Abstract
Tendon-to-bone repair often fails because the functionally-graded attachment is not regenerated during the healing process. Biomimetic scaffolds that recapitulate the unique features of the native tendon-to-bone attachment hold great promise for enhancing the healing process. Among various types of scaffolds that have been developed and evaluated for tendon-to-bone repair, those with gradations (in either a stratified or a continuous fashion) in composition, structure, mechanical properties, and cell phenotype have gained the most attention. In this progress report, we review our recent efforts in the rational design and fabrication of functionally-graded scaffolds based upon electrospun nanofiber mats and inverse opal structures, as well as the evaluation of their applications in augmenting tendon-to-bone repair. We conclude the report with perspectives on the necessary future steps for clinical translation of the scaffolds.
Keywords: Functionally-graded scaffolds, electrospun nanofibers, inverse opal scaffolds, tendon-to-bone insertion, tissue engineering
1. Introduction
Musculoskeletal injuries are a major cause of pain, disability, and lost productivity, leading to significant societal costs.[1] Among them, those occurring at the interface between connective tissue and bone are especially problematic and notable examples include rotator cuff tears in the shoulder, patellar tendon injuries in the knee, gluteal tendon tears in the hip, and the Achilles tendon ruptures in the ankle.[2] In particular, rotator cuff injuries to the shoulder are astonishingly high, affecting 30% of the population over the age of 60.[3] Surgical re-attachment of torn rotator cuff tendons to their bony insertions is typically necessary to recover the shoulder function and it represents one of the most commonly practiced procedures in orthopaedic surgery, with more than 600,000 repairs performed every year in the United States alone.[4] Unfortunately, post-surgical healing is often unsatisfactory, leading to failure rates of 20–94%, depending on the patient population and the extent of the tear.[5,6]
The high failure rates after rotator cuff surgical repair can largely be attributed to a scar-mediated healing process that does not regenerate a functionally-graded transition at the interface.[5,7] As shown in Figure 1A and B, the native attachment of tendon to bone (i.e., the enthesis) involves an insertion site characterized by a complex transitional zone with gradations in composition, structure, mechanical properties, and cell phenotypes.[7–11] Across a distance of hundreds of micrometers, the insertion can be categorized into four compositionally distinct but structurally continuous zones, including tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone, with each zone exhibiting unique features in terms of cell type, extracellular matrix (ECM), collagen alignment, and mineralization (Table 1). The first zone is composed of aligned type I collagen fibers and populated by fibroblasts. Its mechanical properties are similar to those of the tendon mid-substance. The second zone is unmineralized fibrocartilage populated by fibrochondrocytes, and comprised of less aligned types I, II, and III collagen, as well as the proteoglycan aggrecan. The third zone is mineralized fibrocartilage populated by mineralized fibrochondrocytes, and consisted of less aligned type II collagen, type X collagen, and the proteoglycan aggrecan. The fourth zone is the bone proper populated by osteoblasts, osteocytes, and osteoclasts, and composed of mineralized type I collagen. This transitional tissue plays a critical role in eliminating stress concentrations that would otherwise arise from the structural mismatch between tendon (a rope-like tissue with a modulus on the order of 200 MPa) and bone (a hard tissue with a modulus of 20 GPa).[12,13] Once injured, the tendon-to-bone interface is not regenerated during the healing process, even after surgical repair.[14,15] Instead, scar tissue dominates at the interface, giving disorganized ECM rather than the well-aligned collagen fibers found at the native attachment. The scar tissue is an order-of-magnitude weaker than the native tissue and is not well integrated into the bone. Additionally, the poor healing is related to mineral loss at the bone underlying the tendon attachment. Loss of bone begins immediately after the tear of tendon, presumably due to the loss of mechanical loading. Even when surgical repair is performed soon after injury, significant loss is still observed, which is thought to be the increased osteoclast activity in the adjacent bone. Taken together, the root cause for surgical failures of tendon-to-bone repair lies in the non-regenerated tissue interface, which is typically characterized by the aforementioned scar formation and bone loss. As such, augmenting tendon-to-bone repair may be achieved through a combination of strategies that: i) induce the establishment of a graded interface; ii) enhance the formation of bone; and iii) promote the deposition of aligned collagen, all of which would result in better integration and higher attachment strength.
Figure 1.

Gradients at the tendon-to-bone insertion site. A) Supraspinatus tendon-to-bone insertion section from an adult rat stained with toluidine blue. B) Schematic illustration showing the graded, hierarchical structure of the tendon-to-bone insertion. C) Schematic illustrations of functionally-graded scaffolds characterized by stratified or continuous variation. A) Adapted with permission.[5] Copyright 2013, Annual Reviews. B) Adapted with permissions.[7–11] Copyright 2017, Springer Nature; Copyright 2015, ASME; Copyright 2012, PLoS; Copyright 2015, Elsevier; Copyright 2012, Royal Society.
Table 1.
Four compositionally distinct but structurally continuous zones at the tendon-to-bone insertion site.[5]
| Zone | Cell type | ECM | Collagen alignment | Mineral |
|---|---|---|---|---|
| Tendon | Fibroblast | Type I collagen | Aligned | Unmineralized |
| Unmineralized fibrocartilage | Fibrochondrocyte | Type I, II, and III collagen; aggrecan | Less aligned | Unmineralized |
| Mineralized fibrocartilage | Fibrochondrocyte | Type II and X collagen; aggrecan | Less aligned | Mineralized |
| Bone | Osteoblast; osteocyte; osteoclast | Type I collagen | Random | Mineralized |
The path to improved healing may lie in the tissue engineering paradigm, which combines elements of scaffold design, cellular control, and biological signaling to promote regeneration of injured tissues.[16–22] To this end, rational design and fabrication of biomimetic scaffolds have received the most attention for their in-built capability to recapitulate many of the unique features inherent to the native enthesis. Among the various types of scaffolds that have been fabricated and evaluated for tendon-to-bone repair, the most promising ones include gradual variations, either in a stratified or continuous fashion, with respect to composition, structure, mechanical properties, and cell phenotypes (Figure 1C).[23–30] Such biomimetic scaffolds can duplicate various aspects of the native tendon enthesis to promote healing and functional recovery. These materials are typically referred to as functionally-graded scaffolds, which are characterized by gradual spatial variations in composition and/or structure to recapitulate the native interface between soft and hard tissues. To date, a number of approaches have been established for the fabrication of functionally-graded scaffolds,[23,24] including light-based methods,[31,32] sequential deposition and particulate removal,[33,34] 3D printing,[35,36] microfluidics,[27,37,38] electrospinning,[39] freeze-drying,[40] and force-induced sedimentation (e.g., centrifugation,[41] buoyancy,[30] magnetic field,[42] and electric field[43]). In recent years, decellularized scaffolds have emerged as another promising class of biomaterials in tissue engineering.[44–46] These scaffolds are derived from the ECM (e.g., collagen, elastin, proteoglycans, and glycoproteins) of specific tissues and organs by removing cellular components. In contrast to synthetic scaffolds, decellularized scaffolds largely preserve the 3D ultrastructure of the source tissues/organs and the biochemical functions of the source ECM. However, their widespread application is hindered by a number of remaining hurdles, such as the uncontrollable degradation, inadequate mechanical properties, host immune responses, and complicated decellularization procedures. In this regard, functionally-graded scaffolds that are fabricated with tailor-made synthetic materials and customized techniques offer greater potential to meet the needs of tendon-to-bone repair. In this progress report, we focus on our recent activities in the design and fabrication of functionally-graded scaffolds based upon electrospun nanofiber mats and inverse opal structures for augmenting tendon-to-bone repair. Our earlier work focused on scaffolds that were applied as patches over the repaired tendon. More recently, we switched to scaffolds that could be interposed between the repaired tendon and bone. For each type of the scaffold, we begin with a description of how the design recapitulates the physical and/or biochemical cues of the native tendon enthesis. We then discuss fabrication techniques for generating gradients in these scaffolds, followed by biological evaluation of their potential (focusing on in vitro responses). We conclude the report by providing perspectives on ongoing challenges and future directions.
2. Functionally-Graded Nanofiber Scaffolds
Electrospun nanofibers can be fabricated by electrospinning with diameters ranging from tens of nanometers to a few micrometers. The scaffolds have been used to manipulate cell adhesion, growth, migration, elongation, and differentiation by providing specific microenvironments that mimic native ECM.[47–52] Electrospinning is a simple technique that uses the electrostatic repulsion between surface charges to produce nanofibers from a viscoelastic polymer fluid. By varying the setup for electrospinning, the properties of the as-prepared electrospun nanofibers can be easily tailored, including control of the diameter, composition, porosity, and alignment. To match the natural collagen fibers found at the tendon enthesis, the size of the nanofibers should be between 50–500 nm. In terms of materials, both natural polymers (e.g., collagen and gelatin) and synthetic polymers approved by the U.S. Food and Drug Administration (FDA), including poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), should be considered, as they can provide appropriate mechanical properties, programmable biodegradability, and adequate biosafety for human use. Collagen fibers across the tendon enthesis exhibit gradients in structure and composition; non-woven nanofiber mats containing gradations in alignment and porosity should match these features and thereby enable the regulation of cell adhesion and elongation to facilitate tissue repair and regeneration. Apart from modulating the intrinsic properties of the as-prepared electrospun nanofibers, various post-modification approaches have also been developed to create graded biochemical/mechanical cues on electrospun nanofiber mats. These features can be used to manipulate cell adhesion, migration, proliferation, and differentiation. It should be noted that nanofiber mats fabricated using electrospinning are generally less than 1 mm in thickness, and are therefore used as two-dimensional (2D) patches rather than three-dimensional (3D) scaffolds for tendon-to-bone repair.
2.1. Fabrication
We have developed a number of methods for generating gradations in composition, structure, and cell density on electrospun nanofiber mats. Some of these methods are briefly discussed below.
2.1.1. Gradation in Composition
Graded mineralization by regulating the immersion time in a concentrated simulated body fluid.
Stiffness increases by two orders of magnitude from tendon to bone due to the increased concentrations of mineral. A gradient in mineral content, both at the native enthesis and in biomaterial-based replacements, would produce a graded transition in mechanical properties. In this regard, we developed a simple and versatile method for creating a mineral gradient on uniaxially aligned nanofiber mats by varying the deposition time of calcium phosphate from a simulated body fluid that offers calcium and phosphate ions for mineralization (Figure 2A).[53,54] In this approach, the mineralizing solution was added at a constant rate using a syringe pump while the electrospun nanofiber mat was placed in a container at a fixed tilting angle. As the liquid level elevated over time, the electrospun nanofibers positioned at different heights experienced a continuous change in immersion time, with the bottom and top edges corresponding to the longest and shortest periods of time, respectively. Since the deposition of calcium phosphate was positively correlated with the immersion time, a gradient in mineral content could be deliberately generated along the long axis of the nanofiber mat. In particular, the gradient profile could be easily tuned by varying the concentration of the simulated body fluid, the tilting angle of the nanofiber mat, and the feeding rate of the simulated body fluid. Typically, a 10-fold concentrated simulated body fluid is employed, as it can lead to efficient deposition of calcium phosphate within 2–6 h in the presence of sodium bicarbonate. Since cells are able to respond to biochemical and mechanical cues, the gradient in mineral content and stiffness can facilitate graded adhesion, migration, and/or spatial osteogenesis of stem cells as a result of its osteoconductive and osteoinductive properties.
Figure 2.

Schematic illustrations of different methods for the modification of electrospun nanofiber mats with graded functionality. A) Graded mineralization by regulating the immersion time in a concentrated simulated body fluid. B) Graded deposition of bioactive macromolecules by manipulating the deposition time. C) Graded distribution of particles by masked electrospray deposition. D) Gradation in alignment and porosity by regulating solvent exposure. E) Aligned-to-random transition by manipulating the collection manner of electrospun nanofibers. F) Sedimentation of cells onto a surface with a tilting angle.
Graded deposition of bioactive macromolecules by manipulating the deposition time.
In addition to inorganic mineral, we also developed a general strategy to create a gradient in bioactive proteins on electrospun nanofiber mats via graded blocking with a biologically inert protein. This procedure resulted in the formation of a graded biochemical cue to guide cell adhesion, proliferation, and migration (Figure 2B).[55] In this approach, the adsorption sites on the nanofiber mat were first occupied by bovine serum albumin (BSA) in a graded fashion, which was achieved by adopting a method similar to the one described in Figure 2A. In the following step, the nanofiber mat was soaked in a solution of the bioactive protein, filling up the bare regions to generate a gradient that was directionally opposite to that of BSA. In principle, this approach is applicable to any bioactive protein, as long as the gradient profile of BSA has been optimized in advance. In addition, by varying the orientation of nanofibers, both topological and biochemical cues can be established on the same nanofiber mat, and this may elicit a synergistic effect on the regulation of cell behaviors for tendon-to-bone repair.
Graded distribution of particles by masked electrospray deposition.
To create a microenvironment that mimics the natural ECM proteins at the tendon enthesis and thus promotes cell migration and differentiation, we developed a method to deposit particles of natural biomacromolecules (e.g., collagen) on uniaxially-aligned nanofiber mats (Figure 2C).[56,57] In this approach, electrospray, a variant form of electrospinning, was used to coat the surface of a nanofiber mat with a uniform distribution of protein-based particles.[58] To generate a gradient in particle density, we modulated electrospray deposition with a physical mask that moved at a constant rate. Since the particle density was positively correlated with the deposition time, a gradient in particle density along the moving direction was created, and the gradient profile could be easily adjusted by varying the concentration of the solution used for electrospray and/or the moving speed of the mask. This method is applicable to essentially all types of biomacromolecular particles or stimulus-responsive materials.[59] Similarly, the concurrent existence of graded biochemical and topological cues is expected to impose a concerted effect on the regulation of cell behaviors.
2.1.2. Gradation in Structure
Gradation in alignment and porosity by regulating solvent exposure.
Considering that collagen fibrils at the insertion site are uniaxially aligned in the tendon and become disorganized when moving toward the bone, we developed a solvent-exposure method to transform uniaxially aligned electrospun nanofibers into a hierarchical structure with gradations in nanofiber alignment and/or porosity to manipulate cell behaviors (Figure 2D).[60] In a previous study, we found that upon exposure to the vapor of a solvent that was able to swell electrospun nanofibers, the cross points between adjacent nanofibers could be welded with each other in a controlled manner.[61] As such, it was feasible to generate gradients on nanofiber mats in both alignment and porosity by controlling the exposure time and thus the welding extent. For a solvent such as ethanol that is able to form hydrogen bonds with water, it typically exhibits different capabilities to swell or weld nanofibers when supplied as an aqueous solution or a pure vapor. In contrast to its pure vapor, hydrogen bonding with water mitigates the diffusion of the solvent molecules into the nanofibers and thus reduces the extent of welding. Using a method similar to the one described in Figure 2A, a uniaxially aligned nanofiber mat was placed in a sealed container and exposed to the aqueous solution of ethanol fed at a constant rate, leading to a graded transition in both alignment and porosity for the nanofiber mat, with the top and bottom regions exhibiting the highest and lowest extent of welding, respectively, due to the distinct contact time with the aqueous solution and pure vapor of ethanol.
Aligned-to-random transition by manipulating the collection of electrospun nanofibers.
We have also developed a method to directly collect nanofiber mats with both aligned and random sections in the same scaffold (Figure 2E).[62,63] In this new setup, two stapler-shaped metal frames were used as the collectors for electrospinning. The electric field lines split and pointed toward the two opposite edges of the gap, forcing the nanofibers into a uniaxial arrangement across the gap. The electrostatic repulsion between the deposited and the upcoming nanofibers also helped maintain the uniaxial alignment. On the surface of the metal edge, the nanofibers were still collected as a random, nonwoven mat. As a result, both randomly oriented and uniaxially aligned nanofibers coexist within the same scaffold, demonstrating a potential for replicating the gradient in structural organization of collagen fibers at the native tendon enthesis.
2.1.3. Gradation in Cell Density
Sedimentation of cells onto a surface with a tilting angle.
Gradients in cell density and phenotypes represent a unique feature of the tendon-to-bone attachment, with fibroblasts in the tendon, fibrochondrocytes at the interface, and osteoblasts in the bone. The complicated interactions among these cells promote the establishment of structural and compositional gradations necessary for effective attachment. To reproduce this cellular gradient in vitro, we developed an approach to generate a gradient in cell density via simple sedimentation of cells onto a nanofiber mat (Figure 2F).[64] An electrospun nanofiber mat was immersed in a homogenous suspension of cells at a tilting angle. Under the action of gravity, the cells sedimented over time. Because the available number of cells above the nanofibers was linearly dependent on height, a gradient in cell density was generated on the nanofiber mat along the longitudinal direction. The gradient profile could be tuned by varying the concentration of the cell suspension and/or the tilting angle of the nanofiber mat. In particular, this method can be extended for constructing opposite gradients of two types of cells simply by sequentially immersing the nanofiber mat first in a suspension of one cell type and then in the opposing direction in a suspension of a second cell type. Combined with other established approaches for creating gradients in materials structure and composition, a cell-seeded biomimetic scaffold can be developed to augment tendon-to-bone repair.
2.2. Biological Evaluation
This section illustrates how the electrospun nanofiber mats with gradations in composition, structure, and/or cell phenotypes can be used to facilitate tendon enthesis regeneration by acting as a patch to bridge the disparate tendon and bone tissues.
A gradient in mineral content at the native tendon enthesis is critical for dissipating stress concentrations that would otherwise arise between tendon and bone, which differ in modulus by two orders of magnitude.[65] Since the mineral gradient at the native insertion site is not regenerated once ruptured, establishing a mineral gradient on a given scaffold offers one of the most promising strategies to reduce the failure rate of tendon-to-bone repairs. In this regard, we fabricated a uniaxially aligned electrospun nanofiber mat with a gradient in mineral content using the approach shown in Figure 2A. Furthermore, we evaluated its biological effect on the regulation of osteogenic differentiation of adipose-derived mesenchymal stem cells (ASCs).[54] By varying the immersion time, a linear gradient in density and thickness of calcium phosphate was established along the longitudinal direction of the nanofiber mat, as demonstrated by the scanning electron microscopy (SEM) images taken at different positions (Figure 3A). Further quantification of calcium contents by energy-dispersive X-ray spectroscopy (EDX) suggested the presence of a continuous transition from the unmineralized side to the mineralized side (Figure 3B). To evaluate the influence of the mineral gradient on the osteogenic differentiation of ASCs, runt-related transcription factor 2 (Runx2, an essential transcription factor for osteogenic differentiation of stem cells) and osteocalcin (OCN, a noncollagenous protein found in the ECM of bones) were used as an early and a late marker of osteoblast differentiation, respectively, for immunocytochemistry assay. As shown in Figure 3C, the expression of Runx2 and OCN exhibited a graded distribution pattern that was positively correlated with the mineral content, indicating the osteoconductive and osteoinductive effects of calcium phosphate on the osteogenic differentiation of stem cells.
Figure 3.

Uniaxially aligned PLGA nanofibers with graded mineralization for spatial control of osteogenesis. A) SEM images of calcium phosphate coatings on a PLGA nanofiber mat at i) 1 cm, ii) 2 cm, iii) 3 cm, and iv) 5 cm away from the unmineralized end of the scaffold. B) EDX quantification of the gradient in mineral content (n = 3). C) ASCs seeded on the aligned nanofibers with a spatial mineral gradient after culture for 28 days by i–iii) Runx2 and iv–vi) OCN staining, respectively. A–C) Adapted under the terms of the ACS AuthorChoice license.[54] Copyright 2014, American Chemical Society.
To further evaluate the potential of this scaffold for improving tendon-to-bone repair, the scaffold was implanted in a rat model of rotator cuff injury and repair. The unmineralized end of the scaffold was placed over tendon, the mineralized end was placed over bone, and the graded region spanned across the enthesis (Figure 4A).[66] The results shown in Figure 4B–F indicated that scar formation still dominated the repair even in the presence of a scaffold that recapitulated the mineral gradient and the aligned arrangement of the native tendon enthesis. In addition, decreases in trabecular bone and mechanical properties were found for the group that contained cells transduced with the osteogenic factor bone morphogenetic protein 2 (BMP2). Although the results from these in vivo studies were contrary to our hypothesis, we gained a number of insights into the future strategies for improving tendon-to-bone repair. First, the scar-mediated responses overwhelmed the regenerative strategy by recruiting inflammatory cells and presumably flooding the repair site with scar-promoting cytokines. Second, BMP2 was not an effective growth factor for enhancing tendon-to-bone healing, as indicated by the lost bone volume and compromised mechanical properties. Third, the acidic degradation products derived from the dense scaffolding materials could potentially impair tendon-to-bone healing. Fourth, the length scale of the mineral gradient (several millimeters) did not match that of the native tendon-to-bone insertion (tens of microns), and this mismatch may be a critical feature for improving tendon-to-bone repair.
Figure 4.
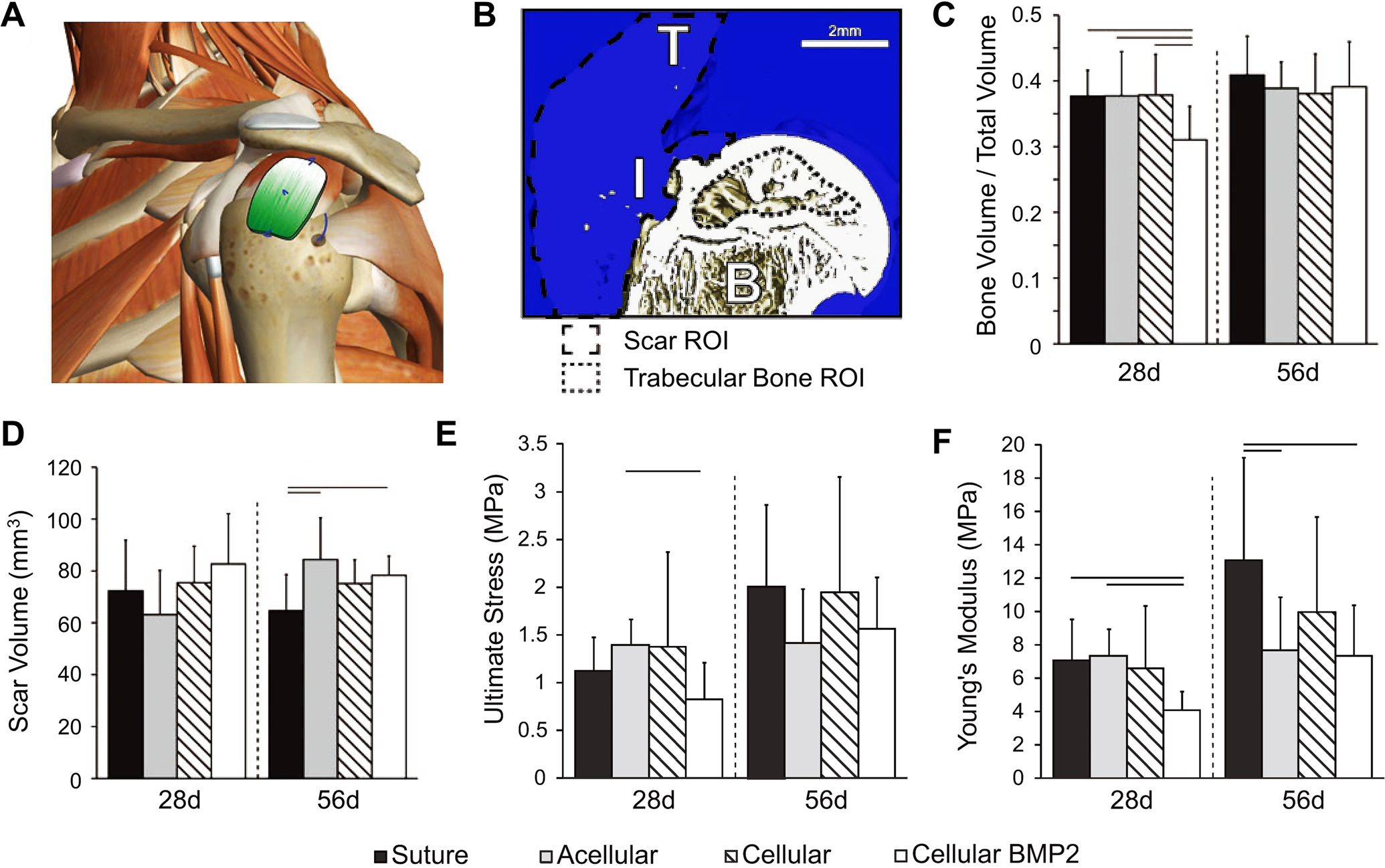
In vivo evaluation of PLGA electrospun nanofiber mats with mineral gradients for tendon-to-bone repair. A) Schematic illustration showing the human shoulder patched with a mineral-graded nanofiber mat. B) 3D reconstruction of the repaired attachment, in which B, I, and T represent bone, insertion, and tendon, respectively. C) Bone volume over total volume, D) scar volume, E) ultimate stress, and F) Young’s modulus as a function of implantation time. Significance is indicated by lines over bars (p < 0.05). For all data shown in C–F), “Suture”, “Acellular”, “Cellular”, and “Cellular BMP2” indicate the group without ASCs and scaffold, the group repaired with a scaffold but without ASCs, the group repaired with ASC-seeded scaffold, and the group repaired with BMP2-transduced ASC-seeded scaffold, respectively. A–F) Adapted with permission.[66] Copyright 2015, Mary Ann Liebert, Inc.
Recruiting appropriate cells to the injured site through graded biochemical cues represents another promising strategy to enhance tendon enthesis repair and regeneration. Taking advantage of the approaches shown in Figure 2B and C, it is possible to generate a gradient in bioactive components to guide cell adhesion, migration, and/or differentiation. To this end, we combined electrospinning and masked electrospray to fabricate uniaxially aligned nanofibers with graded collagen particles, providing a topological pattern together with a graded biochemical cue.[56] The SEM images in Figure 5A (i, ii) indicated that the pristine PCL nanofibers had smooth surfaces. As the collection time was prolonged, an increasing number of collagen particles were found on the nanofibers (Figure 5A, iii–vi). It should be noted that these collagen particles were fused with the surface of the nanofibers due to the presence of residual solvent within the particles. To quantify the graded density of collagen particles, fluorescein isothiocyanate-labeled bovine serum albumin (FITC-BSA) was pre-loaded into the collagen solution for electrospray. As shown in Figure 5B, the relative fluorescence intensity increased linearly along the aligned nanofibers. In addition, the gradient profile could be tuned by varying the moving speed of the mask, as observed from the distinct slopes. As anticipated, bone marrow-derived mesenchymal stem cells seeded on the nanofiber mats with graded collagen particles exhibited a directional migration toward the side with the highest density of collagen particles, giving rise to the generation of a graded cell density as a result of the chemotactic effect of collagen (Figure 5C).
Figure 5.
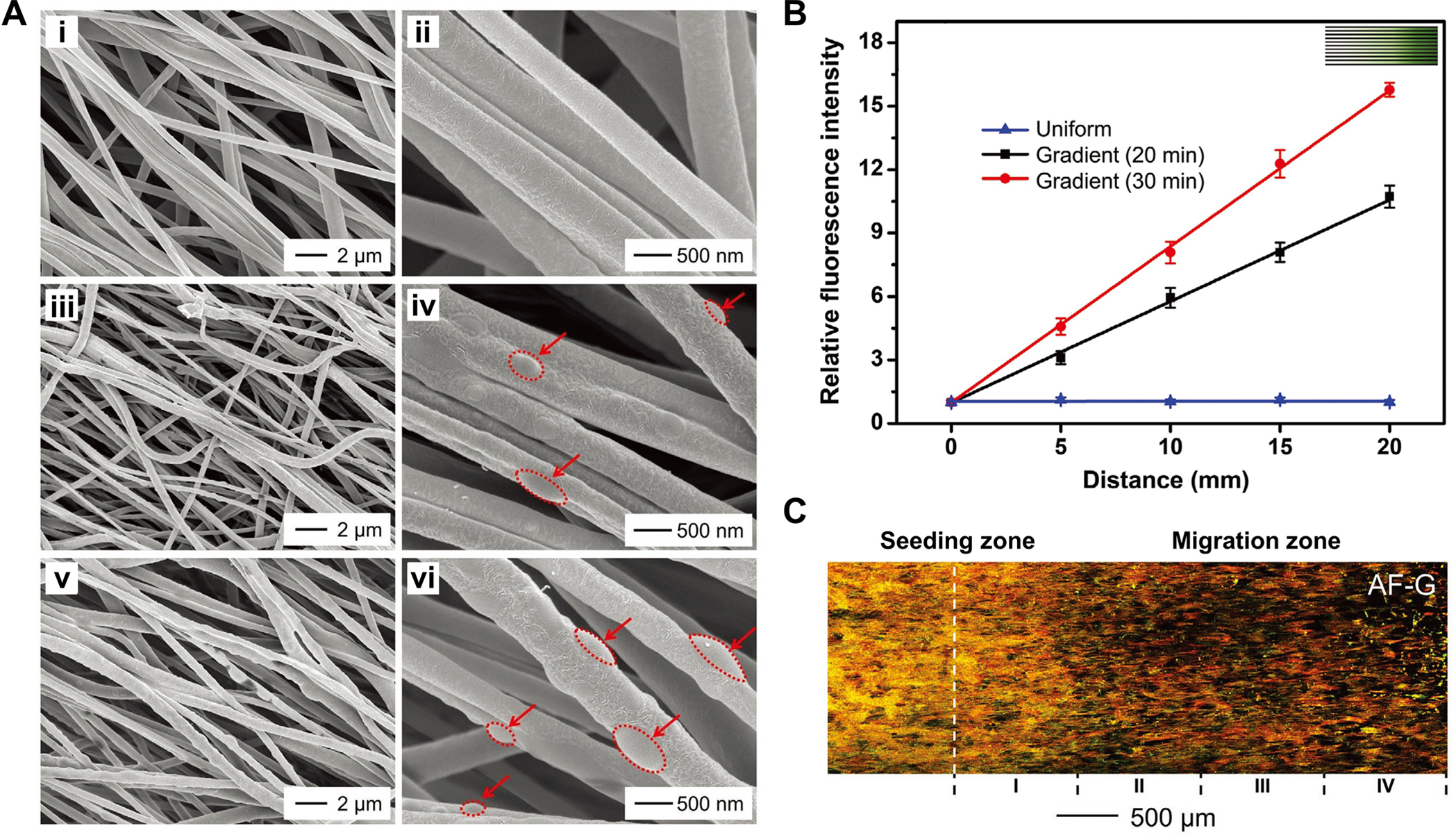
Uniaxially aligned PCL nanofibers with graded density in biomacromolecular particles. A) SEM images of nanofibers at the positions corresponding to collection times of i, ii) 0 min, iii, iv) 10 min, and v, vi) 20 min, respectively, in which the red dotted ellipses outline the collagen particles. B) Relative fluorescence intensity as a function of distance along the gradient of FITC-BSA-loaded collagen particles that were deposited on the nanofibers. C) Fluorescence image showing the migration of bone marrow-derived mesenchymal stem cells on the aligned nanofibers coated with density-graded collagen particles. The actin cytoskeleton and vinculin are shown in red and green, respectively, in which the yellow color corresponds to the overlay of red and green. A–C) Adapted with permission.[56] Copyright 2020, Wiley-VCH.
We also sought to mimic the structural disparity in the translational tissue between tendon and bone. Based on the approaches shown in Figure 2D and E, both the alignment and porosity of electrospun nanofibers could be modulated in a graded fashion. Since collagen fibrils are aligned in the tendon and become randomly distributed in the bone, the fabrication of nanofiber mats with both aligned and random portions within a single scaffold can recapitulate the gradation in fiber orientation at the insertion site. Our results showed that tendon fibroblasts responded to aligned-to-random nanofiber scaffolds by adopting an oriented elongation in the aligned region and a disorganized and rounded morphology in the random region.[62] To introduce more complexity into the structure of nanofiber mats, we further regulated cell behaviors with a nanofiber mat that exhibited gradations in both alignment and porosity.[60] The scaffold was fabricated through a solvent-exposure process (Figure 2D). As shown by the SEM images in Figure 6A–F, a densely-welded structure with low porosity formed at the top region of the nanofiber mat, followed by a smooth change to an aligned structure with high porosity in the bottom region. The corresponding 2D fast Fourier transformation patterns also suggested a gradual transition in orientation for the nanofibers. Bone marrow-derived mesenchymal stem cells were then used to examine biological responses to the hierarchically structured scaffold. As shown in Figure 6G–I, the seeded cells responded to the graded structure by displaying distinct organizations at different locations. Specifically, the cells were elongated and aligned along the direction of the aligned nanofibers in the bottom region. In the middle region, both elongated and disorganized cells were found, corresponding to the transitional places near the bottom and near the top regions, respectively. In the top region, the cells were randomly distributed, without well-defined orientations. Such results demonstrated the potential of a structural gradient for tuning the anisotropy-to-isotropy transition of cells, providing a method to mimic the cellular morphology at the tendon-to-bone interface. In addition to cell elongation and distribution, the sedimentation approach shown in Figure 2F enabled the introduction of gradients in both cell density and cell phenotype on nanofiber mats, holding promise for deciphering the complicated interactions among the different types of cells of the tendon enthesis and the synergistic effects for the re-establishment of the native graded ECM.[64]
Figure 6.
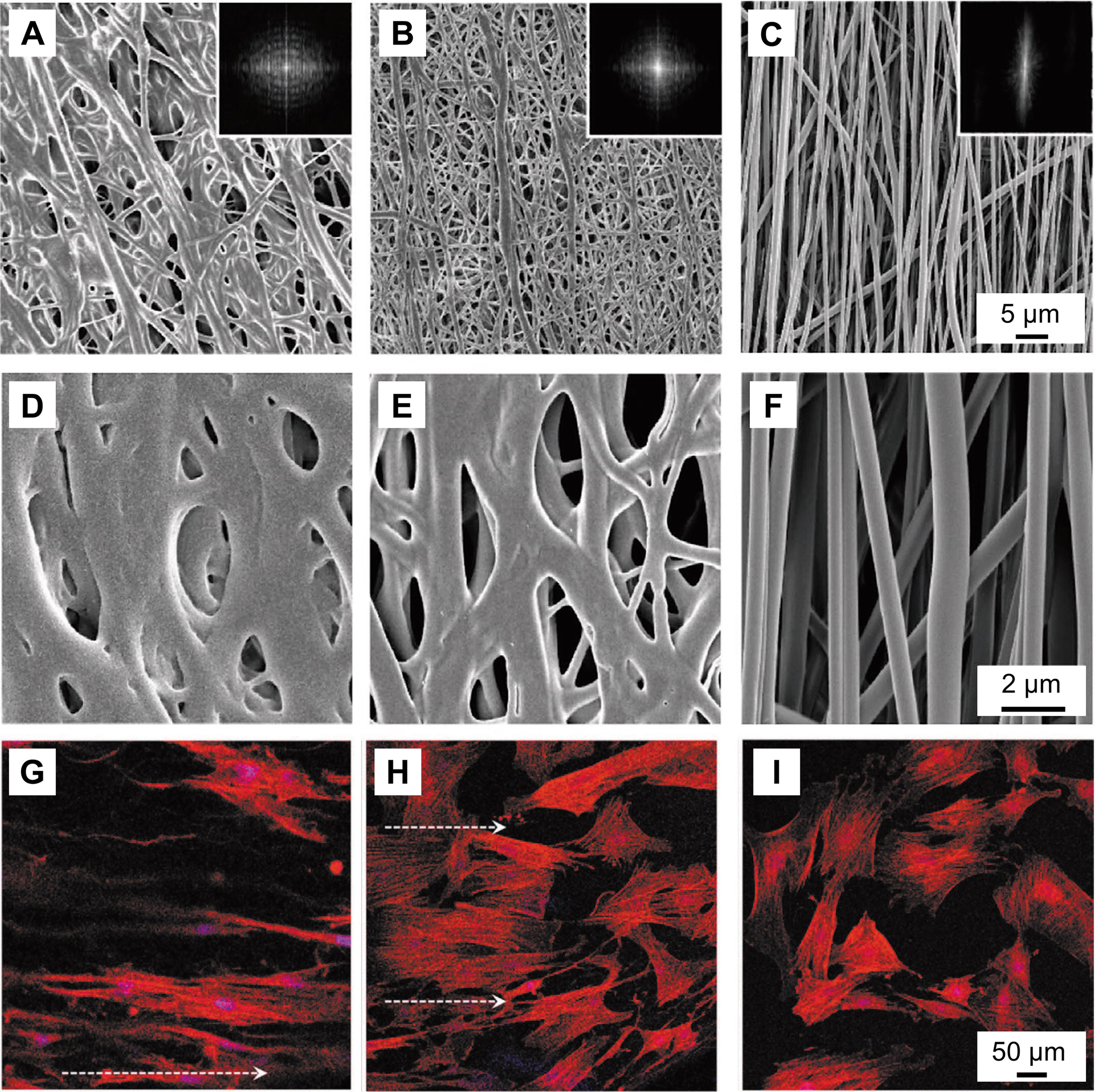
PLGA nanofiber mats with graded changes in porosity and alignment. A–F) SEM images showing a typical mat of uniaxially aligned PLGA nanofibers at the A, D) top, B, E) middle, and C, F) bottom regions after treatment with 70% ethanol solution. The insets show the 2D fast Fourier transformation patterns derived from the corresponding SEM images. G–I) Fluorescence images of bone marrow-derived mesenchymal stem cells cultured on the scaffold at the G) bottom, H) middle, and I) top regions. The actin cytoskeletons and cell nuclei are shown in red and blue, respectively, and the arrows point out the orientation of the aligned nanofibers. A–I) Adapted with permission.[60] Copyright 2019, Wiley-VCH.
3. Functionally-Graded Inverse Opal Scaffolds
Inverse opal scaffolds are fabricated by infiltrating and replicating the void spaces in close-packed lattices of uniform microspheres,[67–72] which are, in turn, fabricated using a microfluidic device.[73,74] Despite 3D printing has enabled the fabrication of scaffolds with well-defined structures,[75,76] the sophisticated and expensive instruments make the approach inaccessible to every laboratory.[67] Furthermore, limits in spatial resolution also make 3D printing impractical for creating complex structures with feature sizes on the micrometer scale.[75] In contrast, due to the fabrication simplicity of inverse opal scaffolds, any laboratory with general materials-processing capabilities is able to produce this class of scaffolds. With their sizes controlled in the range of 10–1000 μm, the microspheres have been successfully constructed from both hydrophilic (e.g., gelatin and chitosan) and hydrophobic (e.g., PLGA and PCL) polymers. As a major advantage over conventional porous scaffolds with non-uniform pores, an inverse opal scaffold contains an ordered array of uniform pores interconnected by windows throughout the sample. The size of the pores is determined by the diameter of the microspheres, while the size of the windows can be easily tuned by varying the experimental conditions such as the annealing temperature. Thanks to the uniformity in pore size and interconnectivity among the pores, inverse opal scaffolds are advantageous in promoting cell infiltration and migration, as well as efficient exchange of nutrients and metabolic wastes. These features promote homogeneous deposition of ECM by ensuring a uniform distribution of cells in the 3D scaffold and eventually facilitate the development of a homogenous tissue. By contrast, due to the absence of windows between adjacent pores, the use of non-uniform scaffolds typically causes inhomogeneous mechanical properties, batch-to-batch variability, and insufficient local tissue formation.[68] Considering the biosafety issue and the requirement on mechanical performance, we mainly focused on the FDA-approved, biodegradable, synthetic polymers such as PLGA and PCL.
Owing to the unique advantages of inverse opal scaffolds with regard to pore uniformity, interconnectivity between the cavities, and long-range ordered structure, a number of biomedical applications have been explored, including cell (co-)culture,[69,77] production of cell spheroids,[70,78] cell migration,[79] neovascularization,[71] cardiac tissue engineering,[80] bone/cartilage/osteochondral tissue engineering,[81–83] neural tissue engineering,[84] and wound healing.[85] Among these applications, inverse opal scaffolds are particularly well-suited for bone tissue engineering because of their structural similarity to natural trabecular bone. For bone regeneration, the size of the pores in a scaffold are typically controlled in the range of 100–400 μm to offer a suitable microenvironment for cell seeding.[86] To better match the composition and mechanical properties of bone, a variety of inorganic materials (e.g., apatite minerals) can be directly incorporated into the scaffolds during their fabrication or added through subsequent growth. Hydroxyapatite (HAp), the primary inorganic component of bone, is commonly used due to its biocompatibility, osteoconductivity, and osteoinductivity, as well as its ability to stiffen a polymer marix.[87,88] Different from the 2D nanofiber mats, inverse opal scaffolds can serve as interpositional scaffolds to bridge tendon and bone and thereby promote integration. In fact, inverse opal scaffolds cannot be directly used for tendon-to-bone repair due to the lack of compositional gradations or aligned structure to guide tendon regeneration. However, as a result of the structural similarities to natural trabecular bone, they are ideal foundations for anchoring stratified scaffolds into bone. In this section, we focus on the fabrication of inverse opal scaffolds with gradations in composition and structure, respectively.
3.1. Fabrication
Mineral gradation by gravity-induced sedimentation and diffusion.
As discussed earlier, a gradient in mineral content at the tendon-to-bone interface can minimize stress concentrations and thus facilitate load transfer. By leveraging gravity-induced sedimentation and diffusion, we fabricated PLGA inverse opal scaffolds featuring mineral gradients (Figure 7A).[89] Specifically, a close-packed lattice of gelatin microspheres was preheated for a specific period of time to initiate partial fusion between adjacent particles, followed by the introduction of a suspension of HAp nanoparticles. Under the action of gravitational force, the HAp nanoparticles settled into the voids among the gelatin microspheres. When held at a temperature above the glass transition temperature of gelatin, the softened gelatin would become sticky. As a result, the HAp nanoparticles tended to adhere to the gelatin microspheres, leading to preferential accumulation of HAp nanoparticles at the top surface of the close-packed lattice. This non-uniform distribution then limited the diffusion of HAp nanoparticles to the regions close to the bottom of the lattice, generating a mineral gradient within the scaffold. Subsequent infiltration of PLGA into the lattice and removal of the gelatin microspheres yielded a PLGA inverse opal scaffold featuring a graded mineral content along the vertical direction. The gradient profile could be conveniently tuned by varying the preheating time. It should be noted that extended preheating may dehydrate the gelatin microspheres and thereby reduce the frictional force between the HAp nanoparticles and gelatin surface, resulting in the complete settlement of HAp nanoparticles rather than the generation of a mineral gradient.
Figure 7.

Schematic illustrations of two methods for generating gradients in inverse opal scaffolds. A) Mineral gradation by gravity-induced sedimentation and diffusion. B) Hierarchical scaffolds with mineral gradation created by layer-by-layer coating.
Hierarchical scaffolds with mineral gradation created by layer-by-layer coating.
The mineral gradient at the native tendon enthesis only spans across a short distance, with a typical length of 20–60 μm regardless of species.[8] For most functionally-graded scaffolds, the mineral gradients are not on a comparable length scale to the native enthesis, leading to unsatisfactory outcomes in vivo. To address this limitation, we developed a layer-by-layer brushing method to create a mineral gradient over an adjustable length scale ranging from tens to one hundred micrometers (Figure 7B).[90] To recapitulate the anatomic structure of the tendon enthesis, the mineral-graded portion was topped with an unmineralized layer containing an array of parallel channels to guide the infiltration of tendon, together with a highly-mineralized inverse opal scaffold as the porous base. The fabrication of such a hierarchical scaffold started from the production of a close-packed lattice of gelatin microspheres, into which a HAp/PLGA composite was then infiltrated. Afterwards, a series of PLGA solutions with decreasing HAp concentrations were brush-coated onto the lattice in a layer-by-layer fashion, with the topmost layer made of pure PLGA. Layer-by-layer coating typically led to the formation of a stratified gradient. However, the brushing of a new layer brought in a small amount of solvent, causing efficient substance exchange and fusion with the existing layer. As such, a linear gradient in mineral content was established. To mimic the parallel orientation of collagen fibers in the tendon and made the entire scaffold interconnected, an array of parallel channels was created via laser machining. After template removal, a hierarchical scaffold was obtained. This scaffold can provide an appropriate microenvironment for tendon-to-bone repair, including unmineralized, aligned channels to guide the insertion of collagen fibrils, a mineral gradient on the order of tens of micrometers to match the native mineral gradient, and a mineralized, porous structure to integrate with the trabecular bone foundation.
3.2. Biological Evaluation
This section discusses the potential applications of inverse opal scaffolds with a graded transition in mineral content in promoting tendon-to-bone regeneration by functioning as an interpositional scaffold to bridge the disparate tissues at the enthesis.
By taking advantage of gravity-induced sedimentation and diffusion of HAp nanoparticles, as shown in Figure 7A, we fabricated a PLGA inverse opal scaffold with a mineral gradient along the vertical direction.[89] The resultant scaffold exhibited a well-defined porous structure with uniform, interconnected pores throughout the sample (Figure 8A). EDX quantification confirmed a mineral gradient along the vertical direction of the cryosectioned samples, with the calcium ratio varying from 0 to 50% (Figure 8B). Microcomputed tomography (micro-CT) further supported the successful establishment of a HAp gradient within the 3D porous scaffold, with the bottom and top surfaces exhibiting the highest and lowest image contrasts, respectively (Figure 8C and D). The incorporation of HAp gradient also resulted in a graded change in mechanical properties for guiding stem cell differentiation. As shown by the Alizarin Red S staining results in Figure 8E and F, after ASCs had been seeded in the scaffold for 21 days, a graded change in the deposition of calcium was observed along the vertical direction, distinct from the control scaffold. In addition, a number of bone-like nodules were found, suggesting osteogenic differentiation of ASCs, as dictated by the mineral gradient. Further semi-quantitative analysis on the mean pixel intensity along the divided region indicated a negative correlation with mineral content (Figure 8G). More importantly, the additional mineralization caused by the differentiated ASCs gave rise to the formation of a smoother mineral gradient, which is promising for the re-establishment of a robust interface at the tendon enthesis.
Figure 8.
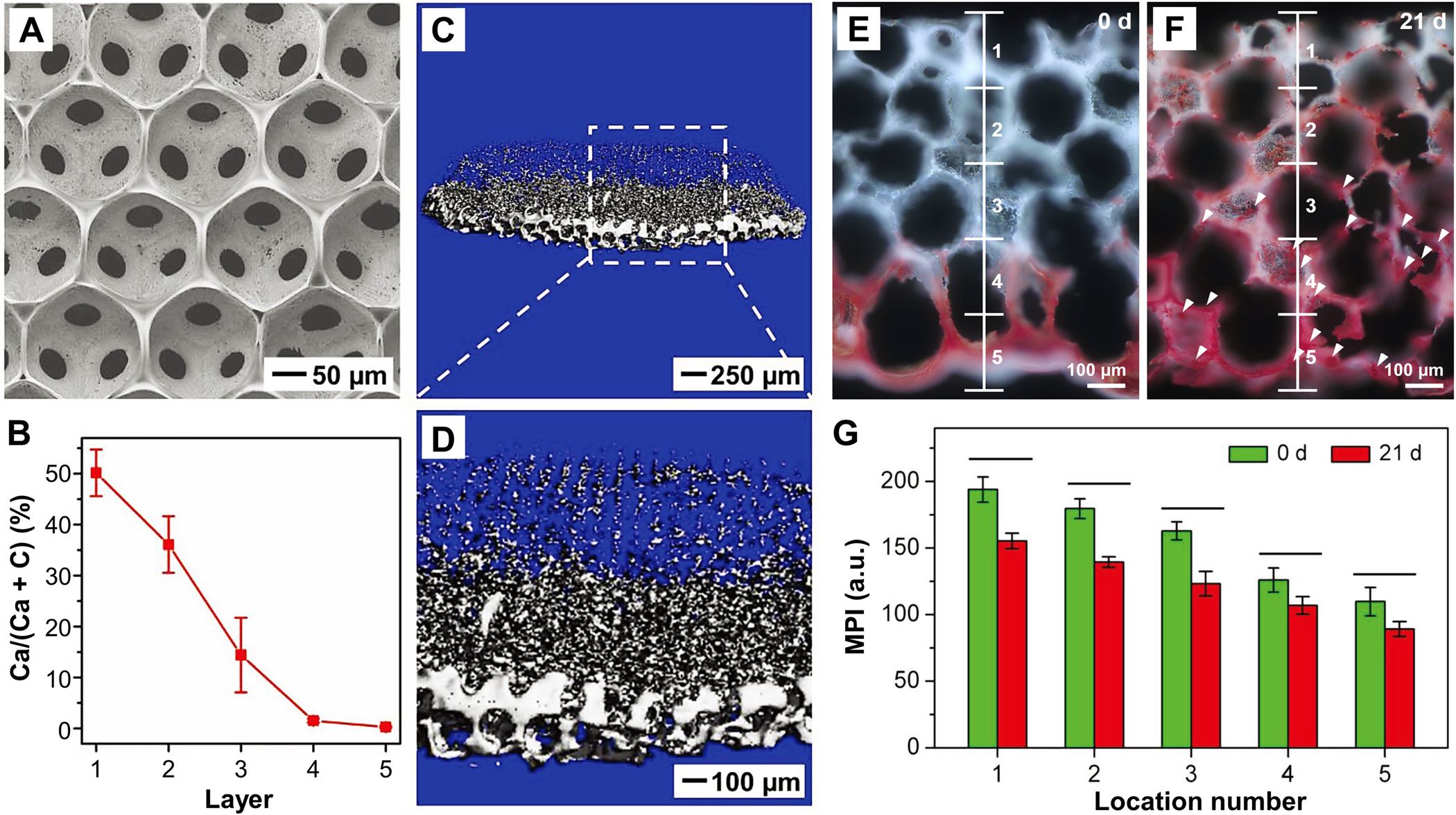
HAp-graded PLGA inverse opal scaffolds for spatial control of osteogenesis. A) SEM image of the scaffold taken from the top view. B) EDX characterization of mineral content along the vertical axis (n = 3). C, D) Micro-CT images of the scaffold at different magnifications. E, F) Calcium deposition on the scaffold seeded with ASCs at 0 and 21 days. Alizarin Red S is shown in red. G) Mean pixel intensity analysis of the images as shown in panels E) and F) (n = 3). Significance is indicated by lines over the bars (p < 0.05). A–G) Adapted with permission.[89] Copyright 2018, Wiley-VCH.
To further recapitulate the characteristics of the native tendon enthesis and create a mineral gradient with a matchable length scale, we fabricated a hierarchically structured scaffold with three functionally distinct but structurally continuous regions, in which the mineral gradient was established through layer-by-layer coating as shown in Figure 7B.[90] EDX mapping confirmed a graded change in calcium and carbon content across the entire thickness (ca. 37 μm), which corresponded to the graded variations in HAp nanoparticle density (Figure 9A). The SEM images in Figure 9B showed a 10 × 10 array of channels. The region between the channels and the inverse opal base was interconnected, ensuring the efficient exchange of nutrients and metabolic wastes across the entire scaffold. In general, the introduction of stiff minerals into a compliant PLGA matrix would improve the mechanical strength. As shown in Figure 9C, the measured local Young’s modulus along the mineral gradient was positively correlated with the density of HAp nanoparticles, with the values varying from 200 MPa in the unmineralized region to 2 GPa in the mineralized region. This result compares favorably to the modulus of the native tendon-to-bone attachment, which changes from 200 MPa in the compliant tendon to 20 GPa in the stiff bone. Among all the scaffolds we have fabricated, the system shown in Figure 9 is most promising in meeting the mechanical needs of the native tissue. The gap in mechanical performance between the mineralized region of the scaffold and bone could be bridged through subsequent biomineralization by the differentiated osteoblasts. To evaluate the biological effect of the mineral gradient on the differentiation of ASCs, both tenogenesis and osteogensis were examined after 28 days of culture, in which scleraxis (Scx) and Runx2 were used as the tendon-specific marker and an early marker of osteogenic differentiation, respectively. As shown in Figure 9D, opposite trends were identified for tenogenesis and osteogenesis, suggesting preferential differentiation of ASCs into tenogenic and osteogenic lineages according to the level of mineralization. In the transitional region, both cell lineages were present, which is particularly important in facilitating functional recovery via heterotypic cellular interactions. This new class of hierarchical scaffolds are expected to promote the production of aligned collagen fibrils, graded mineralization at the interface, and efficient bone regeneration, holding great potential to facilitate the formation of a robust tendon-to-bone attachment in vivo.
Figure 9.
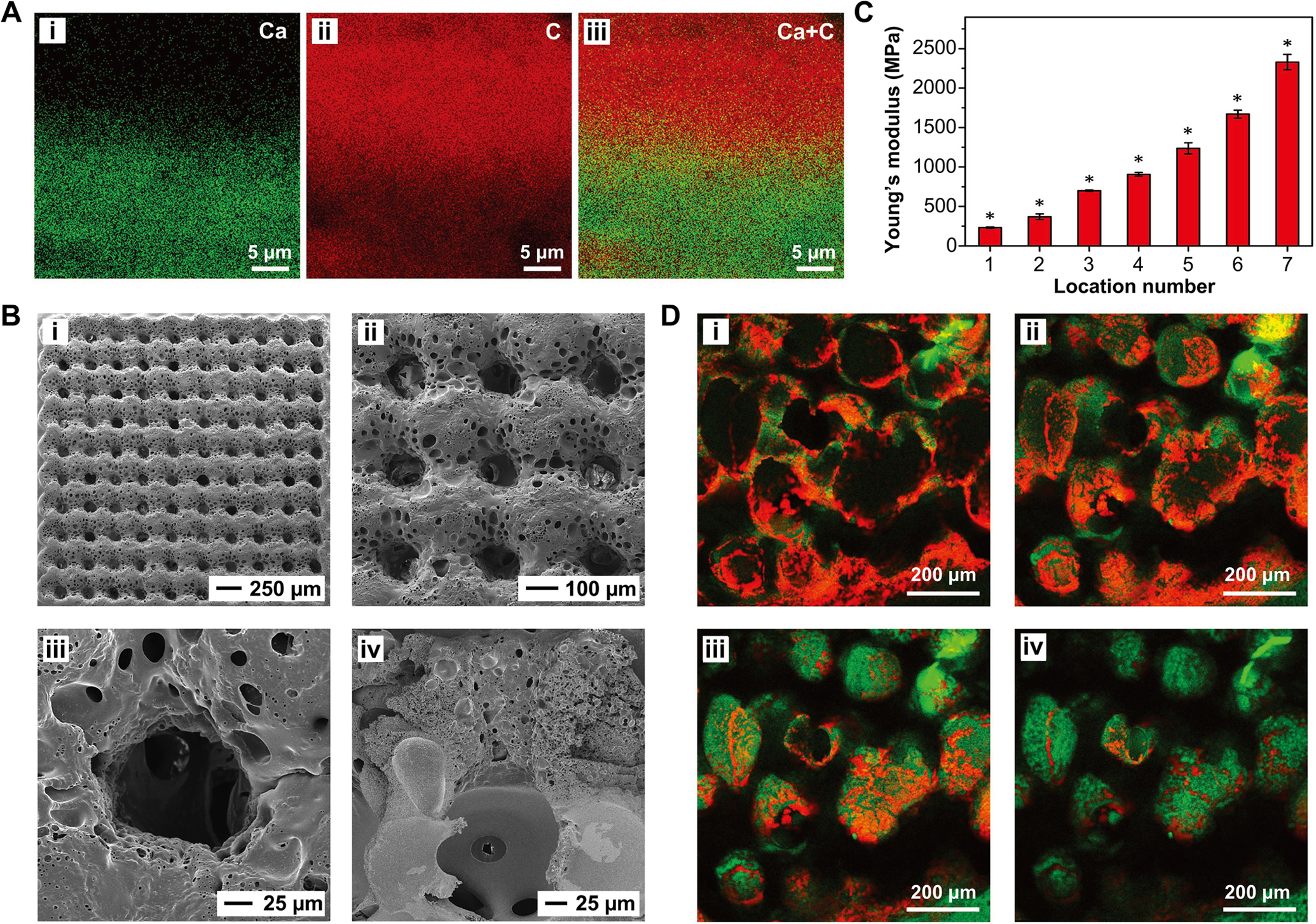
A hierarchically structured PLGA inverse opal scaffold developed for tendon-to-bone repair. A) The distributions of i) calcium, ii) carbon, and iii) calcium + carbon in the cross-section of the scaffold mapped by EDX prior to template removal. B) Local Young’s modulus of seven locations along the mineral gradient from the unmineralized end to the mineralized end (n = 6). Significance for all pairwise comparisons is indicated by * over the bars (p < 0.05). C) SEM images of the scaffold from the i, ii) top and iii, iv) cross-sectional views. D) Scx (red) and Runx2 (green) staining of ASCs seeded in the scaffold after 28 days of culture at the i) unmineralized region, ii) transitional region with less mineral content, iii) transitional region with more mineral content, and iv) highly mineralized region, respectively. A–D) Adapted with permission.[90] Copyright 2018, Wiley-VCH.
4. Conclusion and Perspectives
Functionally-graded scaffolds can replicate some of the unique features of transitional tissues in terms of gradations in composition, structure, mechanical properties, and cell phenotypes. Among various transitional tissues in the musculoskeletal systems, the tendon enthesis plays an important role in minimizing the stress concentrations between the compliant tendon and stiff bone, allowing for effective load transfer without rupture. Both electrospun nanofibers and inverse opals are known for their inherent merits in recapitulating the physical and/or biochemical cues of the native tendon enthesis. We have further enhanced their capability to augment tendon-to-bone repair by developing a number of methods to endow them with gradients in terms of mineral content, growth factors, biomacromolecular particles, alignment, porosity, and cell phenotypes. Determined by their morphology, the electrospun nanofiber mats are typically used as patches over the repaired tendon, while the inverse opal scaffolds can be sandwiched between tendon and bone to better integrate the disparate tissues. By engineering the properties of these scaffolds, we can conveniently manipulate the behaviors of seeded cells, including directed migration and elongation, guided differentiation, and efficient secretion of specific ECM proteins, demonstrating their potential use in augmenting tendon-to-bone repair.
In spite of these advances, a number of challenges remain to be addressed. First, the gradations in most of the functionally-graded scaffolds span across hundreds of micrometers or even several millimeters, creating a major mismatch with the length scale found at the native insertion. In addition to the layer-by-layer coating technique discussed in section 3.1, additional methods with higher precision and controllability should be developed to generate gradation over appropriate length scales. Second, electrospun nanofiber mats are typically composed of densely packed polymer fibers, which inevitably brings in a large quantity of acidic degradation products after implantation and thus compromises tissue healing. Moreover, insufficient cell loading and indirect guidance between the injured tendon and bone also impair tissue repair. In this regard, there is a strong interest to develop 3D electrospun nanofibers with tunable degradation rates and increased cell loading capacities. Third, there is a pressing need to examine the in vivo effects of these scaffolds in appropriate animal models. In fact, many exciting results from in vitro experiments are lost when translated to animal studies because of the complexity of a healing process and, often, the involvement of an inflammatory environment. In view of these possible obstacles, future work should focus on these aspects to determine which factor(s) can facilitate successful clinical translation. Looking ahead, we believe a comprehensive investigation on the structure-property relationships of the scaffolds, preferably tested in animal models, will lead to significant improvement in tendon-to-bone repair.
Acknowledgements
C.Z. acknowledges startup funds from Nankai University and the National Natural Science Foundation of China (52003123). Y.X. acknowledges a research grant from the National Institutes of Health (R01 AR060820) and startup funds from the Georgia Institute of Technology. We are grateful to our coworkers and collaborators for their invaluable contributions to this project.
Biographies

Chunlei Zhu received his B.S. from Jilin University in 2008 and Ph.D. from the Institute of Chemistry, Chinese Academy of Sciences in 2013 with Prof. Shu Wang. He then conducted his postdoctoral research with Prof. Younan Xia at the Georgia Institute of Technology from 2014 to 2017 and Prof. Ben Zhong Tang at the Hong Kong University of Science and Technology from 2017 to 2018. He started his independent academic career in the College of Chemistry at Nankai University in 2018. His current research interests focus on the design and fabrication of functional organic and/or polymeric materials for biomedical applications.

Younan Xia studied at the University of Science and Technology of China (B.S., 1987) and University of Pennsylvania (M.S., 1993) before receiving his Ph.D. from Harvard University in 1996 (with George M. Whitesides). He started as an Assistant Professor of Chemistry at the University of Washington (Seattle) in 1997 and was promoted to Associate Professor and Professor in 2002 and 2004, respectively. He joined the Department of Biomedical Engineering at Washington University in St. Louis in 2007 as the James M. McKelvey Professor. Since 2012, he holds the position of Brock Family Chair and GRA Eminent Scholar in Nanomedicine at the Georgia Institute of Technology.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Chunlei Zhu, Key Laboratory of Functional Polymer Materials of Ministry of Education, State Key Laboratory of Medicinal Chemical Biology, Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China.
Jichuan Qiu, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 30332, USA.
Stavros Thomopoulos, Department of Orthopaedic Surgery, Columbia University, New York, NY 10032, USA; Department of Biomedical Engineering, Columbia University, New York, NY 10027, USA.
Younan Xia, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 30332, USA; School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332, USA.
References
- [1].Briggs AM, Cross MJ, Hoy DG, Sànchez-Riera L, Blyth FM, Woolf AD, March L, Gerontologist 2016, 56, S243. [DOI] [PubMed] [Google Scholar]
- [2].Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GAC, McInnes IB, Rodeo SA, Nat. Rev. Dis. Primers 2021, 7, 1. [DOI] [PubMed] [Google Scholar]
- [3].Dang A, Davies M, Sports Med. Arthrosc. Rev 2018, 26, 129. [DOI] [PubMed] [Google Scholar]
- [4].Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL, J. Shoulder Elbow Surg 2007, 16, 181. [DOI] [PubMed] [Google Scholar]
- [5].Lu HH, Thomopoulos S, Annu. Rev. Biomed. Eng 2013, 15, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K, J. Bone Jt. Surg., Am. Vol 2004, 86A, 219. [DOI] [PubMed] [Google Scholar]
- [7].Genin GM, Thomopoulos S, Nat. Mater 2017, 16, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deymier-Black AC, Pasteris JD, Genin GM, Thomopoulos S, J. Biomech. Eng 2015, 137, 1110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwartz AG, Pasteris JD, Genin GM, Daulton TL, Thomopoulos S, PLoS One 2012, 7, e48630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu Y, Birman V, Demyier-Black A, Schwartz AG, Thomopoulos S, Genin GM, Biophys. J 2015, 108, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alexander B, Daulton TL, Genin GM, Lipner J, Pasteris JD, Wopenka B, Thomopoulos S, Soc JR. Interface. 2012, 9, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maganaris CN, Paul JP, J. Physiol 1999, 521, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rho JY, Kuhn-Spearing L, Zioupos P, Med. Eng. Phys 1998, 20, 92. [DOI] [PubMed] [Google Scholar]
- [14].Thomopoulos S, Williams GR, Soslowsky LJ, J. Biomech. Eng 2003, 125280106. [DOI] [PubMed] [Google Scholar]
- [15].Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH, J. Orthop. Res 2008, 26, 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Langer R, Vacanti JP, Science, 1993, 260, 920. [DOI] [PubMed] [Google Scholar]
- [17].Yang PJ, Temenoff JS, Tissue Eng., Part B 2009, 15, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang X, Bogdanowicz D, Erisken C, Lee NM, Lu HH, J. Shoulder Elbow Surg 2012, 21, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ricchetti ET, Aurora A, Iannotti JP, Derwin KA, J. Shoulder Elbow Surg 2012, 21, 251. [DOI] [PubMed] [Google Scholar]
- [20].Khademhosseini A, Langer R, Nat. Protoc 2016, 11, 1775. [DOI] [PubMed] [Google Scholar]
- [21].Hoffman T, Khademhosseini A, Langer R, Tissue Eng. Part A 2019, 25, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Calejo I, Costa-Almeida R, Reis RL, Gomes ME, Tissue Eng. Part B Rev 2019, 25, 330. [DOI] [PubMed] [Google Scholar]
- [23].Lowen JM, Leach JK, Adv. Funct. Mater 2020, 30, 1909089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li C, Ouyang L, Armstrong JPK, Stevens MM, Trends Biotechnol. 2021, 39, 150. [DOI] [PubMed] [Google Scholar]
- [25].Sola A, Bellucci D, Cannillo V, Biotechnol. Adv 2016, 34, 504. [DOI] [PubMed] [Google Scholar]
- [26].Karpiak JV, Ner Y, Almutairi A, Adv. Mater 2012, 24, 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mahadik BP, Wheeler TD, Skertich LJ, Kenis PJA, Harley BAC, Adv. Healthcare Mater 2014, 3, 449. [DOI] [PubMed] [Google Scholar]
- [28].Wang Z, Wang K, Huang H, Cui X, Shi X, Ma X, Li B, Zhang Z, Tang X, Chiang MYM, Small 2018, 14, 1802717. [DOI] [PubMed] [Google Scholar]
- [29].Wang Z, Shi X, Huang H, Yao C, Xie W, Huang C, Gu P, Ma X, Zhang Z, Chen L-Q, Mater. Horiz 2017, 4, 869. [Google Scholar]
- [30].Li C, Ouyang L, Pence IJ, Moore AC, Lin Y, Winter CW, Armstrong JPK, Stevens MM, Adv. Mater 2019, 31, 1900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jain G, Ford AJ, Rajagopalan P, ACS Biomater. Sci. Eng 2015, 1, 621. [DOI] [PubMed] [Google Scholar]
- [32].Vega SL, Kwon MY, Song KH, Wang C, Mauck RL, Han L, Burdick JA, Nat. Commun 2018, 9, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Q, Lu H, Kawazoe N, Chen G, Mater. Lett 2013, 107, 280. [Google Scholar]
- [34].Nie TT, Xue L, Ge M, Ma HY, Zhang JC, Mater. Lett 2016, 176, 25. [Google Scholar]
- [35].Giachini PAGS, Gupta SS, Wang W, Wood D, Yunusa M, Baharlou E, Sitti M, Menges A, Sci. Adv 2020, 6, eaay0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hardin JO, Ober TJ, Valentine AD, Lewis JA, Adv. Mater 2015, 27, 3279. [DOI] [PubMed] [Google Scholar]
- [37].Costantini M, Jaroszewicz J, Kozoń Ł, Szlązak K, Święszkowski W, Garstecki P, Stubenrauch C, Barbetta A, Guzowski J, Angew. Chem. Int. Ed 2019, 58, 7620. [DOI] [PubMed] [Google Scholar]
- [38].Xin S, Dai J, Gregory CA, Han A, Alge DL, Adv. Funct. Mater 2020, 30, 1907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Horner CB, Maldonado M, Tai Y, Rony RMIK, Nam J, ACS Appl. Mater. Interfaces 2019, 11, 45479. [DOI] [PubMed] [Google Scholar]
- [40].Wang Y, Xu R, Luo G, Lei Q, Shu Q, Yao Z, Li H, Zhou J, Tan J, Yang S, Zhan R, He W, Wu J, Acta Biomater. 2016, 30, 246. [DOI] [PubMed] [Google Scholar]
- [41].Spinnrock A, Schupp D, Cölfen H, Small 2018, 14, 1803518. [DOI] [PubMed] [Google Scholar]
- [42].Zwi-Dantsis L, Wang B, Marijon C, Zonetti S, Ferrini A, Massi L, Stuckey DJ, Terracciano CM, Stevens MM, Adv. Mater 2020, 32, 1904598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xu G, Ding Z, Lu Q, Zhang X, Zhou X, Xiao L, Lu G, Kaplan D, Protein Cell 2020, 11, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rajab TK, O’Malley TJ, Tchantchaleishvili V, Artif. Organs 2020, 44, 1031. [DOI] [PubMed] [Google Scholar]
- [45].Hussey GS, Dziki JL, Badylak SF, Nat. Rev. Mater 2018, 3, 159. [Google Scholar]
- [46].Liao J, Xu B, Zhang R, Fan Y, Xie H, Li X, J. Mater. Chem. B 2020, 8, 10023. [DOI] [PubMed] [Google Scholar]
- [47].Xue J, Wu T, Dai YQ, Xia Y, Chem. Rev 2019, 119, 5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xue J, Xie J, Liu W, Xia Y, Acc. Chem. Res 2017, 50, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu T, Mo X, Xia Y, Adv. Healthcare Mater 2020, 9, 1901761. [Google Scholar]
- [50].Xue J, Pisignano D, Xia Y, Adv. Sci 2020, 7, 2000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Xue J, Wu T, Xia Y, APL Mater. 2018, 6, 120902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu W, Thomopoulos S, Xia Y, Adv. Healthcare Mater 2012, 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y, Nano Lett. 2009, 9, 2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu W, Lipner J, Xie J, Manning CN, Thomopoulos S, Xia Y, ACS Appl. Mater. Interfaces 2014, 6, 2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tanes ML, Xue J, Xia Y, J. Mater. Chem. B 2017, 5, 5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xue J, Wu T, Qiu J, Rutledge S, Tanes ML, Xia Y, Adv. Funct. Mater 2020, 30, 2002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li X, MacEwan MR, Xie J, Siewe D, Yuan X, Xia Y, Adv. Funct. Mater 2010, 20, 1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xie J, Jiang J, Davoodi P, Srinivasan MP, Wang C-H, Chem. Eng. Sci 2015, 125, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xue J, Zhu C, Li J, Li H, Xia Y, Adv. Funct. Mater 2018, 28, 1705563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li H, Wu T, Xue J, Ke Q, Xia Y, Macromol. Rapid Commun 2020, 41, 1900579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li H, Zhu C, Xue J, Ke Q, Xia Y, Macromol. Rapid Commun 2017, 38, 1600723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xie J, Li X, Lipner J, Manning CN, Schwartz AG, Thomopoulos S, Xia Y, Nanoscale 2010, 2, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li D, Wang Y, Xia Y, Nano Lett. 2003, 3, 1167. [Google Scholar]
- [64].Liu W, Zhang Y, Thomopoulos S, Xia Y, Angew. Chem. Int. Ed 2013, 52, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, Thomopoulos S, Biophys. J 2009, 97, 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lipner J, Shen H, Cavinatto L, Liu W, Havlioglu N, Xia Y, Galatz LM, Thomopoulos S, Tissue Eng. Part A 2015, 21, 2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang YS, Zhu C, Xia Y, Adv. Mater 2017, 29, 1701115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang YS, Choi S-W, Xia Y, Soft Matter 2013, 9, 9747. [Google Scholar]
- [69].Choi S-W, Xie J, Xia Y, Adv. Mater 2009, 21, 2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang Y, Xia Y, Adv. Funct. Mater 2012, 22, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Choi S-W, Zhang Y, MacEwan MR, Xia Y, Adv. Healthcare Mater 2013, 2, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhang YS, Cai X, Yao J, Xing W, Wang LV, Xia Y, Angew. Chem. Int. Ed 2014, 53, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Choi S-W, Cheong IW, Kim J-H, Xia Y, Small 2009, 5, 454. [DOI] [PubMed] [Google Scholar]
- [74].Choi S-W, Zhang Y, Xia Y, Adv. Funct. Mater 2009, 19, 2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D, Compos. B Eng 2018, 143, 172. [Google Scholar]
- [76].Murphy SV, Atala A, Nat. Biotechnol 2014, 32, 773. [DOI] [PubMed] [Google Scholar]
- [77].Kim J, Bencherif SA, Li WA, Mooney DJ, Macromol. Rapid Commun 2014, 35, 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shao C, Liu Y, Chi J, Wang J, Zhao Z, Zhao Y, Research 2019, 2019, 9783793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chiu PJ, Mei JC, Huang YC, Yu JS, Microelectron. Eng 2013, 111, 277. [Google Scholar]
- [80].Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD, Proc. Natl. Acad. Sci. USA 2010, 107, 15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sommer MR, Vetsch JR, Leemann J, Müller R, Studart AR, Hofmann S, J. Biomed. Mater. Res. B Appl. Biomater 2017, 105, 2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kuo YC, Tsai YT, Colloids Surf. B 2011, 82, 616. [DOI] [PubMed] [Google Scholar]
- [83].Galperin A, Oldinski RA, Florczyk SJ, Bryers JD, Zhang MQ, Ratner BD, Adv. Healthcare Mater 2013, 2, 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Xia L, Shang Y, Chen X, Li H, Xu X, Liu W, Yang G, Wang T, Gao X, Chai R, Front. Bioeng. Biotechnol 2020, 8, 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Weng W, Chi J, Yu Y, Zhang C, Shi K, Zhao Y, ACS Appl. Mater. Interfaces 2021, 13, 4567. [DOI] [PubMed] [Google Scholar]
- [86].Kim HL, Jung GY, Yoon JH, Han JS, Park YJ, Kim DG, Zhang M, Kim DJ, Mater. Sci. Eng., C 2015, 54, 20. [DOI] [PubMed] [Google Scholar]
- [87].Choi S-W, Zhang Y, Thomopoulos S, Xia Y, Langmuir 2010, 26, 12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nonoyama T, Wada S, Kiyama R, Kitamura N, Mredha MTI, Zhang X, Kurokawa T, Nakajima T, Takagi Y, Yasuda K, Gong JP, Adv. Mater 2016, 28, 6740. [DOI] [PubMed] [Google Scholar]
- [89].Zhu C, Qiu J, Pongkitwitoon S, Thomopoulos S, Xia Y, Adv. Mater 2018, 30, 1706706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhu C, Pongkitwitoon S, Qiu J, Thomopoulos S, Xia Y, Adv. Mater 2018, 30, 1707306. [DOI] [PMC free article] [PubMed] [Google Scholar]


