Abstract
Short chain fructo-oligosaccharides (scFOS) are well-recognized prebiotic fibers. Fossence™ (FOSS) is a scFOS that has been produced from sucrose via a proprietary fermentation process and has not been tested for its digestibility or glucose/insulin response (GR and IR, respectively). The present randomized, controlled, cross-over study was conducted in 3 phases to explore GR and IR to ingestion of FOSS, when replaced by/added to available-carbohydrates (avCHO) among 25 healthy adults (40 ± 14years). In each phase GR and IR elicited by 3–4 test-meals were measured among the fasted recruited subjects. The interventional test meals were as follows: Phase-1, water alone or 10g FOSS or 10g Dextrose in 250ml water; Phase-2, 250ml water containing Dextrose:FOSS (g:g) in the content as 50:0 or 50:15 or 35:0 or 35:15; Phase-3 portions of white-bread (WB) containing avCHO:FOSS (g:g) in the content as 50:0 or 50:15 or 35:0 or 35:15. Blood samples (finger prick method) were collected at fasting and 15, 30, 45, 60, 90 and 120 min after start of test meal ingestion. Plasma glucose and serum insulin were analyzed utilizing standard methods. The primary endpoint was differences in glucose IAUC. All subjects provided their written consent to participate in the study (ClinicalTrials.gov: NCT03755232). The results demonstrated that FOSS, when consumed alone, showed no raise in glycaemia or insulinemia and was statistically equivalent to response of water alone. GR and IR elicited by dextrose:FOSS and WB:FOSS test-meals of Phase 2 and Phase 3, were statistically equivalent to the respective test-meals without FOSS. Result of the 3 phases support the hypothesis that FOSS is resistant to breakdown and is indigestible in the human small-intestine, and therefore can be classified as an unavailable carbohydrate that does not raise post prandial blood glucose or insulin. FOSS, being sweet to taste, may be an acceptable sugar replacer in beverages without compromising their taste and sensory qualities.
Keywords: Short chain fructo-oligosaccharides, Prebiotic fiber, Non-digestible carbohydrates, Glycemic response, Insulin response, Sugar substitute
short chain fructo-oligosaccharides, prebiotic fiber, non-digestible carbohydrates, glycemic response, insulin response, sugar substitute.
1. Introduction
Short-chain fructo-oligosaccharides (scFOS) are non-digestible carbohydrates naturally found in small amounts (0.8–10%) in common foods such as asparagus, artichokes, onions, honey, rye, and wheat (Becker et al., 1977; Shiomi and Izawa 1980; Darbyshire and Henry 1981). Chicory, Jerusalem artichoke and dahlia contain substantial quantities of fructo-oligosaccharides which a high degree of polymerization (DP) and these are referred to as inulin. scFOS can be derived from sucrose enzymatically using ß-fructofuranosidase or fructosyl transferases. The resulting scFOS consist of 1 glucose molecule attached to 2–4 fructose units; GF2 (1-Kestose), GF3 (Nystose) and GF4 (1ß-fructofuranosylnystose) (Ganaie et al., 2014).
ScFOS are prebiotic fibers (Vandenplas et al., 2015; Slavin 2013) that have been studied for health effects including glycemic control (Yamashita et al., 1984; Sheth et al., 2015), gut health (Bouhnik et al., 2004; Bouhnik et al., 2006), mineral absorption (van den Heuvel et al., 1999; van den Heuvel et al., 2009) nonalcoholic fatty liver disease (Loman et al., 2018) and blood lipids (Beserra et al., 2015). Being a prebiotic fiber, scFOS has the ability to increase beneficial colonic bacteria, decrease pathogenic bacteria (Tuohy et al., 2014; Walsh et al., 2014) and increase the production of colonic short-chain fatty acids (SCFA) which, in turn, may influence human health (Ríos-Covián et al., 2016). ScFOS, being resistant to hydrolysis by human digestive enzymes, escape digestion in the small-intestine and enter the colon, ready to be metabolized by the resident gut bacteria. Being unavailable carbohydrates, scFOS do not raise blood glucose. Thus, replacing available-carbohydrate (avCHO) with scFOS in foods reduces acute glycemic responses (Respondek et al., 2014; Lecerf et al., 2015).
Fossence™ (FOSS) is produced from enzymatic modification of sucrose. It is a synthetic product consisting predominantly (93%) of scFOS with DP of 3–5, and is about 30% as sweet as sucrose (Bornet, 1994). Thus, it may be a desirable alternative sweetener if, when incorporated into foods or drinks, it has no impact on postprandial glycaemia. FOSS is not digested by amylase in-vitro, but its digestibility in-vivo is not known, and its effects on postprandial glucose- (GR) and insulin-responses (IR) are unknown. Therefore, the current study, which was designed in 3 phases, was aimed to assess the GR and IR of FOSS. The aim of Phase-1 was to confirm that FOSS is resistant to digestion and would elicit no GR or IR. The aims of Phases-2 and 3 were to confirm, using an avCHO-containing drink (Phase 2) and an avCHO-containing solid food (Phase 3), that FOSS would not increase GR and IR when added to avCHO and, therefore, that replacing avCHO with FOSS would reduce GR and IR by amounts equivalent to those expected from the reduced avCHO intake.
2. Methods
2.1. Study design, subject selection and recruitment
FOSS was compared with an equivalent amount of Dextrose for its glycemic and insulin response, when administered alone (Phase 1), and when 15 g was added to both 35 and 50 g avCHO so as to determine the effects of FOSS when added to avCHO or substituted for 30% of avCHO (Phase 2/Phase 3). Each phase of the study had a randomised, controlled, cross-over design and was conducted at INQUIS Clinical Research, Ltd, Toronto, Canada (INQUIS). For each phase, 13 males and 12 non-pregnant, non-lactating females, were drawn from a pool of 29 volunteers and recruited from a database of individuals who had previously participated in studies at INQUIS and had given permission to be contacted for recruitment for future studies. Participants between the age group of 18–65 years, body mass index between 18 – 30 kg/m2 (height and weight measured using standardized tools at the first visit) no chronic disease such as type-1 or type-2 diabetes mellitus (fasting blood sugar levels <100 mg/dL (or <5.6 mmol/L) as assessed at the first visit) or with no history of cardiovascular, metabolic, respiratory, renal, gastrointestinal or hepatic disease or any other disease affecting their dietary intake were included. Participants with any known food allergies or intolerance or with any strong dislike of or intolerance to sweetened beverages were excluded.
Subjects provided informed consent to participate in each phase of the study using a protocol approved by the Western Institutional Review Board which meets all the requirements of the US Food and Drug Administration (FDA), the Department of Health and Human Services (DHHS), the Canadian Health Protection Branch (HPB), Canadian Institutes of Health Research (CIHR) and the European Community Guidelines (IRB Tracking Number: 20183115). The clinical trial was registered after the recruitment of the participants in ClinicalTrial.gov under the identification number: NCT03755232 (URL: https://clinicaltrials.gov/ct2/show/NCT03755232). The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants provided written informed consent. The consent and screening procedures are described in Supplementary Information.
2.2. Study procedures used in each phase
Eligible participants in each phase were studied on 3 or 4 separate days over periods of 2–5 wk. On each test day, participants came to INQUIS in the morning after a 10–14 h overnight fast (water allowed). Participants were asked to maintain stable dietary and activity habits throughout their participation in the study and to refrain from drinking alcohol, and from unusual levels of food intake or physical activity for 24 h before each test. Participants also refrained from taking any medication for 24 h prior to study visits except those listed in the inclusion/exclusion criteria (Supplementary Information). Any subject who was not feeling well or had not complied with the preceding experimental conditions was rescheduled for another day.
On each test occasion, after subjects were weighed, 2 fasting blood samples for glucose and insulin analysis were obtained by finger-prick 5min apart and, after the 2nd sample, the subject started to consume the test-meal of the respective study Phase. Subjects were asked to consume the entire test-meal evenly over 10min. Test-meal durations over 15 min were considered to be protocol deviations. At the first sip/bite of intake of test meal, timer was started and additional capillary blood samples for glucose and insulin analysis were taken at 15, 30, 45, 60, 90, and 120 min. Each blood sample consisted of 10–12 drops of blood; the first drop was wiped away after which 5–6 drops were placed into an 0.3ml heparin fluoride containing Microvette® 300 FH (Sarstedt AG & Co. KG, Nümbrecht, Germany; product number 20.1307.100) for plasma glucose analysis and 5–6 drops into a 0.3ml Microvette® 100 K3E with a clot activator (Sarstedt product number 20.1278.100) for serum insulin analysis. Before and during the test, a test record was filled out with the participant's initials, ID number, date, body weight, test meal, beverage, time of starting to eat, time it took to eat, time and any unusual activities the previous day. During the 2 h of the test, participants remained seated quietly. After the last blood sample participants were offered a snack and allowed to leave.
2.3. Interventional product
Fossence™ (FOSS) is a scFOS produced from sucrose via a proprietary fermentation process (Tata Chemicals Ltd., Mumbai, Maharashtra, India). It is a mixture of tri-saccharides (GF2), tetra-saccharides (GF3) and penta-saccharides (GF4) of glucose (G) and fructose (F). The glucose at the non-reducing end is attached to fructose through an α(1–2) linkage and the fructose molecules are linked through a β(2-1) linkage. FOSS is a fine white free flowing powder containing about 93% fructo-oligosaccharides and 5.5% other sugars. FOSS is currently US GRAS notified CAS Number: 308066-66-2.
2.4. Randomization
The order of the test meals for each recruited subject in each Phase of the study was randomly determined using an online randomization program (researchrandomizer.org). Test meals were made equi-carbohydrate in arms replacing 30% of the available carbohydrates.
2.4.1. Phase-1 test-meals
The 3 test-meals consisted of 250 ml water alone (Water), or 10g FOSS (F10) or 10g Dextrose (Dex10) (ADM Clintose® A Dextrose, Decatur, IL, USA) dissolved in 250 ml water. Each test-meal was served with an additional 150 ml water to wash out the mouth. The study subjects finished the test meal in the time allocated.
2.4.2. Phase-2 test-meals
The 4 test-meals consisted of 50g Dextrose (Dex50), or Dex50 plus 15g FOSS (Dex50 + F15), or 35g Dextrose (Dex35) or Dex35 plus 15g FOSS (Dex35 + F15) each dissolved in 250 ml water. Each test-meal was served with an additional 150 ml water to wash out the mouth. Total carbohydrates in the test meals Dex50 and Dex35 + F15 were equivalent. The study subjects finished the test meal in the time allocated.
2.4.3. Phase 3 test-meals
The 4 test-meals consisted of 112.5g white-bread (WB) containing 51.9g avCHO (WB50), or WB50 plus 15g FOSS (WB50 + F15), or 76g WB containing 35.1g avCHO (WB35) or WB35 plus 15g FOSS (WB35 + F15). FOSS was carefully sprinkled on the bread portions of the respective test meals. Each test-meal was served with 250 ml water plus an additional 150 ml water to wash out the mouth. WB was baked at INQUIS using the method described in Supplemental Information. Nutritional composition of 100 g of WB, based on proximate analysis, is as follows: 223 kcal, 48.7g carbohydrate, 2.6g dietary fiber, 8.1g protein, 0.6g fat and 46.1g of avCHO (total carbohydrate minus dietary fiber). Total carbohydrates in the test meals WB50 and WB35 + F15 were equivalent. The study subjects finished the test meal in the time allocated.
2.5. Biochemical analysis
2.5.1. Glucose
Immediately after collection, the microvette tubes were centrifuged at 2,000 x g for 5 min and the plasma aliquoted into 2 tubes (1 for back-up) and stored at -70 °C until analysis which was performed within 5 d of collection using a VITROS 350 analyzer (Ortho Clinical Diagnostics, Markham, ON, Canada).
2.5.2. Insulin
Upon collection, the microvette tubes were allowed to clot at room temperature for 30 min, centrifuged at 10,000 x g for 5 min and the plasma aliquoted into 2 tubes (1 for back-up) and stored at -70 °C or -94°F (minus seventy degrees Celsius or minus ninety four degrees Fahrenheit) until analysis using the Human Insulin EIA Kit (Alpco Diagnostics, Salem, NH, USA).
2.6. Calculations
2.6.1. Incremental areas under the curve
Incremental areas under the glucose and insulin response curves, ignoring area below the baseline (iAUC) were calculated by the trapezoid rule (Wolever, 2004). The mean glucose or insulin concentration in the 2 fasting blood samples was taken as the fasting concentration for the purposes of calculating iAUC.
2.6.2. Determination of expected responses
2.6.2.1. Phase 1
To estimate the in-vivo digestibility of FOSS, the Equivalent Glycemic Load (EGL) of F10, defined as the amount of glucose that would elicit the same iAUC as F10 (Wolever et al., 2006a, b), was calculated for each subject as follows (Equation 1):
| (1) |
Eq. (1): Calculation of Equivalent Glycemic Load (EGL).
Where F is the glucose iAUC elicited by F10, W the glucose iAUC after water, and M =(D-W)/10 where D is the glucose iAUC after Dex10. If F < W, then EGL was taken to be zero. The mean of the resulting values was the EGL of FOSS. Since the day-to-day variation of glucose iAUC values elicited by 0–5 g available carbohydrate is 60–100% (Wolever et al., 2006a, b), and since the distribution of the ratio of independently variable values becomes highly skewed to the right as the variation increases (Wolever et al., 1991), EGL values >2SD above the mean were excluded to provide a more accurate and precise estimate. Assuming the absorption 1 molecule of FOSS results in 1 glucose (Glycemic index (GI) = 100) and 3 fructose (GI = 30) molecules, the GI of FOSS was taken to be 0.25 × 100 + 0.75 × 30 = 48. Thus, 2.08g of FOSS (1/0.48) would elicit a glucose response equivalent to 1 g glucose.
2.6.2.2. Phases 2 and 3
If the difference in glucose and insulin responses between the 35g and 50g avCHO test-meals was equivalent to that expected based on previous dose-response studies (Wolever and Bolognesi, 1996; Lee and Wolever, 1998), and if the FOSS containing test-meals (eg. Dex50 + F15 and Dex35 + F15) elicited glucose and insulin responses which were equivalent to those elicited by the respective test-meals without FOSS (eg. Dex50 and Dex35), these, taken together, would constitute evidence that FOSS was indigestible in the human small-intestine.
The Relative Glucose Responses (RGR) and Relative Insulin Responses (RIR) elicited by the 35g avCHO doses in Phase 2 were calculated as follows (Equation 2):
| (2) |
Eq. (2): Equation to calculate Relative Glucose Responses (RGR) and Relative Insulin Responses (RIR) elicited by 35g available carbohydrate.
The analogous calculation was done for the WB test-meals in Phase 3. The RGR (glycemic response = iAUC over 0–2h) elicited by avCHO is proportional to (1-e−0.0222g) where g = grams of avCHO (Wolever, 2006). Thus, the expected glucose iAUC elicited by 35g avCHO, relative to that after 50g avCHO is (Equation 3):
| (3) |
Eq. (3): Calculation of Glucose iAUC elicited by 35g available carbohydrate relative to that after 50g available carbohydrate.
However, the dose-response for insulin is virtually linear (Wolever, 2006) so that the insulin iAUC elicited by 35g avCHO, relative to 50g avCHO would be expected to be 100×[35/50] = 70%. Since the amounts of avCHO in the WB35 and WB50 test-meals were 35.1 vs 51.9g, the glucose and insulin responses elicited by WB35 relative to WB50 are expected to be 79.1% and 67.6%, respectively.
The RGR and RIR of the FOSS-containing test-meals in Phase 2 were calculated as per the following equation (Equation 4):
| (4) |
Eq. (4): Relative Glucose Response and Relative Insulin Response of the FOSS-containing test-meals in Phase 2.
The analogous calculation was done for the WB test-meals in Phase 3. Since it is hypothesized that FOSS was indigestible, the RGR and RIR are expected to be 100%.
2.7. Statistical analysis
2.7.1. Differences between means
2.7.1.1. Phase 1
Glucose and insulin iAUCs and concentrations at the various times were subjected to repeated measures analysis of variance using the General Linear Model (ANOVA) examining for the main effects of test-meal. After demonstrating significant heterogeneity, the significance of differences among individual means was determined using Tukey's test with the significance criterion being 2-tailed p < 0.05.
2.7.1.2. Phases 2 and 3
Glucose and insulin iAUC values and concentrations at the various times were subjected to ANOVA examining for the main effects of avCHO-dose (35g vs 50g) and FOSS (avCHO alone vs avCHO + F) and the avCHO-dose×FOSS interaction. If the avCHO-dose×FOSS interaction was significant, the significance of differences among individual means was determined using Tukey's method with 2-tailed p < 0.05.
2.7.2. Equivalence testing
The observed relative responses were taken to be equivalent to the expected relative responses if the 95% confidence interval (95%CI) of the differences were within ±20% for glucose and ±22.8% for insulin. The rationale for allowing a ±20% difference for glucose is that 20% is generally considered to be a physiologically significant difference in glycemic response by Health Canada which proposed only to allow a glucose reduction claim for a food if the reduction in iAUC was at least 20% (Health Canada, 2013). In addition, low-GI foods must have a GI ≤ 55, while high-GI foods have a GI of ≥70; and 55 is 78.5% of (21.5% less than) 70. A ±22.8% difference for insulin was taken to represent equivalence because a recent inter-laboratory study showed that the mean SD of relative insulin responses is about 14% higher than that of relative glucose responses (Wolever et al., 2019).
2.7.3. Statistical power
Using the t-distribution and assuming an average coefficient of variation (CV) of within-individual variation of 2-hour glucose iAUC values of 23%, n = 25 subjects has 90% power to detect a 22% difference in 2-hour glucose iAUC with 2-tailed p < 0.05.
3. Results
A total of 34 subjects were screened of whom 29 were eligible to participate. The pool of 29 eligible subjects comprised a multiethnic population (Caucasian, 17; East Asian, 3; South East Asian, 3; West Asian, 2; and 1 each of South Asian, Hispanic, African and Indigenous) with 15 male and 14 females aged [Mean ± SD (range)] 38.6 ± 14.3 (20–62) y with BMI 24.4 ± 2.7 (19.1–29.4) kg/m2, fasting serum glucose 4.44 ± 0.46 (3.42–5.57) mmol/L and blood pressure 121 ± 10 (101–138)/75 ± 7 (61–88) mmHg. Twenty-five of these individuals participated in each of the 3 phases of the study. All the test-meals consumed by participants during the 3 phases of the study were well tolerated without any gastrointestinal adverse events. Four adverse events were reported; 3 participants reported having a cold and 1 an eye infection. These events were judged to be mild and unrelated to the study treatments, and those reporting them did not participate in the study until all their symptoms had gone and they had stopped taking any treatments for their condition.
3.1. Phase 1
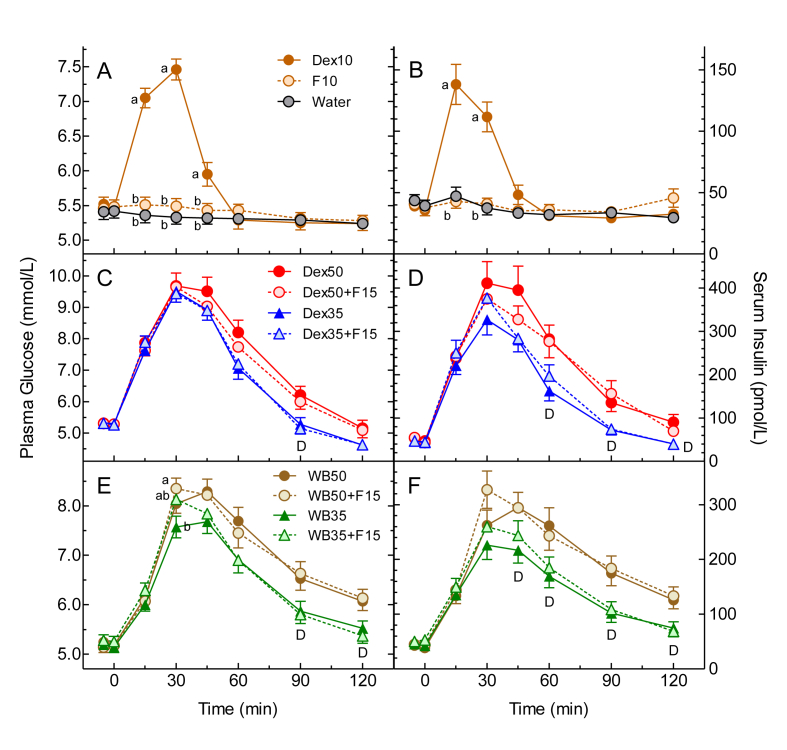
Mean (±SEM) fasting plasma glucose concentrations before Dex10, F10 and Water were similar to each other: 5.50 ± 0.10, 5.50 ± 0.10 and 5.41 ± 0.10 mmol/L, respectively. Plasma glucose after Dex10 was significantly greater than F10 and Water at 15, 30 and 45 min, but Dex10 did not differ significantly from Water at any time after 60min (Figure 1A). Similarly, glucose iAUC after Dex10 was significantly greater compared to that after F10 and Water, with no difference between F10 and Water (Table 1). The mean (95%CI) EGL for F10 was 0.35 (0.09, 0.62) g; after excluding 2 values (2.6 × SD and 3.3 × SD above the mean), EGL for F10 was 0.19 (0.06, 0.32) g. The amount of FOSS that would elicit the same glucose iAUC as 0.19 (0.06, 0.32) g glucose is estimated to be 0.40 (0.12, 0.67) g; this suggests that FOSS contains 96 (99, 93) % unavailable carbohydrate. This corresponds with the scFOS (GF2, GF3 and GF4) content in FOSS of about 93%.
Figure 1.
Glucose and Insulin Responses. Values are means ± SEM for n = 25 subjects. Panels A, C and E show plasma glucose; panels B, D and F show serum insulin. Phase 1 is shown in panels A and B (Dex10 = 10g dextrose, F10 = 10g FOSS), Phase 2 in panels C and D (Dex50 = 50g dextrose; Dex35 = 35g dextrose; F15 = 15g FOSS) and Phase 3 in panels E and F (WB50 = 51.9g available carbohydrate from white bread; WB35 = 35.1g available carbohydrate from white bread; F15 = 15g FOSS). ab means with different letter superscripts differ by Tukey's test, p < 0.05. D = significant main effect of Dose (35g differs from 50g) by ANOVA, p < 0.05.
Table 1.
Phase 1 Glucose and Insulin iAUC and Equivalent Glycemic Load of 10g FOSS.
| Test-Meal | Glucose iAUC (mmol×min/L) | Insulin iAUC (pmol×h/L) | Equivalent Glycemic Load (EGL, g)∗ |
|
|---|---|---|---|---|
| All subjects | Outliers Excluded | |||
| 10g Dextrose | 62.0 ± 4.9a | 50.5 ± 5.3a | - | - |
| Water | 3.3 ± 1.6b | 6.9 ± 2.0b | - | - |
| 10g FOSS | 2.7 ± 0.7b | 11.0 ± 2.4b | 0.35 (0.09, 0.62) | 0.19 (0.06, 0.32) |
Results for iAUC are Mean ± SEM for n = 25 subjects; results for EGL are means (95% confidence intervals).
ab Means with different letter superscripts differ significantly by Tukey's test, p < 0.05.
EGL is defined as the grams of glucose that would elicit the same glycemic response as 10g FOSS. There were 2 outliers excluded (values 2.6 and 3.3 times SD above the mean).
Mean (±SEM) fasting serum insulin concentrations before Dex10, F10 and Water were similar to each other; 37.2 ± 3.6, 39.1 ± 4.2 and 41.5 ± 4.0 pmol/L, respectively. Serum insulin concentrations after Dex10 were significantly greater than F10 and Water at 15 and 30 min, but Dex10 did not differ significantly from Water at any time after that (Figure 1B). Similarly, insulin iAUC after Dex10 was significantly greater than those after F10 and Water, with no difference between iAUC for F10 and Water (Table 1).
3.2. Phase 2
Mean (±SEM) fasting plasma glucose concentrations before Dex50, Dex50 + F15, Dex35 and Dex35 + F15 did not differ significantly from each other (5.26 ± 0.09, 5.27 ± 0.11, 5.24 ± 0.08 and 5.25 ± 0.07 mmol/L, respectively). Plasma glucose concentrations after Dex50 + F15 and Dex35 + F15 were similar to those after Dex50 and Dex35, respectively, at all time points (Figure 1C). The test-meals containing Dex35 elicited lower plasma glucose than those containing Dex50 from 60 to 120 min, but the main effect of dose was only significant at 90 min (Figure 1C). ANOVA of the glucose iAUC values showed a significant main effect of dose, with the Dex35 test-meals eliciting a mean about 20% less than the Dex50 test-meals (Table 2), but there was no significant main effect of FOSS and no avCHO-dose×FOSS interaction. Dex50 elicited a significantly greater glucose iAUC than Dex35 and Dex35 + F15.
Table 2.
Glucose and Insulin iAUC for Phases 2 and 3.
| 50g avCHO | 35g avCHO | Mean (±FOSS) | |||
|---|---|---|---|---|---|
| Phase 2: avCHO source = Glucose | Glucose iAUC (mmol×min/L) | avCHO alone | 269±24a | 199±16b | 234±19~ |
| avCHO+FOSS | 239±16ab | 204±13b | 222±13~ | ||
| Mean (Dose) | 254±19ˆ | 202±13∗ˆ | p=0.438# | ||
| Insulin iAUC (pmol×h/L) | avCHO alone | 364±44a | 235±26b | 299±33~ | |
| avCHO+FOSS | 346±40ab | 267±28ab | 306±29~ | ||
| Mean | 355±36ˆ | 251±24∗ˆ | p=0.579# | ||
| Phase 3: avCHO source = White bread | Glucose iAUC (mmol×min/L) | avCHO alone | 212±18a | 155±12b | 183±13~ |
| avCHO+FOSS | 219±20a | 158±14b | 188±16~ | ||
| Mean | 215±17ˆ | 156±12∗ˆ | p=0.919# | ||
| Insulin iAUC (pmol×h/L) | avCHO alone | 314±33a | 196±22b | 255±27~ | |
| avCHO+FOSS | 324±29a | 210±22b | 267±24~ | ||
| Mean for avCHO | 319±30ˆ | 203±21∗∗ˆ | p=0.960# |
Results are means±SEM for n=25 subjects.
avCHO = available carbohydrate; FOSS = Fossence™; iAUC = incremental area under the curve.
ˆ for 50g and 35g avCHO; ~ for avCHO alone or avCHO+FOSS; # significance of Dose×FOSS interaction from ANOVA.
Significantly different from 50g avCHO; ∗p<0.03; ∗∗p<0.002.
ab Means with different letter superscripts differ significantly by Tukey’s test (p<0.05).
Mean (±SEM) fasting serum insulin concentrations before Dex50, Dex50 + F15, Dex35 and Dex35 + F15 did not differ significantly from each other (50.4 ± 4.4, 49.1 ± 4.4, 45.2 ± 4.3 and 45.5 ± 4.7 pmol/L, respectively). Serum insulin concentrations after Dex50 + F15 and Dex35 + F15 were similar to those after Dex50 and Dex35, respectively, at all time points (Figure 1D). The test-meals containing Dex35 elicited lower serum insulin than those containing Dex50 from 45 to 120 min with the main effect of dose being significant at 60, 90 and 120 min (Figure 1D). ANOVA of the insulin iAUC values showed a significant main effect of avCHO dose, with the Dex35 test-meals eliciting a mean about 30% less than the Dex50 test-meals (Table 2), but there was no significant main effect of FOSS and no avCHO-dose×FOSS interaction. Dex50 elicited a significantly greater insulin iAUC than Dex35.
3.3. Phase 3
Mean (±SEM) fasting plasma glucose concentrations before WB50, WB50 + F15, WB35 and WB35 + F15 did not differ significantly from each other (5.21 ± 0.11, 5.16 ± 0.10, 5.16 ± 0.11 and 5.26 ± 0.11 mmol/L, respectively). Plasma glucose concentrations after WB50 + F15 and WB35 + F15 were similar to those after WB50 and WB35, respectively, at all time points (Figure 1E). At 30 min ANOVA showed a significant main effect of test-meal (p = 0.048) with glucose 30min after WB35 being less than WB50 + F15, but there was no significant main effect of avCHO dose (p = 0.15) or FOSS (p = 0.60) and no avCHO-dose×FOSS interaction (p = 0.078). The test-meals containing WB35 elicited lower plasma glucose than those containing WB50 from 45 to 120 min, but the main effect of avCHO dose was only significant at 90 and 120 min (Figure 1E). ANOVA of the glucose iAUC values showed a significant main effect of avCHO dose, with the WB35 test-meals eliciting a mean about 27% less than the WB50 test-meals (Table 2), but there was no significant main effect of FOSS and no avCHO-dose×FOSS interaction. Glucose iAUC after WB50 was similar to that after WB50 + F15, and both were significantly greater than those after WB35 and WB35 + F15.
Mean (±SEM) fasting serum insulin concentrations before WB50, WB50 + F15, WB35 and WB35 + F15 did not differ significantly from each other (40.4 ± 4.4, 44.3 ± 4.8, 44.0 ± 4.6 and 51.5 ± 5.8 pmol/L, respectively). Serum insulin concentrations after WB50 + F15 and WB35 + F15 were similar to those after WB50 and WB35, respectively, at all time points (Figure 1F). The test-meals containing WB35 elicited lower serum insulin than those containing WB50 from 45 to 120 min with the main effect of avCHO dose being significant at all these times (Figure 1F). ANOVA of the insulin iAUC values showed a significant main effect of avCHO dose, with the WB35 test-meals eliciting a mean about 36% less than the WB50 test-meals (Table 2), but there was no significant main effect of FOSS and no avCHO-dose×FOSS interaction. Insulin iAUC after WB50 was similar to that after WB50 + F15, and both were significantly greater than those after WB35 and WB35 + F15.
3.3.1. Equivalence testing
The glucose and insulin responses elicited by the test-meals containing FOSS, relative to those without FOSS, and the glucose and insulin responses elicited by the test-meals containing 35g avCHO relative to those containing 50g avCHO are shown in Table 3.
Table 3.
Glucose and Insulin Relative Responses for Phases 2 and 3.
| Comparison∗ | All (n=25) | Outliers excluded | Means∗∗ | All (n=25) | Outliers excluded | |
|---|---|---|---|---|---|---|
| Phase 2 Glucose | 100%×Dex35F15/Dex35 | 108.2±6.3 | 105.1±5.71# | 100%×DF/D [100; 80,120] | 99.7±4.7 (89.9, 109.5)ˆ | 99.7±4.70 (89.9, 109.5)# |
| 100%×Dex50F15/Dex50 | 96.9±6.4ˆ | 94.1±5.91# | ||||
| 100%×Dex35/Dex50 | 79.5±5.0ˆ | 77.0±4.61# | 100%×35/50 [80.6; 64.5,96.7] | 82.0±3.3 (75.1, 88.8)ˆ | 78.7±2.62 (73.2, 84.2)# | |
| 100%×Dex35F15/Dex50F15 | 87.7±4.1ˆ | 85.9±3.91# | ||||
| Phase 2 Insulin | 100%×Dex35F15/Dex35 | 133.4±13.9 | 118.5±10.02 | 100%×DF/D [100; 77.2,122.8] | 108.0±6.3 (94.9, 121.1)ˆ | 100.6±4.02 (92.3, 108.9)# |
| 100%×Dex50F15/Dex50 | 102.9±8.9ˆ | 96.3±6.21# | ||||
| 100%×Dex35/Dex50 | 68.1±5.6ˆ | 68.1±5.60# | 100%×35/50 [70, 54,86] | 72.6±3.6 (65.1, 80.0)ˆ | 70.6±3.11 (64.1, 77.0)# | |
| 100%×Dex35F15/Dex50F15 | 83.5±6.4 | 80.5±5.91 | ||||
| Phase 3 Glucose | 100%×WB35F15/WB35 | 107.1±8.0 | 99.0±6.22# | 100%×WBF/WB [100; 80,120] | 105.7±5.6 (94.1, 117.3)ˆ | 105.7±5.60 (94.1, 117.3)# |
| 100%×WB50F15/WB50 | 111.9±8.7 | 105.8±6.51# | ||||
| 100%×WB35/WB50 | 79.8±5.8ˆ | 77.4±5.51# | 100%×35/50 [79.1; 63.3,94.9] | 74.9±3.7 (67.2, 82.6)ˆ | 74.7±3.22 (68.1, 81.3)# | |
| 100%×WB35F15/WB50F15 | 74.6±4.9ˆ | 71.3±3.71# | ||||
| Phase 3 Insulin | 100%×WB35F15/WB35 | 116.2±11.7 | 104.6±9.22 | 100%×WBF/WB [100; 77.2,122.8] | 111.9±6.6 (98.3, 125.5) | 107.3±5.01 (97.0, 117.5)# |
| 100%×WB50F15/WB50 | 120.5±11.9 | 110.9±7.21 | ||||
| 100%×WB35/WB50 | 70.0±5.9ˆ | 63.7±4.32# | 100%×35/50 [67.6; 52.2,83.0] | 64.4±2.9 (58.4, 70.4)ˆ | 64.2±2.52 (59.1, 69.3)# | |
| 100%×WB35F15/WB50F15 | 65.1±5.5ˆ | 62.3±4.91# |
Results are means±SEM for n=25 subjects. 95% confidence intervals (95%CI) shown in (brackets).
∗ iAUC elicited by one test-meal expressed as a % of that after another; test-meal abbreviations: Dex35F15 = 35g dextrose+15g FOSS; Dex35 = 35g dextrose; Dex50F15 = 50g dextrose+15g FOSS, Dex50 = 50g dextrose.
∗∗ iAUC elicited by the mean of 2 test-meals expressed as a % of the mean for 2 test-meals as follows: DF/D=(Dex35F15+Dex50F15)/(Dex35+Dex50), 35/50=(Dex35D+Dex35)/(Dex50F15+Dex50) or (WB35F15+WB35)/(WB50F15+WB50), WBF/WB=(WB35F15+WB50F15)/(WB35+WB50). Expected mean and 95%CI shown in [brackets].
ˆ equivalent to expected (95%CI of n=25 values lie within the equivalence margins).
# 95%CI of values after excluding outliers are within the equivalence margins.
0,1,2 Number of outliers excluded.
3.3.1.1. Effect of FOSS
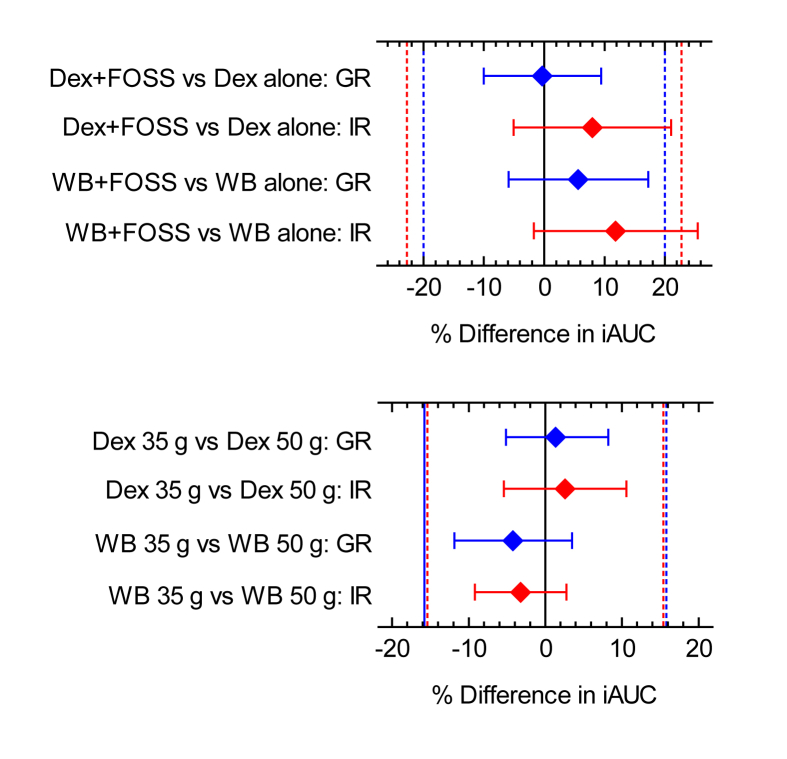
The hypothesis being tested was that the FOSS-containing test-meals would elicit the same glucose and insulin responses as their respective test-meals without FOSS. None of the relative glucose (RGR) or insulin (RIR) responses of the FOSS test-meals differed significantly from 100% either before or after outliers were excluded. Of the 4 individual comparisons for FOSS (Dex35 + F15/Dex35; Dex50 + F15/Dex50; WB35 + F15/WB35 and WB50 + F15/WB50) only 1 (Dex50 + F15/Dex50) demonstrated equivalence for RGR and 1 (Dex50 + F15/Dex50) for RIR. However, when the means of the 2 doses of avCHO were compared, the results demonstrated glucose equivalence for FOSS added to Dex and for FOSS added to WB and insulin equivalence for FOSS added to Dex (Figure 2, top panel). Insulin equivalence for FOS added to WB was demonstrated if 1 outlier was excluded (Table 3).
Figure 2.
Equivalence tests. Dex = dextrose; WB = white bread; FOSS = Fossence™; GR = glycemic response; IR = insulinemic response; iAUC = incremental area under the curve. Top Panel: comparisons of the effects of Dex or WB plus FOSS vs Dex or WB alone on glucose and insulin responses (eg. for Dex, % difference = (100% × Dex + FOSSiAUC/DexiAUC) -100, where Dex + FOSSiAUC = mean iAUC for Dex35 + FOSS15 and Dex50 + FOSS15 doses of Dex + FOSS and DexiAUC = mean iAUC for Dex35 and Dex50). Bottom panel: comparisons of the effects of 35g vs 50g available carbohydrate (avCHO) doses of Dex or WB on glucose and insulin responses (eg. for GR after Dex, % difference = (100% × Dex35iAUC/Dex50iAUC) - 80.6, where Dex35iAUC = mean iAUC for Dex35 + FOSS15 and Dex35; Dex50iAUC = mean iAUC for Dex50 + FOSS15 and Dex50; and 80.6 = the expected relative response; see text under Statistical analysis, Phases 2 and 3). Blue and red diamonds are the mean differences for glucose and insulin, respectively, the error bars are the 95% confidence intervals of the differences after excluding outliers (see Table 3) and the blue and red dashed lines are the equivalence margins (±20% of expected relative GR and ±22.8% of expected relative IR).
3.3.1.2. Effect of avCHO dose
The hypothesis being tested was that the Dex35 and WB35 test-meals, respectively, would elicit 19.4% and 20.9% lower glucose and 30.0% and 32.4% lower insulin responses than the respective Dex50 and WB50 test meals, based on previous dose-response studies (Wolever, 2006). Of the 4 individual comparisons of RGR and 4 of RIR for avCHO dose (Dex35/Dex50; Dex35 + F15/Dex50 + F15; WB35/WB50 and WB35 + F15/WB50 + F15) all demonstrated equivalence of the observed vs the predicted RGRs and 3 for the observed vs the predicted RIRs. When the means of the test-meals with and without FOSS at each level of avCHO were compared, the results demonstrated equivalence for both glucose and insulin for both the Dex and WB test meals without excluding outliers (Table 3; Figure 2, bottom panel).
4. Discussion
The results showed that 10–15g of FOSS elicited no significant increase in postprandial glucose and insulin responses in healthy adults when consumed alone or when added to liquid (dextrose) or solid (white bread) test-meals. FOSS only reduced acute postprandial glucose and insulin responses if used to replace some of the avCHO in foods (WB35 + F15 vs WB50) or drinks (Dex35 + F15 vs Dex50). However, it is not known whether replacing avCHO with FOSS will increase satiety and reduce overall energy intake, factors which may be important in determining the overall effect of such a manoeuvre on health.
Our results confirm and extend the results of a previous study showing that fructo-oligosaccharides ingestion did not raise blood glucose (Hidaka et al., 1986); we found that consuming 10g FOSS did not increase postprandial plasma glucose or serum insulin concentrations and elicited glycemic (GR) and insulinemic (IR) responses which were similar to that elicited by water. This suggests that FOSS is not hydrolyzed by the enzymes in the human small intestine. However, the glycemic responses of individuals vary from day-to-day due to analytical variation, minute-to-minute variation and day-to-day (within-subject) variation (Wolever, Ip & Moghaddam, 2006). To better understand the influence of such variation, the EGL of 10g FOSS was estimated; after excluding 2 extreme outliers the upper limit of the 95% confidence interval of the EGL was 0.32g. This approach allows the inspection with a 95% probability that 10g FOSS elicits a glycemic response which is less than or equal to that elicited by 0.32 g of dextrose. Assuming the GI of FOSS is 48, 0.32g dextrose is equivalent to 0.67g FOSS, and this suggests that at least 93.3% of FOSS is unavailable in the human small intestine. This is very much in line with the composition of FOSS which has 93% scFOS.
The results of Phases 2 and 3 showed that adding 15g FOSS to 35g or 50g avCHO in liquid form (Dex) or solid form (WB) had no significant effect on GR or IR when compared to the responses elicited by 35g or 50g avCHO of Dex alone or WB alone, respectively. It may be noted that a 15g FOSS is equivalent to 30% of the available carbohydrate in the test meals of Phase 2 and 3. However, when comparing the effect of 2 treatments on variable endpoints, such as GR and IR, it is generally impossible to prove the null hypothesis (i.e. that the effects are identical). An experiment can only demonstrate equivalence to within certain confidence limits. We chose 20% as the confidence limits for equivalence testing because <20% reduction in GR is unlikely to be clinically relevant for carbohydrate sources providing a small proportion of dietary avCHO.; e.g. replacing 20% of dietary avCHO with a source eliciting a 20% lower GR would have an overall impact on GR of 0.2 × 0.2 = 0.04 or 4%, and effect size which would require >500 subjects to detect. This may be why Health Canada indicated that, to make a claim for reduced GR, the test food had to reduce GR by at least 20% relative to control (Health Canada, 2013). Since the 95% CI of the differences between avCHO + FOSS and avCHO alone were within the margins of equivalence (±20%) for both liquid (Dex) and solid (WB) forms of avCHO, our results demonstrate adding 15g FOSS to avCHO elicits a glycemic response equivalent to that of the avCHO alone. We also found that adding 15g FOSS to avCHO elicits an equivalent insulin response when added to Dex. However, the results only demonstrated equivalence of the insulin responses when added to WB after 1 outlier was excluded.
Our results showed that the 50Dex and 50WB test-meals, respectively, elicited significantly higher glycemic and insulinemic responses than 35Dex and 35WB and that the observed differences between the 35g and 50g doses were equivalent to those expected based on previous dose-response studies (Wolever and Bolognesi, 1996; Lee and Wolever, 1998). Since we were able to detect an effect on GR and IR of increasing avCHO intake by 15g, the demonstration that adding 15g FOSS to avCHO elicited a GR equivalent to that of avCHO alone also supports the hypothesis that FOSS is an unavailable carbohydrate. Thus, when 15g avCHO from glucose was replaced by 15g FOSS (Dex50 vs Dex35 + F) or when 15g avCHO from WB was replaced by 15g FOSS (WB50 vs WB35 + F), both GR and IR were reduced. The implication of this is that if FOSS replaced some of the avCHO in foods and drinks, the resulting product would elicit lower glucose and insulin responses. However, the magnitude of the reductions in GR and IR would depend on the amount of avCHO in a serving of the product and the proportion of the avCHO replaced by FOSS.
Health agencies around the world recommend limiting the intake of sugars to 10% of total energy (WHO, 2019; USDA, 2019; SACN, 2019) because of the strong association of a high intake of sugar-sweetened beverages with increased risk for obesity (Malik et al., 2013) and type 2 diabetes (Imamura et al., 2016). Sugar-sweetened beverages may contribute to weight gain because the reduced satiety they elicit is insufficient to prevent subsequent excess energy consumption (Malik and Hu, 2019). Dietary fibers such as scFOS have been used to partially replace sugars in foods and beverages (Protonotariou et al., 2012) because they have a sweet taste and a lower energy value than sugars (Roberfroid 2000; Molis et al., 1996). However, the acceptance of sugar-reduced foods is generally less than it is for fat-reduced foods (Biguzzi et al., 2015). This may be because sugars not only provide sweetness but also other functions as structure, volume, flavour and aroma (Bennion and Bamford, 1997; Struck et al., 2014). Being sweet in taste, with 30% of the sweetness of sucrose (Bornet 1994), but having a higher viscosity than sucrose (Won Yun 1996), FOSS may be an acceptable sugar replacer in beverages without compromising their taste and sensory qualities.
The present results support the hypothesis that Fossence™ is an unavailable carbohydrate. If this were true, one implication is that the use of Fossence™ to partially replace glycemic carbohydrates would result in foods and beverages with a reduced GL. The effect of using such foods on the composition of the entire diet is not known and would need to be investigated. However, the consumption of diets with a high glycemic load (GL) have been associated with increased plasma concentrations of the inflammatory marker c-reactive protein (Liu et al., 2002), and increased risk for type 2 diabetes (Bhupathiraju et al., 2014) and coronary heart disease (Liu et al., 2000). Also, there is evidence that diets containing low amounts of available carbohydrate improve glycemic control in diabetes (Huntriss et al., 2018) and reduce body weight, although the long-term effects of such diets on these and other outcomes depend on compliance and whether the dietary carbohydrate is partly replaced by fat or protein (Mansoor et al., 2016; Clifton et al. 2014). The other implication of Fossence™ being an unavailable carbohydrate is the potential physiological impact of its metabolism in the large intestine. Supplementation of the diet with fructo-oligosaccharides of various types has been shown to have laxative effects in constipated elderly subjects (Meksawan et al., 2016) and children (Gordon et al., 2013), to stimulate the growth of colonic bifidobacteria in healthy subjects (Bouhnik et al., 2004, 2006; Tandon et al., 2019) and to increase calcium absorption in healthy adolescent boys (van den Heuvel et al., 1999) and magnesium absorption in adolescent girls with a low calcium intake (van den Heuvel et al., 2009). However, whether these and other health benefits attributed to fructo-oligosaccharides occur with FOSS remains to be determined. The results of our study are limited to FOSS (Fossence) and may not be extrapolated in other fructo-oligosaccharides.
5. Conclusions
We conclude that Fossence™ does not increase the postprandial glucose and insulin levels when consumed alone or when added to avCHO in liquid or solid forms. The results support the hypothesis that Fossence™ is not digested by enzymes in the human small-intestine and therefore can be classified as an unavailable carbohydrate that does not raise post prandial blood glucose or insulin.
Declarations
Author contribution statement
Priyali Shah: Conceived and designed the experiments; Wrote the paper.
Thomas M. S. Wolever, Alexandra L. Jenkins: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Adish Ezatagha, Janice Campbell, Andreea Zurbau: Performed the experiments.
Manish Jain, Ashim Mullick: Conceived and designed the experiments.
Manoj Gote: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Anirban Bhaduri: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by Tata Chemicals Limited, Mumbai, India.
Data availability statement
The data that has been used is confidential.
Declaration of interests statement
The authors declare the following conflict of interests: Priyali Shah, Manish Jain, Manoj Gote, Anirban Bhaduri are employees of Tata Chemicals Limited, India. Ashim Mullick is employee of Tata Consumers Products Limited [former employee of Tata Chemicals Limited, India]. Alexandra Jenkins, Thomas Wolever, Adish Ezatagha, Janice Campbell, Andreea Zurbau; are employees of INQUIS Clinical Research Inc.
Additional information
No additional information is available for this paper.
The clinical trial described in this paper was registered at ClinicalTrial.gov under the registration number NCT03755232.
Acknowledgements
We thank all participants for their time and effort. Authors are thankful to the management of INQUIS Clinical Research Inc., Toronto, Canada for their research facility.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Becker R., Lorenz K., Saunders R.M. Saccharides of maturing triticale, wheat, and rye. J. Agric. Food Chem. 1977;25(5):1115–1118. [Google Scholar]
- Bennion E.B., Bamford G.S.T. sixth ed. Blackie Academic and Professional; London: 1997. Sugars. The Technology of Cake Making; pp. 84–99. [Google Scholar]
- Beserra B.T., Fernandes R., do Rosario V.A., Mocellin M.C., Kuntz M.G., Trindade E.B. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adults patients with overweight or obesity. Clin. Nutr. 2015;34:845–858. doi: 10.1016/j.clnu.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Bhupathiraju S.N., Tobias D.K., Malik V.S., Pan A., Hruby A., Manson J.E., Willett W.C., Hu F.B. Glycemic index, glycemic load, and risk of type 2 daibetes: results from 3 large US cohorts. Am. J. Clin. Nutr. 2014;100(1):218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biguzzi C., Lange C., Schlich P. Effect of sensory exposure on liking for fat- or sugar-reduced biscuits. Appetite. 2015;95:317–323. doi: 10.1016/j.appet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Bornet F.R.J. Undigestible sugars in food products. Am. J. Clin. Nutr. 1994;59(suppl):763S–769S. doi: 10.1093/ajcn/59.3.763S. [DOI] [PubMed] [Google Scholar]
- Bouhnik Y., Raskine L., Simoneau G., Paineau D., Bornet F. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: a dose-response relationship study in healthy humans. Nutr. J. 2006;5(1):8. doi: 10.1186/1475-2891-5-8. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhnik Y., Raskine L., Simoneau G., Vicaut E., Neut C., Flourié B., Brouns F., Bornet F.R. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am. J. Clin. Nutr. 2004;80(6):1658–1664. doi: 10.1093/ajcn/80.6.1658. [DOI] [PubMed] [Google Scholar]
- Clifton P.M., Condo D., Keogh J.B. Long term weight maintenance after advice to consume low carbohydrate, higher protein diets – s systematic review and meta analysis. Nutr. Metabol. Cardiovasc. Dis. 2014;24(3):224–235. doi: 10.1016/j.numecd.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Darbyshire B., Henry R.J. Differences in fructan content and synthesis in some Allium species. New Phytol. 1981;87(2):249–256. [Google Scholar]
- Ganaie M.A., Lateef A., Gupta U.S. Enzymatic trends of Fructooligosaccharides production by microorganisms. Appl. Biochem. Biotechnol. 2014;172(4):2143–2159. doi: 10.1007/s12010-013-0661-9. [DOI] [PubMed] [Google Scholar]
- Gordon M., Naidoo K., Akobeng A.K., Thomas A.G. Cochrane Review: osmotic and stimulant laxatives for the management of childhood constipation. Evid. Base Child Health. 2013;8(1):57–109. doi: 10.1002/ebch.1893. (Review) [DOI] [PubMed] [Google Scholar]
- Health Canada . June 2013. Draft Guidance Document on Food Health Claims Related to the Reduction in Post-Prandial Glycaemic Response.https://www.canada.ca/en/health-canada/services/food-nutrition/public-involvement-partnerships/technical-consultation-draft-guidance-document-food-health-claims-related-post-prandial-glycaemia.html URL: [Google Scholar]
- Hidaka H., Eida T., Takizawa T., Tokunaga T., Tashiro Y. Effects of fructo-oligosaccharides on intestinal flora and human health. Bifidobacteria Microflora. 1986;5:37–50. [Google Scholar]
- Huntriss R., Campbell M., Bedwell C. The interpretation and effect off a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2018;73:311–325. doi: 10.1038/s41430-017-0019-4. [DOI] [PubMed] [Google Scholar]
- Imamura F., O’Connor L., Ye Z., Mursu J., Hayashino Y., Bhupathiraju S.N., Foroughi N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Br. J. Sports Med. 2016;50(8):496–504. doi: 10.1136/bjsports-2016-h3576rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecerf J.M., Clerc E., Jaruga A., Wagner A., Respondek F. Postprandial glycaemic and insulinaemic responses in adults after consumption of dairy desserts and pound cakes containing short-chain fructo-oligosaccharides used to replace sugars. J. Nutr. Sci. 2015;12(4):e34. doi: 10.1017/jns.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.M., Wolever T.M.S. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: comparison with white bread. Eur. J. Clin. Nutr. 1998;52:924–928. doi: 10.1038/sj.ejcn.1600666. [DOI] [PubMed] [Google Scholar]
- Liu S., Manson J.E., Buring J.E., Stampfer M.J., Willett W.C., Ridker P.M. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002;75(3):492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- Liu S., Willett W.C., Stampfer M.J., Hu F.B., Franz M., Sampson L., Hennekens C.H., Manson J.E. A prospective study of dietary glycemic load, carbohydrate intake and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- Loman B.R., Hernandez-Saavendra D., An R., Rector R.S. Prebiotic and protiotic treatment or nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr. Rev. 2018;76:822–839. doi: 10.1093/nutrit/nuy031. [DOI] [PubMed] [Google Scholar]
- Malik V.S., Hu F.B. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11(8):1840. doi: 10.3390/nu11081840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik V.S., Pan A., Willett W.C., Hu F.B. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2013;98(4):1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor N., Vinknes K.J., Veierød M.B., Retterstøl K. Effects of low-carbohydrate v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Br. J. Nutr. 2016;115(3):466–479. doi: 10.1017/S0007114515004699. [DOI] [PubMed] [Google Scholar]
- Meksawan K., Chaotrakul C., Leeaphorn N., Gonlchanvit S., Eiam-Ong S., Kanjanabuch T. Effects of fructo-oligosaccharide supplementation on constipation in elderly continuous ambulatory peritoneal dialysis patients. Perit. Dial. Int. 2016;36(1):60–66. doi: 10.3747/pdi.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molis C., Flourié B., Ouarne F., Gailing M.F., Lartigue S., Guibert A., Bornet F., Galmiche J.P. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am. J. Clin. Nutr. 1996;64(3):324–328. doi: 10.1093/ajcn/64.3.324. [DOI] [PubMed] [Google Scholar]
- Protonotariou S.V., Karali E., Evageliou V., Yanniotis S., Mandala I. Rheological and sensory attributes of cream caramel desserts containing Fructooligosaccharides as substitute sweeteners. Int. J. Food Sci. Technol. 2012;48(3):663–669. [Google Scholar]
- Respondek F., Hilpipre C., Chauveau P., Cazaubiel M., Gendre D., Maudet C., Wagner A. Digestive tolerance and postprandial glycaemic and insulinaemic responses after consumption of dairy desserts containing maltitol and fructo-oligosaccharides in adults. Eur. J. Clin. Nutr. 2014;68(5):575–580. doi: 10.1038/ejcn.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., de los Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M.B. Prebiotics and probiotics: are they functional foods? Am. J. Clin. Nutr. 2000;71:1682S–1687S. doi: 10.1093/ajcn/71.6.1682S. 65. [DOI] [PubMed] [Google Scholar]
- Scientific Advisory Committee on Nutrition . 2019. Carbohydrates and Health. 2015 November 25.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf Available from: [Google Scholar]
- Sheth M., Thakuria A., Chand V., Paban Nath M. Fructo-oligosaccharide (fos)- a smart strategy to modulate inflammatory marker and lipid profile in non-insulin dependent diabetes mellitus (niddm) subjects residing in Assam, India- a randomized control trial. World J. Pharmaceut. Res. 2015;4(5):2673–2678. https://wjpr.net/admin/assets/article_issue/1430827174.pdf [Google Scholar]
- Shiomi N., Izawa M. Purification and characterization of sucrose: sucrose 1-fructosyltransferase from the roots of asparagus (Asparagus officinalis L.) Agric. Biol. Chem. 1980;44(3):603–614. [Google Scholar]
- Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck S., Jaros D., Brennan C.S. Sugar replacement in sweetened bakery goods. Int. J. Food Sci. Technol. 2014;49:1963–1976. [Google Scholar]
- Tandon D., Haque M.M., Gote M., Jain M., Bhaduri A., Dubey A.K., Mande S.S. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci. Rep. 2019 Apr 2;9(1):5473. doi: 10.1038/s41598-019-41837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy K.M., Fava F., Viola R. The way to a man's heart is through his gut microbiota--dietary pro- and prebiotics for the management of cardiovascular risk. Proc. Nutr. Soc. 2014;73(2):172–185. doi: 10.1017/S0029665113003911. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture . 2019. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015 September 7. [Google Scholar]
- van den Heuvel E.G., Muijs T., Brouns F., Hendriks H.F. Short-chain fructo-oligosaccharides improve magnesium absorption in adolescent girls with a low calcium intake. Nutr. Res. 2009;29(4):229–237. doi: 10.1016/j.nutres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- van den Heuvel E.G., Muys T., van Dokkumm W., Schaafsma G. Oligofructose stimulates calcium absorption in adolescents. Am. J. Clin. Nutr. 1999;69(3):544–548. doi: 10.1093/ajcn/69.3.544. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y., Zakharova I., Dmitrieva Y. Oligosaccharides in infant formula: more evidence to validate the role of prebiotics. Br. J. Nutr. 2015;113:1339–1344. doi: 10.1017/S0007114515000823. [DOI] [PubMed] [Google Scholar]
- Walsh C.J., Guinane C.M., O'Toole P.W., Cotter P.D. Beneficial modulation of the gut microbiota. FEBS Lett. 2014;588(22):4120–4130. doi: 10.1016/j.febslet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Wolever T.M.S., Bolognesi C. Source and amount of carbohydrate affect postprandial glucose and insulin in normal subjects. J. Nutr. 1996;126:2798–2806. doi: 10.1093/jn/126.11.2798. [DOI] [PubMed] [Google Scholar]
- Wolever T.M.S. CABI Publishing; Wallingford, UK: 2006. The Glycaemic Index: A Physiological Classification of Dietary Carbohydrate; pp. 66–68. [Google Scholar]
- Wolever T.M.S. Effect of blood sampling schedule and method calculating the area under the curve on validity and precision of glycaemic index values. Br. J. Nutr. 2004;91:295–300. doi: 10.1079/bjn20031054. [DOI] [PubMed] [Google Scholar]
- Wolever T.M.S., Gibbs A.L., Spolar M., Hitchner E.V., Heimowitz C. Equivalent glycemic load (EGL): a method for quantifying the glycemic responses elicited by low carbohydrate foods. Nutr. Metab. 2006;3:33. doi: 10.1186/1743-7075-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolever T.M.S., Ip B., Moghaddam E. Measuring glycaemic responses: duplicate fasting samples or duplicate measures of one fasting sample? Br. J. Nutr. 2006;96:799–802. doi: 10.1017/bjn20061914. [DOI] [PubMed] [Google Scholar]
- Wolever T.M.S., Jenkins D.J.A., Jenkins A.L., Josse R.G. The glycemic index: methodology and clinical implications. Am. J. Clin. Nutr. 1991;54:846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- Wolever T.M.S., Meynier A., Jenkins A.L., Brand-Miller J.C., Atkinson F.S., Gendre D., Leuillet S., Cazaubiel M., Housez B., Vinoy S. Glycemic index and insulinemic index of foods: an interlaboratory study using the ISO 2010 method. Nutrients. 2019;11:2218. doi: 10.3390/nu11092218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won Yun J. Fructooligosaccharides—occurrence, preparation, and application. Enzym. Microb. Technol. 1996;19(2):107–117. [Google Scholar]
- World Health Organization . 2019. Guideline: Sugars Intake for Adults and Children. 2015 September 7.https://apps.who.int/iris/bitstream/handle/10665/149782/9789241549028_eng.pdf?sequence=1 Available from: [PubMed] [Google Scholar]
- Yamashita K., Kawai K., Itakura M. Effects of fructo-oligosaccharides on blood glucose and serum lipids in diabetic subjects. Nutr. Res. 1984;4(6):961–966. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.