Abstract
Background
Pressure ulcers are one of the most common complications of immobility resulting from pressure and shear. Whole-body vibration (WBV) has been shown to increase skin blood flow but little information is known about its effect on pressure ulcers. This study investigated the effects of WBV on wound healing in a mouse pressure ulcer model.
Methods
Two cycles of ischemia-reperfusion were performed by external application of two magnetic plates to dorsal skin to induce stage II pressure ulcers characterized by partial-thickness skin loss with exposed dermis. A total of 32 male ICR mice were randomly and equally divided into untreated control and the WBV groups. Immediately after the completion of 2-cycle ischemia-reperfusion injury, mice in the WBV group participated in a WBV program using a vibrator (frequency 45 Hz, peak acceleration 0.4 g, vertical motion) for 30 min/day and 5 consecutive days/week. At days 7 and 14 post-ulceration, wound closure rate was assessed. Wound tissues were harvested for determination of collagen deposition in Masson's trichrome stained sections, neutrophil infiltration and capillary density in hematoxylin and eosin-stained sections, as well as TNF-α and VEGF levels using ELISA.
Results
TNF-α levels and neutrophil infiltration were significantly decreased in wounds on days 7 and 14 of WBV treatment. Moreover, wound closure rate and collagen deposition were remarkably accelerated on day 14. Tissue VEGF and capillary density were unaffected by WBV at either time point.
Conclusions
These findings suggest that WBV has the potential to promote the healing process of stage II pressure ulcers, as evidenced by attenuation of wound inflammation and enhancement collagen deposition.
Keywords: Whole-body vibration, Pressure ulcer, Ischemia-reperfusion injury, Vascular endothelial growth factor (VEGF), Tumor necrosis factor alpha (TNF-α), Wound healing
Whole-body vibration; Pressure ulcer; Ischemia-reperfusion injury; Vascular endothelial growth factor (VEGF); Tumor necrosis factor alpha (TNF-α); Wound healing
1. Introduction
Pressure ulcers, commonly known as bed sores, are one of the most common complications that occur in immobilized patients as a result of pressure and shear force [1]. The pathophysiology of pressure ulcers involves repeated ischemia-reperfusion injury, impaired vascularization and lymphatics, and cellular distortion, all of which induce the generation of free radicals and inflammatory mediators such as tumor necrosis factor alpha (TNF-α) that cause dysregulated immune response and tissue damage [2, 3, 4, 5, 6, 7]. Based on the extent of tissue loss, pressure ulcers can be divided into 4 stages: stage I non-blanchable erythema of intact skin, stage II partial-thickness skin loss with exposed dermis, stage III full-thickness skin loss, and stage IV full-thickness skin and tissue loss [1]. If improperly treated, pressure ulcers may progress to more severe stages that can result in chronic skin damage, increased risk of wound infection, high medical care costs and a high mortality rate [8, 9]. Management of pressure ulcers includes dressings, microclimate control, nutrition, repositioning and early mobilization, and support surfaces [1]. Nonetheless, development of novel interventions is still needed in an attempt to improve the healing and care of the patients suffering from pressure ulcers. Examples of the advanced treatment modalities currently described in the literature are negative pressure therapy, low-level laser therapy, electrical stimulation, ultrasound and vibration therapy [10, 11, 12, 13].
This study focuses on the utilization of whole-body vibration (WBV), an oscillatory mechanical stimulation using a vibrator. A single bout of vibration with frequency ranging from 30-50 Hz has been proven to increase skin blood flow [14, 15, 16]. Furthermore, WBV has been reported to improve wound healing in only a few clinical and preclinical studies [17, 18, 19]. In elderly patients with stage I pressure ulcers, vibration therapy (47 Hz, 0.18 g) for 15 min 3 times/day increased the number of healed ulcers as assessed by complete disappearance of skin erythema [17]. An experimental study using full-thickness excisional wounds in diabetic mice indicated that whole body low-intensity vibration (45 Hz, 0.4 g) exerted a positive effect on wound healing by enhancing angiogenesis and granulation tissue formation [18]. In another study using foot excisional wounds in neonatal n5-streptozotocin-induced diabetic rats, low magnitude high frequency vibration (35 Hz, 0.3 g) for 20 min/day was able to accelerate foot wound healing and increase blood flow to the wound [19]. However, the roles and underlying mechanisms of WBV in the healing of stage II pressure ulcers have not yet been established.
The purpose of this study was to examine the effects of WBV on the healing of stage II pressure ulcers in a mouse model. We hypothesized that WBV can accelerate the healing process of pressure ulcers by decreasing TNF-α and neutrophil infiltration, as well as increasing VEGF and angiogenesis and collagen deposition in the wound tissues.
2. Materials and methods
2.1. Animals
All animal procedures were conducted in compliance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The experimental protocol was approved by the Institutional Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University. A total of 32 male ICR mice (8 weeks old; body weight 35–40 g) were purchased from the National Laboratory Animal Center, Salaya Campus, Mahidol University, Nakornpathom, Thailand. A 1-week acclimation period was required for the mice before initiation of the experiment. They were housed individually in plastic cages at 25 °C room temperature with a 12-hour light:dark cycle and fed ad libitum. Food consumption and body weight of each mouse were recorded at 4 pm every day.
2.2. Pressure ulcer creation
An ischemia-reperfusion injury model was conducted to create pressure ulcers using a non-invasive method as previously described [2]. Briefly, under sedation with 3–5 min inhalation of 5% isoflurane, dorsal skin of mice was shaved, disinfected, pinched and placed between two external magnets (10 mm diameter, 3 mm thickness, 1.7 g weight, 300 Gauss magnetic force) to compress skin at a pressure of 50 mmHg. Two cycles of ischemia-reperfusion including 16 h of compression and 8 h of compression-release were employed, producing bilateral stage II pressure ulcers. All mice received saline dressings once a day as standard wound care.
2.3. Animals and study design
The animals were randomly divided into two groups (n = 16 each): control group (CON) and whole-body vibration group (WBV). In the CON group, the mice were left in their cages throughout the experiment. Mice in the WBV group were administered a WBV program immediately following completion of 2-cycle ischemia-reperfusion. Each group was subdivided into two subgroups for parameter studies at days 7 and 14 post-ulceration.
At the end of the study period, the animals were sacrificed by cardiac puncture under isoflurane anesthesia. The areas of both left- and right-side wounds of each mouse were measured to assess wound closure rate. Wound tissues were harvested by circular incision about 2 mm distally from the wound edge with an average incision depth of 3 mm. The right-side wound tissues were fixed in 10% formalin, embedded in paraffin blocks and cut into 1-μm-thickness sections before staining for further skin histopathological study and quantification of neutrophil infiltration and capillary density, as well as collagen deposition. The left-side wound tissues were frozen at −80 °C for TNF-α and VEGF analysis. According to our preliminary study, there was no difference between sides of mice in the wound size generated by this ischemia-reperfusion model and the healing rate. Therefore, wound closure was averaged for the left- and right-side wounds, and the wounds were not randomized into histological and protein assays.
2.4. Whole-body vibration program
The vibration intervention consisted of a whole-body low-intensity vibration delivered vertically at 45 Hz with peak acceleration of 0.4 g for 30 min/day and 5 consecutive days/week, which is similar to the vibration intervention method used by Weinheimer-Haus et al. [18]. In the study by Weinheimer-Haus et al., mice were put in an empty plastic cage which was placed on a vibrating plate. In this study, however, the mice were placed directly on a vibrating plate of the vibrator and covered with a clear square plastic cover with ventilation holes to prevent their escape (Figure 1). The area of the plastic cover was 18 × 18 cm2, ensuring that the mice were not restrained while receiving WBV treatment. The vibratory signals were calibrated using computer software every time before use.
Figure 1.
Image of whole-body vibrator. The frequency and vertical-motion acceleration of the vibrator were 45 Hz and 0.4 g, respectively. Mice were placed standing directly on a vibrating plate and covered with a clear rectangular plastic cover with ventilation holes to prevent their escape.
2.5. Assessment of wound closure rate
At days 0, 7 and 14 post-ulceration, both wounds on each mouse were photographed with a digital camera featuring 16.2 megapixels resolution (Sony Cyber-shot™; Sony, Tokyo, Japan). Wound areas were measured using digital image processing and analysis with ImageJ [20], and the areas of both wounds were then averaged for each mouse. The wound closure rate was defined as the percentage reduction in wound size and calculated using the following formula: (area of initial wound – area of final wound) ×100/area of initial wound.
2.6. Skin histopathology and quantification of neutrophil infiltration and capillary density in wound tissues
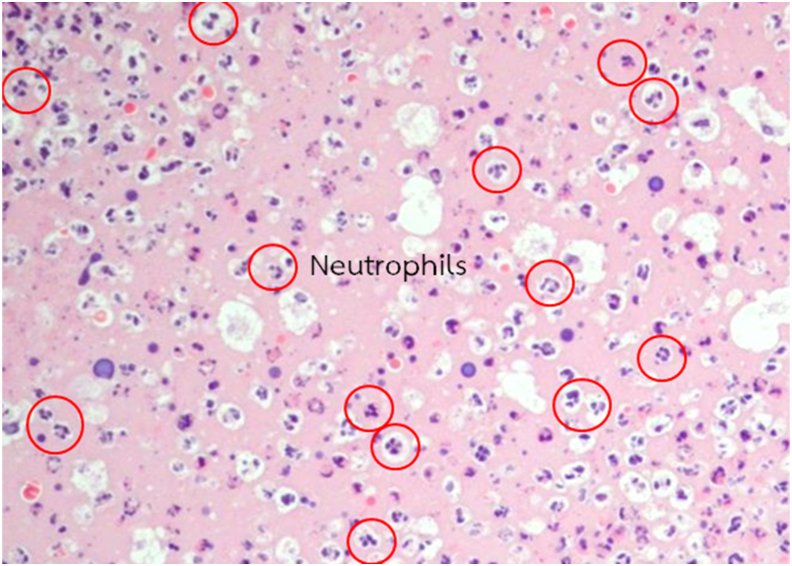
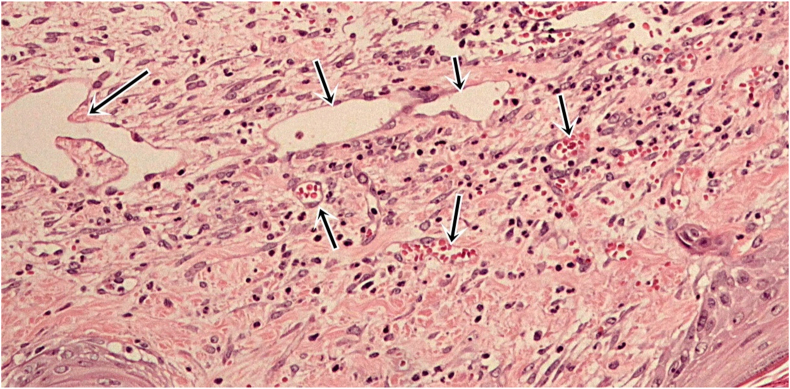
Four tissue sections were cut away from the center of the wound of each mouse and stained with hematoxylin and eosin for histopathological study and quantification of neutrophil infiltration and capillary density. All histopathologic examinations were performed by the same and certified pathologist. Skin histopathology was examined under a light microscope (Olympus BX53) at 100×. Eight frames (2 frames × 4 sections) and 16 frames (4 frames × 4 sections) per mouse were examined for neutrophil infiltration (Figure 2) and capillary density (Figure 3), respectively, under the light microscope at 400×. Neutrophil infiltration was expressed as the number of neutrophils infiltrating the dermis per frame. Capillary density was determined by counting the total number of capillary vessels and expressed as the number of capillaries per frame.
Figure 2.
Morphology of neutrophils in hematoxylin and eosin-stained sections at 400× magnification. Neutrophils are characterized by multilobed nucleus with cytoplasmic purplish granules.
Figure 3.
Morphology of capillaries in hematoxylin and eosin-stained sections at 400× magnification. Capillaries are composed of a thin layer of endothelial cell, surrounded by basement membrane.
2.7. Assessment of collagen deposition
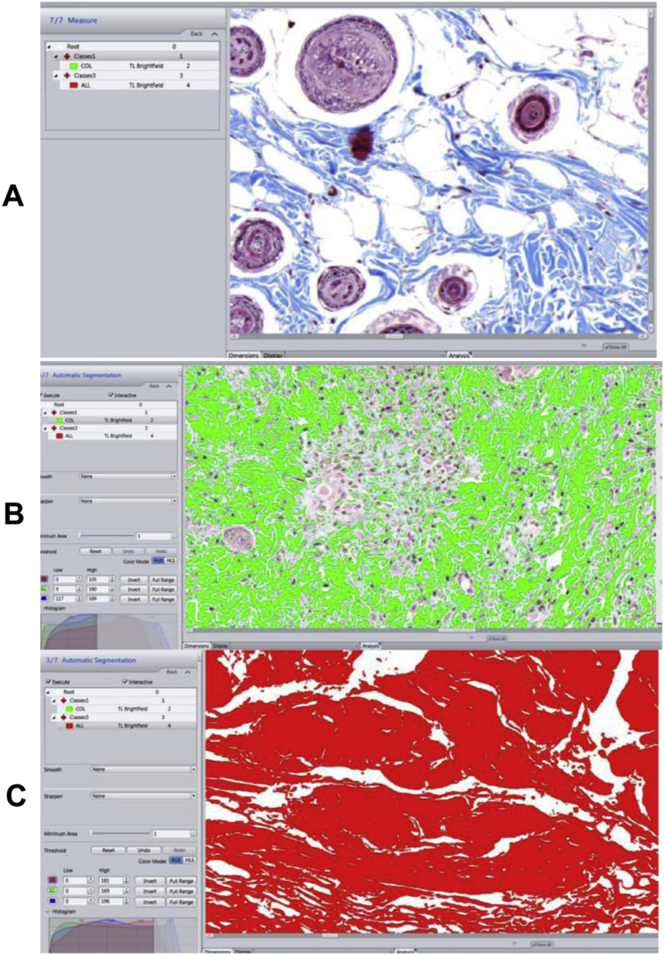
Masson's trichrome staining was performed for collagen identification (International Medical Equipment; IMEB, San Marcos, CA, USA), using the method as previously described [21]. Trichrome stained slides were photographed at 20× using a scanner (Axio Scan.Z1; Carl Zeiss, Jena, Germany) (Figure 4A). Image analysis software ZEN (Carl Zeiss, Jena, Germany) was used to determine the area of blue collagen staining and that of the wound bed, where the segmentation was produced by assigning intensity threshold (Figure 4B,C). Collagen deposition was expressed as the percentage of collagen-stained area relative to total area of the wound bed.
Figure 4.
Analysis of collagen deposition in wounds using image analysis software. (A) Image of Masson's trichrome stained slide at 20× using a scanner (Axio Scan.Z1; Carl Zeiss, Jena, Germany). (B) Intensity threshold was set to determine the area of blue collagen staining as highlighted in green. (C) Determination of the area of the wound bed as highlighted in red.
2.8. Measurement of tissue TNF-α and VEGF levels
Tissue was homogenized in RIPA lysis buffer (Cell Signaling Technology, Danvers, MA, USA) with protease inhibitor cocktail (Sigma Chemical, Saint Louis, MO, USA) and centrifuged at 11,872×g for 10 min. Supernatants were used for quantitative determination of TNF-α and VEGF using ELISA assay kits (R&D Systems, Minneapolis, MN, USA). Levels of tissue TNF-α and VEGF were adjusted for total tissue protein and expressed in pg/mg total protein.
2.9. Statistical analysis
Results are presented as mean and standard deviation (SD). Data were analyzed by one-way analysis of variance (ANOVA) and Mann-Whitney U test. Differences were considered statistically significant at P < 0.05.
3. Results
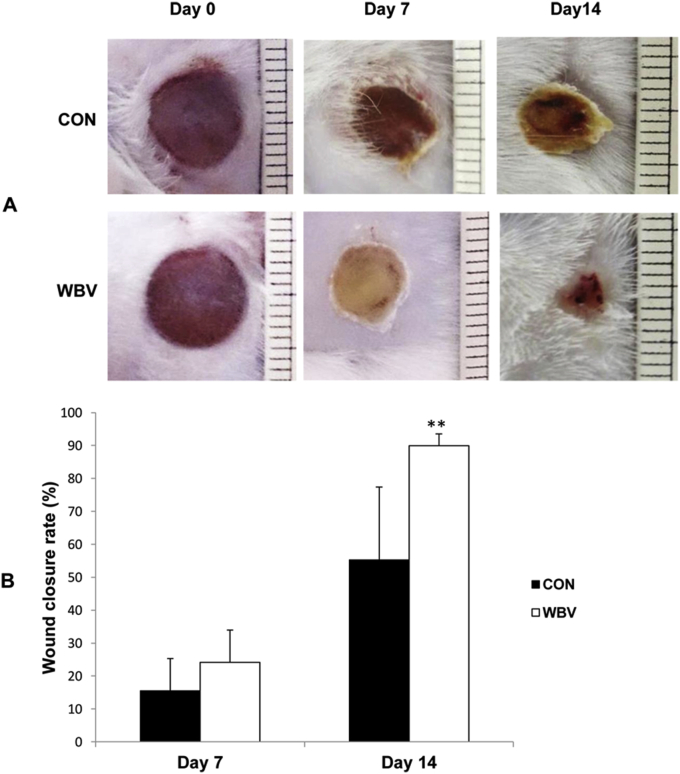
3.1. Wound closure rate
Photographs in Figure 5A show pressure ulcers in the untreated and treated groups at the start and during the experimental periods. There was a notable reduction in wound size, particularly on day 14, following WBV treatment. As shown in Figure 5B, the wound closure rate did not differ between the two groups on day 7 post-ulceration (CON = 15.5 ± 9.8%, WBV = 24.2 ± 9.8%), but was significantly higher on day 14 post-ulceration in the WBV group than in the untreated control group (CON = 55.3 ± 22.1%, WBV = 90.0 ± 3.5%).
Figure 5.
Effect of WBV on wound closure of stage II pressure ulcers. (A) Images of untreated and WBV-treated pressure ulcers at days 0, 7 and 14 post-ulceration. (B) Wound closure rate expressed as percentage reduction in wound area at days 7 and 14 post-ulceration. CON: control group; WBV: vibration group. Data are expressed as mean ± SD. Star indicates a significant difference from CON (P < 0.01).
3.2. Neutrophil infiltration
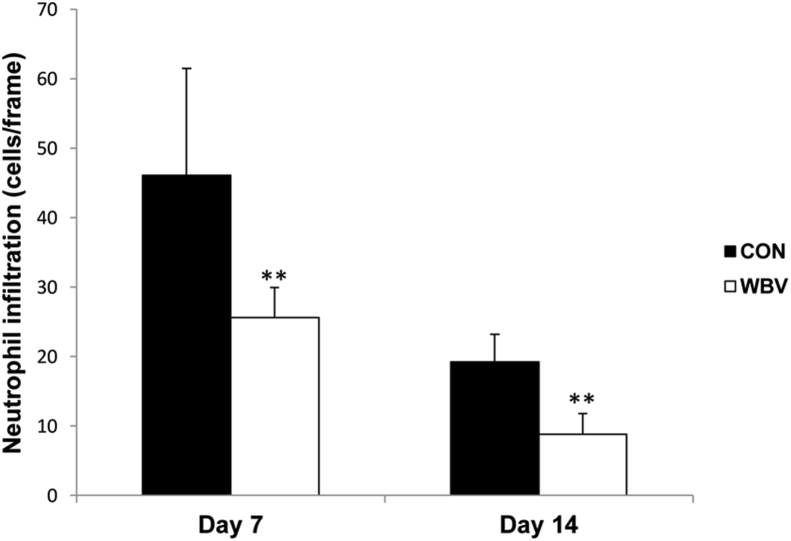
Over days 7 and 14 post-ulceration, neutrophil infiltration was significantly decreased in the WBV group compared with the control group (day 7: CON = 46.1 ± 15.4, WBV = 25.6 ± 4.3 cells/frame; day 14: CON = 19.2 ± 4.0, WBV = 8.8 ± 3.0 cells/frame) (Figure 6).
Figure 6.
Effect of WBV on neutrophil infiltration expressed as average number of neutrophils infiltrated in the dermal layer per frame at days 7 and 14 post-ulceration. CON: control group; WBV: vibration group. Data are expressed as mean ± SD. Star indicates a significant difference from CON (P < 0.01).
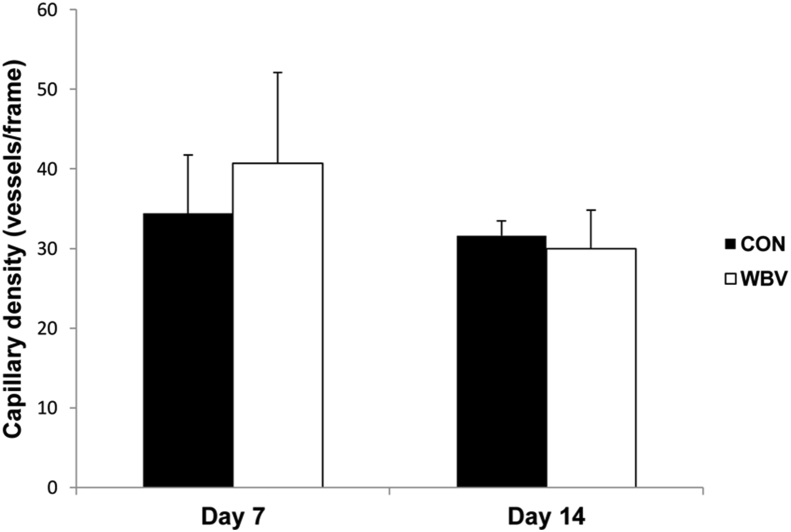
3.3. Capillary density
As depicted in Figure 7, capillary density was not different between both groups at either time point (day 7: CON = 34.4 ± 7.3, WBV = 40.7 ± 11.4 vessels/frame; day 14: CON = 31.6 ± 1.9, WBV = 30 ± 4.8 vessels/frame).
Figure 7.
Effect of WBV on capillary density expressed as average number of capillary vessels per frame at days 7 and 14 post-ulceration. CON: control group; WBV: vibration group. Data are expressed as mean ± SD. There was no significant difference from CON (P > 0.05).
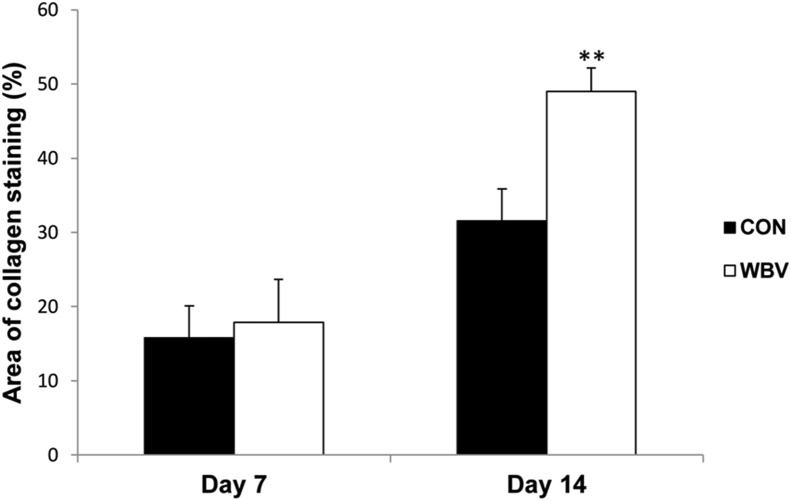
3.4. Collagen deposition
Figure 8 shows that collagen deposition significantly increased in the WBV group on day 14 (CON = 31.6 ± 4.3%, WBV = 49.0 ± 3.2%). However, there was no significant difference between both groups on day 7 (CON = 15.8 ± 2.0%, WBV = 17.9 ± 5.8%).
Figure 8.
Effect of WBV on collagen deposition expressed as percentage of collagen-stained area relative to total area of wound bed at days 7 and 14 post-ulceration. CON: control group; WBV: vibration group. Data are expressed as mean ± SD. Star indicates a significant difference from CON (P < 0.01).
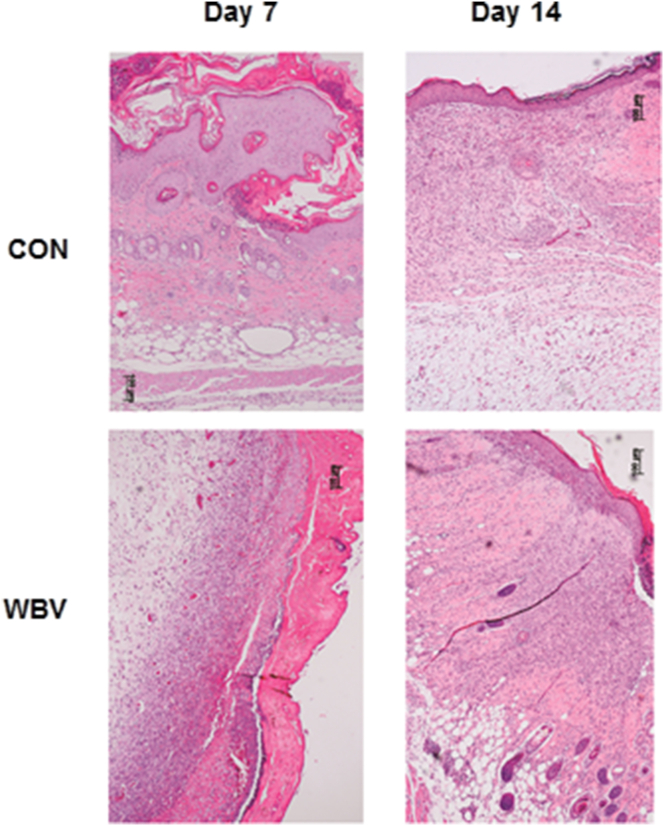
3.5. Skin histopathology
Figure 9 reveals histological features of the ulcers in both groups. On day 7, the untreated ulcer showed a large ulceration, fibrin, and bacterial growth in the epidermal layer, with keratin debris covering the ulcer bed. Moreover, the inflammatory exudate contained numerous lymphocytes, monocytes and macrophages in the dermal layer. There was a mild inflammatory reaction in the hypodermal layer. In WBV-treated ulcers, the lesion characteristics were similar, but less severe than those of untreated ulcers.
Figure 9.
Histopathological features of pressure ulcers in hematoxylin and eosin-stained sections at 100× magnification at days 7 and 14 post-ulceration. CON: control group; WBV: vibration group.
On day 14, polymorphonuclear cell infiltrate, muscle atrophy, and blood vessels had decreased in the untreated ulcers. In addition, epithelialization and a small-sized hemorrhagic crust were observed. In WBV-treated ulcers, similar characteristics were also evident. However, the WBV-treated ulcers displayed more collagen bundles than the untreated ulcer.
3.6. Tissue TNF-α and VEGF levels
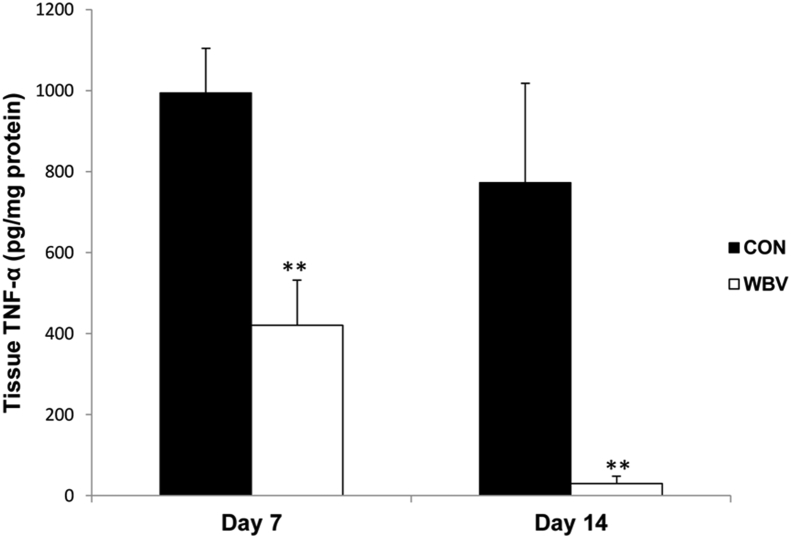
Tissue TNF-α levels of the WBV group were significantly lower than those of the CON group at days 7 and 14 (day 7: CON = 994.1 ± 110.7, WBV = 420.2 ± 111.7 pg/mg protein; day 14: CON = 772.7 ± 245.2, WBV = 30.3 ± 17.7 pg/mg protein) (Figure 10).
Figure 10.
Effect of WBV on tissue TNF-α levels at days 7 and 14 post-ulceration. CON: control group; WBV: vibration group. Data are expressed as mean ± SD. Star indicates a significant difference from CON (P < 0.01).
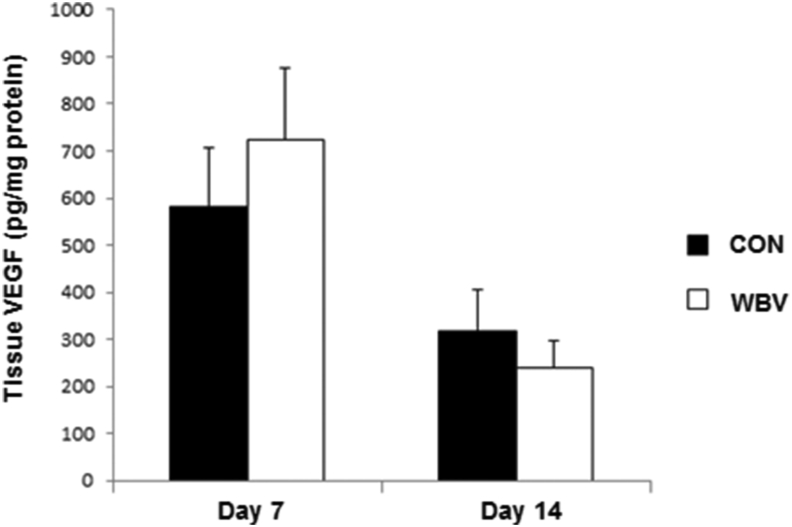
No difference in the levels of tissue VEGF was observed between the two groups on days 7 and 14 (day 7: CON = 581.0 ± 123.4, WBV = 723.9 ± 152.3 pg/mg protein; day 14: CON = 318.1 ± 86.1, WBV = 238.2 ± 58.4 pg/mg protein) (Figure 11).
Figure 11.
Effect of WBV on tissue VEGF levels at days 7 and 14 post-ulceration. CON: control group; WBV: vibration group. Data are expressed as mean ± SD. There was no significant difference from CON (P > 0.05).
4. Discussion
This study investigated effects of WBV on wound healing in a mouse pressure ulcer model. The main finding of this study is that WBV accelerates the healing process of stage II pressure ulcers induced by ischemia-reperfusion injury. Compared with the untreated control, WBV-treated mice exhibited a significant decrease in neutrophil infiltration and TNF-α levels in wounds. There was a remarkable enhancement of wound closure rate and collagen deposition. However, capillary density and tissue VEGF were not increased after WBV treatment.
This study indicated that inflammation of pressure ulcers was suppressed after WBV treatment as demonstrated by a decrease in pro-inflammatory cytokine TNF-α and neutrophil infiltration at days 7 and 14 post-ulceration. Given that prolonged inflammation is a factor in wound chronicity [22, 23], the anti-inflammatory effect of WBV is beneficial to the healing and prevention the severity of pressure ulcers from progressing. This anti-inflammatory effect offers a possible explanation for the reduction of skin redness indicative of inflammation of stage I pressure ulcers after WBV reported by Arashi et al. [17]. The present findings are also in agreement with the study by Weinheimer-Haus et al. showing that neutrophil accumulation and TNF-α mRNA expression were reduced after WBV in CD11b– cells isolated from the wounds at day 7 post-ulceration [18]. In addition to a local anti-inflammatory effect at the wound site, WBV has been shown in one study to improve inflammatory status in the elderly by increasing interleukin-10 mRNA levels while decreasing plasma TNF-α and C-reactive protein levels via downregulation of toll-like receptors 2 and 4 signaling pathways [24]. Thus, a WBV program may reduce inflammation through down-regulation of pro-inflammatory inflammatory cytokines and up-regulation of anti-inflammatory cytokine production.
At day 14 post-ulceration during the proliferative phase of the repair process, WBV was found to stimulate wound closure and collagen deposition. Similarly, Weinheimer-Haus et al. demonstrated that WBV promoted granulation tissue formation and re-epithelialization through local production of pro-healing insulin-like growth factor-1 (IGF-1) in diabetic wounds [18]. IGF-1 is known to activate dermal fibroblast proliferation to synthesize collagen, as well as keratinocyte proliferation and migration, which leads to improved re-epithelialization [25, 26]. In addition, according to Sari et al., vibration therapy was able to prevent the deterioration of compression-induced deep tissue injury in rat skin by inhibiting the activity of matrix metalloproteinases, enzymes responsible for collagen degradation [27]. Besides local effects, WBV has been reported to cause an acute increase in circulating IGF-1 and GH [28, 29]. GH is well recognized to in turn stimulate IGF-1 production by the liver. A recent mechanistic study using human keratinocytes by Kim et al. has indicated that mechanical vibration (45 Hz, 0.8 g) results in F-actin cytoskeletal reorganization despite having no significant effect on cell viability, cell proliferation or cell migration. This action is mediated via activating extracellular signal-regulated kinases (ERK1/ERK2) and upregulating the gene expression levels of heparin-binding epidermal-growth-factor (EGF)-like growth factor (HB-EGF) and EGF receptor (EGFR) [30].
Angiogenesis is another crucial process for providing blood supply for successful healing during the proliferative phase. However, this study revealed no changes in capillary density and tissue VEGF after WBV treatment. Pufe et al. reported that VEGF was not deficient in chronic pressure ulcers and thus proposed that the delayed healing might be attributed to lack of other pro-angiogenic factors [31]. In contrast to pressure ulcers, diabetic wounds are impacted by defective VEGF production, and WBV was found to enhance diabetic wound angiogenesis and VEGF in the studies by Weinheimer-Haus et al. and Yu et al. [18, 19]. The systemic effect of WBV on angiogenesis also seems to be controversial. Since serum VEGF was increased when employing WBV with 90-min cycling exercise in healthy men [32], while no such increase was observed after 6 weeks of WBV treatment applied during resistance exercise [33].
WBV may also speed up wound repair partly through its ability to increase blood flow, which was not assessed in this study. Evidence has indicated that vibration may induce shear stress on endothelial cells, which activates nitric oxide production, which causes vasodilatation [34]. The authors therefore propose that vasodilating effect of WBV is responsible for improving blood supply to the wound sites, which provides tissue oxygenation that contributes to wound repair.
One of the limitations of this study is that the CON mice were left in their cages and not placed on the platform to control for stress of changing the environment. For wound closure measurements, scab may obscure the wound and makes measurement of actual wound size difficult. Lastly, it was conducted in mice, and therefore, the results obtained cannot be generalized as reflecting the effects of WBV on pressure ulcer healing in humans.
5. Conclusion
In summary, WBV improves the healing of stage II pressure ulcers in mice by reducing wound inflammation and enhancing collagen deposition, but not improving angiogenesis. The information gained from these results may provide the basis for further study of WBV as a treatment modality for patients with pressure ulcers.
Additional investigations and clinical studies in humans are warranted, with the aim of exploring the cellular and molecular mechanisms of changes in wound healing, as well as changes in microcirculation and oxidative stress in pressure ulcers following WBV treatment.
Declarations
Author contribution statement
Nattaya Wano: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sompol Sanguanrungsirikul: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Somboon Keelawat: Analyzed and interpreted the data.
Juraiporn Somboonwong: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Ratchadaphiseksompotch Fund from the Faculty of Medicine, Chulalongkorn University [grant number RA58/056], and Scholarship from the Graduate School, Chulalongkorn University to commemorate 72nd Anniversary of his Majesty King Bhumibol Adulyadej.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Edsberg L.E., Black J.M., Goldberg M., McNichol L., Moore L., Sieggreen M. Revised national pressure ulcer advisory panel pressure injury staging system: revised pressure injury staging system. J. Wound Ostomy Cont. Nurs. 2016;43:585–597. doi: 10.1097/WON.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assis de Brito T.L., Monte-Alto-Costa A., Romana-Souza B. Propranolol impairs the closure of pressure ulcers in mice. Life Sci. 2014;100:138–146. doi: 10.1016/j.lfs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Peirce S.M., Skalak T.C., Rodeheaver G.T. Ischemia-reperfusion injury in chronic pressure ulcer formation: a skin model in the rat. Wound Repair Regen. 2000;8:68–76. doi: 10.1046/j.1524-475x.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 4.Gray R.J., Voegeli D., Bader D.L. Features of lymphatic dysfunction in compressed skin tissues - implications in pressure ulcer aetiology. J. Tissue Viability. 2016;25:26–31. doi: 10.1016/j.jtv.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Gefen A., Weihs D. Cytoskeleton and plasma-membrane damage resulting from exposure to sustained deformations: a review of the mechanobiology of chronic wounds. Med. Eng. Phys. 2016;38:828–833. doi: 10.1016/j.medengphy.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Toita R., Shimizu E., Murata M., Kang J.H. Protective and healing effects of apoptotic mimic-induced M2-like macrophage polarization on pressure ulcers in young and middle-aged mice. J. Contr. Release. 2021;330:705–714. doi: 10.1016/j.jconrel.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.H., Mao T.T., Pan Y.Y., Xie H.H., Zhang H.Y., Xiao J., Jiang L.P. Expression and significance of tumor necrosis factor alpha, matrix metalloproteinase 2 and collagen in skin tissue of pressure ulcer of rats. Zhonghua Shao Shang Za Zhi. 2016;32:160–167. doi: 10.3760/cma.j.issn.1009-2587.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Demarré L., Van Lancker A., Van Hecke A., Verhaeghe S., Grypdonck M., Lemey J., Annemans L., Beeckman D. The cost of prevention and treatment of pressure ulcers: a systematic review. Int. J. Nurs. Stud. 2015;52:1754–1774. doi: 10.1016/j.ijnurstu.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Dincer M., Doger C., Tas S.S., Karakaya D. An analysis of patients in palliative care with pressure injuries. Niger. J. Clin. Pract. 2018;21:484–491. doi: 10.4103/njcp.njcp_51_17. [DOI] [PubMed] [Google Scholar]
- 10.Mohammad A.H., Hamed S.A., Abdelghany A.I. Comparison between two different protocols of negative pressure therapy for healing of chronic ulcers. J. Tissue Viability. 2020;29:37–41. doi: 10.1016/j.jtv.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Ruh A.C., Frigo L., Cavalcanti M.F.X.B., Svidnicki P., Vicari V.N., Lopes-Martins R.A.B., Leal Junior E.C.P., De Isla N., Diomede F., Trubiani O., Favero G.M. Laser photobiomodulation in pressure ulcer healing of human diabetic patients: gene expression analysis of inflammatory biochemical markers. Lasers Med. Sci. 2018;33:1651–1671. doi: 10.1007/s10103-017-2384-6. [DOI] [PubMed] [Google Scholar]
- 12.Cavalcanti M.F.X.B., Maria D.A., De Isla N., Leal Junior E.C.P., Joensen J., Bjordal J.M., Lopes-Martins R.A.B., Diomede F., Trubiani O., Frigo L. Evaluation of the proliferative effects induced by low-level laser therapy in bone marrow stem cell culture. Photomed. Laser Surg. 2015;33:610–616. doi: 10.1089/pho.2014.3864. [DOI] [PubMed] [Google Scholar]
- 13.Ennis W.J., Lee C., Gellada K., Corbiere T.F., Koh T.J. Advanced technologies to improve wound healing: electrical stimulation, vibration therapy, and ultrasound – what is the evidence? Plast. Reconstr. Surg. 2016;138(3 Suppl):94S–104S. doi: 10.1097/PRS.0000000000002680. [DOI] [PubMed] [Google Scholar]
- 14.Stewart J.M., Karman C., Montgomery L.D., McLeod K.J. Plantar vibration improves leg fluid flow in perimenopausal women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R623–629. doi: 10.1152/ajpregu.00513.2004. [DOI] [PubMed] [Google Scholar]
- 15.Lohman E.B., 3rd, Petrofsky J.S., Maloney-Hinds C., Betts-Schwab H., Thorpe D. The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med. Sci. Monit. 2007;13:CR71–76. [PubMed] [Google Scholar]
- 16.Maloney-Hinds C., Petrofsky J.S., Zimmerman G. The effect of 30 Hz vs. 50 Hz passive vibration and duration of vibration on skin blood flow in the arm. Med. Sci. Monit. 2008;14:CR112–116. [PubMed] [Google Scholar]
- 17.Arashi M., Sugama J., Sanada H., Konya C., Okuwa M., Nakagami G., Inoue A., Tabata K. Vibration therapy accelerates healing of Stage I pressure ulcers in older adult patients. Adv. Skin Wound Care. 2010;23:321–327. doi: 10.1097/01.ASW.0000383752.39220.fb. [DOI] [PubMed] [Google Scholar]
- 18.Weinheimer-Haus E.M., Judex S., Ennis W.J., Koh T.J. Low-intensity vibration improves angiogenesis and wound healing in diabetic mice. PloS One. 2014;9 doi: 10.1371/journal.pone.0091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu C.O.L., Leung K.S., Jiang J.L., Wang T.B.Y., Chow S.K.H., Cheung W.H. Low-magnitude high-frequency vibration accelerated the foot wound healing of n5- streptozotocin-induced diabetic rats by enhancing glucose transporter 4 and blood microcirculation. Sci. Rep. 2017;7:11631. doi: 10.1038/s41598-017-11934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza R., Koh T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256–264. doi: 10.1016/j.cyto.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Zhao R., Liang H., Clarke E., Jackson C., Xue M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016;17:2085. doi: 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian L.W., Fourcaudot A.B., Yamane K., You T., Chan R.K., Leung K.P. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen. 2016;24:26–34. doi: 10.1111/wrr.12381. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Miguelez P., Fernandez-Gonzalo R., Collado P.S., Almar M., Martinez-Florez S., de Paz J.A., González-Gallego J., Cuevas M.J. Whole-body vibration improves the anti-inflammatory status in elderly subjects through toll-like receptor 2 and 4 signaling pathways. Mech. Aging Dev. 2015;150:12–19. doi: 10.1016/j.mad.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Gillery P., Leperre A., Maquart F.X., Borel J.P. Insulin-like growth factor-I (IGF-I) stimulates protein synthesis and collagen gene expression in monolayer and lattice cultures of fibroblasts. J. Cell. Physiol. 1992;152:389–396. doi: 10.1002/jcp.1041520221. [DOI] [PubMed] [Google Scholar]
- 26.Haase I., Evans R., Pofahl R., Watt F.M. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1 and EGF-dependent signalling pathway. J. Cell Sci. 2003;116(Pt 15):3227–3238. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- 27.Sari Y., Sanada H., Minematsu T., Nakagami G., Nagase T., Huang L., Noguchi H., Mori T., Yoshimura K., Sugama J. Vibration inhibits deterioration in rat deep-tissue injury through HIF1-MMP axis. Wound Repair Regen. 2015;23:386–393. doi: 10.1111/wrr.12286. [DOI] [PubMed] [Google Scholar]
- 28.Cardinale M., Soiza R.L., Leiper J.B., Gibson A., Primrose W.R. Hormonal responses to a single session of wholebody vibration exercise in older individuals. Br. J. Sports Med. 2010;44:284–288. doi: 10.1136/bjsm.2007.043232. [DOI] [PubMed] [Google Scholar]
- 29.Bosco C., Iacovelli M., Tsarpela O., Cardinale M., Bonifazi M., Tihanyi J., Viru M., De Lorenzo A., Viru A. Hormonal responses to whole-body vibration in men. Eur. J. Appl. Physiol. 2000;81:449–454. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- 30.Kim D., Kwon S. Vibrational stress affects extracellular signal-regulated kinases activation and cytoskeleton structure in human keratinocytes. PloS One. 2020;15 doi: 10.1371/journal.pone.0231174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pufe T., Paulsen F., Petersen W., Mentlein R., Tsokos M. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed in chronic sacral pressure ulcers. J. Pathol. 2003;200:130–136. doi: 10.1002/path.1290. [DOI] [PubMed] [Google Scholar]
- 32.Suhr F., Brixius K., de Marées M., Bölck B., Kleinöder H., Achtzehn S. Effects of short-term vibration and hypoxia during high-intensity cycling exercise on circulating levels of angiogenic regulators in humans. J. Appl. Physiol. (1985) 2007;103:474–483. doi: 10.1152/japplphysiol.01160.2006. [DOI] [PubMed] [Google Scholar]
- 33.Beijer A., Rosenberger A., Bolck B., Suhr F., Rittweger J., Bloch W. Whole-body vibrations do not elevate the angiogenic stimulus when applied during resistance exercise. PloS One. 2013;8 doi: 10.1371/journal.pone.0080143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sriram K., Laughlin J.G., Rangamani P., Tartakovsky D.M. Shear-induced nitric oxide production by endothelial cells. Biophys. J. 2016;111:208–221. doi: 10.1016/j.bpj.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.