Abstract
Free fatty acid dysregulation in diabetics may elicit the release of inflammatory cytokines from Müller cells (MC), promoting the onset and progression of diabetic retinopathy (DR). Palmitic acid (PA) is elevated in the sera of diabetics and stimulates the production of the DR-relevant cytokines by MC, including IL-1β, which induces the production of itself and other inflammatory cytokines in the retina as well. In this study we propose that experimental elevation of cytochrome P450 epoxygenase (CYP)-derived epoxygenated fatty acids, epoxyeicosatrienoic acid (EET) and epoxydocosapentaenoic acid (EDP), will reduce PA- and IL-1β-induced MC inflammation. Broad-spectrum CYP inhibition by SKF-525a increased MC expression of inflammatory cytokines. Exogenous 11,12-EET and 19,20-EDP significantly decreased PA- and IL-1β-induced MC expression of IL-1β and IL-6. Both epoxygenated fatty acids significantly decreased IL-8 expression in IL-1β-induced MC and TNFα in PA-induced MC. Interestingly, 11,12-EET and 19,20-EDP significantly increased TNFα in IL-1β-treated MC. GSK2256294, a soluble epoxide hydrolase (sEH) inhibitor, significantly reduced PA- and IL-1β-stimulated MC cytokine expression. 11,12-EET and 19,20-EDP were also found to decrease PA- and IL-1β-induced NFκB-dependent transcriptional activity. These data suggest that experimental elevation of 11,12-EET and 19,20-EDP decreases MC inflammation in part by blocking NFκB-dependent transcription and may represent a viable therapeutic strategy for inhibition of early retinal inflammation in DR.
Subject terms: Drug development, Preclinical research, Translational research, Retinal diseases, Diabetes complications
Introduction
Diabetic retinopathy (DR) is the leading cause of irreversible vision loss among working age Americans, affecting ~ 35% of patients with diabetes mellitus1. As the prevalence of worldwide diabetes increases, the number of people suffering from diabetes-induced vision loss increases as well2. DR pathology is classified in two clinically distinct forms, non-proliferative (NPDR) and proliferative (PDR). NPDR is characterized by the appearance of microaneurysms, focal hemorrhaging, hard exudates beneath the retinal surface and retinal capillary death3,4. The death of retinal capillaries in NPDR can result in vasoregression-promoted ischemia, causing retinal hypoxia that elicits the synthesis and release of vascular endothelial cell growth factor (VEGF)5. Increased levels of retinal VEGF can trigger a vasoproliferative response, transitioning the retina to vision threatening PDR5. Current DR therapies, such as laser photocoagulation or VEGF inhibition, target PDR after irreversible retinal damage has occurred. Therefore, there is an important unmet need to develop a therapy that intervenes prior to PDR onset to preserve retinal function.
DR progression is associated with systemic dyslipidemia, and circulating free fatty acids (FFAs) are known to initiate inflammatory cytokine release6,7. Diabetic mice have over three times the retinal fatty acid content of healthy controls and palmitic acid (PA) is elevated above other FFAs in the circulation and tissues of diabetic patients and experimental models of diabetes8–10. The detrimental effects of FFAs in the diabetic retina has been substantiated in two epidemiological human studies, ACCORD and FIELD, in which the lipid-lowering drug fenofibrate was shown to delay retinopathy progression6,7. Müller cells (MC) are particularly responsive to PA and other FFA8,11. RNA sequencing has shown that PA stimulates a variety of DR-relevant pathways in MC, including NFκB signaling and inflammation, intracellular lipid signaling, angiogenesis, and MAPK signaling, that are not altered by elevated glucose stimulation alone8. It is proposed that diabetes-related dysregulation of PA and other FFAs damage MC, resulting in their production of inflammatory retinal cytokines8,12. These cytokines amplify through autocrine and paracrine mechanisms, reaching levels that promote chronic retinal inflammation5. If these levels are sustained, retinal vascular pathology can ensue, promoting DR progression. In support of this notion, studies in human patients and animal models show that elevated levels of inflammatory cytokines in the vitreous and retina correlate with early DR progression5,12–14. One such cytokine, interleukin 1β (IL-1β), is purported to be a “master regulator” of cytokine-induced inflammation15,16. IL-1β is elevated in DR and induces MC to produce and release itself and other inflammatory cytokines. MC are vital to retinal homeostasis and may become activated in response to diabetes-related metabolic dysfunction. This causes a diversion from their homeostatic functions, promoting DR onset and progression. MC activation is easily assayed by glial fibrillary acidic protein immunostaining of retinal cross-sections and it is one of the earliest observable changes in DR8,17. The foregoing, along with other MC-dependent behaviors, suggests that therapies targeting MC inflammation in DR could potentially preempt PDR and its vision threatening consequences.
Ample data suggest that lipid mediators derived from ω-6 and ω-3 fatty acids regulate diabetes-induced retinal inflammation5,18,19. Arachidonic acid (AA; ω-6) and docosahexaenoic acid (DHA; ω-3) are polyunsaturated fatty acids (PUFAs) found at high abundance in the retina, suggesting their importance in retinal physiology20–22. These PUFAs are metabolized through the cyclooxygenase (COX), lipoxygenase (LOX) or cytochrome P450 epoxygenase (CYP) pathways. Although there are exceptions, AA is metabolized by COX and LOX to yield oxygenated metabolites that are largely pro-inflammatory5,23. For example, it has been shown that COX inhibitors such as aspirin and other NSAIDs reduce DR associated inflammation24. Unlike AA, it has been reported that COX and LOX convert DHA into anti-inflammatory metabolites23. COX converts DHA to hydroxyl DHA, and 15-lipoxygenase (ALOX15) converts DHA to 17S-hydroperoxy-DHA that is further metabolized to yield the D-resolvins25–27. Streptozotocin-induced diabetic rats that received intravitreal injections of resolvin D1 demonstrated reduced levels of retinal IL-1β and NFκB activity26. There is growing interest in the epoxygenation of ω-6 and ω-3 fatty acids by cytochrome P450 epoxygenases (CYPs). CYPs are endoplasmic reticulum membrane-bound monooxygenases that metabolize fatty acids to epoxide derivatives that demonstrate potent anti-inflammatory activities in a variety of biological systems3. CYP2C8, CYP2C9, and CYP2J2 are the most well-characterized human CYPs that epoxygenate AA and DHA to yield epoxyeicosatrienoic acids (EET) and epoxydocosapentaenoic acids (EDP), respectively3,19. AA yields four regioisomers, 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET, while DHA yields six regioisomers, 4,5-EDP, 7,8-EDP, 10,11-EDP, 13,14-EDP, 16,17-EDP, and 19,20-EDP3. We have previously shown that the administration of exogenous 11,12-EET reduces the expression of the leukocyte adhesion protein VCAM1 in human retinal microvascular endothelial cells (hRMEC) activated by TNFα3. These data suggest that increasing EET/EDP levels may be an effective method to reduce DR-related inflammation.
Soluble epoxide hydrolase (sEH) hydrolyzes EET and EDP to their less biologically active diols, dihydroxyeicosatrienoic acid (DHET) and dihydroxydocosapentaenoic acid (DHDP)3. By reducing the half-life of epoxides, sEH decreases their abundance in tissues and thus the potency of their anti-inflammatory activities. Therefore, sEH inhibition presents a rational therapeutic method to elevate epoxide levels and reduce inflammation. sEH inhibitors (sEHi) have been tested in animal models of inflammatory disease to raise EET/EDP levels and mitigate inflammation28. These successful studies have led to clinical trials testing sEH inhibition in diabetes-relevant pathologies, such as impaired glucose tolerance and insulin resistance30. Furthermore, studies that use sEH inhibitors in combination with other pharmacologic strategies to raise EET/EDP levels prove more efficacious than sEHi’s administered alone3. For example, TNFα-induced leukocyte adhesion expression in human retinal endothelial cells was significantly reduced with the administration of sEHi and EET/EDP in combination, but not separately3.
CYP levels are suppressed in diabetic conditions, and patients with NPDR and PDR have reduced levels of EETs observed in the vitreous31,32. It was also found that soluble epoxide hydrolase activity is increased in response to diabetic conditions, contributing to lower epoxygenated fatty acids levels, and creating conditions permissive to inflammation33,34. Thus, pharmacologic manipulations that elevate epoxygenated fatty acids might constitute a rational strategy to reduce retinal inflammation in DR. In this study, we tested the efficacy of increased epoxygenated fatty acid concentrations to mitigate PA- and IL-1β-induced expression of inflammatory cytokines in primary cultures of human Müller cells (hMC). The levels of 11,12-EET and 19,20-EDP were manipulated in hMC cultures via CYP inhibition, exogenous addition of epoxides, and the inhibition of epoxide hydrolysis.
Results
The CYP epoxygenase inhibitor SKF-525a promotes inflammatory cytokine expression in hMC
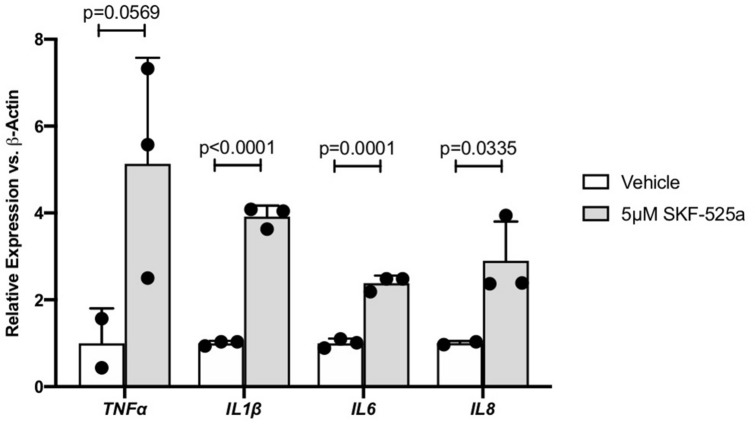
hMC were treated with the CYP epoxygenase inhibitor SKF-525a or vehicle and inflammatory cytokine expression was assayed via qRT-PCR. SKF-525a increased expression of the DR-relevant cytokines TNFα (5.13 fold; p = 0.0569), IL1β (3.92 fold; p < 0.0001), IL6 (2.38 fold; p = 0.0001), and IL8 (2.90 fold; p = 0.0335). Only TNFα did not achieve statistical significance (Fig. 1).
Figure 1.
The effect of CYP inhibitor SKF-525a on Müller cell inflammatory cytokine expression. Human Müller cells were treated with SKF-525a (5.0 μM) for 24 h. After treatment, total RNA was isolated and inflammatory cytokine expression was assayed by qRT-PCR. TNFα, IL1β, IL6 and IL8 expression was increased by SKF-525a, though statistical significance was not achieved for TNFα. Data are displayed as mean ± SD (n = 2 or 3).
11,12-EET, 19,20-EDP or the sEH inhibitor GSK2256294 reduces PA-stimulated inflammatory cytokine expression
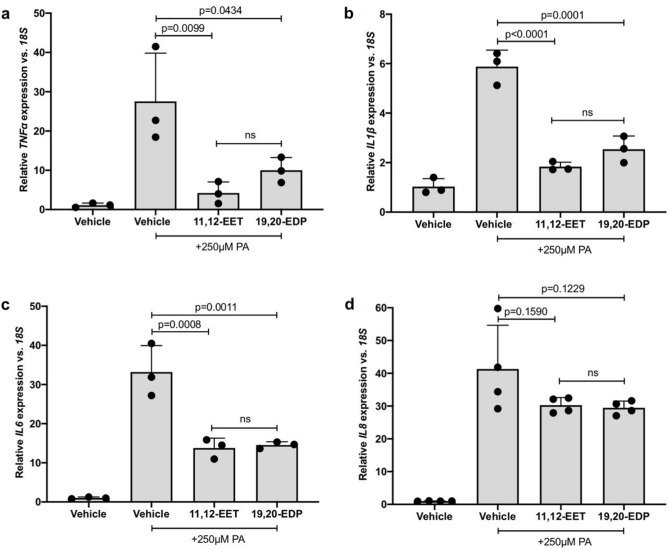
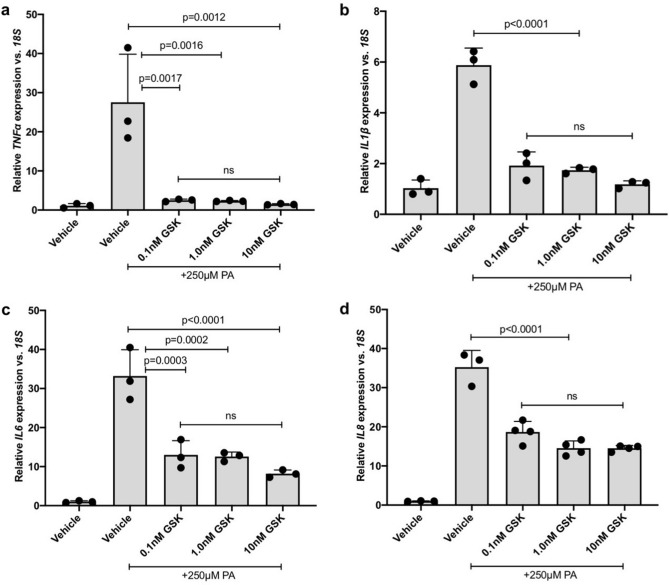
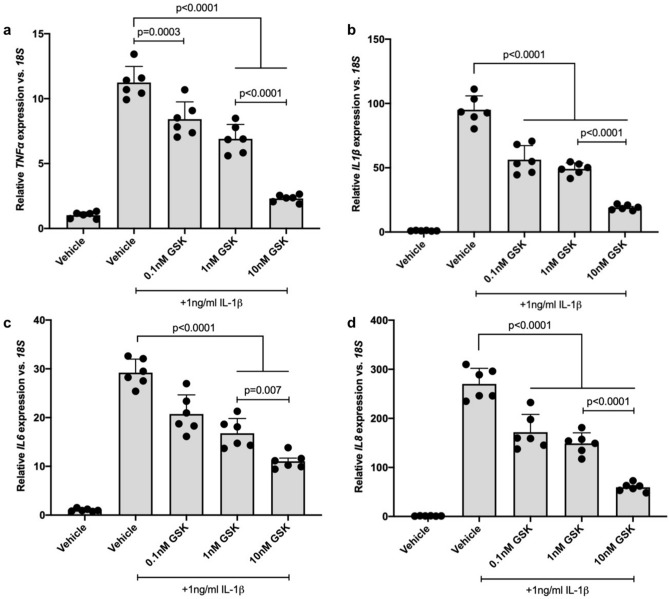
The epoxygenated fatty acids 11,12-EET, 19,20-EDP or the sEH inhibitor, GSK2256294, were tested against PA-induced inflammatory cytokine expression in hMC. hMC were treated with PA in the presence or absence of 11,12-EET, 19,20-EDP or GSK2256294 and inflammatory cytokine expression was assessed by qRT-PCR. 11,12-EET significantly reduced PA-stimulated expression of TNFα by 84.67%, IL1β by 68.72% and IL6 by 58.54% (Fig. 2a–c. p = 0.0099, p < 0.0001, p = 0.0008, respectively). 19,20-EDP significantly reduced PA-stimulated expression of TNFα by 63.67%, IL1β by 56.76%, and IL6 by 56.19% (Fig. 2a–c. p = 0.0434, p = 0.0001, p = 0.0011, respectively). 11,12-EET and 19,20-EDP reduced PA-stimulated IL8 expression by 26.70% and 28.59%, however, statistical significance was not achieved (Fig. 2d). A range of GSK2256294 concentrations were tested (0.1 nM, 1.0 nM, and 10 nM) and GSK2256294 significantly reduced PA-stimulated hMC cytokine expression at each concentration. At the lowest concentration tested, 0.1 nM, GSK2256294 reduced PA-stimulated expression of TNFα by 90.94%, IL1β by 67.31%, IL6 by 60.86%, and IL8 by 47.02% in hMC (Fig. 3a–d. p = 0.0017, p < 0.0001, p = 0.0003, p < 0.0001, respectively). At 1 nM, GSK2256294 reduced PA-stimulated expression of TNFα by 91.64%, IL1β by 70.39%, IL6 by 62.13%, and IL8 by 58.74% in hMC (Fig. 3a–d. p = 0.0016, p < 0.0001, p = 0.0002, p < 0.0001, respectively). At 10 nM, GSK2256294 reduced PA-stimulated expression of TNFα by 94.65%, IL1β by 79.87%, IL6 by 75.36%, and IL8 by 58.83% in hMC (Fig. 3a–d. p = 0.0012, p < 0.0001, p < 0.0001, p < 0.0001, respectively).
Figure 2.
The effect of 11,12-EET and 19,20-EDP on PA-induced inflammatory mediator expression by Müller cells. Human Müller cells were treated with PA (250 μM) for 24 h. 11,12-EET (0.5 μM) or 19,20-EDP (0.5 μM) was added during the final 3 h of treatment. After 24 h, total RNA was isolated and expression was assayed by qRT-PCR. (a) TNFα (b) IL1β, and (c) IL6 expression was significantly decreased by both epoxygenated fatty acids. (d) IL8 expression was reduced but statistical significance was not achieved. Results depicted are representative of three separate experiments. Data are displayed as mean ± SD (n = 3 or 4 for each experiment).
Figure 3.
The effect of sEH inhibitor GSK2256294 on PA-induced inflammatory mediator expression by Müller cells. Human Müller cells were treated with PA (250 μM) or PA plus 0.1 nM, 1.0 nM or 10 nM GSK2256294 (sEH inhibitor). After 24 h, total RNA was isolated, and expression was analyzed by qRT-PCR. (a) TNFα, (b) IL1β, (c) IL6, and (d) IL8 expression was significantly decreased by the addition of the sEH inhibitor at each of the concentrations tested. Results depicted are representative of three separate experiments. Data are displayed as mean ± SD (n = 3 or 4 for each experiment).
11,12-EET, 19,20-EDP or the sEH inhibitor GSK2256294 reduces IL-1β-stimulated inflammatory cytokine expression
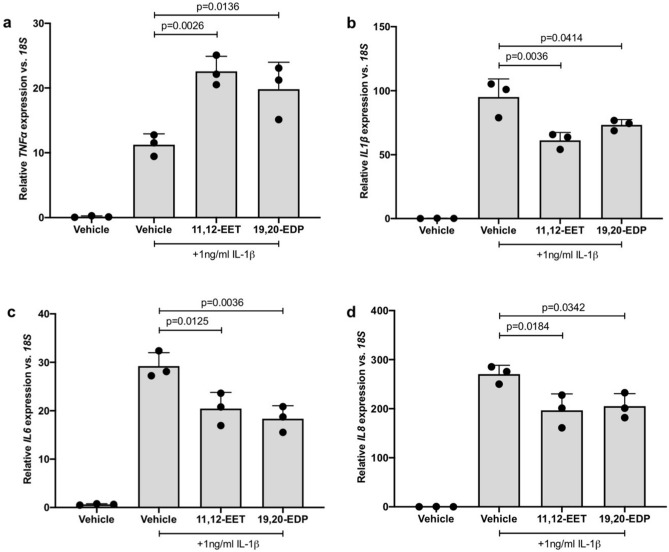
hMC were treated with IL-1β in the presence or absence of 11,12-EET, 19,20-EDP or GSK2256294 to test the effect of each on IL-1β-induced inflammatory cytokine expression. Total RNA was isolated and inflammatory cytokine expression was assessed by qRT-PCR. 11,12-EET significantly reduced IL-1β-stimulated expression of IL1β by 35.65%, IL6 by 30.06%, and IL8 by 27.26% in hMC (Fig. 4b–d. p = 0.0036, p = 0.0125, p = 0.0184, respectively). 19,20-EDP significantly reduced IL-1β-stimulated expression of IL1β by 22.88%, IL6 by 37.18%, and IL8 by 24.10% in hMC (Fig. 4b–d. p = 0.0414, p = 0.0036, p = 0.0342, respectively). TNFα expression, however, was significantly increased by both epoxygenated fatty acids (Fig. 4a. p = 0.0026, p = 0.0136). sEH inhibition was tested at a range of GSK2256294 concentrations (0.1 nM, 1.0 nM, and 10 nM) and at each concentration IL-1β-stimulated cytokine expression was significantly reduced. At the lowest concentration tested, 0.1 nM, GSK2256294 reduced IL-1β-stimulated expression of TNFα by 25.11%, IL1β by 40.78%, IL6 by 29.05%, and IL8 by 36.37% in hMC (Fig. 5a–d. p = 0.0003, p < 0.0001, p < 0.0001, p < 0.0001, respectively). At 1 nM, GSK2256294 reduced IL-1β-stimulated expression of TNFα by 38.56%, IL1β by 48.33%, IL6 by 42.56%, and IL8 by 44.91% in hMC (Fig. 5a–d. p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, respectively). At 10 nM, GSK2256294 reduced IL-1β -stimulated expression of TNFα by 79.45%, IL1β by 79.96%, IL6 by 62.26%, and IL8 by 78.05% in hMC (Fig. 5a–d. p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, respectively).
Figure 4.
The effect of 11,12-EET and 19,20-EDP on IL-1β-induced inflammatory cytokine expression by Müller cells. Human Müller cells were treated with IL-1β (1.0 ng/ml) or IL-1β plus 11,12-EET (0.5 μM) or 19,20-EDP (0.5 μM) for 8 h. Total RNA was isolated and cytokine expression was assayed by qRT-PCR. (a) TNFα expression was significantly elevated while (b) IL1β, (c) IL6, and (d) IL8 expression was significantly decreased by the addition of both exogenous epoxygenated fatty acids. Results depicted are representative of three separate experiments. These data are normalized to induction levels illustrated in Fig. 5. Data are displayed as mean ± SD (n = 3).
Figure 5.
The effect of sEH inhibitor GSK2256294 on IL-1β-induced inflammatory mediator expression by Müller cells. Human Müller cells were treated with IL-1β (1.0 ng/ml) or IL-1β plus 0.1 nM, 1.0 nM, or 10 nM GSK2256294 (sEH inhibitor) for 8 h. Total RNA was isolated and cytokine expression was assayed by qRT-PCR. (a) TNFα, (b) IL1β, (c) IL6, and (d) IL8 expression was significantly decreased at each sEH inhibitor concentration tested. Bars represent mean ± SD (n = 6).
11,12-EET or 19,20-EDP reduces PA- and IL-1β-induced NFκB promoter activity
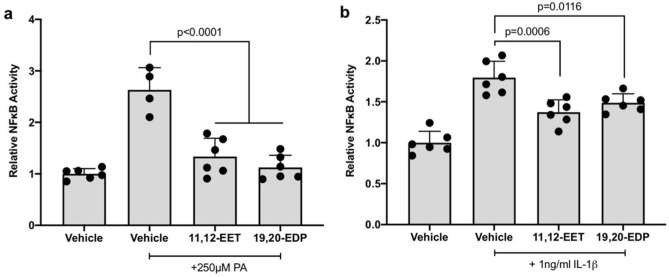
hMC were transfected with a NFκB-luciferase promoter-reporter construct and treated with PA or IL-1β in the presence or absence of 11,12-EET or 19,20-EDP. As shown in Fig. 6, 11,12-EET and 19,20-EDP decreased both PA- and IL-1β-induced NFκB-dependent luciferase activity. 11,12-EET and 19,20-EDP decreased PA-induced reporter activity by 49.2% and 57.3%, respectively (Fig. 6a. p < 0.0001, p < 0.0001), and they decreased IL-1β-induced reporter activity by 23.6% and 17.2%, respectively (Fig. 6b. p = 0.0006, p = 0.0116).
Figure 6.
The effect of 11,12-EET or 19,20-EDP on PA or IL-1β-induced NFκB promoter activity. hMC were transfected with a NFκB-luciferase reporter construct and treated with (a) PA (b) IL-1β or in the presence or absence of 11,12-EET (0.5 μM) or 19,20-EDP (0.5 μM). NFκB activity was determined by measuring the ratio of firefly-to-renilla luciferase luminescence activity. Each bar represents the mean ± SD (n = 4, 5 or 6).
Discussion
EET or EDP in combination with sEHi were previously shown to mitigate several DR-relevant experimental endpoints including: the expression of leukocyte adhesion proteins by hRMEC, peripheral blood monocyte (PBMC) adhesion to hRMEC monolayers, and TNFα-induced retinal leukostasis in mice3. In these studies, epoxygenated fatty acids were determined to act through NFκB-dependent signaling3. The anti-inflammatory potency of these lipid mediators in hRMEC caused speculation of their efficacy in other retinal cell types that are known to contribute to inflammatory conditions, such as glia. MC are potent propagators of preliminary inflammation and serve as a functional link between the neuronal and vascular compartments of the vertebrate retina35. MC span nearly the entire thickness of the retina and control retinal homeostasis including recycling neurotransmitters, maintaining the inner blood-retinal barrier, regulating retinal cation flux, and maintaining photoreceptor function35. MC function in innate immunity36 and some believe that diabetes-induced inflammation causes MC dysfunction, leading them to become destructive and promote DR pathogenesis35. Additionally, changes in MC have been observed prior to the appearance of overt vascular pathology in DR35,37. Consequently, therapeutics that block diabetes-related MC inflammation could prevent or slow the onset and progression of early DR.
11,12-EET and 19,20-EDP were selected for these experiments because both demonstrated efficacy in previous studies3,38. Additionally, 19,20-EDP is the most highly abundant regioisomer in the retina because CYP epoxygenases preferentially mono-oxygenate the terminal double bond of DHA, and sEH hydrolysis of 19,20-EDP is slower compared to the other regioisomers19,39,40. Therefore, 19,20-EDP turnover is presumably lower, enhancing efficacy through increased bioavailability. We chose 11,12-EET because it was one of the most abundant EET regioisomers in tissues and demonstrated potent anti-inflammatory activities in previous studies38,39,41. Notably, compared to other regioisomers, 11,12-EET and 19,20-EDP were also found in higher concentrations in MC-conditioned medium as determined by our mass spectrometric analysis (Supp. Fig. 1). It is important to note that EET and EDP are relatively unstable, and thus sEH inhibitors are a viable therapeutic route to increase epoxide levels and are currently under development for human use28,42,43. With the use of sEH inhibitors, all epoxygenated fatty acid regioisomers would be protected increasing their biological half-lives and activities. Notably, with the application of sEH inhibitors, any efficacy observed against inflammation presumably results from a summed response to all regioisomers, thus diminishing the significance of any single regioisomer’s contribution.
Before testing the effects of increasing epoxygenated fatty acids levels in hMC, we first investigated the effects of their depletion. CYP epoxygenase activity is responsible for converting AA and DHA to regioisomeric EETs and EDPs respectively3. hMC were treated with the broad-spectrum CYP inhibitor SKF-525a to reduce intracellular EET/EDP levels. In the presence of SKF-525a we observed significant increases in the expression levels of the DR-relevant inflammatory cytokines TNFα, IL1β, IL6 and IL8. Others have shown that the proinflammatory effects of SKF-525a in cells are reversed by the addition of exogenous EETs, suggesting SKF-525a acts specifically by EET/EDP depletion38,44,45. Combined, these observations support our hypothesis that EET/EDP depletion, such as that occurring in DR, promotes hMC inflammation (Fig. 1). Exogenous addition of 11,12-EET, 19,20-EDP, and the sEH inhibitor GSK2256294, demonstrated a potent capacity to reduce inflammatory cytokine expression in hMC activated by PA and IL-1β. While previously demonstrated in hRMEC, this is the first report of the anti-inflammatory potential of these agents in retinal glia.
We hypothesize that in the earliest stages of DR pathogenesis, the predominant stimuli are those imposed by metabolic dysfunction such as elevated glucose and/or free fatty acids in the bloodstream and ocular tissues. Abnormal levels of glucose and/or FFA may cause damage to retinal cells that respond by producing and releasing inflammatory cytokines. These cytokines amplify through autocrine and paracrine mechanisms and become the dominant inflammatory stimulus in late-stage DR. Accordingly, we purposefully chose two stimuli, one from each of these stages, to test whether epoxides could continuously intervene, as the weights of these respective stimuli shift along the temporal axis of DR pathogenesis. Past experiments show that, among non-neuronal retinal cells, MC demonstrated the greatest increases in expression and secretion of inflammatory mediators in response to metabolic stimuli. Accordingly, we believe that MC act as the primary driving force of chronic inflammation in DR through their synthesis, release and auto-amplification of inflammatory cytokines that propagate inflammation in neighboring vascular and neuronal cells. While elevated glucose is commonly used to simulate diabetic conditions in vitro, we found that elevated glucose yields little to no response when studying many primary human retinal cells8. However, FFAs reliably and consistently induce inflammation in these cells consistent with DR8. Thus, we studied the response of primary human retinal cells to a free fatty acid, PA, that plausibly models the influence of diabetes-associated dyslipidemia8. We specifically demonstrated the effectiveness of PA as a DR-appropriate stimulus for human Müller cells. We chose to use 250 μM PA because it is physiologically relevant. Analysis of plasma free fatty acids determined PA to be at a concentration of 234.9 + /- 58.1 μmol/l in obese diabetic individuals fasted overnight46. Similar studies aiming to create comprehensive profiles of fatty acids in the plasma of type 2 diabetics have substantiated this finding47, and it is widely accepted that the lipid composition of peripheral tissues often reflect plasma levels. Furthermore, this concentration is within ranges used in studies of other retinal cell behaviors48, as well as other in vitro studies of diabetes49,50. We chose to use 1 ng/ml IL-1β empirically, because this concentration promoted elevated expression of TNFα, IL-1β, IL-6 and IL-8 in hMC cultures like that observed in the vitreous of diabetic patients and retina of experimental diabetes models12,14,32,50–54. Likewise, in vivo, cytokine-producing Müller cells are juxtaposed to vascular and neuronal responder cells, causing local concentrations at surface receptors that are higher than those measured in ocular fluids, retinal lysates and sera. Our chosen concentration of IL-1β is well within the range of those concentrations tested in several published studies16,55 mimicking cytokine amplification via autocrine and paracrine mechanisms. Finally, while reduced IL-1β concentrations could also be relevant to DR inflammation, EET/EDPs and sEH inhibition proved efficacious when tested against our model of severe inflammation induced by 1 ng/ml, suggesting efficacy of this therapeutic strategy over a range of inflammatory conditions that reflect DR onset and progression.
Our data demonstrate that exogenous administration of 11,12-EET and 19,20-EDP significantly decreased hMC cytokine expression induced by the two different inflammatory stimuli, PA and IL-1β. We also demonstrated that these epoxide-dependent activities manifest at the protein level in hMC when using experimental conditions that enhanced the levels and biological half-lives of the epoxides in culture (Supp. Fig. 2). While both epoxygenated fatty acids decreased PA-induced TNFα expression, they exacerbated IL-1β-induced TNFα expression, suggesting a different mechanism of action in the two cases. The exact mechanism of action by which EET and EDP function has yet to be determined, though the results of our NFκB-luciferase experiments indicated that both epoxygenated fatty acids decrease cytokine expression, at least in part, by modulating pathways that converge on NFκB-dependent transcription. NFκB is a pro-inflammatory transcription factor that controls the expression of inflammatory cytokines, and it plays an important, well recognized role in early DR pathogenesis12. Similar findings were obtained in our previous studies using human retinal microvascular endothelial cells, and there is ample precedent for this mechanism occurring in other cells and tissues3,56. Saturated fatty acids activate toll-like receptors expressed by MC that are upstream of NFκB-dependent transcription57,58. Additionally, the canonical IL-1β signaling pathway includes NFκB activation5. Therefore, we speculate that EET and EDP decrease IL1β, IL6, IL8 and PA-induced TNFα mRNAs in part by an NFκB-dependent mechanism, while another signaling mechanism becomes overriding in the case of IL-1β-induced effects on TNFα mRNA. We do not consider this observation a deterrent to this therapeutic approach because we have previously shown that EET and EDP decrease TNFα-induced leukocyte adhesion functions in hRMEC3. Therefore, any potentially detrimental effects of MC-derived TNFα on the retinal endothelium would be mitigated downstream.
We also tested the capacity of sEH inhibitor GSK2256294 to reduce inflammatory cytokines in PA- and IL-1β-treated hMC. GSK2256294 blocks the hydrolysis of endogenous EET/EDP, raising their endogenous cellular concentrations to therapeutic levels. The results of several studies indicate that sEH inhibition is a promising therapeutic modality in a wide variety of systems. In our studies, we observed a consistent reduction of cytokine mRNAs across all GSK2256294 concentrations tested (0.1 nM, 1.0 nM, and 10 nM). Interestingly, while hMC responded to sEH inhibition alone, hRMEC do not, suggesting that hMC may be the main sight of bioactive sEH that affects paracrine EET/EDP. Similarly, others have shown that sEH is more highly expressed in MC compared to other retinal cells types3,59. While GSK2256294 potently inhibits sEH activity in HMC, it is important to note that it can exert off-target effects related to the end points explored in this study. For instance, sEH inhibition has been correlated with increased concentrations of lipoxin A4, an anti-inflammatory compound that resolved vascular damage and inflammation60. However, in the present study, this metabolite was not detected when queried in the conditioned medium of MC by mass spectrometric analysis. sEH is constitutively expressed in the retina and is elevated in diabetic murine retina, human retina and in human vitreous3,59. sEH activity in diabetes is thought to be responsible for pericyte loss and endothelial barrier dysfunction by promoting the production of pro-inflammatory diol 19,20-DHDP, the hydrolysis product of 19,20-EDP59. 19,20-DHDP alters the localization of cholesterol-binding proteins in the cell membrane, disrupting pericyte-endothelial cell junctions and inter-endothelial cell junctions59. Like the expression of sEH, the accumulation of 19,20-DHDP is significantly increased in samples from patients with diabetic retinopathy59. To ensure that potential activity from vicinal diols did not confound any of the cytokine measurements observed in our experiments, we treated hMC with 11,12-DHET and 19,20-DHDP. Neither lipid metabolite increased any of the inflammatory cytokines that were assayed in this study.
Mimicking a chronic, multifaceted disease like DR is a challenge in vitro, but in vitro experiments remain crucial tools to dissect the mechanisms of disease in a controlled, step-wise fashion. We used primary human Müller cells in order to maintain physiological relevance in our studies and to more easily translate our findings to future clinical trials in humans. Our proposed therapeutic strategy provides a unique advantage in translation to the clinic because it relies on manipulation of an endogenous system, allowing for protection throughout multiple stages of DR progression, while at the same time minimizing toxicity. Current mainstream therapies focus on mediating late-stage DR morbidities directly associated with vision loss, while herein we propose a strategy that would focus on chronic retinal inflammation in early-stage DR, before irreversible damage has commenced. Our results confirm the anti-inflammatory effects of epoxide elevation in hMC, paving the way for directed in vivo studies. In future studies, we hope to confirm the therapeutic potential of systemically administered epoxides over longer time spans of pathogenesis in in vivo models of DR. These studies will be enabled by the recent development of water-soluble analogues of the epoxygenated fatty acids, as they will overcome issues of hydrophobicity and turnover of the parent EET/EDPs, enhancing their systemic circulation and bioavailability61. In conclusion, our data indicate that therapeutic manipulations to increase retinal levels of epoxygenated fatty acids offer the potential to be highly efficacious in the treatment of DR.
Methods
Human Müller cell culture
Human tissue samples were obtained courtesy of the Advancing Sight Network, Birmingham Alabama. All experiments were approved and performed in accordance with guidelines by the Vanderbilt University Medical Center Institutional Biosafety Committee. Human Müller cells (hMC) were isolated from human donor tissue (NDRI, Philadelphia, PA, USA) within 24 h postmortem. The retinas were dissected from the eyecups and dissociated in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies; Carlsbad, CA) containing trypsin and collagenase (Worthington Biochemical Corp; Lakewood, NJ). Following incubation in dissociation medium, cells were grown in DMEM containing 10% fetal bovine serum (FBS) (R&D Systems; Minneapolis, MN) and 1X antibiotic/antimycotic solution (Thermo Fisher Scientific Asheville LLC; Asheville, NC). Cells were incubated at 37 °C, 5% CO2, 20.9% O2, and 95% relative humidity. Collectively, these conditions favor the survival of MC over other retinal cell types62. If needed, cultures were policed for removal of non-MC or colonies of pure MC were sub-cloned into a new dish. Final MC purity of cultures was > 97% and was determined by immunohistochemistry IHC with antibodies against cellular retinaldehyde-binding protein (CRALBP), glutathione synthetase (GS), and glial fibrillary acidic protein (GFAP). Passages 4 to 6 were used for all experiments.
Human Müller cell treatment (SKF-525a, PA, IL-1β, 11,12-EET, 19,20-EDP, GSK2256294)
In preparation for treatment, hMC were seeded in 6-well dishes and grown to 70% confluence using 10% FBS-containing DMEM culture medium. Culture media were changed to serum-reduced conditions (2% FBS) for 12 h before treatment. Cells were treated with SKF-525a (5.0 μM; ENZO Life Science, Farmingdale, NY, USA) or vehicle for 24 h. Experiments using PA as a stimulus are described as follows. Cells were treated for 24 h in 2% FBS medium with BSA-bound palmitic acid (PA; 250 μM; Sigma-Aldrich; St Louis, MO) or fatty acid-free BSA vehicle (100 mg/ml in PBS; Sigma-Aldrich; St Louis, MO). BSA-bound PA was prepared by dissolving PA in EtOH at 200 mM. This PA/EtOH solution was mixed for 2 h at 37 °C with 100 mg/ml BSA in PBS to yield 5 mM PA before dilution to the final concentration of 250 μM in culture media. hMC treated with BSA-bound PA were co-treated during the final 3 h of the 24-h PA treatment with 0.1 nM, 1.0 nM, or 10 nM GSK2256294 (sEH inhibitor; Axon Medchem LLC; Reston, VA); 11,12-EET (0.5 μM; Cayman Chemical; Ann Arbor, MI); or 19,20-EDP (0.5 μM; Cayman Chemical; Ann Arbor, MI). Experiments using IL1β as a stimulus are described as follows. Cells were treated for 8 h in 2% FBS-containing DMEM culture medium supplemented with 1.0 ng/ml of human recombinant protein IL-1β (R&D Systems; Minneapolis, MN) and vehicle, 0.1 nM, 1.0 nM, or 10 nM GSK2256294; 11,12-EET (0.5 μM); or 19,20-EDP (0.5 μM). In experiments using GSK2256294, cells were pretreated with corresponding concentrations for 2 h before treatment with IL-1β. In all experiments, epoxygenated fatty acid concentrations (0.5 μM) were based on our previously published studies and literature precedents.
Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) of IL-1β, IL-6, IL-8 and TNFα mRNAs
After treatment, cells were washed twice with cold PBS, lysed with RNeasy Lysis Buffer (RLT; Qiagen; Germantown, MD), and total RNA was isolated using an RNeasy Mini kit (Qiagen; Germantown, MD). RNA was reverse transcribed to cDNA using the High-Capacity cDNA Archive Kit (Applied Biosystems; Waltham, MA). qRT-PCR was performed in duplicate by co-amplification of cDNA vs. 18S using gene-specific TaqMan Gene Expression Assays (Applied Biosystems). The delta Ct method was used to determine relative expression of the targeted mRNA normalized to 18S levels. These commercial assays were performed according to the manufacturer’s protocol.
NFκB promoter assay
hMC were seeded on 96-well black-walled, clear bottom plates. Each well was transfected with NFκB-luciferase promoter-reporter, negative control, or positive control constructs, from the Cignal NFκB Reporter Assay (Qiagen). Seventy-five μL of fresh 10% medium was added to each well 30 min prior to transfection. A transfection mixture was prepared in a separate PCR tube, consisting of 200 ng of construct, 1.8μL of Targefect solution A (Targeting Systems; El Cajon, CA), and 3.6μL Virofect (Targeting Systems) in 50μL of Optimem (Life Technologies). Fifteen tube inversions were performed between the additions of each reagent, and the transfection mixture was incubated at 37 °C for 25 min before use. Fifty μL of the transfection mixture was added per well of cultured hMC. Twelve hours after transfection, cells were washed and treated with fresh 10% medium for 12 h. Twenty-four hours post-transfection, cells were treated with vehicle, IL-1β (1.0 ng/ml) or PA-BSA (250 μM) in the presence or absence of 11,12-EET (0.5 μM) or 19,20-EDP (0.5 μM) for 4 h and 8 h respectively. Luciferase activity was quantified using the Dual-Glo Luciferase Assay System (Promega; Madison, WI), according to the manufacturer’s protocol. Data are reported as the relative ratio of firefly-to-renilla luciferase.
Statistical analysis
Data were analyzed using Prism software (GraphPad; La Jolla, CA). T Test and ANOVA with Tukey’s multiple comparisons post-hoc test were used to evaluate significant differences among treatment groups. Values of p < 0.05 were considered statistically significant.
Consent for publication
All authors consent for publication.
Supplementary Information
Acknowledgements
This work was supported by NIH grants R01 EY007533, R01 EY023397, T32 EY021453, T32 EY007135, a grant from the Carl Marshall Reeves and Mildred Almen Reeves Foundation, and Research to Prevent Blindness, Inc.
Author contributions
Work conceived by M.E.C., C.D.O. and J.S.P. Experiments performed by M.E.C., M.J.K., G.W.M. and C.D.O. Data graphed and analyzed by C.D.O. G.W.M. and C.D.O. wrote the manuscript. Project financed by J.S.P. All authors edited and approved final manuscript.
Data availability
Data and materials will be available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Supplementary Information file published with this Article contained errors. In the Supplementary Figure 2, the scale of the y-axis in Panel (a) was incorrect. Furthermore, under the subheading ‘Soluble Protein Quantification Method’, “Secreted protein concentrations are reported as pg/mL/µg total cellular protein.” now reads: “Secreted protein concentrations are reported as pg/mL.”
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Cayla D. Ontko and Megan E. Capozzi.
Change history
9/16/2021
A Correction to this paper has been published: 10.1038/s41598-021-98425-7
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-89000-1.
References
- 1.Yau JWY, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N. Engl. J. Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Capozzi ME, Hammer SS, McCollum GW, Penn JS. Epoxygenated fatty acids inhibit retinal vascular inflammation. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep39211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, K. What Is Diabetic Retinopathy? American Academy of Ophthalmology (2019).
- 5.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group. TASG. AES Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keech A, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 8.Capozzi ME, Giblin MJ, Penn JS. Palmitic acid induces müller cell inflammation that is potentiated by co-treatment with glucose. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-23601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegde KR, Varma SD. Electron impact mass spectroscopic studies on mouse retinal fatty acids. Ophthalmic Res. 2009;42:9–14. doi: 10.1159/000219679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chorváthová V, Ondreicka R. The fatty acid composition of the tissues of streptozotocin-diabetic rats. Physiol. Bohemoslov. 1982;32:466–475. [PubMed] [Google Scholar]
- 11.Capozzi, M. E., McCollum, G. W., Cousins, D. B. & Penn, J. S. Linoleic Acid is a Diabetes-relevant Stimulator of Retinal Inflammation in Human Retinal Muller Cells and Microvascular Endothelial Cells. J Diabetes Metab7, (2016). [DOI] [PMC free article] [PubMed]
- 12.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy. J. Clin. Cell. Immunol. 2011;1:1–12. doi: 10.4172/2155-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammad G, Mairaj Siddiquei M, Imtiaz Nawaz M, Abu El-Asrar AM. The ERK1/2 inhibitor U0126 attenuates diabetes-induced upregulation of MMP-9 and biomarkers of inflammation in the retina. J. Diabetes Res. 2013;2013:1–9. doi: 10.1155/2013/658548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu A, Krady J, Levison S. Interleukin-1: a master regulator of neuroinflammation. J. Neurosci. Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Costa M, Gerhardinger C. IL-1β is upregulated in the diabetic retina and retinal vessels: Cell-specific effect of high glucose and IL-1β autostimulation. PLoS ONE. 2012;7:1–8. doi: 10.1371/journal.pone.0036949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Investig. Ophthalmol. Vis. Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- 18.Gong Y, et al. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am. J. Clin. Nutr. 2017;106:16–26. doi: 10.3945/ajcn.117.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougle DR, et al. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA. 2017;114:E6034–E6043. doi: 10.1073/pnas.1610325114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim AS, et al. A lipidomic screen of hyperglycemia-treated hrecs links 12/15-lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase. J. Lipid Res. 2015;56:599–611. doi: 10.1194/jlr.M056069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naveh-Floman N, Weissman C, Belkin M. Arachidonic acid metabolism by retinas of rats with streptozotocin-induced diabetes. Curr. Eye Res. 1984;3:1135–1139. doi: 10.3109/02713688409000813. [DOI] [PubMed] [Google Scholar]
- 22.Lecomte M, Paget C, Ruggiero D. Docosahexaenoic acid is a major n-3 polyunsaturated fatty acid in bovine retinal microvessels. J. Neurochem. 1996;66:2160–2167. doi: 10.1046/j.1471-4159.1996.66052160.x. [DOI] [PubMed] [Google Scholar]
- 23.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta Proteins Proteomics. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Liu H, Rojas M, Caldwell RW, Caldwell RB. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy. 2011;3:609–628. doi: 10.2217/imt.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, et al. ω-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113–115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin Y, Chen F, Wang W, Wang H, Zhang X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol. Vis. 2017;23:242–250. [PMC free article] [PubMed] [Google Scholar]
- 27.Demarquoy J, Borgne FL. Biosynthesis, metabolism and function of protectins and resolvins. Clin. Lipidol. 2014;9:683–693. [Google Scholar]
- 28.Anandana S-K, et al. 1-(1-Acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea (AR9281) as a potent, selective, and orally available soluble epoxide hydrolase inhibitor with efficacy in rodent models of hypertension and dysglycemia. Bioorg Med Chem Lett. 2011;21:983–988. doi: 10.1016/j.bmcl.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luther, J. M. Soluble Epoxide Hydrolase Inhibition and Insulin Resistance. ClincalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03486223?term=soluble+epoxide+hydrolase&draw=2&rank=2 (2020).
- 30.Green, J. Arete Therapeutics Initiates Phase IIa Clinical Trial for AR9281, a Novel s-EH Inhibitor to Treat Type 2 Diabetes Phase II Study in Patients with Impaired Glucose Tolerance Designed to Confirm Therapeutic Activity. BioSpace.
- 31.Tsai SH, Hein TW, Kuo L, Yang VC. High glucose impairs EDHF-mediated dilation of coronary arterioles via reduced cytochrome P450 activity. Microvasc. Res. 2011;82:356–363. doi: 10.1016/j.mvr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Schwartzman ML, et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010;59:1780–1788. doi: 10.2337/db10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettaieb A, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J. Biol. Chem. 2013;288:14189–14199. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo P, et al. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J. Pharmacol. Exp. Ther. 2010;334:430–438. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coughlin BA, Feenstra DJ, Mohr S. Müller cells and diabetic retinopathy. Vis. Res. 2017;139:93–100. doi: 10.1016/j.visres.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A, Pandey RK, Miller LJ, Singh PK, Kanwar M. Müller glia in retinal innate immunity: a perspective on their roles in endophthalmitis. Crit. Rev. Immunol. 2013;33:119–135. doi: 10.1615/critrevimmunol.2013006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 38.Node K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, et al. Müller glia cells regulate notch signaling and retinal angiogenesis via the generation of 19,20-dihydroxydocosapentaenoic acid. J. Exp. Med. 2014;211:281–295. doi: 10.1084/jem.20131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morisseau C, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bystrom J, et al. Endogenous epoxygenases are modulators of monocyte/macrophage activity. PLoS ONE. 2011;6:4–11. doi: 10.1371/journal.pone.0026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, et al. Mechanisms of vascular dysfunction in COPD and effects of a novel soluble epoxide hydrolase inhibitor in smokers. Chest. 2017;151:555–563. doi: 10.1016/j.chest.2016.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Therapeutics, A. Arete Therapeutics Presents Positive Clinical and Preclinical Data for AR9281. La Merie Buisness Intelligencehttps://pipelinereview.com/index.php/2009060927441/Small-Molecules/Arete-Therapeutics-Presents-Positive-Clinical-and-Preclinical-Data-for-AR9281.html (2009).
- 44.Kozak W, Aronoff DM, Boutaud O, Kozak A. 11,12-Epoxyeicosatrienoic acid attenuates synthesis of prostaglandin E2 in rat monocytes stimulated with lipopolysaccharide. Exp. Biol. Med. 2003;228:786–794. doi: 10.1177/15353702-0322807-03. [DOI] [PubMed] [Google Scholar]
- 45.Knickle LC, Bend JR. Bioactivation of arachidonic acid by the cytochrome P450 monooxygenases of guinea pig lung: The orthologue of cytochrome P450 2B4 is solely responsible for formation of epoxyeicosatrienoic acids. Mol. Pharmacol. 1994;45:1273–1280. [PubMed] [Google Scholar]
- 46.Clore JN, Allred J, White D, Li J, Stillman J. The role of plasma fatty acid composition in endogenous glucose production in patients with type 2 diabetes mellitus. Metabolism. 2002;51:1471–1477. doi: 10.1053/meta.2002.35202. [DOI] [PubMed] [Google Scholar]
- 47.Yi L, et al. Simultaneously quantitative measurement of comprehensive profiles of esterified and non-esterified fatty acid in plasma of type 2 diabetic patients. Chem. Phys. Lipids. 2007;150:204–216. doi: 10.1016/j.chemphyslip.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Jump DB, Grant MB, Esselman WJ, Busik JV. Dyslipidemia, but not hyperglycemia, induces inflammatory adhesion molecules in human retinal vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2003;44:5016–5022. doi: 10.1167/iovs.03-0418. [DOI] [PubMed] [Google Scholar]
- 49.Coll T, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 2008;283:11107–11116. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 50.Dyntar D, et al. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 2001;50:2105–2113. doi: 10.2337/diabetes.50.9.2105. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Liu H, Al-Shabrawey M, Caldwell R, Caldwell R. Inflammation and diabetic retinal microvascular complications. J. Cardiovasc. Dis. Res. 2011;2:96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, Z. et al. Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. J. Diabetes Res.2015, (2015). [DOI] [PMC free article] [PubMed]
- 53.Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn. J. Ophthalmol. 2011;55:256–263. doi: 10.1007/s10384-011-0004-8. [DOI] [PubMed] [Google Scholar]
- 54.McAuley AK, et al. Vitreous biomarkers in diabetic retinopathy: A systematic review and meta-analysis. J. Diabetes Complications. 2014;28:419–425. doi: 10.1016/j.jdiacomp.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Natoli R, et al. Microglia-derived IL-1β promotes chemokine expression by Müller cells and RPE in focal retinal degeneration. Mol. Neurodegener. 2017;12:1–11. doi: 10.1186/s13024-017-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Wang B, Ding H, Wang DW, Zeng H. A potential therapeutic effect of CYP2C8 overexpression on anti-TNF-α activity. Int. J. Mol. Med. 2014;34:725–732. doi: 10.3892/ijmm.2014.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang S, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar, A. & Shamsuddin, N. Retinal muller glia initiate innate response to infectious stimuli via toll-like receptor signaling. PLoS One7, (2012). [DOI] [PMC free article] [PubMed]
- 59.Hu J, Dziumbla S, Lin J, Bibli S-I, Zukunft S, de Mos J, Awwad K, Frömel T, Jungmann A, Devraj K, Cheng Z, Wang L, Fauser S, Eberhart CG, Sodhi A, Hammock BD, Lie I. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature. 2017;552:248–252. doi: 10.1038/nature25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ono E, et al. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am. J. Respir. Crit. Care Med. 2014;190:886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan MAH, et al. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013;27:2946–2956. doi: 10.1096/fj.12-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro 1. An improved method for isolation and culture. Exp. Eye Res. 1990;51:119–129. doi: 10.1016/0014-4835(90)90063-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials will be available upon request.