Abstract
Main conclusion
Transgenic Arabidopsis thaliana and Populus alba plants overexpressing the zinc transporter ScZRC1 in shoots exhibit Zn tolerance. Increased Zn concentrations were observed in shoots of P. alba, a species suitable for phytoremediation.
Abstract
Genetic engineering of plants for phytoremediation is worth to consider if genes leading to heavy metal accumulation and tolerance are expressed in high biomass producing plants. The Saccharomyces cerevisiae ZRC1 gene encodes a zinc transporter which is primarily involved in the uptake of Zn into the vacuole. The ZRC1 gene was expressed in the model species A. thaliana and P. alba (cv. Villafranca). Both species were transformed with constructs carrying ScZRC1 under the control of either the CaMV35S promoter for constitutive expression or the active promoter region of the tobacco Rubisco small subunit (pRbcS) to limit the expression to the above-ground tissues. In hydroponic cultures, A. thaliana and poplar ScZRC1-expressing plants accumulated more Zn in vegetative tissues and were more tolerant than untransformed plants. No differences were found between plants carrying the CaMV35::ScZRC1 or pRbcS::ScZRC1 constructs. The higher Zn accumulation in transgenic plants was accompanied by an increased superoxide dismutase (SOD) activity, indicating the activation of defense mechanisms to prevent cellular damage. In the presence of cadmium in addition to Zn, plants did not show symptoms of metal toxicity, neither in hydroponic cultures nor in soil. Zn accumulation increased in shoots, while no differences were observed for Cd accumulation, in comparison to control plants. These data suggest that ectopic expression of ScZRC1 can increase the potential of poplar for the remediation of Zn-polluted soils, although further tests are required to assay its application in remediating multimetal polluted soils.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00425-021-03634-z.
Keywords: Heavy metals, Metal accumulation in plants, Phytoremediation, Populus alba, ZRC1 transporter
Introduction
Environmental heavy metal pollution is a worldwide issue that arises mainly from industrial activities, mining and agricultural practices (Nriagu and Pacyna 1988). The heavy metal pollution can cause a significant decrease in plant yield and can be dangerous for all living organisms. Plants are likely the entry point of heavy metals into the food chain, with subsequent concerns for animal and human health (Peralta-Videa et al. 2009). Environmental research aims to both, avoid pollution and remediate already contaminated lands, with the least possible impact on the ecosystem. However, biological remediation of metal-polluted soils can prove challenging, since contrary to organic compounds, biodegradation of heavy metals is impossible and hence they continuously accumulate in the environment (Shim et al. 2013; He et al. 2015a, b). To this respect, plants can be exploited to help detoxifying polluted soils, due to their ability in the uptake and root-to-shoot transport of heavy metals. This approach is known as phytoremediation (Salt et al. 1998; McGrath and Zhao 2003). It has attracted attention during the last decades as a non-invasive and cost-effective strategy, alternative or complementary to currently adopted chemical and physical approaches (Pilon-Smits 2005; Zhao et al. 2009). Phytoremediation encompasses two main processes: (i) pollutant containment and stabilization, when the vegetation covers the polluted areas, preventing migration to ground waters and nearby areas, thus reducing the inlet of toxic metals into the food chain (Raskin and Ensley 2000) and (ii) extraction in which the plant species accumulate pollutants in harvestable tissues. The latter has the advantage that the heavy metal is permanently removed, and in some cases, can be recovered from the plant tissues (Elekes 2014). Ideally, at the end of the process the concentration of heavy metals in the shoot biomass should be higher than that present in the soil.
Zinc (Zn) is an essential nutrient for all organisms and is implicated in many biological processes. Among prokarya and eukarya, between 4 and 10% of the genomes encodes Zn proteins (Andreini et al. 2006). This metal is required in the catalytic processes of several metalloenzymes and is a highly effective structural cofactor of many proteins (Krämer and Clemens 2005; Maret 2009). However, despite its essential role, Zn can be toxic when present in excess. Therefore, the intracellular Zn concentration has to be tightly controlled to avoid toxicity and competition with other metal cations that would result in impaired uptake and inhibition of the function of non-Zn proteins by occupying binding sites (Sinclair and Krämer 2012). Zn is usually abundant in the mineral component of soils. Its levels have increased through human activities such as mining and smelting, and agricultural practices such as the intensive use of phosphate-based fertilizers or the application of sewage sludge (Chaney 1993). Organisms have evolved mechanisms of Zn homeostasis to ensure, to some extent, adequate intracellular levels in response to environmental changes in Zn content; however, Zn excess can prove very detrimental for their metabolism and physiology. In plants, toxicity symptoms include reduced yield and stunted growth, although crops differ markedly in their susceptibility to Zn toxicity (Broadley et al. 2007). To approach this problem, the application of phytoremediation to Zn-polluted soils can prove beneficial. Metal hyperaccumulators such as Arabidopsis halleri and Noccaea caerulescens have the capacity to remove Zn from soil, but their small biomass makes them unsuitable for phytoremediation purposes. The ideal species should grow fast, have a deep and extensive root apparatus along with a consistent development of biomass and, not least, it must tolerate heavy metal pollution (Halimaa et al. 2014).
Poplars (Populus spp.) are fast growing trees, characterized by deep root system and large aerial biomass, which can be obtained within few growing seasons in the temperate zone (Yadav et al. 2010; Bredemeier et al. 2015). They are easily propagated, have the aptitude to be coppiced (Rockwood et al. 2004), and have well-established industrial uses such as bioenergy, pulp and paper production, as well as ecological applications in the form of windbreaks and shelterbelts (da Ros and Mansfields 2020). Most importantly, as poplars are not a source of food, the risk of heavy metals entering the food chain is low. Populus spp. have been tested for their heavy metal tolerance and accumulation. It was demonstrated that intra-population genetic variability could affect heavy metal accumulation: clones tolerant to copper (Cu) and Zn showed very different content of Cd, lead (Pb), Zn and iron (Fe), and different ability of metal translocation (Baldantoni et al. 2014). In trials applying nutrient films with a mix of trace elements, a screen of 21 clones of poplar revealed variability between clones in element accumulation and underlined the effect of pH and multi-elemental pollution on the growth of the above-ground biomass (Migeon et al. 2012). Cu, Cd, Pb and Zn remediation by Populus inoculated with arbuscular mycorrhizae was also demonstrated (Bissonnette et al. 2010). Many other works have compared the response of poplar clones in hydroponic medium for selecting the best genotype for phytoremediation (Vassilev et al. 2005; Dos Santos Utmazian et al. 2007; Zacchini et al. 2009). In addition, the genome sequencing of Populus trichocarpa (Tuskan et al. 2006), and recently whole-genome resequencing of more than 500 individuals of the same species, revealed a high degree of adaptive traits across a wide range of latitude (Evans et al. 2014). All these features make Populus spp. an appropriate genus to be considered in phytoremediation approaches. Moreover, the established genetic transformation protocols (Balestrazzi et al. 2000) allow to improve useful traits such as heavy metal tolerance and accumulation. For instance, the transformation of yellow poplar (Liriodendron tulipifera) with the bacterial merA gene provided a system for mercury (Hg) remediation of polluted sites by the conversion of highly toxic Hg(II) to less toxic elemental Hg(0) (Rugh et al. 1998). Transgenic poplar lines were also designed for the remediation of organic xenobiotics, by overexpressing gamma-glutamylcysteine synthetase for treating chloroacetanilide herbicides (Gullner et al. 2001). More recently, poplar was engineered to improve Cd phytoremediation by introducing the yeast vacuolar Cd-transporter ScYCF1. Plants overexpressing ScYCF1 displayed greater tolerance to Cd and their shoots contained five times more Cd than wild-type plants (Shim et al. 2013). Usually, the excess of heavy metals, both nutrients and toxic ions, is stored in the vacuole (Martinoia et al. 2012), making this compartment a good target to deliver potentially toxic heavy metals. Accumulating metals into the vacuole could increase tolerance and, indirectly, phytoremediation potential (Tong et al. 2004).
The objective of this work was to engineer Arabidopsis thaliana, as model species, and white poplar (Populus alba L. clone Villafranca) with the introduction of the Saccharomyces cerevisiae ZRC1 gene to increase the Zn accumulation potential. This poplar clone, obtained at the Poplar Research Institute, Casale Monferrato (Italy), was registered for commercial use in Italy in 1989; it is used for reforestation in the plains along rivers, showing good biomass production and remarkable resprouting ability after coppicing (Confalonieri et al. 2000). In addition to these features, this clone was selected as the most tolerant and best accumulating in a Zn dose–response study with four commercial clones (Romeo et al. 2014). The ScZRC1 gene encodes a high-affinity vacuolar Zn transporter belonging to the CDF (Cation Diffusion Facilitator) transporter family, primarily involved in the uptake of Zn into the vacuole (MacDiarmid et al. 2003), and, to a lesser extent, Cd and nickel (Ni) (MacDiarmid et al. 2002). The transgenic lines were designed to express ScZRC1 under the control of the CaMV35S constitutive promoter, to have protein expression and hence metal accumulation in the whole plant, or driven by a light-inducible promoter (pRbcS) to direct metal accumulation mainly to the aerial tissues. Results of this work demonstrated that the engineered plants perform better in term of stress tolerance and biomass production even in control conditions and are efficient in the accumulation of Zn, shedding lights on their possible consideration into phytoremediation application.
Materials and methods
Plant materials, metal treatment and growth conditions
White poplar (Populus alba L. clone Villafranca), kindly provided by Dr. G. Nervo (Research Unit for Intensive Wood Production, Casale Monferrato, Alessandria, Italy), was propagated in vitro. Plants of A. thaliana (Col 0) were cultured on a MS (Murashige and Skoog 1962) medium, whereas poplar cuttings were maintained on a WPM medium (Lloyd and McCown 1981) in a growth chamber under 16-h light/8-h dark regime at 22 °C/18 °C (light intensity of 80 to 120 μmol m−2 s−1). For in vitro culturing, seeds of wild type and transformed A. thaliana were surface-sterilized with 70% ethanol for 1 min, and then with 10% sodium hypochlorite containing 0.03% TritonX-100 for 15 min, before being rinsed three times with sterile water. Sterile seeds were sown on solid MS medium supplemented with 30 g L−1 sucrose (with the addition of 100 mg L−1 kanamycin for the selection of transformed lines) and vernalized for 2 days at 4 °C prior to be transferred to the growth chamber. Alternatively, plants were grown in hydroponic culture in Hoagland’s solution (Hoagland and Arnon 1950) in the growth chamber, under controlled conditions. Two-week-old Arabidopsis plants were submitted to the following treatments: (i) control conditions in standard Hoagland’s solution (0.7 µM Zn) and (ii) with the addition of 20 µM ZnSO4. This Zn concentration was chosen as high but below toxicity levels and, therefore, tolerated by the non-tolerant species A. thaliana (Fasani et al. 2017). Two-week-old (roughly 10 cm tall) in vitro propagated poplar plants were transferred for 2 weeks in Hoagland’s solution, and thereafter submitted to metal treatment with 500 µM ZnSO4 (Romeo et al. 2014) or 10 μM CdSO4 and 250 μM ZnSO4 for further 3 weeks. In this case, plant conditions before treatment were adopted as control condition. For the in soil experiment on poplar, plastic pots were filled with 750 g of a mixture (1:1) peat: sand and saturated with Hoagland’s solution (avoiding leakage) with additional CdSO4 and ZnSO4. The obtained artificially polluted soil was characterized by pH 6.7 and a metal contamination corresponding to 10 mg kg−1 CdSO4 and 300 mg kg−1 ZnSO4, consistently with the concentration reported in literature for artificially contaminated soils (Guerra et al. 2011). Two-week-old in vitro propagated poplar plants were transferred into normal non-contaminated soil and acclimated for 2 weeks. Afterwards, plants were transferred to the above-described contaminated pots and grown for further 3 weeks, watered every week with an equal amount of water, avoiding leakage from the pots.
Plasmid DNA constructs and plant transformation
The ScZRC1 full-length coding sequence was amplified from the genomic DNA of S. cerevisiae; the gene-specific primers for the amplification include the restriction sites for the subsequent cloning into the destination vector, XbaI and SacI, at the 5′-end (forward 5′-TCTAGAATGATCACCGGTAAAGAATTGA-3′ and reverse 5′-CTCGAGTTACAGGCAATTGGAAGTATTG-3′). The amplified fragment was subjected to digestion with XbaI and SacI and subsequent ligation into the plant expression vector pMD1 (Sendín et al. 2012), under the control of the CaMV35S promoter. In addition to the constitutive promoter, a construct was produced to limit the expression to the above-ground tissues. In this case, the CaMV35S promoter in the above-mentioned construct was replaced with the 1050 bp-long regulatory region upstream the Rubisco small subunit (RbcS) gene. To obtain this, the Rbcs promoter was amplified from tobacco genomic DNA (Nicotiana tabacum cv. Petit Havana SR1, Uozumi et al. 1994; Cui et al. 2015) by means of specific primers which include the restriction sites HindIII and XbaI at the 5′-end (forward 5′-AAGCTTAAGCTTGTGGGAACGAGATAA-3′ and reverse 5′-TCTAGATGTTAATTACACTTAGACAGAAAG-3′). The amplified fragment was cloned into the pMD1 vector containing the ScZRC1 sequence, by HindIII and XbaI restriction (the two restriction sites flank the CaMV35S sequence in the pMD1 vector) and subsequent ligation, replacing the CaMV35S sequence. The recombinant plasmids were checked by sequencing, transformed into Agrobacterium tumefaciens strain EHA105 and used to transform A. thaliana and white poplar. The former was transformed using the floral dip method (Zhang et al. 2006), whereas poplar transformation was accomplished by co-cultivation for 8–10 min of an A. tumefaciens solution with leaf discs of in vitro plantlets, followed by regeneration as previously described (Fan et al. 2015). Transformation was confirmed in both species by PCR analysis on genomic DNA and evaluation of transgene expression. In all subsequent experiments, wild-type plants were adopted as control in both poplar and A. thaliana.
Genomic DNA isolation, RNA extraction and cDNA synthesis
Genomic DNA was extracted from plant tissues using the Qiagen Genomic DNA Extraction Kit (Qiagen, Hilden, Germany). Total RNA was extracted from fresh tissues with TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA). After DNase treatment, first-strand cDNA was synthesized using SuperScript III Reverse Transcriptase kit (Thermo Fisher Scientific) before real-time reverse transcription polymerase chain reaction (real-time RT-PCR) experiments.
Expression analysis in transgenic A. thaliana and poplar plants
Expression of target genes in the Arabidopsis and poplar transgenic lines was assessed by real-time RT-PCR performed with a StepOne Plus Real-Time PCR System (Applied Biosystems) using KAPA SYBR FAST ABI Prism 2X qPCR Master Mix (Kapa Biosystems, Wilmington, MA, USA). Each reaction (40 amplification cycles) was carried out in triplicate and a melting curve analysis was used to confirm the amplification of specific targets. Specific primers were used to specifically amplify ScZRC1 (forward 5′-AGCTCCGCCAAGCTGATAAG-3′ and reverse 5′-TCTGCGAATATCCTCATTAACA-3′) to determine the expression level of the transgene. As for poplar metal transporters, specific forward and reverse primers were used to specifically amplify PaMTP1 (5′-CTATCCACGAACTGCATATATG-3′ and 5′-CATTGTCTAGCACCATGTCTG-3′), PaNRAMP1.3 (5′-GAAACAAGGAGGCTACACATC-3′ and 5′-TCCTCTGAGGCAACTGCATG-3′), PaPCR2 (5′-TGGTTCATTGCTGCTGTGAGT-3′ and 5′-TACGCTGCGATTCTTCTTCTC-3′) and PaHMA4 (5′-TCAGAGGTTCCTTTGATCGAG-3′ and 5′-CCTTAACAATTTGGAGCTGAGA-3′). Control genes for the analysis were selected as follows (forward and reverse primers are indicated): for Arabidopsis, the actin gene (5′-ATCCCAGTTGCTGACAATTC-3′ and 5′-GACCCGCCATACTGGTGTGAT-3′); for poplar, actin (5′-GAACTACGAGCTACCTGATG-3′ and 5′-CTTCCATTCCGATGAGCGAT-3′) and ubiquitin genes (5′-AAACAGCTTGAAGATGGAAGGA-3′ and 5′-TGTCTGAACTCTCCACCTCC-3′).
Subcellular localization of ScZRC1 in A. thaliana protoplasts
To test the subcellular localization of ScZRC1 in plant, the expression vector pMD1-35S::ScZRC1-eGFP was produced by fusing the coding sequence of ScZRC1 (amplified with the primers 5′-TCTAGAATGATCACCGGTAAAGAATTGA-3′ as forward and 5′-GTCGACCAGGCAATTGG AAGTATTGCA-3′ as reverse primers) in frame to the N-terminus of the reporter eGFP (amplified by PCR using 5′-GTCGACGTGAGCAAGGGCGAGGAGC-3′ as forward and 5′-GAGCTCTTACTTGTACAGCTCGTCCATG-3′ as reverse primers). As a control, a second expression vector pMD1-35S::eGFP was created by introducing the eGFP sequence (amplified by PCR using 5′-TCTAGAATGGTGAGCAAGGGCGAGGAGC-3′ as forward and 5′-GAGCTCTTACTTGTACAGCTCGTCCATG-3′ as reverse primers). A. thaliana protoplasts were then isolated and transfected with the two prepared constructs (pMD1-35S::ScZRC1-eGFP and pMD1-35S::eGFP) by PEG-mediated transfection, as previously described (Yoo et al. 2007). Images were taken with a Leica TCS SP5 confocal microscope using a 488 nm argon laser for the detection of GFP in the range of 496–555 nm, and both 488 nm and 561 nm lasers for the chloroplast auto-fluorescence detection at 651–800 nm.
In-gel superoxide dismutase (SOD) activity assay and nitroblue tetrazolium (NBT) staining
Native soluble proteins were extracted from leaves harvested from poplar plants cultivated in hydroponic culture in control medium and supplemented with 500 µM ZnSO4 grinding fresh tissues in 0.15 M Tris, pH 7.5. Three independent poplar transgenic lines carrying 35S::ScZRC1 or pRbcS::ScZRC1 were analysed. After centrifugation, the supernatant was recovered and the total protein content was estimated using the DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were separated at 4 °C by native 10% poly-acrylamide gel electrophoresis (PAGE) in Tris-glycine native buffer (25 mM Tris, 0.192 M glycine). SOD activity was then determined in-gel as described by Beauchamp and Fridovich (1971). The band intensity was determined by scanning the gels and processing images with Quantity OneR software v4.4.1 (Bio-Rad); intensities were normalized on actual protein loading, as estimated by Coomassie staining of a replica gel. O2− in plants was detected by treating leaves with NBT and visualizing the blue spots according to Rao and Davis (1999).
Quantification of metal content
Analysis of metal content was performed on plants grown in hydroponic culture and soil mesocosmos. Three independent transgenic lines carrying 35S::ScZRC1 or pRbcS::ScZRC1 for both species were analysed. Ten plants for wild type and each transgenic A. thaliana line were considered. Regarding poplar, four wild-type plants and each transgenic line were considered, either (i) grown in hydroponic culture on Hoagland’s solution treated with 500 μM ZnSO4 or 10 μM CdSO4 and 250 μM ZnSO4 or (ii) kept in soil artificially contaminated with 10 mg kg−1 CdSO4 and 300 mg kg−1 ZnSO4. The shoots and roots of A. thaliana plants and leaves, stems and roots of poplars were harvested. Samples were weighted and washed twice with ice-cold bidistilled water; shoots and roots were then oven dried at 60 °C for 72 h and ground to powder. Metal content was measured using inductively coupled plasma mass spectrometry (ICP-MS; EPA 6010C, 2007) after microwave-assisted acid digestion (EPA 3051A, 2007). Replica pots, filled with artificially contaminated soil (with 10 mg kg−1 CdSO4 and 300 mg kg−1 ZnSO4) were considered as non-planted control. At the end of the experiments, when plants were harvested, this control soils was air dried. Metal phytoavailability was determined by quantification by ICP-MS of Zn and Cd in solution upon CaCl2 extraction, which was demonstrated to provide the most useful indications of metal phytoavailability for studied elements (Qasim et al. 2005): 100 mL of 0.01 M CaCl2 were added to 10 g of soil, shaken for 2 h at 20 °C. The liquid to solid ratio of 10 was high enough to avoid sample heterogeneities (Qasim et al. 2005).
Determination of chlorophylls
Fresh leaves were weighed, frozen and ground to a powder in liquid nitrogen, and chlorophylls were extracted with 80% acetone saturated with Na2CO3. Cell debris was removed by centrifugation at 10,000×g, 4 °C for 10 min, and the absorbance was determined at 750, 646.6 and 663.6 nm. The concentration of Chl a and b was determined as previously described (Porra 2002).
Statistical analysis
Data in histograms are represented as mean ± standard deviation. Statistical significance of experimental data was evaluated using GraphPad Prism 7 (GraphPad Software). All analyses on A. thaliana, as well as chlorophyll content and expression analysis on poplar were subjected to a two-way analysis of variance (ANOVA) followed by a post hoc Bonferroni test. The remaining experiments on poplar were evaluated by means of a one-way ANOVA followed by a post hoc Tukey’s test. Statistically significant variations at P < 0.05 are marked with letters, the same letter corresponding to non-statistically significant differences.
Results and discussion
The ZRC1 gene encodes a transporter protein of the CDF family (Paulsen and Saier 1997). In S. cerevisiae, it transports Zn into the vacuole, reducing its concentration in the cytosol and protecting the cell from excess Zn (Miyabe et al. 2001; MacDiarmid et al. 2003). The overproduction of ZRC1 conferred resistance to Zn excess, and the increased expression of ZRC1 is a homeostasis mechanism in Zn stress tolerance (MacDiarmid et al. 2003). In the present study, A. thaliana was transformed with constructs carrying the ScZRC1 coding sequence under the control of the CaMV35S promoter conferring constitutive expression, or under the control of the light-inducible promoter of the tobacco small subunit of Rubisco (pRbcS), to test the expression of ScZRC1 only in the above-ground plant tissues. The promising results regarding Zn accumulation and tolerance in A. thaliana have brought us to investigate the effects of the expression of this transporter in the poplar clone Villafranca, a good candidate for metal phytoextraction due to its fast and consistent biomass production (Romeo et al. 2014).
ScZRC1 localized to the vacuolar membrane in the plant cell
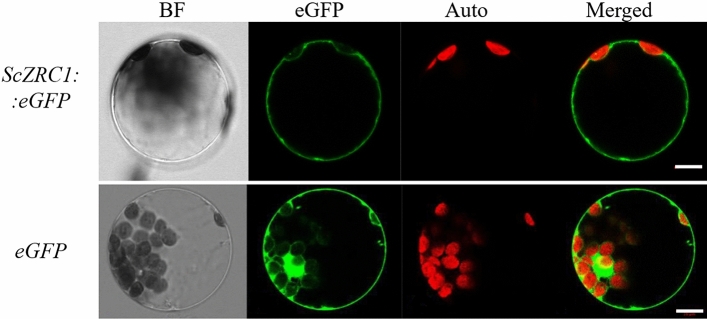
To investigate the localization of the ScZRC1 protein in the plant cell, the construct carrying the eGFP sequence fused to the 3′-terminus of ScZRC1 coding sequence was used for transient expression in A. thaliana protoplasts. Microscopic analysis of GFP fluorescence shows a cytosolic distribution of the eGFP alone, and a vacuolar membrane localization for the fusion protein ScZRC1::eGFP (Fig. 1), reflecting the tonoplast localization of the protein in yeast (Miyabe et al. 2001).
Fig. 1.
Subcellular localization of ScZRC1 in plant. ScZRC1::eGFP fusion protein (upper panel) and eGFP alone (cytoplasmic control, lower panel) were transiently expressed in A. thaliana leaf protoplasts by PEG-mediated transfection, and analyzed using fluorescence microscopy. BF, picture taken upon illumination in bright field; eGFP, fluorescence of the fusion protein ScZRC1::eGFP; Auto, chloroplasts revealed by chlorophyll autofluorescence; Merged, the two signals for eGFP and Auto were merged in the same image. Scale bar = 10 µm
ScZRC1 enhances Zn tolerance and accumulation in A. thaliana
Several A. thaliana p35S::ScZRC1 and pRbcS::ScZRC1 transgenic lines were obtained and the expression pattern of ScZRC1 was assessed by real-time RT-PCR in roots and shoots of plants maintained in hydroponic culture. ScZRC1 expression was confirmed in the whole plants for p35S::ScZRC1 transgenic lines, whereas it was restricted to the shoots for plants harbouring the pRbcS::ScZRC1 (Suppl. Fig. S1). Three independent transgenic lines for each construct were then chosen for further analyses.
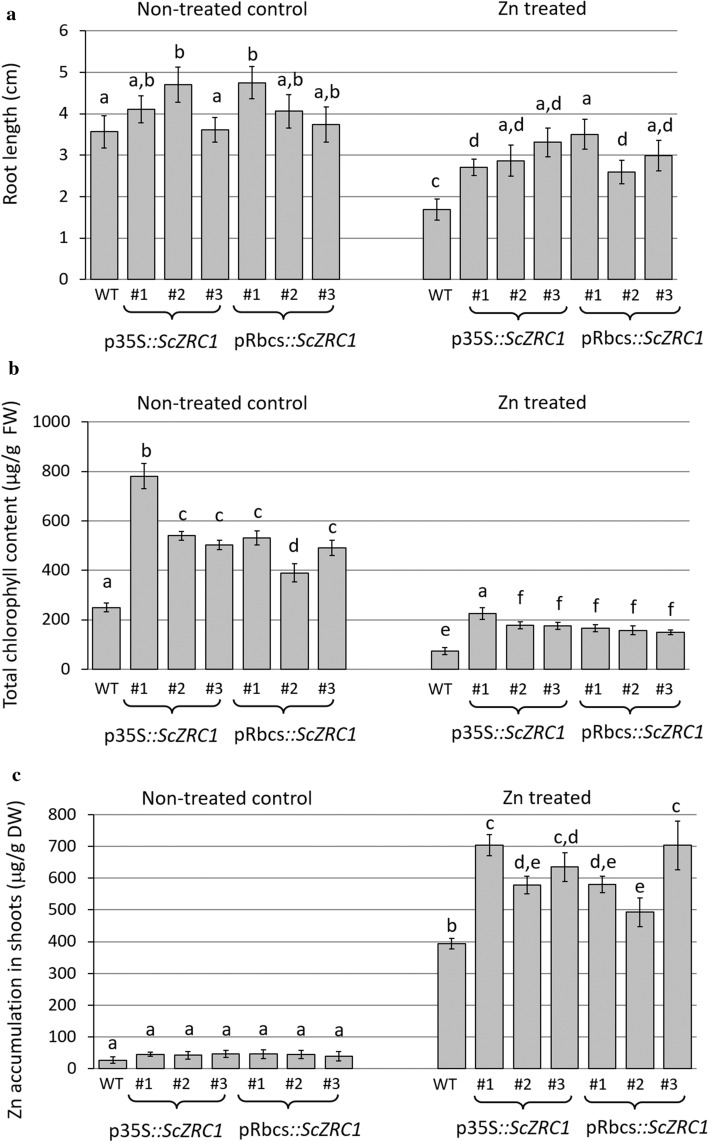
First of all, we evaluated the difference in Zn tolerance of transgenic lines by measuring root length and total chlorophyll content of plants after exposure to 0.7 μM ZnSO4 (control condition) and 20 μM ZnSO4 (excess Zn condition). Differences between wild type and transgenic lines were observed both in the absence and presence of Zn treatment (Fig. 2). In control conditions root length was not significantly different, with exception of one p35S::ScZRC1 line, while upon treatment with excess Zn, root growth in all transgenic plants was significantly less inhibited than in wild-type plants (Fig. 2a). The chlorophyll content in all transgenic lines was significantly higher than in the wild type in both control and Zn treated conditions, even though the Zn treatment induced a marked and comparable decrease in chlorophylls in all genotypes (Fig. 2b). No differences were detected in either parameters between plants carrying p35S::ScZRC1 or pRbcS::ScZRC1 with the exception of line #1 p35S::ScZRC1 and line #2 pRbcS::ScZRC1. The tested Zn tolerance indicators showed that Arabidopsis transgenic lines performed better than wild type even in control condition. In this respect, we speculate that the presence of the ScZRC1 transporter in the cellular tonoplast may contribute to improve/reinforce Zn acquisition and/or distribution. In addition, the effects of the transgene in Zn excess support the hypothesis that ScZRC1 confers enhanced tolerance to Zn excess. Overall, this transporter likely cooperates with other metal transporters, aiding metal homeostasis at least in the conditions tested.
Fig. 2.
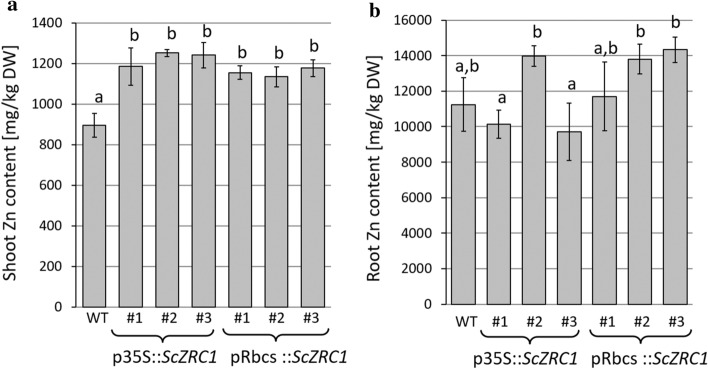
Analysis of Zn tolerance and accumulation in wild type (WT) and ScZRC1-expressing A. thaliana plants. Seeds were germinated on standard MS solid medium and grown in a growth chamber for 2 weeks. Plantlets were transferred in hydroponics for two further weeks, in standard Hoagland’s solution (non-treated control) and in Hoagland’s solution amended with 20 µM ZnSO4 (Zn treated). To estimate Zn tolerance, root length (a) and total chlorophyll content (b) were measured. Zn accumulation was determined in shoots (c). Different letters above the histograms indicate statistical significance, evaluated by two-way ANOVA followed by a post hoc Bonferroni test (P < 0.05, n = 30 for root length and n = 4 pools for chlorophyll content and Zn accumulation)
Next, Zn accumulation was investigated in shoots of control plants and in ScZRC1-expressing transgenic lines. In control conditions, four out of six transgenic lines showed a significant increase of Zn accumulation with respect to wild-type plants. Under Zn excess, significantly more Zn accumulated in shoots of all but one of the Arabidopsis ScZRC1-expressing lines when compared to control plants (Fig. 2c). On the same samples Fe concentration was measured to verify whether the Zn excess could interfere and compete with Fe uptake and transport; no significant differences in Fe content were found in shoots between control plants and lines expressing ScZRC1 (data not shown).
Altogether these results showed that ScZRC1 is localized to the tonoplast and hence its role in Zn tolerance may be exerted by vacuolar sequestration of the metal, analogously to its native role in yeast (Miyabe et al. 2001; MacDiarmid et al. 2003). As other members of the CDF family, such as MTPs in plants (van der Zaal et al. 1999; Arrivault et al. 2006), ScZRC1 enhances Zn accumulation both when constitutively expressed and when under the control of a light-inducible promoter. The significant accumulation of Zn in shoots of transgenic lines indicates that the production of ZRC1 in plants enhanced the long-distance Zn transport. Similar performances for Zn tolerance and accumulation were observed when the expression of ScZRC1 was either under the control of a constitutive promoter or regulated by a light inducible promoter. Since the constitutive expression may adversely affect plant growth or determine abnormal development (Hsieh et al. 2002; Takasaki et al. 2010), the possibility of driving gene expression under the control of an inducible promoter received particular attention.
Highly efficient Zn accumulation in plants and storage capability allow to remove Zn from contaminated soils through a phytoremediation process. Considering this last aspect, we moved from Arabidopsis to Populus, analysing the effect of the expression of ScZRC1 in transgenic poplar (a species which is worldwide recognized for its exploitation for phytoremediation, Dos Santos Utmazian et al. 2007). With this aim, we assessed Zn accumulation and tolerance in the presence of high Zn concentration in transgenic poplar lines harbouring p35S::ScZRC1 and pRbcS::ScZRC1.
ScZRC1 enhances Zn tolerance in P. alba
ScZRC1 transcript abundance was analysed in different lines harbouring the p35S::ScZRC1 or pRbcS::ScZRC1 insert; for each construct, the three lines displaying the highest ScZRC1 expression were selected for further trials. The different expression localization of ScZRC1 determined by the 35S promoter or the light-inducible Rbcs promoter, tested by RT-PCR, is reported in Suppl. Fig. S1. All transgenic plants were similar to wild-type plants in appearance. To test whether ScZRC1 expression in poplar can improve Zn tolerance and accumulation, plants propagated by cutting and maintained in vitro were moved to hydroponic culture on Hoagland’s solution for 2 weeks, and then treated with 500 µM ZnSO4 for further 3 weeks before proceeding to the phenotypic assays.
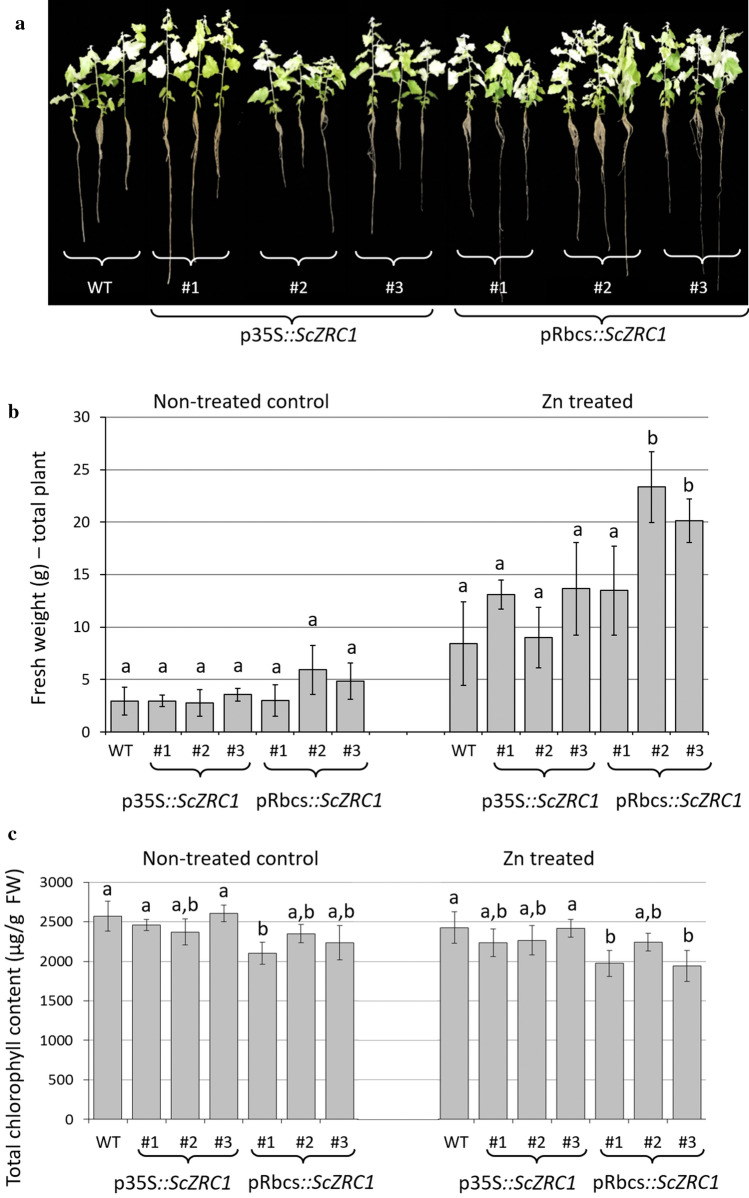
Transgenic plants obtained after transformation with both constructs did not show distinguishable differences in visual appearance with wild-type plants after the treatment with 500 µM ZnSO4 (Fig. 3a). Due to the inaccuracy in measuring root length in micropropagated poplar plants, we decided to consider the plant fresh weight and chlorophyll content as indicators for tolerance. Fresh weight was measured on the same plants before and after the Zn treatment. No differences were observed between control and ScZRC1-expressing lines or between plants carrying the p35S::ScZRC1 and pRbcS::ScZRC1 cassettes for lines #2 and #3 of pRbcs::ScZRC1 which appear to be taller before the treatment and upon Zn treatment (Fig. 3b). In addition, regarding chlorophyll content, similar results were found: the total amount of chlorophyll was unchanged before and after the Zn treatment, with the exception of lines 1 and 3 of pRbcs::ScZRC1, which show a small decrease in chlorophyll concentration when compared to wild type control, but no changes upon Zn treatment (Fig. 3c).
Fig. 3.
Zn tolerance of poplar plants, wild type (WT) and transgenic harboring p35S::ScZRC1 and pRbcs::ScZRC1, grown hydroponically for 2 weeks in Hoagland’s solution and for three further weeks in Hoagland’s solutions supplemented with 500 μM ZnSO4. Plants were evaluated before and after Zn treatment. General phenotype a total fresh weight of the plant b and total chlorophyll content c were measured to determine Zn tolerance. Different letters above the histograms indicate statistical significance, evaluated by one-way ANOVA followed by a post hoc Tukey’s test (P < 0.05, n = 5) for fresh weight analysis and by two-way ANOVA followed by a post hoc Bonferroni test (P < 0.05, n = 5) for chlorophyll content
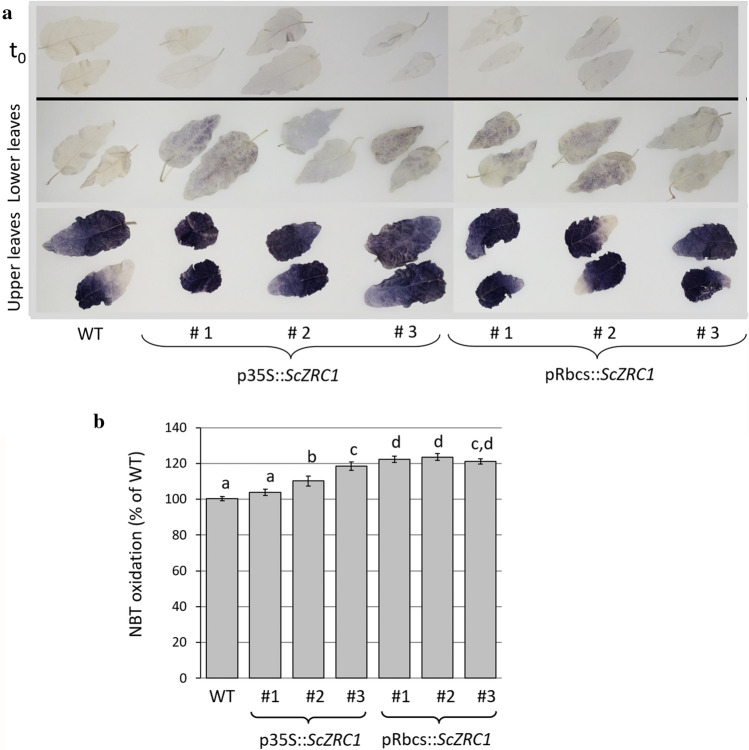
Further analyses were performed to determine the potential increase in reactive oxygen species (ROS) in leaves by nitroblue tetrazolium (NBT) staining which detects the superoxide anion radical (). In all plants maintained in hydroponic culture and treated for 3 weeks with 500 µM ZnSO4, an evident increase in oxidative stress was observed on upper leaves as effect of the light intensity in the growth chamber, whereas a pale colouring indicating a slight superoxide anion accumulation was seen in the lower leaves of all plants expressing ScZRC1 (Fig. 4a). In addition, the sensitivity to ROS on the same plants was measured by detecting in-gel SOD activity, on total protein extracts from leaves. This analysis showed a slight but significant increase of SOD activity in all ScZRC1-expressing lines but one (Fig. 4b). These data indicate that the high Zn accumulation in shoots of transgenic lines stimulates a moderate production of free radicals, imposing oxidative stress and leading to the activation of antioxidant defence mechanisms that help contain the cellular damage, as reported also in other species (Małecka et al. 2019). Therefore, ScZRC1-expressing plants might have acquired a more efficient antioxidant system with increased activity of antioxidant enzymes, which in turn is associated with tolerance to high Zn concentrations.
Fig. 4.
Oxidative stress in poplar plants, wild type (WT) and transgenic harboring p35S::ScZRC1 and pRbcs::ScZRC1, grown hydroponically for 2 weeks in Hoagland’s solution and for three further weeks in Hoagland’s solutions supplemented with 500 μM ZnSO4. a Nitroblue tetrazolium (NBT) analysis on leaves of plants before (t0, upper panel) and after Zn treatment (two lower panels). Zn-treated lower leaves (shaded by the other leaves) and upper leaves (in full light) were collected and analyzed. b In-gel SOD activity, measured on total protein extracts from leaves of Zn-treated plants. SOD activity is expressed as a percentage of NBT oxidation in comparison to the WT value. Results are derived from densitometric analysis of visibly clear bands in an in-gel activity assay. Each measurement was performed in triplicate. Band intensity was normalized on the actual protein loading. Different letters indicate significantly different values, evaluated by one-way ANOVA followed by a post hoc Tukey’s test (P < 0.05, n = 5)
ScZRC1 enhances Zn accumulation in P. alba
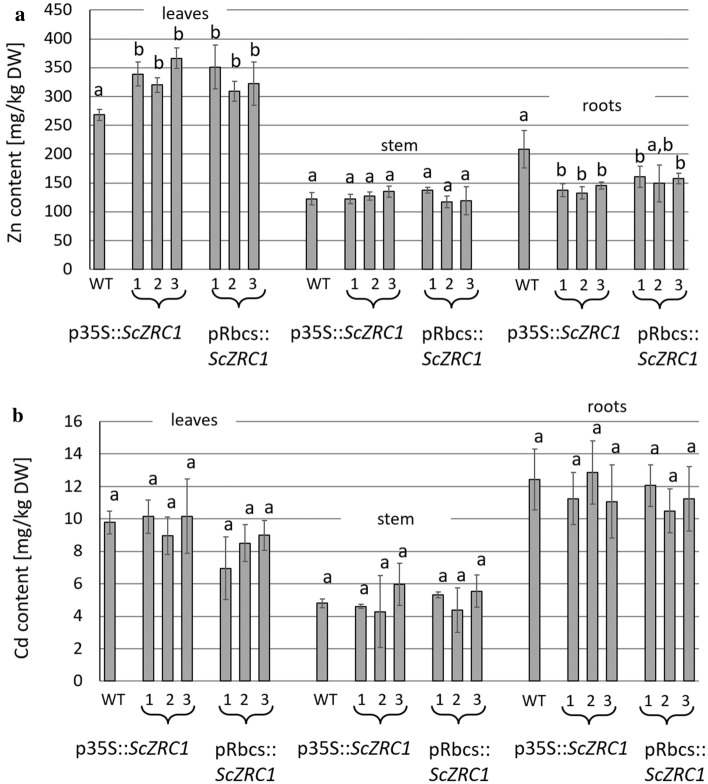
Zn content was analysed in the shoots and roots of plants grown in hydroponic solution. All transgenic poplar lines, regardless of which promoter the ScZRC1 expression was driven by, accumulated significantly higher levels of Zn in their shoot than the untransformed control (Fig. 5a), whereas no clear differences were found in Zn concentration in roots between control plants and ScZRC1-expressing lines (Fig. 5b). It is worth noting that the poplar clone Villafranca, chosen for this study, was the least damaged and showed the highest Zn tolerance and accumulation capacity, among four commercial clones, when grown in modified Hoagland's nutrient solution under Zn excess for 4 weeks (Romeo et al. 2014). Thus, the absence of toxicity symptoms after exposure to 500 µM ZnSO4 for 3 weeks, observed even in wild-type plants matches the intrinsic tolerance of the Villafranca clone to high Zn concentrations. The poplar lines expressing ScZRC1 were able to take up, transport from root to shoot and accumulate more Zn in the aerial part than the wild type and possess the characteristics that increase the potential of poplar for phytoextraction of Zn from polluted soils. Such enhanced Zn accumulation in shoots of transgenic individuals prompted us to test the potential accumulation capacity of these plants in the presence of multimetallic mixtures, as often happens in polluted soils. Therefore, poplar plants were tested both in mesocosmos pots filled with an artificially contaminated soil substrate (10 mg kg−1 CdSO4 and 300 mg kg−1 ZnSO4) and in hydroponic culture (10 μM CdSO4 and 250 μM ZnSO4), considering that these metals are frequently co-pollutants and share several chemical properties (Chaney 2010). Even in the presence of both, Zn and Cd plants did not show visible symptoms of metal toxicity, further supporting the intrinsic metal tolerance of this poplar clone (data not shown). After 3 weeks of growth in the contaminated soil substrate, poplar plants harbouring the ScZRC1 gene accumulated higher amounts of Zn in the shoot, particularly in leaf tissues, while Zn accumulation in roots decreased in transgenic individuals when compared to wild-type controls (Fig. 6a). The Zn translocation factor, calculated as the ratio between metal concentration in shoots and metal concentrations in roots, is increased in plants harboring ScZCR1 (Table 1). These evidences point to an enhanced effectiveness of the root-to-shoot transport of Zn due to the expression of this vacuolar yeast Zn transporter. The enhanced Zn accumulation capacity is also demonstrated by the increased total Zn accumulated by the plant tissues, as reported in Table 2.
Fig. 5.
Analysis of Zn content in shoots a and roots b of poplar plants, wild type (WT) and transgenic harboring p35S::ScZRC1 and pRbcs::ScZRC1, measured after 3 weeks of growth in Hoagland’s solution upon addition of 500 µM ZnSO4. Different letters above the histograms indicate statistical significance, evaluated by one-way ANOVA followed by a post hoc Tukey’s test (P < 0.05, n = 5)
Fig. 6.
Analysis of Zn (a) and Cd (b) in leaves, stems and roots of poplar plants, WT and ScZRC1-expressing plants. Metals were analyzed after 3 weeks of growth in mesocosmos pots filled with soil artificially contaminated with 10 mg kg−1 CdSO4 and 300 mg kg−1 ZnSO4. Different letters above the histograms indicate statistical significance, evaluated by one-way ANOVA followed by a post hoc Tukey’s test (P < 0.05, n = 5)
Table 1.
Zn and Cd translocation factors
| Genotype | Zn translocation factor | Cd translocation factor | ||
|---|---|---|---|---|
| WT | 1.31a | ± 0.25 | 0.83a | ± 0.18 |
| p35S::ScZRC1 #1 | 2.47b | ± 0.05 | 0.7a,b | ± 0.02 |
| p35S::ScZRC1 #2 | 2.43b | ± 0.29 | 0.59a | ± 0.44 |
| p35S::ScZRC1 #3 | 2.52b | ± 0.23 | 0.91a | ± 0.54 |
| pRbcs::ScZRC1 #1 | 2.18c | ± 0.03 | 0.57a,b | ± 0.10 |
| pRbcs::ScZRC1 #2 | 2.13b,c | ± 0.57 | 0.95a | ± 0.40 |
| pRbcs::ScZRC1 #3 | 2.04c | ± 0.02 | 0.85a | ± 0.34 |
Zn and Cd translocation factors (means ± SD) were calculated as the ratio between metal concentration in shoots and metal concentrations in roots of poplar plants (three lines each transgenic genotpye indicated as #1, #2 and #3) grown in mesocosmos pots filled with an artificially contaminated soil substrate (10 mg kg−1 CdSO4 and 300 mg kg−1 ZnSO4). The translocation factor is a measure for the effectiveness of the root-to-shoot transport of a metal ion. Different letters (a, b, c) within a column indicate significant differences among the genotypes (P < 0.05, n = 5)
Table 2.
Total metals in harvestable parts
| Genotype | plant dry weight (g) | Zn content (µg) | Cd content (µg) | |||
|---|---|---|---|---|---|---|
| WT | 0.87 | ± 0.21 | 185.66a | ± 6.66 | 6.91a | ± 0.62 |
| p35S::ScZRC1 #1 | 0.96 | ± 0.21 | 248.56b | ± 59.13 | 7.07a | ± 3.11 |
| p35S::ScZRC1 #2 | 1.02 | ± 0.25 | 244.3b | ± 48.13 | 6.16a | ± 2.92 |
| p35S::ScZRC1 #3 | 0.93 | ± 0.13 | 247.51b | ± 33.45 | 7.51a | ± 3.56 |
| pRbcs::ScZRC1 #1 | 0.87 | ± 0.17 | 239.15b | ± 26.42 | 5.66a | ± 2.07 |
| pRbcs::ScZRC1 #2 | 1.05 | ± 0.05 | 246.87b | ± 12.84 | 7.29a | ± 0.12 |
| pRbcs::ScZRC1 #3 | 1.08 | ± 0.28 | 254.13b | ± 57.96 | 7.97a | ± 0.89 |
Poplar biomass and Zn and Cd content in total harvestable tissues, leaves plus stems (means ± SD). The numbers #1, #2 and #3 indicate three different lines used. Different letters (a and b) within a column indicate significant differences among the genotypes (P < 0.05, n = 5)
Differently, the expression of ScZRC1, either constitutive or circumscribed to the green tissue, did not result in an altered accumulation of Cd (Fig. 6b). The translocation factor associated to this metal was unchanged between wild type and transgenic plants (Table 1). In the experiment conducted in hydroponic culture, with double contamination of 10 μM CdSO4 and 250 μM ZnSO4, no differences were observed in Zn shoot accumulation between control and transgenic lines (Suppl. Fig. S2), whereas the amount of Zn in roots was significantly higher in wild-type plants than in ScZRC1-expressing plants. Likewise, measurement of Cd concentration in these plants revealed similar values of Cd content in shoots of control and transgenic lines, while a significantly lower Cd accumulation was measured in roots of five out of six transgenic lines compared to wild-type control (Suppl. Fig. S2).
Interestingly, the full potential of poplar plants in accumulating Zn was not reached in soil conditions. Even if the concentration of total Zn in soil is much higher than the concentration in hydroponic solution, the bioavailability of this metal in soil is greatly decreased by the interaction between the metal ions and the soil matrix itself (Rooney et al. 2006). Bioavailable metals in the pot mesocosmos were estimated as 3.30 ± 0.24 mg kg−1 for Zn and 0.10 ± 0.05 mg kg−1 for Cd. Such bioavailability, significantly lower than that of the liquid nutrient solution, reflects in the difference in accumulated metals between hydroponic conditions and soil. Metal concentrations in shoots of plants grown in hydroponic culture are up to four times higher for Zn and more than 20 times higher for Cd than in soil, and the difference is even greater for roots (Fig. 6 and Suppl. Fig. S2). The data obtained in hydroponics, apparently contrasting with the results achieved in soil, suggest a competition between Zn and Cd, at least when plants are cultured in hydroponic solution, and the availability of the two metals is greatly higher than in soil. There are evidences that Zn and Cd uptake and distribution in plants can be at least partially competitive (Noraho and Gaur 1995; Queiroz Santos et al. 2014; Fontanili et al. 2016). Moreover, it has been highlighted in previous works that root-to-shoot translocation (here estimated as translocation factor) is strongly dependent on the growth conditions and the metal availability and generally inversely proportional to the latter (Talke et al. 2006; Fernandez et al. 2012; van der Ent et al. 2013; Kozhevnikova et al. 2017), consistently with what was observed in this analysis. It must also be considered that strategies based on hydroponic culture with high metal concentrations can lead to the saturation of the plant system, thus flattening the differences in metal accumulation (van der Ent et al. 2013). Despite these flaws of hydroponics, both the strategies tested support a greater effectiveness of ScZRC1-expressing plants in Zn translocation from roots to shoots, which becomes manifest in a higher Zn accumulation in shoots in the first analysis and a lower Zn and Cd retention in roots in the second. Therefore, the potential of ScZRC1-expressing poplar plants for phytoremediation is evident, but their efficiency has to be assessed case-to-case in field soils contaminated with multiple heavy metals. Under in-field conditions, characterized by a multi-year timeframe, the biomass developed by poplar plants, the deeper root apparatus and the interactions of roots with rhizosphere microorganisms will influence the remediation capacity, allowing to drive conclusions on the applicability in field of these transgenic clones.
Influence of ScZRC1 on the expression of metal transporters in Populus alba
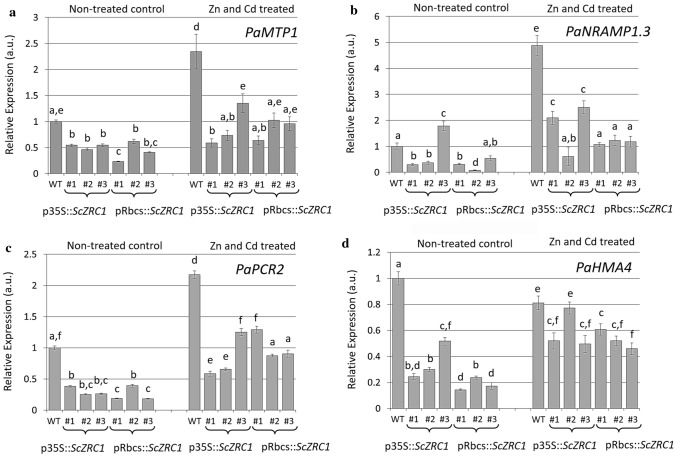
As described in the previous paragraph, the results achieved for metal accumulation in poplar suggest a competition between Zn and Cd or possibly other ions in the nutrient medium, and a possible interference in root-to-shoot transport. To understand if metal homeostasis as a whole is altered by the introduction of ScZRC1, we monitored the expression modulation of the genes encoding four poplar metal transporters in ScZRC1 transgenic plants grown in hydroponic condition with both Zn and Cd present in the medium (Fig. 7). For this analysis we selected poplar homologues of the following genes: (i) the metal tolerance protein 1 (MTP1), localized in the tonoplast and involved in Zn tolerance by metal sequestration into the vacuole (Gustin et al. 2009; Podar et al. 2012); (ii) the natural resistance-associated macrophage protein 1.3 (NRAMP1.3), whose homologous AtNRAMP1 of Arabidopsis is located in the plasma membrane and controls entry of different elements in the cytosol (Cailliatte et al. 2010; Castaings et al. 2016); (iii) the plant cadmium resistance protein 2 (PCR2) and (iv) the P-type heavy metal ATPase 4 (HMA4) which are also located in the plasma membrane, where they translocate cytosolic bivalent cations (e.g., Cd2+ / Zn2+) for xylem loading (Hanikenne et al. 2008; Song et al. 2010). These genes are modulated in response to Cd treatment in poplar lines overexpressing γ-glutamylcysteine synthetase (He et al. 2015a, b). As reported in Fig. 7, increased transcription for PaMTP1, PaNRAMP1.3 and PaPCR2 was detected in leaves of wild-type plants when exposed to high Zn and Cd, in comparison to control conditions. The expression of ScZRC1 in transgenic lines induced a reduction in the expression of all the four considered transporters also in control conditions, with a slight induction upon metal treatment. With reference to existing literature, the analysis of metal transporters highlights a variable behaviour, highly dependent on the species analysed, the organ examined and the treatment applied. For instance, the expression of MTP1 decreases in roots of Populus tremula × Populus alba upon Cd treatment for 80 days (He et al. 2015a, b), while it is enhanced by Zn or Cd treatment in roots of the metal hyperaccumulator Noccaea caerulescens (Ganges ecotype) (Martos et al. 2016). In the present experiment, PaMTP1 upregulation in wild-type plants under metal treatment is consistent with its role in Zn detoxification. Moreover, the significantly lower levels measured in ScZRC1-expressing plants in both control and treated conditions can be easily explained by the likely overlapping roles of ScZRC1 and PaMTP1 in metal vacuolar storage (Fig. 7a). As for PaNRAMP1.3, variable results have been reported in previous studies. For example, NRAMP1.3 upregulation in roots was induced by ABA treatment in Populus × canescens, whereas its level in shoots decreased upon Pb exposure regardless of exogenous ABA addition (Shi et al. 2019). Moreover, NRAMP1.3 upregulation in roots was detected in poplar plants overexpressing the γ-glutamylcysteine synthetase, and upon Cd treatment such induction was enhanced more in transgenic individuals than in wild-type plants (He et al. 2015a, b). In A. thaliana, PCR2 plays a role in Zn detoxification and root-to-shoot transport thus conferring tolerance to excess Zn and Cd (Song et al. 2010), while in Populus × canescens, its transcription was induced by Zn excess in roots but not in shoots (Shi et al. 2015).
Fig. 7.
Real-time RT-PCR analysis on the expression of poplar transporters PaMTP1 (a), PaNRAMP1.3 (b), PaPCR2 (c) and PaHMA4 (d) in leaves of wild type and ScZRC1-expressing plants, exposed to high Zn and Cd in comparison to control conditions. The expression levels were calculated using the 2−ΔΔCT method, relative to the expression level in leaves from WT plants upon untreated conditions. Different letters indicate statistical significance, evaluated by two-way ANOVA followed by a post hoc Bonferroni test (P < 0.05, n = 3)
Conversely to the other genes tested, mRNA level of PaHMA4 did not change in leaves of wild-type plants exposed to Zn and Cd excess. On the other hand, a slight increase was observed in all ScZRC1-expressing lines upon Zn and Cd treatment compared to control condition (Fig. 7d). In A. thaliana, HMA4 plays an essential role in root-to-shoot translocation of Zn and Cd, to maintain Zn homeostasis and Cd detoxification (Hussein et al. 2004). In poplar cells, this transporter is implicated in pumping Zn2+ out of the cytosol; in this species, a down-regulation of HMA4 mRNA levels was reported in response to excess Zn (Adams et al. 2011).
Overall, this transcriptional analysis suggests an alteration in the global metal homeostasis, as highlighted by the lower mRNA levels of the four transporter tested in plants expressing ScZRC1 than in wild type. Therefore, it may be hypothesised that ScZRC1 collaborates with other transporters in the management of Zn and Cd excess; in particular, due to the similarity in localization and activity, a functional overlapping can be suggested with PaMTP1.
Conclusions
In summary, ScZRC1 is a vacuolar transporter able to confer Zn tolerance and accumulation in shoot when expressed in plants. The increased Zn concentration in the shoot was observed in particular in poplar, a species suitable for phytoremediation. In a comparison with other clones, the Villafranca used in this study performed better in terms of Zn tolerance and accumulation (Romeo et al. 2014). The experiments conducted in the present study suggest that it is possible to even increase the potential of this clone for the remediation of Zn polluted soils. However, the results achieved when Cd was present in addition to Zn indicate that case-to-case field trials, over a multi-year timeframe allowing substantial plant growth, are required when dealing with multimetal polluted soils.
Author contribution statement
GDC, FM: conception and design of the study, acquisition of data, analysis and interpretation of data; GDC, AM, EF, GV and AF: drafting the article and revising it critically for important intellectual content; AF: Funding acquisition; GDC, FM, AM, EF, GV and AF final approval of the version to be submitted.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. S1 Expression of ScZRC1 in transgenic Arabidopsis and poplar lines. Fig. S2 Quantification of Zn and Cd in shoots and roots of poplar plants grown in hydroponic conditions (DOCX 669 KB)
Acknowledgements
The authors wish to thank Dr. G. Nervo (Research Unit for Intensive Wood Production, Casale M., Alessandria, Italy) for providing in vitro propagated white poplar plants.
Abbreviations
- eGFP
Enhanced green fluorescent protein
- HM
Heavy metal ATPase
- MTP1
Metal tolerance protein 1
- NRAMP
Natural resistance-associated macrophage protein
- PCR2
Cadmium resistance protein 2
- RbcS
Rubisco small subunit
- SOD
Superoxide dismutase
- ZRC1
Zinc/cadmium resistance protein
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. Funding for F. M.’s PhD was from MIUR (the Italian Ministry of University and Research).
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giovanni DalCorso and Flavio Martini are contributed equally to the work.
References
- Adams JP, Adeli A, Hsu CY, Harkess RL, Page GP, dePamphilis C, Yuceer C. Poplar maintains zinc homeostasis with heavy metal genes HMA4 and PCS1. J Exp Bot. 2011;62:3737–3752. doi: 10.1093/jxb/err025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3179. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- Arrivault S, Senger T, Krämer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 2006;46:861–879. doi: 10.1111/j.1365-313X.2006.02746.x. [DOI] [PubMed] [Google Scholar]
- Baldantoni D, Cicatelli A, Bellino A, Castiglione S. Different behaviours in phytoremediation capacity of two heavy metal tolerant poplar clones in relation to iron and other trace elements. J Environ Manage. 2014;146:94–99. doi: 10.1016/j.jenvman.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Carbonera D, Confalonieri M. Agrobacterium tumefaciens-mediated transformation of elite white poplar (Populus alba L.) and regeneration of transgenic plants. J Genet Breed. 2000;54:263–270. doi: 10.1007/s002990000230. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bissonnette L, St-Arnaud M, Labrecque M. Phytoextraction of heavy metals by two Salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial. Plant Soil. 2010;332:55–67. doi: 10.1007/s11104-009-0273-x. [DOI] [Google Scholar]
- Bredemeier M, Busch G, Hartmann L, Jansen M, Richter F, Lamersdorf NP. Fast growing plantations for wood production—integration of ecological effects and economic perspectives. Front Bioeng Biotech. 2015;3:72. doi: 10.3389/fbioe.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell. 2010;22:904–917. doi: 10.1105/tpc.109.073023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaings L, Caquot A, Loubet S, Curie C. The high-affinity metal transporters NRAMP1 and IRT1 team up to take up iron under sufficient metal provision. Sci Rep. 2016;6:37222. doi: 10.1038/srep37222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney RL. Zinc phytotoxicity. In: Robson AD, editor. Zinc in soils and plants. Dordrecht, NL: Springer; 1993. pp. 135–150. [Google Scholar]
- Chaney RL. Cadmium and zinc. In: Hooda PS, editor. Trace elements in soils. Hoboken, New Jersey, USA: Blackwell Publishing Ltd; 2010. pp. 409–439. [Google Scholar]
- Confalonieri M, Belenghi B, Balestrazzi A, Negri S, Facciotto G, Schenone G, Delledonne M. Transformation of elite white poplar (Populus alba L.) cv. ‘Villafranca’ and evaluation of herbicide resistance. Plant Cell Rep. 2000;19:978–982. doi: 10.1007/s002990000230. [DOI] [PubMed] [Google Scholar]
- Cui XY, Chen ZY, Wu L, Liu XQ, Dong YY, Wang FW, Li HY. RbcS SRS4 promoter from Glycine max and its expression activity in transgenic tobacco. Genet Mol Res. 2015;14(3):7395–7405. doi: 10.4238/2015.July.3.15. [DOI] [PubMed] [Google Scholar]
- Da Ros LM, Mansfield SD. Biotechnological mechanism for improving plant remobilization of phosphorus during leaf senescence. Plant Biotech J. 2020;18:470–478. doi: 10.1111/pbi.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Utmazian MN, Wieshammer G, Vega R, Wenzel WW. Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ Pollut. 2007;148:155–165. doi: 10.1016/j.envpol.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Elekes CC. Eco-technological solutions for the remediation of polluted soil and heavy metal recovery. In: Hernández-Soriano MC, editor. Environmental risk assessment of soil contamination. London, UK: IntechOpen Ltd; 2014. pp. 309–335. [Google Scholar]
- Evans LM, Slavov GT, Rodgers-Melnick E, Martin J, Ranjan P, Muchero W, Brunner AM, Schackwitz W, Gunter L, Chen J-G, Tuskan GA, DiFazio SP. Population genomics of Populus trichocarpa identifies signatures of selection and adaptive trait associations. Nat Gen. 2014;46:1089–1096. doi: 10.1038/ng.3075. [DOI] [PubMed] [Google Scholar]
- Fan D, Liu T, Li C, Jiao B, Li S, Hou Y, Luo K. Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Sci Rep. 2015;5:12217. doi: 10.1038/srep12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasani E, DalCorso G, Varotto C, Li M, Visioli G, Mattarozzi M, Furini A. The MTP1 promoters from Arabidopsis halleri reveal cis-regulating elements for the evolution of metal tolerance. New Phytol. 2017;214:1614–1630. doi: 10.1111/nph.14529. [DOI] [PubMed] [Google Scholar]
- Fernàndez J, Zacchini M, Fleck I. Photosynthetic and growth responses of Populus clones Eridano and I-214 submitted to elevated Zn concentrations. J Geochem Explor. 2012;123:77–86. doi: 10.1016/j.gexplo.2012.01.010. [DOI] [Google Scholar]
- Fontanili L, Lancilli C, Suzui N, Dendena B, Yin YG, Ferri A, Ishii S, Kawachi N, Lucchini G, Fujimaki S, Sacchi GA. Kinetic analysis of zinc/cadmium reciprocal competitions suggests a possible Zn-insensitive pathway for root-to-shoot cadmium translocation in rice. Rice. 2016;9:1–13. doi: 10.1186/s12284-016-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra F, Gainza F, Pérez R, Zamudio F. Phytoremediation of heavy metals using poplars (Populus spp.): a glimpse of the plant responses to copper, cadmium and zinc stress. In: Golubev IA, editor. Handbook of phytoremediation. New York USA: Nova Science Publishers Inc.; 2011. pp. 387–413. [Google Scholar]
- Gullner G, Kömives T, Rennenberg H. Enhanced tolerance of transgenic poplar plants overexpressing gamma-glutamylcysteine synthetase towards chloroacetanilide herbicides. J Exp Bot. 2001;52:971–979. doi: 10.1093/jexbot/52.358.971. [DOI] [PubMed] [Google Scholar]
- Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 2009;57:1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- Halimaa P, Lin YF, Ahonen VH, Blande D, Clemens S, Gyenesi A, Haikio E, Karenlampi SO, Laiho A, Aarts MGM, Pursiheimo JP, Schat H, Shmidt H, Toumainen MH, Tervahauta AI. Gene expression differences between Noccaea caerulescens ecotypes help to identify candidate genes for metal phytoremediation. Environ Sci Technol. 2014;48(6):3344–3353. doi: 10.1021/es4042995. [DOI] [PubMed] [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer, Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- He Z, Shentu J, Yang X, Baligar VC, Zhang T, Stoffella PJ. Heavy metal contamination of soils: sources, indicators, and assessment. J Environ Indic. 2015;9:17–18. [Google Scholar]
- He J, Li H, Ma C, Zhang Y, Polle A, Rennenberg H, Cheng X, Luo Z-B. Overexpression of bacterial γ-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015;205(1):240–254. doi: 10.1111/nph.13013. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular 347. Berkeley, CA, USA: College of Agriculture, University of California
- Hsieh TH, Lee JT, Charng YY, Chan MT. Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiol. 2002;130:618–626. doi: 10.1104/pp.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, Young J, Camakaris J, Harper JF, Cobbett CS. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell. 2004;16:1327–1339. doi: 10.1105/tpc.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikova AD, Seregin IV, Gosti F, Schat H. Zinc accumulation and distribution over tissues in Noccaea caerulescens in nature and in hydroponics: a comparison. Plant Soil. 2017;411(1–2):5–16. doi: 10.1007/s11104-016-3116-6. [DOI] [Google Scholar]
- Krämer U, Clemens S. Functions and homeostasis of zinc, copper, and nickel in plants. In: Tamas MJ, Martinoia E, editors. Molecular biology of metal homeostasis and detoxification. Berlin, Heidelberg: Springer; 2005. pp. 215–271. [Google Scholar]
- Lloyd G, McCown BH (1981) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. In: Combined Proceedings, International Plant Propagators’ Society 30: 421–427
- MacDiarmid CW, Milanick MA, Eide DJ. Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J Biol Chem. 2002;277(42):39187–39194. doi: 10.1074/jbc.M205052200. [DOI] [PubMed] [Google Scholar]
- MacDiarmid CW, Milanick MA, Eide DJ. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J Biol Chem. 2003;278(17):15065–15072. doi: 10.1074/jbc.M300568200. [DOI] [PubMed] [Google Scholar]
- Małecka A, Konkolewska A, Hanć A, Barałkiewicz D, Ciszewska L, Ratajczak E, Staszak AM, Kmita H, Jarmuszkiewicz W. Insight into the phytoremediation capability of Brassica juncea (v. Malopolska): metal accumulation and antioxidant enzyme activity. Int J Mol Sci. 2019;20(18):4355. doi: 10.3390/ijms20184355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals. 2009;22:149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- Martinoia E, Meyer S, De Angeli A, Nagy R. Vacuolar transporters in their physiological context. Annu Rev Plant Biol. 2012;63:183–213. doi: 10.1146/annurev-arplant-042811-105608. [DOI] [PubMed] [Google Scholar]
- Martos S, Gallego B, Sáez L, López-Alvarado J, Cabot C, Poschenriede C. Characterization of zinc and cadmium hyperaccumulation in three Noccaea (Brassicaceae) populations from non-metalliferous sites in the eastern Pyrenees. Front Plant Sci. 2016;7:128. doi: 10.3389/fpls.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath SP, Zhao FJ. Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol. 2003;14:277–282. doi: 10.1016/S0958-1669(03)00060-0. [DOI] [PubMed] [Google Scholar]
- Migeon A, Richaud P, Guinet F, Blaudez D, Chalot M. Hydroponic screening of poplar for trace element tolerance and accumulation. Int J Phytoremed. 2012;14:350–361. doi: 10.1080/15226514.2011.620651. [DOI] [PubMed] [Google Scholar]
- Miyabe S, Izawa S, Inoue Y. The Zrc1 is involved in zinc transport system between vacuole and cytosol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2001;282(1):79–83. doi: 10.1006/bbrc.2001.4522. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Noraho N, Gaur JP. Effect of cations, including heavy metals, on cadmium uptake by Lemna polyrhiza L. Biometals. 1995;8(2):95–98. doi: 10.1007/BF00142006. [DOI] [Google Scholar]
- Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air water and soils by trace metals. Nature. 1988;333:134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Paulsen IT, Saier MJ. A novel family of ubiquitous heavy metal ion transport proteins. J Membr Biol. 1997;156:99–103. doi: 10.1007/s002329900192. [DOI] [PubMed] [Google Scholar]
- Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int J Biochem Cell Biol. 2009;41(8–9):1665–1677. doi: 10.1016/j.biocel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits E. Phytoremedaition. Annu Rev Plant Biol. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- Podar D, Scherer J, Noordally Z, Herzyk P, Nies D, Sanders D. Metal selectivity determinants in a family of transition metal transporters. J Biol Chem. 2012;287:3185–3196. doi: 10.1074/jbc.M111.305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 2002;73:149–156. doi: 10.1023/A:1020470224740. [DOI] [PubMed] [Google Scholar]
- Qasim B, Motelica-Heino M, Joussein E, Soubrand M, Gauthier A. Potentially toxic element phytoavailability assessment in TECHNOSOLS from former smelting and mining areas. Environ Sci Pollut Res. 2015;22:5961–5974. doi: 10.1007/s11356-014-3768-9. [DOI] [PubMed] [Google Scholar]
- Queiroz Santos AC, Maria de Aguiar Accioly A, do Araujo Nascimento CW, dos Santos NM, de Chaves Melo EE, de Lima Xavier BT. Competitive absorption of cadmium, zinc, and lead by Velvet Bean (Stizolobium aterrimum) and metal distribution among soil fractions. Comm Soil Sci Plant Anal. 2014;45(11):1499–1510. doi: 10.1080/00103624.2014.904333. [DOI] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ensley BD, editors. Phytoremediation of toxic metals: using plants to clean up the environment. New York, USA: Wiley; 2000. [Google Scholar]
- Rockwood DL, Naidu CV, Carter DR, Rahmani M, Spriggs TA, Lin C, Alker GR, Isebrands JS, Segrest SA. Short rotation woody crops and phytoremediation: opportunities for agroforestry? Agrofor Syst. 2004;61:51–63. doi: 10.1023/B:AGFO.0000028989.72186.e6. [DOI] [Google Scholar]
- Romeo S, Francini A, Ariani A, Sebastiani L. Phytoremediation of Zn: identify the diverging resistance, uptake and biomass production behaviours of poplar clones under high zinc stress. Water Air Soil Pollut. 2014;225:1813. doi: 10.1007/s11270-013-1813-9. [DOI] [Google Scholar]
- Rooney CP, Zhao FJ, McGrath SP. Soil factors controlling the expression of copper toxicity to plants in a wide range of European soils. Environ Toxicol Chem. 2006;25(3):726–732. doi: 10.1897/04-602r.1. [DOI] [PubMed] [Google Scholar]
- Rugh CL, Senecoff JF, Meagher RB, Scott A, Merkle SA. Development of transgenic yellow poplar for mercury phytoremediation. Nat Biotechnol. 1998;16:925–928. doi: 10.1038/nbt1098-925. [DOI] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Sendín LN, Filippone MP, Orce IG, Rigano L, Enrique R, Peña L, Vojnov AA, Marano MR, Castagnaro AP. Transient expression of pepper Bs2 gene in Citrus limon as an approach to evaluate its utility for management of citrus canker disease. Plant Pathology. 2012;61:648–657. doi: 10.1111/j.1365-3059.2011.02558.x. [DOI] [Google Scholar]
- Shi WG, Li H, Liu TX, Polle A, Peng CH, Luo ZB. Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus × canescens exposed to excess zinc. Plant Cell Environ. 2015;38:207–223. doi: 10.1111/pce.12434. [DOI] [PubMed] [Google Scholar]
- Shi WG, Liu W, Yu W, Zhang Y, Ding S, Li H, Mrak T, Kraigher H, Luo ZB. Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus × canescens. J Hazard Mater. 2019;362:275–285. doi: 10.1016/j.jhazmat.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Shim D, Kim S, Choi YI, Song WY, Park J, Youk ES, Jeong SC, Martinoia E, Noh EW, Lee Y. Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemosphere. 2013;90(4):1478–1486. doi: 10.1016/j.chemosphere.2012.09.044. [DOI] [PubMed] [Google Scholar]
- Sinclair SA, Krämer U. The zinc homeostasis network of land plants. Biochim Biophys Acta. 2012;1823:1553–1567. doi: 10.1016/j.bbamcr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Song WY, Choi KS, Kim DY, Geisler M, Park J, Vincenzetti V, Schellenberg M, Kim SH, Lim YP, Noh EW, Lee Y, Martinoia E. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell. 2010;22:2237–2252. doi: 10.1105/tpc.109.070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki H, Maruyama K, Kidokoro S, Ito Y, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K, Nakashima K. The abiotic stress- responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol Genet Genomics. 2010;284:173–183. doi: 10.1007/s00438-010-0557-0. [DOI] [PubMed] [Google Scholar]
- Talke IN, Hanikenne M, Krämer U. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2006;142(1):148–167. doi: 10.1104/pp.105.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong YP, Kneer R, Zhu YG. Vacuolar compartmentalization: a second generation approach to engineering plants for phytoremediation. Trends Plant Sci. 2004;9(1):7–9. doi: 10.1016/j.tplants.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Tuskan GA, DiFazio S, Jansson S, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- Uozumi N, Inoue Y, Yamazaki K, Kobayashi T. Light activation of expression associated with the tomato rbcS promoter in transformed tobacco cell line BY-2. J Biotechnol. 1994;36(1):55–62. doi: 10.1016/0168-1656(94)90023-x. [DOI] [PubMed] [Google Scholar]
- van der Ent A, Baker AJ, Reeves RD, Pollard AJ, Schat H. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil. 2013;362(1):319–334. doi: 10.1007/s11104-012-1287-3. [DOI] [Google Scholar]
- van der Zaal BJ, Neuteboom LW, Pinas JE, Chardonnens AN, Schat H, Verkleij JAC, Hooykaas PJJ. Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. 1999;119:1047–1055. doi: 10.1104/pp.119.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev A, Perez-Sanz A, Semane B, Carleer R, Vangronsveld J. Cadmium accumulation and tolerance of two Salix genotypes hydroponically grown in the presence of cadmium. J Plant Nutr. 2005;28:2159–2177. doi: 10.1080/01904160500320806. [DOI] [Google Scholar]
- Yadav R, Arora P, Kumar S, Chaudhury A. Perspectives for genetic engineering of poplars for enhanced phytoremediation abilities. Ecotoxicology. 2010;19:1574. doi: 10.1007/s10646-010-0543-7. [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- Zacchini M, Pietrini F, Mugnozza GS, Iori V, Pietrosanti L, Massacci A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut. 2009;197:23–34. doi: 10.1007/s11270-008-9788-7. [DOI] [Google Scholar]
- Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath S. Biofortification and phytoremediation. Curr Opin Plant Biol. 2009;12:373–380. doi: 10.1016/j.pbi.2009.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1 Expression of ScZRC1 in transgenic Arabidopsis and poplar lines. Fig. S2 Quantification of Zn and Cd in shoots and roots of poplar plants grown in hydroponic conditions (DOCX 669 KB)