among kidney failure patients. This larger effect size observed in Hong Kong could potentially be explained by the higher levels of air pollution persisting in the region in comparison to the Unites States. Furthermore, patients with kidney failure in Hong Kong was quite different from those in the Unites States in terms of primary renal diagnosis.[32] Another recent study investigating short-term effect of wildfire smoke conducted among hemodialysis patients in the United Sates (US) found that a 10 μg/m3 increase in wildfire PM2.5 was associated with 1.04-fold (95%CI:1.01–1.17) increase in the same-day mortality.[33] All these findings supports that air pollution is a potential threat to the health of patients with kidney failure.
Many of the studies found linear or supralinear (characterized by decrease slope with increased exposure level) concentration-response associations between PM2.5 and mortality, where increased air pollution associated mortality risk occurred even at very low levels of PM2.5.[3, 29, 34–36] Specifically, a study conducted among the Medicare population found that per 10 μg/m3 increase in PM2.5 was linearly associated with 7.3% (95%CI:7.1–7.5%) increase in all-cause mortality without any thresholds[3] while another study conducted among a Canadian census cohort observed that PM2.5 was associated with a steep increase in relative mortality risk at levels below 5 μg/m3, with associations attenuating at higher levels.[29] However, in this study, the reverse was observed; we found that there was increased mortality risk only when PM2.5 was >12 μg/m3, and no association when PM2.5 was at lower levels among the overall study population. Previous studies suggested that air pollution could affect health by inducing systematic inflammation.[1] When compared to general population, older patients with kidney failure are more likely to have a high inflammatory state.[37, 38] It is possible that, at low levels of PM2.5, the proinflammatory effect from air pollution is small relative to the already high state of inflammation experienced by this vulnerable population, such that there is no observed increase in mortality risk. However, when the PM2.5 increased to a specific level, the effect was large enough to lead to adverse outcomes among older patients with kidney failure. This could also explain the comparable or even stronger association we observed at higher levels of air pollution than in other US studies.[3, 28, 30] Additionally, this potential mechanism was also supported by our findings of significant exposure-outcome associations in regions with highest levels of PM2.5 (Great Lakes and Far West).
In this study, patients >75 years of age with kidney failure were more vulnerable to air pollution compared to their younger counterparts (65–75 years), such that elevated mortality risk was observed even at low levels of PM2.5. This finding is in line with other studies demonstrating the susceptibility of the oldest older adults in general to harmful effects of air pollution,[1, 16, 17] supporting current EPA recommendations for them to take extra precautions during high pollution days.[39] A previous study had found that older adults (aged≥65) who were frail were more vulnerable to the adverse effects of air pollution than their non-frail counterparts.[40] The higher vulnerability to air pollution among older dialysis patients observed in our study may be attributable to frailty, a condition characterized by higher vulnerability to stressors,[41] considering that the prevalence of frailty increases with age and more than 25% of the kidney transplant candidates aged≥65 years-old were frail.[42–44] Meanwhile, the higher prevalence of comorbidities including cardiovascular disease and respiratory disease among older patients initiating dialysis could also contribute to the vulnerability of this population.[1]
In this population of older dialysis patients, we found that the effect of air pollution on mortality differed by racial/ethnic groups, with older non-Hispanic Black patients being more vulnerable to the effects of PM2.5 at levels above the current EPA standard, and non-Hispanic White patients at increased risk of mortality even at levels below the current EPA standard. One potential cause for this difference could be the geographical distribution of racial/ethnic groups as the composition of particulate matter varies greatly by region[45] and the particle compositions were found to be associated with mortality.[46, 47] Furthermore, Black patients are also more likely to be disproportionately affected by other risk factors such as low SES, which may increase their vulnerability to environmental stressors and thus contribute to this observed difference, yet the role of race/ethnicity on the association between air pollution and mortality remains inconclusive.
Older patients initiating dialysis are a vulnerable group with high baseline mortality,[18] such that even a small increase in relative risk would result in a relatively large increase in absolute mortality. Consequently, it is especially critical for this population to avoid unnecessary excess mortality risk from PM2.5 exposure. Although air pollution has long been considered to be a threat to health, recent studies on air quality awareness among US adults found that only 29.2% of participants believed that air pollution was bad and 14.7% would change their behavior when there was low air quality; similar results were found among participants with heart disease.[48, 49] Under these circumstances, there is a need for healthcare providers, like geriatricians, to discuss the potential harms of air pollution with their vulnerable older patients and provide recommendations for behavioral changes. This study identified different subgroups of older patients initiating dialysis with varying vulnerability to air pollution, which could help geriatricians and nephrologists target air pollution exposure mitigation strategies for older patients most in need of them.
Our study has the strength of using the geographically diverse, registry-based data capturing the majority of older patients with kidney failure in the US. Data from a national registry database allowed us to adjusted for key health-related factors which were most relevant to older patients initiating dialysis. Additionally, the large sample size enabled us to do individual analysis in different subgroups and regions.
This study has its limitations in the measurements of exposure and confounders and ascertainment of outcomes. Air pollution concentration was assessed at the ZIP code level and we were not able to account for the indoor air pollution; this could result in potential exposure misclassification as variations in PM2.5 can exist within a ZIP code and this level may not reflect all locations where older patients may spend time. Even within the same area, individual exposure of PM2.5 could be affected by many factors such as proximation to major road ways and outdoor activity level. Due to the limited availability of other data, we only investigated the effect of PM2.5 in this study and was not able to control for other pollutants such as ozone and NO2. Therefore, part of the observed effect of might be contributed to other types of pollutants. However, previous studies suggested that further controlling for other pollutants did not significantly change the estimated effects of PM2.5.[3, 50] We only have the ZIP code and health-related information for the patient at the time of listing, such that we were not able to account for time varying exposure of PM2.5 and other time-varying confounding. The ZIP code information was obtained solely from USRDS patient profile and we were not able to confirm whether the patients actually resided within the ZIP code area at the time of dialysis initiation and during the follow-up. However, the ZIP code data had been used to link ZIP code-level exposure in other studies [51, 52] and this is the only data we have regarding the patients’ area of residence. For SES confounders, we were only able to measure at ZIP code level, which could lead to residual confounding. In terms of outcome ascertainment, the large number of missingness in cause-of-death data limited our ability to investigate and interpret the association between PM2.5 and cause-specific mortality.
In conclusion, ambient air pollution, measured by PM2.5, was associated with all-cause mortality among older patients initiating dialysis. More notably, increased mortality risk was observed among patients aged>75 years, even at levels below current EPA National Ambient Air Quality Standard for PM2.5. Our results suggested that the current EPA standard for PM2.5 might not be enough to protect the most vulnerable populations. Geriatricians and nephrologists should consider ambient air pollution as an important environmental exposure and implement ambient air pollution exposure mitigation strategies for their vulnerable older patients and actively engage in advocacy for population-level solutions.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
FUNDING
Funding for this study was provided in part by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and the National Institute on Aging (NIA); grant numbers R01DK120518 (PI: Mara McAdams-DeMarco), R01AG055781 (PI: Mara McAdams-DeMarco), K01AG064040 (PI: Chu), and K24AI144954 (PI: Dorry Segev).
Footnotes
No authors report a conflict of interest.
DATA AVAILABILITY STATEMENT
The data underlying this article were provided by USRDS under license / by permission. Data will be shared on request to the corresponding author with permission of USRDS.
Reference
- 1.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78. [DOI] [PubMed] [Google Scholar]
- 2.Correia AW, Pope CA 3rd, Dockery DW, Wang Y, Ezzati M, Dominici F. Effect of air pollution control on life expectancy in the United States: an analysis of 545 U.S. counties for the period from 2000 to 2007. Epidemiology. 2013;24(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376(26):2513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120(7):965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343(24):1742–9. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg MS, Burnett RT, Stieb DM, Brophy JM, Daskalopoulou SS, Valois MF, et al. Associations between ambient air pollution and daily mortality among elderly persons in Montreal, Quebec. Sci Total Environ. 2013;463–464:931–42. [DOI] [PubMed] [Google Scholar]
- 7.Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am J Epidemiol. 2017;186(8):961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Estimates of the 2016 global burden of kidney disease attributable to ambient fine particulate matter air pollution. BMJ Open. 2019;9(5):e022450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum MF, Surapaneni A, Stewart JD, Liao D, Yanosky JD, Whitsel EA, et al. Particulate Matter and Albuminuria, Glomerular Filtration Rate, and Incident CKD. Clin J Am Soc Nephrol. 2020;15(3):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afsar B, Elsurer Afsar R, Kanbay A, Covic A, Ortiz A, Kanbay M. Air pollution and kidney disease: review of current evidence. Clin Kidney J. 2019;12(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemmar A, Karaca T, Beegam S, Yuvaraju P, Yasin J, Hamadi NK, et al. Prolonged Pulmonary Exposure to Diesel Exhaust Particles Exacerbates Renal Oxidative Stress, Inflammation and DNA Damage in Mice with Adenine-Induced Chronic Renal Failure. Cell Physiol Biochem. 2016;38(5):1703–13. [DOI] [PubMed] [Google Scholar]
- 13.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol. 2018;29(1):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, et al. Long-Term Exposure to Ambient Fine Particulate Matter and Renal Function in Older Men: The Veterans Administration Normative Aging Study. Environ Health Perspect. 2016;124(9):1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lue SH, Wellenius GA, Wilker EH, Mostofsky E, Mittleman MA. Residential proximity to major roadways and renal function. J Epidemiol Community Health. 2013;67(8):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samoli E, Peng R, Ramsay T, Pipikou M, Touloumi G, Dominici F, et al. Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA study. Environ Health Perspect. 2008;116(11):1480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibuakuu M, Michos ED, Navas-Acien A, Jones MR. Air Pollution and Cardiovascular Disease: A Focus on Vulnerable Populations Worldwide. Curr Epidemiol Rep. 2018;5(4):370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2020;75(1S1):A6–A7. [DOI] [PubMed] [Google Scholar]
- 19.US Environmental Protection Agency, and US Environmental Protection Agency, Office of Air Quality Planning and Standards, Outreach and Information Division. “Air Quality Index: A guide to air quality and your health.” EPA-456/F-14-002. Research Triangle Park, NC: (2014). [Google Scholar]
- 20.U.S. Renal Data System. 2019 Researcher’s Guide to the USRDS Database. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2019. [Google Scholar]
- 21.van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, et al. Global Annual PM2.5 Grids from MODIS, MISR and SeaWiFS Aerosol Optical Depth (AOD) with GWR, 1998–2016. Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC); 2018. [Google Scholar]
- 22.van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, et al. Global Estimates of Fine Particulate Matter Using a Combined Geophysical-Statistical Method with Information from Satellites. Environmental Science & Technology. 2016;50(7):3762. [DOI] [PubMed] [Google Scholar]
- 23.Center for International Earth Science Information Network - CIESIN - Columbia University. 2018. Gridded Population of the World, Version 4 (GPWv4): Population Count, Revision 11. Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC). 10.7927/H4JW8BX5. Accessed 20th March 2020. [DOI] [Google Scholar]
- 24.US Census Bureau. “TIGER/Line Shapefiles.” The United States Census Bureau, www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html. Accessed 20th March 2020. [Google Scholar]
- 25.2014 American Community Survey 5-year estimates, prepared by the US Census Bureau 2015. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed 20th March 2020.
- 26.StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- 27.R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- 28.Pope CA 3rd, Lefler JS, Ezzati M, Higbee JD, Marshall JD, Kim SY, et al. Mortality Risk and Fine Particulate Air Pollution in a Large, Representative Cohort of U.S. Adults. Environ Health Perspect. 2019;127(7):77007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappin AJ, Christidis T, Pinault LL, Crouse DL, Brook JR, Erickson A, et al. Examining the Shape of the Association between Low Levels of Fine Particulate Matter and Mortality across Three Cycles of the Canadian Census Health and Environment Cohort. Environ Health Perspect. 2019;127(10):107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston GD, Ahn J, Cromar KR, Shao Y, Reynolds HR, Jerrett M, et al. Ambient Particulate Matter Air Pollution Exposure and Mortality in the NIH-AARP Diet and Health Cohort. Environ Health Perspect. 2016;124(4):484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ran J, Sun S, Han L, Zhao S, Chen D, Guo F, et al. Fine particulate matter and cause-specific mortality in the Hong Kong elder patients with chronic kidney disease. Chemosphere. 2020;247:125913. [DOI] [PubMed] [Google Scholar]
- 32.Leung CB, Cheung WL, Li PK. Renal registry in Hong Kong-the first 20 years. Kidney Int Suppl (2011). 2015;5(1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi Y, Kshirsagar AV, Wade TJ, Richardson DB, Brookhart MA, Wyatt L, et al. Mortality in US Hemodialysis Patients Following Exposure to Wildfire Smoke. J Am Soc Nephrol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz J, Coull B, Laden F, Ryan L. The effect of dose and timing of dose on the association between airborne particles and survival. Environ Health Perspect. 2008;116(1):64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope CA 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120(11):941–8. [DOI] [PubMed] [Google Scholar]
- 36.Pope CA 3rd, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119(11):1616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004;65(3):1031–40. [DOI] [PubMed] [Google Scholar]
- 38.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107(1):87–92. [DOI] [PubMed] [Google Scholar]
- 39.US Environmental Protection Agency, and US Environmental Protection Agency, Office of Air Quality Planning and Standards, Outreach and Information Division. “Air Quality Index: A guide to air quality and your health.” EPA-456/F-14–002. Research Triangle Park, NC: (2014). [Google Scholar]
- 40.Eckel SP, Louis TA, Chaves PH, Fried LP, Margolis AH. Modification of the association between ambient air pollution and lung function by frailty status among older adults in the Cardiovascular Health Study. Am J Epidemiol. 2012;176(3):214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. [DOI] [PubMed] [Google Scholar]
- 43.Haugen CE, Thomas AG, Chu NM, Shaffer AA, Norman SP, Bingaman AW, et al. Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplant. 2020;20(4):1170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu NM, Chen X, Norman SP, Fitzpatrick J, Sozio SM, Jaar BG, et al. Frailty Prevalence in Younger End-Stage Kidney Disease Patients Undergoing Dialysis and Transplantation. American journal of nephrology. 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19(5):680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kioumourtzoglou MA, Austin E, Koutrakis P, Dominici F, Schwartz J, Zanobetti A. PM2.5 and survival among older adults: effect modification by particulate composition. Epidemiology. 2015;26(3):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirabelli MC, Ebelt S, Damon SA. Air Quality Index and air quality awareness among adults in the United States. Environ Res. 2020;183:109185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mirabelli MC, Boehmer TK, Damon SA, Sircar KD, Wall HK, Yip FY, et al. Air Quality Awareness Among U.S. Adults With Respiratory and Heart Disease. Am J Prev Med. 2018;54(5):679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer PH, Marra M, Ameling CB, Velders GJM, Hoogerbrugge R, de Vries W, et al. Particulate air pollution from different sources and mortality in 7.5 million adults - The Dutch Environmental Longitudinal Study (DUELS). Sci Total Environ. 2020;705:135778. [DOI] [PubMed] [Google Scholar]
- 51.Agunbiade A, Dasgupta A, Ward MM. Racial/Ethnic Differences in Dialysis Discontinuation and Survival after Hospitalization for Serious Conditions among Patients on Maintenance Dialysis. J Am Soc Nephrol. 2020;31(1):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51(4):563–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.