Sir,
Many hospital outbreaks of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have been reported worldwide, involving patients and/or healthcare workers (HCWs) [1,2]. Variant of concern 202012/01 (VOC-202012/01) lineage B.1.1.7, responsible for an important epidemic resumption in the UK in November 2020, seems to be more transmissible [3] and more lethal [4] than the pre-existing SARS-CoV-2 variants (non-VOC). In the current context of the emergence of this new variant in France [5], the aim of this observational study was to assess whether transmission control measures were effective to control hospital VOC-202012/01 outbreaks.
Assistance publique-Hôpitaux de Paris (AP-HP) is a network of 38 university-affiliated public hospitals, spread over Paris and its suburbs, with a total of 20,000 beds and employing 100,000 HCWs.
The coronavirus disease 2019 (COVID-19) transmission prevention strategy is based on universal (HCWs and patients) continuous use of medical masks, use of FFP2 masks during aerosol-generating procedures, contact and droplet precautions in suspected or confirmed cases of COVID-19, limitation of the number of visitors per patient (one visitor/day), and screening of any patients admitted to hospital and any HCWs or patients with symptoms that may be COVID-19 related. Social distancing during breaks (especially meals) is also promoted. HCWs and patients experiencing contact with a confirmed COVID-19 case without wearing a medical mask are tested for SARS-CoV-2, even if asymptomatic, with repeated screening on Day 7. Outbreak control measures include weekly screening of asymptomatic contacts (patients and/or HCWs), cohorting of patient cases and furloughing HCW cases for 10 days, stopping visits and joint activities, and reinforcing COVID-19 prevention transmission measures.
A case was defined as any patient or HCW with a positive result on SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) assay. A VOC-202012/01 case was defined as any case with specific mutations identified through specific RT-PCR or genotyping of the spike protein gene [6]. An outbreak was defined as at least two cases (patients and/or HCWs) linked by time (within 7 days), geographic location and possible contact. An outbreak was considered to be controlled after 14 days without any new cases.
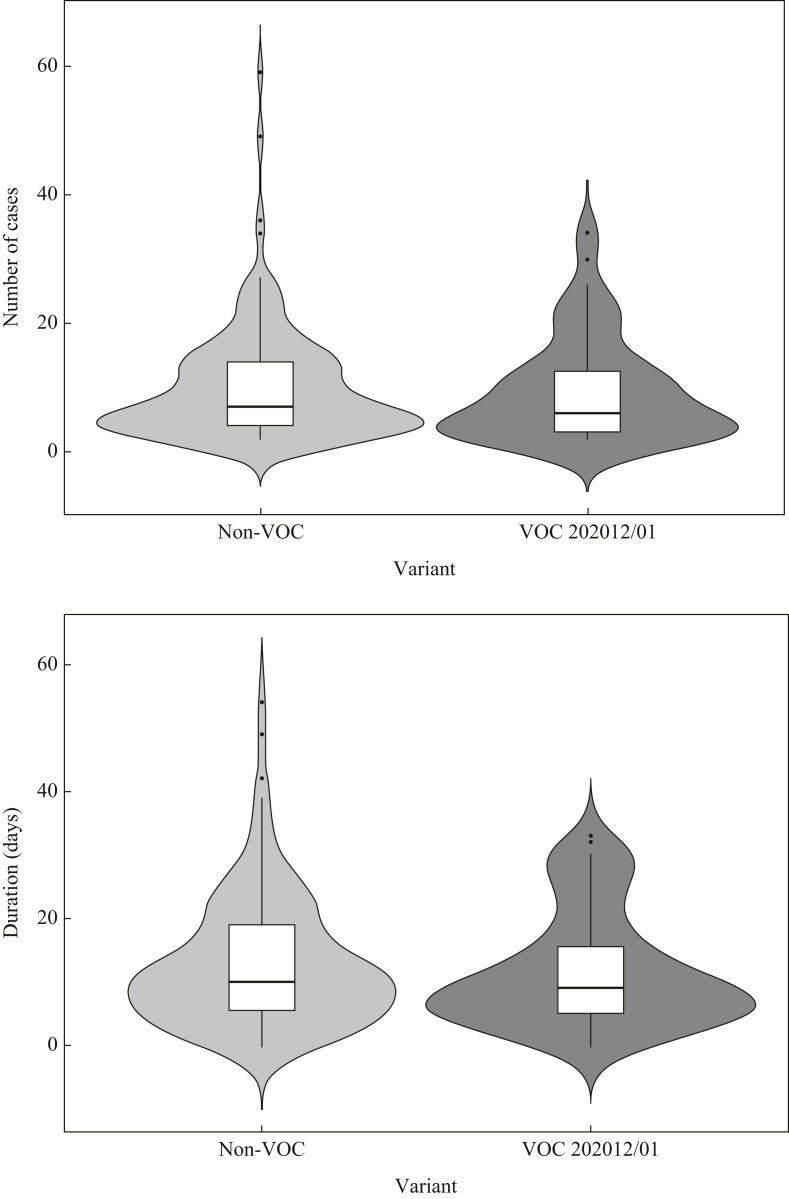
For each outbreak from 20th December 2020 (first outbreak of VOC-202012/01 in AP-HP hospitals) to 21st February 2021, the following data were collected: variant type, number of cases in patients and HCWs, and outbreak duration (time between first and last cases). The number of cases and the outbreak duration in the non-VOC and VOC-202012/01 groups were compared using the Wilcoxon rank sum test (P=0.05). Analyses were performed using R Version 4.0.3.
Among the 218 outbreaks identified during this period (145 non-VOC and 73 VOC-202012/01), 1013 patients and 1166 HCWs were involved. The median number of cases involved in outbreaks was 7.0 [interquartile range (IQR) 4.0–14.0] and 6.0 [IQR 3.0–12.5] in non-VOC and VOC-202012/01 outbreaks, respectively (P=0.35) (Figure 1 ). The median number of patients was 3.0 (IQR 0.0–8.0) in non-VOC outbreaks and 2.0 (IQR 0–6.5) in VOC-202012/01 outbreaks (P=0.23), and the median number of HCWs was 4.0 (IQR 2.0–7.5) in non-VOC outbreaks and 5.0 (IQR 2.0–7.5) in VOC-202012/01 outbreaks (P=0.54). The median outbreak duration was 10.0 (IQR 5.5–19.0) and 9.0 (IQR 5.0–15.5) days in non-VOC and VOC-202012/01 outbreaks, respectively (P=0.41) (Figure 1).
Figure 1.
Comparison of the number of cases and duration in hospital outbreaks of pre-existing severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants [non-variants of concern (non-VOC)] and SARS-CoV-2 VOC-202012/01. Horizontal lines on the violin plots correspond from bottom to top of box: 25th, 50th and 75th percentiles. The whiskers extend to the minimum and maximum values. In addition, a rotated kernel density plot on each side shows peaks in the data.
This study had several limitations, including the lack of sequencing completeness. Another limitation is that HCW compliance with the prevention measures was not assessed, and may have been more rigorous with VOC-202012/01 cases, although the time frame in which the sequencing results were available makes this unlikely. In contrast, contact screening of exposed HCWs and patients may have been more extensive due to the fear of rapid dissemination of VOC-202012/01, and this could have explained a higher number of cases in VOC-202012/01 outbreaks, thus reinforcing that the transmission of VOC was not higher than the transmission of non-VOC in this hospital network. Unfortunately, the numbers of screened individuals in both groups are not known.
Finally, despite its higher transmissibility and its rapid spread in France, these results suggest that VOC-202012/01 was not associated with higher intrahospital diffusion compared with non-VOC in the hospital network, thanks to strict infection control measures.
Acknowledgements
The authors wish to thank all the AP-HP virologists and infectious diseases teams involved in the diagnosis and care of COVID-19 cases, all AP-HP healthcare workers and the patients.
The AP-HP COVID-19 Infection Prevention and Control Group: F. Espinasse, M.C. Gramer, Hôpital Ambroise Paré, Boulogne; M. Lepainteur, Hôpital Antoine Béclère, Clamart; D. Seytre, J.R. Zahar, Hôpital Avicenne, Bobigny; S. Nerome, C. Ciotti, I. Garrigues, Hôpital Beaujon, Clichy; M.L. Delaby, Hôpital Maritime, Berck sur Mer; N. Fortineau, S. Ouzani, M. Khecharem, Hôpital Bicêtre, Kremlin-Bicêtre; J.C. Lucet, S. Kernéis, S. Géra, G. Bendjelloul, Hôpital Bichat, Paris; L. Vaillant, M. Vanderbrugghe, Hôpital Bretonneau, Paris; V. Goldstein, C. Loison, S. Borde, Hôpital Charles Foix, Ivry Sur Seine; V. Moulin, C. Leboydre, Hôpital Corentin Celton, Paris; V. Derouin, Hôpital Broca, Paris; A. Casetta, L. Meyer, Hôpital Cochin, Paris; A. Akpabie, Hôpital Emile Roux, Limeil-Brévannes; N. Kassis-Chikhani, Hôpital Européen Georges Pompidou, Paris; A. Maurand, Hôpital Georges Clémenceau, Champcueil; M. Silvie, Hôpital Marin d’Hendaye, Hendaye; J.W. Decousser, F. Fourreau, B. Hacquin, Hôpital Henri Mondor, Créteil; A. Tackin, Hospitalisation á domicile, Paris; A. Lomont, Hôpital Jean Verdier, Bondy; N. Sabourin, Hôpital Joffre-Dupuytren, Draveil; R. Amarsy, S. Roulleau, Y. Boufflers, Hôpital Lariboisière, Paris; N. Idri, Hôpital Louis Mourier, Colombes; P. Frange, Hôpital Necker, Paris; P. Baune, Hôpital Paul Brousse, Villejuif; J. Robert, N. Osinski, C. Tamames, J. Auraix, N. Forest, Hôpital Pitié-Salpêtrière, Paris; E. Pierson, Hôpital Paul Doumer, Liancourt; C. Lawrence, C. Flament, Hôpital Raymond Poincaré, Garches; G. Rolland Hôpital René Muret, Sevran; P. Mariani, K. Belhacel, Hôpital Robert Debré, Paris; B. Salauze, Hôpital Rothschild, Paris; F. Barbut, S. Jolivet, N. Audrain, Hôpital Saint Antoine, Paris; I. Simon, L. Turpin, Hôpital Sainte Périne, Paris; M. Rouveau, M. Thegat Le Cam, C. Eble, W. Zebiche Hôpital Saint Louis, Paris; V. Simha, C. Grudzien, Hôpital Maritime, San Salvadour; M. Denis, E. Le-Roux, Hôpital Tenon, Paris; S. Angerand, Hôpital Trousseau, Paris; and C. Charpinet, Hôpital Vaugirard, Paris.
Contributor Information
AP-HP COVID-19 Infection Prevention and Control Group:
F. Espinasse, M.C. Gramer, M. Lepainteur, D. Seytre, J.R. Zahar, S. Nerome, C. Ciotti, I. Garrigues, M.L. Delaby, N. Fortineau, S. Ouzani, M. Kecharem, J.C. Lucet, S. Kernéis, S. Géra, G. Bendjelloul, L. Vaillant, M. Vanderbrugghe, V. Goldstein, C. Loison, S. Borde, V. Moulin, C. Leboydre, V. Derouin, A. Casetta, L. Meyer, A. Akpabie, N. Kassis-Chikhani, A. Maurand, M. Silvie, J.W. Decousser, F. Fourreau, B. Hacquin, A. Tackin, A. Lomont, N. Sabourin, R. Amarsy, S. Roulleau, Y. Boufflers, N. Idri, P. Frange, P. Baune, J. Robert, N. Osinski, C. Tamames, J. Auraix, N. Forest, E. Pierson, C. Lawrence, C. Flament, G. Rolland, P. Mariani, K. Belhacel, B. Salauze, F. Barbut, S. Jolivet, N. Audrain, I. Simon, L. Turpin, M. Rouveau, M.T. Le Cam, C. Eble, W. Zebiche, V. Simha, C. Grudzien, M. Denis, E. Le-Roux, S. Angerand, and C. Charpinet
Author contributions
Concept and design: Duverger, Souyri, Fournier.
Acquisition analysis, or interpretation of data: All authors.
Statistical analysis: Monteil.
Drafting of the manuscript: Duverger, Fournier.
Critical revision of the manuscript for important intellectual content: All authors.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Abbas M., Robalo Nunes T., Martischang R., Zingg W., Iten A., Pittet D., et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:1–13. doi: 10.1186/s13756-020-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paltansing S., Sikkema R.S., de Man S.J., Koopmans M.P.G., Oude Munnink B.B., de Man P. Transmission of SARS-CoV-2 among healthcare workers and patients in a teaching hospital in the Netherlands confirmed by whole-genome sequencing. J Hosp Infect. 2021;110:178–183. doi: 10.1016/j.jhin.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:1–10. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaymard A., Bosetti P., Feri A., Destras G., Enouf V., Andronico A., et al. Early assessment of diffusion and possible expansion of SARS-CoV-2 lineage 20I/501Y.V1 (B.1.1.7, variant of concern 202012/01) in France, January to March 2021. Eurosurveillance. 2021;26:1–6. doi: 10.2807/1560-7917.ES.2021.26.9.2100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frampton D., Rampling T., Cross A., Bailey H., Heaney J., Byott M., et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis. 2021;3099:1–11. doi: 10.1016/S1473-3099(21)00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]