Abstract
Objective
To investigate the prevalence of depressive symptoms and its association with clinical and psychological factors in patients with rheumatoid arthritis (RA) in Germany and in Brazil.
Method
A convenience sample of 267 RA patients, 176 from Germany (age 62.4 ± 12.3 years) and 91 from Brazil (age 56.3 ± 12.6 years), was used in this cross-sectional study. The following questionnaires were used: Beck Depression Inventory (BDI), painDETECT test, Perceived Stress Questionnaire, fatigue questionnaire (FACIT), Health Assessment Questionnaire Disability Index (HAQ-DI), and the SF–36 questionnaires (Short-Form 36 Health Survey). Disease activity score (DAS 28-CRP) and visual analogue scale (VAS) for pain were also evaluated. Statistical analysis is based on comparison of means and proportions. Statistical significance for non-normal data was evaluated by non-parametrical tests.
Results
Depressive symptoms were more prevalent in the Brazilian sample (44% vs 22.9%, p = 0.025). Compared to German patients, the Brazilian ones also experienced more pain (current pain status on VAS: 4.67 ± 3.4 vs 3.67 ± 2.31 respectively, p < 0.01), were physically more limited (1.89 ± 1.85 vs 1.01 ± 0.75, p = 0.012), and had higher C-reactive protein levels (7.78 ± 18.3 vs 5.82 ± 10.45, p = 0.028). Despite receiving a more intensive treatment, German patients presented similar disease activity when compared to Brazilian patients (DAS28-CRP: Brazil 3.4 ± 1.5 vs Germany 3.3 ± 1.3, p = 0.307).
Conclusion
Depressive symptoms are frequent in RA patients from different countries and interact with psychological disorders and the experience of pain. They contribute negatively to their well-being suggesting the need for psychoeducational strategies.
|
Key Points • New psychoeducational strategies for RA management.• Higher inflammation marker in rheumatoid arthritis patients is associated with depression.• Medical treatment in RA influences depressive symptoms.• Depressive symptoms are dependent on population group.• High disease activity is related to depression. |
Keywords: Rheumatoid arthritis, Depressive symptoms, Psychoeducational strategies
Background
Rheumatoid arthritis (RA) is a chronic inflammatory disease of unknown aetiology. It leads to pain and swelling and may progress to destruction of peripheral joints. Although a variety of conventional synthetic, biological, and more recently developed, targeted synthetic disease modifying anti-rheumatic drugs (cs, b, tsDMARD) exist, the treatment of the disease is still a challenge, requiring close monitoring for a long follow-up period [1]. The female predominance in younger ages diminishes with rising age and vanishes in people aged 75 years and above [2]. The prevalence of RA differs worldwide, as do the incidence rates, ranging from the median annual incidence of 13.4 cases per 10,000 inhabitants in Brazil to 38 cases per 10,000 inhabitants (range 31 to 45) for North American countries. In North European countries, RA is known with an incidence of 29 cases per 100, 000 inhabitants (range 24 to 36) [3, 4].
RA is associated with psychological disorders, especially depression [5]. About 13–20% of RA patients worldwide have clinically relevant depression compared with 6% in the general population [6, 7]. It was shown that depression in RA patients has a high variability between countries ranging from 2% in Morocco up to 33% in the USA [8]. Mental disorders including depression are the most frequent comorbidities in RA [9]. In clinical practice, depressive symptoms and corresponding disorders are common in RA with a recent meta-analysis reporting an estimated 16.8% of RA patients as having a major depressive disorder [10]. The coexistence of immune-mediated inflammatory diseases with depression has been recognized for a long time [11]. However, until now, it is not clear if inflammation markers can also be used as markers for depression. Also unclear is the influence of medications on RA patients with depressive symptoms. Thus, detecting and addressing depression in RA patients are highly relevant and need to be part of optimal care of these patients [12]. Therefore, we studied the prevalence of depressive symptoms and their associations with parameters of disease activity, severity, and psychological factors in a cohort of RA patients from Germany and Brazil.
Material and methods
Study design and settings
This was a cross-sectional study carried out in two RA outpatient clinics: one from Leipzig, Germany, in 2011 and 2012, and one from Rio de Janeiro, Brazil, in 2013. Data were collected from medical records and from questionnaires applied during the scheduled visit for routine care.
Patients
RA was classified according to the 2010 American College of Rheumatology (ACR)/European League against Rheumatism (EULAR) Classification Criteria for Rheumatoid Arthritis [13]. Patients of both sexes and age ≥ 18 years were included. Exclusion criterion was the presence of a chronic degenerative neurological disease. All patients provided written informed consent. The study was approved by the medical ethics committee of the University of Leipzig.
Variables
Demographic and lifestyle variables
Demographic data were collected during the medical visit and included age, sex, marital status, history of having children, and employment status. Only German patients were asked about their smoking habits.
RA disease activity and psychological evaluation
RA characterization
The current RA disease activity was determined by the disease activity score based on a 28 joint assessment and C-reactive protein level (DAS28-CRP), performed as routine care. Disease duration was verified during the medical visit. The characterization of erosive disease was based on the results of X-ray of hands and feet described in the medical records. The presence of rheumatoid factor or anti-citrullinated protein antibodies was also verified from medical records. ACPA was rarely measured in the Brazilian cohort because of the unavailability of the test. C-reactive protein (CRP) was collected when the required equipment was available during the medical visit. Ongoing RA treatment was assessed through levels of dosage of glucocorticoids, conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs), and biological DMARD (bDMARDs).
The functional disability was assessed by the Health Assessment Questionnaire Disability Index (HAQ-DI) [14]. Pain intensity during the prior week (current pain) was evaluated using a 10 cm visual analogue scale (VAS) anchored by two verbal descriptors “no pain” (score of 0) to “worst possible pain” (score 10). Other parameters were worst pain ever (10 cm VAS) and the average of pain in the last 4 weeks (10 cm VAS).
Depression characterization
Depressive symptoms were evaluated by the Beck Depression Inventory (BDI) [15], with a score of less than 13 being considered as normal, 14–19 points being coded as mild or probable depression, 20–28 points being classified as moderate or definite depression, and more than 28 points being valued as severe depression [16]. Special training sessions on how to work with the Beck Depression Inventory were held by a psychiatrist prior to the study period at participating sites.
Additional questionnaires
Quality of life was evaluated by the Short-Form Health Survey (SF-36) [17]. Additionally, we utilized the painDETECT test [18] for the measurement of neuropathic pain; if the resulting score is higher than 18 points, the classifier for neuropathic pain is coded as ‘positive’ (probability > 90%). Stress was evaluated by the Perceived Stress Questionnaire (PSQ) [19]: more than seventy-five points is an index for stress [20]. Fatigue was estimated with the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-Fatigue Scale) questionnaire [21]. All questionnaires were administered in the patients’ native languages and had been previously validated.
Statistical methods
First, we compared the two populations concerning sociodemographic, clinical, incapacity, and treatment characteristics. We used means and standard deviations for the continuous variables and proportion for the nominal ones. The same tests were used for comparing depression symptoms, pains, stress, fatigue, and physical and mental health components of the SF-36. Kolmogorov-Smirnov test and graphics were used to inspect normal distribution of continuous variables. For non-normal distribution, we used the Mann-Whitney U Test instead of Student t test for statistical significance. In cases of more than two groups, Kruskal-Wallis test was used to compare the mean values. The statistical significance for nominal variables was tested by the Chi-square and the Fisher’s test. p values were two-sided and considered statistically significant if ≤ 0.05. Spearman correlation was estimated for the correlation between psychological markers and depressive symptoms.
The analyses were performed using IBM SPSS Statistics 20 Windows (SPSS Inc., Chicago, Illinois, USA) and Excel Windows (Microsoft® GmbH, Unterschleißheim).
Results
Participants
The demographic characteristics of RA patients are presented in Table 1. Participants in the Brazilian sample were significantly younger than members of the German group and had more female and single patients. Distribution of employment status was similar between the samples.
Table 1.
Characteristics of German und Brazilian RA patients (mean ± SEM or n (%))
| German patients n = 176 | Brazilian patients n = 91 | p | |
|---|---|---|---|
| Age, years | 62.43 ± 12.33 | 56.3 ± 12.62 | < 0.001 |
| Age > 65 years | 87 (49.4%) | 24 (26.3%) | < 0.001 |
| Women | 138 (78.4%) | 84 (92.3%) | < 0.001 |
| Smoking | 16 (12.9%) | No information | |
| Marital status | |||
| Married | 114 (64.8%) | 32 (35.2%) | 0.001 |
| Single | 19 (10.8%) | 32 (35.2%) | 0.001 |
| Divorced | 9 (5.1%) | 13 (14.3%) | 0.001 |
| Widow | 26 (14.8%) | 13 (14.3%) | 0.001 |
| Having children | 140 (79.5%) | 66 (74.2%) | 0.013 |
| Laboral situation | |||
| Employed | 56 (31.8%) | 32 (35.2%) | 1.00 |
| Retired | 116 (65.9%) | 59 (64.8%) | 0.283 |
| Incapacitated | 4 (2.2%) | 0 | |
| RA duration, years | 14.35 ± 10.41 | 15.9 6 ± 10.28 | 0.234 |
| Positive rheumatoid factor | 104/148 (70.3%) | 18/31 (60.0%) | 0.042 |
| Positive ACPA | 91/125 (72.8%) | 5/29 (17.2%) | 0.000 |
| Erosive disease | 70 (51.1%) | 40 (43.9%) | 0.011 |
| DAS28-CRP | 3.30 ± 1.35 | 3.42 ± 1.51 | 0.307 |
| CRP, mg/L | 5.82 ± 10.45 | 7.78 ± 18.3 | 0.028 |
| HAQ-DI | 1.01 ± 0.75 | 1.89 ± 1.85 | 0.012 |
| HAQ-DI > 0.5 | 65.5% | 64.1% | 0.908 |
| Treatment | |||
| Glucocorticoid (Gc), low dose | 10 (5.7%) | 2 (3.4%) | < 0.001 |
| csDMARD ± Gc | 68 (48.2%) | 44 (74.6%) | 0.003 |
| bDMARD + csDMARD + Gc | 33 (23.4%) | 5 (8.5%) | 1.00 |
| bDMARD + csDMARD | 5 (3.5%) | 4 (6.8%) | 1.00 |
| bDMARD ± Gc | 19 (13.5%) | 3 (5.6%) | 1.00 |
bDMARD biological disease modifying anti-rheumatic drug; CRP C-reactive protein; csDMARD conventional synthetic DMARD; DAS disease activity score; HAQ-DI Health Assessment Questionnaire Disability Index; SEM standard error of the mean
Most critically, there is no statistically significant difference between German and Brazilian RA patients with respect to mean disease duration, disease activity (DAS28-CRP), and percentage of patients with erosive disease (Table 1). In contrast, the mean of CRP level was 1.76 mg/L higher in Brazilian patients (p = 0.028) who had also a 0.88-point higher scores of disability score (HAQ-DI) compared to German patients (p = 0.012).
Concerning anti-rheumatic therapy, more German patients received low doses of glucocorticoid (5.5% vs 3.4%), while csDMARD (with or without glucocorticoid) were more prescribed to Brazilian patients (74.6% vs 48.2%). More patients in Germany (23.4%, compared to Brazil’s 8.5%) receive a combination therapy (biologics, csDMARD, and low dose prednisolone), but this difference is not statistically significant (p > 0.05) (Table 1).
Main results
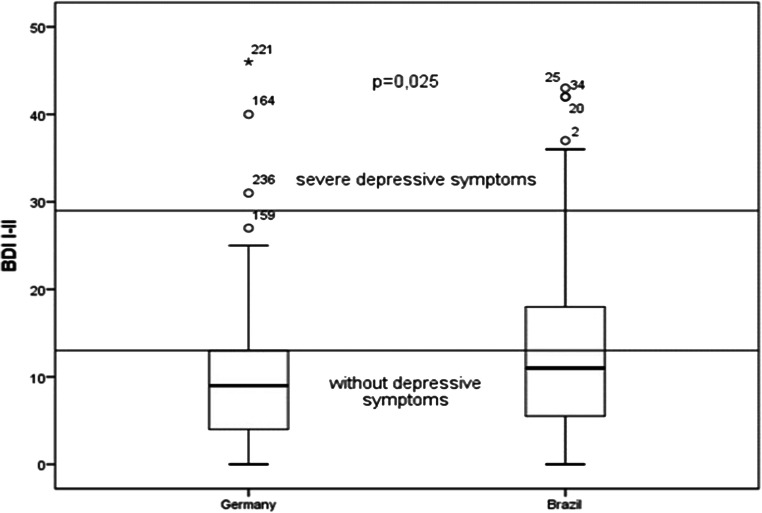
BDI average scores are higher among Brazilian patients (13.0 versus 9.82; = 0.025 (Table 2 and Fig. 1). The percentage of RA patients with depressive symptoms (BDI score ≥ 13 points) in Brazil is almost twice as high as in Germany (44% vs 24.3%; p = 0.001) (Table 2). Although there were differences in the severity of depressive symptoms between the two groups, these differences did not reach statistical significance, probably due to sample size.
Table 2.
Psychological characteristics of German and Brazilian RA patients
| Parameters | German Patients n = 176 | Brazilian Patients n = 91 | p |
|---|---|---|---|
| BDI, mean ± SEM* | 9.82 ± 7.3 | 13.0 ± 10.2 | 0.025** |
| BDI score, n (%)* | |||
| ≤ 13 (normal) | 131 (75.7%) | 51 (56.0%) | 0.001 |
| 14–19 (mild or probable depression) | 25 (14.5%) | 21 (23.1%) | 0.40*** |
| 20–28 (moderate depression) | 14 (8.1%) | 12 (13.2%) | |
| ≥ 29 (severe depression) | 3 (1.7%) | 7 (7.7%) | |
| painDETECT, mean ± SEM | 18.3 ± 6.29 | 20.54 ± 10.0 | 0.002** |
| Neuropathic pain (painDETECT score > 18), n (%) | 85 (48.3) | 53 (57.6) | 0.001 |
| VAS for current pain | 3.67 ± 2.3 | 4.67 ± 3.4 | 0.016 |
| VAS for worst pain | 5.42 ± 2.67 | 6.56 ± 3.5 | < 0.001 |
| VAS for pain in the last 4 weeks | 4.04 ± 2.04 | 4.59 ± 3.18 | 0.067 |
| Perceived Stress Questionnaire (PSQ) | 55.2 ± 16.5 | 37.10 ± 6.73 | < 0.001** |
| Fatigue (FACIT-F) | 37.8 ± 10.8 | 37.2 ± 10.0 | 0.484 |
| Physical health component summary-SF-36 (PCS) | 32.4 ± 19.43 | 33.7 ± 11.9 | 0.559 |
| Mental health component summary-SF-36 (MCS) | 51.4 ± 11.4 | 46.5 ± 12.2 | 0.002** |
BDI Beck Depression Inventory; SF-36 Short-Form Health Survey; VAS visual analogue scale
*Three missing patients in German sample; **statistical significance was calculated using the Mann-Whitney U test; ***p value for comparing the three levels of severity between countries
Fig. 1.
Difference in depression score between German and Brazilian patients; BDI, Beck Depression Inventory
The frequency of depressive symptoms measured on the BDI score was twice as high in Brazilian women (45%) compared to German women (24%) (p = 0.02).
Brazilian patients scored significantly higher on the pain scales than German patients (Table 2). The VAS score was 1 point higher (p = 0.016) for current pain and 1.14 point higher for worst pain (p < 0.001). There was also a difference of 2.24 points for the painDETECT, being higher in the Brazilian sample (p = 0.002), leading to a prevalence of neuropathic pain (painDETECT score > 18 points) of 57.6% of Brazilian patients against 48.3% in German patients (p = 0.001).
There is no difference between the two groups concerning the prevalence of fatigue symptoms (Table 2). However, Brazilian patients had lower scores for the psychological quality of life (SF-36—mental health component summary (MCS)) compared to German patients (46.6 vs. 51.4; p = 0.002).
German patients who took glucocorticoids had significantly (p = 0.030) more depressive symptoms (83.8%) than the group without depressive symptoms (67.3%).
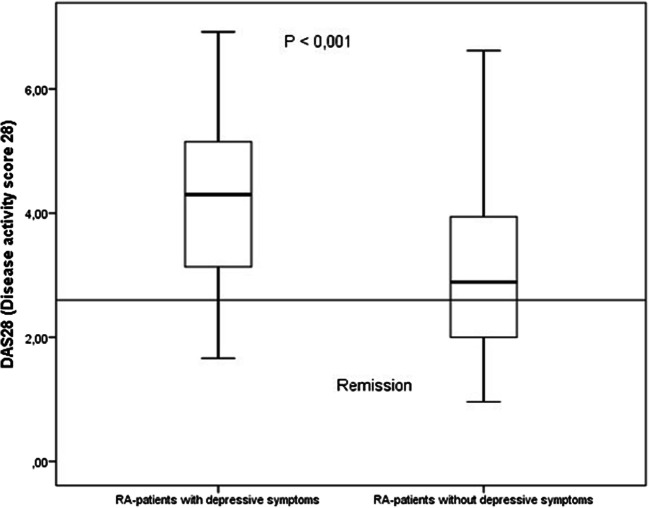
There was a significant correlation (p < 0.001) between depressive symptoms and disease activity in both groups of RA patients (Fig. 2). Interestingly, 12.6% of German patients with depressive symptoms were in clinical remission (DAS28-CRP < 2.3). While 26.8% of German patients with minimal to moderate depressive symptoms had active disease, only 5% without depressive symptoms had disease activity.
Fig. 2.
DAS28-CRP in German RA patients with and without depression
Table 3 depicts the correlation coefficients of BDI with different variables, stratified by country. These correlations were positive for pain variables, HAQ-DI, disease activity, and stress. In the case of SF-36, the correlation was inverse for both dimensions (physical and psychological). In the case of CRP, there was a small positive correlation for German patients, but no correlation (p = 0.553) for the Brazilian ones.
Table 3.
Correlations between BDI and other variables
| German patients | Brazilian patients | |||
|---|---|---|---|---|
| Correlation | p value | Correlation | p value | |
| Pain-DETECT | 0.392 | < 0.001 | 0.520 | < 0.001 |
| Current VAS | 0.473 | < 0.001 | 0.599 | < 0.001 |
| Worst VAS | 0.471 | < 0.001 | 0.598 | < 0.001 |
| 4 Weeks VAS | 0.487 | < 0.001 | 0.498 | < 0.001 |
| HAQ-DI | 0.446 | < 0.001 | 0.552 | < 0.001 |
| DAS28-CRP | 0.404 | < 0.001 | 0.302 | 0.041 |
| PSQ | 0.656 | < 0.001 | 0.621 | < 0.001 |
| MCS–SF-36 | − 0.583 | < 0.001 | − 0.759 | < 0.001 |
| PCS–SF-36 | − 0.651 | < 0.001 | − 0.330 | 0.012 |
| CRP | 0.226 | 0.003 | − 0.107 | 0.553 |
MCS–SF-36 mental health component summary of the Short-Form Health Survey
PCS–SF-36 physical health component summary of the Short-Form Health Survey
Discussion
In RA patients, the prevalence of depressive symptoms is rather high with varying results among different studies. Depending on the criteria used for depression, the prevalence was calculated between 16.8 and 38, 8%. Furthermore, depression was associated with poorer RA outcome [22].
Our study showed a markedly high prevalence of depressive symptoms in RA patients, particularly in the Brazilian cohort (every second patient), indicating a need for screening during routine clinical practice and corresponding follow-up procedures in case of depressive symptoms. Most of our patients showed mild depressive symptoms, while 17.5% of all patients had depressive symptoms so severe that they require special healthcare support in a psychiatric clinic.
Pain symptoms play an important role in the well-being of patients with rheumatic diseases [23]. Our study has demonstrated the association between depressive symptoms and chronic pain in Brazilian as well as in German RA patients [24]. This is consistent with other studies on chronic pain patients, e.g. in Brazil, where depression was observed in 35.2% of patients with chronic pain and was associated with lower quality of life in physical, mental, emotional, and social domains [25].
In our cohorts, RA disease activity was associated with depressive symptoms in both RA populations. Inflammation and trophic factors (brain-derived neurotrophic factor [BDNF], vascular endothelial growth factor, glial cell line-derived neurotrophic factor, and insulin-like growth factor-1) are associated with depression in the general population. One study showed that RA disease activity (DAS 28-CRP) and severity of fatigue were associated with the presence and intensity of depressive symptoms. [26].
In German RA patients, depressive symptoms are more prevalent among prednisolone users (83.8% vs. 67.3%). A previous study reported that RA patients with pain receive more medications despite a low disease activity, which was due to depression [27]. In addition to this stands the fact that glucocorticoids can lead to depression. Here, the hypothalamic-pituitary-adrenal axis plays a major role since 80% of patients with depressive symptoms suffer from hyperactivity of the HPA axis with a higher production of glucocorticoids [28, 29].
On the other hand, depression has a significant influence upon achieving remission in RA patients. Data from the British Society for Rheumatology Biologics Register showed that the presence of depression symptoms at biologic treatment initiation was associated with 20–40% reduced odds of achieving a good treatment response within 1 year. Experiencing symptoms of depression at the start of biologic treatment may reduce the odds of achieving a good treatment response and reduce the probability of disease control over time. Patients with a history of depression or reporting symptoms of depression according to the EuroQol five-dimension scale showed reduced improvement in tender and swollen joints, patient global assessment, and erythrocyte sedimentation rate (ESR). Therefore, depression should be managed as part of routine clinical care to optimize treatment outcomes in RA [30].
In turn, however, adequate anti-rheumatic therapy might help to tackle depressive symptoms in RA patients [31]. In this respect, the difference in the therapeutic setting between Brazilian and German RA patients might partly explain the differences observed in the percentage of RA patients with depressive symptoms. Of interest, the prevalence for depression in the general population is higher in Brazil than in Germany. Ours well as other studies could demonstrate that in Brazil the percentage of patients receiving a biologic therapy is lower compared to the German cohort [32]. It is true that it represents a considerable economic burden to increase the number of RA patients receiving therapy with biologics in Latin America. However, it has been calculated that the expanded use of biologic agents will result in cumulative cost net savings due to reduced indirect costs of RA [33]. Still, the compliance rate of an anti-TNF therapy is rather low in Brazil, i.e. after 1 year, 48.2% of RA patients continued using anti-TNF (± csDMARD) therapy, and at the end of the second year, only 23.1% of anti-TNF (± csDMARD) users were still on the medication [34]. There exists a difference in the healthcare systems, which might contribute to the observed differences. Due to different levels of access to public healthcare systems and greater economic inequality in Brazil, access to expensive drugs is limited. In Germany, the time between onset of symptoms and presentation to a rheumatologist could be reduced to 9 months [35, 36]. In contrast, recent studies in Brazil demonstrated a huge variation of the interval between onset of symptoms and diagnosis of a rheumatic disease, ranging in the REAL study from one to 457 months (median 12 months) and a mean of 28 months in a study from the country’s south [32, 37]. Both studies show that almost 80% of the RA patients in Brazil were of middle-low or low socioeconomic status, and the delay in diagnosing RA was associated with lower socioeconomic status and lower education level of the patients [38]. The sociodemographic comparison between our patient populations revealed differences for age and marital status of the patients, both potential influences for depressive symptoms in RA patients. In a population of older Brazilian adults, lower emotional support and depressive symptoms have been independently predictive for subsequent disability over a long term [39]. In addition, survival of married patients was longer due to a better life balance and a healthier lifestyle compared to single patients. In RA patients, social and emotional support is an important factor to reduce pain and depression [40].
Limitations
Our study is an observational study with a few limitations. It was not possible to match the cohorts for socioeconomic factors. We used statistical tests and variables that were not adjusted for age, sex, or demographic status due to the relatively low number of RA patients. Furthermore, we could not evaluate the role of fibromyalgia as a differential diagnosis in our study. However, it might be difficult to differentiate between chronic pain, depressive symptoms, and fibromyalgia which have to be investigated in upcoming studies.
Conclusion
Our data corroborate the idea that there are significant differences of psychological factors and prevalence of depressive symptoms between various countries. Our study indicates that RA-related depressive symptoms contribute to diminished psychological well-being in RA patients and points to the need for psychoeducational strategies that specifically target depression as part of an overall RA management program [41]. In this respect, our significant results should be confirmed by further studies.
Acknowledgements
Thank you for the special training by Dr. Med. Daniela Morf (psychiatrist) to work with the Beck Depression Inventory in the respective participating site.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials
Compliance with ethical standards
Conflict of interest
ABVS has received speaking fees from Abbvie; the remaining authors have no conflict of interest, financial, or otherwise.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (N 4431/2011, at 12.04.2011).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Code availability
IBM SPSS Statistics 20 Windows, Excel Windows, Open Office.org.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Harriet Morf, Email: harriet.morf@uk-erlangen.de.
Geraldo da Rocha Castelar-Pinheiro, Email: castelar@uerj.br.
Ana Beatriz Vargas-Santos, Email: anabvargas@gmail.com.
Christoph Baerwald, Email: christoph.baerwald@medizin.uniklinik-leipzig.de.
Olga Seifert, Email: olga.seifert@medizin.uniklinik-leipzig.de.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Krasselt M, Baerwald C. Sex, symptom severity, and quality of life in rheumatology. Clin Rev Allergy Immunol. 2017;9:1–16. doi: 10.1007/s12016-017-8631-6. [DOI] [PubMed] [Google Scholar]
- 3.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182–188. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.David JM, Mattei RA, Mauad JL, de Almeida LG, Nogueira MA, Menolli PV, Menolli RA. Clinical and laboratory features of patients with rheumatoid arthritis diagnosed at rheumatology services in the Brazilian municipality of Cascavel, PR, Brazil. Rev Bras Reumatol. 2013;53(1):57–65. doi: 10.1016/s2255-5021(13)70006-3. [DOI] [PubMed] [Google Scholar]
- 5.Englbrecht M, Alten R, Aringer M, Baerwald CG, Burkhardt H, Eby N, Flacke JP, Fliedner G, Henkemeier U, Hofmann MW, Kleinert S, Kneitz C, Krüger K, Pohl C, Schett G, Schmalzing M, Tausche AK, Tony HP, Wendler J. New insights into the prevalence of depressive symptoms and depression in rheumatoid arthritis - implications from the prospective multicenter VADERA II study. PLoS One. 2019;14(5):e0217412. doi: 10.1371/journal.pone.0217412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheehy C, Murphy E, Barry M. Depression in rheumatoid arthritis- underscoring the problem. Rheumatology (Oxford) 2006;45(11):1325–1327. doi: 10.1093/rheumatology/kel231. [DOI] [PubMed] [Google Scholar]
- 7.Mella LF, Bértolo MB, Dalgalarrondo P. Depressive symptoms in rheumatoid arthritis. Rev Bras Psiquiatr. 2010;32(3):257–263. doi: 10.1590/S1516-44462010005000021. [DOI] [PubMed] [Google Scholar]
- 8.Covic T, Cumming SR, Pallant JF, Manolios N, Emery P, Conaghan PG, Tennant A. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the depression, anxiety and stress scale (DASS) and the hospital, anxiety and depression scale (HADS) BMC Psychiatry. 2012;12:6. doi: 10.1186/1471-244X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, Casado G, Detert J, el-zorkany B, Emery P, Hajjaj-Hassouni N, Harigai M, Luo S-F, Kurucz R, Maciel G, Mola EM, Montecucco CM, McInnes I, Radner H, Smolen JS, Song Y-W, Vonkeman HE, Winthrop K, Kay J. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA) Ann Rheum Dis. 2014;73:62–68. doi: 10.1136/annrheumdis-2013-204223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overman CL, Jurgens MS, Bossema ER, Jacobs JW, Bijlsma JW, Geenen R. Change of psychological distress and physical disability in patients with rheumatoid arthritis over the last two decades. Arthritis Care Res (Hoboken). 2014;66(5):671–678. doi: 10.1002/acr.22211. [DOI] [PubMed] [Google Scholar]
- 11.Nerurkar L, Siebert S, McInnes IB, Cavanagh J (2018) Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiat 6(2):164–173 [DOI] [PubMed]
- 12.Gåfvels C, Hägerström M, Nordmark B, Wändell PE. Psychosocial problems among newly diagnosed rheumatoid arthritis patients. Clin Rheumatol. 2012;31(3):521–529. doi: 10.1007/s10067-011-1894-z. [DOI] [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology /European league against rheumatism collaborative initiative. Arthritis Rheumatol. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 14.Bruce B, Fries JF. The health assessment questionnaire (HAQ) Clin Exp Rheumatol. 2005;23:S14–S18. [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-2. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 16.Krug HE, Woods SR, Mahowald ML. The importance of identifying depression in patients with rheumatoid arthritis: evaluation of the beck depression inventory. J Clin Rheumatol. 1997;3(5):248–257. doi: 10.1097/00124743-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 17.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 19.Faro A. Analise Fatorial Confirmatoria das Tres Versoes da perceived stress scale (PSS): um Estudo Populacional. Psychol/Psicol Reflexao Crit. 2015;28(1):21–30. doi: 10.1590/1678-7153.201528103. [DOI] [Google Scholar]
- 20.Spielberger CD. Preliminary manual for the state-trait personality inventory. Tampa: University of South Florida; 1996. [Google Scholar]
- 21.Lai JS, Cella D, Chang CH, Bode RK, Heinemann AW. Item banking to improve, shorten and computerized self-reported fatigue: an illustration of steps to create a core item bank from the FACIT-fatigue scale. Qual Life Res. 2003;12(5):485–501. doi: 10.1023/A:1025014509626. [DOI] [PubMed] [Google Scholar]
- 22.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2013;52(12):2136–2148. doi: 10.1093/rheumatology/ket169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips K, J Clauw D (2013) Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum 65(2): 291–302 [DOI] [PMC free article] [PubMed]
- 24.Pollard LC, Choy EH, Gonzalez J, Khoshaba B, Scott DL. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford) 2006;45(7):885–889. doi: 10.1093/rheumatology/kel021. [DOI] [PubMed] [Google Scholar]
- 25.Morete MC, Solano JPC, Boff MS, Filho WJ, Ashmawi HA. Resilience, depression, and quality of life in elderly individuals with chronic pain followed up in an outpatient clinic in the city of São Paulo, Brazil. J Pain Res. 2018;11:2561–2566. doi: 10.2147/JPR.S166625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheon YH, Lee SG, Kim M, Kim HO, Sun Suh Y, Park KS, Kim RB, Yang HS, Kim JM, Son CN, Kyoung Park E, Kim SH, Lee SI. The association of disease activity, pro-inflammatory cytokines, and neurotrophic factors with depression in patients with rheumatoid arthritis. Brain Behav Immun. 2018;73:274–281. doi: 10.1016/j.bbi.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Rathbun AM, Reed GW, Harrold LR. The temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence and response: a systematic review. Rheumatol (Oxf, Engl) 2013;52(10):1785–1794. doi: 10.1093/rheumatology/kes356. [DOI] [PubMed] [Google Scholar]
- 28.De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. In: nature reviews. Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 29.Kajiyama Y, Iijima Y, Chiba S, Furuta M, Ninomiya M, Izumi A, et al. Prednisolone causes anxiety- and depression-like behaviors and altered expression of apoptotic genes in mice hippocampus. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34(1):159–165. doi: 10.1016/j.pnpbp.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Matcham F, Davies R, Hotopf M, Hyrich KL, Norton S, Steer S, Galloway J. The relationship between depression and biologic treatment response in rheumatoid arthritis: an analysis of the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2018;57(5):835–843. doi: 10.1093/rheumatology/kex528. [DOI] [PubMed] [Google Scholar]
- 31.Miwa Y, Ikari Y, Hosonuma M, Hatano M, Hayashi T, Kasama T, Sanada K. A study on characteristics of rheumatoid arthritis patients achieving remission in depression with 6 months of bDMARDs treatment. Eur J Rheumatol. 2018;5(2):111–114. doi: 10.5152/eurjrheum.2018.17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Rocha Castelar-Pinheiro G, Vargas-Santos AB, de Albuquerque CP, Bértolo MB, Júnior PL, Giorgi RDN, Radominski SC, Resende Guimarães MFB, Bonfiglioli KR, Sauma MFLDC, Pereira IA, Brenol CV, Coutinho ESF, da Mota LMH. The REAL study: a nationwide prospective study of rheumatoid arthritis in Brazil. Adv Rheumatol. 2018;58(1):9. doi: 10.1186/s42358-018-0017-9. [DOI] [PubMed] [Google Scholar]
- 33.Tundia N, Kotze PG, Rojas Serrano J, Mendes de Abreu M, Skup M, Macaulay D, Signorovitch J, Chaves L, Chao J, Bao Y. Economic impact of expanded use of biologic therapy for the treatment of rheumatoid arthritis and Crohn's disease in Argentina, Brazil, Colombia, and Mexico. J Med Econ. 2016;19(12):1187–1199. doi: 10.1080/13696998.2016.1209508. [DOI] [PubMed] [Google Scholar]
- 34.Acurcio FA, Machado MA, Moura CS, Ferre F, Guerra AA, Jr, Andrade EI, Cherchiglia ML, Rahme E. Medication persistence of disease-modifying antirheumatic drugs and anti-tumor necrosis factor agents in a cohort of patients with rheumatoid arthritis in Brazil. Arthritis Care Res (Hoboken) 2016;68(10):1489–1496. doi: 10.1002/acr.22840. [DOI] [PubMed] [Google Scholar]
- 35.Lorenz HM, Wendler J, Krause A. Improvement of prognosis by timely treatment: requirement: initial presentation within 6 weeks. Z Rheumatol. 2019;78:396–403. doi: 10.1007/s00393-019-0607-x. [DOI] [PubMed] [Google Scholar]
- 36.da Mota LM, Brenol CV, Palominos P, Pinheiro GR. Rheumatoid arthritis in Latin America: the importance of an early diagnosis. Clin Rheumatol. 2015;34(Suppl 1):S29–S44. doi: 10.1007/s10067-015-3015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomes RKS, de Linhares AC, Lersch LS. Prevalence and factors associated with diagnosis of early rheumatoid arthritis in the south of Brazil. Adv Rheumatol. 2018;58(1):35. doi: 10.1186/s42358-018-0034-8. [DOI] [PubMed] [Google Scholar]
- 38.Das Chagas Medeiros MM, Ferraz MB, Quaresma MR. The effect of rheumatoid arthritis on the quality of life of primary caregivers. J Rheumatol. 2000;27(1):76–83. [PubMed] [Google Scholar]
- 39.Torres JL, Castro-Costa E, Mambrini JVM, Peixoto SWV, Diniz BSO, Oliveira C, Lima-Costa MF. Depressive symptoms, emotional support and activities of daily living disability onset: 15-year follow-up of the Bambuí (Brazil) cohort study of aging. Cad Saude Publ. 2018;34(7):e00141917. doi: 10.1590/0102-311x00141917. [DOI] [PubMed] [Google Scholar]
- 40.Reese JB, Somers TJ, Keefe FJ, Mosley-Williams A, Lumley MA. Pain and functioning of rheumatoid arthritis patients based on marital status: is a distressed marriage preferable to no marriage? J Pain. 2010;11(10):958–964. doi: 10.1016/j.jpain.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albano MG, Giraudet-Le Quintrec JS, Crozet C, d'Ivernois JF. Characteristics and development of therapeutic patient education in rheumatoid arthritis: analysis of the 2003-2008 literature. Joint Bone Spine. 2010;77(5):405–410. doi: 10.1016/j.jbspin.2010.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials