Abstract
Background
During the coronavirus disease 2019 (COVID-19) pandemic, health care workers (HCWs) have been obliged to wear personal protective equipment (PPE). We assessed the impact of PPE use on HCWs’ physical health and we examined factors related to a greater risk of adverse events due to PPE use.
Methods
We applied the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines and the Cochrane criteria. We searched PubMed, Medline, Scopus, ProQuest, CINAHL, and medRxiv from January 1, 2020 to December 27, 2020.
Results
Our review included 14 studies with 11,746 HCWs. The estimated overall prevalence of adverse events among HCWs was 78% with a range from 42.8% to 95.1% among studies. Among others, the following factors were related to the risk of adverse events among HCWs due to PPE use: obesity, diabetes mellitus, smoking, pre-existing headache, longer duration of shifts wearing PPE, increased consecutive days with PPE, and increased exposure to confirmed or suspected COVID-19 patients.
Conclusions
The frequency of adverse events among HCWs due to PPE use is very high. Healthcare facilities should take the necessary precautions and change the working conditions during the COVID-19 pandemic to prevent adverse events associated with PPE use and minimize harm to HCWs.
Key Words: Adverse events, SARS-CoV-2, Risk factors, Health care staff, Headaches, PPE
Background
Health care workers (HCWs) can be exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) through clinical settings or community transmission and are essential workers at risk for coronavirus disease 2019 (COVID-19). According to the Centers for Disease Control and Prevention (CDC) during February 12-July 16, 2020, in the USA, 11% of patients had been identified as HCWs,1 while during March 1-May 31, 2020, among hospitalized adults, 5.9% were HCWs.2 A meta-analysis3 found that the prevalence of hospitalization among HCWs infected with COVID-19 is 15.1% and the mortality is 1.5%, while another meta-analysis4 found that the proportion of SARS-CoV-2 positive HCWs among all COVID-19 patients is 10.1% and the mortality is significantly lower in HCWs as compared to that of all patients (0.3% vs 2.3%). According to an analysis that included studies only in Australia between January 25th and July 8th, HCWs were 2.69 times more likely to contract COVID-19 than the general population.5 Also, the seroprevalence of SARS-CoV-2 antibodies among HCWs is high (8.7%) especially in North America (12.7%) compared to Europe (8.5%), Africa (8.2), and Asia (4%).6
During the COVID-19 pandemic, HCWs caring for patients with COVID-19 in high-risk clinical settings such as isolation wards, intensive care units, emergency rooms, and general medical wards have been obliged to wear personal protective equipment (PPE). PPE includes equipment or specific clothing (eg, respiratory and eye protection, gown and gloves) that protects HCWs against infectious materials.7 The necessity of PPE to prevent transmission of viruses to HCWs has already proven during the severe acute respiratory distress syndrome (SARS)8 and the Ebola epidemic.9 During the COVID-19 pandemic, HCWs have to wear PPE unceasingly for more than 6-8 hours in a shift. Moreover, inappropriate PPE reuse (eg, donning of a used PPE item without contamination) due to global PPE shortages remains affecting HCWs and patients’ safety and the sustainability of health care systems.10, 11, 12, 13 Under these circumstances, World Health Organization diffuses recommendations for optimizing PPE use by HCWs caring for suspected or confirmed COVID-19 patients especially in countries with severe PPE shortages.7
Several studies have already shown that adverse reactions from PPE use among HCWs are common including dermatitis, allergy, atopy, facial itch, acne, rash, etc.14, 15, 16, 17, 18 Considering the long-time wearing of PPE among HCWs and PPE shortages during the COVID-19 pandemic, we anticipated a high incidence of physical health problems due to PPE use among HCWs. To our knowledge, the overall impact of PPE use on HCWs’ physical health during the COVID-19 pandemic is unknown. Thus, the primary aim of this systematic review and meta-analysis was to assess the impact of PPE use on HCWs’ physical health during the COVID-19 pandemic. The secondary objective was to examine factors related to a greater risk of adverse events among HCWs due to PPE use.
Methods
Data sources and strategy
We applied the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines19 and the Cochrane criteria20 for this systematic review and meta-analysis. We searched PubMed, Medline, Scopus, ProQuest, CINAHL, and preprint services (medRxiv) from January 1, 2020 to December 27, 2020. Also, we examined reference lists of all relevant articles and we removed duplicates. We applied the following filters during the search in the databases: humans, English language, and journal article. We used the following strategy searching in title/abstract query: (("health care worker*" OR "healthcare worker*" OR "healthcare personnel" OR "health care personnel" OR "health personnel" OR "health care professional*" OR "healthcare professional*" OR staff OR "nursing staff" OR professional* OR worker* OR doctor* OR physician* OR clinician* OR nurs* OR midwives OR midwife* OR paramedic* OR practitioner*) AND ("personal protective equipment")) AND (COVID-19 OR COVID19 OR COVID OR SARS-CoV* OR "Severe Acute Respiratory Syndrome Coronavirus*" OR coronavirus*). The study protocol was registered with PROSPERO (CRD42021228221).
Selection and eligibility criteria
Two independent reviewers performed study selection and discrepancies were resolved by a third, senior reviewer. We initially screened title and abstract of the records and then full-text. We included studies that examine the impact of PPE use on HCWs’ physical health during the COVID-19 pandemic. Also, we included studies examining factors related to a greater risk of adverse events among HCWs due to PPE use. We examined articles that were published in English, except reviews, qualitative studies, protocols, case reports, editorials, and letters to the Editor. All types of HCWs directly involving in the management of COVID-19 patients were accepted for inclusion, while we excluded studies with health care students and the general population. Also, we excluded studies that examined the effects of PPE use on the psychological or mental health of HCWs.
Data extraction and quality assessment
We extracted the following data from each study: authors, location, sample size, age, gender, study design, sampling method, assessment of the adverse events, response rate, data collection time, type of publication (journal or preprint service), number and type of adverse events among HCWs, factors related to a greater risk of adverse events, and the level of analysis (univariate or multivariable).
Two reviewers used the Joanna Briggs Institute critical appraisal tools to assess the quality of studies as poor, moderate, or good.21 Regarding cross-sectional studies, an 8-point scale is used with a score of ≤3 refers to poor quality, a score of 4-6 points refers to moderate quality, and a score of 7-8 points refers to good quality.
Statistical analysis
For each study, we extracted the sample size and adverse events that occurred among HCWs due to PPE use. We initially calculated the prevalence of any adverse event and the 95% confidence interval (CI) for each included study. Then, we transformed these prevalences with the Freeman-Tukey Double Arcsine method before pooling.22 Moreover, we pooled the results for adverse events that occurred among HCWs at least in three studies. We assessed between-studies heterogeneity with the Hedges Q statistic and I2 statistics. I2 values higher than 75% indicate high heterogeneity, while a P-value <.1 for the Hedges Q statistic indicates statistically significant heterogeneity.23 A random effect model was applied to estimate pooled effects since the heterogeneity between results was very high.23 A leave-one-out sensitivity analysis was performed to determine the influence of each study on the overall effect. We used a funnel plot and the Egger's test to assess the publication bias with a P value <.05 indicating publication bias.24 A priori, we considered gender, age, sample size, the continent that studies were conducted, studies quality, study design, assessment of the outcome, data collection time, and publication type (journal or preprint service) as sources of heterogeneity. Due to the limited data and limited variability of some of these variables, we decided to perform meta-regression analysis and subgroup analysis considering gender, sample size, studies quality, and data collection time as sources of heterogeneity. We did not perform a meta-analysis for the factors related to the occurrence of adverse events among HCWs since the data were very limited and highly heterogeneous. We used the OpenMeta[Analyst] to perform meta-analysis.25
Results
Identification and selection of studies
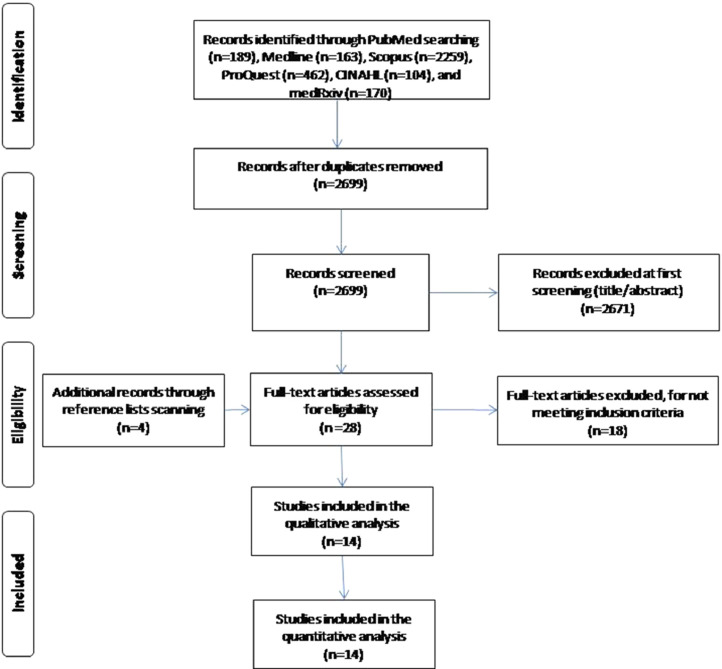
Flowchart of the literature search is summarized in PRISMA format and it is shown in Figure 1 . We initially identified 2,699 potential records through PubMed, Medline, Scopus, ProQuest, CINAHL, and medRxiv removing duplicates. After the screening of the titles and abstracts, we removed 2,671 records and we added 4 more records found by the reference lists scanning. Finally, we included 14 studies26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 in this meta-analysis that met our inclusion criteria.
Fig 1.
Flowchart of the literature search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Characteristics of the studies
Main characteristics of the studies included in our systematic review and meta-analysis are shown in Table 1 . A total of 11,746 HCWs from 16 countries were included in this review. Number of HCWs in studies ranged from 40 to 4,306, while females’ percentage ranged from 46.0% to 91.8%. The majority of studies were conducted in Asia (n = 10),26 , 28 , 30, 31, 32, 33 , 35 , 36 , 38 , 39 2 studies were conducted in Europe,34 , 37 one study was conducted in South America,27 and one study included HCWs from 10 countries.29 All studies were cross-sectional, while 13 studies26, 27, 28, 29, 30 , 32, 33, 34, 35, 36, 37, 38, 39 used a convenience sample method and one study31 used a purposeful sampling method. Assessment of adverse events was self-reported through questionnaires in 13 studies,26, 27, 28, 29, 30, 31, 32, 33, 34, 35 , 37, 38, 39 while in one study36 a clinical diagnosis was performed. All studies were published in journals and seven studies26 , 28 , 29 , 31 , 32 , 38 , 39 reported response rate.
Table 1.
Main characteristics of the studies included in this systematic review
| Reference | Location | Sample size (n) | Age, mean (SD) | Females (%) | Study design | Sampling method | Assessment of the adverse events | Response rate (%) | Data collection time | Publication in |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al. 202026 | China | 960 | 33 (23-43)a | 64.3 | Cross-sectional | Convenience sampling | Self-reported | 27.6 | April 21 to May 15 | Journal |
| Coelho et al. 202027 | Brazil | 1106 | 34.1 (8.9) | 83.6 | Cross-sectional | Convenience sampling | Self-reported | NR | May 15-20 | Journal |
| Çağlar et al. 202028 | Turkey | 315 | 31.6 (4.6) | 50.5 | Cross-sectional | Convenience sampling | Self-reported | 43.4 | August 01 to September 01 | Journal |
| Tabah et al. 202029 | Australia, Italy, United Kingdom, France, Libya, Portugal, Austria, Argentina, Netherlands, Belgium | 2711 | 41 (34-49)a | 46.0 | Cross-sectional | Convenience sampling | Self-reported | 56.0 | March 30 to April 20 | Journal |
| Jiang et al. 202030 | China | 4306 | 32.5 (7.1) | 88.0 | Cross-sectional | Convenience sampling | Self-reported | NR | February 8-22 | Journal |
| Hu et al. 202031 | China | 61 | 20-29 years:26.3%; 30-39 years:67.2%; 40-49 years:4.9%; 50-59:1.6% | 91.8 | Cross-sectional | Purposeful sampling | Self-reported | 93.8 | February | Journal |
| Ong et al. 202032 | Singapore | 158 | 21-40 years:87.3%; >40:12.7% | 70.3 | Cross-sectional | Convenience sampling | Self-reported | 98.7 | February 26 to March 8 | Journal |
| Metin et al. 202033 | Turkey | 526 | 34 (7) | 69.2 | Cross-sectional | Convenience sampling | Self-reported | NR | April 05-12 | Journal |
| Guertler et al. 202034 | Germany | 40 | 32 (6.9) | 52.5 | Cross-sectional | Convenience sampling | Self-reported | NR | April 01-14 | Journal |
| Yildiz et al. 202035 | Turkey | 553 | 20-30 years:62.4%; 31-40 years:23.5%; 41-50 years:12.7%; >50:1.4% | 70.0 | Cross-sectional | Convenience sampling | Self-reported | NR | April 15 to May 15 | Journal |
| Singh et al. 202036 | India | 43 | 32.8 (14.5) | 90.7 | Cross-sectional | Convenience sampling | Clinical diagnosis | NR | March 24 to April 16 | Journal |
| Battista et al. 202037 | Italy | 185 | 32.6 (8.3) | 68.6 | Cross-sectional | Convenience sampling | Self-reported | NR | April 20 to May 04 | Journal |
| Lin et al. 202038 | China | 376 | 32.2 (6.5) | 77.7 | Cross-sectional | Convenience sampling | Self-reported | 37.6 | February 6-11 | Journal |
| Zuo et el. 202039 | China | 404 | NR | 75.2 | Cross-sectional | Convenience sampling | Self-reported | 69.8 | February 01-28 | Journal |
Median (interquartile range).
NR = not reported.
Quality assessment
Quality assessment of cross-sectional studies included in this systematic review is shown in Table 2 . Quality was poor in 9 studies26 , 29 , 31 , 33, 34, 35, 36, 37 , 39 and moderate in 5 studies.27 , 28 , 30 , 32 , 38
Table 2.
Quality of cross-sectional studies included in this systematic review
| Zhao et al. 202026 | Coelho et al. 202027 | Çağlar et al. 202028 | Tabah et al. 202029 | Jiang et al. 202030 | Hu et al. 202031 | Ong et al. 202032 | Metin et al. 202033 | Guertler et al. 202034 | Yildiz et al. 202035 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Were the criteria for inclusion in the sample clearly defined? | Χ | X | X | X | X | |||||
| 2. Were the study subjects and the setting described in detail? | Χ | X | X | X | X | X | X | X | X | |
| 3. Was the exposure measured in a valid and reliable way? | ||||||||||
| 4. Were objective, standard criteria used for measurement of the condition? | ||||||||||
| 5. Were confounding factors identified? | X | X | X | X | ||||||
| 6. Were strategies to deal with confounding factors stated? | X | X | X | X | ||||||
| 7. Were the outcomes measured in a valid and reliable way? | ||||||||||
| 8. Was appropriate statistical analysis used? | X | X | X | X | X | X | X | X | X | X |
| Total quality | Poor | Moderate | Moderate | Poor | Moderate | Poor | Moderate | Poor | Poor | Poor |
| Singh et al. 202036 | Battista et al. 202037 | Lin et al. 202038 | Zuo et el. 202039 | |||||||
| 1. Were the criteria for inclusion in the sample clearly defined? | X | |||||||||
| 2. Were the study subjects and the setting described in detail? | X | X | X | |||||||
| 3. Was the exposure measured in a valid and reliable way? | ||||||||||
| 4. Were objective, standard criteria used for measurement of the condition? | ||||||||||
| 5. Were confounding factors identified? | X | Χ | ||||||||
| 6. Were strategies to deal with confounding factors stated? | X | Χ | ||||||||
| 7. Were the outcomes measured in a valid and reliable way? | X | |||||||||
| 8. Was appropriate statistical analysis used? | X | X | X | Χ | ||||||
| Total quality | Poor | Poor | Moderate | Poor |
Meta-analysis
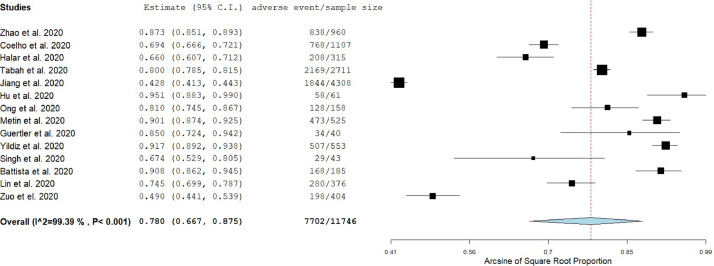
A random effect model was applied to estimate the pooled prevalence of adverse events since the heterogeneity between results was very high (I2 = 99.39, P value for the Hedges Q statistic <0.001). The estimated overall prevalence of adverse events among HCWs was 78% (95% CI: 66.7%-87.5%; Fig 2 ). Prevalence among studies ranged from 42.8%30 to 95.1%.31 A leave-one-out sensitivity analysis showed that no single study had a disproportional effect on the pooled prevalence, which varied between 76.4% (95% CI: 64.5%-86.4%), with Hu et al.31 excluded, and 80.3% (95% CI: 73.8%-86.1%) with Jiang et al.30 excluded (Web Fig 1). A publication bias was potential since P value for Egger's test was <.05 and the shape of the funnel plot was asymmetrical (Web Fig 2).
Fig 2.
Forest plot of the prevalence of adverse events among health care workers.
According to subgroup analysis, the prevalence of adverse events was higher for the studies with poor quality (83.5% [95% CI: 75.4%-90.2%], I2 = 97.64) compared to those with moderate quality (67.1% [95% CI: 50.4%-81.8%], I2 = 99.13). Meta-regression analysis identified that increased sample size was related to decreased prevalence of adverse events among HCWs (P< .001; Web Fig 3). Also, the prevalence of adverse events was independent of the gender distribution (P= .32), and data collection time (P= .63).
Adverse events among HCWs due to personal protective equipment use during COVID-19 pandemic are listed in Table 3 . We pooled the results for adverse events that occurred among HCWs at least in three studies and the results are presented in Table 4 . According to the pooled results, the adverse events that occurred more often were headache (55.9% [95% CI: 35.8%-75.0%]), dry skin (54.4% [95% CI: 25.4%-81.8%]), dyspnoea (53.4% [95% CI: 27.2%-78.6%]), pressure injuries (40.4% [95% CI: 27.7%-53.8%]), itching (39.8% [95% CI: 16.2%-66.3%]), hyperhidrosis (38.5% [95% CI: 15.3%-64.9%]), and dermatitis (31.0% [95% CI: 11.1%-55.5%]).
Table 3.
Adverse events among health care workers due to personal protective equipment use during the COVID-19 pandemic in the studies included in this systematic review
| Reference | Any adverse event | Dry skin | Pressure injuries | Headache | Dermatitis | Allergy | Rash | Itching | Pain |
|---|---|---|---|---|---|---|---|---|---|
| Zhao et al. 202026 | 838 (87.3) | 199 (20.7) | 516 (53.8) | 146 (15.2) | 162 (16.9) | 222 (23.1) | |||
| Coelho et al. 202027 | 768 (69.4) | 768 (69.4) | |||||||
| Çağlar et al. 202028 | 208 (66.0) | 115 (36.5) | 64 (20.3) | ||||||
| Tabah et al. 202029 | 2169 (80.0) | 1088 (40.1) | 696 (25.7) | ||||||
| Jiang et al. 202030 | 1844 (42.8) | 1293 (30.0) | |||||||
| Hu et al. 202031 | 58 (95.1) | 15 (24.6) | 42 (68.9) | 7 (11.5) | 10 (16.4) | 17 (27.9) | 7 (11.5) | ||
| Ong et al. 202032 | 128 (81.0) | 128 (81.0) | |||||||
| Metin et al. 202033 | 473 (90.1) | 473 (90.1) | 379 (72.5) | 100 (19.1) | 100 (19.1) | ||||
| Guertler et al. 202034 | 34 (85.0) | 32 (82.1) | 13 (32.5) | 6 (15.0) | 8 (20.5) | 12 (30) | |||
| Yildiz et al. 202035 | 507 (91.7) | 507 (91.7) | 410 (74.1) | 494 (89.3) | |||||
| Singh et al. 202036 | 29 (67.4) | 16 (37.2) | 11 (25.6) | 27 (62.8) | 17 (39.5) | 3 (7.0) | 21 (48.8) | 29 (67.4) | |
| Battista et al. 202037 | 168 (90.8) | 54 (29.2) | 118 (63.8) | 93 (50.3) | |||||
| Lin et al. 202038 | 280 (74.5) | 258 (68.6) | |||||||
| Zuo et el. 202039 | 198 (49.0) | 47 (11.6) | 76 (18.8) | 50 (12.4) | 60 (14.9) | 13 (3.2) | |||
| Reference | Hyperhidrosis | Dyspnoea | Others | ||||||
| Zhao et al. 202026 | 593 (61.8) | Skin squeeze; 598 (62.3), dizziness; 555 (57.8), maceration; 142 (14.8), conjunctivitis; 61 (6.4), extreme exhaustion; 598 (62.3) | |||||||
| Coelho et al. 202027 | |||||||||
| Çağlar et al. 202028 | 79 (25.1) | ||||||||
| Tabah et al. 202029 | 1266 (46.7) | Extreme exhaustion; 412 (15.2) | |||||||
| Jiang et al. 202030 | Skin tears; 86 (0.2), moisture-associated skin damage; 465 (10.8) | ||||||||
| Hu et al. 202031 | Skin desquamation; 6 (9.9), moisture-associated skin damage; 16 (26.2) | ||||||||
| Ong et al. 202032 | |||||||||
| Metin et al. 202033 | 75 (14.3) | Stomatitis; 100 (19.1), acne; 207 (39.4) | |||||||
| Guertler et al. 202034 | 5 (12.5) | Asthma; 3 (7.5) | |||||||
| Yildiz et al. 202035 | Constipation; 145 (26.2), urine-related problems; 225 (40.7), nutritional disorders; 257 (46.5), sleep disorders; 309 (55.9), dehydration; 439 (79.4) | ||||||||
| Singh et al. 202036 | 37 (86.1) | Acne; 5 (11.6), nausea; 5 (11.6) | |||||||
| Battista et al. 202037 | 136 (73.5) | Sneezing; 92 (49.7), acne; 79 (42.7), panic attack; 17 (9.2), ocular symptoms; 83 (44.9) | |||||||
| Lin et al. 202038 | Maceration; 199 (52.9), erythema; 227 (60.4) | ||||||||
| Zuo et el. 202039 | Discomfort; 90 (22.3), erythema; 51 (12.6), burning; 15 (3.7) |
Values are expressed as n (%).
Values are expressed as n (%).
Table 4.
Meta-analysis for the adverse events among health care workers due to personal protective equipment use during the COVID-19 pandemic
| Adverse event | No. of studies | Pooled prevalence | 95% confidence interval | I2 | P value for the Hedges Q statistic |
|---|---|---|---|---|---|
| Dry skin | 8 | 54.4 | 25.4 – 81.8 | 99.60 | <.001 |
| Pressure injuries | 7 | 40.4 | 27.7 – 53.8 | 99.12 | <.001 |
| Headache | 6 | 55.9 | 35.8 – 75.0 | 99.32 | <.001 |
| Dermatitis | 6 | 31.0 | 11.1 – 55.5 | 99.09 | <.001 |
| Allergy | 5 | 16.4 | 13.2 – 19.8 | 47.76 | .105 |
| Rash | 5 | 21.4 | 15.1 – 28.5 | 90.04 | <.001 |
| Itching | 5 | 39.8 | 16.2 – 66.3 | 97.62 | <.001 |
| Pain | 4 | 35.5 | 0.3 – 88.1 | 99.73 | <.001 |
| Hyperhidrosis | 4 | 38.5 | 15.3 – 64.9 | 98.98 | <.001 |
| Dyspnoea | 3 | 53.4 | 27.2 – 78.6 | 98.81 | <.001 |
Risk factors for adverse events
Eleven studies26, 27, 28, 29, 30, 31, 32, 33 , 35 , 38 , 39 investigated risk factors for adverse events among HCWs due to personal protective equipment use during the COVID-19 pandemic (Table 5 ). Six studies21 , 27 , 28 , 32 , 38 , 39 used multivariable models to eliminate confounding factors, while all studies except one33 measured the occurrence of any adverse event as the dependent variable.
Table 5.
Factors related with a greater risk of adverse events among health care workers due to personal protective equipment use during the COVID-19 pandemic in the studies included in this systematic review
| Reference | Factors | Level of analysis |
|---|---|---|
| Zhao et al. 202026 |
|
Univariate |
| Coelho et al. 202027 |
|
Multivariable |
| Çağlar et al. 202028 |
|
Multivariable |
| Tabah et al. 202029 |
|
Univariate |
| Jiang et al. 202030 |
|
Multivariable |
| Hu et al. 202031 |
|
Univariate |
| Ong et al. 202032 |
|
Multivariable |
| Metin et al. 202033 | Dermatitis
|
Univariate |
| Yildiz et al. 202035 |
|
Univariate |
| Lin et al. 202038 |
|
Multivariable |
| Zuo et el. 202039 |
|
Multivariable |
BMI: body mass index; CI: confidence interval; HCWs: health care workers; OR: odds ratio; PPE: personal protective equipment.
We found that demographic, clinical, and job characteristics were related to the risk of adverse events among HCWs due to PPE use.
Regarding gender, four studies31 , 33 , 35 , 38 found that females had a higher risk of adverse events with ORs ranging from 1.87 to 3.20, while one study30 found the opposite (OR:1.54 for males). A higher proportion of nurses were typically females and this possible confounder could be a reason that females found at higher risk of adverse events. Moreover, four studies27 , 31 , 33 , 35 showed that younger age was associated with increased risk of adverse events, while one study26 showed the opposite. Among HCWs, nurses and physicians were at a greater risk of developing adverse events.26
Several clinical characteristics of the HCWs affected the occurrence of adverse events. In particular, comorbidity such as diabetes mellitus, obesity, pre-existing headache, and smoking significantly increased the risk of adverse events.28 , 32 , 33 Similar, heavy sweating was a risk factor for adverse events.30
We found that job characteristics affected adverse events in a significant way. The longer duration of shifts wearing PPE, the greater the risk of adverse events with ORs ranging from 1.24 to 4.26.27, 28, 29, 30 , 32 , 38 Two studies27 , 38 found that shifts >6 hours was a risk factor, while 2 studies32 , 39 found a different cut-off point of 4 hours. Moreover, increased consecutive days with PPE26 , 28 and higher grade of PPE30 , 39 significantly increased risk of adverse events among HCWs. Our review showed that increased exposure to confirmed or suspected COVID-19 patients,26 , 33 , 38 working in hospitals with a more severe epidemic,38 and no use of prevention inputs27 increased the probability of adverse events.
Discussion
To our knowledge, this is the first systematic review and meta-analysis that investigates the impact of PPE use on HCWs’ physical health during the COVID-19 pandemic. Also, we searched for risk factors related to adverse events among HCWs.
We found that the overall prevalence of adverse events among HCWs was very high (78%) with a wide range from 42.8% to 95.1% among studies. PPE use among HCWs is related to skin reactions such as dermatitis, allergy, atopy, facial itch, acne, rash.14, 15, 16, 17, 18 HCWs wear PPE items for long periods of time due to the shortage of PPE especially at the beginning of the COVID-19 pandemic and the increased workload in healthcare facilities.40 , 41 This scenario increases considerably the risk of adverse events such as skin reactions. The problem is further complicated by the lack of training and awareness among HCWs about the use of PPE.42 , 43 During the COVID-19 pandemic, HCWs have to encounter several challenges regarding PPE such as donning (putting on) and doffing (taking off) equipment in the appropriate way, wearing PPE items for long periods of time, difficulties in communication with patients and colleagues etc.44 , 45 HCWs should undergo compulsory training on the correct use of PPE and guidelines should emphasize on the correct use of PPE using video training and simulations than traditional methods of teaching.7 , 45
According to our results, the most prevalent physical complaint from the use of PPE was headaches. Previous studies confirm that headaches are common among HCWs when the filtering facepiece respirator is used especially for a prolonged period.46, 47, 48 It is well known that headaches could arise from the continuous pressure of pericranial soft issues by putting on objects with tight straps around the head, for example, helmets, hats, goggles.49, 50, 51 Also, breathing discomfort due to filtering facepiece respirator has also been reported in the literature confirming our finding that dyspnoea is a common adverse event among HCWs due to PPE use.52, 53, 54 Α survey among dental professionals during the COVID-19 pandemic found that the prolonged use of filtering facepiece 2 (FFP2) respirators was related to moderate breathing difficulties.55 Moreover, increased levels of anxiety and stress among HCWs during the pandemic56 , 57 may contribute to breathing difficulties.
We found that skin reactions (eg, dry skin, itching, dermatitis, and rash) were the most frequent adverse events that HCWs encountered. While increased use of gloves and filtering facepiece respirators and excessive sanitizing of hands among HCWs are indispensable to prevent transmission of SARS-CoV-2, they also have negative implications leading to a removal of normal bacterial flora and a disruption of the natural protective skin barrier.58, 59, 60 In that case, the frequency and the severity of occupational skin diseases increase.61, 62, 63
Adverse events caused by PPE use are a comprehensive effect with sociodemographic, clinical, and job characteristics as the contributing factors. Regarding the sociodemographic factors, we found that gender, age, and type of occupation affect the impact of PPE use on HCWs’ physical health. The effect of gender and age is controversial. In particular, 4 studies31 , 33 , 35 , 38 found that adverse events are more common among females and one study30 found the opposite. A multicenter survey in China64 found a higher prevalence of pressure injuries in male hospitalized patients while another study with outpatients in Turkey65 found that acne, hand eczema, and urticaria are more common in females and seborrheic dermatitis is more common in males. Differences in hormones, genetic factors, activity levels, hygiene behavior and use of skin care products could explain differences in skin reactions among males and females HCWs. Regarding age, four studies27 , 31 , 33 , 35 found that younger age is related to a greater risk of skin reactions, while one study26 found the opposite. Several studies found that skin reactions are more frequent in young adults.65, 66, 67
According to our review, comorbidity is a risk factor for new-onset symptoms from the PPE use. In particular, obesity, smoking, diabetes mellitus, and pre-existing headache were related to increased odds of adverse events. Obesity and smoking decrease cardiopulmonary capacity causing dyspnea.68 , 69 Obese individuals and smokers could face more symptoms because of the use of filtering facepiece respirators without valve that brings difficulties in breathing. Laferty and McKay70 found that filtering facepiece respirators cause breathing resistance resulting on a decrease in SpO2 and an increase in CO2 levels. Moreover, isolation gowns cover the entire body causing heavy sweating and continuous dehydration especially among smokers and obese individuals. A scoping review55 among dental professionals has revealed moderate breathing difficulties due to the use of filtering facepiece respirators, while the prolonged duration of respirators usage was related to headaches. This finding is confirmed by a study46 that was conducted during the SARS pandemic and found that 37.3% of HCWs who were filtering facepiece respirators developed headaches. This percentage was even higher (81%) in a study32 that was conducted during the COVID-19 pandemic and found also that the odds of headache were 4.2 times higher in HCWs with pre-existing headache than among those without a pre-existing headache. Likelihood of developing headache was greater among HCWs with a long-term utilization of filtering facepiece respirators.32 Prolonged use of filtering facepiece respirators could result in hypercapnia and hypoxemia which led to headache.31
Seven studies27, 28, 29, 30 , 32 , 38 , 39 in our review found that the duration of PPE use is an important risk factor for adverse events among HCWs. The literature comes to an agreement with this finding since Lim et al.46 during the SARS pandemic revealed that the increased duration of filtering facepiece respirator use is related to headaches development, while Shenal et al.48 found a relation between prolonged wear of respiratory protection and discomfort. Also, longer wearing time of filtering facepiece respirators, surgical masks and goggles compress cheeks, ears, nose bridge, and forehead which could be the main cause of skin and pressure injuries on the head and face.71 Additionally, the longer the wearing time of PPE items, the more the sweaty with heavy sweating stimulates the skin causing redness, itching and pain.30 The problem is further complicated by the increased consecutive days with PPE leading to more adverse skin effects among HCWs.26 , 28 Moreover, increased exposure to confirmed or suspected COVID-19 patients is related to increased wearing time of PPE because of the contagiousness of SARS-CoV-2.26 , 33 , 38 In light of the above situations, daily wearing time of PPE items among HCWs should be decreased to protect them and avoid the adverse impact of PPE use.
Filtering facepiece respirators are more likely to cause adverse events such as breathing difficulties, headaches, panic attacks, and pressure related symptoms especially in case of prolonged use.31 , 32 , 37 , 46 , 55 , 70 Also, Ong et al.32 found that face shields cause headaches due to pain, pressure or compression from this PPE, while Battista et al.37 found that face shields cause several symptoms, for example, nasal/facial pain, redness zygoma and nosebridge, and auricular pain. Moreover, head itching was more common among HCWs wearing caps,37 while skin related symptoms (eg, dry skin, itching, and rash) were more frequent in case of HCWs with latex gloves.31
Our study has several limitations introducing bias. First, 10 out of 14 included studies were conducted in Asia and thus further studies should be performed worldwide, allowing us to generalize the results. Also, quality of studies was poor (in 9 studies) or moderate (in 5 studies), while adverse events were more frequent in studies with poor quality compared to those with moderate quality. There is a need to perform more valid studies since studies with poor quality may inflate the results. In the same way, the fact that the assessment of adverse events was self-reported in 13 out of 14 studies may introduce information bias that exaggerates the frequency of adverse events. This bias could be eliminated with a clinical diagnosis of adverse events due to PPE use. Variability in study designs and populations introduces high heterogeneity in our meta-analysis. We applied a random effect model and we performed subgroup and meta-regression analysis to overcome this issue. We searched six databases and the reference lists of the studies included in our review but always there is a probability to omit relevant studies. Data regarding the factors that were related to a greater risk of adverse events were scarce and only 6 studies used multivariable analysis to eliminate confounders. Also, causal inferences between risk factors and adverse events are impossible since all studies were cross-sectional. Thus, studies with more appropriate design (eg, cohort studies and case-control studies) and more sophisticated analysis should be conducted to infer more valid results regarding risk factors for adverse events due to PPE use.
Conclusion
n conclusion, the frequency of adverse events among HCWs due to PPE use is very high, while there are several sociodemographic, clinical and job risk factors for these events. The COVID-19 pandemic continues to threaten public health, and adverse events frequency and severity among HCWs may get worse. PPE among HCWs is imperative to avoid the widespread diffusion of SARS-CoV-2 but could be harmful due to the long-term utilization. Thus, organizations worldwide should publish guidelines for the appropriate PPE use to prevent these adverse events especially in countries with PPE shortages. Healthcare facilities should take the necessary precautions and change the working conditions during the COVID-19 pandemic (eg, regular breaks, shorter shifts, adequate supply of PPE, air-conditioning, prophylactic dressing, better material, proper fitting masks, and reduction in wearing time of PPE) to prevent adverse events associated with PPE use and minimize harm to HCWs. Creating a secure and safe work environment for HCWs could lead to better management of the COVID-19 pandemic and an increase in work performance. Since skin reactions are the more frequent adverse events, policymakers should pay attention to skin hygiene and skin protection including use of skin or sealant protector, protection of injured areas, no use of oily products, wipe of skin to remove sweat, and removal of the masks as frequent as possible. HCWs’ training about appropriate PPE use and knowledge of skin hygiene is of utmost importance. HCWs should recognize symptoms and signs of initial tissue damages adopting then preventive measures to avoid more severe injuries. For example, dry skin and dehydration-induced dermatoses could be avoided with adequate hydration, while moisturizers could help to restore the integrity of skin barrier.
Footnotes
Author contributions: P.G, I.V. and D.K. were responsible for the conception and design of the study. P.G, I.V., D.F., A.B. were responsible for the acquisition, analysis and interpretation of data. All the authors drafted the article or revised it critically for important intellectual content, and provided final approval of the version to be submitted.
Conflicts of interest: None.
Funding: None.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2021.04.084.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.CDC COVID-19 Response Team Characteristics of Health Care Personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477–481. doi: 10.15585/mmwr.mm6915e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kambhampati AK, O'Halloran AC, Whitaker M, et al. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 States, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1576–1583. doi: 10.15585/mmwr.mm6943e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gholami M, Fawad I, Shadan S, et al. COVID-19 and healthcare workers: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:335–346. doi: 10.1016/j.ijid.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID-19 in health care workers – a systematic review and meta-analysis. Am J Emerg Med. 2020;38:1727–1731. doi: 10.1016/j.ajem.2020.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley AL, Stone H, Nguyen PY, Chughtai AA, MacIntyre CR. Estimating the burden of COVID-19 on the Australian healthcare workers and health system during the first six months of the pandemic. Int J Nurs Stud. 2021;114 doi: 10.1016/j.ijnurstu.2020.103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2020. World Health Organization. Rational use of personal protective equipment for COVID-19 and considerations during severe shortages. [Google Scholar]
- 8.Moore D, Gamage B, Bryce E, Copes R, Yassi A. Protecting health care workers from SARS and other respiratory pathogens: organizational and individual factors that affect adherence to infection control guidelines. Am J Infect Control. 2005;33:88–96. doi: 10.1016/j.ajic.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer WA, Hynes NA, Perl TM. Protecting health care workers from Ebola: personal protective equipment is critical but is not enough. Ann Intern Med. 2014;161:753–754. doi: 10.7326/M14-1953. [DOI] [PubMed] [Google Scholar]
- 10.Park C-Y, Kim K, Roth S, Beck S, Kang JW, Tayag MC, et al. 2020. Global Shortage of Personal Protective Equipment amid COVID-19: Supply Chains, Bottlenecks, and Policy Implications. Asian Development Bank. [Google Scholar]
- 11.Cohen J, Rodgers Y, van der M. Contributing factors to personal protective equipment shortages during the COVID-19 pandemic. Prev Med. 2020;141 doi: 10.1016/j.ypmed.2020.106263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowan NJ, Laffey JG. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic – Case study from the Republic of Ireland. Sci Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Gupta P, Jha R. COVID-19: impact on health supply chain and lessons to be learnt. J Health Manag. 2020;22:248–261. [Google Scholar]
- 14.Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non-glove personal protective equipment-related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Derm. 2019;80:217–221. doi: 10.1111/cod.13177. [DOI] [PubMed] [Google Scholar]
- 15.Foo CCI, Goon ATJ, Leow Y-H, Goh C-L. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome ? A descriptive study in Singapore. Contact Derm. 2006;55:291–294. doi: 10.1111/j.1600-0536.2006.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mekonnen TH, Yenealem DG, Tolosa BM. Self-report occupational-related contact dermatitis: prevalence and risk factors among healthcare workers in Gondar town, Northwest Ethiopia, 2018—a cross-sectional study. Environ Health Prev Med. 2019;24:11. doi: 10.1186/s12199-019-0765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Safety equipment: when protection becomes a problem. Contact Derm. 2019;81:130–132. doi: 10.1111/cod.13254. [DOI] [PubMed] [Google Scholar]
- 18.Higgins CL, Palmer AM, Cahill JL, Nixon RL. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Derm. 2016;75:213–222. doi: 10.1111/cod.12616. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group The PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, et al. 2nd ed. WILEY Blackwell; New Jersey: 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 21.dos Santos WM, SR Secoli, Püschel VA de A. The Joanna Briggs Institute approach for systematic reviews. Rev Latino-Am Enfermagem. 2018;26 doi: 10.1590/1518-8345.2885.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Liang W, Luo Y, et al. Personal protective equipment protecting healthcare workers in the Chinese epicentre of COVID-19. Clin Microbiol Infect. 2020;26:1716–1718. doi: 10.1016/j.cmi.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coelho M, Cavalcante VMV, Moraes JT, et al. Pressure injury related to the use of personal protective equipment in COVID-19 pandemic. Rev Bras Enferm. 2020;73 doi: 10.1590/0034-7167-2020-0670. [DOI] [PubMed] [Google Scholar]
- 28.Çağlar A, Kaçer İ, Hacımustafaoğlu M, Öztürk B, Öztürk K. Symptoms associated with personal protective equipment among frontline healthcare professionals during the COVID-19 pandemic. Disaster Med Public Health Prep. 2020;19:1–4. doi: 10.1017/dmp.2020.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabah A, Ramanan M, Laupland KB, et al. Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): An international survey. J Crit Care. 2020;59:70–75. doi: 10.1016/j.jcrc.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Q, Song S, Zhou J, et al. The prevalence, characteristics, and prevention status of skin injury caused by personal protective equipment among medical staff in fighting COVID-19: a multicenter, cross-sectional study. Adv Wound Care. 2020;9:357–364. doi: 10.1089/wound.2020.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu K, Fan J, Li X, Gou X, Li X, Zhou X. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine. 2020;99:e20603. doi: 10.1097/MD.0000000000020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong JJY, Bharatendu C, Goh Y, et al. Headaches associated with personal protective equipment - a cross-sectional study among frontline healthcare workers during COVID-19. Headache. 2020;60:864–877. doi: 10.1111/head.13811. [DOI] [PubMed] [Google Scholar]
- 33.Metin N, Turan Ç, Utlu Z. Changes in dermatological complaints among healthcare professionals during the COVID-19 outbreak in Turkey. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29:115–122. [PubMed] [Google Scholar]
- 34.Guertler A, Moellhoff N, Schenck TL, et al. Onset of occupational hand eczema among healthcare workers during the SARS-CoV -2 pandemic: comparing a single surgical site with a COVID-19 intensive care unit. Contact Derm. 2020;83:108–114. doi: 10.1111/cod.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yildiz C, Kaban H, Tanriverdi FŞ. COVID-19 pandemic and personal protective equipment: evaluation of equipment comfort and user attitude. Arch Environ Occup Health. 2020:1–8. doi: 10.1080/19338244.2020.1828247. [DOI] [PubMed] [Google Scholar]
- 36.Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing Coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:e378–e380. doi: 10.1111/jdv.16628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battista RA, Ferraro M, Piccioni LO, Malzanni GE, Bussi M. Personal Protective Equipment (PPE) in COVID 19 pandemic: related symptoms and adverse reactions in healthcare workers and general population. J Occup Environ Med. 2021;63:e80–e85. doi: 10.1097/JOM.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin P, Zhu S, Huang Y, et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol. 2020;183:190–192. doi: 10.1111/bjd.19089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuo Y, Hua W, Luo Y, Li L. Skin reactions of N95 masks and medial masks among healthcare personnel: a self-report questionnaire survey in China. Contact Derm. 2020;83:145–147. doi: 10.1111/cod.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed J, Malik F, Bin Arif T, et al. Availability of Personal Protective Equipment (PPE) among US and Pakistani doctors in COVID-19 pandemic. Cureus. 2020;12:e8550. doi: 10.7759/cureus.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakim M, Khattak FA, Muhammad S, et al. Access and use experience of personal protective equipment among frontline healthcare workers in Pakistan during the COVID-19 emergency: a cross-sectional study. Health Secur. 2020;19:140–149. doi: 10.1089/hs.2020.0142. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . 2018. Preferred product characteristics for personal protective equipment for the health worker on the frontline responding to viral hemorrhagic fevers in tropical climates. Geneva. [Google Scholar]
- 43.Lakshmi P, Jennifer H.G, Stanly A.M, Mary C. A study on personal protective equipment use among health care providers, Tamil Nadu. Int J Community Med Public Health. 2018;5:1771. [Google Scholar]
- 44.Liu Q, Luo D, Haase JE, et al. The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. Lancet Glob Health. 2020;8:e790–e798. doi: 10.1016/S2214-109X(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwu CJ, Jordan P, Jaca A, Iwu CD, Schutte L, Wiysonge CS. Cochrane corner: personal protective equipment for preventing highly infectious diseases such as COVID-19 in healthcare staff. Pan Afr Med J. 2020;37:148. doi: 10.11604/pamj.2020.37.148.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim ECH, Seet RCS, Lee K-H, Wilder-Smith EPV, Chuah BYS, Ong BKC. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand. 2006;113:199–202. doi: 10.1111/j.1600-0404.2005.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radonovich LJ, Cheng J, Shenal BV, Hodgson M, Bender BS. Respirator tolerance in health care workers. JAMA. 2009;301:36–38. doi: 10.1001/jama.2008.894. [DOI] [PubMed] [Google Scholar]
- 48.Shenal BV, Radonovich LJ, Cheng J, Hodgson M, Bender BS. Discomfort and exertion associated with prolonged wear of respiratory protection in a health care setting. J Occup Environ Hyg. 2012;9:59–64. doi: 10.1080/15459624.2012.635133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahmani Z, Kochanek A, Astrup JJ, Poulsen JN, Gazerani P. Helmet-induced headache among Danish military personnel. Scand J Public Health. 2017;45:818–823. doi: 10.1177/1403494817731417. [DOI] [PubMed] [Google Scholar]
- 50.Krymchantowski AV. Headaches due to external compression. Curr Pain Headache Rep. 2010;14:321–324. doi: 10.1007/s11916-010-0122-x. [DOI] [PubMed] [Google Scholar]
- 51.O'Brien JC. Swimmer's headache, or supraorbital neuralgia. Baylor Univ Med Center Proc. 2004;17:418–419. doi: 10.1080/08998280.2004.11928006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoo K-L, Leng P-H, Ibrahim IB, Lim T. The changing face of healthcare worker perceptions on powered air-purifying respirators during the SARS outbreak. Respirology. 2005;10:107–110. doi: 10.1111/j.1440-1843.2005.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rebmann T, Carrico R, Wang J. Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am J Infect Control. 2013;41:1218–1223. doi: 10.1016/j.ajic.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chughtai AA, Stelzer-Braid S, Rawlinson W, et al. Contamination by respiratory viruses on outer surface of medical masks used by hospital healthcare workers. BMC Infect Dis. 2019;19:491. doi: 10.1186/s12879-019-4109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farronato M, Boccalari E, Del Rosso E, Lanteri V, Mulder R, Maspero C. A scoping review of respirator literature and a survey among dental professionals. IJERPH. 2020;17:5968. doi: 10.3390/ijerph17165968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W-R, Wang K, Yin L, et al. Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychother Psychosom. 2020;89:242–250. doi: 10.1159/000507639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elkholy H, Tawfik F, Ibrahim I, et al. Mental health of frontline healthcare workers exposed to COVID-19 in Egypt: a call for action. Int J Soc Psychiatry, in press. [DOI] [PubMed]
- 58.Warner RR, Boissy YL, Lilly NA, et al. Water disrupts stratum corneum lipid lamellae: damage is similar to surfactants. J Invest Dermatol. 1999;113:960–966. doi: 10.1046/j.1523-1747.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- 59.de Almeida e Borges LF, Silva BL, Gontijo Filho PP. Hand washing: changes in the skin flora. Am J Infect Control. 2007;35:417–420. doi: 10.1016/j.ajic.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Khosrowpour Z, Ahmad Nasrollahi S, Ayatollahi A, Samadi A, Firooz A. Effects of four soaps on skin trans-epidermal water loss and erythema index. J Cosmet Dermatol. 2019;18:857–861. doi: 10.1111/jocd.12758. [DOI] [PubMed] [Google Scholar]
- 61.Hamnerius N, Svedman C, Bergendorff O, Björk J, Bruze M, Pontén A. Wet work exposure and hand eczema among healthcare workers: a cross-sectional study. Br J Dermatol. 2018;178:452–461. doi: 10.1111/bjd.15813. [DOI] [PubMed] [Google Scholar]
- 62.Visscher MO, Randall Wickett R. Hand hygiene compliance and irritant dermatitis: a juxtaposition of healthcare issues. Int J Cosmet Sci. 2012;34:402–415. doi: 10.1111/j.1468-2494.2012.00733.x. [DOI] [PubMed] [Google Scholar]
- 63.Malik M, English J. Irritant hand dermatitis in health care workers. Occup Med (Lond) 2015;65:474–476. doi: 10.1093/occmed/kqv067. [DOI] [PubMed] [Google Scholar]
- 64.Jiang Q, Li X, Qu X, et al. The incidence, risk factors and characteristics of pressure ulcers in hospitalized patients in China. Int J Clin Exp Pathol. 2014;7:2587–2594. [PMC free article] [PubMed] [Google Scholar]
- 65.Bilgili ME, Yildiz H, Sarici G. Prevalence of skin diseases in a dermatology outpatient clinic in Turkey. A cross-sectional, retrospective study. J Dermatol Case Rep. 2013;7:108–112. doi: 10.3315/jdcr.2013.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeichner JA, Baldwin HE, Cook-Bolden FE, Eichenfield LF, Fallon-Friedlander S, Rodriguez DA. Emerging issues in adult female acne. J Clin Aesthet Dermatol. 2017;10:37–46. [PMC free article] [PubMed] [Google Scholar]
- 67.Romero FR, Haddad GR, Miot HA, Cataneo DC. Palmar hyperhidrosis: clinical, pathophysiological, diagnostic and therapeutic aspects. An Bras Dermatol. 2016;91:716–725. doi: 10.1590/abd1806-4841.20165358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med. 2017;108:212–228. doi: 10.23736/S0026-4806.17.05022-4. [DOI] [PubMed] [Google Scholar]
- 69.Mukamal KJ. The effects of smoking and drinking on cardiovascular disease and risk factors. Alcohol Res Health. 2006;29:199–202. [PMC free article] [PubMed] [Google Scholar]
- 70.Laferty E, McKay R. Physiologic effects and measurement of carbon dioxide and oxygen levels during qualitative respirator fit testing. J Chem Health Saf. 2006;13:22–28. [Google Scholar]
- 71.Tan KT, Greaves MW. N95 acne. Int J Dermatol. 2004;43:522–523. doi: 10.1111/j.1365-4632.2004.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.