Abstract
Limited research has investigated the development of auditory ERPs in young children, and particularly how stimulus intensity may affect these auditory ERPs. Previous research has also yielded inconsistent findings regarding differences in the development of auditory ERPs in autism and typical development. Furthermore, stimulus intensity may be of particular interest in autism insofar as autistic people may have atypical experiences of sound intensity (e.g., hyperacusis). Therefore, the present study examined associations between age and ERPs evoked by tones of differing intensities (50, 60, 70, and 80 dB SPL) in a large sample of young children (2 – 5 years) with and without an autism diagnosis. Correlations between age and P1 latencies were examined, while cluster-based permutation testing was used to examine associations between age and neural response amplitudes, as well as group differences in amplitude, over all electrode sites in the longer time window of 1 – 350 ms. Older autistic participants had faster P1 latencies, but these effects only attained significance over the right hemisphere in response to soft 50 dB sounds. Autistic participants had slower P1 responses to 80 dB sounds over the right hemisphere. Over the scalp regions associated with the later N2 response, more negative response amplitudes (that is, larger N2 responses) were observed in typically-developing than autistic participants. Furthermore, continuous associations between response amplitudes and age suggested that older typically-developing participants exhibited stronger N2 responses to all intensities, though this effect may have at least in part reflected the absence of small positive voltage deflections in the N2 latency window. Age was associated with amplitudes of responses to 50 dB through 70 dB sounds in autism, but in contrast to Typical Development (TD), little evidence of relationships between age and amplitudes in the N2 latency window was found in autism in the 80 dB condition. Although caution should be exercised in interpretation due to the cross-sectional nature of this study, these findings suggest that developmental changes in auditory responses may differ across diagnostic groups in a manner that depends on perceived loudness and/or stimulus intensity.
Keywords: Development, cortical auditory event-related potentials (ERPs), autism, P1, N2
1. Introduction
Although a number of studies have examined age-related changes in children’s cortical auditory event-related potentials (ERPs; e.g., Čeponienė, Rinne, & Näätänen, 2002; Ponton, Eggermont, Khosla, Kwong, & Don, 2002; Shafer, Yu, & Wagner, 2015), large gaps remain in this literature. In particular, a review by Wunderlich and Cone-Wesson (2006) identifies two major areas wherein further developmental research on auditory ERPs is required: the development of cortical auditory responses in toddlers and younger children (ages 1 – 6); and the effects of variations in stimulus properties on children’s auditory ERPs. For example, although the effects of varying stimulus intensity have been investigated in a number of ERP studies with adults (e.g., Hegerl, Gallinat, & Mrowinski, 1994; Schechter & Buchsbaum, 1973, Tlumak, Durrant, & Delgado, 2016), there is little research regarding the development of intensity-dependency in auditory ERPs. Furthermore, there is evidence that intensity-dependent responses may be abnormal in autism spectrum development (ASD1; Bruneau, Roux, Adrien, & Barthélémy, 1999; Bruneau, Bonnet-Brilhault, Gomet, Adrien, & Barthélémy, 2003). Further, there is evidence of differences in intensity-dependent responses across different parts of the autistic population, such as autistic children with and without disproportionate megalencephaly (De Meo-Monteil et al., 2019).
Despite historical inattention, atypical responses to aspects of the sensory environment (hereafter referred to as “sensory features” of the ASD phenotype) are increasingly recognized as a major part of the ASD phenotype. Not only does a recent review and meta-analysis on sensory issues in ASD highlight this increasing recognition, it also indicates that autistic people differ from controls in levels of sensory over-responsivity, under-responsivity, and seeking (Ben-Sasson, Gal, Fluss, Katz-Zetler, & Cermak, 2019). Notably, the atypical autistic sensory phenotype can include increased sensitivity to sound and hyperacusis (Khalfa et al., 2004). It therefore seems reasonable to ask whether altered intensity-dependent ERPs in autism might be related to autistic people’s atypical sensory behaviours and experiences. Indeed, when both autistic and typically-developing children are clustered on the basis of the intensity-dependency of their auditory ERPs, autistic participants sorted into different clusters differ in caregiver-reported levels of auditory distractibility/filtering (Dwyer et al., 2020). Unfortunately, many autistic people describe ways that their sensory features can lead to substantial distress or disability in everyday environments constructed without the sensory needs of autistic people in mind (see, e.g., Danker, Strnadová, & Cumming, 2019; Madriaga, 2010; Maloret & Scott, 2018). This real-world significance highlights the need for further research regarding autistic sensory processing and its development.
1.1. Maturation of Cortical Auditory ERPs in Typical Development (TD)
In young children, the fronto-central topography of auditory ERPs can be described in terms of the auditory P1 and N2 components (Čeponiene; Lepistö, Alku, Aro, & Näätänen, 2003; Ponton et al., 2002; Shafer et al., 2015). Generally, it is only later in development, at around ages 10-11, that the fronto-central N1 (or N1b) and P2 components emerge to form the canonical adult sequence P1-N1b-P2-N2 (Gilley, Sharma, Dorman, & Martin, 2005; Ponton et al., 2002; cf. Wunderlich, Cone-Wesson, & Shepherd, 2006). Before this age, the P1 is the main cortical auditory response at ~100-150 ms. P1 latency generally decreases with age (Ponton et al., 2002, Gilley et al., 2005; Shafer et al., 2015; cf. Wunderlich et al., 2006), while P1 amplitudes may show small increases with age until the age of four (Shafer et al., 2015) or even ages 5-6 (Gilley et al., 2005), after which P1 amplitudes appear fairly stable until the emergence of the N1 (Ponton et al., 2002). The later N2 response does not appear to undergo significant maturation among children aged similarly to those in the present study, with no age-related changes being found in N2 amplitudes or latencies (Shafer et al., 2015). However, Ponton et al. (2002) suggest that N2 latencies increase in slightly older school-age children, while N2 amplitudes begin declining after emergence of the auditory P2.
There are also temporal auditory ERPs in young children. These include the negative Na (Pang & Taylor, 2000; Shafer et al., 2015), positive Ta (Ponton et al., 2002; Shafer et al., 2015), negative Tb (Bruneau et al., 1999; Pang & Taylor, 2000; Ponton et al., 2002; Shafer et al., 2015), and positive TP200 (Ponton et al., 2002) waves. The Ta and Tb responses might only emerge around the age of four (Shafer et al., 2015). Overall, however, such temporal ERPs may be visible over mastoid sites used in linked mastoid references, and thus be subtracted from other sites when a mastoid reference is employed.
1.2. Intensity-Dependency of Cortical Auditory ERPs in Typically Developing Children
In Typical Development (TD), little is known about how children’s cortical auditory potentials are affected by stimulus intensity and even less regarding how these intensity-dependent responses change over the course of development. Davies, Chang, and Gavin (2010) found that P1 amplitudes were greater to 70 compared to 50 dB sounds in children with or without sensory processing disorders. In another series of studies similar in design to the current study, Bruneau and colleagues (1997, 1999, 2003) examined both fronto-central and temporal responses to tones of 50, 60, 70, and 80 dB SPL in children aged 4-8. Latency and amplitude of the child fronto-central N1 may (Bruneau et al., 1999) or may not (Bruneau et al., 1997) have been modulated by intensity. However, the child Tb was larger and faster to louder stimuli (Bruneau et al., 1997, 1999, 2003). These effects did not vary between 4-6- and 6-8-year-olds (Bruneau et al., 1997).
De Meo-Monteil et al. (2019) examined intensity-dependent ERP global field power (GFP) in 2-4-year-olds in a subset (both ASD and TD) of the dataset to be reported here. Reporting on analyses of their typically-developing individuals, De Meo-Monteil and colleagues found that stimulus intensity modulated latencies of the GFP peak of the main auditory response, as well as GFP amplitudes 55-115ms after stimulus onset. Based on results obtained by Shafer et al. (2015) it seems plausible that these effects reflect loudness-dependency in the cortical auditory P1 though the spatial location of the effects in De Meo-Monteil et al cannot be determined on the basis of the GFP. Finally, Dwyer et al. (2020) normalized GFP in the full study sample referred to in De Meo-Monteil et al. to specifically explore inter-individual differences in strengths of relative responses to different intensities, then clustered participants on the basis of these normalized GFP values. Dwyer et al. found clusters showing markedly discrepant intensity-dependent patterns, highlighting the importance of individual differences in intensity dependency. Apart from Bruneau et al. (1997), who compared children aged 4-6 to children aged 6-8 without finding differences in intensity-dependency between age groups, the authors of the present study are unaware of any direct investigations of changes in the loudness-dependency of children’s auditory ERPs over the course of development.
1.3. Cortical Auditory ERPs in Autism
Auditory electrophysiological and magnetoencephalographic responses have been explored in ASD by a number of studies. Some of these studies have suggested altered latencies (e.g., Matsuzaki et al., 2018; Vlaskamp et al., 2017) or amplitudes (e.g., Donkers et al., 2015; Vlaskamp et al., 2017) of cortical auditory responses. Studies have also examined some behavioural, cognitive, and perceptual correlates of these responses in ASD. For example, M50 (corresponding to P1) latencies might be particularly delayed in minimally-verbal and nonspeaking autistic people (Roberts et al., 2019), P1 amplitudes might be linked to sensory sensitivity (Karhson & Golob, 2016), and reduced N2 amplitudes may be linked to sensory hyporesponsiveness (Donkers, Carlson, Schipul, Belger, & Baranek, 2020).
Only a few studies have specifically examined intensity-dependent responses, but these do provide some reason to believe that intensity-dependency at the group/population level may be atypical in autism. Bruneau et al. (1999) found that the intensity-dependency of fronto-central N1 did not differ between ASD and TD (or intellectual disability); however, left temporal Tb amplitudes did not vary with intensity in ASD, in contrast to TD. Bruneau et al. (2003) replicated this finding. Furthermore, De Meo-Monteil et al. (2019) found that intensity-dependency of cortical auditory responses differed even between distinct parts of the autistic constellation.2 Autistic people with disproportionate megalencephaly (DM) have been described as a subgroup within ASD (see Amaral et al., 2017), and De Meo-Monteil explored loudness-dependent auditory ERPs using GFP in 2-4-year-old typically-developing children and separate autistic groups with and without DM, all individuals from a subset of the present study. From 55-115ms (likely overlapping with the P1), autistic children without DM showed no significant intensity-dependency of GFP responses, in contrast to autists with DM, who displayed a more TD-like pattern of intensity-dependency. However, from 145-195 ms, of all three groups, only autistics with DM displayed significant intensity-dependency of GFP amplitudes. The importance of inter-individual variability of loudness-dependent auditory processing in autism is further supported by results from Dwyer et al. (2020), who, as noted earlier, clustered participants according to loudness-dependency of auditory ERPs. All clusters contained a mixture of participants from different diagnostic groups, but autistic participants were significantly more likely to display patterns of increasing amplitude with loudness from 50 dB to 60 dB to 70 dB (but not 80 dB), and autists with this pattern of responses appeared to have higher scores on a measure of cognitive ability. Meanwhile, autists with particularly strong 80 dB responses had more parent-reported auditory filtering/distractibility problems.
Developmental changes in intensity-dependent auditory ERPs have not been investigated in autism, except indirectly by Dwyer et al. (2020), who did not find any evidence that autistic participants in different clusters varied from one another in age.
In terms of developmental change in auditory ERPs and magnetoencephalographic responses more generally, different studies have yielded different conclusions, with some studies suggesting age differences present in TD are not apparent or attenuated in ASD (e.g., Edgar et al., 2015; Gage, Siegel, & Roberts, 2003; Roberts et al., 2010; Stephen et al., 2017), while other studies find age effects in both TD and ASD (e.g., Orekhova et al., 2008; Port et al., 2016).
One could also ask about the neurobiological correlates of these responses in ASD. Studies in older children suggest the relationship between white matter microstructure and the latency of the M50 (corresponding to P1) might be less apparent in autism than TD (Roberts et al., 2013, 2020). However, it should be noted that autistic children in the age range of the present study may tend to demonstrate increased fractional anisotropy – more directed/restricted white matter diffusion – relative to typical development (e.g., Andrews et al., 2019), the opposite of the pattern often reported in older autistic individuals (e.g., Haigh et al., 2020). Furthermore, Roberts et al. (2020) report a subgroup of autistic individuals with reduced GABA concentrations and slower M50 latencies, although they found no overall association between GABA and M50 latency. While clearly quite tentative, this finding would seem to both emphasize the neurobiological heterogeneity inherent to ASD and raise the possibility that atypical balances of excitation and inhibition in ASD (see Sohal & Rubenstein, 2019) could affect ERP latencies in at least particular subgroups, even if group differences in relevant neurotransmitter levels cannot be consistently detected (see Kolodny et al., 2020). However, it is again unclear how this might relate to participants in the age range of the present study.
Overall, there is evidence that both auditory responses in general, and more specifically, intensity-dependency of auditory ERPs, are atypical in ASD. However, while some studies suggest that the developmental trajectory of these auditory responses in ASD differs from TD, this literature is not consistent. Furthermore, the extant research literature can provide very little information regarding development of intensity-dependency of auditory responses in ASD.
1.4. Present Study
The present study explored cross-sectional associations between intensity-dependent auditory ERPs and chronological age in a large sample of autistic and typically-developing participants from the Autism Phenome Project (APP) at the UC Davis MIND Institute. Latencies of the P1 main auditory response were quantified separately in each hemisphere using an approach that allows for inter-individual variability in the precise location of this response over lateralized fronto-central channels; associations between these latency measurements and chronological age, as well as ASD-TD group differences, are then examined. Furthermore, associations between chronological age and response amplitudes in all channels are examined over a wider, 1 – 350 ms time window using cluster-based nonparametric permutation tests (Maris & Oostenveld, 2007), as are differences between ASD and TD groups.
We hypothesized:
That P1 latencies would, in general, be shorter in older participants across both hemispheres and groups;
That ERP amplitudes obtained from scalp regions and time windows typically associated with the P1 positivity in children would be greater in older participants across both hemispheres and groups; and
Based on results obtained by Shafer et al. (2015), that there would be no age-related changes in amplitude of fronto-central responses in the time window of the N2 negativity in typically-developing participants.
2. Methods
2.1. Participants
Attempts were made to collect ERP data from 243 autistic and 96 typically-developing children, aged between 2 and 5 years, participating in the APP. Autistic participants were required to meet DSM-IV/Collaborative Programs of Excellence in Autism criteria for a Pervasive Developmental Disorder and pass ADOS-G (Lord et al., 2000) cut-off scores as well as cut-offs for either the social or communication subscales of the ADI-R (Lord, Rutter, & Le Couteur, 1994). Participants were primarily recruited from the MIND Institute’s Subject Tracking System, other research studies, community advertising, and mailing lists from commercial databases. Typically-developing participants did not have first-degree autistic relatives and were screened for autism using the Social Communication Questionnaire (Berument et al., 1999). Further information about the APP and participant recruitment can be found in previous publications (e.g., Libero et al., 2016; Nordahl et al., 2011). Participants were excluded due to: noisy data, an insufficient number of acceptable-quality trials (<400; ~100/condition), an excessive number of poor-quality channels (> 6-7), or the presence of neuroanatomical abnormalities revealed by magnetic resonance imaging collected in the APP. One participant entered the study in the typically-developing group but was diagnosed with autism at a later APP time-point; this participant’s data are also excluded. The final sample of children with usable electrophysiological data included a total of 81 typically-developing participants (52 male) and 130 autistic participants (110 male) (Table 1). Cognitive ability was measured with the Mullen Scales of Early Learning (MSEL; Mullen, 1995); note that only the visual reception, fine motor, expressive language, and receptive subscales, and not the gross motor subscale, were administered in this study. Adaptive functioning was assessed with the parent-report form of the Vineland Adaptive Behavior Scales, Second Edition (VABS-II; Sparrow, Cichetti, & Balla, 2005). Anxiety and ADHD characteristics were measured on the preschool-age Childhood Behavior Checklist (CBCL; Achenbach & Rescorla, 2000). Sleep quality was measured using the Children’s Sleep Habits Questionnaire (CSHQ; Owens, Spirito, & McGuinn, 2000). Families received a gift card in return for their participation in the study.
Table 1.
Characteristics of typically-developing (n=81) and autistic participants (n=130) with usable electrophysiological data. Welch’s t-test was used to examine group differences in age, DQ, CBCL, CSHQ, and VABS scores. Fisher’s exact test was used to compare participants based on sex, megalencephaly status, and medication status (medicated vs. unmedicated). A Wilcoxon-Mann-Whitney rank-sum test was used to examine group differences in income brackets, entered as an ordinal variable with values of 1 – 7.
| TD | ASD | p | |||
|---|---|---|---|---|---|
| Mean (SD) |
Range | Mean (SD) |
Range | ||
| Chronological Age (months) | 37.09 (6.46) | 25.80 – 56.33 | 38.50 (6.02) | 25.50 – 54.87 | .12 |
| MSEL Developmental Quotient (DQ) | 106.37 (11.58) | 79.89 – 128.62 | 65.25 (20.91) | 30.39 – 138.66 | <.0001 |
| MSEL Verbal DQ | 107.97 (12.70) | 81.26 – 149.47 | 58.90 (26.17) | 19.31 – 148.81 | <.0001 |
| MSEL Non-Verbal DQ | 104.77 (13.88) | 71.49 – 129.96 | 71.60 (18.58) | 36.39 – 136.93 | <.0001 |
| VABS Adaptive Behaviour Composite | 111.22 (12.00) | 82.00 – 135.00 | 75.41 (11.00) | 53.00 – 104.00 | <.0001 |
| CBCL DSM-Oriented Anxiety T-score | 51.69 (3.44) | 50.00 – 67.00 | 55.77 (8.88) | 50.00 – 93.00 | <.0001 |
| CBCL DSM-Oriented ADHD T-score | 52.96 (4.68) | 50.00 – 76.00 | 58.72 (7.21) | 50.00 – 76.00 | <.0001 |
| CSHQ Total Score | 41.78 (5.88) | 32.00 – 55.00 | 44.33 (8.03) | 33.00 – 70.00 | .03 |
| Megalencephaly status1 N (%) |
6 (8.82%) DMega | 11 (10.89%) DMega | .41 | ||
| 57 (83.82%) normal range | 87 (86.14%) normal range | ||||
| 5 (7.35%) DMicro | 3 (2.97%) DMicro | ||||
| Sex N (%) |
52 (64.20%) male | 110 (84.62%) male | .0008 | ||
| 29 (35.80%) female | 20 (15.38%) female | ||||
| Medication status2 N (%) |
9 (11.84%) taking psychoactive medications | 23 (19.17%) taking psychoactive medications | .23 | ||
| Annual income brackets (brackets on left, number reporting each on right) | < $10,000 | 2 | < $10,000 | 1 | .04 |
| $10,000 - $29,999 | 13 | $10,000 - $29,999 | 5 | ||
| $30,000 - $49,999 | 9 | $30,000 - $49,999 | 7 | ||
| $50,000 - $74,999 | 25 | $50,000 - $74,999 | 12 | ||
| $75,000 - $99,999 | 23 | $75,000 - $99,999 | 10 | ||
| $100,000 - $149,999 | 17 | $100,000 - $149,999 | 25 | ||
| > $150,000 | 17 | > $150,000 | 14 | ||
Disproportionate megalencephaly (DMega) is defined as a total cerebral volume (TCV) to height ratio > 1.5 standard deviations above the TD mean in the entire APP study, including participants without usable ERPs (see Libero et al., 2016, for details). Disproportionate microcephaly (DMicro) is a ratio > 1.5 standard deviations below the typical mean. Megalencephaly status was only calculated from participants with usable ERPs, cerebral volume (from MRI), and height data from Time 1 of the APP (n = 68 TD, 101 ASD).
Medication status at the exact time of the ERP visit is unknown. However, data regarding medications taken in the last 3 days before a phlebotomy appointment within 6 months of the time of the ERP are available from 196 participants (n = 76 TD, 120 ASD), offering some indication as to the likely medication status of participants at the time of the ERP. Note that a liberal criterion was used to determine whether medications were psychoactive, based on medications either having brain-based biological mechanisms and/or being demonstrated in double-blind studies to have behavioural/psychological effects; accordingly, for example, pain relievers, many allergy medications, and insulin were classified as psychoactive.
2.2. Electroencephalography (EEG) Task
The experimental setup and data collection have been previously described in De Meo-Monteil et al, 2019 and Dwyer et al (2020). Briefly, participants were seated on a caregiver’s lap in a dimly-lit, audiometrically-quiet room. Sony MDR-222KD headphones were calibrated and used to present 50 ms complex tones (mixed sine waves at 249, 616, 788, 1042, 1410, 1952, and 2749 Hz) with a random ISI of 1-2s. Stimulus intensity randomly varied in loudness (50 dB, 60 dB, 70 dB, and 80 dB SPL); tones of the same intensity were never presented twice in succession. While they passively listened to these tones, participants watched a video of their or their caregiver’s choice, set to a low audio volume. Breaks were included as required. Approximately 1200 trials (~300 trials/condition) were collected from each participant.
2.3. EEG Data Acquisition and Processing
EEG data were collected with 61-channel EASYCAP system (www.easycap.de) using a Compumedics Neuroscan Synamp II amplifier. Data were sampled at a rate of 1000Hz with Cz as a reference. Data were then average-referenced and band-pass filtered offline with a low cut-off of 0.4 Hz (12dB/octave roll-off). Epochs (spanning −200ms to 900ms, including 300ms necessary for subsequent independent components analysis) were screened and extreme amplitudes removed using the artifact scan tool of BESA 5.2 (www.besa.de); amplitude thresholds were adjusted manually to optimize retention of usable data and rejection of extreme artefacts (e.g., temporary channel disconnection, gross movements). Mean amplitude thresholds were 316.76 μV (SD = 98.74) in ASD and 303.81 μV (SD = 95.09) in TD. Data were then inspected and clear artefacts not removed by the amplitude threshold were rejected manually. On average, in the ASD group, 23% of trials were removed in this process, compared to 19% in the TD group (see also Table 2). As the signal-to-noise ratio increases in proportion to the square root of the number of trials (Luck, 2014), this suggests signal-to-noise ratio in the ASD group is, in all four conditions, ~96% of the signal-to-noise ratio in the TD group.
Table 2.
Mean and standard deviation of number of trials retained in final averages after all data processing was completed in each intensity condition from autistic (n=130) and typically-developing (n=81) participants.
| 50 dB | 60 dB | 70 dB | 80 dB | |
|---|---|---|---|---|
| ASD | 221.33 (50.29) | 212.33 (51.97) | 225.64 (49.76) | 217.24 (49.77) |
| TD | 240.07 (53.51) | 229.49 (54.05) | 244.00 (54.35) | 234.63 (53.07) |
| Welch’s p | .01 | .02 | .02 | .02 |
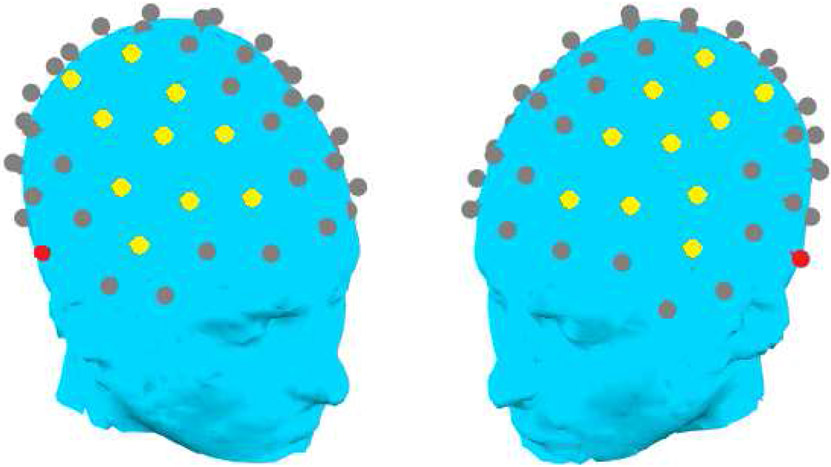
Given the study’s goal of exploring heterogeneity and individual differences in electrophysiological data, we sought to maximize the ERP signal to noise ratio by removing putatively non-neural signal sources from the data. To accomplish this, the remaining epochs were submitted to Second-Order Blind source Identification (SOBI; Belouchrani, Abed-Meraim, Cardoso, & Moulines, 1997; Tang, Sutherland, & McKinny, 2005) independent components analysis. A semi-automatic artifact removal tool (SMART, https://stanford.edu/~saggar/Software.html) was used to provide outputs depicting signal source topography, spectra, autocorrelation, and time series; these, in addition to channel-by-channel time series, were used as a basis for manual classification of sources as being of putatively neural or non-neural origin (such as EMG, EOG, and blinks). SOBI was performed separately on the first and second halves of the data from each participant, consistent with recommendations (Luck, 2014). Additional details regarding artifact removal using SOBI and SMART are discussed in Saggar et al. (2012). Artifact-free trials were then reconstructed from the putatively neural SOBI signal sources. Separate averages for each of the four loudness conditions were computed for each subject. Data from excluded channels were then interpolated using a 3-dimensional spline (Perrin, Pernier, Bertrand, Giard, & Echallier, 1987) as implemented in CARTOOL (Brunet et al., 2011). Epochs (now spanning 100 ms pre-stimulus onset to 599 ms post-stimulus onset after reconstruction) were filtered (second-order Butterworth with −12 dB/octave roll/off; 40 Hz low-pass; 60 Hz notch) and baseline-corrected using the pre-stimulus period in CARTOOL. Finally, epochs were re-referenced to an average of the left and right hemisphere channels closest to the mastoids, corresponding to TP9 and TP10 in the 10-10 system (Figure 1).
Figure 1.
Fronto-central channels over each hemisphere selected as the measurement region for the P1 response (in yellow) and reference channels (in red).
2.4. EEG Data Analysis
2.4.1. P1 Main Auditory Response Latencies.
In order to minimize the confounding influence of variations in scalp topography on the measurement of inter-individual differences in amplitudes and latencies of the P1 main auditory response, we used pre-defined time windows and interrogated responses over a broad scalp area over frontocentral regions of the right and left hemispheres. Within these areas, we used the location of maximum amplitude as our measure of P1. Time windows were drawn, separately in each condition, as ±50ms centered around the greatest positive peak in any channel in the target regions, irrespective of hemisphere, observed in the grand-averaged data combined across groups within the two frontocentral regions, yielding the following windows applied to both hemispheres: 71 – 171ms (50 dB), 62 – 162ms (60 dB), 49 – 149ms (70 dB), and 44 – 144ms (80 dB). Frontocentral channels in each hemisphere, but not channels from the edge of the electrode cap, were included in the measurement region (Figure 1). Separately for each hemisphere, loudness condition, and participant, the channel with the most positive response, as averaged across the measurement window, was selected. The distributions of P1 maxima in each group are shown in Figure 2. After the maximum channel was identified in each participant, it was averaged with immediately adjacent channels falling within the limits of the measurement region (a total of three to six channels, depending on location of the maximum channel). This average was then used to measure latency using the 50% area latency approach (see Luck, 2014), which selects as an estimate of latency the time point that divides the area of the waveform within the time window into equal halves on each side of itself. Area amplitude was also extracted and is presented in Appendix A. Correlations between chronological age and latencies, and ASD-TD group differences in latencies, were then examined in each hemisphere, intensity condition, and diagnostic group; the Holm-Bonferroni approach was used to correct for multiple comparisons. Only participants who exhibited the canonical P1 positivity in at least one loudness condition and one hemisphere were included in the analysis. To accomplish this, participants were excluded from the P1 analysis in any given condition and over either hemisphere if the mean positive area of the waveform in the time window of interest was less than the standard deviation of amplitudes observed at different time points across the waveform’s baseline (−100 – 0ms); on average, 6.38 participants in the ASD group and 0.63 in the TD group were excluded in each condition and hemisphere (see Table 4 for numbers of included participants).
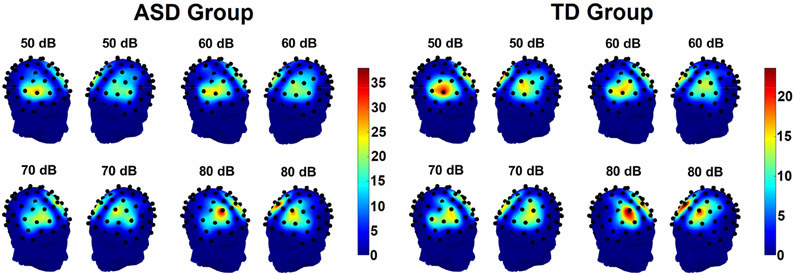
Figure 2.
Heatmap showing the distributions of locations of P1 maxima in fronto-central channels in each hemisphere for individual participants in the ASD group (left) and the TD group (right). Heatmap scales refer to the number of participants with P1 maxima at each site, note that scales are adjusted so that proportions of total group size are equal across groups.
2.4.2. Timewise Amplitudes in All Channels.
We explored relations between response amplitude and age using a non-parametric cluster-based permutation test (Maris & Oostenveld, 2007), which was used to investigate age-related changes in amplitudes at every channel and at each time point over the first 350 ms post-stimulus. Separately for each group and loudness condition, the linear correlation between chronological age and response amplitude, as well as the t-statistics associated with the correlations, were calculated at every channel and time-point between 1 – 350 ms using FieldTrip (Oostenveld, Fries, Maris, & Schoffelen, 2011). Furthermore, independent samples t-tests were used to compare amplitudes between the ASD and TD groups at every channel and time point from 1 – 350 ms. To control for the numerous multiple comparisons generated by examining effects over a large number of electrodes and time-points, spatially and temporally contiguous “clusters” of t-values exceeding an initial two-tailed probability threshold of .05 were summed. Channels were not included in these clusters unless at least two other adjacent channels at the same time point also exceeded the initial probability threshold. To determine final statistical significance, the summed t-statistics from the obtained clusters were compared against the null distribution of summed cluster t-statistics generated through 10,000 random permutations of the data. Essentially, this procedure discards any correlations or group differences that are not stronger and/or more spatio-temporally sustained than would be expected from chance alone.
As the linked mastoid reference used in the present study employs channels canonically associated with temporal auditory responses such as Tb and TP200, we also computed and examined group differences in the reference-independent current source density. These results are presented in Appendix B.
3. Results
3.1. P1 Latencies
P1 latencies over each hemisphere and in each intensity condition were compared across groups using Welch’s t-tests (Table 3). After Holm-Bonferroni corrections for multiple comparisons were applied, autistic participants had significantly slower P1 latencies to 80 dB sounds over the right hemisphere, p = .04. Several other trends no longer attained significance after correction.
Table 3.
Mean and standard deviation of P1 area latency in each diagnostic group, along with Welch’s t-tests comparing groups. In cases where uncorrected p-values are statistically significant, p-values are provided both before correction and after Holm-Bonferroni correction for eight multiple comparisons.
| ASD | TD | Welch’s t-test | |||
|---|---|---|---|---|---|
| Mean (SD) |
Mean (SD) |
Welch’s t (df) |
p, before correction |
p, after correction |
|
| 50 dB Left | 124.06 (8.62) |
122.37 (6.70) |
1.56 (195.93) |
.11 | N/A |
| 50 dB Right | 123.78 (9.23) |
122.77 (7.05) |
0.88 (196.46) |
.38 | N/A |
| 60 dB Left | 114.11 (9.25) |
112.29 (7.32) |
1.55 (191.39) |
.12 | N/A |
| 60 dB Right | 114.11 (9.84) |
111.56 (7.05) |
2.17 (202.55) |
.03 | .19 |
| 70 dB Left | 104.04 (9.16) |
101.51 (8.08) |
2.06 (181.01) |
.04 | .20 |
| 70 dB Right | 103.83 (9.14) |
101.59 (9.16) |
1.70 (171.24) |
.09 | N/A |
| 80 dB Left | 101.35 (9.05) |
98.64 (8.26) |
2.22 (181.87) |
.03 | .20 |
| 80 dB Right | 100.88 (9.40) |
97.29 (8.36) |
2.87 (182.46) |
.005 | .04 |
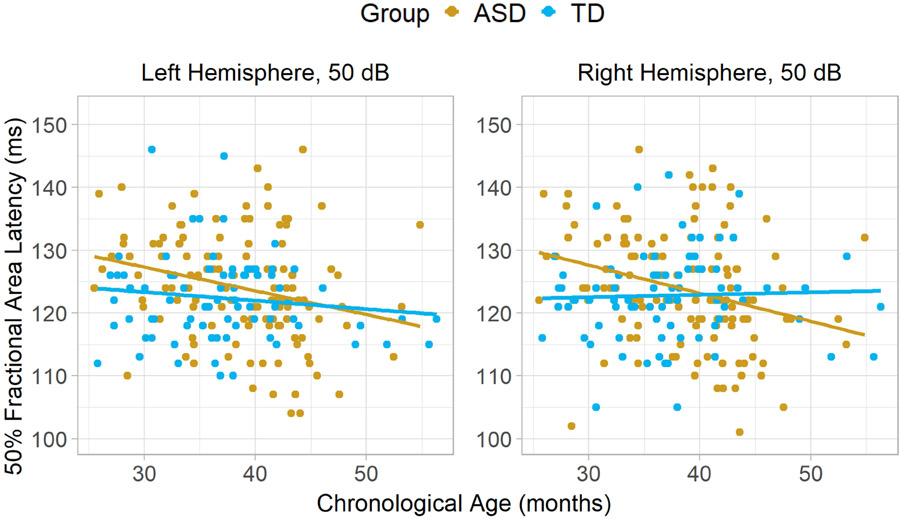
After Holm-Bonferroni correction for sixteen comparisons, one association between P1 latency and chronological age attained significance, specifically, in the ASD group over the right hemisphere in the 50 dB condition; faster latencies were observed in older participants, corrected p = .02 (Figure 3, Table 4). An analysis controlling for MSEL Developmental Quotient (calculated as mental age/chronological age*100) was also examined, but the partial correlation remained significant after applying correction for 16 comparisons, p = .02. However, after multiple comparison correction, no correlations between P1 latency and chronological age exceeded significance threshold in TD.
Figure 3.
Scatterplots depicting correlations between chronological age and P1 latency to 50 dB sounds, separately in each diagnostic group and over each hemisphere. In TD, there was little evidence of faster latencies in older participants over the right hemisphere, r = .04, or the left, r = −.13. In ASD, there was a significant effect over the right hemisphere, r = −.28, and a trend over the left, , r = −.26.
Table 4.
Pearson’s correlation coefficients, along with p-values, reflecting associations between age and P1 latencies in each group, hemisphere, and loudness condition. In cases where uncorrected p-values are statistically significant, p-values are provided both before correction and after Holm-Bonferroni correction for sixteen multiple comparisons. The number of participants remaining after exclusion is noted.
| ASD | TD | |||||||
|---|---|---|---|---|---|---|---|---|
| n | r |
p, before correction |
p, after correction |
n | r |
p, before correction |
p, after correction |
|
| 50 dB Left | 122 | −.26 | .004 | .06 | 81 | −.13 | .25 | N/A |
| 50 dB Right | 121 | −.29 | .001 | .02 | 81 | .04 | .75 | N/A |
| 60 dB Left | 123 | −.17 | .06 | N/A | 79 | −.23 | .04 | .48 |
| 60 dB Right | 126 | −.23 | .01 | .15 | 81 | −.08 | .48 | N/A |
| 70 dB Left | 123 | −.13 | .15 | N/A | 79 | −.16 | .15 | N/A |
| 70 dB Right | 122 | −.08 | .39 | N/A | 81 | −.09 | .41 | N/A |
| 80 dB Left | 126 | −.04 | .64 | N/A | 81 | −.00 | .99 | N/A |
| 80 dB Right | 126 | −.16 | .08 | N/A | 80 | .06 | .60 | N/A |
3.2. Timewise Amplitudes in All Channels
3.2.1. Between-Group Comparisons.
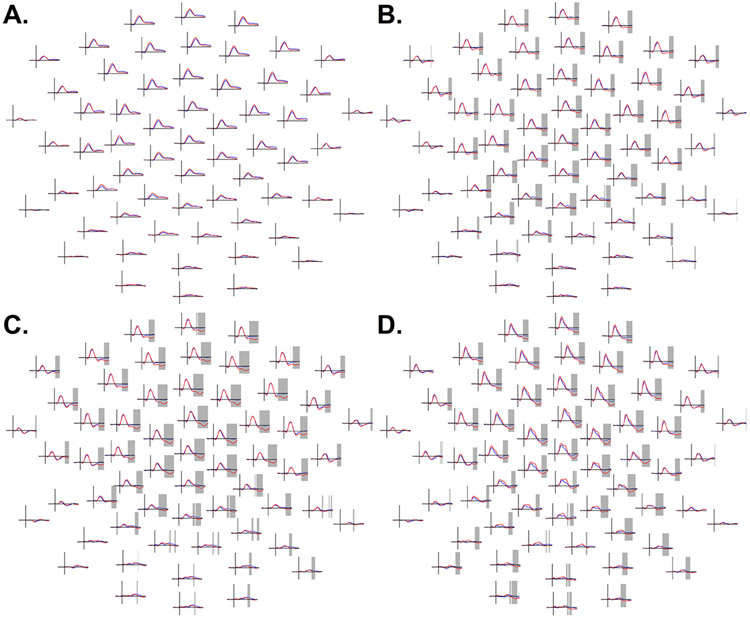
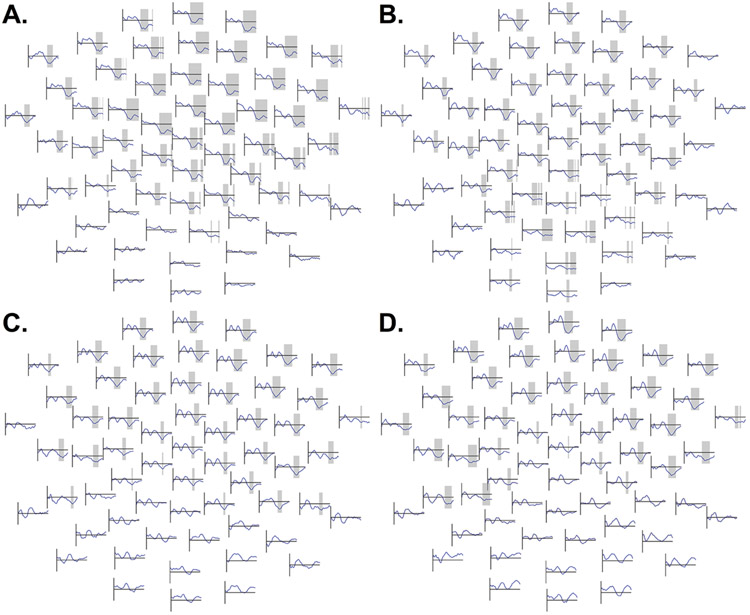
Despite a trend, autistic and typically-developing participants did not significantly differ in their responses to 50 dB tones, lowest p = .06 (Figure 4A). However, in the 60 dB condition, autistic participants displayed more positive voltages over a cluster of predominantly fronto-central (and some posterior) channels spanning 253 – 350 ms, p = .02 (Figure 4B), suggesting reduced N2 amplitudes. In the 70 dB condition (198 – 350 ms, p = .001, Figure 4C) and the 80 dB condition (192 – 350 ms, p = .002, Figure 4D), similar fronto-central clusters suggesting reduced N2 amplitudes in ASD also extended farther over posterior channels.
Figure 4.
A (top left). ERP amplitudes in the 50 dB condition between autistic participants (ASD, blue) and typically-developing participants (TD, red) from 100 ms before stimulus presentation to 350 ms post-stimulus; time points from 1 to 350 ms were compared using cluster-based permutation tests. B (top right). Comparison of ERP amplitudes across groups in the 60 dB condition. C (bottom left). Group differences in 70 dB condition. D (bottom right). Group differences in 80 dB condition. Frontal channels are shown at the top of the figure and posterior channels at the bottom of the figure. Periods of significant effects obtained in the cluster-based permutation test comparing group amplitudes between 1 ms and 350 ms are highlighted in grey. In the 60, 70, and 80 dB conditions, a widely-distributed but predominantly frontocentral cluster of channels evidenced a significant group difference, with less negative amplitudes in ASD, in the approximate time range of the canonical N2 response. The Y-axis (vertical line on each subplot) ranges from −4 to +8 μV.
3.2.2. Correlations with Age.
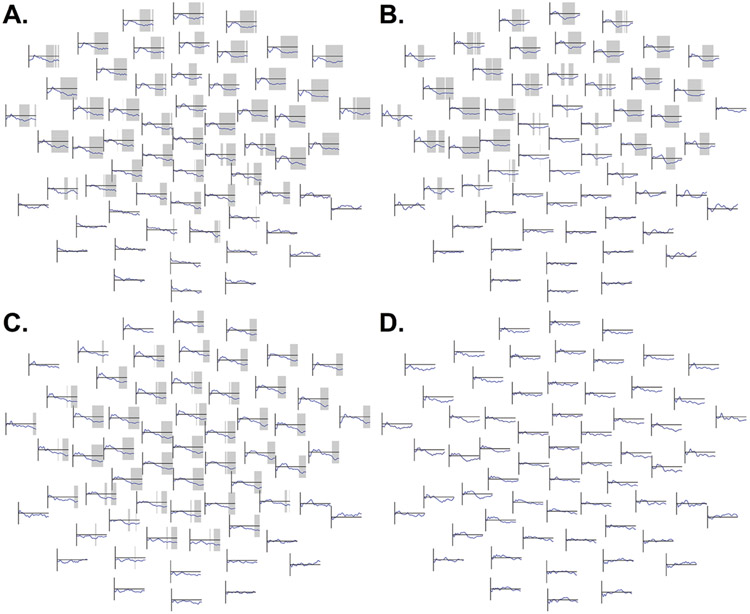
In TD, a negative correlation between 50 dB amplitudes and age was observed over fronto-central channels between 199 – 350 ms, p = .002 (Figure 5A, Figure C.1), such that older participants had more negative responses in the latency and topography range of the N2. The ASD group showed a similar effect cluster spanning 151 – 350 ms, p = .005 (Figure 6A, Figure C.2).
Figure 5.
In the TD group, waveforms at each electrode depicting, separately in each loudness condition, correlation coefficients between age and ERP amplitudes at each time point between 1 – 350ms. Time windows with effects that were significant in the non-parametric permutation test are highlighted. The Y-axis ranges from −.50 to +.50; this is the range of the vertical line to the left of each channel subplot. Each y-value represents the correlation coefficient at a specific point in time. Frontal channels are shown at the top and posterior channels at the bottom of each panel.
A (top left). Waveforms depicting, in the TD group, correlation coefficients between age and 50 dB responses in each channel at each time point between 1 – 350ms. A frontocentral negative correlation, indicating amplitudes are more negative in older participants, was observed in the approximate latency range of the frontocentral N2 response.
B (top right). Waveforms depicting, in the TD group, correlation coefficients between age and 60 dB responses. In this condition, a negative correlation was observed over a cluster of channels extending not only over fronto-central but also posterior scalp.
C (bottom left). Waveforms depicting, in the TD group, correlation coefficients between age and 70 dB responses. A frontocentral negative correlation, indicating amplitudes are more negative in older participants, was observed in the approximate latency range of the frontocentral N2 response.
D (bottom right). Waveforms depicting, in the TD group, correlation coefficients between age and 80 dB responses. A frontocentral negative correlation, indicating amplitudes are more negative in older participants, was observed in the approximate latency range of the frontocentral N2 response.
Figure 6.
In the ASD group, waveforms at each electrode depicting, separately in each loudness condition, correlation coefficients between age and ERP amplitudes at each time point between 1 – 350ms. Time windows with effects that were significant in the non-parametric permutation test are highlighted. The Y-axis ranges from −.50 to +.50; this is the range of the vertical line to the left of each channel subplot. Each y-value represents the correlation coefficient at a specific point in time. Frontal channels are shown at the top and posterior channels at the bottom of each panel.
A (top left). Waveforms depicting, in the ASD group, correlation coefficients between age and 50 dB responses. A negative correlation was observed over a cluster of fronto-central channels, indicating amplitudes are more negative in older participants, in the approximate latency range of the frontocentral N2 response.
B (top right). Waveforms depicting, in the ASD group, correlation coefficients between age and 60 dB responses. A negative correlation was observed over a cluster of fronto-central channels, indicating amplitudes are more negative in older participants, in the approximate latency range of the frontocentral N2 response.
C (bottom left). Waveforms depicting, in the ASD group, correlation coefficients between age and 70 dB responses. A negative correlation was observed over a cluster of fronto-central channels, indicating amplitudes are more negative in older participants, in the approximate latency range of the frontocentral N2 response.
D (bottom right). Waveforms depicting, in the ASD group, correlation coefficients between age and 80 dB responses. No effects attained statistical significance after the cluster-based permutation test.
In the TD group, a negative correlation between amplitude and age in the 60 dB condition was observed over a cluster of both fronto-central and posterior channels spanning 201 – 349 ms, p = .01 (Figure 5B, Figure C.3). The ASD group exhibited a similar effect over a fronto-central cluster spanning 146 – 350 ms, p = .02 (Figure 6B, Figure C.4).
Likewise, a negative correlation was observed in the 70 dB condition in the TD group over a cluster spanning 196 – 304 ms, p = .02 (Figure 5C, Figure C.5), and in the ASD group over a cluster spanning 216 – 350 ms, p = .008 (Figure 6C, Figure C.6).
Similarly, in 80 dB, a significant negative correlation was observed in TD over a cluster of fronto-central channels spanning 178 – 330 ms, p = .009 (Figure 5D, Figure C.7). However, in the ASD group, no correlations in the 80 dB condition approached statistical significance (Figure 6D, Figure C.8).
4. Discussion
In this cross-sectional study of large samples of young autistic and typically-developing children, we examined associations between chronological age and latencies of the P1 auditory electrophysiological response to sounds of 50, 60, 70, and 80 dB. P1 latency differences between autistic and typically-developing groups were also investigated. Furthermore, time- and electrode-wise group differences and age correlations were investigated over all channels in a time window from 1 – 350 ms using cluster-based permutation testing (Maris & Oostenveld, 2007), which led to significant findings in the latency range of the N2 response.
4.1. P1 Latencies
After correction, the present study revealed significantly slower P1 responses to 80 dB sounds over the right hemisphere in ASD. This is consistent with prior findings of slower P1 latencies in ASD (reviewed by Williams et al., 2020). That this group difference was evoked by 80 dB stimuli need not suggest that group differences are intensity-dependent: the sharper waveforms evoked by high-intensity 80 dB tones could have made detection of ASD-TD group differences easier than at lower intensities, and there were some trending effects at lower intensities that became nonsignificant after correction.
Furthermore, over the right hemisphere, P1 latencies to 50 dB sounds were significantly associated with age in the ASD group. In this condition, older autistic participants had faster P1 responses; this association remained significant after controlling for overall cognitive ability. In contrast, the present study found relatively little evidence of associations between P1 latencies and chronological age in typically-developing participants.
There appear to be two possible interpretations of this finding. First, although conducting partial correlations controlling MSEL DQ did not eliminate the observed associations, it is still possible that older autistic participants might come from a different subpopulation of autistic individuals than younger autistic participants, due to any other systematic factor that might have delayed diagnosis of these older individuals. Alternatively, it is possible that the present study identifies an actual trajectory of auditory response maturation in ASD, or at least within a part of the heterogeneous autistic constellation. As the present study is cross-sectional, it is difficult to determine which of these explanations might be most accurate, but prior literature (e.g., Shafer et al., 2015) does suggest P1 response latencies change with age and we expected to find such effects in both groups from the present study. The lack of TD effects in the present study could reflect aspects of study design; notably, Shafer et al. employed speech stimuli rather than complex tones, and these stimuli were presented at much shorter interstimulus intervals (350 ms) than those in present study (1-2 s). However, the multiple comparison correction used in the present study did reduce our power to detect such effects.
4.2. Timewise Amplitudes in All Channels
Significant amplitude differences between autism and TD were observed in the 60 dB, 70 dB and 80 dB loudness conditions, although not in the 50 dB loudness condition (Figure 4). These differences were observed using cluster-based permutation testing, and it should be noted that the exact spatiotemporal boundaries of observed effects should not be interpreted literally (see Sassenhagen & Draschkow, 2019, for further discussion). Briefly, the exact boundaries of the significant “cluster” effect in the permutation test reflect nothing more than statistical thresholding: they are simply the points at which effects happened to sufficiently exceed an initial criterion for inclusion in a “cluster.”3 However, if significant effects are observed, it is often still reasonable to conclude that a true effect exists somewhere in their approximate spatiotemporal vicinity. In these data, robust group differences in the 60 dB through 80 dB conditions were most prominent fronto-centrally in the approximate latency range of the N2 response, and they appear to have reflected greater (more negative) N2 responses in ASD than TD.
One complication in the interpretation of these group differences comes from the reference scheme used in the present study. Prior studies have described temporal responses such as the Tb and TP200 in young children, and the linked mastoid reference used in the present study may have subtracted these temporal responses from all channels. Importantly, prior research suggests Tb amplitudes may be attenuated in ASD (Williams et al., 2020). To ensure that we could interpret our effects in light of these patterns, we examined reference-independent current source density (Appendix B). In these data, clusters consistent with reduced N2 responses in autistic participants were indeed observed over the right hemisphere in the 70 and 80 dB conditions, but clusters suggestive of reduced positive-going temporal TP200 responses to 80 dB tones in autistic participants were also observed. Thus, while group differences in the N2 itself likely do exist, the subtraction of the TP200 response may have enhanced them further. TP200 subtraction might also help account for the group differences observed over posterior channels in the linked-mastoid data. No cluster that appeared to reflect Tb group differences attained significance in the current source density data.
Regardless, the present study offers an unusual demonstration of robust ASD-TD amplitude differences in basic cortical sensory responses in a large sample, although attenuated N2 responses in ASD are also shown in prior studies with smaller samples (e.g., Donkers et al., 2020; Orekhova et al., 2009).
Unexpectedly, the amplitudes of responses in approximate spatiotemporal vicinity of the N2 were also found to be significantly associated with age. Younger typically-developing participants appeared to have less negative fronto-central responses to sounds of all intensities (Figure 5). While the most parsimonious interpretation of these data may be that younger typically-developing participants had smaller N2 responses than their older counterparts, it should be noted that the canonical N2 negativity is hardly visible in the 50 dB condition (as shown in Figures B.1-B.2). Meanwhile, it is of interest to note that younger typically-developing participants exhibited, particularly in the 60 dB and 70 dB conditions (Figures C.3, C.5), positive voltage deflections over some frontal-central channels. In older participants, these small positive deflections are absent, with their latency range instead appearing solely as part of the larger N2 negativity. Thus, instead of the present study’s findings reflecting an increasingly strong N2 in older TD participants, it is possible that a positive-going response may have been superimposed over the N2 in younger participants; in older participants, this positive-going response may be diminished relative to the N2 or absent.
Interestingly, although autistic participants displayed similar negative correlations between fronto-central ERP amplitudes and age in the 50 dB through 70 dB conditions (Figures 6A-6C), correlations between age and amplitudes of responses to 80 dB sounds did not approach significance in autistic participants (Figure 6D).
The observed correlations between age and response amplitudes in the spatiotemporal range of the N2 violated the present study’s hypothesis 3, that response amplitudes in the N2 window would be unrelated to age, which was based on prior research (Shafer et al., 2015). Again, it is possible that this difference in results reflects factors such as the longer and random ISIs employed in the present study or the present study’s use of tones rather than speech stimuli. It is also possible that age effects observed in this study could reflect the use of a mastoid reference. It is less likely that the present study’s use of a cluster-based permutation testing approach identified effects outside the recording sites used by Shafer et al., as their frontal recording sites appear to fall within the area where N2 effects were observed in the present study. However, even if N2 amplitudes increase with age in preschool years, or even if a positivity in the same latency range disappears, prior research (e.g., Ponton et al., 2002) suggests that responses in the N2 range are stable in much of middle childhood, after which the strength of the N2 diminishes with the emergence of the auditory P2 response. Thus, the effects seen in this study could represent the first part of an overall (inverted) U-shaped developmental trajectory of N2 amplitudes.
Unexpectedly, the cluster-based permutation test did not reveal associations between age and amplitudes in the spatiotemporal range of the P1 main auditory response. This was again contrary to our expectations, as we hypothesized that response amplitudes in the P1 window would increase in older participants. While one might argue that the cluster-based permutation test might not have been sensitive enough to detect subtle associations between P1 amplitudes and age, it is notable that a supplementary analysis examining area amplitude in the measurement windows used for analysis of P1 latencies revealed no significant associations in any loudness condition, diagnostic group, or hemisphere even before multiple comparison corrections were applied (Appendix A).
4.3. Limitations and Future Directions
The chief limitation of the present study is its cross-sectional nature, especially insofar as the ASD group is concerned. Factors such as age of diagnosis, use of autism supports and services, and so forth could be systematically related to whether participants can be found and included in studies. For example, autistic children diagnosed early appear to obtain lower scores on tests of language and cognitive ability (Zwaigenbaum et al., 2019), and nonspeaking and minimally-verbal autistic people have displayed slower auditory ERP response latencies in prior research (Roberts et al., 2019). Although the present study’s significant P1 age effects in ASD were not driven by cognitive ability, it is still conceivable that another unmeasured confound might have affected them. Furthermore, even if the present study’s cross-sectional data are not confounded by other variables, other variables might still increase noise. To address this limitation, the authors are currently collecting a new longitudinal dataset using the same experimental paradigm employed in this study: specifically, presentation of large numbers of 50 dB, 60 dB, 70dB, and 80 dB tones. It is also noteworthy that many more autistic participants were eliminated from this sample due to poor data than TD individuals, which could also contribute towards the sample being non-representative.
Furthermore, the cluster-based permutation testing approach did not allow for measurement of the latencies of later ERP responses such as the N2. Thus, it is possible that latency differences rather than amplitude differences might have driven obtained effects. However, visual inspection of channel-by-channel waveforms in Figures 4-6 and Figures C.1-C.8 counts against this interpretation. Moreover, as noted earlier, the exact spatiotemporal boundaries of cluster-based permutation effects cannot be taken literally (Maris & Oostenveld, 2007; Sassenhagen & Draschkow, 2019); however, we believe that the data-driven approach, which allows effects to appear if and approximately where and when the data indicate they exist, may in fact be advantageous.
In the present study, the need to record EEG from a neurodevelopmentally diverse sample of young children required the simultaneous viewing of a film with low soundtrack volume (a common procedure; see, e.g., Gilley et al., 2005; Wunderlich, Cone-Wesson, & Shepherd, 2006). It is possible that, owing to differential gating effects of visual input on auditory ERPs, the developmental trajectories observed in ASD and TD may reflect differences in the developmental trajectory of cross-modal and/or selective attention between groups. Studies do suggest atypical attentional and cross-modal processing in ASD (Bonneh et al., 2008; Brandwein et al., 2013; Keehn et al., 2016).
Furthermore, auditory thresholds were not measured in the present study, owing to the difficulty of collecting high-quality audiometric data in such young participants. Prior research does suggest that hearing acuity may be heterogeneous in autism samples and reduced in many autistic individuals (e.g., Demopoulos & Lewine, 2016; Rosenhall, Nordin, Sandström, Ahlsén, & Gillberg, 1999) and it is unclear how such differences might impact the electrophysiological group differences observed in this study. We also observed a modest group difference in caregiver-reported income, and we likewise cannot exclude the possibility that this may have affected our results. Moreover, given the heterogeneity of autism, it is possible that still other variables might have influenced our results: some of the present study findings might reflect the influence of co-occurring conditions and characteristics rather than autism per se.
Finally, the present study appears to suggest group differences in amplitudes of ERP responses to 60 dB through 80 dB sounds over the spatiotemporal region of the N2 response. However, it has also been noted that positive voltage deflections might be superimposed over the N2 in younger participants. Further research analyzing response patterns at the individual level will be required to disambiguate whether heterogeneity, and positive voltage deflections of this type, might contribute to these group mean-level differences between ASD and TD. The present study’s use of a linked mastoid reference should also be borne in mind; any reference could change the ERP patterns observed over the scalp and complicate interpretation of effects in relation to underlying generators and prior literature.
4.4. Summary and Conclusions
The present study suggests that P1 amplitudes are stable over preschool age ranges, while right hemisphere P1 latencies to soft sounds may show some declines with age in autistic participants. No latency age effects attained significance in TD, and P1 latencies only significantly differed between ASD and TD to 80 dB sounds after correction. However, there were robust differences between ERP amplitudes of autistic and typically-developing participants around the spatiotemporal region of the canonical N2 negativity: specifically, typically-developing participants apparently exhibited larger negative N2 amplitudes in the 60 dB through 80 dB conditions, though supplementary results suggest larger temporal TP200 in TD may have contributed to this pattern as well. Finally, based on correlations between amplitudes and age, older typically-developing participants appeared to have increased N2 amplitudes (or a diminished positive voltage deflection in the same latency range as the N2) in all conditions. In contrast, associations between amplitudes around the N2 spatiotemporal region and age in ASD were only observed in the 50 dB through 70 dB conditions, not to 80 dB stimuli.
The present study uses a number of strategies to enhance the quality of research of inter-individual differences in ERPs. In addition to having a very large sample size, having large numbers of trials, and using SOBI ICA to remove artefacts from recordings, we employed data analysis procedures specifically aiming to focus on inter-individual variability in neural responses of interest. P1 latencies were quantified using a measurement approach allowing for different sites to be used in order to reduce confounds from variations in scalp topographies, while amplitudes of later responses were examined using a cluster-based permutation testing procedure that does not require a priori definition of ERP components.
Supplementary Material
Faster P1 to 50 dB tones over right hemisphere in older ASD participants
In TD participants, no association between P1 latency and age
Larger N2 to 60 through 80 dB sounds in TD than ASD participants
In ASD, unlike TD, no association between age and N2 amplitudes to 80 dB sounds
Acknowledgements
We wish to gratefully acknowledge all of the children and families who generously devoted considerable time and effort to participating in this large study, which included many other components besides ERP data collection. We warmly acknowledge the MIND Institute APP implementation and assessment team for their neuropsychological screening work and for coordinating the logistics of ERP appointments with participants’ families. We thank all of the research assistants and junior specialists for their help with EEG data collection and processing (including Sarah Abedi, Margarita Beransky, Costanza Columbi, Sam Cheyette, Sharon Corina, Tucker Fisher, Sevan K. Harootonian, David Horton, Ryan Hubbard, Anne Kalomiris, Sarabeth Maciey, Lindsey Marcelino, Joshua Martin, Saloni Mathur, Thomas McLennan, Tracy Riggins, and Ashley Stark). We also thank Manish Saggar and Iman Mohammadrezazadeh for software development, and Yukari Takarae for scientific support. We thank Dr. Tal Kenet, of the Harvard Medical School Department of Pediatric Neurology, who provided the stimuli used in this study.
Funding: this work was supported by the UC Davis MIND Institute, by the Robert Shoes Fund, by Scott & Jennifer Fearon, by the UC Davis Deans’ Distinguished Graduate Fellowship, by the NIH [grant number 1R01 MH089626-01], the Swiss National Science Foundation [grant number P2LAP3_164911], the NIMH [grant number U24MH081810], and by an Autism Center of Excellence grant awarded by the NICHD [grant number P50 HD093079].
Footnotes
Kenny and colleagues (2016) report that only a minority of autistic people endorse the use of the term “disorder” to describe their neurotype, and even fewer endorse the use of the term “condition”. For this reason, the present paper uses “autism spectrum development” as an alternative. Furthermore, Kenny et al. found that many autistic people prefer identity-first language (e.g., “autistic”) to person-first language (e.g., “person with autism), a preference which is respected in this paper, although it should be noted that this point remains controversial among autistic people (Bury, Jellett, Spoor, & Hedley, 2020).
Regarding the term “constellation,” see Fletcher-Watson & Happé (2019, pp. 34-35).
Arguably, similar cautions apply in conventional parametric analyses, where the boundaries of regions and times of interest are established by experimenter decisions, and thus cannot be taken as the exact spatiotemporal boundaries of any effects that are observed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms and profiles. Burlington, VT: University of Vermont. [Google Scholar]
- Amaral DG, Li D, Libero L, Solomon M, Van de Water J, Mastergeorge A, … Nordahl CW (2017). In pursuit of neurophenotypes: The consequences of having autism and a big brain. Autism Research, 10(5), 711–722. doi: 10.1002/aur.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DS, Lee JK, Solomon M, Rogers SJ, Amaral DG, & Nordahl CW (2019). A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. Journal of Neurodevelopmental Disorders, 11, 32. doi: 10.1186/s11689-019-9291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouchrani A, Abed-Meraim K, Cardoso J-F, & Moulines E (1997). A blind source separation technique using second-order statistics. IEEE Transactions on Signal Processing, 45(2), 434–444. doi: 10.1109/78.554307 [DOI] [Google Scholar]
- Ben-Sasson A, Gal E, Fluss R, Katz-Zetler N, & Cermak SA (2019). Update of a meta-analysis of sensory symptoms in ASD: A new decade of research. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-019-04180-0 [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, & Bailey A (1999). Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry, 175, 444–451. 10.1192/bjp.175.5.444 [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Belmonte MK, Pei F, Iversen PE, Kenet T, Akshoomoff N, … Merzenich MM (2008). Cross-modal extinction in a boy with severely autistic behaviour and high verbal intelligence. Cognitive Neuropsychology, 25(5), 635–652. doi: 10.1080/02643290802106415 [DOI] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Butler JS, Russo NN, Altschuler TS, Gomes H, & Molholm S (2013). The development of multisensory integration in high-functioning autism: High-density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs. Cerebral Cortex, 23(6), 1329–1341. doi: 10.1093/cercor/bhs109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau N, Bonnet-Brilhault F, Gomot M, Adrien JL, & Barthélémy C (2003). Cortical auditory processing and communication in children with autism: Electrophysiological/behavioral relations. International Journal of Psychophysiology, 51(1), 17–25. doi: 10.1016/S0167-8760(03)00149-1 [DOI] [PubMed] [Google Scholar]
- Bruneau N, Roux S, Adrien JL, & Barthélémy C (1999). Auditory associative cortex dysfunction in children with autism: Evidence from late auditory evoked potentials (N1 wave-T complex). Clinical Neurophysiology, 110(11), 1927–1934. doi: 10.1016/S1388-2457(99)00149-2 [DOI] [PubMed] [Google Scholar]
- Bruneau N, Roux S, Guérin P, Barthélémy C, Lelord G, 1997. Temporal prominence of auditory evoked potentials (N1 wave) in 4-8-year-old children. Psychophysiology 34 (1), 32–38. doi: 10.1111/j.1469-8986.1997.tb02413.x [DOI] [PubMed] [Google Scholar]
- Brunet D, Murray MM, & Michel CM (2011). Spatiotemporal analysis of multichannel EEG: CARTOOL. Computational Intelligence and Neuroscience, 2011: 813870. doi: 10.1155/2011/813870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury SM, Jellett R, Spoor JR, & Hedley D (2020). “It defines who I am” or “it’s something I have”: What language do [autistic] Australian adults [on the autism spectrum] prefer? Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-020-04425-3 [DOI] [PubMed] [Google Scholar]
- Čeponiene R, Lepistö T, Alku P, Aro H, & Näätänen R (2003). Event-related potential indices of auditory vowel processing in 3-year-old children. Clinical Neurophysiology, 114(4), 652–661. 10.1016/S1388-2457(02)00436-4 [DOI] [PubMed] [Google Scholar]
- Čeponiene R, Rinne T, Näätänen R, 2002. Maturation of cortical sound processing as indexed by event-related potentials. Clinical Neurophysiology 113, 870–882. doi: 10.1016/S1388-2457(02)00078-0 [DOI] [PubMed] [Google Scholar]
- Danker J, Strnadová I, & Cumming TM (2019). Picture my well-being: Listening to the voices of students with autism spectrum disorder. Research in Developmental Disabilities, 89, 130–140. doi: 10.1016/j.ridd.2019.04.005 [DOI] [PubMed] [Google Scholar]
- Davies PL, Chang WP, & Gavin WJ (2010). Middle and late latency ERP components discriminate between adults, typical children, and children with sensory processing disorders. Frontiers in Integrative Neuroscience, 4, 16. doi: 10.3389/fnint.2010.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meo-Monteil R, Nordahl CW, Amaral DG, Rogers SJ, Harootonian SK, Martin J, … Saron CD (2019). Differential altered auditory event-related potential responses in young boys on the autism spectrum with and without disproportionate megalencephaly. Autism Research, 12(8), 1236–1250. doi: 10.1002/aur.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos C, & Lewine JD (2016). Audiometric profiles in autism spectrum disorders: Does subclinical hearing loss impact communication? Autism Research, 9(1), 107–120. doi: 10.1002/aur.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FCL, Carlson M, Schipul SE, Belger A, & Baranek GT (2020). Auditory event-related potentials and associations with sensory patterns in children with autism spectrum disorder, developmental delay, and typical development. Autism, 24(5), 1093–1110. doi: 10.1177/1362361319893196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FCL, Schipul SE, Baranek GT, Cleary KM, Willoughby MT, Evans AM, … Belger A (2015). Attenuated auditory event-related potentials and associations with atypical sensory response patterns in children with autism. Journal of Autism and Developmental Disorders, 45(2), 506–523. doi: 10.1007/s10803-013-1948-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer P, Wang X, De Meo-Monteil R, Hsieh F, Saron CD, & Rivera SM (2020). Defining clusters of young autistic and typically-developing children based on loudness-dependent auditory electrophysiological responses. Molecular Autism, 11, 48. 10.1186/s13229-020-00352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Fisk CL, Berman JI, Chudnovskaya D, Liu S, Pandey J, … Roberts TPL (2015). Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Molecular Autism, 6, 69. 10.1186/s13229-015-0065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher-Watson S, & Happé F (2019). Autism: A new introduction to psychological theory and current debate. Abingdon, UK: Routledge. [Google Scholar]
- Gage NM, Siegel B, & Roberts TPL (2003). Cortical auditory system maturational abnormalities in children with autism disorder: An MEG investigation. Developmental Brain Research, 144, 201–209. doi: 10.1016/S0165-3806(03)00172-X [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, & Martin K (2005). Developmental changes in refractoriness of the cortical auditory evoked potential. Clinical Neurophysiology, 116(3), 648–657. doi: 10.1016/j.clinph.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Haigh SM, Keller TA, Minshew NJ, & Eack SM (2020). Reduced white matter integrity and deficits in neuropsychological functioning in adults with autism spectrum disorder. Autism Research, 13(5), 702–714. doi: 10.1002/aur.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerl U, Gallinat J, & Mrowinski D (1994). Intensity dependence of auditory evoked dipole source activity. International Journal of Psychophysiology, 17(1), 1–13. doi: 10.1016/0167-8760(94)90050-7 [DOI] [PubMed] [Google Scholar]
- Karhson DS, & Golob EJ (2016). Atypical sensory reactivity influences auditory attentional control in adults with autism spectrum disorders. Autism Research, 9(10), 1079–1092. doi: 10.1002/aur.1593 [DOI] [PubMed] [Google Scholar]
- Keehn B, Nair A, Lincoln AJ, Townsend J, & Müller RA (2016). Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Developmental Cognitive Neuroscience, 17, 46–56. doi: 10.1016/j.dcn.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny L, Hattersley C, Molins B, Buckley C, Povey C, Pellicano E, 2016. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism 20 (4), 442–462. doi: 10.1177/1362361315588200 [DOI] [PubMed] [Google Scholar]
- Khalfa S, Bruneau N, Rogé B, Georgieff N, Veuillet E, Adrien J-L, … Collet L (2004). Increased perception of loudness in autism. Hearing Research, 198(1–2), 87–92. doi: 10.1016/j.heares.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Kolodny T, Schallmo M-P, Gerdts J, Edden RAE, Bernier RA, & Murray SO (2020). Concentrations of cortical GABA and glutamate in young adults with autism spectrum disorder. Autism Research, 13(7), 1111–1129. doi: 10.1002/aur.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libero LE, Nordahl CW, Li DD, Ferrer E, Rogers SJ, & Amaral DG (2016). Persistence of megalencephaly in a subgroup of young boys with autism spectrum disorder. Autism Research, 9(11), 1169–1182. doi: 10.1002/aur.1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Linda L, Cook EH Jr., Leventhal, Bennett L, DiLavore PC, … Rutter M (2000). The Autism Diagnostic Observation Schedule - Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. doi: 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. doi: 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Luck SJ (2014). An Introduction to the Event-Related Potential Technique (2nd ed.). Cambridge, MA: MIT Press. [Google Scholar]
- Madriaga M (2010). “I avoid pubs and the student union like the plague”: Students with Asperger syndrome and their negotiation of university spaces. Children’s Geographies, 8(1), 39–50. doi: 10.1080/14733280903500166 [DOI] [Google Scholar]
- Maloret P, & Scott T (2018). Don’t ask me what’s the matter, ask me what matters: Acute mental health facility experiences of people living with Autism Spectrum Conditions. Journal of Psychiatric and Mental Health Nursing, 25(1), 49–59. doi: 10.1111/jpm.12438 [DOI] [PubMed] [Google Scholar]
- Maris E, & Oostenveld R (2007). Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods, 164(1), 177–190. doi: 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Matsuzaki AJ, Ku M, Berman JI, Blaskey L, Bloy L, Chen Y, … Roberts TP (2018). Abnormal auditory mismatch fields in adults with autism spectrum disorder. Neuroscience Letters, 698, 140–145. doi: 10.1016/j.neulet.2018.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning (AGS ed.). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, … Amaral DG (2011). Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proceedings of the National Academy of Sciences, 108(50), 20195–20200. doi: 10.1073/pnas.1107560108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, & Schoffelen J-M (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011: 156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, & Elam M (2008). Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neuroscience Letters, 434(2), 218–223. doi: 10.1016/j.neulet.2008.01.066 [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofiev AO, Nygren G, Gillberg C, & Elam M (2009). The right hemisphere fails to respond to temporal novelty in autism: Evidence from an ERP study. Clinical Neurophysiology, 120(3), 520–529. doi: 10.1016/j.clinph.2008.12.034 [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, & McGuinn M (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, (8), 1043–1051. doi: 10.1093/sleep/23.8.1d [DOI] [PubMed] [Google Scholar]
- Pang EW, & Taylor MJ (2000). Tracking the development of the N1 from age 3 to adulthood: An examination of speech and non-speech stimuli. Clinical Neurophysiology, 111(3), 388–397. doi: 10.1016/S1388-2457(99)00259-X [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertnard O, Giard MH, & Echallier JF (1987). Mapping of scalp potentials by surface spline interpolation. Electroencephalography and Clinical Neurophysiology, 66(1), 75–81. doi: 10.1016/0013-4694(87)90141-6 [DOI] [PubMed] [Google Scholar]
- Ponton C, Eggermont J, Khosla D, Kwong B, & Don M (2002). Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clinical Neurophysiology, 113, 407–420. doi: 10.1016/S1388-2457(01)00733-7 [DOI] [PubMed] [Google Scholar]
- Port RG, Edgar JC, Ku M, Bloy L, Murray R, Blaskey L, … Roberts TPL (2016). Maturation of auditory neural processes in autism spectrum disorder - A longitudinal MEG study. NeuroImage: Clinical, 11, 566–577. doi: 10.1016/j.nicl.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Bloy L, Ku M, Blaskey L, Jackel CR, Edgar JC, & Berman JI (2020). A multimodal study of the contributions of conduction velocity to the auditory evoked neuromagnetic response: Anomalies in autism spectrum disorder. Autism Research, 13(10), 1730–1745. doi: 10.1002/aur.2369 [DOI] [PubMed] [Google Scholar]
- Roberts TPL, Lanza MR, Della J, Qasmieha S, Hines K, Blaskey L, … Berman JI (2013). Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Research, 1537, 79–85. doi: 10.1016/j.brainres.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Matsuzaki J, Blaskey L, Bloy L, Edgar JC, Kim M, … Embick D (2019). Delayed M50/M100 evoked response component latency in minimally verbal/nonverbal children who have autism spectrum disorder. Molecular Autism, 10, 34. doi: 10.1186/s13229-019-0283-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Sandström M, Ahlsén G, & Gillberg C (1999). Autism and hearing loss. Journal of Autism and Developmental Disorders, 29(5), 349–357. doi: 10.1023/A:1023022709710 [DOI] [PubMed] [Google Scholar]
- Saggar M, King BG, Zanesco AP, MacLean KA, Aichele SR, Jacobs TL, … & Saron CD (2012). Intensive training induces longitudinal changes in meditation state-related EEG oscillatory activity. Frontiers in Human Neuroscience, 6: 256. doi: 10.3389/fnhum.2012.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter G, & Buchsbaum M (1973). The effects of attention, stimulus intensity, and individual differences on the average evoked response. Psychophysiology, 10(4), 392–400. doi: 10.1111/j.1469-8986.1973.tb00797.x [DOI] [PubMed] [Google Scholar]
- Sassenhagen J, & Draschkow D (2019). Cluster-based permutation tests of MEG/EEG data do not establish significance of effect latency or location. Psychophysiology, 56(6): e13335. doi: 10.1111/psyp.13335 [DOI] [PubMed] [Google Scholar]
- Shafer VL, Yu YH, & Wagner M (2015). Maturation of cortical auditory evoked potentials (CAEPs) to speech recorded from frontocentral and temporal sites: Three months to eight years of age. International Journal of Psychophysiology, 95(2), 77–93. doi: 10.1016/j.ijpsycho.2014.08.1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, & Rubenstein JLR (2019). Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Molecular Psychiatry, 24, 1248–1257. doi: 10.1038/s41380-019-0426-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cichetti DV, & Balla DA (2005). Vineland adaptive behavior scales (2nd ed.). Minneapolis, MN: NCS Pearson. [Google Scholar]
- Stephen JM, Hill DE, Peters A, Flynn L, Zhang T, & Okada Y (2017). Development of auditory evoked responses in normally developing preschool children and children with autism spectrum disorder. Developmental Neuroscience, 39(5), 430–441. doi: 10.1159/000477614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC; Sutherland MT, & McKinney CJ (2005). Validation of SOBI components from high-density EEG. NeuroImage, 25(2), 539–553. doi: 10.1016/j.neuroimage.2004.11.027 [DOI] [PubMed] [Google Scholar]
- Tlumak A, Durrant J, & Delgado R (2016). The effect of stimulus intensity and carrier frequency on auditory middle- and long-latency evoked potentials using a steady-state-response approach. American Journal of Audiology, 25(1), 62–74. doi: 10.1044/2016_AJA-15-0061 [DOI] [PubMed] [Google Scholar]
- Vlaskamp C, Oranje B, Madsen GF, Møllegaard Jepsen JR, Durston S, Cantio C, … Bilenberg N (2017). Auditory processing in autism spectrum disorder: Mismatch negativity deficits. Autism Research, 10(11), 1857–1865. doi: 10.1002/aur.1821 [DOI] [PubMed] [Google Scholar]
- Williams ZJ, Abdelmessih PG, Key AP, & Woynaroski TG (2020). Cortical auditory processing of simple stimuli is altered in autism: A meta-analysis of auditory evoked responses. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. doi: 10.1016/j.bpsc.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich JL, & Cone-Wesson BK (2006). Maturation of CAEP in infants and children: A review. Hearing Research, 212(1–2), 212–223. doi: 10.1016/j.heares.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK, & Shepherd R (2006). Maturation of the cortical auditory evoked potential in infants and young children. Hearing Research, 212(1–2), 185–202. doi: 10.1016/j.heares.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Duku E, Fombonne E, Szatmari P, Smith IM, Bryson SE, … Bruno R (2019). Developmental functioning and symptom severity influence age of diagnosis in Canadian preschool children with autism. Paediatrics and Child Health, 24(1), e57–e65. doi: 10.1093/pch/pxy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.