Abstract
Purpose
Papillorenal syndrome (PAPRS) is a rare inherited disorder often involves abnormalities of eye and kidney. Paired box 2 (PAX2) gene, which is widely expressed in the development of the organs including kidney, ureter, eye, ear, and central nervous system has been considered an underlying cause of PAPRS. The present work aims to further our understanding of PAX2 gene and PAPRS by reporting a family with PAPRS associated with a novel PAX2 mutation and describing ocular manifestation of PAX2 mutation in previous literatures.
Observation
We herein present a family with PAPRS presented with typical congenital optic disc defects and mild renal dysplasia. Through screening of candidate genes based on the next-generation sequencing, the heterozygous PAX2 mutation c.175C > T (p. Arg59Trp) was identified which had never been reported.
Conclusions
The study expands the genetic and clinic spectrum of PAPRS. Further review of detailed ocular manifestation and genotypes of PAX2 mutation in previous study improves the recognition of the ocular phenotypes’ spectrum, assists in the identification of PAPRS. Moreover, this study reveals that PAPRS is a systemic disorder with heterogeneous diverse phenotypes, and shows the importance of gene panel sequencing in the diagnosis of PAPRS which could achieve high diagnostic rates.
Keywords: Papillorenal syndrome, PAX2 gene, Mutation, Optic disc, Ophthalmic manifestations
1. Introduction
Papillorenal syndrome (PAPRS; MIM 120330), also known as renal-coloboma syndrome is a rare autosomal dominant disease characterized by abnormal optic nerve and renal findings. In 1977, PAPRS was first described in a family with diverse manifestations of eye and kidney by Rieger.1 According to previous studies, commonly observed ocular manifestations of PAPRS are various, including optic nerve coloboma, morning glory anomaly, and excavation of the optic disc. Other infrequent findings may include keratopathy, scleral staphyloma, optic nerve cyst, microphthalmia, and pigmentary macular dysplasia.2 Kidney disorders like renal hypoplasia, multicystic kidney, oligomeganephronia, and vesicoureteric reflux leading to end-stage renal disease are always represented in patients with PAPRS. About one-half of patients who have findings of optic nerve malformation and renal dysplasia harbor a mutation in the Paired box 2 (PAX2; MIM 167409) gene.3 PAX2 is a nuclear transcription factor gene abundantly expressed in the kidney, eye, cochlea, pancreas, and central nervous system during embryogenic development.4 The mutations in PAX2 gene have been recognized as a critical cause of PAPRS.
Here, we present three patients in a family consist of three generations, who was suspected as PAPRS based on their ocular manifestations, though their renal abnormalities are insignificant. The diagnosis was further confirmed by detection of a novel PAX2 heterozygous mutation using the next-generation sequencing.
2. Case report
Patient 1 (Fig. 1, II:1), a 46 years old female, was admitted to our hospital because of diminution of vision in the right eye for one year in March 2016. The best corrected visual acuity (BCVA) of her right eye was 0.15 and 0.6 in the left eye. There was no obvious abnormity found in the examination of anterior segment examination, pupillary reflexes, and external ocular eye movements. Fundus examinations (Fig. 2 A, B) revealed bilateral optic nerve abnormalities with temporal optic disc pit (ODP)——an oval, gray-white depression with absent of central retinal vessels and numerous cilioretinal vessels. Meanwhile the right eye presented with maculopathy including cystic edema and retinal detachment (RD). The spectral domain optic coherence tomography (SD-OCT) (Heidelberg Engineering, Heidelberg, Germany) examination (Fig. 3 A, C) of the right eye showed temporal ODP, macular cystic edema, retinal detachment and disruption of IS/OS. And temporal ODP, retina fuzzy border, and discontinuity of IS/OS were found in SD-OCT of the left eye (Fig. 3 B, D). The ultrasound of the right eye also showed retina edema and detachment. The systemic evaluation was unremarkable. In previous medical history, the patient underwent right mastofibroma resection six years ago and hysteromyomectomy four years ago. The diagnosis of the patient was amblyopia, bilateral ODP, cystoid macular edema and serous retinal detachment of right eye. Then, the patient was treated with a pars plana vitrectomy (PPV), internal limiting membrane (ILM) peeling, and fluid-gas exchange with C3F8 in the right eye. As shown in Fig. 3, the RD and macular edema were completely recovered in two years with an improved BCVA (OD 0.3 OS 0.8).
Fig. 1.
The pedigree of the family in this study.
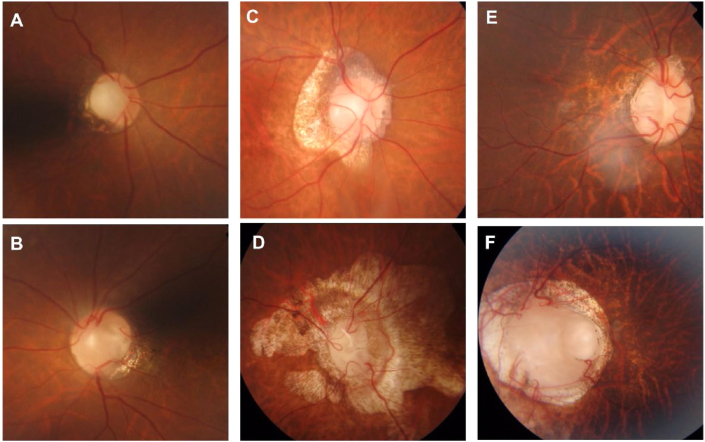
Fig. 2.
Fundus images of the patients.
(A, B) Fundus image of patient 1 showing the abnormal appearance of bilateral optic discs with temporal optic disc pit (ODP). The absence of central retinal vessels and numerous cilioretinal vessels.
(C, D) Fundus image of patient 2 showing the presence of optic disc central excavation and chorioretinal atrophic lesion in both eyes, more prominent in left eye(D).
(E, F) Fundus image of patient 3. A typical optic nerve coloboma in the left eye could be observed (F).
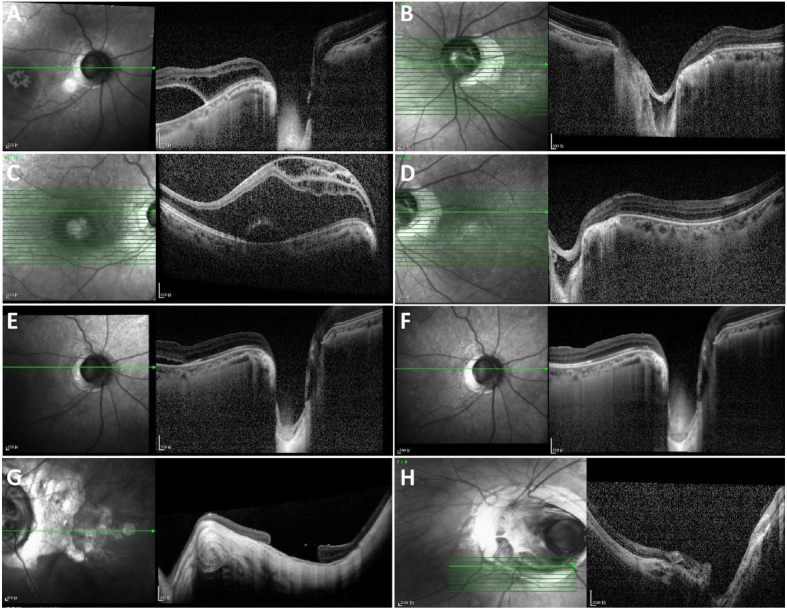
Fig. 3.
SD-OCT images of the patients.
(A, C) SD-OCT images in the right eye of patient 1 showing severe retinoschisis in the whole macular area which termed to ODP maculopathy and evident neurosensory detachment.
(B, D) A temporal ODP, retina fuzzy border, and discontinuity of IS/OS are presenting in SD-OCT in the left eye of patient 1.
(E) The macular cystic edema had significant improvement but neurosensory detachment remained in the right eye of patient 1 in one year after surgery.
(F) The RD and macular edema were completely recovered in the right eye of patient 1 in 2 years after surgery.
(G) SD-OCT images of the patient 2 showing a macular hole in the left eye.
(H) SD-OCT images of the patient 3 showing the optic nerve coloboma in the left eye.
Of note, her mother (Fig. 1, I:1) also suffered from ocular disease. In July 2010, patient 2 (Fig. 1, I:1), a 67 years old female, who complained about floater accompanied by blurred vision of left eye for one year was admitted to our hospital. The BCVA was 0.4 of her right eye and HM/BE of the left eye. Under fundus examination, as shown in Fig. 2, the left eye showed retinal proliferative membrane, RD with obviously elevated retina, and abnormal appearance of the optic disc with a central excavation and chorioretinal atrophy. The Optic disc central excavation and chorioretinal atrophic lesion were also noted in the right eye but much milder. The SD-OCT revealed a macular hole (MH) in the left eye (Fig. 3). She had a history of high myopia for 30 years. Previously she underwent a surgery of phacoemulsification with intraocular lens (IOL) implantation in the left eye for cataract in 2008. She was treated with PPV, photocoagulation, and silicone oil injection in the left eye during hospitalization. Seven months later, the silicone oil was removed. In the last follow-up visit, the OCT scan showed the macular hole of the left eye did not fully recover but remained stable and BCVA achieved a slight improvement (OD 0.5 OS FC/BE).
Patient 3 (Fig. 1, III:1), the daughter of patient 1, was born in 1998. She was diagnosed as strabismus shortly after birth. The strabismus surgery and amblyopia training were performed in the left eye when she was six years old while no other medical history of interest. The ophthalmologic examination revealed the congenital abnormality of her left optic nerve presented with optic nerve coloboma——a bowl-shaped inferior excavation with enlarged optic nerve head (Fig. 2 E, F). Up to now, she was under close follow-up and remained a stable vision——BCVA of right eye is 1.0 with −1.00D myopic correction and 0.9 of left eye with −6.25D myopic correction without any complication.
We assumed the existence of a hereditary cause associated with optic disc abnormality among this family. After obtaining informed consent, the genomic DNA from affected patients (patient 1–3) in this family was extracted from peripheral blood according to standard procedures. Mutations were screened among 792 candidate genes using the custom-designed Target_Eye_792_V2 chip (BGI-Shenzhen, China). The detailed procedure was described previously.5 The genetic study revealed that all three patients carried the heterozygous PAX2 mutation c.175C > T (p. Arg59Trp), consisting of a nucleotide exchange from C to G at position 175 in exon 2 of PAX2 gene, leading to a substitution of the arginine to tyrosine at position 59 of the protein (p. Arg59Trp). The missense alteration was predicted a pathogenic effect by all four software (SIFT/LRT/Mutation Taster/FATHMM). To the best of our knowledge, this variant has not been previously reported in the literature.
To further evaluate the condition of the patients, routine urinalysis, kidney functions, and abdominal ultrasound scans were performed, even though none of them complained of symptoms associated with renal disorders. As shown in Table 1, there were no renal abnormality except for patient 2 presented with multiple renal cysts and patient 3 had mild elevated BUN (8.9mmol/L, normal range: 2.5–6.4 mmol/L).
Table 1.
Laboratory findings and renal abnormity of patients at present.
| Patient (No.) | Gender | age (years) | BUN (mmol/L) | CREA (umol/L) | Renal gross morphology | PU | GU | Gout |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 46 | 4.0 | 61 | Not significant | – | – | – |
| 2 | F | 73 | 6.7 | 101 | Multiple renal cysts | – | – | – |
| 3 | F | 20 | 8.9 | 99 | NA | – | – | – |
| Normal range | 2.5–6.4 | 50–110 | – | – | – | – |
F: female; BUN: Blood Urea Nitrogen; CREA: Creatine; NA: not available; PU: proteinuria; GU, glycosuria.
3. Discussion
Papillorenal syndrome is an autosomal dominant congenital disorder mainly manifesting by ocular and renal abnormalities due to the mutation of PAX2 gene. There is currently a lack of the exact incidence of PAPRS, because of its rare incidence and only a few cases have been reported. 92% of individuals with positive PAX2 mutation were reported to have renal disorders and 77% of individuals with positive PAX2 mutation had ophthalmologic diseases.6 We reviewed and summarized reported cases with PAX2 gene related optic involvement from 2003 to 2019, as depicted in supplementary table 1, to demonstrate the reported detailed ocular manifestations of PAPRS and genotype of PAX2 mutation. The most common ocular findings in these reports were, in descending order, optic nerve coloboma (n = 22), excavation of the optic disc or pit (n = 15), optic nerve hypoplasia (n = 15), abnormal retinal vessels, strabismus (n = 13), and abnormal retinal vessel (n = 12).
In this study, patient 1 was admitted to our hospital for retinal detachment related to temporal optic disc pits. As one kind of maculopathy, the serous RD was observed in 63% of eyes with temporally located ODP.7 The existence of acquired type of pits was revealed by the prevalence of ODP was 0.19% which increased with age and higher in patients with high tension glaucoma.8 Though the fundus manifestation of patient 2 was kind of similar to the myopia-related fundus, the optic disc central excavation could be observed. What's more, the daughter of patient 1 (patient 3) presented with optic nerve coloboma in the left eye which was a classic finding of congenital optic malformations. Thus, we arranged a follow-up genetic testing for this family. To our knowledge, the phenotype of patient 2 in this case report, a macular hole, has not been previously reported in PAPRS, which we suggest probably could be a complication of high myopia.
Through the genetic testing based on the next generation sequencing (NGS) for this family, a new PAX2 heterozygous mutation was detected. All three affected patients in this family were observed with a missense mutation of PAX2, that is C to G at position 175 in exon 2, which changed arginine to tyrosine at position 59 of the protein. The human PAX2 gene encoding a conserved 128 amino acid paired box domain is located on chromosome 10 close to the bands q24 and q25, consists of 12 exons.9 The PAX2 gene is expressed in primitive cells of the kidney, ureter, eye, ear, and central nervous system during fetal development. Based on currently reported literatures, various mutation types have been detected in PAX2, containing missense, in-frame deletion, in-frame duplication, and nonsense mutations.10 Variants of PAX2 causing PAPRS have been identified in the exons encoding the DNA binding domain (exons 2, 3, and 4), the octapeptide domain (exon 5), the homeodomain (exon 7), and the transactivation domain (exons 8,9,10 and 11).6 The PAX2 mutation detected in our patients occurred in the paired domain, known for its DNA-binding properties.
In terms of the type and location of PAX2 mutation, no exact correlation between genotype and phenotype has emerged. Whereas a previous study9 suggested that PAX2 mutations in exons 2,3 and 4 were associated with both abnormality of eye and kidney, while mutations in exons 7 and 9 were related to typical renal dysplasia and milder ocular manifestations. The onset syndromes of the family identified in this study were various ocular disorders while the renal alterations were asymptomatic that had not been diagnosed before the detection of the PAX2 mutation. Thus, c.175C > T of PAX2 gene may cause the PAPRS characterized by evident ocular disorders with milder renal abnormalities. However, regular long-term follow-up of renal function for these patients is still necessary due to renal dysfunction can develop at different ages.11
Even though patients in our study possessed the same variant of PAX2, ocular manifestations of them were diverse such as amblyopia, strabismus, optic pit or excavation, optic nerve coloboma, retinal detachment and maculopathy (macular cystic edema and MH). The phenomenon that one kind of variant of PAX2 presented with diverse phenotypes has been widely reported, ranging from Iwafuchi et al.12 reported a single family with PAX2 mutation c.76dupG presented with various renal phenotypes including FSGS and CAKUT to Deng et al.13 identified inconsistent manifestations among children with PAX2-related disorder. Therefore, environmental or epigenetic modifiers should be considered and further investigated.
4. Conclusion
This study identified a novel PAX2 mutation among a family diagnosed with PAPRS. Detailed ocular manifestations related to PAX2 mutation of this family and previous literatures were described and reviewed. Thus, this study may help in identifying more individuals with pathogenic variants of PAX2 by typical fundus abnormalities and hopefully to improve prognosis of them by early detection and intervention of renal disorders. Moreover, for patients suspected of congenital abnormal optic disc, thorough inquiry of the family history should be conducted and the gene screen is recommended.
Patient consent
The patients provided consent for the publication of this case report in writing. This report does not contain any personal identifying information.
Declaration of competing interest
No conflict of interest exists.
Acknowledgement and Disclosures
We sincerely thank the patients and their families for their participation and support in this study. In addition, we would like to thank BGI-Shenzhen for their technical support of the gene test. The authors declare that no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. All authors attest that they meet the current ICMJE criteria for Authorship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2021.101091.
Contributor Information
Shixue Liu, Email: lsx12312233@163.com.
Peijun Zhang, Email: peijun_zhang@aliyun.com.
Jihong Wu, Email: jihongwu@fudan.edu.cn.
Qing Chang, Email: qngchang@aliyun.com.
Funding
This study was supported by the research Grant (18411965100) from the Science and Technology Commission of Shanghai Municipality.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rieger G. [On the clinical picture of Handmann's anomaly of the optic nerve Morning glory syndrome? (author's transl)] Klin Monbl Augenheilkd. 1977;170(5):697–706. [PubMed] [Google Scholar]
- 2.Schimmenti L.A. Renal coloboma syndrome. Eur J Med Genet. 2011;19(12):1207–1212. doi: 10.1038/ejhg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wall P.B., Traboulsi E.I. Congenital abnormalities of the optic nerve: from gene mutation to clinical expression. Curr Neurol Neurosci Rep. 2013;13(7) doi: 10.1007/s11910-013-0363-2. [DOI] [PubMed] [Google Scholar]
- 4.Hoefele J., Gabert M., Heinrich U. A novel interstitial deletion of 10q24.2q24.32 in a patient with renal coloboma syndrome. Eur J Med Genet. 2012;55(3):211–215. doi: 10.1016/j.ejmg.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Wang D.D., Gao F.J., Hu F.Y. Next-generation sequencing-aided precise diagnosis of Stickler syndrome type I. Acta Ophthalmol. 2020;98(4):e440–e446. doi: 10.1111/aos.14302. [DOI] [PubMed] [Google Scholar]
- 6.Bower M., Salomon R., Allanson J. Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat. 2012;33(3):457–466. doi: 10.1002/humu.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown G.C., Shields J.A., Goldberg R.E. Congenital pits of the optic nerve head. II. Clinical studies in humans. Ophthalmology. 1980;87(1):51–65. doi: 10.1016/s0161-6420(80)35278-0. [DOI] [PubMed] [Google Scholar]
- 8.Healey P.R., Mitchell P. The prevalence of optic disc pits and their relationship to glaucoma. J Glaucoma. 2008;17(1):11–14. doi: 10.1097/IJG.0b013e318133fc34. [DOI] [PubMed] [Google Scholar]
- 9.Laimutis K., Jackson C., Xu X. Typical renal-coloboma syndrome phenotype in a patient with a submicroscopic deletion of the PAX2 gene. Am J Med Genet. 2012;158A(6):1437–1441. doi: 10.1002/ajmg.a.35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossanti R., Morisada N., Nozu K. Clinical and genetic variability of PAX2-related disorder in the Japanese population. J Hum Genet. 2020;65(6):541–549. doi: 10.1038/s10038-020-0741-y. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Zhai S-b, Zhao L-y, Zhang Y., Sun B-c, Ma Q-s. New PAX2 heterozygous mutation in a child with chronic kidney disease: a case report and review of the literature. BMC Nephrol. 2018;19 doi: 10.1186/s12882-018-1044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwafuchi Y., Morioka T., Morita T. Diverse renal phenotypes observed in a single family with a genetic mutation in paired box protein 2. Case Rep Nephrol Dial. 2016;6(1):61–69. doi: 10.1159/000445679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng H., Zhang Y., Xiao H. Diverse phenotypes in children with PAX2-related disorder. Mol Genet Genom Med. 2019;7(6) doi: 10.1002/mgg3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.