Abstract
Objective:
While food protein-induced enterocolitis syndrome (FPIES) was first described approximately 50 years ago, and research is increasing, there are still significant unmet needs in FPIES. This article catalogs areas of progress and areas for further research.
Data sources:
Through our personal experiences caring for patients with FPIES, our personal research, and review of the existing FPIES literature as indexed in PubMed, we explored what is known and what is needed in FPIES .
Study Selections:
These studies have improved knowledge of FPIES, defined phenotypes, allowed for better informed management of FPIES, and laid the groundwork for further research.
Results:
Further research is needed in the areas of prevalence, natural history, trigger foods, threshold doses, how and when to perform oral food challenges, and immunopathogenesis of this disorder. Development of a biomarker and determination of the best method to treat reactions is also needed. Furthermore, FPIES has a substantial psychosocial and economic impact on families and more research is needed into developing and implementing ameliorating strategies.
Conclusion:
By partnering together, healthcare providers, advocacy organizations and families can continue to advance our understanding and improve care of patients and families living with FPIES.
Keywords: anxiety, diagnosis, epidemiology, food protein-induced enterocolitis syndrome, FPIES, management, natural history, psychosocial, quality of life
INTRODUCTION
Food protein-induced enterocolitis syndrome (FPIES) is a non-IgE, cell-mediated food allergic disorder typically manifesting with protracted vomiting, often followed by diarrhea. In some cases, this can be accompanied by lethargy, pallor, hypotension, and metabolic derangements.1 Although FPIES typically is a disease of childhood, presenting in infancy and resolving by grade-school, onset in older ages including adults is increasingly being recognized.2
Accurately identifying FPIES food triggers is crucial for the health of the patient, but can be difficult in the absence of diagnostic tests. Current FPIES management relies on allergen avoidance, treatment of reactions, and periodic re-evaluations with an allergist who can perform supervised oral food challenges (OFC) to monitor for resolution. Like other chronic illnesses, FPIES requires daily management, with patients and families needing to remain vigilant for food exposures and reaction symptoms. FPIES reactions can be unpredictable in occurrence and severity. The chronic, unpredictable, and uncertain nature of FPIES can impact patient and family psychosocial functioning and quality of life.3, 4
In this article, we will review the progress made in achieving our current understanding of FPIES phenotypes, pathophysiology, and management. We will also discuss the unmet needs and opportunities for healthcare providers, patients, and families to partner together to expand knowledge and search for answers.
A BRIEF HISTORY OF FPIES
Emergence of FPIES in the medical literature
The first descriptions of FPIES in the medical literature emerged in the 1960s and 1970s.5-8 Reports detailed unusual cases of infants with delayed enterocolitis 1-4 hours after ingesting cow’s milk (CM), soy, or rice. 5-8 In following decades, increasing cases of FPIES have been reported to more foods.9, 10 Reports of a more protracted presentation of infant enterocolitis have also emerged, which is now recognized as chronic FPIES.
Getting an International Classification of Diseases (ICD)-10 code
Significant progress has been made in getting FPIES recognized in the medical community. The first official FPIES ICD-10 code (K52.21) took effect in 2016. Attaining the ICD-10 code was the result of a multi-year undertaking, with partnership between the International Food Protein-Induced Enterocolitis Syndrome Association (I-FPIES) lay organization and the American Academy of Allergy, Asthma & Immunology (AAAAI) medical organization.
This achievement was crucial to be able to perform large-scale chart review studies based on medical coding to better define the epidemiology of FPIES and lay the groundwork for future prospective studies. Collaborations like these between lay and medical organizations have been instrumental in pushing forward critically needed areas of FPIES awareness and research.
DEFINING FPIES PHENOTYPES
To be able to properly diagnose, manage, and ultimately study a disorder, defining diagnostic criteria is essential. In 2017, nearly 50 years after the first FPIES cases were reported, the first International Consensus Guidelines for the Diagnosis and Management of Food Protein–induced Enterocolitis Syndrome was published by a workgroup of the AAAAI.1 This landmark document proposed diagnostic criteria and defined clinical phenotypes for acute and chronic FPIES.
Acute FPIES
Acute FPIES is characterized by repetitive vomiting within 1-4 hours of culprit food ingestion, and may be associated with lethargy, hypotonia, pallor, hypothermia, and/or diarrhea. Acute FPIES reactions manifest when the trigger food is ingested intermittently or after a period of avoidance. Reactions typically resolve within 24 hours and patients are healthy between episodes.1, 11
Chronic FPIES
Chronic FPIES is characterized by frequent vomiting and diarrhea over days to weeks, associated with poor weight gain or weight loss. Chronic FPIES reactions manifest when the trigger food is ingested regularly. Symptoms typically resolve within days to up to 2 weeks after elimination of the trigger food.12
Until recently, chronic FPIES was reported exclusively in infants less than 4 months old fed with CM or soy formulas.1, 5, 6, 12, 13 To date, there are no reports of pediatric chronic FPIES presenting outside of early infancy or triggered by solid foods. A recent report describes the first known case of adult chronic FPIES in a 20-year-old man, triggered by egg, beef, and salmon.14 The list of foods causing chronic FPIES may expand as awareness increases. As infant feeding recommendations have evolved to promote earlier introduction of solid foods,15, 16 additional foods triggers of chronic FPIES may emerge. Further studies are needed to characterize the prevalence, risk factors, natural history, and trigger foods in chronic FPIES. Mechanistic studies will be critical to understand why patients present with acute versus chronic FPIES to specific foods. Factors such as timing of food introduction, types of food introduced, and intestinal microbiome and permeability may be important in determining whether a patient presents with acute versus chronic FPIES.
Atypical FPIES
Atypical FPIES is diagnosed in the setting of a clinical history consistent with FPIES and positive specific IgE (sIgE) to the trigger food. Up to 25% of patients with FPIES have detectable food sIgE.9 Patients with atypical FPIES may have a more protracted course, with prolonged time to resolution. Some patients may develop IgE-mediated food allergy reactions, including anaphylaxis, upon re-exposure to the culprit food. Additionally, some patients with “typical” FPIES without evidence of IgE sensitization may subsequently develop IgE sensitization and convert from FPIES to IgE-mediated food allergy. This has been described in infants initially presenting with CM FPIES.1, 2, 11, 17 More studies are needed to better characterize this disease phenotype and identify risk factors and mechanisms for conversion from one type of food allergy to another.
Adult FPIES
For decades, FPIES was thought to be a disorder of infants and young children. Recently, new-onset FPIES in adults was described. The most common presentation in adults is acute FPIES to fish or shellfish in those who were previously tolerant.18-22 The list of acute FPIES trigger foods in adults is expanding.20, 21 A recent report describes the first known case of adult chronic FPIES.14 These adult FPIES cases have further expanded the FPIES clinical phenotype, and challenged our understanding of epidemiology and mechanisms. To date, there have been no reports of atypical FPIES in adults and no large studies on the natural history. We need prospective studies to assess prevalence, risk factors, natural history, triggers, and comorbid conditions. Studies of the immunologic mechanisms of adult FPIES, and genetic and environmental factors associated with this disorder are also important.
FPIES FOOD TRIGGERS
Common food triggers
The most common FPIES triggers reported in the literature are highlighted in Table 1.9, 10, 13, 20, 21, 23-33 The differences in FPIES food triggers in these studies are notable. Common food triggers differ between countries as well as over time. There are also differences between pediatric and adult FPIES triggers. These variations may be due to study design, geography, racial/ethnic differences, ages of patients, FPIES phenotype, and yet-to-be discovered genetic and/or epigenetic factors.1 Factors that may affect geographic variations are feeding practices including age of weaning and type of first food introduction, and differences in microbiome. Common FPIES food triggers may change over time due to factors such as increased awareness of FPIES and potential food triggers, as well as changes in feeding behaviors. With new recommendations to introduce solid foods including peanuts into infants’ diets,15, 16 it will be interesting to see if other common FPIES food triggers emerge. Large, multicenter studies are needed to better define common and uncommon food triggers of both acute and chronic FPIES in children and adults in different geographic regions.
Table 1.
Common FPIES food triggers-selected studies.
| Reference | Country | Study design | Number of patients |
Ages of patients | Food triggers | Number of food triggers |
|---|---|---|---|---|---|---|
| Mehr et al, 200927 | Australia | Retrospective, single center | 35 | Children | Rice-40% Soy-34% Cow’s milk-20% Oat-6% Sweet potato-6% |
1 food-83% 2 foods-17% Range 1-2 foods |
| Mehr et al, 201726 | Australia | Retrospective, multi-center | 230 | Children | Any grain-52% Rice-45% Cow’s milk-33% Egg-12% Any meat-10% Any fruit-10% Oat-9% Any vegetable-8% Chicken-8% Soy-5% Fish-5% |
1 food-68% 2 foods-20% 3 foods-7% ≥4 foods-6% Range 1-≥4 foods |
| Tan et al, 201421 | Australia | Retrospective, single center | 31 | Adults | Crustacean-26% Mollusk-16% Egg-16% Fish-10% |
1 food-84% 2 foods-13% 3 foods-3% Range 1-3 foods |
| Du et al, 201820 | Canada | Retrospective, single center | 20 | Adults | Shellfish-65% Cow’s milk-20% Wheat-20% Egg-15% |
Did not evaluate |
| Xepapadaki et al, 201933 | Greece | Retrospective, single center | 72 | Children | Cow’s milk-46% Fish-35% Rice-10% Egg-7% |
Did not evaluate |
| Sopo et al, 201231 | Italy | Retrospective, multicenter | 66 | Children | Cow’s milk-65% Fish-12% Egg-6% |
1 food-85% 2 foods-14% 3 foods-1% Range 1-3 foods |
| Vazquez-Ortiz et al, 201732 | Spain | Retrospective, single center | 81 | Children | Fish-54% Cow’s milk-26% Egg-10% Rice-7% |
1 food-99% 2 foods-0% 3 foods-1% Range 1-3 foods |
| Alonso, et al, 201923 | Spain | Prospective, single center | 8 | Children | Cow’s milk-50% Fish-38% Egg-13% |
1 food-100% |
| Diaz et al, 201913 | Spain | Retrospective, multicenter | 120 | Children | Cow’s milk-37% Fish-33% Egg-11% Rice-10% |
1 food-84% ≥2 foods-16% Range 1-8 foods |
| Ludman et al, 201425 | UK | Retrospective, single center | 54 | Children | Cow’s milk-46% Fish-15% Egg-13% Soy-11% Wheat-11% Chicken-7% Banana-6% Oat-6% |
1 food-70% ≥2 foods-30% Range 1-≥2 foods |
| Sicherer et al, 199830 | USA | Retrospective, single center | 20 | Children | Soy-65% Cow’s milk-60% Rice-5% Pea-5% Turkey-5% Chicken-5% |
1 food-45% 2 foods-55% Range 1-2 foods |
| Fogg et al, 200624 | USA | Retrospective, single center | 19 | Children | Cow’s milk-68% Soy-63% Rice-16% Oat-16% Wheat-5% Egg-5% |
1 food-42% 2 foods-47% 3 foods-5% 4 foods-5% Range 1-4 foods |
| Nowak-Wegrzyn et al, 200328 | USA | Retrospective, single center | 44 | Children | Cow’s milk-66% Soy-54% Rice-23% Oat-20% |
1 food-48% 2 foods-32% 3 foods-7% 4 foods-2% 5 foods-2% 6 foods-2% 7 foods-2% Range 1-7 foods |
| Ruffner et al, 201329 | USA | Retrospective, single center | 462 | Children | Cow’s milk-67% Soy-41% Rice-19% Oat-16% Wheat-10% Egg-11% Any vegetable-11% Corn-8% Any fruit-8% Any meat and/or fish-6% |
1-2 foods-70% 3-6 foods-25% ≥7 foods-5% Range 1-≥7 foods |
| Caubet et al, 20149 | USA | Mixed prospective and retrospective, single center | 160 | Children and adults | Cow’s milk-44% Any grain-44% Soy-41% Rice-23% Oat-16% Fish and/or shellfish-7% |
1 food-65% 2 foods-26% ≥3 foods-9% Range 1-10 foods |
| Maciag et al, 202010 | USA and multinational (did not specify other countries) | Retrospective, multicenter | 441 | Children | Any grain-60% Cow’s milk-53% Any vegetable-43% Any fruit-38% Soy-37% Oat-37% Rice-34% Egg-23% Any meat-18% Sweet potato-17% Wheat-16% Avocado-13% Peanut-12% Any poultry-12% Banana-12% Peas-9% Fish-9% Beef-9% Any tree nut-8% Apple-8% Corn-7% Chicken-7% Squash-6% Shellfish-6% White potato-5% |
1 food group-31% 2 food groups-17% 3 food groups-17% 4 food groups-12% 5 food groups-7% 6 food groups-6% ≥7 food groups-10% Range 1-13 food groups |
Food triggers are listed in descending order. Only foods reported avoided by ≥5% of the subjects within each study are reported.
Number of food triggers
Patients and families who experience an FPIES reaction to one food often wonder whether they should be concerned for developing reactions to others. Studies evaluating number of FPIES food triggers are summarized in Table 1. Single food FPIES rates ranged from 31-100%, with the majority of both pediatric and adult patients reacting to a single food.9, 10, 13, 21, 23-32 Single food FPIES is less common in the US compared to other countries. For patients with FPIES to multiple foods, the number of additional trigger foods ranged from 2-13.9, 10, 13, 21, 24-32
Few studies have evaluated risk factors for developing multiple food FPIES. In an Australian infant cohort, younger age at first reaction and FPIES to fruits or vegetables were risk factors.26 In another Australian cohort, children with FPIES to rice were more likely to have multiple food FPIES compared to those with FPIES to CM or soy.34 Maciag et al. reported FPIES in a first-degree relative was associated with greater risk.10
FPIES in breastfed infants
FPIES typically presents once CM- or soy-based formulas and/or solid foods are introduced into an infant’s diet. There are rare cases of FPIES in exclusively breastfed infants, triggered by culprit food proteins present in breastmilk.2, 35 Both acute and chronic FPIES to breastmilk have been reported, however these studies are limited to case reports and small case series.35 While CM is the most commonly reported trigger, soy, chicken and grains have also been reported, and some causes remain unknown.2, 35 Age of onset ranges from 15 days to 6 months, with mean 3 months.35 A case of possible fetal-onset FPIES was reported, suspected due to CM in the mother’s diet.36 Larger studies are needed to define the prevalence of FPIES in exclusively breastfed infants and identify trigger foods.
Guidance for nutrition and feeding
Current recommendations for new food introduction in patients with FPIES are based solely on expert opinion.1, 19 The majority of breastfed infants do not react to food proteins in breastmilk, so routine maternal dietary elimination of trigger foods is not recommended if the infant is thriving and asymptomatic.1 While most infants are able to successfully breastfeed, some require maternal elimination diet or transition to a hypoallergenic formula.1
Patient with FPIES are at risk for nutritional deficiencies, food aversion, and poor weight gain, even following elimination of trigger foods. Feeding skills begin developing in infancy. Disruption of normal feeding skill development by FPIES symptoms or dietary interventions such as avoidance diets can create feeding difficulties, including aversions to various tastes and textures and impaired acquisition of eating behaviors and feeding skills.37 Experiencing repeated FPIES reactions when first introducing foods can cause eating to be regarded as an unpleasant experience, leading to food aversions. A retrospective study of 203 patients with FPIES found higher rates of food aversion in patients with ≥3 compared to 1-2 food triggers (P=0.006) and increased risk of poor weight gain in FPIES triggered by CM (P=0.02) and banana (P=0.002).38
Knowledge gaps exist in defining “high-risk” and “low-risk” FPIES foods, recognizing patients at risk for multiple food FPIES, and identifying risk factors and developing effective interventions for feeding and nutritional deficits. These are important areas of unmet need, as they have implications for developing evidence-based guidance on infant feeding, dietary and nutritional recommendations, and patient and family mental health. Large, multicenter studies across a range of age groups and countries are needed to address these questions from a research perspective. Improving access to multidisciplinary teams involving Nutrition, Feeding Therapy, and Psychology is needed to improve patient management.
RESOURCES FOR PATIENTS, FAMILIES, AND HEALTHCARE PROVIDERS
Unmet needs for patients and families
Patients and families with FPIES face many struggles, including obstacles in establishing diagnosis and challenges in management due to lack of FPIES awareness among many medical providers.39, 40 In a 2018-2019 survey of I-FPIES members,3 caregivers indicated they felt most confident getting information about their child’s FPIES from websites, followed by pediatrician/allergist, and general practitioner nurse/family doctor (unpublished data). This highlights the important role of internet resources, but also an opportunity for healthcare providers to become more valued sources of information.
Confidence and knowledge gaps for healthcare providers
In a 2014 survey of AAAAI members, nearly one-third reported poor familiarity with FPIES, and there was considerable variability in evaluation and management practices.39 One significant leap forward was the publication of the 2017 International Consensus Guidelines,1 which was created to assist healthcare professionals care for patients with FPIES and addressed a knowledge gap by providing widely-available, evidence-based diagnostic and management guidelines. Follow up studies are needed to determine whether this has subsequently improved awareness and standardized practice in the medical community. Increased FPIES programming at allergy meetings is another method to raise awareness among healthcare providers.
Resources for patients, families, and healthcare providers
Selected online resources are provided in Table 2. These websites offer educational materials, webinars, and links to consensus guidelines and research studies, providing useful information to healthcare providers to increase knowledge and competence. These websites are also valuable and trusted resources for patient and families with FPIES, offering written and recorded educational materials, connections to the FPIES community and research studies, support groups, and search functions for finding a local allergist. The internet offers a unique platform for FPIES providers, researchers, patients, and families to come together to raise awareness, provide support, and increase research efforts in FPIES. This opportunity to connect online is especially important during the current COVID-19 pandemic, when in-person contact is limited.
Table 2.
List of suggested online resources.
| Organization | Website | Comments |
|---|---|---|
| Allergy & Asthma Network | www.allergyasthmanetwork.org | Educational resources, online events, and e-newsletter. Information available in English and Spanish. |
| Allergy Eats | www.allergyeats.com | Leading guide to allergy-friendly restaurants in the US. Phone app available. |
| American Academy of Allergy Asthma & Immunology (AAAAI) | www.aaaai.org | Educational materials, practice resources, research updates, and search function to find a local allergist. Information available in English and Spanish. |
| American College of Allergy, Asthma & Immunology (ACAAI) | www.acaai.org | Educational materials, practice resources, and research updates. |
| Food Allergy Research and Education (FARE) | www.foodallergy.org | Nonprofit organization and largest private funder of food allergy research. Resources for children, teenagers, and adults about managing food allergies. |
| International FPIES Association (I-FPIES) | www.fpies.org | Nonprofit organization providing FPIES education, support, and advocacy for patients and families. “FPIES University” section on their website is a free video library for the FPIES community and healthcare providers. |
| Kids with Food Allergies | www.kidswithfoodallergies.org | A division of the Asthma and Allergy Foundation of America (AAFA), the US’s oldest and leading asthma and allergy not-for-profit organization. Online and local support groups available. |
| The FPIES Foundation | www.fpiesfoundation.org | Nonprofit organization offering tools for FPIES education, support, and advocacy for families and the medical community. |
UNCERTAINTIES IN DIAGNOSIS AND MANAGEMENT
The footprint of FPIES in the medical literature has expanded (Figure 1), but our understanding of FPIES is still in its infancy and many unanswered questions remain. We do not know what factors modulate the development or resolution of FPIES, or why some patients develop FPIES to one food and others to multiple foods. Improved understanding of various cohorts such as mild/severe reactions, single/multiple foods, higher/lower threshold doses and tolerance of foods like CM and egg in baked forms is needed.41 A phenotypic assessment of individuals with acute FPIES, chronic FPIES, atypical FPIES, FPIES during breastfeeding, and adult FPIES may improve understanding of the pathophysiology, risk factors, and modulating factors. If we could accurately determine phenotype, this knowledge could influence the rate and location at which we introduce foods, perform food challenges, and allow for avoiding the minimum number of triggers for the shortest time necessary and could reduce the negative nutritional and psychosocial impacts of avoidance diets.42
Figure 1. FPIES publications by date.
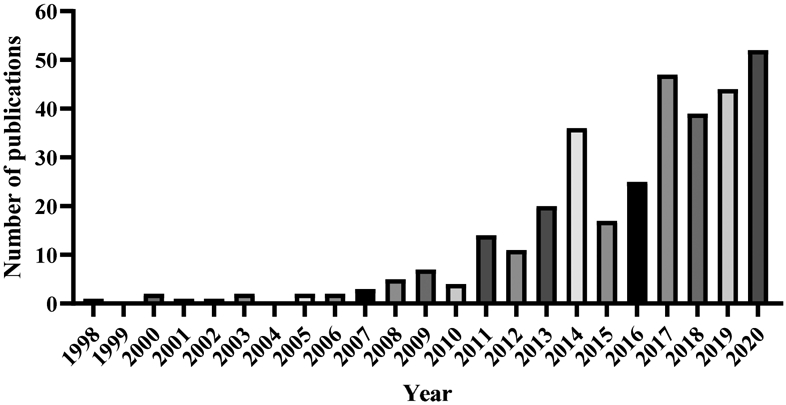
A PubMed literature search was conducted using the search terms FPIES OR “food protein-induced enterocolitis syndrome.” All publications through December 2020 are shown, totaling 335.
Need for a diagnostic test
The differential diagnosis of acute FPIES is broad.1 There is no diagnostic test or procedure that is pathognomonic of this diagnosis. Atopy patch testing has been evaluated in several small studies as a means to identify possible food triggers.24, 29, 43 However, due to conflicting results, no specific recommendations on the utility of atopy patch tests has been made.1 FPIES remains a clinical diagnosis based on a high index of suspicion.1 Some laboratory tests can be helpful in diagnosing an acute episode.1 In chronic FPIES, symptom resolution with trigger avoidance is suggestive of the diagnosis, and OFC after this period of avoidance could be considered to confirm the diagnosis.1 Given the lack of diagnostic testing, low awareness, and broad differential diagnosis, studies of FPIES in the acute setting may be helpful in developing validated differentiating factors and could lead to development of noninvasive biomarkers with the goal of establishing an early and accurate diagnosis.
FPIES pathophysiology
We need a better understanding of the pathophysiology of FPIES. FPIES has traditionally been thought of as a T cell-mediated, non-IgE-mediated food allergy. However, some individuals develop concomitant sIgE (atypical FPIES),30 and sensitized individuals with initial typical FPIES reactions may subsequently develop IgE-mediated food reactions.9
A recent study examining the role of humoral and cellular responses in FPIES did not find a difference in the T cell profiles with casein stimulation in patients with active CM FPIES versus control subjects. Casein stimulation in CM FPIES led to higher levels of IL-9 and lower levels of IL-10 versus those with IgE-mediated CM allergy. Active CM FPIES versus resolved showed higher baseline tryptase levels and lower IL-10. There was no change in tryptase levels after reactive food challenges; however, IL-9 and IL-10 levels increased significantly.44
Similar to IgE-mediated food allergy, a Th2-biased immune response is seen in FPIES and activation of T cells may play a role in gastrointestinal inflammation. The impact of antigen exposure on T cell activation is unclear.19 Other innate immune cells are activated in FPIES reactions, including monocytes, natural killer cells, neutrophils, and eosinophils.19, 45 A more comprehensive understanding of the pathophysiology of FPIES may help with developing biomarkers to identify which foods a person is likely to react to, confirm diagnosis, and predict resolution.
Standardizing FPIES OFCs
OFCs are performed when the diagnosis is unclear and to assess for FPIES resolution. There is no standard, validated protocol. Protocols in the literature vary in regard to dosing, timing of dose administration, duration of observation and whether a full serving is administered after an asymptomatic period of observation.1, 7, 46-49 As patients do not always react on the first exposure to food, there is some concern that a one-day OFC does not suffice to confirm resolution.
Another unresolved concern is where the OFC should be conducted (e.g., at home, outpatient clinic, hospital-based ambulatory, or hospital inpatient setting). This results from lack of predictive factors for OFC outcome and severity, including need for intravenous fluid (IVF) resuscitation.50 Risk is likely impacted by the reason for OFC, time since last reaction, age of the patient, and severity of previous reactions.41 While up to 50% of OFC require IVF,47 there is data that home introduction with oral rehydration has been successful.51 There is no consensus regarding need for placement of an intravenous line prior to outpatient OFC, although IVF should be available. It seems reasonable that more severe reactions and younger children would direct a more conservative approach.
Ultimately, the decision to perform a food challenge must include family preference, physician resources to manage OFC reactions, severity of initial reaction, nutritional adequacy of the diet, implicated food, and data on natural history in a given country.1
FPIES treatments
We need further studies on FPIES treatment. Oral rehydration or breastfeeding is recommended for mild symptoms and IVF for moderate to severe reactions.1 Although steroids have been recommended for severe reactions, this has never been formally studied.52 Ondansetron, a serotonin 5-HT3 receptor antagonist, has been used in infants >6 months old for acute FPIES reactions during OFCs with success, however this has not been studied in a randomized, placebo controlled trial.53, 54 Ondansetron has not been systematically studied for home use in the setting of accidental exposure or in adult FPIES.
We do not know whether there are threshold doses for triggering acute or chronic FPIES reactions or which patients might tolerate foods in extensively baked forms.1 Miniscule55 to significant exposures have been reported to lead to reactions.31, 51 There are case reports of patients with acute CM and egg FPIES tolerating these items in baked forms.56 Does tolerance of baked foods portend a more favorable prognosis and accelerate the rate of resolution as in IgE-mediated food allergy?57
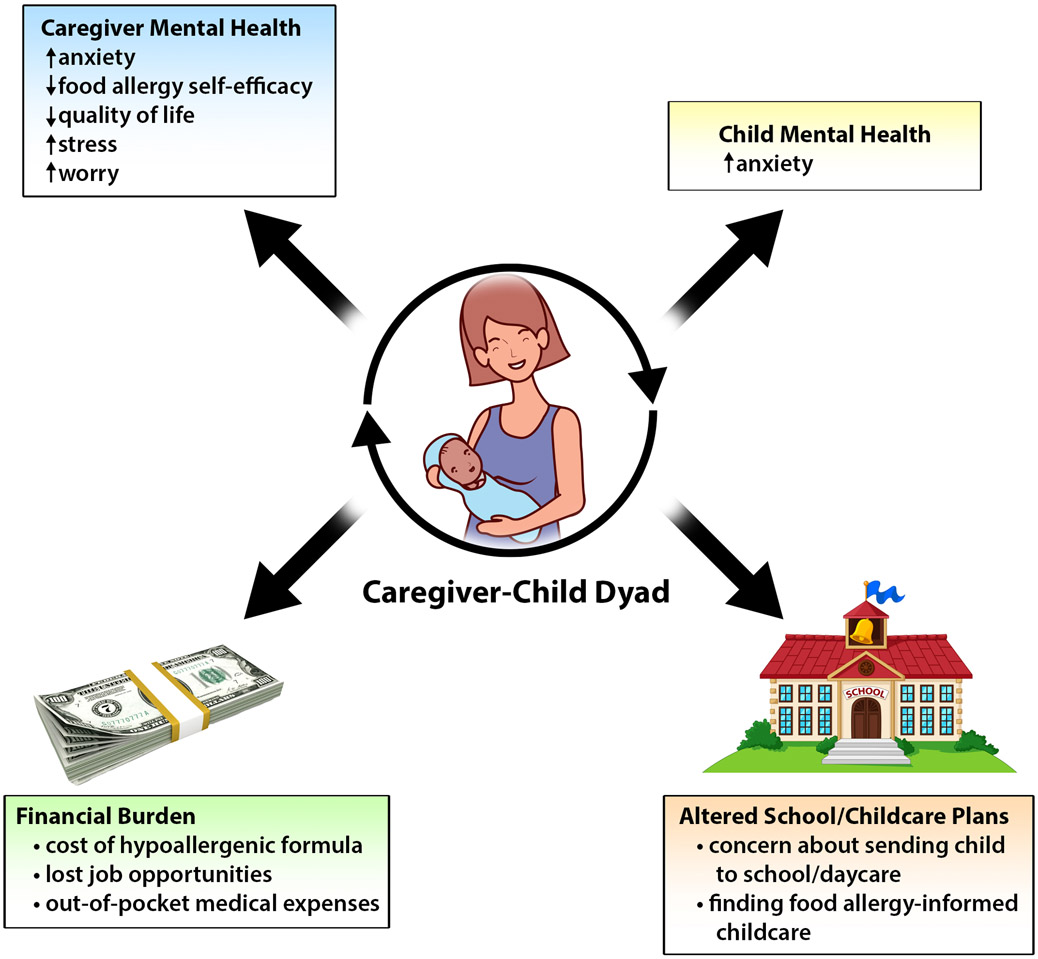
PSYCHOSOCIAL IMPACT
Patients and families with FPIES face unique challenges that can lead to a psychosocial distress (Figure 2). A 2014 article on FPIES written from a parent’s perspective discusses the psychosocial challenges of seeking a diagnosis.40
Figure 2. Psychosocial impact of FPIES on patients and families.
Summary of the psychosocial impacts of FPIES on patients and their caregivers. FPIES is associated with significant mental health burden, altered school/daycare plans due to related to FPIES, and increased financial burden.
Impact on children
The only study to date evaluating psychosocial function of children with FPIES was conducted by Maciag et al., with responses representing 100 children.3 Caregivers’ ratings of their preschoolers’ separation anxiety, obsessive-compulsive disorder (OCD), and general anxiety symptoms were higher than published norms (P<0.05). However, older children’s self-reported panic, OCD, general anxiety, and total anxiety symptoms were lower than published norms (P<0.05). Conversely, caregivers’ ratings of their older children’s separation anxiety, general anxiety, and total anxiety were higher than published norms (P<0.05).
Impact on caregivers
Greenhawt et al. performed an exploratory validation study of the Food Allergy Quality of Life-Parental Burden (FAQL-PB) questionnaire for use in FPIES.4 Caregivers of children with FPIES had worse HRQOL compared to those with IgE-mediated food allergy (P<0.001). Female caregivers had worse HRQOL than male caregivers (P < 0.001). Caregivers of children with both solid and liquid FPIES reported worse HRQOL than those with a single trigger food (P=0.01).
Maciag et al. conducted a subsequent larger study of 410 I-FPIES caregiver-members in 2018-2019 utilizing multiple validated measures and found a high psychosocial burden among caregivers and affected children.3 On the FAQL-PB, caregivers reported highest levels of burden in restaurant choice, vacation plans, and social engagement, similar to Greenhawt et al.4 Maciag et al. found that caregivers reported moderate stress and worry. Parental confidence in ability to measure their child’s food allergy was significantly lower in caregivers of children with FPIES compared with IgE-mediated food allergy (P<0.001). In the Maciag et al. FPIES cohort, there was a positive correlation between caregiver HRQOL, stress, worry, and anxiety, and these correlated negatively with food allergy self-efficacy. Avoiding a greater number of food groups was associated with lower caregiver HRQOL and food allergy self-efficacy, and higher total anxiety among preschool- and elementary school-aged children. CM FPIES was also associated with lower caregiver food allergy-related self-efficacy and HRQOL, and higher stress.3
Further studies are needed in more diverse populations, as well as studies designed to improve self-efficacy in caregivers.
FPIES and school/daycare attendance
In the studies by Maciag et al.,3, 10 46% of families (N=187/403) reported their children with FPIES did not attend school/daycare, and of those not attending, 54% did not attend because of concern for FPIES. Avoiding multiple food groups was associated with increased likelihood of not attending school/daycare (P=0.001).10 Caregivers whose children were not attending school/daycare due to FPIES had poorer HRQOL, higher stress, greater worry, and higher total anxiety among preschool-aged children, compared with caregivers whose children were not attending for other reasons.3
We need to advocate for our patients and increase education for schools and daycares about FPIES triggers, and recognition and initial management of reactions.41 This may reduce the psychosocial burden experienced by families with FPIES and allow for increased comfort sending their children to school/daycare. The FPIES community and researchers can collaborate to identify patients and families at risk and develop ways to alleviate this burden.
Financial burden
The economic burden to families with food allergies is significant.58, 59 To our knowledge, there are no studies focused on direct and indirect costs of FPIES. Investigation is needed to determine the cost burden of food allergy for families that considers opportunity costs, lost productivity, direct out-of-pocket costs, dietary costs including cost of hypoallergenic formulas, and food allergy-informed childcare arrangements. Costs of visits with dieticians, feeding specialists, social workers and psychologists should also factor into these calculations. This knowledge could help develop policies to ensure adequate insurance coverage for patients across the socioeconomic spectrum and minimize disparities.
CONCLUSION
Since half a century ago when FPIES was first described, many advances have been made in understanding the clinical phenotypes, epidemiology, and pathophysiology. The establishment of the first FPIES ICD-10 code in 2016 and publication of the first International Consensus Guidelines in 2017 have laid the groundwork for further, urgently-needed FPIES research. However, many unanswered questions remain, summarized in Table 3. A major challenge of FPIES is that it is an uncommon and incompletely understood type of food allergy. However, this challenge has catalyzed the establishment of a powerful and tireless FPIES community, joining patients, families, and healthcare providers. By partnering together, this community is increasing FPIES awareness through outreach and education and advancing the pace of FPIES research to address these questions urgently in need of answers.
Table 3.
Assessment of unmet needs in FPIES.
| FPIES PHENOTYPES |
|---|
| Characterize chronic FPIES |
| Characterize atypical FPIES |
| Identify risk factors and mechanisms for conversion from FPIES to IgE-mediated food allergy |
| Characterize acute and chronic FPIES in adults |
| FPIES FOOD TRIGGERS |
| Identify prevalence of common FPIES food triggers |
| Understand geographic differences in FPIES food triggers |
| Identify risk factors for developing FPIES to single versus multiple foods |
| Determine impact of updated infant feeding recommendations on FPIES food triggers |
| Develop evidence-based groupings of “high-risk” and “low-risk” foods to guide infant feeding and dietary expansion |
| Characterize nutritional burden of FPIES and provide guidance for prevention and management |
| RESOURCES FOR PATIENTS, FAMILIES, AND HEALTHCARE PROVIDERS |
| Increase community education (e.g., schools, daycares, etc.) and awareness |
| Develop a written FPIES Action Plan for use in schools and daycares |
| Increase FPIES programming at allergy conferences |
| DIAGNOSIS AND MANAGEMENT |
| Identify factors that modulate the likelihood of developing FPIES |
| Identify factors that accelerate resolution of FPIES |
| Determine FPIES pathophysiology |
| Develop biomarker for diagnosis, resolution and food trigger identification |
| Standardize FPIES food challenges, including dosing and location |
| Determine evidence-based treatments for FPIES reactions |
| Develop a multidisciplinary care team approach |
| PSYCHOSOCIAL IMPACT |
| Characterize the psychosocial impact on children and adults in more diverse populations |
| Develop strategies to improve self-efficacy |
| Characterize financial burden |
Key messages:
The number of studies and publications related to FPIES has steadily increased over the past 50 years.
There are multiple unmet needs regarding FPIES.
Further research is needed to understand the pathophysiology of FPIES and to develop a biomarker to aid in diagnosis, resolution and food trigger identification.
A better understanding of FPIES prevalence, phenotypes, modifying factors and treatments is needed.
An FPIES diagnosis is associated with significant psychosocial and financial burden and research is needed to identify ameliorating factors.
Collaboration between clinicians, researchers, families and patient and support groups is needed to accelerate research and knowledge acquisition regarding FPIES.
Acknowledgments
Funding: This research is supported by the National Institutes of Health K23 AI143962 (Bartnikas), K24 AI106822 (Phipatanakul), and The Allergy and Asthma Awareness Initiative, Inc. (Phipatanakul).
Abbreviations/Acronyms:
- AAAAI
American Academy of Allergy, Asthma & Immunology
- CM
cow’s milk
- FAQL-PB
Food Allergy Quality of Life-Parental Burden
- FPIES
food protein-induced enterocolitis syndrome
- HRQOL
health related quality of life
- ICD
International Classification of Diseases
- I-FPIES
International Food Protein-Induced Enterocolitis Syndrome Association
- IVF
intravenous fluids
- OCD
obsessive-compulsive disorder
- OFC
oral food challenge
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Bartnikas received travel support from the International FPIES Association. Drs. Bartnikas, Bingemann and Nowak-Wegrzyn are unpaid medical advisors to the International FPIES Association. Ms. Schultz is founder and chair of the International FPIES Association. Dr. Bartnikas is an unpaid medical advisory to the Allergy and Asthma Foundation of America-New England chapter. The other authors declare no conflicts of interest.
References
- 1.Nowak-Wegrzyn A, Chehade M, Groetch ME, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017;139:1111–1126 e1114. [DOI] [PubMed] [Google Scholar]
- 2.Baker MG, Nowak-Wegrzyn A Food protein-induced enterocolitis syndrome: epidemiology and comorbidities. Curr Opin Allergy Clin Immunol. 2020;20:168–174. [DOI] [PubMed] [Google Scholar]
- 3.Maciag MC, Herbert LJ, Sicherer SH, et al. The Psychosocial Impact of Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol Pract. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhawt M, Schultz F, DunnGalvin A A validated index to measure health-related quality of life in patients with food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2016;137:1251–1253 e1255. [DOI] [PubMed] [Google Scholar]
- 5.Powell GK Enterocolitis in low-birth-weight infants associated with milk and soy protein intolerance. J Pediatr. 1976;88:840–844. [DOI] [PubMed] [Google Scholar]
- 6.Gryboski JD Gastrointestinal milk allergy in infants. Pediatrics. 1967;40:354–362. [PubMed] [Google Scholar]
- 7.Powell GK Milk- and soy-induced enterocolitis of infancy. Clinical features and standardization of challenge. J Pediatr. 1978;93:553–560. [DOI] [PubMed] [Google Scholar]
- 8.Ikola RA Severe intestinal reaction following ingestion of rice. Am J Dis Child. 1963;105:281–284. [DOI] [PubMed] [Google Scholar]
- 9.Caubet JC, Ford LS, Sickles L, et al. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J Allergy Clin Immunol. 2014;134:382–389. [DOI] [PubMed] [Google Scholar]
- 10.Maciag MC, Bartnikas LM, Sicherer SH, et al. A Slice of Food Protein-Induced Enterocolitis Syndrome (FPIES): Insights from 441 Children with FPIES as Provided by Caregivers in the International FPIES Association. J Allergy Clin Immunol Pract. 2020;8:1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard SA, Pecora V, Fiocchi AG, Nowak-Wegrzyn A Food protein-induced enterocolitis syndrome: a review of the new guidelines. World Allergy Organ J. 2018;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger T, Feuille E, Thompson C, Nowak-Wegrzyn A Chronic food protein-induced enterocolitis syndrome: Characterization of clinical phenotype and literature review. Ann Allergy Asthma Immunol. 2016;117:227–233. [DOI] [PubMed] [Google Scholar]
- 13.Diaz JJ, Espin B, Segarra O, et al. Food Protein-induced Enterocolitis Syndrome: Data From a Multicenter Retrospective Study in Spain. J Pediatr Gastroenterol Nutr. 2019;68:232–236. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Delgado P, Ruano-Zaragoza M, Gutierrez A, Lopez F, Fernandez J Chronic adult food protein-induced enterocolitis syndrome. Ann Allergy Asthma Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- 15.Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134:1016–1025 e1043. [DOI] [PubMed] [Google Scholar]
- 16.Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessel A, Dalal I The pendulum between food protein-induced enterocolitis syndrome and IgE-mediated milk allergy. Acta Paediatr. 2011;100:e183–185. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes BN, Boyle RJ, Gore C, Simpson A, Custovic A Food protein-induced enterocolitis syndrome can occur in adults. J Allergy Clin Immunol. 2012;130:1199–1200. [DOI] [PubMed] [Google Scholar]
- 19.Nowak-Wegrzyn A, Berin MC, Mehr S Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol Pract. 2020;8:24–35. [DOI] [PubMed] [Google Scholar]
- 20.Du YJ, Nowak-Wegrzyn A, Vadas P FPIES in adults. Ann Allergy Asthma Immunol. 2018;121:736–738. [DOI] [PubMed] [Google Scholar]
- 21.Tan JA, Smith WB Non-IgE-mediated gastrointestinal food hypersensitivity syndrome in adults. J Allergy Clin Immunol Pract. 2014;2:355–357 e351. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Delgado P, Caparros E, Moreno MV, Cueva B, Fernandez J Food protein-induced enterocolitis-like syndrome in a population of adolescents and adults caused by seafood. J Allergy Clin Immunol Pract. 2019;7:670–672. [DOI] [PubMed] [Google Scholar]
- 23.Alonso SB, Ezquiaga JG, Berzal PT, et al. Food protein-induced enterocolitis syndrome: Increased prevalence of this great unknown-results of the PREVALE study. J Allergy Clin Immunol. 2019;143:430–433. [DOI] [PubMed] [Google Scholar]
- 24.Fogg MI, Brown-Whitehorn TA, Pawlowski NA, Spergel JM Atopy patch test for the diagnosis of food protein-induced enterocolitis syndrome. Pediatr Allergy Immunol. 2006;17:351–355. [DOI] [PubMed] [Google Scholar]
- 25.Ludman S, Harmon M, Whiting D, du Toit G Clinical presentation and referral characteristics of food protein-induced enterocolitis syndrome in the United Kingdom. Ann Allergy Asthma Immunol. 2014;113:290–294. [DOI] [PubMed] [Google Scholar]
- 26.Mehr S, Frith K, Barnes EH, Campbell DE, Group FS Food protein-induced enterocolitis syndrome in Australia: A population-based study, 2012-2014. J Allergy Clin Immunol. 2017;140:1323–1330. [DOI] [PubMed] [Google Scholar]
- 27.Mehr S, Kakakios A, Frith K, Kemp AS Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics. 2009;123:e459–464. [DOI] [PubMed] [Google Scholar]
- 28.Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics. 2003;111:829–835. [DOI] [PubMed] [Google Scholar]
- 29.Ruffner MA, Ruymann K, Barni S, Cianferoni A, Brown-Whitehorn T, Spergel JM Food protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol Pract. 2013;1:343–349. [DOI] [PubMed] [Google Scholar]
- 30.Sicherer SH, Eigenmann PA, Sampson HA Clinical features of food protein-induced enterocolitis syndrome. J Pediatr. 1998;133:214–219. [DOI] [PubMed] [Google Scholar]
- 31.Sopo SM, Giorgio V, Dello Iacono I, Novembre E, Mori F, Onesimo R A multicentre retrospective study of 66 Italian children with food protein-induced enterocolitis syndrome: different management for different phenotypes. Clin Exp Allergy. 2012;42:1257–1265. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Ortiz M, Machinena A, Dominguez O, et al. Food protein-induced enterocolitis syndrome to fish and egg usually resolves by age 5 years in Spanish children. J Allergy Clin Immunol Pract. 2017;5:512–515 e511. [DOI] [PubMed] [Google Scholar]
- 33.Xepapadaki P, Kitsioulis NA, Manousakis E, Manolaraki I, Douladiris N, Papadopoulos NG Remission Patterns of Food Protein-Induced Enterocolitis Syndrome in a Greek Pediatric Population. Int Arch Allergy Immunol. 2019;180:113–119. [DOI] [PubMed] [Google Scholar]
- 34.Mehr SS, Kakakios AM, Kemp AS Rice: a common and severe cause of food protein-induced enterocolitis syndrome. Arch Dis Child. 2009;94:220–223. [DOI] [PubMed] [Google Scholar]
- 35.Baldo F, Bevacqua M, Corrado C, et al. FPIES in exclusively breastfed infants: two case reports and review of the literature. Ital J Pediatr. 2020;46:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichimura S, Kakita H, Asai S, et al. A Rare Case of Fetal Onset, Food Protein-Induced Enterocolitis Syndrome. Neonatology. 2019;116:376–379. [DOI] [PubMed] [Google Scholar]
- 37.Chehade M, Meyer R, Beauregard A Feeding difficulties in children with non-IgE-mediated food allergic gastrointestinal disorders. Ann Allergy Asthma Immunol. 2019;122:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su KW, Patil SU, Stockbridge JL, et al. Food aversion and poor weight gain in food protein-induced enterocolitis syndrome: A retrospective study. J Allergy Clin Immunol. 2020;145:1430–1437 e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenhawt M, Bird JA, Nowak-Wegrzyn AH Trends in Provider Management of Patients with Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol Pract. 2017;5:1319–1324 e1312. [DOI] [PubMed] [Google Scholar]
- 40.Schultz F, Westcott-Chavez A Food protein-induced enterocolitis syndrome from the parent perspective. Curr Opin Allergy Clin Immunol. 2014;14:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown-Whitehorn TF, Cianferoni A Food Protein Induced Enterocolitis (FPIES) : Diagnosis and Management. Cham, Switzerland: Springer International Publishing AG; 2019. [Google Scholar]
- 42.Wang J, Fiocchi A Unmet needs in food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol. 2014;14:206–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarvinen KM, Caubet JC, Sickles L, Ford LS, Sampson HA, Nowak-Wegrzyn A Poor utility of atopy patch test in predicting tolerance development in food protein-induced enterocolitis syndrome. Ann Allergy Asthma Immunol. 2012;109:221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caubet JC, Bencharitiwong R, Ross A, Sampson HA, Berin MC, Nowak-Węgrzyn A Humoral and cellular responses to casein in patients with food protein-induced enterocolitis to cow's milk. J Allergy Clin Immunol. 2017;139:572–583. [DOI] [PubMed] [Google Scholar]
- 45.Goswami R, Blazquez AB, Kosoy R, Rahman A, Nowak-Wegrzyn A, Berin MC Systemic innate immune activation in food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2017;139:1885–1896 e1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jarvinen KM, Nowak-Wegrzyn A Food protein-induced enterocolitis syndrome (FPIES): current management strategies and review of the literature. J Allergy Clin Immunol Pract. 2013;1:317–322. [DOI] [PubMed] [Google Scholar]
- 47.Sicherer SH Food protein-induced enterocolitis syndrome: case presentations and management lessons. J Allergy Clin Immunol. 2005;115:149–156. [DOI] [PubMed] [Google Scholar]
- 48.Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, et al. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–383. [DOI] [PubMed] [Google Scholar]
- 49.Bird JA, Leonard S, Groetch M, et al. Conducting an Oral Food Challenge: An Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. J Allergy Clin Immunol Pract. 2020;8:75–90 e17. [DOI] [PubMed] [Google Scholar]
- 50.Wang KY, Lee J, Cianferoni A, et al. Food Protein-Induced Enterocolitis Syndrome Food Challenges: Experience from a Large Referral Center. J Allergy Clin Immunol Pract. 2019;7:444–450. [DOI] [PubMed] [Google Scholar]
- 51.Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M The prevalence and natural course of food protein-induced enterocolitis syndrome to cow's milk: a large-scale, prospective population-based study. J Allergy Clin Immunol. 2011;127:647–653 e641-643. [DOI] [PubMed] [Google Scholar]
- 52.Sicherer SH Food protein-induced enterocolitis syndrome: clinical perspectives. J Pediatr Gastroenterol Nutr. 2000;30 Suppl:S45–49. [DOI] [PubMed] [Google Scholar]
- 53.Holbrook T, Keet CA, Frischmeyer-Guerrerio PA, Wood RA Use of ondansetron for food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2013;132:1219–1220. [DOI] [PubMed] [Google Scholar]
- 54.Miceli Sopo S, Bersani G, Monaco S, et al. Ondansetron in acute food protein-induced enterocolitis syndrome, a retrospective case-control study. Allergy. 2017;72:545–551. [DOI] [PubMed] [Google Scholar]
- 55.Bansal AS, Bhaskaran S, Bansal RA Four infants presenting with severe vomiting in solid food protein-induced enterocolitis syndrome: a case series. J Med Case Rep. 2012;6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miceli Sopo S, Buonsenso D, Monaco S, Crocco S, Longo G, Calvani M Food protein-induced enterocolitis syndrome (FPIES) and well cooked foods: A working hypothesis. Allergologia et immunopathologia. 2013;41:346–348. [DOI] [PubMed] [Google Scholar]
- 57.Upton J, Nowak-Wegrzyn A The Impact of Baked Egg and Baked Milk Diets on IgE- and Non-IgE-Mediated Allergy. Clinical reviews in allergy & immunology. 2018;55:118–138. [DOI] [PubMed] [Google Scholar]
- 58.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026–1031. [DOI] [PubMed] [Google Scholar]
- 59.Dyer AA, Negris OR, Gupta RS, Bilaver LA Food allergy: how expensive are they? Curr Opin Allergy Clin Immunol. 2020;20:188–193. [DOI] [PubMed] [Google Scholar]