SUMMARY
The ancient, dynamic, and multifaceted functions of the mitochondrial network are essential for organismal homeostasis and contribute to numerous human diseases. As central hubs for metabolism, ion transport, and multiple macromolecular synthesis pathways, mitochondria establish and control extensive signaling networks to ensure cellular survival. In this review, we explore how these same mitochondrial functions also participate in the control of regulated cell death (RCD). We discuss the complementary essential mito-chondrial functions as compartments that participate in the production and presentation of key molecules and platforms that actively enable, initiate, and execute RCD.
Discussions on the involvement of mitochondria in regulated cell death (RCD) usually focus on how they are permeabilized or damaged to produce an irreversible juncture that pushes a stressed cell to die. But is there more? Should a mechanistic discussion about RCD begin, rather than end, with the contributions of mitochondria? Millions of years ago, following the engulfment of the protomitochondrion by a protoeukaryote, these organelles lost most of their genome; yet, they maintained the machinery for oxidative metabolism (Zimorski et al., 2014). In parallel, mitochondria supported a myriad of unique functions that may have established the cellular underpinnings for interconnected RCD mechanisms.
In this review, we examine the mechanistic connections between mitochondrial biology and RCD in a broad context. Our goal here is to parse mitochondrial biology by suborganellar compartments and relevant signaling pathways and to highlight their impact within diverse RCD mechanisms. We aim to move the focus away from the concept that mitochondria merely serve as downstream targets of RCD signaling (Bock and Tait, 2020). Indeed, as we will discuss, they participate in the production and presentation of key macromolecules and platforms that actively enable, initiate, and execute RCD. Of course, mitochondrial biology is complex and captures macromolecular synthesis, sorting, and assembly—all of which are essential to the underpinnings of a functional organelle and homeostasis. But here we strive to go beyond these elemental components to discuss the mechanistic contributions of mitochondria to RCD signaling. Starting with the matrix and working outward, we present concepts and ideas—supported by literature and interpretations—that highlight the need to more closely investigate how mitochondrial biology contributes to the cellular mechanisms that dictate RCD. Since mitochondrial biology is cross-disciplinary, it is not possible to discuss every aspect within the scope of this review. We have therefore omitted certain topics, such as mitochondrial DNA maintenance and organelle quality control (we point the interested reader to recent reviews on these topics, such as Anderson and Haynes, 2020; Lawless et al., 2020.)
THE FUNDAMENTALS OF MITOCHONDRIAL BIOLOGY
When considering mitochondrial contributions to cell biology, it is essential to appreciate mitochondrial architecture as a key feature that dictates their function (Figures 1 and 2). Mitochondria are double-membrane organelles, and the two proteolipid membranes, the outer and inner mitochondrial membranes (OMM and IMM, respectively) establish at least five compartments that provide unique biochemical environments. Starting from outside a mitochondrion and moving inwards, these compartments include the OMM, IMS (intermembrane space), IMM, cristae, and matrix (Jakobs et al., 2020; Scheffler, 2008).
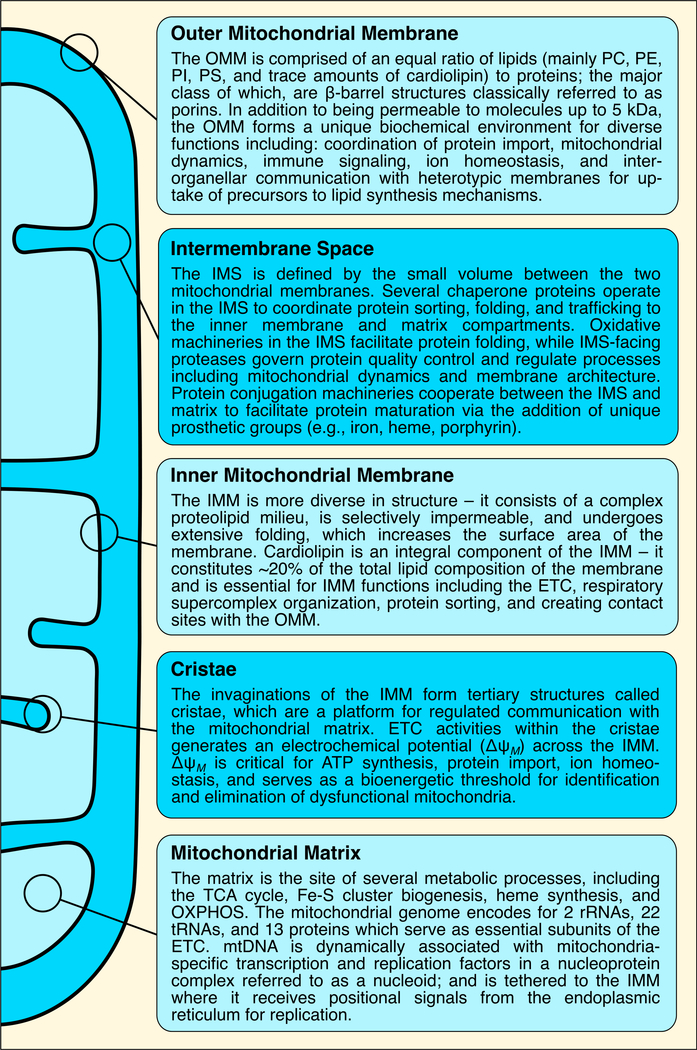
Figure 1. Definitions of the mitochondrial compartments.
A summary of the fundamental contributions of each mitochondrial compartment to mitochondrial biology and function. Abbreviations: ETC, electron transport chain; IMM, inner mitochondrial membrane; IMS, intermembrane space; mtDNA, mitochondrial DNA; OMM, outer mitochondrial membrane; OXPHOS, oxidative phosphorylation; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; TCA, tricarboxylic acid.
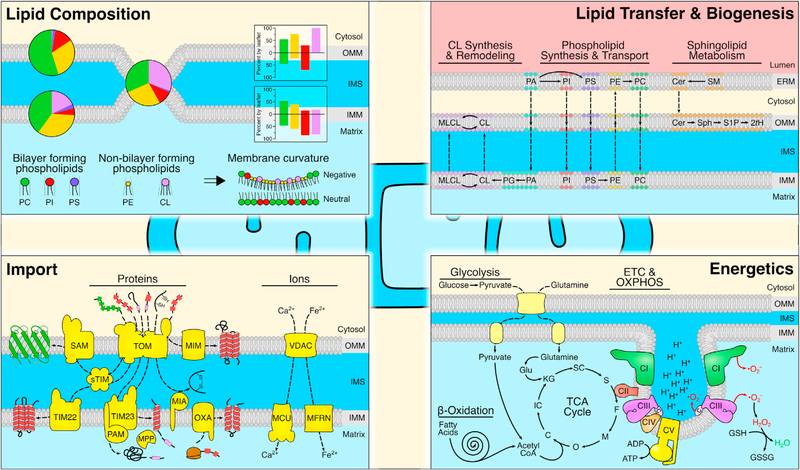
Figure 2. Fundamental mitochondrial functions and pathways.
(Upper left) Lipid composition of the mitochondrial membranes dictates organelle function. Major bilayer forming phospholipids (PC, PI, PS), along with additional lipid species (PE, CL), are differentially represented in the OMM and IMM (pie charts), and asymmetric between inner and outer leaflets of each membrane (histograms). The OMM/IMM contact site generates an additional unique lipid environment. Lipid composition and membrane contact sites contribute to overall membrane curvature. (Upper right) Structural and signaling lipids originate from the ER membrane (ERM; or other organelles—e.g., lysosomes) and are transferred to mitochondria where resident enzymes increase lipid diversity. Solid and dashed arrows denote reactions and transport, respectively. (Bottom left) Protein and ion import into mitochondria are mediated by a diverse system of specific carriers, enzymes, and chaperones, which deliver clients into the correct compartments. (Bottom right) Mitochondrial bioenergetics are based on the import of substrates into the mitochondrial matrix, which hosts several metabolic pathways (TCA cycle, beta-oxidation of fatty acids, and glutaminolysis) that provide essential cofactors to the ETC. Complexes I, III, and IV pump protons into the IMS, thus establishing ΔψM for CV-mediated ATP generation. Oxygen radicals from the ETC may be scavenged by GSH to create GSSG and H2O. Abbreviations: 2tH, 2-trans-hexadecenal; ADP/ATP, adenosine di-/tri-phosphate; C, citrate; CI–CV, complex I–V; Cer, ceramide; CL, cardiolipin; ETC, electron transport chain; F, fumarate; Glu, glutamate; GSH/GSSG, reduced/oxidized glutathione; IC, isocitrate; KG, α-ketoglutarate; M, malate; MCU, mitochondrial calcium uniporter; MFRN, mitoferrin; MIA, mitochondrial IMS assembly; MIM, mitochondrial import machinery 1-containing complex; MLCL, monolysocardiolipin; MPP, mitochondrial processing peptidase; O, oxaloacetate; OXA, oxidase assembly; OXPHOS, oxidative phosphorylation; PA, phosphatidic acid; PAM, presequence translocase-associated import motor; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; S, succinate; S1P, sphingosine-1-phosphate; SAM, sorting and assembly machinery; SC, succinyl-CoA; SM, sphingomyelin; Sph, sphingosine; sTIM, small TIM; TIM, translocase of the inner membrane; TOM, translocase of the outer membrane; VDAC, voltage-dependent anion channel.
The IMM and OMM are composed of unique lipids and proteins that permit the localization and function of various carriers, channels, and sorting machineries that position the metabolic enzymes, assembly factors, transcriptional complexes, and translational components that ensure organelle homeostasis (Friedman and Nunnari, 2014; Giacomello et al., 2020). The IMM and OMM create a compartment referred to as the IMS, which biochemically tethers IMM/OMM functions through the coordinated activity of protein translocation, folding, and posttranslational events. The IMM is organized into structures referred to as cristae, which are the primary site of electron (e−) transport by the respiratory complexes (CI–CIV; the e− transport chain, [ETC]) and ATP synthesis (F0F1-ATP Synthase/CV). Cristae are dynamic subcompartments of the IMM that increase surface area and tightly assemble the mitochondrial complexes into efficient ATP-generating machines. Finally, the matrix is a complex mixture of proteins and enzymes that consist of the tricarboxylic acid cycle (TCA) and that mediate oxidative phosphorylation (OXPHOS), and many other metabolic processes. The matrix also hosts the mitochondrial genome (mtDNA), which is dynamically associated with mitochondria-specific transcription and replication factors in a nucleoprotein complex referred to as a nucleoid. mtDNA-encoded proteins are directly integrated within the respiratory complexes and are essential for efficient ATP generation; mutations/deletions in mitochondrially encoded genes lead to stress accumulation and to decreased energy production (Patananan et al., 2016).
ATP generation through the establishment of a proton gradient and electrochemical potential (ΔψM) across the IMM is both a chief function and key sentinel of the mitochondrial network. While the proton gradient provides a means to convert ADP and Pi to ATP, ΔψM is broadly required for both ion homeostasis and protein import into the mitochondrial network (Song et al., 2021). More than a thousand proteins are translated on cytosolic ribosomes and destined for mitochondrial localization; these proteins support all intra-mitochondrial metabolism (e.g., lipid synthesis and Fe–S cluster formation). Not only are ΔψM and protein import coupled for organelle integrity, but aberrations in either process are sentinels for mitochondrial quality control signaling (Anderson and Haynes, 2020; Pickles et al., 2018). While establishing the biochemical compartments that drive ΔψM, protein import, and the interconnected metabolic pathways for biosynthesis and signaling, mitochondria also communicate with each other. The notion of static, independent organelles is archaic; mitochondria undergo dynamic processes of fusion and division to equilibrate membranes and macromolecules within the mitochondrial network (Gao and Hu, 2021). These processes are referred to as mitochondrial dynamics and require a series of large GTPases such as dynamin-related protein 1 (DRP1) that are either soluble and translocate to the OMM to initiate mitochondrial division or are membrane-anchored to promotefusion, e.g., mitofusins (MFN1 and 2). Not onlyisthismachinery essential for mitochondrial dynamics; it also appears to function in parallel with the tethering of complexes that communicate with other organelles, including the endoplasmic reticulum (ER) and lysosome (Lackner, 2019).
REGULATED-CELL-DEATH PATHWAYS
In this review, we consider ten RCD pathways, each of which has signal transduction networks, regulatory aspects, and death initiation and/or execution phases that appear to be reliant on key aspects of mitochondrial biology (Galluzzi et al., 2018). These pathways are: (1) apoptosis—the death-receptor “extrinsic” pathway; (2) apoptosis—the mitochondrial “intrinsic” pathway; (3) entosis; (4) ferroptosis; (5) mitochondrial permeability transition-driven necrosis; (6) necroptosis; (7) NETotic cell death, (8) oxytosis, (9) parthanatos, and (10) pyroptosis (see Table 1 and Box 1). In our discussions of RCD, there are three critical points to recognize. First, multiple RCD pathways allow for a cell to die following physiological (e.g., developmental) or stress-induced (e.g., infection) signals. Second, prodeath signals are rarely “pure inducers” of one particular RCD pathway; therefore, clear delineations of mechanisms and mitochondrial contributions are often perplexing (Green, 2019). Third, few RCD pathways are thoroughly examined with biochemical, cellular, and genetic models to establish widely accepted mechanisms (e.g., apoptosis). As such, recently described RCD pathways (e.g., ferroptosis and entosis) require an open mind when considering both the limited mechanistic insights and how to interpret this information within a mitochondrial context.
Table 1.
Key aspects of regulated cell-death pathways
| RCD | Inducer | Mediator | Amplifier | Effector | Phenotype |
|---|---|---|---|---|---|
| Apoptosis (extrinsic) | DRs | DISC | CASP-8/-10 (sometimes MOMP) | CASP-3/-6/-7 | organelle breakdown, condensed nucleus, cleaved DNA, apoptotic bodies |
| Apoptosis (intrinsic) | DNA damage, metabolic stress | BCL-2 family | MOMP, apoptosome, CASP-9 | CASP-3/-6/-7 | organelle breakdown, condensed nucleus, cleaved DNA, apoptotic bodies |
| Entosis | loss of cell-matrix interactions | Cell-cell junctions | actin, actomyosin | LAP machinery | engulfment by non-phagocytic neighbor |
| Ferroptosis | cystine starvation, xc− inhibition | GSH depletion, GPX4 exhaustion | LOXs, ROS | PUFA peroxides, aliphatic aldehydes | necrotic, mitochondria alterations (shrinkage, rupture, disordered cristae) |
| MPT-Driven necrosis | cytosolic Ca2+, oxidative damage | mPTP containing cyclophilin D | increased IMM permeability | OMM rupture | necrotic, mitochondrial swelling and rupture |
| NETosis | activation-induced ROS production | NADPH oxidase complex, ROS, H2O2 | MPO | elastase | DNA decondensation, membrane degradation, NET release |
| Necroptosis | DRs, PRRs | DISC/RIP1, TRIF, inhibited CASPs | RIP3 | MLKL pore, ATP depletion? | necrotic (cell swelling, membrane rupture, DAMP release) |
| Oxytosis | glutamate overload, cystine starvation | GSH depletion, GPX4 deficiency | LOXs, ROS, Ca2+ influx | calpains, AIF, PUFA peroxides | necrotic, mitochondria alterations (shrinkage, rupture, disordered cristae) |
| Parthanatos | DNA damage, ROS, increased Ca2+ | PARP1 | PAR polymers, calpains | AIF | large-scale DNA fragmentation, mitochondrial permeability |
| Pyroptosis | intracellular PRRs | inflammasome | CASP-1, CASP-4/-5/-11 | gasdermins | chromatin condensation, cellular swelling, PM permeabilization |
Common inducers, key molecular and mechanistic hallmarks, and characterizations of distinct phenotypes of each RCD pathway. For more detailed definitions of these pathways, see Box 1. Abbreviations: AIF, apoptosis-inducing factor; BCL-2, B cell lymphoma 2; CASP, caspase; DAMP, damage-associated molecular patterns; DISC, death-inducing signaling complex; DR, death receptor; GPX4, glutathione peroxidase 4; GSH, glutathione; ICD, immunogenic cell death; IMM, inner mitochondrial membrane; LAP, LC3-associated phagocytosis; LOX, lipid oxidase; MLKL, mixed lineage kinase domain-like protein; MOMP, mitochondrial outer membrane permeabilization; MPO, myeloperoxidase; MPT, mitochondrial transition pore; NET, neutrophil extracellular traps; OMM, outer mitochondrial membrane; PARP1, poly(ADP-ribose) polymerase 1; PM, plasma membrane; PRR, pattern recognition receptors; PTP, permeability transition pore; PUFA, poly-unsaturated fatty acid; RIP, receptor interacting protein; ROS, reactive oxygen species; TLR, Toll-like receptor; TRIF, TIR-domain-containing adapter-inducing interferon-β.
Box 1. Definitions of regulated cell-death pathways.
Apoptosis (extrinsic pathway): cells engage this pathway of apoptosis in response to extracellular ligands binding to “death receptors” on the cell surface (e.g., TNFR1, Fas, TRAIL). Oligomerized receptors recruit adaptor proteins and form intracellular signaling complexes (i.e., DISC). Recruited initiator caspases-8 (or −10) undergo proximity-mediated cleavage and activation and perpetuate the proapoptotic signal by activating executioner caspases. In some cell types, initiator caspases engage BCL-2 family proteins to induce MOMP and amplify the caspase cascade.
Apoptosis (intrinsic pathway): prolonged cell stress (e.g., DNA damage, metabolic stress, macromolecular damage) signals through this pathway of apoptosis. Signal transduction is mediated by the BCL-2 family of proteins resulting in MOMP and release of apoptogenic factors from the mitochondrial IMS into the cytosol (e.g., cytochrome c, SMAC). Following MOMP, the assembled apoptosome recruits and activates initiator caspase-9 to induce the caspase cascade and cellular dismantling.
Entosis: epithelial cells undergoing this type of RCD are internalized and cannibalized by nonphagocytic neighbor cells. Entosis is triggered by disrupted cell-matrix interactions (e.g., integrin signaling), mediated through cadherin- and catenin-containing junctions between cells and driven by actin and actomyosin. Engulfed cells are eliminated using a specific autophagy-related process called LC3-associated phagocytosis and degraded in endolysosomes.
Ferroptosis: an iron-dependent pathway of RCD that is characterized by severe lipid peroxidation mediated by PUFA-targeted LOXs and is inhibited by iron chelators or lipophilic antioxidants. Primarily characterized in oncogenic RAS models, ferroptosis requires glutathione depletion, which can be induced via inhibition of GPX4, the cystine/glutamate transporter (xc−), or indirectly through cystine starvation. Ferroptotic cells manifest as a necrotic phenotype with mitochondrial alterations (shrinkage, disorganized cristae, electron-dense ultrastructure, rupture of the OMM).
MPT-driven necrosis: the sudden increased permeability of the IMM to small solutes results in ΔψM dissipation, respiratory dysfunction, and osmotic breakdown of mitochondrial membranes during this RCD. It is suggested that permeability is mediated by a cyclophilin D-containing pore complex at the junctions between IMM and OMM, but the identification of additional essential contributors remains elusive. MPT is induced by severe oxidative damage or cytosolic Ca2+ and has been suggested to involve several solute carriers.
Necroptosis: death receptors (e.g., TNFR1, Fas) or PRRs (e.g., TLR3, TLR4, ZBP1) can initiate this RCD when caspases are inhibited. Signaling is mediated by RIP3 activation, which catalyzes the phosphorylation of MLKL to form oligomeric membrane pores resulting in a necrotic phenotype (e.g., loss of plasma membrane integrity, release of intracellular macromolecules) with an ensuing inflammatory response. It is widely believed that MLKL pores in the plasma membrane execute necroptosis by disrupting ion homeostasis and osmotic pressure resulting in membrane rupture.
NETosis: in response to pathogenic insult, neutrophils engage this RCD resulting in cellular lysis and extrusion of neutrophil extracellular traps (NETs), a meshwork of chromatin, histones, and proteins from the cytoplasm and granules. Formation of NETs is initiated by activation-induced ROS production, enzymatic decondensation of DNA, and degradation of intracellular membranes. NETs support host-defense by limiting spread of pathogens (e.g., immobilization, inactivation, aggregation) and inducing inflammation (via NET-containing DAMPs).
Oxytosis: an RCD pathway in cells of the CNS that mechanistically overlaps with ferroptosis (characterized by mitochondria dysfunction, ROS production, and lipid peroxidation). Glutathione depletion, caused by glutamate overload, cystine starvation, or direct inhibition of cystine/glutamate transporter (xc−), results in GPX4 deficiency and subsequent LOX-mediated PUFA peroxidation. Unlike ferroptosis, oxytosis is believed to include cGMP-induced Ca2+ influx and subsequent activation of Ca2+-dependent enzymes; however, this distinction may be cell type-dependent.
Parthanatos: an RCD pathway defined by hyperactivation of PARP-1, subsequent depletion in NAD+ and ATP during PAR polymer formation, and downstream mitochondrial defects including ΔψM dissipation. Parthanatos is executed by AIF, which is bound by PARP-1-generated polymers resulting in translocation to the nucleus and large-scale DNA fragmentation. AIF proteins may be activated by additional mechanisms and have a role in other RCD pathways.
Pyroptosis: in response to cellular infection, non-apoptotic caspases (canonically caspase-1, but caspases-4, −5, and −11 have also been implicated) mediate this form of RCD. PRR signaling leads to formation of the inflammasome (e.g., NLRP3, NLRC4), activation of caspase-1, and cleavage-mediated activation of gasdermins, which form pores in the plasma membrane. Additionally, caspase-1 activation results in cytokine secretion (IL-1β and IL-18) which has autocrine and paracrine consequences within innate immunity.
THE MATRIX
Cellular mechanisms of amino-acid transport, redox, and iron metabolism are ancient systems that converge within the mitochondrial matrix. Stressors that directly impinge on these systems are likely to signal for cellular demise via the same homeostatic mechanisms that are necessary to maintain life. One example of this scenario is ferroptosis, which activates following the inhibition of system cystine/glutamate transporter (xc−) or direct cystine depletion (Dixon et al., 2012). The function of system xc− is to exchange cystine for glutamate, which is essential for providing cysteine in glutathione (GSH) biosynthesis. As system xc− is a cystine-glutamate antiporter, TCA-mediated loss of glutamate production via mitochondrial glutaminase 2 may stall system xc− function, along with reducing the mitochondrial capacity to fuel OXPHOS, leading to enhanced reactive oxygen species (ROS) production. In parallel, cells deliver iron to the mitochondrial network via cooperation between transferrin and its plasma-membrane-bound receptor (Sheftel et al., 2007). Once iron is released into the mitochondrial network, it is utilized for the synthesis of heme and Fe-S cluster formation, which cooperate to create a functioning ETC and ΔψM.
By definition, ferroptosis is a cell-death mechanism that is characterized by the requirement for iron-dependent lipid peroxidases that directly compromise cellular membranes, leading to death. When cells no longer have access to cystine, due to either loss of glutamate or the pharmacological inhibition of system xc− with erastin, ΔψM substantially increases. As a consequence, cells exhaust their GSH pool, resulting in enhanced mitochondrial ROS, abrogated redox maintenance, and unopposed lipid oxidation. On the flip side, ferroptosis susceptibility and inhibition are mediated by glutathione peroxidase 4 (GPX4), which is an antioxidant enzyme that mitigates ferroptosis by reducing oxidized lipids and oxygen radicals generated by CI and CIII (Yang et al., 2014). Together, the pro- and antiferroptotic signaling machinery co-exist within the matrix, and agents that selectively inhibit either component give rise to expected cellular outcomes. There are likely to be broad differences in the extent of matrix contributions to ferroptosis sensitivity within a given cell type. For example, cardiomyocytes deficient in apoptosis and necroptosis signaling robustly activate ferroptosis, which is readily blocked by mitochondria-targeted antioxidants. This contrasts with pancreatic-cancer cells, which demonstrate minimal protection with similar treatment (Fang et al., 2019; Kasukabe et al., 2016). Differences between iron metabolism/storage also influence ferroptosis susceptibility, as mitochondrial ferritin, which is highly expressed in tissues that robustly consume O2, prevents ferroptosis in neuroblastoma cells (Wang et al., 2016). These data suggest that mitochondria control ferroptosis based on the signals they produce (i.e., ROS) and regulate (i.e., iron storage). However, a substantial body of literature has demonstrated that mitophagy-induced mitochondrial depletion does not impact the ability of RAS-driven tumor cells to undergo ferroptosis; of note, these cells exhibited increased sensitivity to GPX4-targeting inducers and decreased sensitivity to methods that target xc−. This might reflect a nonessential contribution of mitochondria to ferroptosis (Gaschler et al., 2018). A molecularly similar RCD pathway, termed oxytosis, was identified prior to ferroptosis in CNS cells subjected to glutamate overload or cystine withdrawal (Lewerenz et al., 2018). While these inducers of oxytosis affect system xc− and cellular GSH (similar to erastin-induced ferroptosis), and are suggestive of overlapping pathways, there is a debate as to whether the two pathways are one and the same. Of particular note, oxytosis and ferroptosis are studied and characterized in different cellular models, and these studies have come to different conclusions regarding how they are influenced by mitochondria. This debate may reveal that the role of mitochondria in ferroptosis differs in RAS-driven tumor cells compared with cells with higher metabolic requirements (Lewerenz et al., 2018).
Another ancient mitochondrial-signaling mechanism that originates from the matrix is based on ion transport, and this pathway centers on calcium (Ca2+). Decades of effort have revealed how Ca2+ is imported, stored, and participates in RCD, and we will discuss these topics in the relevant sections. Here, our focus is on the matrix, where Ca2+ originating from the ER is readily transported across the IMM via the mitochondrial Ca2+ uniporter (MCU) (Marchi et al., 2020). Matrix Ca2+ levels influence several cellular processes, including three mitochondrial dehydrogenases (i.e., pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase) that fuel the TCA leading to NADH production, OXPHOS, and NADPH. There is also evidence that Ca2+-mediated activation of CV enhances the proton motive force supporting ATP generation. When Ca2+ overloads the matrix, this enables of a large channel to open, which is known as the mitochondrial permeability transition pore (mPTP) (Baines et al., 2005). The mPTP transverses the IMM and releases small peptides and solutes into the IMS (and beyond), which causes mitochondrial ROS and ΔψM loss (Izzo et al., 2016). mPTP activation impacts on ischemia-reperfusion injury in heart and skeletal muscle but extends into the apoptosis and necroptosis literature in the presence and absence of caspase activity, respectively (Lemasters et al., 1999). In parallel, high Ca2+ also interacts with cardiolipin (CL) to promote CII disassembly, ROS, and apoptosis (Petrosillo et al., 2006). These data suggest that mitochondrial [Ca2+] must be properly controlled as the signal produced by Ca2+ levels to initiate death is not “on” or “off” but results from the hyperaccumulation and gain-of-function of an otherwise prosurvival signaling component. Indeed, it appears that numerous proteins and lipids in the matrix have evolved or maintained Ca2+-sensing properties that are revealed when [Ca2+] exceeds levels compatible with survival. Multiple forms of RCD may be triggered in this setting, depending on cell type and the composition of RCD machineries.
Necroptosis has been described to have fundamental roles in matrix functions but there is no agreement as to whether they are essential or not. Tumor necrosis factor (TNF)-mediated necroptosis is associated with ETC dysfunction and ROS bursts; the use of antioxidants (e.g., BHA) and loss-of-function models of NADPH oxidases have suggested mechanistic contributions (Kim et al., 2007; Zhang et al., 2017). However, further analyses have revealed that BHA has alternative activities, such as altering the ETC, which complicates interpretations. The contributions of mitochondrial ROS to necroptosis have also been debated, but TNF-induced necroptosis is attenuated by mPTP inhibition in cultured cells and in zebrafish [refs?]. Little is known about how the necroptosis machinery and mitochondria communicate, but RIP1/RIP3/MLKL may interact with the mitochondrial divi-sion machinery, as well as with glutamate dehydrogenase I, py-ruvate dehydrogenase, and/or B cell lymphoma 2 (BCL-2) family members (Wang et al., 2012; Yang et al., 2018). Despite the above, several studies report evidence that argues against mito-chondrial contributions. The use of cell lines deficient for mito-chondria or Drp1−/− revealed that mitochondria have no influ-ence on necroptosis (Moujalled et al., 2014; Tait et al., 2013). Yet, other evidence has indicated that Ca2+- and sepsis-induced RIP3 activation leads to decreased ΔψM, ETC loss, enhanced ROS, and eventual ATP decline in an MLKL-independent manner; preventing these changes preserved survival (Sun et al., 2017; Sureshbabu et al., 2018). Perhaps these data reveal that mitochondria have the potential to receive/transduce pronecroptotic signals when they are evaluated using specific inducers and primary cell models. As such, reaching generalized conclusions from studying canonical necrosome components/signaling in transformed cell lines may not be prudent.
Matrix functions are intimately linked with the maintenance and utilization of mtDNA, the direct role of which in RCD is highlighted by NETosis. Neutrophils utilize NETosis to trap and kill extracellular pathogens (Papayannopoulos, 2018). Since its initial description, NETosis has been linked to ROS bursts and to DNA release, but the contributions of mitochondria to this RCD pathway have remained obscure. More recent studies have revealed that NETosis activation promotes increased mitochondrial respiration and ROS generation, which stimulate the positioning of mitochondria to the plasma membrane, and the extracellular extrusion of mtDNA (Itagaki et al., 2015; Lood et al., 2016). Mitochondrial ROS is also activated by intracellular Ca2+, which is thought to promote mPTP opening, mitochondrial swelling, and commitment to NETosis (Vorobjeva et al., 2020). In systemic lupus erythematosus, an additional relationship between mitochondrial ROS and mtDNA release has been revealed with the discovery that oxidized mtDNA is a more potent inducer of proinflammatory cytokines (Lood et al., 2016; Zhong et al., 2018). In essence, mtDNA oxidation, perhaps due to passive ETC inefficiency or the direct hyperactivation of ROS, likely balances the physiological (i.e., pathogen clearance) and pathological (i.e., autoimmunity) outcomes of NETosis. A means to mitigate against ROS production from these situations is controlled by the mitochondrial sirtuin, Sirt3. Sir3 deacetylates and activates the enzyme manganese super oxide dismutase (MnSOD) to directly degrade ROS within the matrix. This pathway is implicated in organismal homeostasis (i.e., longevity), multiple diseases (e.g., cancer metastasis), and therapeutics (i.e., cardiovascular disease) with links to RCD in each scenario (Kenny et al., 2019)
THE INNER MITOCHONDRIAL MEMBRANE AND CRISTAE
The “powerhouse” aspects of mitochondrial biology are always related to the IMM, while the “gates of death” are uniquely positioned away at the OMM. In this section, we propose that these distinctions may not be entirely correct. While there are numerous synthetic, metabolic, and sorting machineries within the IMM that are crucial for powering a cell, we propose that the IMM also controls death in at least three ways: (1) via oxidized mitochondrial lipids that influence RCD signaling; (2) via ETC assembly/functions that alter cellular stress pathways; and (3) via dynamic IMM components that establish the RCD threshold. In addition, the IMM is assembled into convoluted structures called cristae. As such, we will also highlight the influence of cristae in RCD processes, where appropriate.
Phospholipids are synthesized by the ER and transported to mitochondria for sorting. A subset of these lipids, CL and phosphatidylethanolamine (PE), are directly synthesized within the IMM from phosphatidylglycerol (PG) and phosphatidylserine (PS), respectively. Mitochondrial CL and PE biosynthesis are essential for IMM function and organismal development; these lipids cooperate with the mitochondrial contact site and cristae organizing system (MICOS) and the protein import machinery (e.g., TOM, SAM, and Mia40) to construct cristae junctions compatible with optimal respirasome formation and function. CL and PE oxidation directly alters polarity, leading to metabolic disruptions that exacerbate lipid peroxidation and mitochondrial decline (Horvath and Daum, 2013). But do these pathways actively signal within RCD mechanisms or are they circumstantial? There are suggestions that mitochondrial Ca2+ alters CL-mediated IMM curvature and that metabolic perturbations lead to oxidized CL accumulation, which influences mPTP opening and cell-death sensitivity. These events establish a potential connection between Ca2+ signaling, CL-maintained IMM function, and mPTP-driven necrosis (Petrosillo et al., 2006). CL also interacts with multiple components of the ETC and ATP synthase to provide stabilization, dimerization, catalytic function, and proton translocation (Guo et al., 2017; Wu et al., 2016). Curiously, CL interacts with cyt c via two distinct binding sites: an electrostatic interaction between a lysine and the CL phosphate head and another between an asparagine and an unsaturated acyl chain of CL (Tuominen et al., 2002). When CL is oxidized due to metabolic perturbations or alterations in REDOX, cyt c loses its affinity for CL, which may be a key point of regulation for commitment to the mitochondrial pathway of apoptosis (Kagan et al., 2005). In parallel, CL promotes the peroxidase activity of cyt c by destabilizing the hemeprotein, and this leads to further CL oxidation, likely amplifying mitochondrial dysfunction and the likelihood of cyt c release. These interactions might explain how bioenergetic crises, CL alterations, and cyt c redistributions might not constitute linear paths in RCD pathways but paths that are nevertheless influenced by the peripheral activation of proapoptotic BCL-2 proteins versus mPTP opening, thus determining the RCD pathway. Likewise, lipid peroxidation linked to ferroptosis initiation and execution might be directly impacted by mitochondrial PE metabolism, suggesting that cells with uniquely high mitochondrial volumes (and larger pools of mitochondrial PE) might be more susceptible to mitochondrial contributions to ferroptosis (Kagan et al., 2017).
The above pathways are governed by mitochondrial ROS generation, of which ~85% originates from the ETC. Efficient e− transport prevents mitochondrial ROS generation, while sluggish, poorly assembled, and/or oxidized ETC complexes promote ROS and bioenergetic crises. Indeed, most ETC regulation occurs within the mitochondrial network, but not exclusively. For example, cytosolic proteins, e.g., murine double minute 2 (MDM2) are described to sequester ETC assembly factors, e.g., NADH-ubiquinone oxidoreductase 75-kDa subunit (NDUFS1), factors which have dire consequences for bioenergetics (Elkholi et al., 2019). In this setting, cells with sequestered NDUFS1 disassemble ETC super-complexes between CI and CIII, and this leads to ROS production and sensitization to the mitochondrial pathway of apoptosis. MDM2 gain-of-function is sensed by the mitochondrial network to induce apoptosis, and in some circumstances mPTP. By contrast, MDM2 inhibition enhances ferroptosis suppressor protein 1 (FSP1) function and production of co-enzyme Q10 (an endogenous antioxidant found in the IMM), leading to decreased ferroptosis sensitivity (Bersuker et al., 2019; Venkatesh et al., 2020). Similarly, CI-deficient cells are sensitized to apoptosis and/or ischemia-reperfusion injury (likely through mPTP) and re-establish CI function through the enhanced assembly or heightened production of NADPH and GSH, which protect from cellular stress (Balsa et al., 2020). In a cancer setting, the pharmacological inhibition of CI is associated with ΔψM loss, ROS, lipid peroxidation, mPTP opening, and death (Basit et al., 2017). Moreover, enhanced antioxidants, mitochondrial fusion, or GPX4 prevent tumor-cell death, suggesting direct links to mPTP, necroptosis, pyroptosis, and ferroptosis. Together, these observations reveal that cytosolic interactions with ETC biology serve as mitochondrial sentinels for numerous RCD mechanisms. While CI subunits impact numerous forms of RCD, their regulation also influences RCD kinetics, likely influencing the inflammatory environment. While less attention has been focused on CII, parallels to ROS generation, RCD, and rescue by prosurvival pathways have been described (Jiang et al., 2016).
IMM architecture and cristae maintenance are dynamic determinants of RCD. One classic example is depicted by optic atrophy protein 1 (OPA1), a mitochondrial large GTPase that controls IMM fusion and cristae organization. OPA1 has numerous isoforms that are either within the IMM (i.e., long OPA1) or are soluble within the IMS but associated with cristae junctions (i.e., short OPA1), following their proteolytic cleavage by ATP-dependent peptidases and proteases (e.g., OMA1/YMEL1) (MacVicar and Langer, 2016). A relationship between OPA1 and RCD begins with cyt c availability following BCL-2 antagonist killer (BAK)/BCL-2 associated X protein (BAX)-mediated mitochondrial outer membrane permeabilization (MOMP). While cyt c shuttles e− within the IMS, this occurs mostly within the cristae, leaving most of cyt c not readily available to initiate apoptosis following MOMP. To circumvent this problem, cristae reorganize in a proapoptotic BCL-2-family dependent manner (e.g., BCL-2 interacting mediator of cell death [BIM], BAK) and widen their junctions to allow most of the cyt c to escape the IMS, thereby ensuring productive caspase activation (Frezza et al., 2006; Jiang et al., 2014; Scorrano et al., 2002; Yamaguchi et al., 2008). Indeed, OPA1 overexpression preserves cristae architecture and prevents apoptosis following certain cellular stresses (Varanita et al., 2015). In contexts where cyt c-mediated caspase activation is not achieved, cells may still engage other cell death pathways since mitochondrial function is disrupted and soluble IMS proteins are mislocalized.
OPA1 interacts with numerous proteins unrelated to mitochondrial dynamics, such as solute carriers (e.g., SLC25A) and ETC components. It is thought that these interactions allow for tighter cristae junctions to form, supporting ATP synthase assembly and ATP-linked respiration (Patten et al., 2014). When mitochondrial energy substrates are limited, long- and short-OPA1 isoforms dynamically “seal” cristae junctions to increase ATP-producing capacity. Likewise, OPA1 gain-of-function preserves ΔψM following mitochondrial toxin exposure or ETC dysfunction (Wu et al., 2019). The ability to sense metabolic stress also positions OPA1 to influence IMM swelling following ischemia/reperfusion injury and is linked to Ca2+ homeostasis, foreshadowing mPTP and necrosis regulation. Mutations in Parkinson’s patients also support the above notions that OPA1 functionally interacts with cristae to not only regulate ETC function and efficiency, but to increase ROS and stress, which ultimately sets the cellular threshold to numerous RCD pathways (Iannielli et al., 2018). For example, the OPA1G488R mutation leads to mitochondrial stress accumulation, RIP1/RIP3/MLKL expression, and necroptosis execution. Taken together, homeostatic OPA1 functions establish a cellular environment that is metabolically plastic, efficient, and responsive to bioenergetic demands. Yet these pathways also harbor the capacity to mediate ROS production, Ca2+ handling, mitochondrial swelling, and cyt c availability, supporting diverse RCD mechanisms. Furthermore, OPA1 deficiency in certain cell types contributes to mtDNA maintenance and a failure to induce NETosis, indicating that OPA1 has roles both in cell autonomous RCD and in broader organism-wide roles (Amini et al., 2018).
THE INTERMEMBRANE SPACE
The first clues of mitochondrial involvement in RCD arose from the identification that cyt c is released from the IMS to initiate caspase activation. Since then, it is generally accepted that the bulk of soluble IMS proteins gain access to the cytosol during early commitment stages of the mitochondrial pathway of apoptosis. Nevertheless, RCD is influenced by these proteins on both sides of the OMM and in multiple RCD pathways. While there are many proteins worthy of discussion here (such as cyt c, SMAC, HtrA2/OMI, and AIF), we will focus on cyt c and apoptosis-inducing factor (AIF) as they are the most prevalent in the literature.
Cyt c mRNA is translated on cytosolic ribosomes, transported into mitochondria, and processed via heme addition. It then immediately participates within the ETC. Beyond biosynthesis, cyt c is also dynamically regulated within the IMS to suppress or promote its prodeath potential. For example, cyt c is reduced and inactivated by the glutathione produced by glucose metabolism via the pentose-phosphate pathway, which is observed in both healthy neurons and cancer cells (Vaughn and Deshmukh, 2008). The dual roles of cyt c are highlighted by a murine model in which a respiratory-proficient, yet apoptosis-deficient, mutant of cyt c phenocopies the genetic loss of Caspase9 and Apaf1 in neurons (Hao et al., 2005). Furthermore, families with mutations that lead to alterations in either e− shuttling capacity or heme quality corroborate the relationships between oxidation and apoptosis. As these mutant cyt c proteins enable ROS production and various mitochondrial bioenergetic stresses, it is reasonable to predict they also set thresholds for mPTP and necrosis. Post-translational modifications, including tyrosine phosphorylation and nitration, impact the binding of cyt c to CL and CIV and reduce its cooperation with APAF-1 in caspase activation (García-Heredia et al., 2010; Yu et al., 2008). Most of these studies do not provide clear evidence as to which kinases are responsible for cyt c phosphorylation, but AMPK is implicated, suggesting that metabolic signals result in cyt c phosphorylation prior to mitochondrial localization or inadvertently disable released cyt c from engaging in apoptosis when survival conditions are favored (Mahapatra et al., 2017; Pecina et al., 2010; Yu et al., 2008).
The role of AIF in cell death originates within the mitochondrial pathway of apoptosis as an IMS protein capable of inducing morphological changes when added to isolated nuclei (Susin et al., 1999). AIF is a flavoprotein with NADH oxidase activity that is encoded by the nuclear genome; upon mitochondrial import, it undergoes maturation and association with the IMS-facing side of the IMM. Early studies suggested that AIF participates in DNA cleavage and nuclear condensation following MOMP, which is presumed to occur following its cleavage by either a mitochondrial or cytosolic protease. However, murine models of Aif deficiency have revealed that AIF has integrated roles in mitochondrial biology and homeostasis, as its loss is associated with oxidative stress (Vahsen et al., 2004). Since the whole-body deletion of Aif is embryonically lethal in mice, its tissue-specific ablation has been used to investigate its role in mitochondria. T cells deficient for Aif display proliferation defects associated with CI, CIII, and CIV subunit downregulation, OXPHOS deficiencies, and a failure to engage the mitochondrial bioenergetics necessary for survival (Milasta et al., 2016). These phenotypes are potentially explained by AIF interacting with coiled-coil-helix-coiled-coil-helix domain containing protein 4 (CHCHD4), a component of the IMS mitochondrial-import machinery that catalyzes the oxidation of precursor proteins through the disulfide relay system and assists with e− transfer to CIV (Hangen et al., 2015; Meyer et al., 2015). AIF appears to restrict CHCHD4 degradation, allowing for client ETC proteins to properly localize; additional pathways related to MCU and Ca2+ uptake are subject to CHCHD4 modification (Petrungaro et al., 2015). Based on these observations, AIF appears to influence a spectrum of mitochondrial functions, converging upon bioenergetics, ROS, and Ca2+ signaling, to establish stress thresholds leading to apoptosis, to mPTP, and potentially to other RCD pathways. Indeed, high levels of genomic stress promote parthanatos, which is mediated by AIF release from mitochondria following their permeabilization, but in a manner that is unknown, yet unique from the mitochondrial pathways of apoptosis (i.e., without BAX or caspase requirements).
THE OUTER MITOCHONDRIAL MEMBRANE
Let us return to the “gates of death” concept of the OMM—a term that was adopted after the initial discovery of MOMP. Although the OMM is quite porous, this membrane physically segregates an arsenal of prodeath factors from their cooperating partners and targets within the cell. Yet over the years, we have learned that the OMM is not simply a passive membrane that undergoes MOMP when proapoptotic BCL-2 members accumulate and collaborate. Instead, the OMM serves as a signaling platform that functionally enables multiple RCD pathways by creating a unique biochemical environment that is permissive for key mediators of RCD. Here, we discuss OMMs’ contributions to maintaining life and initiating death in the context of organelle composition (i.e., lipids and proteins), shape, and dynamics.
While OMM resident proteins are usually β-barrels, the BCL-2 family comprises α-helical species that constitutively localize to the OMM (e.g., BCL-2 and BAK), retro-translocate from the cytosol to mitochondria to cytosol (e.g., BAX), or are cytosolic until activated (e.g., BID) (Birkinshaw and Czabotar, 2017). These differences are determined by three regions: (1) the carboxyl-transmembrane α-helix 9, which does not occupy the hydrophobic groove thus supporting OMM insertion; (2) the mid-structure α-helical bundle, which defines the “BCL-2 core,” which transiently associates with the OMM until fully activated; and (3) conformation changes mediated by activation/cleavage, which reveal the BH3 domain and target the OMM directly or via protein-protein interactions (Birkinshaw and Czabotar, 2017). Indeed, additional levels of complexity are determined by the presence of CL, the OMM’s curvature, and mitochondrial contact sites with other organelles (e.g., ER and lysosomes). Certainly, these three factors cooperate to produce a dynamic biochemical and biophysical environment that has evolved to support BCL-2 family function. A repertoire of OMM factors recruit, bind, and differentially influence the BCL-2 family; for example, MTCH2 binds and accelerates BID activation, and while CL performs similar proapoptotic functions to MTCH2, it is likely that additional OMM proteins behave similarly (Raemy et al., 2016; Zaltsman et al., 2010). By contrast, a host of viral proteins localize to the OMM during infection and inhibit BAX activation, demonstrating conserved and pathological mechanisms of OMM contributions to apoptosis. Additionally, while most of the literature interrogates BCL-2 family function at the OMM, MCL-1 is implicated in regulating IMM organization and ATP synthase assembly, while BCL-xL is suggested to regulate IMM permeability through interactions with ATP synthase, impacting mPTP and necroptosis (Alavian et al., 2011; Anilkumar et al., 2020; Chen et al., 2011; Perciavalle et al., 2012).
CL in the OMM is mostly present at the ER-OMM contact sites and mitochondria-associated membranes, which establish the basis for both mitochondrial lipid synthesis and lipid composition. There is significant focus on CL as a mediator of RCD. This is because CL (1) binds BCL-2 proteins, enabling the mitochondrial recruitment of activated BID and BAX; (2) promotes VDAC-regulated death; (3) serves as an OMM docking site for the NLRP3 inflammasome, contributing to pyroptosis initiation; and (4) enhances gasdermin family function in permeabilizing the OMM to further enhance pyroptosis signaling (Iyer et al., 2013; Kuwana et al., 2002; Lutter et al., 2000; Rogers et al., 2019). As mentioned earlier, CL undergoes oxidation, which also unleashes a membrane-permeabilization function of BFL-1 to amplify cyt c release (Flores-Romero et al., 2019). However, numerous lipid species impact on OMM participation in RCD. For example, ceramides interact with VDAC½ to induce RCD, which signals through mPTP to independently impact on apoptosis and necrosis (Dadsena et al., 2019). Cellular lipids are broadly impacted by the TCA cycle, which regulates the production of palmitate and fatty acid species that are essential mediators of pyroptosis and ferroptosis. The OMM also initiates death by directly responding to changes in sphingolipids, and mitochondria are described to actively maintain lipid environments that are essential for mediating proapoptotic BCL-2 family function. For example, sphingosine-1-PO4 and its metabolite, trans-2-hexedecenal, promote BAK- and BAX-dependent MOMP by supporting oligomerization; additionally, reciprocal interactions also exist where BCL-2 family members in the OMM (e.g., BAK) promote sphingolipid generation (i.e., long chain ceramide production) to alter the cellular death threshold (Chipuk et al., 2012; Siskind et al., 2010). While most RCD literature on the role of OMM lipids is based on positive mediators of death, several lipids, including PE and cholesterol, are described to interact with the cell-death machinery in both prodeath and prosurvival contexts, which are likely determined by cell type and stress stimulus.
Relationships between mitochondrial dynamics and the BCL-2 family highlight both prosurvival and prodeath interplay. DRP1, MFN1, and MFN2—three proteins that function at the OMM—are described to regulate proapoptotic BCL-2 proteins, but not all results are in agreement. MFN1 and MFN2 overexpression decreases apoptotic sensitivity, and their removal lowers the apoptotic threshold; MFN1 phosphorylation promotes BAK oligomerization, while soluble BAX promotes mitochondrial fusion via MFN2 homotypic complexes (Hoppins et al., 2011; Pyakurel et al., 2015; Sugioka et al., 2004). The complexity increases when considering DRP1, as it shuttles between the cytosol and OMM and, at the time of MOMP, accumulates at ER-OMM contact sites. These sites create unique biochemical environments that promote Ca2+ and lipid transfer and are essential in determining where mitochondrial division occurs (Voutsinas et al., 2018). Opposing reports suggest that DRP1 is either essential or not for mediating cell death, but most data indicate that BAK/BAX-dependent MOMP occurs independently of DRP1. While MOMP occurs without DRP1, IMS protein release is differentially impacted as cyt c is delayed yet SMAC is normal; additional studies have revealed that DRP1 at the OMM controls cristae junctions and IMM architecture via the OMM adaptor proteins MiD49 and MiD51 (Estaquier and Arnoult, 2007; Otera et al., 2016). DRP1 post-translational modifications are essential to these mechanisms, as phosphorylation (e.g., serine 616 phosphorylation) and SUMOylation promote mitochondrial accumulation and the function of DRP1 oligomers (Taguchi et al., 2007; Zunino et al., 2007). For example, mitochondria-associated protein ligase (MAPL) is activated following cellular stress and SUMOylates DRP1 to promote its localization at ER-OMM contact sites, which results in cristae-junction reorganization and prodeath signaling between the organelles (Prudent et al., 2015). DRP1 is also targeted by multiple signaling pathways, as is the case in cancer where oncogenes cause chronic DRP1-dependent mitochondrial division and chemoresistance. One explanation of this phenotype is that positive membrane curvature fails to support the stable insertion of BAX alpha-helix 9 into the OMM; while re-establishing mitochondrial fusion and decreasing positive membrane curvature increases cellular sensitivity to chemotherapeutics, indicating that this pathway offers therapeutic potential (Kashatus et al., 2015; Renault et al., 2015; Serasinghe et al., 2015).
Additional aspects of OMM biology hint at its broader participation in RCD. For example, OMM recruitment and localization of the activated caspase-8/death-inducing signaling complex (DISC) mediates BID cleavage following death receptor (DR) ligation and DNA damage, suggesting that CL and/or undefined factors participate in the extrinsic pathway of apoptosis (Chandra et al., 2004; Schug et al., 2011). MLKL might also directly interact with mitochondria to initiate necroptosis, perhaps in a manner dependent upon DRP1 remodeling of the OMM-ER contact sites, but as yet no mechanisms for this are known (Wang et al., 2014; Zhang et al., 2019). Biophysical tethering between the OMM and lysosomes via transient receptor potential mucolipin (TRPML1) and VDAC1 is a newly described means to regulate intracellular Ca2+ homeostasis and mitochondrial Ca2+ uptake; while there are no insights into the contribution of biophysical tethering to RCD, it is rational to speculate that TRPML1 alters sensitivity to mPTP and related outcomes (Peng et al., 2020). Finally, a more dynamic relationship between the OMM and Rab5+ endolysosomes was recently described to promote BAX-dependent MOMP and apoptosis, perhaps by altering OMM protein/lipid composition or influencing additional factors, such as membrane curvature, to support BAX activation (Wang et al., 2020).
MITOCHONDRIAL CONTROL OF RCD PATHWAYS AND THE FUTURE
Since the first indications of RCD as a homeostatic mechanism were described by Kerr, Wyllie, and Currie in 1972, and the identification of mitochondria as essential mediators of apoptosis in 1990s, the contributions of mitochondrial biology to RCD signaling have expanded to capture nearly every aspect of this organelle’s function within a formidable list of RCD pathways (Kerr et al., 1972). Mitochondria are curious strongholds of cell biology as they guard the mediators of life and death within their structure. Indeed, some molecules (e.g., cyt c) are essential for life and death—and their redistribution to initiate cellular demise reveals the complex nature by which signaling diversity arises from a single peptide. Each compartment of the mitochondrial network directly sets the cellular threshold leading to death while also signaling to multiple RCD machineries; likewise, some RCD pathways are under the control of each mitochondrial compartment via diverse mechanisms, for example, apoptosis (Figure 3). It should be noted that the integration of metabolism within RCD reveals how metabolites (e.g., ROS and TCA intermediates) act as rheostats between survival and death. Some cell-death mechanisms, like entosis, are so intimately linked to general mitochondrial biology that it seems nearly absurd to investigate their specific contributions. Most importantly, what does the future hold for expanding our understanding of mitochondrial contributions to cell death? While numerous aspects of mitochondrial biology have been explored, numerous long-outstanding questions remain: (1) Do mtDNA or quality control machineries directly signal to RCD pathways to provide signaling diversity like other mitochondrial factors. (2) And does a higher dependence on mitochondrial bioenergetics in some cell lineages affect the requirement for mitochondria in RCD pathways? A barrier and/or challenge to these and many more related questions is the development of modeling systems and techniques that enable the experimental differentiation between stress signaling that promotes repair and active stress that promotes death, and how or if these converge to communicate with the RCD machinery. What we do know is that the relationships between mitochondria and RCD are fundamental for understanding the role of this organelle in biology and that there are prognostic and therapeutic opportunities related to these relationships that could have an impact on human disease.
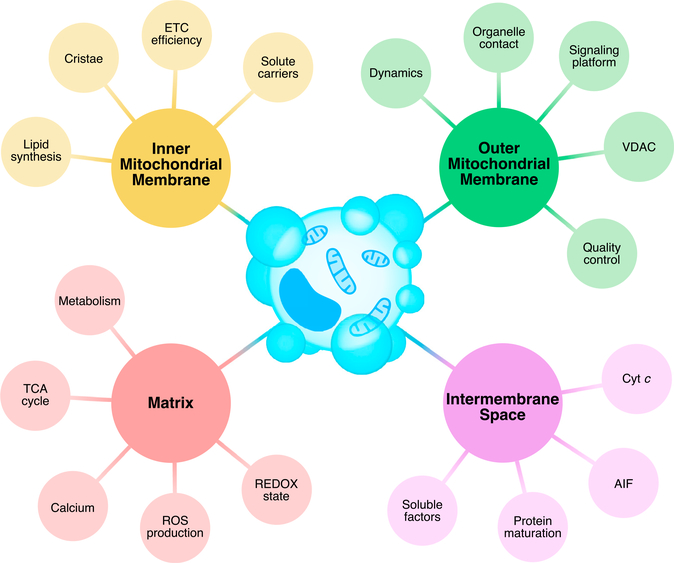
Figure 3. Multiple mitochondrial compartments control apoptosis via diverse mechanisms.
Mitochondrial compartments (OMM, IMS, IMM, and matrix) maintain organelle function and homeostasis while harboring the potential to enable, initiate, and execute apoptosis. A brief summary of each mitochondrial compartment’s activities and/or signaling pathways that directly communicate to the apoptosis machinery are indicated. Detailed descriptions are provided in the text. Abbreviations: AIF, apoptosis-inducing factor; cyt c, cytochrome c; ETC, electron transport chain; ROS, reactive oxygen species; TCA, tricarboxylic acid; VDAC, voltage-dependent anion channel.
ACKNOWLEDGMENTS
We acknowledge this manuscript highlights only a small fraction of literature dedicated to this topic and appreciate the efforts of all laboratories not mentioned. This work was supported by NIH grants R01 CA157740 (J.E.C.), R01 CA206005 (J.E.C.), and R01 CA259110 (J.E.C.); an American Cancer Society Research Scholar Award; a Leukemia & Lymphoma Society Career Development Award; a Collaborative Pilot Award from the Melanoma Research Alliance; and the Tisch Cancer Institute Cancer Center Support grant (P30 CA196521).
REFERENCES
- Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, Lazrove E, Nabili P, Flaherty B, Graham M, et al. (2011). Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol 13, 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini P, Stojkov D, Felser A, Jackson CB, Courage C, Schaller A, Gelman L, Soriano ME, Nuoffer JM, Scorrano L, et al. (2018). Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat. Commun 9, 2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NS, and Haynes CM (2020). Folding the mitochondrial UPR into the integrated stress response. Trends Cell Biol. 30, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar U, Khacho M, Cuillerier A, Harris R, Patten DA, Bilen M, Iqbal MA, Guo DY, Trudeau LE, Park DS, et al. (2020). MCL-1Matrix maintains neuronal survival by enhancing mitochondrial integrity and bioenergetic capacity under stress conditions. Cell Death Dis. 11, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662. [DOI] [PubMed] [Google Scholar]
- Balsa E, Perry EA, Bennett CF, Jedrychowski M, Gygi SP, Doench JG, and Puigserver P (2020). Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat. Commun 11, 2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basit F, van Oppen LM, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, Grefte S, Kopitz C, Heroult M, Willems PH, and Koopman WJ (2017). Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 8, e2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkinshaw RW, and Czabotar PE (2017). The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin. Cell Dev. Biol 72, 152–162. [DOI] [PubMed] [Google Scholar]
- Bock FJ, and Tait SWG (2020). Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol 21, 85–100. [DOI] [PubMed] [Google Scholar]
- Chandra D, Choy G, Deng X, Bhatia B, Daniel P, and Tang DG (2004). Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol. Cell. Biol 24, 6592–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, et al. (2011). Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J. Cell Biol 195, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, and Green DR (2012). Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148, 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadsena S, Bockelmann S, Mina JGM, Hassan DG, Korneev S, Razzera G, Jahn H, Niekamp P, Müller D, Schneider M, et al. (2019). Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun 10, 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkholi R, Abraham-Enachescu I, Trotta AP, Rubio-Patiño C, Mohammed JN, Luna-Vargas MPA, Gelles JD, Kaminetsky JR, Serasinghe MN, Zou C, et al. (2019). MDM2 integrates cellular respiration and apoptotic signaling through NDUFS1 and the mitochondrial network. Mol. Cell 74, 452–465.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaquier J, and Arnoult D (2007). Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 14, 1086–1094. [DOI] [PubMed] [Google Scholar]
- Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. (2019). Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U S A 116, 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Romero H, Landeta O, Ugarte-Uribe B, Cosentino K, García-Porras M, García-Sáez AJ, and Basañez G (2019). BFL1 modulates apoptosis at the membrane level through a bifunctional and multimodal mechanism showing key differences with BCLXL. Cell Death Differ. 26, 1880–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, and Scorrano L (2006). OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189. [DOI] [PubMed] [Google Scholar]
- Friedman JR, and Nunnari J (2014). Mitochondrial form and function. Nature 505, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, and Hu J (2021). Mitochondrial fusion: the machineries in and out. Trends Cell Biol. 31, 62–74. [DOI] [PubMed] [Google Scholar]
- García-Heredia JM, Díaz-Moreno I, Nieto PM, Orzáez M, Kocanis S, Teixeira M, Pérez-Payá E, Díaz-Quintana A, and De la Rosa MA (2010). Nitration of tyrosine 74 prevents human cytochrome c to play a key role in apoptosis signaling by blocking caspase-9 activation. Biochim. Biophys. Acta 1797, 981–993. [DOI] [PubMed] [Google Scholar]
- Gaschler MM, Hu F, Feng H, Linkermann A, Min W, and Stockwell BR (2018). Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem. Biol 13, 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Pyakurel A, Glytsou C, and Scorrano L (2020). The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol 21, 204–224. [DOI] [PubMed] [Google Scholar]
- Green DR (2019). The coming decade of cell death research: five riddles. Cell 177, 1094–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Zong S, Wu M, Gu J, and Yang M (2017). Architecture of human mitochondrial respiratory megacomplex I2III2IV2. Cell 170, 1247–1257.e12. [DOI] [PubMed] [Google Scholar]
- Hangen E, Féraud O, Lachkar S, Mou H, Doti N, Fimia GM, Lam NV, Zhu C, Godin I, Muller K, et al. (2015). Interaction between AIF and CHCHD4 regulates respiratory chain biogenesis. Mol. Cell 58, 1001–1014. [DOI] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, et al. (2005). Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell 121, 579–591. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, and Nunnari J (2011). The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol. Cell 41, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath SE, and Daum G (2013). Lipids of mitochondria. Prog. Lipid Res 52, 590–614. [DOI] [PubMed] [Google Scholar]
- Iannielli A, Bido S, Folladori L, Segnali A, Cancellieri C, Maresca A, Massimino L, Rubio A, Morabito G, Caporali L, et al. (2018). Pharmacological inhibition of necroptosis protects from dopaminergic neuronal cell death in Parkinson’s disease models. Cell Rep. 22, 2066–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, Sandler N, Grimm MJ, Segal BH, Otterbein LE, and Hauser CJ (2015). Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One 10, e0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. (2013). Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo V, Bravo-San Pedro JM, Sica V, Kroemer G, and Galluzzi L (2016). Mitochondrial permeability transition: new findings and persisting uncertainties. Trends Cell Biol. 26, 655–667. [DOI] [PubMed] [Google Scholar]
- Jakobs S, Stephan T, Ilgen P, and Brüser C (2020). Light microscopy of mitochondria at the nanoscale. Annu. Rev. Biophys 49, 289–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Jiang H, Shen Z, and Wang X (2014). Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc. Natl. Acad. Sci. U S A 111, 14782–14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Li L, Ying Z, Pan C, Huang S, Li L, Dai M, Yan B, Li M, Jiang H, et al. (2016). A small molecule that protects the integrity of the electron transfer chain blocks the mitochondrial apoptotic pathway. Mol. Cell 63, 229–239. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol 13, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, et al. (2005). Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol 1, 223–232. [DOI] [PubMed] [Google Scholar]
- Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, Counter CM, and Kashatus DF (2015). Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol. Cell 57, 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasukabe T, Honma Y, Okabe-Kado J, Higuchi Y, Kato N, and Kumakura S (2016). Combined treatment with cotylenin A and phenethyl isothiocyanate induces strong antitumor activity mainly through the induction of ferroptotic cell death in human pancreatic cancer cells. Oncol. Rep 36, 968–976. [DOI] [PubMed] [Google Scholar]
- Kenny TC, Gomez ML, and Germain D (2019). Mitohormesis, UPRmt, and the complexity of mitochondrial DNA landscapes in cancer. Cancer Res. 79, 6057–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, and Currie AR (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Morgan MJ, Choksi S, and Liu ZG (2007). TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 26, 675–687. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, and Newmeyer DD (2002). Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342. [DOI] [PubMed] [Google Scholar]
- Lackner LL (2019). The expanding and unexpected functions of mitochondria contact sites. Trends Cell Biol. 29, 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless C, Greaves L, Reeve AK, Turnbull DM, and Vincent AE (2020). The rise and rise of mitochondrial DNA mutations. Open Biol. 10, 200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, Nishimura Y, Nieminen AL, and Herman B (1999). Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J. Bioenerg. Biomembr 31, 305–319. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Ates G, Methner A, Conrad M, and Maher P (2018). Oxytosis/ferroptosis-(re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Front. Neurosci 12, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, and Kaplan MJ (2016). Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med 22, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Fang M, Luo X, Nishijima M, Xie X, and Wang X (2000). Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol 2, 754–761. [DOI] [PubMed] [Google Scholar]
- MacVicar T, and Langer T (2016). OPA1 processing in cell death and disease - the long and short of it. J. Cell Sci 129, 2297–2306. [DOI] [PubMed] [Google Scholar]
- Mahapatra G, Varughese A, Ji Q, Lee I, Liu J, Vaishnav A, Sinkler C, Kapralov AA, Moraes CT, Sanderson TH, et al. (2017). Phosphorylation of cytochrome c threonine 28 regulates electron transport chain activity in kidney: IMPLICATIONS FOR AMP kinase. J. Biol. Chem 292, 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Giorgi C, Galluzzi L, and Pinton P (2020). Ca2+ fluxes and cancer. Mol. Cell 78, 1055–1069. [DOI] [PubMed] [Google Scholar]
- Meyer K, Buettner S, Ghezzi D, Zeviani M, Bano D, and Nicotera P (2015). Loss of apoptosis-inducing factor critically affects MIA40 function. Cell Death Dis. 6, e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milasta S, Dillon CP, Sturm OE, Verbist KC, Brewer TL, Quarato G, Brown SA, Frase S, Janke LJ, Perry SS, et al. (2016). Apoptosis-inducing-factor-dependent mitochondrial function is required for T cell but not B cell function. Immunity 44, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moujalled DM, Cook WD, Murphy JM, and Vaux DL (2014). Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis. 5, e1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Miyata N, Kuge O, and Mihara K (2016). Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J. Cell Biol 212, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V (2018). Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol 18, 134–147. [DOI] [PubMed] [Google Scholar]
- Patananan AN, Wu TH, Chiou PY, and Teitell MA (2016). Modifying the mitochondrial genome. Cell Metab. 23, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten DA, Wong J, Khacho M, Soubannier V, Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM, Trinkle-Mulcahy L, et al. (2014). OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 33, 2676–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina P, Borisenko GG, Belikova NA, Tyurina YY, Pecinova A, Lee I, Samhan-Arias AK, Przyklenk K, Kagan VE, and Hüttemann M (2010). Phosphomimetic substitution of cytochrome C tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry 49, 6705–6714. [DOI] [PubMed] [Google Scholar]
- Peng W, Wong YC, and Krainc D (2020). Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. U S A 117, 19266–19275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, et al. (2012). Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat. Cell Biol 14, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo G, Casanova G, Matera M, Ruggiero FM, and Paradies G (2006). Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett. 580, 6311–6316. [DOI] [PubMed] [Google Scholar]
- Petrungaro C, Zimmermann KM, Küttner V, Fischer M, Dengjel J, Bogeski I, and Riemer J (2015). The Ca(2+)-dependent release of the Mia40-induced MICU1-MICU2 dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab. 22, 721–733. [DOI] [PubMed] [Google Scholar]
- Pickles S, Vigié P, and Youle RJ (2018). Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol 28, R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent J, Zunino R, Sugiura A, Mattie S, Shore GC, and McBride HM (2015). MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol. Cell 59, 941–955. [DOI] [PubMed] [Google Scholar]
- Pyakurel A, Savoia C, Hess D, and Scorrano L (2015). Extracellular regulated kinase phosphorylates mitofusin 1 to control mitochondrial morphology and apoptosis. Mol. Cell 58, 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemy E, Montessuit S, Pierredon S, van Kampen AH, Vaz FM, and Martinou JC (2016). Cardiolipin or MTCH2 can serve as tBID receptors during apoptosis. Cell Death Differ. 23, 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault TT, Floros KV, Elkholi R, Corrigan KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla JJ, Buettner C, et al. (2015). Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol. Cell 57, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, and Alnemri ES (2019). Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun 10, 1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler IE (2008). Mitochondria, Second Edition (Wiley-Liss; ). [Google Scholar]
- Schug ZT, Gonzalvez F, Houtkooper RH, Vaz FM, and Gottlieb E (2011). BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 18, 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, and Korsmeyer SJ (2002). A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2, 55–67. [DOI] [PubMed] [Google Scholar]
- Serasinghe MN, Wieder SY, Renault TT, Elkholi R, Asciolla JJ, Yao JL, Jabado O, Hoehn K, Kageyama Y, Sesaki H, and Chipuk JE (2015). Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol. Cell 57, 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel AD, Zhang AS, Brown C, Shirihai OS, and Ponka P (2007). Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 110, 125–132. [DOI] [PubMed] [Google Scholar]
- Siskind LJ, Mullen TD, Romero Rosales K, Clarke CJ, Hernandez-Corbacho MJ, Edinger AL, and Obeid LM (2010). The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J. Biol. Chem 285, 11818–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Herrmann JM, and Becker T (2021). Quality control of the mitochondrial proteome. Nat. Rev. Mol. Cell Biol 22, 54–70. [DOI] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, and Tsujimoto Y (2004). Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J. Biol. Chem 279, 52726–52734. [DOI] [PubMed] [Google Scholar]
- Sun W, Wu X, Gao H, Yu J, Zhao W, Lu JJ, Wang J, Du G, and Chen X (2017). Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells. Free Radic. Biol. Med 108, 433–444. [DOI] [PubMed] [Google Scholar]
- Sureshbabu A, Patino E, Ma KC, Laursen K, Finkelsztein EJ, Akchurin O, Muthukumar T, Ryter SW, Gudas L, Choi AMK, and Choi ME (2018). RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight 3, e98411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. (1999). Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446. [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, and Mihara K (2007). Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem 282, 11521–11529. [DOI] [PubMed] [Google Scholar]
- Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, et al. (2013). Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 5, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen EK, Wallace CJ, and Kinnunen PK (2002). Phospholipid-cytochrome c interaction: evidence for the extended lipid anchorage. J. Biol. Chem 277, 8822–8826. [DOI] [PubMed] [Google Scholar]
- Vahsen N, Candé C, Brière JJ, Bénit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, et al. (2004). AIF deficiency compromises oxidative phosphorylation. EMBO J. 23, 4679–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V, Civiletto G, Pesce P, et al. (2015). The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 21, 834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AE, and Deshmukh M (2008). Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat. Cell Biol 10, 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh D, O’Brien NA, Zandkarimi F, Tong DR, Stokes ME, Dunn DE, Kengmana ES, Aron AT, Klein AM, Csuka JM, et al. (2020). MDM2 and MDMX promote ferroptosis by PPARalpha-mediated lipid remodeling. Genes Dev. 34, 526–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjeva N, Galkin I, Pletjushkina O, Golyshev S, Zinovkin R, Prikhodko A, Pinegin V, Kondratenko I, Pinegin B, and Chernyak B (2020). Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim. Biophys. Acta Mol. Basis Dis 1866, 165664. [DOI] [PubMed] [Google Scholar]
- Voutsinas N, Lekperic S, Barazani S, Titano JJ, Heiba SI, and Kim E (2018). Treatment of primary liver tumors and liver metastases, part 1: nuclear medicine techniques. J. Nucl. Med 59, 1649–1654. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, and Wang X (2014). Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146. [DOI] [PubMed] [Google Scholar]
- Wang TS, Coppens I, Saorin A, Brady NR, and Hamacher-Brady A (2020). Endolysosomal targeting of mitochondria is integral to BAX-mediated mitochondrial permeabilization during apoptosis signaling. Dev. Cell 53, 627–645.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, Yu P, Shi ZH, Wu WS, Gao G, and Chang Y-Z (2016). The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front. Aging Neurosci 8, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiang H, Chen S, Du F, and Wang X (2012). The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148, 228–243. [DOI] [PubMed] [Google Scholar]
- Wu M, Gu J, Guo R, Huang Y, and Yang M (2016). Structure of mammalian respiratory supercomplex I1III2IV1. Cell 167, 1598–1609.e10. [DOI] [PubMed] [Google Scholar]
- Wu W, Zhao D, Shah SZA, Zhang X, Lai M, Yang D, Wu X, Guan Z, Li J, Zhao H, et al. (2019). OPA1 overexpression ameliorates mitochondrial cristae remodeling, mitochondrial dysfunction, and neuronal apoptosis in prion diseases. Cell Death Dis. 10, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, and Newmeyer DD (2008). Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol. Cell 31, 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wang Y, Zhang Y, He X, Zhong CQ, Ni H, Chen X, Liang Y, Wu J, Zhao S, et al. (2018). RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol 20, 186–197. [DOI] [PubMed] [Google Scholar]
- Yu H, Lee I, Salomon AR, Yu K, and Hüttemann M (2008). Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim. Biophys. Acta 1777, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, Vaz FM, De Leonardis F, Fiermonte G, Palmieri F, et al. (2010). MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat. Cell Biol 12, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Che L, He C, Huang J, Guo N, Shi J, Lin Y, and Lin Z (2019). Drp1 and RB interaction to mediate mitochondria-dependent necroptosis induced by cadmium in hepatocytes. Cell Death Dis. 10, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ, Chen X, Cai Q, Yang ZH, Huang D, Wu R, and Han J (2017). RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun 8, 14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, Wong J, Ding S, Seki E, Schnabl B, et al. (2018). New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimorski V, Ku C, Martin WF, and Gould SB (2014). Endosymbiotic theory for organelle origins. Curr. Opin. Microbiol 22, 38–48. [DOI] [PubMed] [Google Scholar]
- Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, and McBride HM (2007). The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J. Cell Sci 120, 1178–1188. [DOI] [PubMed] [Google Scholar]