Abstract
Body homeostasis is predominantly controlled by hormones secreted by endocrine organs. The central nervous system contains several important endocrine structures, including the hypothalamic-pituitary axis. Conventionally, neurohormones released by the hypothalamus and the pituitary gland (hypophysis) have received much attention owing to the unique functions of the end hormones released by their target peripheral organs (e.g., glucocorticoids released by the adrenal glands). Recent advances in mouse genetics have revealed several important metabolic functions of hypothalamic neurohormone-expressing cells, many of which are not readily explained by the action of the corresponding classical downstream hormones. Notably, the newly identified functions are better explained by the action of conventional neurotransmitters (e.g., glutamate and GABA) that constitute a neuronal circuit. In this review, we discuss the regulation of appetite and metabolism by hypothalamic neurohormone-expressing cells, with a focus on the distinct contributions of neurohormones and neurotransmitters released by these neurons.
Subject terms: Hypothalamus, Homeostasis
Metabolism: Dual function for neurohormone-producing cells in the brain
Signaling molecules produced by the brain’s hypothalamus function both as neurotransmitters (within the central nervous system) and hormones (throughout the rest of the body) to regulate appetite and metabolism. Jong-Woo Sohn and colleagues from the Korea Advanced Institute of Science and Technology in Daejeon, South Korea, summarize the well-established ways in which certain hypothalamic cells interact with parts of the pituitary gland in the brain to control the activity of hormones involved in feeding behaviors and energy balances.The same cells can also impact appetite and metabolism in non-hormonal ways. New research has shown that neurohormone-producing cells in the hypothalamus can form connections with appetite-associated neurons and communicate via neurotransmitters. A deeper understanding of this process could lead to new therapies for obesity, diabetes and other metabolic disorders.
Introduction
The hypothalamus is a vital region of the brain that regulates whole-body homeostasis. Hypothalamic neuroendocrine cells control homeostasis through the production and secretion of neurohormones into the general circulation1. Similar to other endocrine organs, some hypothalamic neuroendocrine cells secrete end hormones (e.g., oxytocin [OXT] and vasopressin [VP]), which are delivered to their target organs, where they produce specific effects. Other types of hypothalamic neuroendocrine cells secrete releasing hormones, such as corticotropin-releasing hormone (CRH). Releasing hormones are secreted into the hypophyseal portal system and consequently excite a second population of neuroendocrine cells in the anterior pituitary gland (adenohypophysis) to secrete stimulating hormones, such as adrenocorticotropic hormone (ACTH). This type of functional connection between hypothalamic and pituitary neuroendocrine cells is referred to as the hypothalamic-pituitary (HP) axis, which constitutes a major component of the hypothalamic neuroendocrine system.
The HP axis provides important humoral responses to challenges such as stress and cold. The HP-adrenal (HPA) axis releases glucocorticoids from the adrenal cortex in response to stress, and the HP-thyroid (HPT) axis releases thyroid hormones from the thyroid gland in response to cold2,3. Hormones released by the HPA and HPT axes were also shown to regulate appetite and metabolism. Glucocorticoids were shown to increase appetite and affect glucose homeostasis4, and thyroid hormones tend to promote appetite, increase heat generation, and reduce body weight5. Human diseases associated with dysfunction of the HPA axis (e.g., Cushing syndrome) and the HPT axis (e.g., Graves’ disease) are characterized by abnormal feeding behavior and/or perturbed metabolism, which confirms the above-mentioned effects of these hormones6,7. Additionally, CRH and thyrotropin-releasing hormone (TRH) are reported to control appetite and metabolism8–10. These findings highlight the potential importance of hypothalamic CRH and TRH neurons as well as the HPA and HPT axes in the regulation of energy homeostasis and metabolism.
All neurons that express neurohormones are not “neuroendocrine” cells; some neurohormone-expressing neurons (e.g., CRH and TRH neurons) do not project to the median eminence11,12. Therefore, neurohormone-expressing cells comprise neuroendocrine and non-neuroendocrine cells, and the term “neuroendocrine cells” may only be used to refer to neurons that project to the median eminence and release neurohormones. However, a subset of CRH neurons located in the paraventricular nucleus of the hypothalamus (PVH) labeled with peripherally injected fluorogold also showed retrograde labeling of fluorescent beads injected into the lateral hypothalamic area (LHA)13. These results suggest that neuroendocrine and non-neuroendocrine cells are not always completely segregated within a single population of neurohormone-expressing cells. However, throughout this review, we use the expression “neuroendocrine cells” only to refer to neuroendocrine neurohormone-expressing cells.
The non-neuroendocrine neurohormone-expressing cells of the hypothalamus constitute a neural circuitry that utilizes conventional neurotransmitters. For example, previous studies have reported that TRH neurons within the PVH form glutamatergic synapses on orexigenic (appetite-promoting) agouti-related peptide (AgRP) neurons within the arcuate nucleus of the hypothalamus (ARH), the stimulation of which increases appetite14. Moreover, PVH CRH neurons send glutamatergic fibers to neurons of the LHA, which is reportedly involved in stress behaviors13. OXT neurons within the PVH were also shown to transmit glutamatergic projections to the lateral parabrachial nucleus (PBN) to regulate fluid intake15. Therefore, it is important to consider the neural (non-neuroendocrine) circuitry collectively with the neuroendocrine axis to gain a deeper and complete understanding of the role of hypothalamic neurohormone-expressing cells.
In this review, we summarize the endocrine function of selected hypothalamic neurohormone-expressing cells and the relevant HP axis that regulates appetite and metabolism. We also discuss recent studies that investigated the role of conventional neurotransmitters released by these neurons.
Hypothalamic neuroendocrine cells and metabolic function
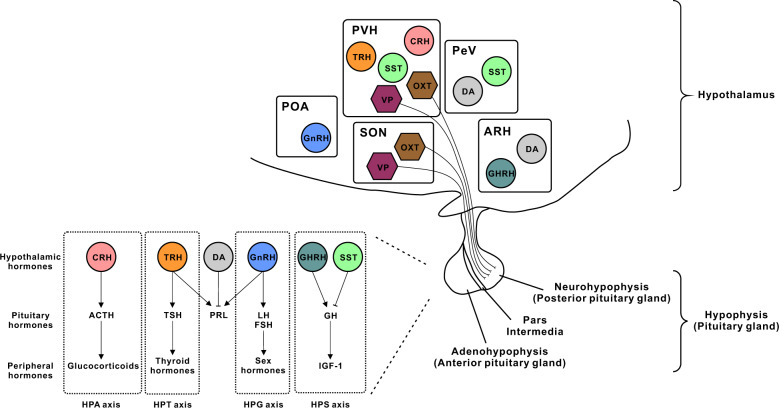
Hypothalamic neuroendocrine cells release neurohormones that regulate the homeostatic function of the pituitary gland (hypophysis) (Fig. 1). Usually, hypothalamic neuroendocrine cells are classified depending on the mode of control of pituitary hormone secretion. A specific class of hypothalamic neuroendocrine cells synthesizes and transports hormones to axon terminals, which constitute the posterior pituitary gland (neurohypophysis). These posterior pituitary hormones include OXT and VP (also called arginine VP [AVP] or anti-diuretic hormone [ADH]) (Fig. 1). Neuroendocrine cells that release OXT and VP are located in both the PVH and the supraoptic nucleus (SON)16. OXT causes contraction of the smooth muscles of the uterus and mammary gland, and VP acts on the kidneys to promote water reabsorption17. These posterior pituitary hormones and the OXT and VP neurons are also known to control appetite and metabolism. For example, OXT administration causes anorexia18, and hypothalamic lesions of OXT neurons result in hyperphagia and obesity19. OXT neurons within the PVH were shown to be inhibited by AgRP neurons within the ARH20. OXT also produces several beneficial metabolic effects, such as an increase in energy expenditure and promotion of lipolysis18. On the other hand, experimental evidence suggests that VP suppresses food intake21. Recent studies in mouse models have reported that chemogenetic activation of VP neurons within the PVH induced anorexia, whereas chemogenetic inhibition of these neurons partially reversed anorexia induced by MTII, a melanocortin-4 receptor (MC4R) agonist22. Reportedly, chemogenetic activation of VP neurons located in the PVH, SON, and suprachiasmatic nucleus reduces food intake in rats23, further supporting the anorexigenic (appetite-suppressing) property of VP neurons.
Fig. 1. Hypothalamic neuroendocrine cells and hormones.
Hypothalamic neuroendocrine cells regulate the adenohypophysis via the HP axis and the neurohypophysis through their axons. Note that the axons of the OXT and VP neurons constitute the neurohypophysis. The axons of other hypothalamic neurons are not shown for clarity. See the text for abbreviations.
Another class of hypothalamic neuroendocrine cells regulates the secretion of hormones from the adenohypophysis via the hypophyseal portal system. These anterior pituitary hormones include CRH, TRH, growth hormone-releasing hormone (GHRH), somatostatin (SST), gonadotropin-releasing hormone (GnRH), and dopamine (DA)1 (Fig. 1). CRH and TRH neurons within the PVH activate the HPA and HPT axes to stimulate the release of ACTH and thyroid-stimulating hormone (TSH), respectively, from the adenohypophysis. The release of ACTH results in the release of glucocorticoids from the adrenal gland, and the release of TSH induces secretion of thyroid hormones from the thyroid gland1. The HP-somatotropic (HPS) axis is bidirectionally controlled by hypothalamic neuroendocrine cells with opposite functions. This axis is upregulated by GHRH neurons in the ARH, which initiates the release of growth hormone (GH) from the adenohypophysis and insulin-like growth factor-1 (IGF-1) from the liver24,25. Conversely, SST neurons in the periventricular nucleus (PeV) and PVH downregulate the HPS axis by inhibiting GH secretion25,26. The HP-gonadal (HPG) axis is stimulated by GnRH neurons in the preoptic area (POA), which causes secretion of follicle-stimulating hormone (FSH)/luteinizing hormone (LH) and sex hormones1. DAergic neurons in the PeV and ARH downregulate prolactin (PRL) release1,27, whereas several neurohormones including TRH and GnRH trigger PRL release28,29. In contrast to DA, which is an amine, hypothalamic neurohormones are peptide hormones.
Hormones of the HP axes are associated with multiple metabolic functions. For example, estradiol is a gonadal steroid hormone released by stimulation of the HPG axis. Studies have shown that food intake is reduced close to the time of ovulation and that postmenopausal women gain body weight; therefore, estradiol was proposed to exert anti-obesity effects30. Later, investigators attributed the anti-obesity effects of estradiol to estrogen receptor-α expressed by pro-opiomelanocortin (POMC) neurons within the ARH and steroidogenic factor-1 neurons within the ventromedial nucleus of the hypothalamus (VMH)31. As mentioned earlier, hormones controlled by the HPA and HPT axes are known to regulate appetite and metabolism4,5,8,9. Compared with the functions of hormones released by the neurohypophysis and the other HP axes, the “classical” function of glucocorticoids and thyroid hormones is more relevant to the regulation of energy balance and glucose homeostasis. Interestingly, glucocorticoids and thyroid hormones promote food intake4,5, whereas CRH and TRH produce anorexigenic effects8,9. These findings suggest a complex nature of appetite and metabolism regulation by the HPA and HPT axes. Recent studies have observed that both CRH and TRH neurons within the PVH release glutamate to regulate homeostasis13,14, which highlights the non-neuroendocrine function of these neurohormone-expressing cells. Therefore, investigators believe that the “neuroendocrine” subsets of CRH and TRH neurons utilize the HPA and HPT axes, whereas other “non-neuroendocrine” cell populations affect appetite and metabolism via neural circuitry. In the following sections, we review the available literature to discuss the role of CRH neurons/the HPA axis and TRH neurons/the HPT axis in the regulation of appetite and metabolism, with a focus on the differences in the functions of each hormone and distinctions between neural and endocrine regulation.
Role of the HPA axis and CRH neurons
CRH is a 41-amino acid peptide that is widely expressed throughout the body, including the brain32,33. Available evidence suggests that in addition to playing a pivotal role in stress-related responses34, CRH actively participates in the regulation of energy metabolism35. Most CRH neurons that control the HPA axis are located within the PVH, particularly in the lateral part36. CRH neurons within the PVH may affect appetite and metabolism either intrinsically or via HPA axis stimulation37,38. Additionally, CRH neurons located outside the hypothalamus may function independent of the HPA axis. We discuss the control of appetite and metabolism by the HPA axis and the CRH neurons expressed throughout the brain.
The HPA axis
Glucocorticoids are secreted from the adrenal cortex following HPA axis activation. Corticosterone (CORT), which is the major glucocorticoid in rodents, induces hyperphagia and increases fat mass and body weight39–42 (Table 1). Chronic administration of exogenous CORT concomitant with high-fat diet (HFD) feeding did not cause further increases in fat mass and body weight43,44, although obesity secondary to HFD feeding is associated with elevated blood CRH, ACTH, and CORT levels. Exogenous CORT administration induced whitening of brown adipose tissue (BAT), which is associated with downregulation of uncoupling protein-1 (UCP-1) expression, as well as expansion of white adipose tissue (WAT)42. CORT administration was shown to elevate plasma triglyceride (TG) levels and increase the liver TG content39,42, which was associated with increased expression of genes involved in hepatic fatty acid metabolism. CORT injections also tended to increase the blood free fatty acid (FFA) level, although the difference was not statistically significant42. However, energy expenditure was unchanged in mice after CORT administration, regardless of whether the mice were fed a HFD or a chow diet40,43. Locomotive activity remained unchanged43 or was decreased39 after exogenous CORT administration. These results suggest that CORT-induced obesity is largely due to increased appetite rather than changes in energy consumption. Studies suggest that the orexigenic effects of glucocorticoids are at least partially mediated by modulation of POMC and AgRP neurons within the ARH41,45.
Table 1.
Metabolic effects of exogenous HPA axis hormones.
| Hormone | Application routes (animal model) | Food intake | Body weight/fat and lean mass | Energy expenditure parameters | Glucose balance | Lipid metabolism | Ref. |
|---|---|---|---|---|---|---|---|
| CORT |
In drinking water (mouse) |
↑ |
↑ Body weight ↑ Gonadal WAT mass |
↓ Locomotive activity |
↑ Insulin ↔ Glucose (after 2 weeks) ↑ Glucose (after 4 weeks) |
↑ Blood TG | 39 |
|
In drinking water (mouse) |
N.D. | ↔ Body weight |
↔ VO2 ↔ Locomotive activity |
↔ GTT ↔ ITT ↔ Insulin |
N.D. | 43 | |
|
In drinking water (mouse) |
↑ |
↑ Body weight ↑ Fat mass ↓ Lean mass |
↔ RER | N.D. | N.D. | 40 | |
| Via pellet implantation (mouse) | ↑ |
↓ Body weight gain ↑ BAT and WAT mass (↓ UCP-1) |
N.D. | ↔ Glucose |
↑ Blood TG ↑ Liver TG ↔ Blood FFA (a non-significant increase) |
41,42 | |
| ACTH |
s.c. injection (rat) |
↓ | ↓ Body weight | N.D. | ↔ Glucose |
↔ Blood TG ↔ Blood FFA ↔ Blood Total cholesterol ↔ Blood HDL cholesterol ↑ Blood LDL cholesterol |
47,51 |
| CRH |
i.c.v. injection (rat) |
↓ |
↓ Body weight ↓ Retroperitoneal and epididymal fat pads |
↑ VO2 ↓ RER ↑ Locomotive activity ↑ Sympathetic activity |
↔ Glucose | N.D. | 8,35,52,53 |
|
PVH injection (rat) |
↓ | N.D. | N.D. | N.D. | N.D. | 54 | |
|
LS injection (rat) |
↓ | ↔ Body weight | N.D. | N.D. | N.D. | 55 |
N.D. not determined, see text for abbreviations.
Previous studies have also reported the effects of exogenous CORT on glucose metabolism. It is well known that glucocorticoid-induced gluconeogenesis and glycogenolysis result in increased blood glucose levels38. Dexamethasone (a glucocorticoid analog) has been reported to reduce insulin secretion from the isolated pancreas46. However, the effects of glucocorticoids on whole-body glucose homeostasis are equivocal. It was shown that CORT administration for 1 to 2 weeks did not affect glucose homeostasis39,42; however, CORT administration for 4 weeks caused hyperglycemia with increased insulin levels39, although glucose homeostasis was unaffected after 22 weeks of CORT treatment43. These findings suggest that although glucocorticoids may cause hyperglycemia, they appear to produce variable effects on long-term glucose homeostasis depending on the experimental conditions.
Interestingly, the effects of ACTH on appetite and energy consumption contrasted with those induced by glucocorticoids (Table 1). For example, subcutaneous ACTH injections administered for a month caused anorexia and reduced body weight47. In addition, treatment of primary cultures of BAT and WAT with ACTH led to elevated UCP-1 levels48. The effects of ACTH on lipid and glucose metabolism were also different from those produced by glucocorticoids. ACTH was shown to cause a transient increase in insulin secretion from the isolated pancreas49 and release of FFAs from isolated epididymal fat tissue50 under ex vivo conditions. However, ACTH injections administered in vivo for 3 consecutive days did not affect blood glucose, TG and FFA levels51. Blood levels of total cholesterol and high-density lipoprotein (HDL) cholesterol were also unchanged by in vivo ACTH injections, although this treatment increased plasma CORT and low-density lipoprotein (LDL) cholesterol levels51. Currently, no plausible explanation is available for the disparate observations; however, these results could be attributed to the possibility that exogenous ACTH affects the HPA axis and glucocorticoid secretion under some experimental conditions. Further studies utilizing knockout or knockdown of endogenous ACTH, as described below for CRH, are required to resolve this issue.
The effects of exogenous CRH on energy homeostasis are also markedly different from those of CORT, although these effects are consistent with those of exogenous ACTH administration (Table 1). For example, intracerebroventricular (i.c.v.) administration of CRH resulted in reduced appetite, decreased body weight gain, reduced fat pad weight, and increased sympathetic activity without any effects on blood glucose levels8,52,53. Another study demonstrated that i.c.v. injections of CRH increased oxygen consumption (VO2) and locomotion concomitant with a decrease in the respiratory exchange ratio (RER)35. CRH injections into the PVH reduced food intake in rats54, and CRH injections into the lateral septal area (LS) caused anorexia in food-deprived mice with no effects on body weight55. However, CRH-deficient mice did not show any defects in feeding behavior56,57 or even showed anorexigenic phenotypes58,59, which suggests that experiments using exogenous CRH may not accurately predict the metabolic effects of endogenous CRH. These results also emphasize the necessity of loss-of-function experiments to corroborate the findings of experiments using exogenous hormones to investigate the role of the HPA axis in the regulation of appetite and metabolism.
CRH neurons
Studies in mice showed that chemogenetic inhibition or toxin-induced ablation of PVH CRH neurons did not alter acute or chronic food intake or body weight60,61 (Table 2). Interestingly, increased excitatory synaptic input onto PVH CRH neurons was associated with the anorexigenic effects of glucagon-like peptide-1 (GLP-1) signaling that originates in the nucleus tractus solitarius (NTS), and chemogenetic inhibition of PVH CRH neurons significantly attenuated the anorexigenic effects of GLP-1 signaling62. In this study, optogenetic stimulation of PVH CRH neurons, which presumably mimics the effects of GLP-1 signaling, suppressed food intake62. In another study, impaired activity of PVH CRH neurons exaggerated neuropeptide Y (NPY)-induced hyperphagia63. It was recently reported that fasting-induced activation of AMP-activated kinase (AMPK) causes increased activity of PVH CRH neurons, which contributes to a preference for a high-carbohydrate diet (nutrition) over a HFD (palatability) under fasting conditions64. A more recent study demonstrated that HFD feeding blunted the responsiveness of PVH CRH neurons and that mice with blunted PVH CRH neuronal responsiveness were more likely to develop HFD-induced obesity65. Overall, these studies suggest that PVH CRH neurons may participate in the fine tuning of food intake rather than control food intake per se. It is currently unknown whether appetite modulation by PVH CRH neurons occurs via the action of hormones or neurotransmitters. Considering the conflicting reports on the role of endogenous CRH in appetite regulation56–59, it can be inferred that the observed alterations in food intake are largely mediated by glutamate, which is a classical neurotransmitter released by PVH CRH neurons. Notably, defective glutamatergic neurotransmission from single-minded-1 neurons, which constitute the largest proportion of PVH neurons, was shown to cause obesity66. PVH CRH neurons send monosynaptic projections to various brain regions13,67 and polysynaptic projections to peripheral tissues and organs that regulate metabolism, such as WAT and the liver68. Therefore, defective glutamatergic neurotransmission from PVH CRH neurons is also likely to play a role in the pathophysiology of obesity.
Table 2.
Metabolic phenotypes of CRH and TRH neuronal activity modulation.
| Target nucleus | Neuronal population (animal model) | Modulation of neuronal activity (viral construct) | Involved neural circuitry | Observed phenotype | Ref. |
|---|---|---|---|---|---|
| PVH |
CRH neuron (Crh-ires-cre mouse) |
Chemogenetic inhibition (AAV8-DIO-hM4Di-mCherry) |
N.D. | ↔ Food intake | 60 |
|
CRH neuron (Crh-ires-cre mouse) |
Neuronal ablation (AAV-DJ-CMV-DIO-eGFP-2A-TeNT) |
N.D. |
↔ Food intake ↔ Body weight |
61 | |
|
CRH neuron (Crh-ires-cre mouse) |
Chemogenetic activation (AAV-hSyn-DIO-hM3Dq-mCherry) |
N.D. | ↓ Food intake | 62 | |
|
Chemogenetic inhibition (AAV-hSyn-DIO-hM4Di-mCherry) |
N.D. | ↓ Anorexia by GLP-1 | |||
|
TRH neuron (Trh-ires-cre mouse) |
Chemogenetic activation (AAV8-DIO-hM3Dq-mCherry) |
Glutamatergic innervation of AgRP neurons within the ARH | ↑ Food intake | 14 | |
| CeA |
CRH neuron (Crh-ires-cre mouse) |
Optogenetic activation (AAV2-EF1α-DIO-ChR2-EYFP) |
N.D. | ↔ Food intake | 73 |
|
CRH neuron (Crh-ires-cre mouse) |
Chemogenetic activation (AAV2-hSyn-DIO-hM3Dq-mCherry) |
N.D. |
↔ Food intake (basal) ↓ Food intake (with stress) |
77 | |
| BNST |
CRH neuron (Crh-ires-cre mouse) |
Chemogenetic activation (AAV5-EF1α-DIO-hM3Dq-mCherry) |
N.D. |
↔ Food intake ↔ Body weight |
78 |
N.D. not determined, see text for abbreviations.
Outside the PVH, CRH is highly expressed by neurons of the central amygdala (CeA) and the bed nucleus of the stria terminalis (BNST)69. Conventionally, CRH neurons in the CeA and BNST have received considerable attention for their roles in the development of fear and anxiety70,71. Available evidence suggests that CRH neurons in these regions also participate in the control of appetite and metabolism. A recent study demonstrated that lentiviral knockdown of CRH in the CeA resulted in elevation of the basal but not the stress-induced CORT level72, which suggests that CeA CRH neurons downregulate the basal (non-stress) activity of the HPA axis. These neurons do not directly project to the median eminence; therefore, it is possible that CRH neurons in the CeA inhibit PVH CRH neurons via an unidentified neural circuitry. A previous study reported that photostimulation of CeA CRH neurons did not change food consumption under either fed or fasting conditions73 (Table 2). Moreover, administration of RO27-3225 (an MC4R agonist) and ghrelin failed to induce the expression of Fos, a marker for increased activity, in these neurons74. These results suggest that CeA CRH neurons do not control food intake. However, CRH expression was selectively decreased in rats, which developed compulsive eating upon introduction of palatable food after conditioning with prolonged intermittent presentation of a chow diet and palatable food75. In addition, fast-refeeding paradigms caused elevated CeA CRH neuronal activity in mice fed either a chow diet or a HFD74,76. A more recent study showed that chemogenetic activation of CeA CRH neurons exacerbated novelty-induced suppression of food consumption, whereas food intake and locomotion in the home cage remained unaffected77. Therefore, it can be concluded that CRH neurons in the CeA are dispensable for feeding behavior under normal conditions, although they respond to some stressful conditions to control food intake.
Chemogenetic activation of BNST CRH neurons did not change body weight or food intake78, similar to the findings associated with CeA CRH neurons (Table 2). However, optogenetic stimulation of BNST GABAergic neuronal projections resulted in feeding phenotypes. It was previously shown that optogenetic stimulation of BNST GABAergic neurons (VgatBNST neurons) to LHA projections induced food consumption in fed mice, whereas optogenetic inhibition of this circuit diminished food consumption in fasted mice79. Similarly, food consumption was increased by optogenetic stimulation of axon terminals of VgatBNST neurons innervating PBN neurons in sated mice80. Notably, this manipulation increased the consumption of salty and bitter-tasting food80. BNST CRH neurons are primarily GABAergic; however, it is unclear whether stimulation and inhibition of BNST CRH neuronal projections to the above-mentioned brain areas also affect feeding behavior under comparable experimental conditions.
Role of the HPT axis and TRH neurons
Thyroid hormones released from the thyroid gland include thyroxine (T4), which is converted by the action of deiodinase enzymes into the bioactive form triiodothyronine (T3). Thyroid hormones affect the metabolic rate and synthesis of proteins essential for thermogenesis in cold environments predominantly via peripheral mechanisms81, but they may also affect feeding behavior via their actions on specific areas of the brain82. TRH neurons reportedly regulate appetite and metabolism directly via neural circuitry within the brain14,83. Currently, there is little direct evidence that TRH neurons control appetite and metabolism indirectly through the HPT axis. Below, we discuss the metabolic function of thyroid hormones, TSH, TRH, and TRH neurons in the brain.
Thyroid hormones and TSH
Type 2 deiodinase (D2) is an important enzyme that catalyzes the conversion of endogenous T4 to T3. Local production of T3 in the hypothalamus is controlled by tanycytes, which are glial cells that express D284,85. Fasting is reported to cause a significant increase in hypothalamic T3 and D2 mRNA levels/activity86–88. Conversely, D2-null mice showed reduced food intake in response to a fast-refeeding paradigm, but normal food intake was restored after exogenous T3 injections89. Therefore, increased production of hypothalamic T3 secondary to D2 activity is important for increased food intake in response to acute food deprivation.
Considering that body weight is usually reduced in hyperthyroidism90, increased food intake secondary to the action of thyroid hormones was previously considered a compensatory mechanism for the increased energy consumption in these patients. However, a direct action of T3 on hypothalamic neurons was suggested in a study in which exogenous T3 significantly increased food intake whether it was injected subcutaneously or directly into the VMH, whereas T3 injections into the ARH did not alter food intake86 (Table 3). However, subsequent studies demonstrated that food intake was unaffected by T3 injections into the VMH91,92. In another study, T3 was shown to activate UCP-2 and induce mitochondrial proliferation in orexigenic NPY/AgRP neurons within the ARH, which reportedly contributed to rebound feeding after food deprivation89. T3-induced stimulation of food intake was also attributed to enhanced hypothalamic AMPK activity93, although the specific neuronal population involved remains unclear. Therefore, current experimental evidence suggests that T3 increases food intake via actions in the medial basal region of the hypothalamus, including the VMH and ARH, but further studies are warranted to accurately identify the contributory neuronal populations and the relevant cellular mechanisms.
Table 3.
Metabolic effects of exogenous HPT axis hormones.
| Hormone | Application routes (animal model) | Food intake | Body weight | Energy expenditure parameters | Glucose balance | Lipid metabolism | Ref. |
|---|---|---|---|---|---|---|---|
| T3 |
s.c. injection (rat) |
↑ | ↔ |
↔ VO2 (single or short-term injections) ↑ VO2 (long-term injections) |
N.D. | N.D. | 86 |
|
i.c.v. injection (rat) |
↔ | ↓ | ↑ BAT thermogenesis | N.D. |
↔ Blood TG ↔ Blood FFA ↑ Liver TG |
91,92 | |
|
VMH injection (rat) |
↔ or ↑ | ↓ |
↑ BAT thermogenesis ↓ RER ↓ Locomotive activity |
N.D. |
↑ Blood TG ↑ Hepatic lipogenesis |
86,91,92 | |
| TRH |
s.c. injection (rat) |
↓ | ↔ | ↑ Body temperature | N.D. | N.D. | 98 |
|
i.c. injection (rat) |
↓ | N.D. | N.D. | N.D. | N.D. | 99 | |
|
i.c.v. injection (rat) |
↓ | N.D. | N.D. | ↑ Glucose | N.D. | 9,99,100 | |
|
i.c.v. injection (hamster) |
N.D. | N.D. | ↑ BAT temperature | N.D. | N.D. | 101 | |
|
PVH injection (rat) |
N.D. | N.D. | ↑ Body temperature | ↑ Glucose | N.D. | 102 | |
|
VMH injection (rat) |
↔ | N.D. | ↑ Locomotive activity | N.D. | N.D. | 104 | |
|
VMH injection (hamster) |
N.D. | N.D. | ↑ BAT temperature | N.D. | N.D. | 101 | |
|
DMH injection (hamster) |
N.D. | N.D. | ↑ BAT temperature | N.D. | N.D. | 101 | |
|
LHA injection (rat) |
↓ | N.D. | ↔ Locomotive activity | N.D. | N.D. | 104 | |
|
LHA injection (hamster) |
N.D. | N.D. | ↔ BAT temperature | N.D. | N.D. | 101 |
N.D. not determined, see text for abbreviations.
Thyroid hormones increase thermogenesis and energy consumption primarily via peripheral mechanisms94. However, i.c.v. injections as well as chronic subcutaneous injections of exogenous T3 were shown to increase energy expenditure in rats86,91,92 (Table 3), which suggests the involvement of central mechanisms. Furthermore, T3 injections into the VMH were shown to suppress AMPK function to increase sympathetic activity and promote BAT thermogenesis91,92. Notably, T3-induced suppression of AMPK signaling within the VMH increased vagal activity to increase hepatic lipogenesis and blood TG levels, whereas the decreased RER suggested that BAT utilized blood TGs for thermogenesis92. Overall, it appears that T3 suppresses AMPK signaling within the VMH, which at least partially contributes to T3-mediated promotion of thermogenesis via both the sympathetic and parasympathetic nervous systems.
Currently, limited data are available regarding the role of TSH in the regulation of metabolism. A recent clinical study reported that serum cholesterol and TG levels were positively correlated with TSH levels in children and adolescents with subclinical hypothyroidism, a condition characterized by elevated TSH but normal T4 levels95,96. This finding suggests that elevated TSH levels may be an early indicator of an adverse lipid profile. Another study showed that TSH receptor stimulation may promote WAT and BAT adipogenesis97. Therefore, it is reasonable to conclude that TSH may regulate fat metabolism and increase fat mass, although these effects appear to contradict the effects of thyroid hormones. Further studies are essential to conclusively establish the role of TSH.
TRH and TRH neurons
A previous study showed that subcutaneous injections of exogenous TRH decreased food intake and increased body temperature in rats98 (Table 3). Additionally, intracranial (i.c.) and i.c.v. TRH injections suppressed food intake and increased BAT temperature and blood glucose levels9,99–101. TRH injections into the PVH caused a prompt increase in body temperature and blood glucose as well as CORT levels102. These studies suggest that TRH may inhibit food intake and reduce body weight, as well as stimulate metabolism, via central mechanisms. Exogenous TRH and T3 produced similar effects to increase energy expenditure; however, the anorexigenic effects of exogenous TRH were not consistent with the orexigenic effects of exogenous T3. Perhaps the anorexigenic effects of TRH are independent of the HPT axis; previous studies demonstrated that TRH-induced suppression of food intake was not accompanied by effects on TSH or thyroid hormone levels9,103. Interestingly, TRH injections into different areas of the hypothalamus yielded distinct phenotypes. For example, TRH injections into the VMH stimulated locomotion in rats; however, this effect was not observed after injections into the LHA104. TRH injections into the LHA reduced food intake; however, injections into the VMH did not affect food consumption104. A study performed in hamsters showed an increase in BAT temperature after TRH microinjections into the dorsomedial nucleus of the hypothalamus (DMH) or VMH but not into the LHA101. It is possible that exogenous TRH injected into a specific brain area can spread to adjacent brain sites; therefore, these findings should be confirmed using more sophisticated methods, such as studies in genetically engineered mice and neuron-specific viral injections.
Studies have investigated the cellular and circuit-level mechanisms underlying the development of in vivo phenotypes after exogenous TRH injections. Earlier research showed that TRH administration stimulated glucose-responsive neurons within the VMH105,106, which may explain the anorexigenic effects of i.c.v. TRH9,99,100. In another study, i.c.v. injections of TRH increased histamine turnover in the tuberomammillary nucleus, PVH, and VMH in rats, and the anorexigenic effects of i.c.v. TRH were attenuated in histamine-depleted rats and histamine H1 receptor-null mice107. TRH was also shown to suppress the activity of melanin-concentrating hormone (MCH) neurons in the LHA indirectly by increasing synaptic inhibition through activation of local GABAergic neurons108, which suggests that the metabolic effects of TRH can be attributed to inhibition of MCH neuronal activity. In this study, TRH was shown to excite the hypocretin/orexin neurons in the LHA, although it produced no effect on the activity of POMC or NPY neurons within the ARH108. Therefore, multiple cellular and synaptic mechanisms are implicated in the in vivo phenotypes resulting from exogenous TRH injections. Considering the TRH-induced alterations in the activity of multiple neuronal populations, future experiments should focus on assigning specific neuronal populations to distinct phenotypes of TRH administration.
TRH neurons are located in multiple hypothalamic areas, including the PVH, DMH, VMH, and LHA109. It was previously shown that chemogenetic activation of PVH TRH neurons stimulates feeding via their excitatory glutamatergic projections to the orexigenic AgRP neurons within the ARH14 (Table 2). Additionally, PVH TRH neurons project to the brain stem and spinal cord and activate UCP-1 in BAT to control thermogenesis and body temperature83. PVH TRH neurons are reported to function as targets of major metabolic signals, such as leptin and melanocortin110. In rat models, leptin was shown to regulate TRH production via a combination of a direct action on PVH TRH neurons and an indirect mechanism by which leptin first modulates the activity of ARH neurons, which consequently project to PVH TRH neurons111. Leptin was also reported to directly activate PVH TRH neurons and increase serum T4 levels in fasted mice112. However, a recent study reported that only a few PVH TRH neurons express leptin receptors and that PVH TRH neurons receive few axon fibers originating from ARH POMC neurons in mice113. It is, therefore, reasonable to infer that leptin-induced regulation of PVH TRH neurons shows species-specific differences. MC4R is expressed by most TRH neurons within the PVH114. PVH TRH neurons were shown to receive synaptic input from the POMC and NPY/AgRP neurons within the ARH, constituting the central melanocortin pathway115–117. In vivo studies have also confirmed that i.c.v. injections of α-melanocyte-stimulating hormone can prevent the reduction in Trh gene expression that occurs during fasting114,118. Therefore, the central melanocortin pathway originating from the ARH appears to control PVH TRH neurons via MC4Rs, although the physiological significance of MC4Rs expressed by TRH neurons remains to be determined.
Currently, little is known regarding the function of TRH neurons located outside the PVH. TRH neurons in the LHA were shown to receive input from the POMC and NPY/AgRP neurons within the ARH, which suggests that the activity of TRH neurons in the LHA is affected by the metabolic state of the organism119. However, no studies have investigated the metabolic functions of TRH neurons in the LHA. Further studies are warranted to accurately delineate the functions of TRH neurons located in the above-mentioned hypothalamic areas.
Concluding remarks
Hypothalamic neurohormone-expressing cells control appetite and metabolism via hormones and neurotransmitters. Therefore, the mechanisms underlying their physiological functions are significantly more complicated than those underlying humoral or neural regulation. Various genetic tools are currently available to investigate the neural circuitry associated with various animal behaviors; these tools include genetically engineered mouse models and optogenetic, chemogenetic, and in vivo Ca2+ imaging techniques. In fact, these cutting-edge techniques have revealed several previously unknown mechanisms underlying homeostatic regulation by hypothalamic neurohormone-expressing cells at the molecular, cellular, and circuit levels. To date, these techniques have been applied primarily to understand the contribution of non-neuroendocrine or neural mechanisms to feeding behavior and metabolism. Currently available data regarding the contribution of endocrine mechanisms are derived mainly from experiments using more conventional methods, such as fluorogold labeling, immunostaining, and hormone measurements. Notably, the neuroendocrine effects of these cells are potentially more powerful and long-lasting than the non-neuroendocrine effects; therefore, it is necessary to address this issue using novel techniques with the understanding that these techniques could lead to major scientific breakthroughs. Therefore, further research is warranted to develop effective strategies to evaluate the endocrine and neural functions of hypothalamic neurohormone-expressing cells of interest to determine the relative importance of each aspect in the regulation of feeding behavior and metabolism. This information will enable scientists to gain a deeper understanding of the functions of neurohormone-expressing cells and facilitate the development of novel therapeutic strategies for obesity and metabolic diseases.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2016R1C1B2006614, NRF-2019R1A2C2005161 to J.-W. S.) funded by the Korean Ministry of Science and ICT.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Eun-Seon Yoo, Jieun Yu
References
- 1.Clarke IJ. Hypothalamus as an endocrine organ. Compr. Physiol. 2015;5:217–253. doi: 10.1002/cphy.c140019. [DOI] [PubMed] [Google Scholar]
- 2.Chiamolera MI, Wondisford FE. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150:1091–1096. doi: 10.1210/en.2008-1795. [DOI] [PubMed] [Google Scholar]
- 3.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthon BS, MacDonald-Wicks LK, Wood LG. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr. Res. 2014;34:179–190. doi: 10.1016/j.nutres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2009;94:3121–3131. doi: 10.1210/jc.2009-0612. [DOI] [PubMed] [Google Scholar]
- 7.Brent GA. Clinical practice. Graves’ Dis. N. Engl. J. Med. 2008;358:2594–2605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 8.Levine AS, Rogers B, Kneip J, Grace M, Morley JE. Effect of centrally administered corticotropin releasing factor (CRF) on multiple feeding paradigms. Neuropharmacology. 1983;22:337–339. doi: 10.1016/0028-3908(83)90249-6. [DOI] [PubMed] [Google Scholar]
- 9.Vijayan E, McCann SM. Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH) Endocrinology. 1977;100:1727–1730. doi: 10.1210/endo-100-6-1727. [DOI] [PubMed] [Google Scholar]
- 10.Hill JW. PVN pathways controlling energy homeostasis. Indian J. Endocrinol. Metab. 2012;16:S627. doi: 10.4103/2230-8210.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quaresma PGF, Dos Santos WO, Wasinski F, Metzger M, Donato J Jr. Neurochemical phenotype of growth hormone-responsive cells in the mouse paraventricular nucleus of the hypothalamus. J. Comp. Neurol. 2020;529:1228–1239. doi: 10.1002/cne.25017. [DOI] [PubMed] [Google Scholar]
- 12.Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J. Comp. Neurol. 2009;516:423–441. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- 13.Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat. Commun. 2016;7:11937. doi: 10.1038/ncomms11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krashes MJ, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan PJ, Ross SI, Campos CA, Derkach VA, Palmiter RD. Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat. Neurosci. 2017;20:1722–1733. doi: 10.1038/s41593-017-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J. Clin. Endocrinol. Metab. 1999;84:4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- 17.Watts AG. Great expectations: anticipatory control of magnocellular vasopressin neurons. Neuron. 2017;93:1–2. doi: 10.1016/j.neuron.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017;13:700. doi: 10.1038/nrendo.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer A, Langhans W, Scharrer E. Vasopressin reduces food intake in goats. Q. J. Exp. Physiol. 1989;74:465–473. doi: 10.1113/expphysiol.1989.sp003294. [DOI] [PubMed] [Google Scholar]
- 22.Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol. Metab. 2014;3:209–215. doi: 10.1016/j.molmet.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura M, et al. Activation of endogenous arginine vasopressin neurons inhibit food intake: by using a novel transgenic rat line with DREADDs system. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-16049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemmons DR. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J. Clin. Invest. 2004;113:25–27. doi: 10.1172/JCI200420660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodier PM, Kates B, White WA, Phelps CJ. Birthdates of the growth hormone releasing factor cells of the rat hypothalamus: an autoradiographic study of immunocytochemically identified neurons. J. Comp. Neurol. 1990;291:363–372. doi: 10.1002/cne.902910304. [DOI] [PubMed] [Google Scholar]
- 26.Gillies G. Somatostatin: the neuroendocrine story. Trends Pharm. Sci. 1997;18:87–95. doi: 10.1016/s0165-6147(96)01032-2. [DOI] [PubMed] [Google Scholar]
- 27.Szarek E, Cheah PS, Schwartz J, Thomas P. Molecular genetics of the developing neuroendocrine hypothalamus. Mol. Cell Endocrinol. 2010;323:115–123. doi: 10.1016/j.mce.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell RE, Rodgers-Neame NT, Bradley EL, Jr., Asch RH. Regulation of human prolactin secretion by gonadotropin-releasing hormone in vitro. Fertil. Steril. 1986;46:26–31. [PubMed] [Google Scholar]
- 29.Kanasaki H, et al. Interactions between two different G protein-coupled receptors in reproductive hormone-producing cells: the role of PACAP and its receptor PAC1R. Int. J. Mol. Sci. 2016;17:1635. doi: 10.3390/ijms17101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butera PC. Estradiol and the control of food intake. Physiol. Behav. 2010;99:175–180. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muglia LJ, Jenkins NA, Gilbert DJ, Copeland NG, Majzoub JA. Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J. Clin. Invest. 1994;93:2066–2072. doi: 10.1172/JCI117201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and á-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 34.Majzoub JA. Corticotropin-releasing hormone physiology. Eur. J. Endocrinol. 2006;155:S71–S76. [Google Scholar]
- 35.Semjonous NM, et al. Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance. Int. J. Obes. 2009;33:775–785. doi: 10.1038/ijo.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biag J, et al. Cyto‐and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol. Behav. 2008;94:169–177. doi: 10.1016/j.physbeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Rabasa C, Dickson SL. Impact of stress on metabolism and energy balance. Curr. Opin. Behav. Sci. 2016;9:71–77. [Google Scholar]
- 39.Karatsoreos IN, et al. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151:2117–2127. doi: 10.1210/en.2009-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luijten IH, et al. Glucocorticoid-induced obesity develops independently of UCP1. Cell Rep. 2019;27:1686–1698. doi: 10.1016/j.celrep.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 41.Perry RJ, et al. Leptin’s hunger-suppressing effects are mediated by the hypothalamic–pituitary–adrenocortical axis in rodents. Proc. Natl Acad. Sci. 2019;116:13670–13679. doi: 10.1073/pnas.1901795116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Beukel JC, et al. Cold exposure partially corrects disturbances in lipid metabolism in a male mouse model of glucocorticoid excess. Endocrinology. 2015;156:4115–4128. doi: 10.1210/en.2015-1092. [DOI] [PubMed] [Google Scholar]
- 43.Bates HE, et al. Gipr is essential for adrenocortical steroidogenesis; however, corticosterone deficiency does not mediate the favorable metabolic phenotype of Gipr−/− mice. Diabetes. 2012;61:40–48. doi: 10.2337/db11-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H-C, et al. NAFLD aggravates septic shock due to inadequate adrenal response and 11β-HSDs dysregulation in rats. Pharmaceutics. 2020;12:403. doi: 10.3390/pharmaceutics12050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gyengesi E, et al. Corticosterone regulates synaptic input organization of POMC and NPY/AgRP neurons in adult mice. Endocrinology. 2010;151:5395–5402. doi: 10.1210/en.2010-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambillotte C, Gilon P, Henquin J-C. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J. Clin. Investig. 1997;99:414–423. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alario P, Gamallo A, Beato M, Trancho G. Body weight gain, food intake and adrenal development in chronic noise stressed rats. Physiol. Behav. 1987;40:29–32. doi: 10.1016/0031-9384(87)90181-8. [DOI] [PubMed] [Google Scholar]
- 48.Schnabl K, Westermeier J, Li Y, Klingenspor M. Opposing actions of adrenocorticotropic hormone and glucocorticoids on UCP1-mediated respiration in brown adipocytes. Front. Physiol. 2019;9:1931. doi: 10.3389/fphys.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sussman KE, Vaughan GD. Insulin release after ACTH, glucagon and adenosine-3′-5′-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967;16:449–454. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- 50.ZINDER O, SHAPIRO B. Effect of cell size on epinephrine-and ACTH-induced fatty acid release from isolated fat cells. J. Lipid Res. 1971;12:91–95. [PubMed] [Google Scholar]
- 51.Xu N, Hurtig M, Ekstrom U, Nilsson-Ehle P. Adrenocorticotrophic hormone retarded metabolism of low-density lipoprotein in rats. Scand. J. Clin. Lab. Invest. 2004;64:217–222. doi: 10.1080/00365510410005730. [DOI] [PubMed] [Google Scholar]
- 52.Arase K, York D, Shimizu H, Shargill N, Bray G. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am. J. Physiol.-Endocrinol. Metab. 1988;255:E255–E259. doi: 10.1152/ajpendo.1988.255.3.E255. [DOI] [PubMed] [Google Scholar]
- 53.Egawa M, Yoshimatsu H, Bray G. Effect of corticotropin releasing hormone and neuropeptide Y on electrophysiological activity of sympathetic nerves to interscapular brown adipose tissue. Neuroscience. 1990;34:771–775. doi: 10.1016/0306-4522(90)90181-3. [DOI] [PubMed] [Google Scholar]
- 54.Krahn DD, Gosnell BA, Levine AS, Morley JE. Behavioral effects of corticotropin-releasing factor: localization and characterization of central effects. Brain Res. 1988;443:63–69. doi: 10.1016/0006-8993(88)91598-3. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Kotz CM. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am. J. Physiol.-Regulatory Integr. Comp. Physiol. 2002;283:R358–R367. doi: 10.1152/ajpregu.00558.2001. [DOI] [PubMed] [Google Scholar]
- 56.Gay J, Kokkotou E, O’Brien M, Pothoulakis C, Karalis KP. Corticotropin-releasing hormone deficiency is associated with reduced local inflammation in a mouse model of experimental colitis. Endocrinology. 2008;149:3403–3409. doi: 10.1210/en.2007-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weninger SC, Muglia LJ, Jacobson L, Majzoub JA. CRH-deficient mice have a normal anorectic response to chronic stress. Regul. Pept. 1999;84:69–74. doi: 10.1016/s0167-0115(99)00070-1. [DOI] [PubMed] [Google Scholar]
- 58.Jacobson L. Lower weight loss and food intake in protein-deprived, corticotropin releasing hormone-deficient mice correlate with glucocorticoid insufficiency. Endocrinology. 1999;140:3543–3551. doi: 10.1210/endo.140.8.6910. [DOI] [PubMed] [Google Scholar]
- 59.Jeong KH, Sakihara S, Widmaier EP, Majzoub JA. Impaired leptin expression and abnormal response to fasting in corticotropin-releasing hormone-deficient mice. Endocrinology. 2004;145:3174–3181. doi: 10.1210/en.2003-1558. [DOI] [PubMed] [Google Scholar]
- 60.Garfield AS, et al. A neural basis for melanocortin-4 receptor–regulated appetite. Nat. Neurosci. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 2019;29:681–694. doi: 10.1016/j.cmet.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. 2017;96:897–909. doi: 10.1016/j.neuron.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menzaghi F, Heinrichs SC, Pich EM, Tilders FJ, Koob GF. Functional impairment of hypothalamic corticotropin-releasing factor neurons with immunotargeted toxins enhances food intake induced by neuropeptide Y. Brain Res. 1993;618:76–82. doi: 10.1016/0006-8993(93)90431-l. [DOI] [PubMed] [Google Scholar]
- 64.Okamoto S, et al. Activation of AMPK-regulated CRH neurons in the PVH is sufficient and necessary to induce dietary preference for carbohydrate over fat. Cell Rep. 2018;22:706–721. doi: 10.1016/j.celrep.2017.11.102. [DOI] [PubMed] [Google Scholar]
- 65.Zhu C, et al. Disrupted hypothalamic CRH neuron responsiveness contributes to diet-induced obesity. EMBO Rep. 2020;21:e49210. doi: 10.15252/embr.201949210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y, et al. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 2013;18:860–870. doi: 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang R, et al. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry. 2017;22:733–744. doi: 10.1038/mp.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stanley S, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl Acad. Sci. 2010;107:7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kono J, et al. Distribution of corticotropin-releasing factor neurons in the mouse brain: a study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Struct. Funct. 2017;222:1705–1732. doi: 10.1007/s00429-016-1303-0. [DOI] [PubMed] [Google Scholar]
- 70.Daniel SE, Rainnie DG. Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2016;41:103–125. doi: 10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pomrenze MB, et al. A corticotropin releasing factor network in the extended amygdala for anxiety. J. Neurosci. 2019;39:1030–1043. doi: 10.1523/JNEUROSCI.2143-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol. Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 73.Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 2014;17:1240–1248. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Francesco PN, et al. Neuroanatomical and functional characterization of CRF neurons of the amygdala using a novel transgenic mouse model. Neuroscience. 2015;289:153–165. doi: 10.1016/j.neuroscience.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cottone P, et al. CRF system recruitment mediates dark side of compulsive eating. Proc. Natl Acad. Sci. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol. Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 77.Kreifeldt, M. et al. Chemogenetic stimulation of mouse central amygdala corticotropin-releasing factor neurons: Effects on cellular and behavioral correlates of alcohol dependence. Preprint at bioRxiv10.1101/2020.02.07.939496 (2020).
- 78.Giardino WJ, et al. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 2018;21:1084–1095. doi: 10.1038/s41593-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luskin, A. T. et al. Extended amygdala-parabrachial circuits alter threat assessment to regulate feeding. Sci. Adv. 7, eabd3666 (2021). [DOI] [PMC free article] [PubMed]
- 81.Iwen KA, Oelkrug R. & Brabant, G. Effects of thyroid hormones on thermogenesis and energy partitioning. J. Mol. Endocrinol. 2018;60:R157–R170. doi: 10.1530/JME-17-0319. [DOI] [PubMed] [Google Scholar]
- 82.Herwig A, Ross AW, Nilaweera KN, Morgan PJ, Barrett P. Hypothalamic thyroid hormone in energy balance regulation. Obes. Facts. 2008;1:71–79. doi: 10.1159/000123428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res. 1980;198:190–195. doi: 10.1016/0006-8993(80)90354-6. [DOI] [PubMed] [Google Scholar]
- 84.Tu HM, et al. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- 85.Diano S, Leonard JL, Meli R, Esposito E, Schiavo L. Hypothalamic type II iodothyronine deiodinase: a light and electron microscopic study. Brain Res. 2003;976:130–134. doi: 10.1016/s0006-8993(03)02692-1. [DOI] [PubMed] [Google Scholar]
- 86.Kong WM, et al. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology. 2004;145:5252–5258. doi: 10.1210/en.2004-0545. [DOI] [PubMed] [Google Scholar]
- 87.Diano S, Naftolin F, Goglia F, Horvath TL. Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology. 1998;139:2879–2884. doi: 10.1210/endo.139.6.6062. [DOI] [PubMed] [Google Scholar]
- 88.Coppola A, et al. Suppression of hypothalamic deiodinase type II activity blunts TRH mRNA decline during fasting. FEBS Lett. 2005;579:4654–4658. doi: 10.1016/j.febslet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 89.Coppola A, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 91.Lopez M, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez-Sanchez N, et al. Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 2017;26:212–229. doi: 10.1016/j.cmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ishii S, et al. Triiodothyronine (T3) stimulates food intake via enhanced hypothalamic AMP-activated kinase activity. Regul. Pept. 2008;151:164–169. doi: 10.1016/j.regpep.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- 95.Marras V, et al. Thyroid function in obese children and adolescents. Horm. Res. Paediatr. 2010;73:193–197. doi: 10.1159/000284361. [DOI] [PubMed] [Google Scholar]
- 96.Jin HY. Prevalence of subclinical hypothyroidism in obese children or adolescents and association between thyroid hormone and the components of metabolic syndrome. J. Paediatr. Child Health. 2018;54:975–980. doi: 10.1111/jpc.13926. [DOI] [PubMed] [Google Scholar]
- 97.Draman MS, et al. The role of thyrotropin receptor activation in adipogenesis and modulation of fat phenotype. Front. Endocrinol. (Lausanne) 2017;8:83. doi: 10.3389/fendo.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi YH, Hartzell D, Azain MJ, Baile CA. TRH decreases food intake and increases water intake and body temperature in rats. Physiol. Behav. 2002;77:1–4. doi: 10.1016/s0031-9384(02)00784-9. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki T, Kohno H, Sakurada T, Tadano T, Kisara K. Intracranial injection of thyrotropin releasing hormone (TRH) suppresses starvation-induced feeding and drinking in rats. Pharm. Biochem. Behav. 1982;17:249–253. doi: 10.1016/0091-3057(82)90078-8. [DOI] [PubMed] [Google Scholar]
- 100.Marubashi S, Kunii Y, Tominaga M, Sasaki H. Modulation of plasma glucose levels by thyrotropin-releasing hormone administered intracerebroventricularly in the rat. Neuroendocrinology. 1988;48:640–644. doi: 10.1159/000125075. [DOI] [PubMed] [Google Scholar]
- 101.Shintani M, Tamura Y, Monden M, Shiomi H. Thyrotropin-releasing hormone induced thermogenesis in Syrian hamsters: site of action and receptor subtype. Brain Res. 2005;1039:22–29. doi: 10.1016/j.brainres.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Z, et al. Administration of thyrotropin-releasing hormone in the hypothalamic paraventricular nucleus of male rats mimics the metabolic cold defense response. Neuroendocrinology. 2018;107:267–279. doi: 10.1159/000492785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morley JE, Levine AS. Thyrotropin releasing hormone (TRH) suppresses stress induced eating. Life Sci. 1980;27:269–274. doi: 10.1016/0024-3205(80)90147-2. [DOI] [PubMed] [Google Scholar]
- 104.Shian LR, Wu MH, Lin MT, Ho LT. Hypothalamic involvement in the locomotor stimulant or satiety action of thyrotropin-releasing hormone and amphetamine. Pharmacology. 1985;30:259–265. doi: 10.1159/000138076. [DOI] [PubMed] [Google Scholar]
- 105.Ishibashi S, Oomura Y, Okajima T. Facilitatory and inhibitory effects of TRH on lateral hypothalamic and ventromedial neurons. Physiol. Behav. 1979;22:785–787. doi: 10.1016/0031-9384(79)90249-x. [DOI] [PubMed] [Google Scholar]
- 106.Kow LM, Pfaff DW. Neuropeptides TRH and cyclo(His-Pro) share neuromodulatory, but not stimulatory, action on hypothalamic neurons in vitro: implication for the regulation of feeding. Exp. Brain Res. 1987;67:93–99. doi: 10.1007/BF00269457. [DOI] [PubMed] [Google Scholar]
- 107.Gotoh K, et al. Hypothalamic neuronal histamine mediates the thyrotropin-releasing hormone-induced suppression of food intake. J. Neurochem. 2007;103:1102–1110. doi: 10.1111/j.1471-4159.2007.04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, van den Pol AN. Thyrotropin-releasing hormone (TRH) inhibits melanin-concentrating hormone neurons: implications for TRH-mediated anorexic and arousal actions. J. Neurosci. 2012;32:3032–3043. doi: 10.1523/JNEUROSCI.5966-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joseph-Bravo P, Jaimes-Hoy L, Charli JL. Regulation of TRH neurons and energy homeostasis-related signals under stress. J. Endocrinol. 2015;227:X1. doi: 10.1530/JOE-14-0593e. [DOI] [PubMed] [Google Scholar]
- 110.Harris M, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J. Clin. Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perello M, Stuart RC, Nillni EA. The role of intracerebroventricular administration of leptin in the stimulation of prothyrotropin releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2006;147:3296–3306. doi: 10.1210/en.2005-1533. [DOI] [PubMed] [Google Scholar]
- 112.Ghamari-Langroudi M, et al. Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Mol. Endocrinol. 2010;24:2366–2381. doi: 10.1210/me.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campos AMP, et al. Differences between rats and mice in the leptin action on the paraventricular nucleus of the hypothalamus: Implications for the regulation of the hypothalamic-pituitary-thyroid axis. J. Neuroendocrinol. 2020;32:e12895. doi: 10.1111/jne.12895. [DOI] [PubMed] [Google Scholar]
- 114.Kishi T, et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 115.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 116.Mihaly E, et al. Hypophysiotropic thyrotropin-releasing hormone-synthesizing neurons in the human hypothalamus are innervated by neuropeptide Y, agouti-related protein, and alpha-melanocyte-stimulating hormone. J. Clin. Endocrinol. Metab. 2000;85:2596–2603. doi: 10.1210/jcem.85.7.6662. [DOI] [PubMed] [Google Scholar]
- 117.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 118.Fekete C, et al. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J. Neurosci. 2000;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Horjales-Araujo E, Hellysaz A, Broberger C. Lateral hypothalamic thyrotropin-releasing hormone neurons: distribution and relationship to histochemically defined cell populations in the rat. Neuroscience. 2014;277:87–102. doi: 10.1016/j.neuroscience.2014.06.043. [DOI] [PubMed] [Google Scholar]