Abstract
Objectives:
Intensive interdisciplinary pain treatment (IIPT) programs have been shown to restore function, improve coping, and reduce pain in adolescents with chronic pain. Yet, little is known about patients’ sleep during IIPT and whether or not improvements in pain treatment outcomes are associated with changes in sleep pre-to-post IIPT treatment. The objectives of the current study were to describe sleep among adolescents entering IIPT and examine associations between sleep parameters and IIPT treatment effects.
Methods:
Self-reported sleep measures and clinical outcomes (e.g., functional disability, coping, average pain), were collected from 44 adolescents (mean age=14.57, 68.2% female) at admission and discharge from an inpatient IIPT program. Wrist-worn actigraphy data and sleep diaries from participants’ first week and last week in the program were analyzed to characterize sleep parameters.
Results:
Participants self-reported poor sleep/wake patterns, high levels of insomnia symptoms, and sub-clinical problems with daytime sleepiness upon admission into IIPT, although actigraphic indices of sleep from the first week of IIPT admission were only just under clinical guidelines for healthy adolescent sleep. Better self-reported sleep quality assessed via aggregated sleep diaries from the first week was associated with improvement in average pain and disability over the course of the program. Furthermore, improvements in insomnia symptoms and daytime sleepiness throughout the program were positively correlated with concurrent improvements in functional disability and coping.
Discussion:
Taken together, results suggest that sleep may be associated with IIPT treatment effects and pave the way for future research to continue examining these relationships.
Keywords: Actigraphy, Chronic Pain, Functional Disability, Pain Rehabilitation, Pediatric Pain
Introduction
Approximately 30% of children and adolescents experience pain lasting three months or longer (1,2) with approximately 5% suffering from pain that interferes with daily functioning (2,3). Without proper treatment, pediatric chronic pain often persists into adulthood (4–6). Fortunately, effective treatments for chronic pain exist, including intensive interdisciplinary pain treatment (IIPT) within an inpatient or day hospital treatment setting. IIPT is defined by interdisciplinary treatment focused on functional restoration, reduction of pain-related disability, and improved coping skills (7). IIPT programs have been shown to lead to robust improvements in disability, psychological outcomes, and pain (7–13), yet, little is known about factors associated with treatment effects of IIPT.
Known interactions between sleep and pain suggest sleep could be an important factor affecting treatment outcomes in youth with chronic pain. Sleep disruption, restriction, and deprivation can alter perception and modulation of pain in experimental settings (14–19). Clinically, youth with chronic pain report more problematic sleep habits (20), poorer sleep (21,22), and greater daytime sleepiness than pain-free peers (23). Actigraphy studies find youth with pain have frequent nighttime awakenings and inefficient sleep compared to healthy controls (21). Additionally, day-to-day variability in sleep can significantly impact daily pain levels (24). Youth report higher pain levels on days following nights when they spent more time awake – but pain levels do not predict the following night’s sleep as strongly (25,26).
Despite strong relationships between sleep and pain, only one published pediatric study has examined how changes in sleep are associated with underlying IIPT treatment effects (10). In that study, significant improvements in patients’ sleep habits, sleep duration, night awakenings, and daytime sleepiness were observed throughout the IIPT program and were maintained up to 3 months post treatment. Youths’ sleep habits at discharge correlated with improvements in functional disability at discharge, and decreased night awakenings throughout the program were associated with lower functional disability and pain during post treatment assessments. These results are promising but some methodological limitations of the study are noteworthy. This study was conducted in a day treatment setting which did not control patients’ home sleep environments, and sleep outcomes were exclusively self-reported. The current study builds on these limitations by examining sleep parameters in an inpatient IIPT program that provides a controlled sleep environment (i.e., single-bed hospital room with scheduled wake and bed times; see Setting and Procedures for more information) using a combination of self-report and actigraphic measures.
The current study had three aims. First, we sought to characterize subjective and objective sleep parameters among youth prior to admission to inpatient IIPT and during their first week in IIPT. We hypothesized that self-report measures characterizing sleep prior to admission and objective sleep estimates (actigraphy) measured during their first week in the program would reflect poor sleep (i.e., irregular sleep/wake patterns, poor sleep hygiene, high levels of insomnia symptoms and daytime sleepiness, high levels of waking after sleep onset and sleep variability, short total sleep time) relative to published sleep norms or clinical cut-points. Second, we sought to test whether subjective and objective measures of sleep were associated with changes in key clinical outcomes (i.e., functional disability, coping, pain) throughout the program. Third, we sought to test whether changes in subjective and objective sleep measured during IIPT correlated with changes in clinical outcomes during IIPT. Based on longitudinal studies showing that changes in sleep lead to future changes in pain (25), we hypothesized that self-report sleep measures characterizing patients’ subjective sleep experience prior to admission and objective estimates of sleep measured during the first week in the program would be associated with clinical outcomes during the program, and that improvements in sleep would be correlated with improvements in clinical outcomes.
Methods
Participants
Participants were 44 pediatric patients 9 – 18 years of age admitted into the Functional Independence ReSToration (FIRST) program, an inpatient IIPT program at Cincinnati Children’s Hospital (https://www.cincinnatichildrens.org/service/f/functional-independence), between January 2018 and June 2019. To be eligible for admission into the FIRST program, patients needed a diagnosis of chronic pain confirmed by a physician using comprehensive diagnostic testing, medical clearance to engage in physical activity, and failure to progress in outpatient treatment for pain (i.e., medical, psychological, physical therapy) or lack of access to comprehensive pain therapy in an outpatient setting. Patients were discharged from the FIRST program upon meeting program goals for clinical improvement (e.g., ≥25% improvement in functional disability) and/or individualized goals for functional restoration (e.g., ambulating independently, return to school/sports). In the current sample, the average length of hospital admission was approximately 20 days, with an observed minimum of 11 days and an observed maximum of 32 days.
Settings and Procedures
The FIRST program is a Commission on the Accreditation of Rehabilitation Facilities (CARF) accredited inpatient IIPT program that aims to restore functional ability and increase self-management and coping skills among children and adolescents with debilitating chronic pain conditions. A detailed description of the FIRST program including treatment outcomes and trajectory of recovery over time is published elsewhere (13). Patients maintained a highly structured schedule and participated in four hours of daily interdisciplinary treatment (i.e., psychology, physical therapy, occupational therapy), one hour of school instruction, one hour of self-directed exercise, and up to two additional hours of recreational therapy, massage therapy, music therapy, and child life intervention. Youth were under medical supervision by pain management and pediatric rehabilitation physicians, nurse practitioners, and nurses. Upon admission into and discharge from the FIRST program, patients completed the battery of self-report measures assessing pain, sleep, disability, and coping described below. During their time in the program patients continuously wore a wrist-based actigraphy watch and completed a sleep diary each morning (further described in methods). All procedures pertaining to the study were approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center (IRB#2015-8104).
There were several considerations made to support a good sleep environment and adherence to basic sleep hygiene rules that patients followed in the FIRST program. During hospitalization, patients slept in their own single-bed hospital room. Patients were encouraged to bring their own pillows and blankets from home for comfort. Each room had at least one window to the outside and one window to the hallway. The windows had curtains or blinds which patients controlled. Patients were also able to control the temperature in their room. During the day, patients were expected to keep the lights on in the room and keep their bed in an upright chair position. At night, they were allowed to position their bed in a typical flat position for sleeping. Lights needed to be off no later than 10 pm but patients were allowed to go to bed as early as 8 pm if desired. Patients were not allowed to have screen time (e.g., TV, phones, computers, tablets) after 10 PM and were asked to refrain from screen time 30 minutes prior to going to bed. Patients woke up between 7:00 and 7:30 am in order to be ready for therapies at 8:15 am. Naps were not allowed. Patients were allowed to have one parent stay overnight with them in their hospital room where they slept on a pull-out couch. Parents had to abide by the same rules as patients (e.g., lights on during the day, no daytime napping, lights out at 10 pm). Nurses rounded during the night but only entered patients’ rooms if a medication was required to be administered. Patient’s vital signs were not monitored during the night unless medically indicated. If the patient was struggling with sleep, strategies to improve sleep hygiene were addressed in psychology sessions.
Materials
The questionnaires and assessment instruments used to measure study outcomes are described below. Table 1 illustrates when each measure was collected.
Table 1.
Data Collection Schedule for all Study Variables.
| Collection Period | ||||
|---|---|---|---|---|
| Admission | First 7 Nights | Last 7 Nights | Discharge | |
| Sleep Variables | ||||
| Sleep/Wake Patterns (ASWS) | X | |||
| Sleep Hygiene (ASHS) | X | |||
| Insomnia Symptoms (PISI) | X | X | ||
| Daytime Sleepiness (ESS) | X | X | ||
| Sleep Quality (Daily Diary) | X | X | ||
| Actigraphy Measures | X | X | ||
| Clinical Outcome Variables | ||||
| Functional Disability (FDI) | X | X | ||
| Pain Coping (PCQ) | X | X | ||
| Pain Intensity (NRS) | X | X | ||
Note: ASWS = Adolescent sleep wake scale; ASHS = Adolescent sleep hygiene scale; ESS = Epworth sleep scale for children; FDI = Functional disability inventory; NRS = Numeric rating scale; PCQ = Pain coping questionnaire; PISI = Pediatric insomnia severity index.
Demographics
Sex, age, ethnicity/race, and pain diagnosis were collected as part of routine clinical care and extracted from the patient’s medical record post discharge from the program.
Self-Report Sleep Measures
Behavioral Sleep/Wake Patterns.
Sleep/wake patterns were assessed at admission via the Adolescent Sleep Wake Scale (ASWS; 20). The ASWS has been well-validated for assessing behavioral sleep/wake patterns in healthy adolescents and in those with chronic pain ages 12-18 years (27). ASWS data from patients ages 11 and under were not analyzed (n = 7, 15.9%). The ASWS consists of 33 patient-report items examining the frequency of sleep behaviors over the last month across five domains (i.e., going to bed, falling asleep, maintaining sleep, reinitiating sleep, returning to wakefulness). Each item was scored using a 6-point scale with anchors of 1 (“Always”) to 6 (“Never”). A total score was computed by averaging all items, with higher scores reflecting better sleep/wake patterns. Only the total score was analyzed for the current study. Internal consistency for the scale was α = .86.
Sleep hygiene.
The Adolescent Sleep Hygiene Scale (ASHS; 20) was administered at admission to assess frequency of sleep-promoting and sleep-inhibiting behaviors occurring over the past month (e.g. “I do things in bed that keep me awake”). The ASHS has been well-validated for assessing sleep hygiene in healthy adolescents and in those with chronic pain ages 12-18 (28). ASHS data from patients ages 11 and under were not analyzed (n = 7, 15.9%). The ASHS consists of 33 patient reported items scored on a 6-point scale with anchors of 1 (“Always”) to 6 (“Never”). A total score was computed by averaging all items on the scale, with higher scores reflecting better sleep hygiene. Internal consistency for the full scale was α = .75.
Insomnia symptoms.
The Pediatric Insomnia Severity Index (PISI; 29,30) was administered upon admission and at discharge to assess insomnia symptoms in the past week. The PISI is a 6-item measure with demonstrated reliability and validity as a measure of insomnia severity within the context of clinical care for youths 18 years of age and younger (29,30). The PISI assesses sleep domains most relevant to clinical treatment: sleep onset problems (e.g., “It takes me longer than 30 minutes to fall asleep after going to bed”), sleep maintenance problems (e.g., “During the night I wake up more than once”), daytime sleepiness (e.g., “I feel sleepy during the day”), and nocturnal sleep duration (e.g., “How many hours of sleep do you get on most nights?”). The total nocturnal sleep item is rated on a 0–5 point scale with each rating designating an estimate of total hours slept on most nights (0 = greater than 9 hours of sleep, 5 = less than 5 hours of sleep). The remaining five items are rated on a Likert scale with anchors of 0 (“Never”) to 5 (“Always”). Thus, scores range from 0 to 30, with higher scores indicative of more severe insomnia symptomatology. Internal consistency for the scale was α = .78 at admission and α = .77 at discharge.
Daytime sleepiness.
The Epworth Sleepiness Scale for Children (ESS; 31) was administered at admission and discharge to assess patient’s level of daytime sleepiness over the last week. The ESS asks patients how likely they would be to fall asleep while performing each of eight different activities (e.g., “sitting and eating a meal,” “sitting and watching TV or a video”). For each of the activities, patients rate the likelihood of falling asleep using a 4-point Likert scale ranging from 0 (“Would never fall asleep”) to 3 (“High chance of falling asleep”). A total score is computed by summing all eight items, with higher scores representing greater levels of daytime sleepiness. The ESS has demonstrated validity for assessing daytime sleepiness in pediatric populations (19). Internal consistency for the scale was α = .77 at admission and α = .78 at discharge.
Actigraphy-derived Sleep Measures
Patients wore an actigraph (Actiwatch-2, Phillips Respironics) on their non-dominant wrist throughout their time in the program; for this manuscript, data were only analyzed from the first seven nights and last seven nights in the program. Actigraphy data was binned into 30 second segments (epochs). Each epoch was scored as either sleep or wake by the proprietary Actiware algorithm (Phillips Respironics, Bend, OR; Version 6.0.9), based on the amplitude and frequency of movement detected during that 30-second epoch. Time of sleep onset and offset were also determined by criteria specific to the proprietary algorithm. The sleep variables listed below were derived and averaged across the first seven nights following admission date, as well as the last seven nights prior to discharge. If patients had four or more nights of actigraphy available at either time point, an average was taken using all available nights. If patients had less than four nights available, actigraphy data for that time point was considered missing. If patients were in the program for fewer than 14 nights (n = 4, all of who were in the program for 11 nights), the first seven nights were averaged for the ‘admission’ time point and the last 4 nights were averaged for ‘discharge’ time point.
Total Sleep Time (TST, min) was calculated as number of minutes scored as sleep between the algorithm-defined time of initial sleep onset (first time the patient fell asleep for the night) and algorithm-defined sleep offset (time the patient woke up in the morning without falling back asleep). Internal consistency of total sleep time across the first seven nights was α = .68 and across the last seven nights was α = .84.
Wake After Sleep Onset (WASO, min) was calculated as the number of minutes spent awake during the night between the time of sleep onset and sleep offset. Internal consistency of WASO across the first seven nights was α = .87 and across the last seven nights was α = .89.
Sleep Onset Intraindividual Variability was calculated by taking each patient’s personal standard deviation in sleep onset (first time patient fell asleep for the night) across each seven-night monitoring period. Because this measure was a standard deviation of the seven nights of data, reliability for this measure could not be computed.
Sleep Offset Intraindividual Variability was calculated by taking each patient’s personal standard deviation in sleep offset (time patient woke up in the morning without falling back asleep) across each 7 seven-morning monitoring period. Because this measure was a standard deviation of the seven days of data, reliability for this measure could not be computed.
Daily Diary Sleep Measures
Sleep quality.
As part of the daily sleep diary, patients were asked to rate their sleep quality for the previous night using an 11-point Likert scale ranging from 0 (“Extremely poor sleep”) to 10 (“Extremely good sleep”). Responses were averaged across the first seven nights after admission and the last seven nights prior to discharge for each patient. If patients had less than four nights of diaries at a given time point, data for that time point was considered missing. Internal consistency of responses across the first seven nights of collection was α = .89 and across the last seven nights was α = .86.
Clinical Outcome Measures
Functional Disability.
Patients self-reported their perceived functional disability at admission and discharge using the Functional Disability Inventory (FDI; 32). The FDI consists of 15 items assessing difficulty due to physical health in completing activities in home, school, recreational, and social domains over the past few days. Responses were rated on a 5-point Likert scale ranging from 0 (“No trouble”) to 4 (“Impossible). Higher scores represent greater perceived functional disability. The FDI is well-validated in pediatric chronic pain populations (33). Among patients in the FIRST program, internal consistency of the measure was α = .86.
Coping.
Patients self-reported their perceived ability to emotionally manage pain over the past few days using the 3-item Pain Coping Questionnaire (PCQ; 34). Each item was scored on a 5-point Likert scale with scores ranging from 1 (“Never”) to 5 (“Very often”). Higher scores represent greater/better perceived pain coping efficacy, and the PCQ has been validated in pediatric chronic pain populations (34). Among patients in the FIRST program, internal consistency of the measure was α = .68.
Pain Intensity.
Pain intensity at admission and discharge was assessed using the Numeric Rating Scale-11 (NRS; 35). Children rated average pain levels over the last few days on an 11-point scale ranging from 0 (“No pain”) to 10 (“Most pain possible”). The NRS is well validated for assessing pain in pediatric patients with chronic pain conditions (35,36).
Power Analyses and Data Analysis Plan
For the current analyses, all available data obtained during the study period (January 2018 through June 2019) were utilized. Because this was a clinical sample, it was not possible to recruit additional participants. A sample size of 44 has power of 0.72 to detect effects of f = 0.39 or larger.
Prior to primary analyses, actigraphy-derived and daily diary sleep data were averaged across the first seven nights and last seven nights for each patient. For clinical outcome measures, a difference was taken between the admission and discharge scores for each measure (i.e., disability, coping, pain). For self-reported sleep variables collected at admission and discharge (PISI, ESS) a similar difference score was computed. These computed differences between admission and discharge indicated total change in each of these variable throughout the program; these variables were coded so that positive numbers indicated improvement over the course of the program, and negative numbers indicated worsening over the program. Once variables were computed, data were checked for outliers using a criteria of +/− 3 SD and skewness using a criteria of skew/kurtosis scores of +/− 1.5. Data were visually checked for missingness. Handling of outliers, non-normality, and missingness is described in the results section below.
Confirming changes in clinical outcome and sleep in the FIRST program.
After preliminary inspection of data, to replicate previous studies demonstrating the effectiveness of IIPT programs in general (7) and the FIRST program specifically (13), paired-samples t-tests were used to compare changes in pain, disability, and coping between admission and discharge. In addition, paired-samples t-tests were used to compare changes in sleep variables at admission and discharge. Effect sizes were computed using Hedges’ g, which is more conservative than Cohen’s D for small sample sizes (.2 = small effect; .5 = medium effect, .8 = large effect; 37).
Aim 1.
The first aim of the study was to characterize self-reported sleep prior to admission and actigraphic sleep during the first week in the inpatient IIPT program. Descriptive statistics of mean, standard deviation, and minimum and maximum observed values were computed for each variable. To contextualize the findings, means from the current sample were compared to published norms or clinical cut-points from relevant adolescent populations. When means and standard deviations from published norms were available, independent sample t-tests were used to compare the means from the current sample to the means from the reference sample.
Aim 2.
The second aim of the study was to determine if sleep prior to admission and/or during the first week of the program (i.e., actigraphic measures) was associated with changes in disability, coping, or pain during the program. Separate models were run for each of the three outcomes and each of the nine sleep variables (i.e., ASWS total score, ASHS total score, PISI, ESS, daily diary sleep quality, actigraphy-derived WASO, total sleep time, sleep onset consistency, sleep offset consistency). For each model, a residualized change approach with hierarchical linear regressions was used. The first step included the clinical outcome at admission and covariates of age, sleep medication use (0 = not on any medication; 1 = on medication) and duration of IIPT in days. The second step included the sleep variable of interest. The dependent variable was the clinical outcome at discharge. A Holm Bonferroni (38) correction was used to protect against Type 1 error from multiple comparisons (27 total models; 9 independent variables × 3 outcome variables). The Holm-Bonferroni correction consists of sorting all 27 p-values in order of smallest to largest, and each p-value is considered significant if it is larger than α/(number of test – 1). For example, the fourth largest p-value in the list would be significant at the p = .017 level or lower, because α/(number of test – 1) would equal .05/(4-1), which equals .017.
Aim 3.
The final aim of the study was to determine if changes in sleep correlated with concurrent changes in disability, coping, or pain over the course of the program. To examine these correlations, changes in each of the sleep variables was correlated with changes in each of the clinical outcomes using a Pearson’s correlation coefficient. Then, partial correlations were computed to remove the variance associated with the three covariates (i.e., age, medication use, program duration).
All cleaning and extraction of actigraphy data was conducted using Actiware software and extracted to SPSS for data analyses. All data analyses were conducted using SPSS Version 25 (IBM, Amonk, New York).
Results
Demographic Data
Demographic information for the final sample is reported in Table 2. Average age of the sample at admission was 14.57 years (SD = 2.68), and the sample was 68.2% female. The sample was primarily Caucasian (81.8%), and the most frequent primary diagnoses were fibromyalgia and Ehlers-Danlos syndrome (27.3% each).
Table 2.
Demographics of the Final Sample (N = 44).
| N | Mean (SD, Min/Max) or % | |
|---|---|---|
| Age at Admission | 14.57 (2.68, 9-18) | |
| Number of days in program | 20.02 (4.82, 11-32) | |
| Sex (n and % Female) | 30 | 68.2% |
| Ethnicity/Racial | ||
| Caucasian Non-Hispanic | 36 | 81.8% |
| African-American Non-Hispanic | 3 | 6.8% |
| Hispanic/Latino | 1 | 2.3% |
| Mixed Ethnicity/Other | 4 | 9.1% |
| Primary Diagnosis | ||
| Fibromyalgia/diffuse amplified pain | 12 | 27.3% |
| Ehlers-Danlos syndrome | 12 | 27.3% |
| Headache/migraine | 2 | 4.5% |
| Complex regional pain syndrome | 5 | 11.4% |
| Conversion disorder | 1 | 2.3% |
| Functional abdominal pain | 3 | 6.8% |
| Other | 9 | 20.5% |
| Sleep Medication | ||
| Melatonin | 15 | 34.1% |
| Trazodone | 2 | 2.3% |
Outliers and Missing Data
One patient had two admissions into the program. Only data from their most recent admission were used. Seven patients were aged 9, 10, or 11 and thus had missing ASHS and ASWS data. Two additional patients did not complete the ASHS or ASWS due to time constraints resulting from a developmental delay or language barrier.
PISI data was missing from one participant at admission and from two participants at discharge. ESS data was missing from one participant at admission and discharge.
Actigraphy data for the first week in the program were available for all patients. Forty-two patients had all seven nights of data available; one patient had six nights of data available, and one patient had four nights of data available.
Actigraphy data for the final week was missing from two patients. An additional patient only had six nights of data available, and four patients had four nights of data available (due to program duration of 11 days, as described above). Missing actigraphy data at both time points was due to technical failure or to the participant forgetting to wear their watch to bed.
Three patients were missing daily diary data over the first week. Seven patients were missing daily diary data for the last week.
There were no other missing data. Outliers were not identified on any variable. The number of cases available for each variable are reported in Table 3.
Table 3.
Descriptive Statistics of Sleep and Clinical Outcomes for the Final Sample
| Admission or First Week in Program | Discharge or Last Week in Program | |||||
|---|---|---|---|---|---|---|
| n | Mean (SD, min-max value) | n | Mean (SD, min-max value) | p | Hedges’ G | |
| Sleep Outcomes – Self-Report | ||||||
| Sleep/WakePatterns (ASWS) | 35 | 3.57 (0.58, 2.59-4.86) | - | - | - | - |
| Sleep Hygiene (ASHS) | 35 | 4.52 (0.51, 3.38-5.86) | - | - | - | - |
| Insomnia Symptoms (PISI) | 43 | 17.12 (6.69, 1-30) | 42 | 12.00 (5.91, 1-26) | <.001 | 0.80 |
| Daytime Sleepiness (ESS) | 43 | 8.00 (4.55, 0-19) | 43 | 5.58 (4.56, 0-16) | <.001 | 0.53 |
| Sleep Quality (Diaries) ǂ | 42 | 5.69 (1.70, 1.40-10.00) | 37 | 5.58 (1.52, 2.0-10.0) | .66 | 0.07 |
| Sleep Outcomes – Actigraphy | ||||||
| WASO (min) ǂ | 44 | 38.54 (21.67, 15.93-126.34) | 42 | 38.54 (14.26, 18.21-81.00) | .93 | 0.01 |
| Total Sleep Time (hours) ǂ | 44 | 7.96 (0.82, 5.61-9.59) | 42 | 7.73 (0.79, 5.65-10.16) | .07 | 0.28 |
| Sleep Onset Variability (min) ǂ | 44 | 37.98 (31.31, 2.0-147.00) | 42 | 38.48 (24.13, 9.52-116.12) | .93 | 0.02 |
| Sleep Offset Variability (min) ǂ | 44 | 32.82 (31.57, 8.0-174.00) | 42 | 37.04 (24.84, 6.52-105.50) | .50 | 0.15 |
| Clinical Outcomes | ||||||
| Disability (FDI) | 44 | 32.25 (9.38, 10-52) | 44 | 12.86 (7.73, 0-29) | <.001 | 2.24 |
| Coping (PCQ) | 44 | 8.27 (1.99, 3-13) | 44 | 10.59 (2.51, 5-15) | <.001 | 1.02 |
| Pain Intensity (NRS) | 44 | 6.20 (1.83, 2-10) | 44 | 5.61 (2.22, 0-10) | .042 | 0.29 |
Note: ASWS = Adolescent sleep wake scale; ASHS = Adolescent sleep hygiene scale; ESS = Epworth sleep scale for children; FDI = Functional disability inventory; NRS = Numeric rating scale; PCQ = Pain coping questionnaire; PISI = Pediatric insomnia severity index.
Measure averaged over first seven nights in program (admission) and last seven nights in program (discharge).
Changes in Clinical Outcomes and Sleep Over the FIRST Program
Replicating the previous literature (7,12), the current sample reported significant decreases in functional disability (Hedges’ g = 1.73), improvements in coping (Hedges’ g = 0.85), and reductions in average pain (Hedges’ g = 0.32) over the course of the program (see Table 3). There were also significant reductions in insomnia symptoms (Hedges’ g = 0.89) and daytime sleepiness (Hedges’ g = 0.72) over the course of the program, although there were not significant changes in sleep quality assessed via diaries or in actigraphy measures (see Table 3). The results were similar when controlling for program duration (in number of days).
Aim 1: Description of Self-report and Actigraphic Sleep Parameters at Admission
Aim 1 was to characterize subjective and objective sleep parameters among youth upon admission to inpatient IIPT. Patients self-reported poor sleep/wake patterns assessed via the ASWS (mean ASWS Total Score = 3.57). However, patients reported slightly better sleep hygiene assessed via the ASHS as compared to a healthy adolescent reference sample (20) (mean = 4.52 in the current sample vs. 4.0 in the reference sample, t(605) = 4.94, p <.001). As a group, patients reported sub-clinical problems with daytime sleepiness (mean ESS = 8.00; ESS>10 is reference cut-point for clinically significant sleepiness; 39). Patients also reported insomnia severity comparable to adolescents presenting for clinical insomnia treatment (mean PISI = 18.8 in clinical reference sample vs 17.12 in the current sample, t(509) = 1.68, p = .11; 29). Over the first week in the program, patients tended to report fair sleep quality (mean = 5.69, 0 to 10 scale) in their daily sleep diaries. Actigraphy revealed that patients slept just under clinical guidelines of 8-10 hours for adolescent sleep (mean total sleep time in the current sample = 7.96 hours; 40), spent just over 30 minutes awake after achieving first sleep onset (mean wake after sleep onset = 38 minutes), and had bed/wake times that were minimally variable – falling within about a 30-minute window day-to-day.
Aim 2: Associations of Sleep Parameters Measured at Admission with Changes in Clinical Outcomes
Aim 2 was to test whether patients’ sleep measured at admission was associated with changes in key clinical outcomes (i.e., functional disability, coping, pain) throughout the program. Table 4 contains results from the adjusted model examining associations between sleep parameters at admission with clinical outcomes. After controlling for outcome variables at admission (e.g., disability, coping, pain), age, sleep medication use, and program duration, better self-reported sleep quality assessed via the daily diaries over the first week was associated with improvements in average pain levels and disability over the course of the program. Additionally, worse self-reported sleep hygiene at admission was associated with improvements in average pain over the course of the program; however, this association was not significant after applying the Holm Bonferroni correction to control for Type 1 error.
Table 4.
Associations between Sleep Variables Collected at Admission and Clinical Outcome at Discharge, Controlling for Age, Sleep Medication Use, and Program Duration.
| FDI at Discharge | Coping at Discharge | Average Pain at Discharge | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variable | β | t | p | R2 | β | t | p | R2 | β | t | p | R2 |
| Sleep/wake patterns total score (ASWS)* | ||||||||||||
| Step 1: Outcome at admission | 0.25 | 1.42 | .17 | .14 | 0.54 | 3.24 | .003 | .28 | 0.84 | 8.51 | <.001 | .72 |
| Age | 0.20 | 1.14 | .26 | 0.000 | 0.002 | .99 | −0.14 | −1.35 | .19 | |||
| Sleep medication use (Y/N) | −0.22 | −1.22 | .23 | 0.03 | 0.18 | .86 | −0.002 | −0.02 | .99 | |||
| Program duration (in days) | 0.21 | 1.20 | .24 | −0.09 | −0.56 | .58 | −0.04 | −0.34 | .74 | |||
| Step 2: ASWS total score at admission | −0.11 | −0.54 | .60 | .15 | −0.03 | −0.18 | .86 | .28 | 0.05 | 0.45 | .66 | .72 |
| Sleep hygiene total score (ASHS)* | ||||||||||||
| Step 1: Outcome at admission | 0.25 | 1.42 | .17 | .14 | 0.54 | 3.24 | .003 | .28 | 0.84 | 8.51 | <.001 | .72 |
| Age | 0.20 | 1.14 | .26 | 0.000 | 0.002 | .99 | −0.14 | −1.35 | .19 | |||
| Sleep medication use (Y/N) | −0.22 | −1.22 | .23 | 0.03 | 0.18 | .86 | −0.002 | −0.02 | .99 | |||
| Program duration (in days) | 0.21 | 1.20 | .24 | −0.09 | −0.56 | .58 | −0.04 | −0.34 | .74 | |||
| Step 2: ASHS total score at admission | 0.28 | 1.56 | .13 | .21 | −0.13 | −0.78 | .44 | .30 | 0.24 | 2.20 | .04 | .76 |
| Insomnia symptoms (PISI)* | ||||||||||||
| Step 1: Outcome at admission | 0.19 | 1.05 | .30 | .05 | 0.37 | 2.23 | .03 | .14 | 0.61 | 4.90 | <.001 | .41 |
| Age | 0.17 | 0.98 | .33 | −0.14 | −0.85 | .40 | 0.21 | 1.60 | .12 | |||
| Sleep medication use (Y/N) | −0.11 | −0.64 | .53 | 0.05 | 0.29 | .78 | −0.12 | −0.98 | .33 | |||
| Program duration (in days) | 0.11 | 0.66 | .51 | 0.05 | 0.30 | .76 | −0.06 | −0.42 | .68 | |||
| Step 2: PISI total score at admission | 0.06 | 0.29 | .78 | .06 | 0.09 | 0.53 | .60 | .14 | 0.95 | 0.62 | .54 | .42 |
| Daytime sleepiness (ESS)* | ||||||||||||
| Step 1: Outcome at admission | 0.19 | 1.05 | .30 | .05 | 0.37 | 2.23 | .03 | .14 | 0.61 | 4.90 | <.001 | .41 |
| Age | 0.17 | 0.98 | .33 | −0.14 | −0.85 | .40 | 0.21 | 1.60 | .12 | |||
| Sleep medication use (Y/N) | −0.11 | −0.64 | .53 | 0.05 | 0.29 | .78 | −0.12 | −0.98 | .33 | |||
| Program duration (in days) | 0.11 | 0.66 | .51 | 0.05 | 0.30 | .76 | −0.06 | −0.42 | .68 | |||
| Step 2: ESS total score at admission | 0.08 | 0.53 | .60 | .06 | −0.16 | −1.04 | .31 | .16 | 0.08 | 0.65 | .52 | .42 |
| Sleep quality (daily diaries)ǂ | ||||||||||||
| Step 1: Outcome at admission | 0.19 | 1.06 | .30 | .05 | 0.37 | 2.26 | .03 | .14 | 0.61 | 4.78 | <.001 | .41 |
| Age | 0.17 | 1.01 | .32 | −0.14 | −0.84 | .41 | 0.21 | 1.57 | .13 | |||
| Sleep medication use (Y/N) | −0.09 | −0.51 | .62 | 0.04 | 0.26 | .79 | −0.13 | −0.97 | .34 | |||
| Program duration (in days) | 0.06 | 0.35 | .73 | 0.05 | 0.30 | .77 | −0.04 | −0.33 | .74 | |||
| Step 2: Sleep Quality at admission | −0.37 | −2.22 | .03 | .16 | 0.22 | 1.32 | .20 | .17 | −0.46 | −4.02 | <.001 | .59 |
| Total sleep time (actigraphy)ǂ | ||||||||||||
| Step 1: Outcome at admission | 0.21 | 1.20 | .24 | .08 | 0.36 | 2.26 | .03 | .13 | 0.61 | 4.97 | <.001 | .42 |
| Age | 0.20 | 1.26 | .22 | −0.22 | −1.37 | .18 | 0.20 | 1.58 | .12 | |||
| Sleep medication use (Y/N) | −0.13 | −0.80 | .43 | 0.09 | 0.56 | .58 | −0.12 | −0.95 | .35 | |||
| Program duration (in days) | 0.14 | 0.85 | .40 | −0.04 | −0.22 | .82 | −0.07 | −0.51 | .61 | |||
| Step 2: Total sleep time at admission | 0.15 | 0.96 | .35 | .11 | −0.03 | −0.16 | .87 | .13 | −0.14 | −1.15 | .26 | .44 |
| WASO (actigraphy)ǂ | ||||||||||||
| Step 1: Outcome at admission | 0.21 | 1.20 | .24 | .08 | 0.36 | 2.26 | .03 | .13 | 0.61 | 4.97 | <.001 | .42 |
| Age | 0.20 | 1.26 | .22 | −0.22 | −1.37 | .18 | 0.20 | 1.58 | .12 | |||
| Sleep medication use (Y/N) | −0.13 | −0.80 | .43 | 0.09 | 0.56 | .58 | −0.12 | −0.95 | .35 | |||
| Program duration (in days) | 0.14 | 0.85 | .40 | −0.04 | −0.22 | .82 | −0.07 | −0.51 | .61 | |||
| Step 2: WASO at admission | 0.17 | 1.08 | .29 | .11 | −0.07 | −0.47 | .64 | .14 | −0.05 | −0.42 | .68 | .42 |
| Sleep onset variability (actigraphy)ǂ | ||||||||||||
| Step 1: Outcome at admission | 0.21 | 1.20 | .24 | .08 | 0.36 | 2.26 | .03 | .13 | 0.61 | 4.97 | <.001 | .42 |
| Age | 0.20 | 1.26 | .22 | −0.22 | −1.37 | .18 | 0.20 | 1.58 | .12 | |||
| Sleep medication use (Y/N) | −0.13 | −0.80 | .43 | 0.09 | 0.56 | .58 | −0.12 | −0.95 | .35 | |||
| Program duration (in days) | 0.14 | 0.85 | .40 | −0.04 | −0.22 | .82 | −0.07 | −0.51 | .61 | |||
| Step 2: Sleep onset var. at admission | −0.11 | −0.67 | .51 | .09 | −0.07 | −0.43 | .67 | .14 | 0.04 | 0.31 | .76 | .42 |
| Sleep offset variability (actigraphy)ǂ | ||||||||||||
| Step 1: Outcome at admission | 0.21 | 1.20 | .24 | .08 | 0.36 | 2.26 | .03 | .13 | 0.61 | 4.97 | <.001 | |
| Age | 0.20 | 1.26 | .22 | −0.22 | −1.37 | .18 | 0.20 | 1.58 | .12 | |||
| Sleep medication use (Y/N) | −0.13 | −0.80 | .43 | 0.09 | 0.56 | .58 | −0.12 | −0.95 | .35 | |||
| Program duration (in days) | 0.14 | 0.85 | .40 | −0.04 | −0.22 | .82 | −0.07 | −0.51 | .61 | |||
| Step 2: Sleep offset var. at admission | 0.08 | 0.45 | .66 | .09 | −0.03 | −0.17 | .87 | .13 | −0.05 | −0.35 | .73 | .42 |
Note: Bolded results indicate statistical significance at Step 2. All models were run independently using linear regression. Age, sleep medication status (Yes [1]/No [0]), and program duration in days were included as covariates in all models. Unadjusted models are available upon request to the corresponding author.
ASHS, Adolescent sleep hygiene scale; ASWS, Adolescent sleep wake scale; ESS, Epworth sleep scale for children; FDI, Functional disability inventory; NRS, Numeric rating scale; PCQ, Pain coping questionnaire; PISI, Pediatric insomnia severity index; WASO, Wake after sleep onset.
Measured at admission
Averaged over first seven days in program (admission)
Aim 3: Correlating Changes in Sleep with Improvement in Clinical Outcomes
Aim 3 was to test whether changes in sleep correlated with concurrent changes in clinical outcomes during IIPT. Table 5 and Figure 1 reveal that while controlling for relevant covariates, improvements in insomnia symptoms throughout the program were strongly positively correlated with concurrent improvements in functional disability and coping throughout the program. Similar associations, albeit not as strong, were seen for improvements in daytime sleepiness correlating with concurrent improvements in functional disability and coping.
Table 5.
Correlations Between Changes in Sleep Variables and Changes in Clinical Outcomes, With and Without Controlling for Covariates of Age, Sleep Medication Use, and Program Duration.
| Improvement in Disability (FDI)# | Improvements in Coping (PCQ)# | Improvements in Pain (NRS)# | |
|---|---|---|---|
| r, p | r, p | r, p | |
| Improvements in Insomnia Symptoms (PISI) | |||
| Unadjusted | .58, <.001 | .72, <.001 | .33, .03 |
| Controlling for covariates | .49, <.01 | .73, <.001 | .23, .16 |
| Improvements in Daytime Sleepiness (ESS) | |||
| Unadjusted | .35, .02 | .41, <.01 | .18, .25 |
| Controlling for covariates | .42, <.01 | .45, <.01 | .22, .18 |
Note: Bolded results indicate statistical significance. Age, sleep medication use (Yes [1]/No [0]), and program duration in days were included as covariates. Unadjusted models are available upon request to the corresponding author.
ESS, Epworth sleep scale for children; FDI, Functional disability inventory; NRS, Numeric rating scale; PCQ, Pain coping questionnaire; PISI, Pediatric insomnia severity index.
Correlations between actigrapgy and sleep quality measures with clinical outcomes were not computed because there were no significant changes in actigraphy or sleep quality from admission to discharge.
Variables were calculated from a difference between admission and discharge scores and were coded so that positive scores represent improvement over the program and negative scores indicate worsening over the program.
Figure 1.
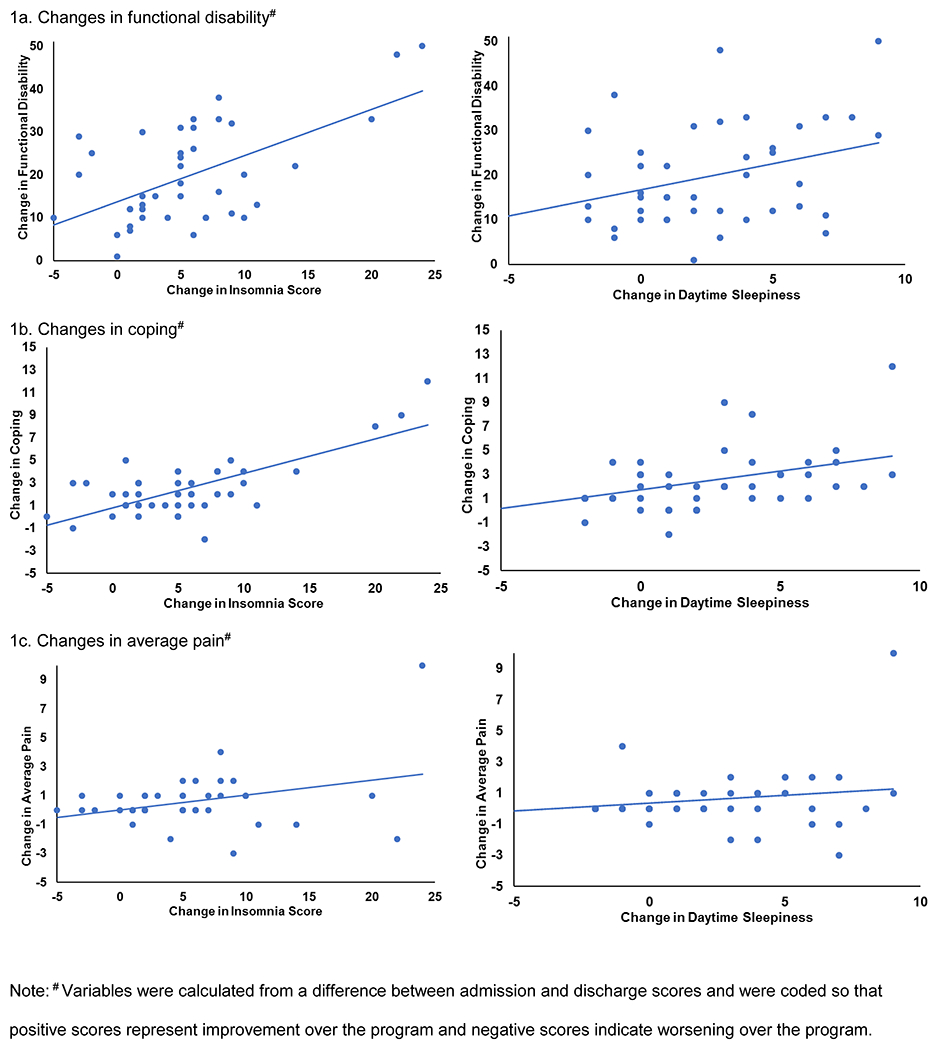
Scatterplots of improvement in insomnia symptoms and daytime sleepiness against improvement in a) functional disability b) coping and c) average pain.
Discussion
The current study sought to characterize self-report and actigraphic sleep parameters among pediatric patients with severely disabling chronic pain undergoing inpatient IIPT, test whether sleep variables at admission were associated with changes in clinical outcomes, and determine whether changes in sleep correlated with concurrent changes in disability, coping, and/or pain during inpatient IIPT. Study hypotheses were partially supported. Results demonstrated that patients self-reported poor sleep parameters prior to admission into IIPT but had generally healthy actigraphic markers of sleep during the first week following admission. Improvement in some self-reported sleep parameters during treatment were associated with clinical improvements in disability, pain, and coping. To our knowledge, these are the first data to examine both self-reported and actigraphic indices of sleep in a pediatric chronic pain population undergoing IIPT.
The interrelationships between poor sleep and higher pain are well established in adolescent and adult chronic pain populations (21,22), and, thus, we expected to find that adolescent patients presenting to the IIPT program would report disrupted sleep. Indeed, when assessed at admission, patients self-reported problematic sleep/wake patterns, sub-clinical levels of daytime sleepiness, but high levels of insomnia symptoms relative to healthy reference samples (ASWS; ASHS) and a clinical reference sample (PISI). These findings suggest that perceptions of poor sleep are part of the clinical panorama for children presenting to IIPT programs for chronic pain.
Despite poor self-reported sleep at admission, we did not find significantly disrupted actigraphic indices of sleep during the seven nights following admission. Actigraphy data suggested patients were obtaining near adequate sleep based on the recent American Academy of Sleep Medicine consensus guidelines (8-10 hours of sleep per night for adolescents; 40) and had consistent sleep onset and sleep offset times. One possible explanation for the discrepancy between self-reported and actigraphic data is that these data were from different measurement time points, with the questionnaires assessing sleep in the days, weeks, or month prior to admission, and actigraphy reflecting sleep in the first week following admission. The strict environmental control and schedule maintained during the FIRST program may have restricted the range for actigraphic indices and may have exacerbated differences between the patients’ subjectively reported sleep experience prior to program entry and actigraphic sleep measured during the program. Discrepancies between self-reported and actigraphic indices of sleep are common in sleep research, with self-report being more strongly correlated with negative chronic pain outcomes than actigraphic indices of sleep (41). Still, more work is needed to determine the causes of this discrepancy, and to determine which measure type (self-report or actigraphy) is more appropriate for specific clinical outcomes.
Assessments of certain sleep variables measured at admission were found to be associated with changes in clinical outcomes over the course of the program. Specifically, better patient-perceived sleep quality as reported on the daily diaries over the first seven days in the program were associated with the greater improvements in average pain and disability throughout the program. It may be that those who perceive having better sleep also have more positive perceptions regarding other outcomes (i.e., pain, disability) because they may have a more positive outlook in general. A second possibility is that better sleep quality early on in the program was associated with a change in an unmeasured variable (e.g., fatigue), and that the associations between sleep quality and pain throughout the program were mediated by this variable (e.g., fatigue). Other potential mediators of this relationship include physical activity, diet, and social interactions with peers. Future work examining the mediators and moderators of the sleep-pain relationship is critically important.
The third aim of the study examined how improvements in sleep over the course of the program were correlated with concurrent improvements in clinical outcomes. Improvements in disability and coping were strongly correlated with concurrent improvements in daytime sleepiness and insomnia symptoms; correlations between these variables and changes in average pain were less robust and not significant. These correlations were only found between self-report measures of sleep and clinical outcomes; actigraphic indices of sleep did not change over this time period. As previously discussed, because patients were generally sleeping well in the first week following admission, there may have simply been less room for improvement in total sleep time, wake after sleep onset, and sleep consistency. Future studies with baseline home-based actigraphic information are needed and would help answer questions about whether the objective sleep measures found here represent improvements in sleep from baseline. These results complement those reported by Logan and colleagues, who found that sleep habits at discharge correlated with concurrently-measured functional disability (10). Taken together, results suggest that treatment outcomes from IIPT are associated with improvements in the subjective experience of sleep.
The mechanisms underlying these relationships remain an important area for future research. Sleep could enhance program success by increasing emotion regulation ability (42), improving encoding and rehearsal of information (43), reducing fatigue (44), reducing perceived intensity of pain (45), or via numerous other mechanisms. Improvements in sleep may also change physiology in ways that promote health, for example, it may decrease inflammation and improve immunity among other benefits (46). The specific mechanism by which better sleep promotes better treatment outcomes are beyond the scope of this paper but, if discovered, have the potential to improve the treatment effects from IIPT.
Clinical implications
Clinically, the findings from the current study have important implications. Given the significant improvements in insomnia symptoms and daytime sleepiness observed over the program, it may be that inpatient IIPT programs – aside from serving to reduce functional disability associated with pain – also serve as sleep interventions due to the focus on functional improvement and following a wake and sleep schedule. Another important clinical implication pertains to the value of assessing actigraphic indices of sleep in IIPT. Even though evidence for change in actigraphy parameters between the first week and last week of the program were not found, incorporating actigraphy into IIPT and other chronic pain treatment programs may prove beneficial. Actigraphy provides clinically-relevant descriptive information for both the clinician and patient and can be used to illustrate to the patient how certain aspects of sleep (i.e., sleep latency, WASO) map to their subjective experience of sleep and pain (47). Actigraphy may be particularly helpful for visually illustrating to patients which elements of their sleep are under their control (e.g. time they get into and out of bed), and which are not under their control (e.g. time they actually fall asleep, sleep quality). Receiving this visual feedback that they are taking control of their sleep/wake patterns can be empowering for youth who may previously have felt that they had little control over their sleep. Actigraphy data can also be used to facilitate important conversations between the patients and the providers regarding sleep and its potential relation to pain.
Clinically, for children with chronic pain, targeting and treating problems with sleep may be just as important as reducing pain to achieve long term improvements in function (48). Because sleep behavior can be modifiable, incorporating sleep intervention into IIPT may offer another angle of treatment that could improve patients’ outcomes. Monitoring sleep during IIPT may teach patients that they are improving their sleep even if they continue to subjectively experience sleep challenges (e.g., perceived sleepiness, insomnia). In this way, objective sleep information could allow patients to see evidence of changes in sleep (even if they do not perceive such changes), which could encourage ongoing adoption of healthy sleep habits (e.g. good sleep hygiene, consistent sleep/wake times). Taken together, patients likely stand to make significant improvements in long-term pain and function when they improve their sleep.
Limitations, strengths, and conclusion
The current study is not without limitations. First, the sample size was small and the clinical population was unique (i.e., adolescents with chronic pain; 27% of population had Ehlers-Danlos Syndrome); as such, results may not generalize to other chronic pain populations or age groups. Future work should aim to replicate these findings with a larger sample size which would allow for construction of structural equation models to parsimoniously explore the relationships between sleep variables and treatment outcomes. Second, the significant findings observed between self-reported sleep and self-reported outcomes may be due in part to method variance. Third, the measurement period captured by the different sleep measures was variable, with some assessing sleep in the last month, others in the last week, and others in the last night. These time frames should be taken into consideration when interpreting results. Finally, the current study averaged across the first week and last week in the program but did not look at daily associations between sleep, pain, and outcomes. This approach was primarily chosen because other clinical outcomes were not measured daily (e.g., pain, disability); however, greater temporal resolution between these outcomes and sleep variables should be examined in the future.
In conclusion, the results from this study have clinical relevance and are among the first to describe actigraphic indices of sleep among youth with chronic pain during IIPT, and to specifically examine associations of sleep parameters to changes in clinical outcomes in an inpatient IIPT program. The results provide compelling evidence and pave the way for future research to more thoroughly examine the pathways by which sleep (or perceptions of sleep) exert influence on treatment outcomes.
Acknowledgments
Conflict of Interest and Source of Funding: The authors declare no conflicts of interest. Funding for the second author (Kendra Krietsch) of this project was, in part, supported by an NIH training grant (T32DK063929). The CCTST at Cincinnati Children’s Hospital Medical Center is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant 2UL1TR001425-05A. The CTSA program is led by the NIH’s National Center for Advancing Translational Sciences (NCATS). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgments
The authors would like to thank Victor Schneider for his help collecting and cleaning the actigraphy data. The authors would also like to thank all the FIRST patients and their families for their willingness to contribute to research. Funding for the second author (Kendra Krietsch) of this project was, in part, supported by an NIH training grant (T32DK063929). The CCTST at Cincinnati Children’s Hospital Medical Center is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant 2UL1TR001425-05A. The CTSA program is led by the NIH’s National Center for Advancing Translational Sciences (NCATS). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors do not have any conflicts of interest.
References
- 1.Fuss S, Pagé MG, Katz J. Persistent pain in a community-based sample of children and adolescents: sex differences in psychological constructs. Pain Res Manag 2011;16(5):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain 2011;152(12):2729–38. [DOI] [PubMed] [Google Scholar]
- 3.Huguet A, Miró J. The severity of chronic pediatric pain: an epidemiological study. The Journal of Pain 2008;9(3):226–36. [DOI] [PubMed] [Google Scholar]
- 4.Brna P, Dooley J, Gordon K, Dewan T. The prognosis of childhood headache: a 20-year follow-up. Arch Pediatr Adolesc Med 2005;159(12):1157–60. [DOI] [PubMed] [Google Scholar]
- 5.Kashikar-Zuck S, Cunningham N, Peugh J, Black WR, Nelson S, Lynch-Jordan AM, et al. Long-term outcomes of adolescents with juvenile-onset fibromyalgia into adulthood and impact of depressive symptoms on functioning over time. Pain 2019;160(2):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashikar-Zuck S, King C, Ting TV, Arnold LM. Juvenile fibromyalgia: different from the adult chronic pain syndrome? Curr Rheumatol Rep. 2016;18(4):19. [DOI] [PubMed] [Google Scholar]
- 7.Hechler T, Kanstrup M, Holley AL, Simons LE, Wicksell R, Hirschfeld G, et al. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics 2015;136(1):115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce B, Ale C, Sperry J, Weiss K, Harrison T, Luedtke C. Can a pediatric pain rehabilitation program achieve and maintain important health outcomes? The Journal of Pain. 2015;16(4):S105. [Google Scholar]
- 9.Bruce BK, Ale CM, Harrison TE, Bee S, Luedtke C, Geske J, et al. Getting back to living. The Clinical Journal of Pain 2017;33(6):535–542. [DOI] [PubMed] [Google Scholar]
- 10.Logan DE, Sieberg CB, Conroy C, Smith K, Odell S, Sethna N. Changes in sleep habits in adolescents during intensive interdisciplinary pediatric pain rehabilitation. J Youth Adolescence 2015;44(2):543–55. [DOI] [PubMed] [Google Scholar]
- 11.Stahlschmidt L, Zernikow B, Wager J. Satisfaction with an intensive interdisciplinary pain treatment for children and adolescents. The Clinical Journal of Pain 2018;34(9):795–803. [DOI] [PubMed] [Google Scholar]
- 12.Stahlschmidt L, Zernikow B, Wager J. Specialized rehabilitation programs for children and adolescents with severe disabling chronic pain: indications, treatment and outcomes. Children 2016;3(4):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams SE, Homan KJ, Crowley SL, Pruitt DW, Collins AB, Deet ET, et al. The impact of spatial distribution of pain on long-term trajectories for chronic pain outcomes after intensive interdisciplinary pain treatment. The Clinical Journal of Pain. 2020;36(3):181–188. [DOI] [PubMed] [Google Scholar]
- 14.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain 2009;145(1):136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain 2005;119(1):56–64. [DOI] [PubMed] [Google Scholar]
- 16.Kundermann B, Hemmeter-Spernal J, Huber MT, Krieg J- C, Lautenbacher S. Effects of total sleep deprivation in major depression: overnight improvement of mood is accompanied by increased pain sensitivity and augmented pain complaints. Psychosomatic Medicine 2008;70(1):92–101. [DOI] [PubMed] [Google Scholar]
- 17.Kundermann B, Spernal J, Huber MT, Krieg J-C, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosomatic Medicine 2004;66(6):932–937. [DOI] [PubMed] [Google Scholar]
- 18.Ødegård SS, Omland PM, Nilsen KB, Stjern M, Gravdahl GB, Sand T. The effect of sleep restriction on laser evoked potentials, thermal sensory and pain thresholds and suprathreshold pain in healthy subjects. Clinical Neurophysiology 2015;126(10):1979–87. [DOI] [PubMed] [Google Scholar]
- 19.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep 2007;30(4):494–505. [DOI] [PubMed] [Google Scholar]
- 20.LeBourgeois MK, Giannotti F, Cortesi F, Wolfson AR, Harsh J. The relationship between reported sleep quality and sleep hygiene in italian and american adolescents. Pediatrics 2005;115(Supplement 1):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain 2007;23(9):812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valrie CR, Bromberg MH, Palermo T, Schanberg LE. A systematic review of sleep in pediatric pain populations. J Dev Behav Pediatr 2013;34(2):120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law EF, Dufton L, Palermo TM. Daytime and nighttime sleep patterns in adolescents with and without chronic pain. Health Psychology 2012;31(6):830–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzierzewski JM, Williams JM, Roditi D, Marsiske M, McCoy K, McNamara J, et al. Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: evidence of covariation over time. Journal of the American Geriatrics Society 2010;58(5):925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. The Journal of Pain 2013;14(12):1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewandowski AS, Palermo TM, Motte SD la, Fu R Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain 2010;151(1):220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Essner B, Noel M, Myrvik M, Palermo T. Examination of the factor structure of the adolescent sleep–wake scale (ASWS). Behavioral Sleep Medicine 2015;13(4):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palermo TM, Wilson AC, Lewandowski AS, Toliver-Sokol M, Murray CB. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain 2011;152(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byars K, Simon S. Practice patterns and insomnia treatment outcomes from an evidence-based pediatric behavioral sleep medicine clinic. Clinical Practice in Pediatric Psychology 2014;2(3):337–49. [Google Scholar]
- 30.Byars KC, Simon SL, Peugh J, Beebe DW. Validation of a brief insomnia severity measure in youth clinically referred for sleep evaluation. J Pediatr Psychol 2017;42(4):466–75. [DOI] [PubMed] [Google Scholar]
- 31.Janssen KC, Phillipson S, O’Connor J, Johns MW. Validation of the Epworth Sleepiness Scale for children and adolescents using Rasch analysis. Sleep Medicine 2017;33:30–5. [DOI] [PubMed] [Google Scholar]
- 32.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol 1991;16(1):39–58. [DOI] [PubMed] [Google Scholar]
- 33.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain 2006;121(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid GJ, Gilbert CA, McGrath PJ. The pain coping questionnaire: preliminary validation. Pain 1998;76(1):83–96. [DOI] [PubMed] [Google Scholar]
- 35.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the numerical rating scale (NRS-11) for children’s self-reports of pain intensity. Pain 2009;143(3):223–7. [DOI] [PubMed] [Google Scholar]
- 36.Castarlenas E, Jensen MP, von Baeyer CL, Miró J. Psychometric properties of the numerical rating scale to assess self-reported pain intensity in children and adolescents: a systematic review. Clin J Pain 2017;33(4):376–83. [DOI] [PubMed] [Google Scholar]
- 37.Goulet-Pelletier J- C, Cousineau D. A review of effect sizes and their confidence intervals, part I: the Cohen’s d family. TQMP 2018;14(4):242–65. [Google Scholar]
- 38.Holm. A simple sequentially rejective multiple test procedure. Scand J Statistics 1979, 6, 65–70. [Google Scholar]
- 39.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- 40.Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American academy of sleep medicine. J Clin Sleep Med 2016;12(6):785–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boggero IA, Schneider VJ, Thomas PL, Nahman-Averbuch H, King CD. Associations of self-report and actigraphy sleep measures with experimental pain outcomes in patients with temporomandibular disorder and healthy controls. J Psychosomatic Res 2019;123:109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry 2013;55(2):180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stickgold R Parsing the role of sleep in memory processing. Current Opinion in Neurobiology 2013;23(5):847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boggero IA, Rojas-Ramirez MV, Carlson CR. All fatigue is not created equal: the association of fatigue and its subtypes on pain interference in orofacial pain. Clin J Pain 2017;33(3):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonelli G, Mantua J, Gad M, St Pierre M, Moore L, Yarnell AM, et al. Sleep extension reduces pain sensitivity. Sleep Medicine 2019;54:172–6. [DOI] [PubMed] [Google Scholar]
- 46.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 2015;66:143–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an american academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 2018;14(7):1209–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanstrup M, Holmström L, Ringström R, Wicksell RK. Insomnia in paediatric chronic pain and its impact on depression and functional disability. European Journal of Pain 2014;18(8):1094–102. [DOI] [PubMed] [Google Scholar]


