Abstract
Healthcare workers (HCWs) are at an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel virus that causes Coronavirus Disease (COVID-19). We aim to assess the seroprevalence of SARS-CoV-2 IgG among healthcare workers and compare risk-factors between seropositive and seronegative HCWs. In this observational study, serum samples were collected from HCWs between July 13th to 26th, 2020 at Boston Medical Center (BMC). Samples were subsequently tested for SARS-CoV-2 IgG antibody using the Abbott SARS-CoV-2 IgG assay. Participants also answered a questionnaire capturing data on demographics, history of COVID-19 symptoms, occupation, infection prevention and control measures. Overall, 95 of 1743 (5.5%) participants tested positive for SARS-CoV-2 IgG. Of these, 1.8% of the participants had mild or no COVID-19 symptoms and did not require a diagnostic test. Seropositivity was not associated with gender, occupation, hand hygiene and personal protective equipment (PPE) practices amongst HCWs. However, lack of physical distancing among health care workers in work areas and break room was associated with seropositivity (p = 0.05, p = 0.003, respectively). The majority of the HCWs are negative for SARS-CoV-2 IgG. This data highlights the need to promote infection prevention measures, and the importance of distance amongst co-workers to help mitigate infection rates.
Subject terms: SARS-CoV-2, Epidemiology
Introduction
Healthcare workers (HCWs) are at an increased risk for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a novel virus that causes Coronavirus Disease 2019 (COVID-19). COVID-19 has infected nearly 7.75 million and caused the deaths of over 214,000 people in the United States as of October 11th, 2020. As of July 16th, 2020, 100,570 HCWs with confirmed COVID-19 and 641 deaths were reported to the United States Centers of Disease Control (CDC)1,2. This comprised 22% of cases reported to the CDC3. It is important to understand the prevalence and risk factors amongst HCWs as it can inform infection prevention and control measures.
The first case of COVID-19 in Massachusetts was reported on February 1st, 20204. The number of cases in the state grew quickly due to a widely attended scientific conference, considered to be a superspreading event, and Massachusetts experienced a surge in mid-April, 20205. Boston Medical Center (BMC), a 514-bed academic medical center, historically the safety-net hospital for the city of Boston, experienced a steep rise in cases during early April to mid-May. At peak, BMC, averaged over 30 COVID-19 admissions per day with a hospital census of over 230 SARS-CoV-2 positive patients. At peak our institution experienced a 53% positivity rate for diagnostic testing; by July, positive test rates were less than two percent.
Seroprevalence studies can assist in estimating the proportion of a population that has been infected. It provides a better estimate of population level-data by capturing individuals with mild or no symptoms and others who never underwent diagnostic testing. This is especially important for COVID-19 because people with asymptomatic infections are thought to make up a majority of SARS-CoV-2 infections, but are less likely to present for diagnostic testing6. Assessing the cumulative prevalence is critical to understanding disease transmission rates.
HCWs spend a significant amount of time in a high-risk setting. Once infected, they can spread SARS-CoV-2 to patients, colleagues, and members of the larger community. Literature suggests the general population has a wide seroprevalence range, between 2.7–16.6%, and HCW range from 1.3–22.0%2,7–13. A recent report from Asian countries reported that HCWs constituted over 20% presumptive occupation related cases14. In July, the Boston community prevalence was reported to be over 116,000 confirmed and probable cases15. However, the disease burden amongst HCWs in Boston remains unknown. It is important to understand disease prevalence and characteristics amongst HCWs as it can identify areas or personnel that are at increased risk. It can also inform infection control policy in the hospital setting to mitigate infection rates.
We aim to assess the seroprevalence of SARS-CoV-2 among healthcare workers at BMC and compare characteristics, including demographics, occupation, COVID-19 symptoms, and infection prevention and control measures taken between seropositive and seronegative HCWs.
Methods
Study design and study population
We conducted a cross-sectional study at BMC (July 13th to July 26th, 2020) to detect SARS-CoV-2 Immunoglobulin G (IgG) antibodies in HCWs. Eligible participants worked at BMC during the initial COVID-19 surge at BMC (March 13th to May 31st, 2020). BMC, located in Boston, Massachusetts, has approximately 7442 employees. This project was approved by the Institutional Review Board at BMC. All methods were performed in accordance with relevant guidelines and regulations.
All eligible study participants were offered SARS-CoV-2 IgG antibody test. Eligible HCWs were at least 18 years old and worked physically on the BMC campus during the study period. Participants were recruited via a multi-pronged approach including email communications, physical flyers in employee-only spaces on campus, advertisement on the hospital’s internal website, announcements at a hospital-wide town hall, and at department-level meetings.
Potential participants opted-in to the study by following the link in recruitment materials to a REDCap (Research Electronic Data Capture) survey16. Using this platform, participants filled out a pre-screening questionnaire, and completed an electronic informed consent form if deemed eligible. Participants then provided the following information in online self-administered surveys: sociodemographic data (age, sex, height, weight), occupation, self-report of COVID-19 related symptoms, known COVID exposure, prior SARS-CoV-2 RT-PCR test result, if any, and infection prevention and control measures. All SARS-CoV-2 IgG levels were obtained from participants between July 13th–26th, 2020. A subset of participants who were also eligible for an additional study were scheduled for a phone-based questionnaire with the same questions captured by the electronic version. Consent and questionnaire were available in English, Spanish and Haitian-Creole, reflecting the predominant languages spoken by BMC staff.
RT-PCR measurement
BMC HCWs with suspicion for COVID-19 were tested for SARS-CoV-2 infection in accordance with hospital policy via a RT-PCR assay. Study participants self-reported their SARS-CoV-2 RT-PCR test history and corresponding test result.
SARS-CoV-2 antibody measurement
IgG antibody analyses were performed by the clinical pathology laboratory at BMC. Serum samples were run on the Abbott Architect i2000 Instrument using the Abbott SARS-CoV-2 IgG assay per the manufacturer’s instructions (SARS-CoV-2 IgG; Abbott Laboratories, Abbott Park, IL). This assay is a chemiluminescent microparticle immunoassay for detection of IgG antibody in human serum against the SARS-CoV-2 nucleoprotein. Samples were interpreted as positive (index value > = 1.4) or negative (index value < 1.4) based on the index values reported by the instrument. Qualitative results were used in the analyses.
Low and high level of quality control (QC), as supplied by the manufacturer, were run twice daily during the study period. The inter-day coefficient of variation (CV) for low and high levels were 14.30%, and 2.78%, respectively.
Statistical analysis
All questionnaire data were collected in REDCap. Non-English questionnaire data responses were manually translated to English. Categorical variables are presented as counts. We tested the association between serology status and variables of interest with either a Chi-Square or Fisher’s Exact test. Missing data ranged from 0.1 to 5.3%. If the missing data for any variable was less than five percent, it was excluded from analysis. Otherwise, it is indicated in the tables. Risk ratios (RR) and 95% confidence Intervals (CI) were calculated using the epitools add-on package in R. A P-value of < 0.05 was considered statistically significant, and all tests were two-sided. Analyses were performed in R Version R-1.3.1056 (R Foundation for Statistical Computing)17. Graphical representations were performed on GraphPad Prism software version 9.0 (GRAPH PAD software Inc, California, USA).
Results
Baseline characteristics
All BMC employees that worked on campus during the surge were invited to participate, with 2228 completing the initial screening agreement. Ten participants were ineligible as they did not work on campus during the first surge or were not healthcare providers at BMC. Of the 2218 eligible HCWs, 1989 (89.6%) individuals went on to provide informed consent. Of those who provided informed consent, 246 (12.3%) individuals did not complete the survey and/or get a blood draw. A total of 1743 individuals with complete data were included in the analysis (Fig. 1).
Figure 1.
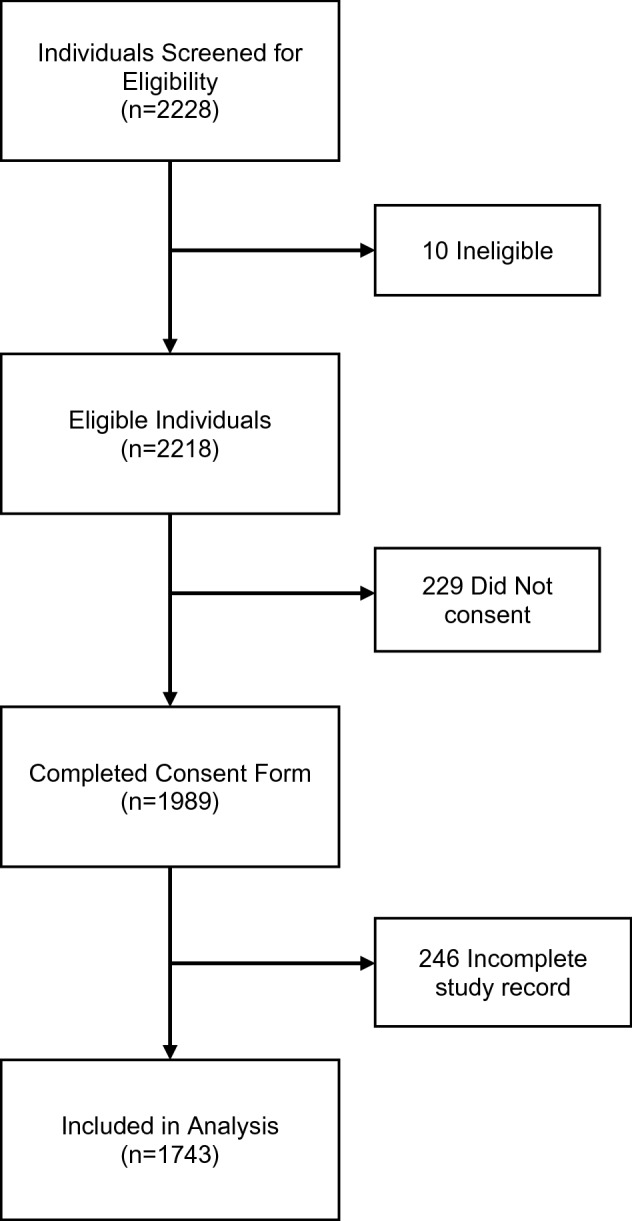
Study enrollment.
The mean age of participants was 38.9 years (SD 15.2 years). Participants were predominantly female (74.8%), half self-reported as overweight or obese (49.5%). A majority (74.5%) of the participants identified as White. The remaining identified as either Asian, Black, or Hispanic (9.2%, 8.1%, and 8.2%, respectively).
Most of the enrolled participants were nurses (n = 716, 41.1%) or physicians (n = 523, 30.0%). Administrative staff and patient/non-patient facing allied-health professionals comprised of 8.3%, 12.7% and 6.4%, respectively, of the total participants (Table 1). Facilities management and support services, which encompass HCWs from environmental services, housekeeping, valet, public safety represented 1.4% of the total participants.
Table 1.
Study demographics.
| Variable | Total n (column %) |
SARS-CoV-2 IgG Status | p-value† (all data) |
RR (95% CI) (all data) |
|
|---|---|---|---|---|---|
| Positive n (row %) |
Negative n (row %) |
||||
| Total | 1743 | 95 (5.5%) | 1648 (94.5%) | ||
| Sex | 0.55† | ||||
| Female | 1304 (74.8%) | 75 (5.8%) | 1229 (94.2%) | 1 (referent) | |
| Male | 432 (24.8%) | 20 (4.6%) | 412 (95.4%) | 0.79 (0.49–1.29) | |
| Nonbinary/third gender | 3 (0.2%) | 0 | 3 (100%) | 0 (0.00–NaN**) | |
| Age (years) | p < 0.001†* | ||||
| < 20 | 93 (5.3%) | 16 (17.2%) | 77 (82.8%) | 1 (referent) | |
| 20–29 | 334 (19.2%) | 17 (5.1%) | 317 (94.9%) | 0.28 (0.15–0.54)* | |
| 30–39 | 534 (30.6%) | 22 (4.1%) | 512 (95.9%) | 0.23 (0.12–0.42)* | |
| 40–49 | 322 (18.5%) | 15 (4.7%) | 307 (95.3%) | 0.26 (0.13–0.50)* | |
| 50–59 | 294 (16.9%) | 20 (6.8%) | 274 (93.2%) | 0.38 (0.20–0.70)* | |
| 60–69 | 155 (8.9%) | 5 (3.2%) | 150 (96.8%) | 0.18 (0.07–0.47)* | |
| > 69 | 11 (0.6%) | 0 | 11 (100%) | 0.00 (0.00–NaN**) | |
| BMI | 0.02†* | ||||
| Underweight | 30 (1.7%) | 0 | 30 (100%) | 0.00 (0.02–5.56) | |
| Normal | 843 (48.4%) | 39 (4.6%) | 804 (95.4%) | 1 (referent) | |
| Overweight | 529 (30.3%) | 25 (4.7%) | 504 (95.3%) | 1.00 (0.62–1.71) | |
| Obese | 335 (19.2%) | 30 (9.0%) | 305 (91.0%) | 1.97 (1.25–3.32)* | |
| Hispanic/LatinX | 0.05† | ||||
| Yes | 143 (8.2%) | 13 (9.1%) | 130 (90.9%) | 1.79 (1.02–3.14)* | |
| No | 1594 (91.5%) | 80 (5.0%) | 1514 (95.0%) | 1 (referent) | |
| Race | 0.54† | ||||
| Asian | 160 (9.2%) | 10 (6.3%) | 150 (93.8%) | 1.27 (0.66–2.42) | |
| Black | 142 (8.1%) | 11 (7.7%) | 131 (92.3%) | 1.57 (0.85–2.92) | |
| White | 1299 (74.5%) | 63 (4.8%) | 1236 (95.2%) | 1 (referent) | |
| Native American/Pacific Islander | 7 (0.4%) | 0 | 7 (100%) | 0.00 (0.00–NaN**) | |
| Other | 112 (6.4%) | 5 (4.5%) | 107 (95.5%) | 0.91 (0.37–2.20) | |
| Smoking | 0.33† | ||||
| Yes | 47 (2.7%) | 4 (8.5%) | 43 (91.5%) | 1.55 (0.59–4.04) | |
| No | 1675 (96.1%) | 91 (5.4%) | 1584 (94.6%) | 1 (referent) | |
| Occupation | 0.0028†* | ||||
| Administrative | 145 (8.3%) | 7 (4.8%) | 138 (95.2%) | 1 (referent) | |
| Allied health—non-patient facing | 112 (6.4%) | 1 (0.9%) | 111 (99.1%) | 0.16 (0.02–1.31) | |
| Allied health—patient facing | 222 (12.7%) | 19 (8.6%) | 203 (91.4%) | 1.56 (0.67–3.62) | |
| Facilities management/support services | 25 (1.4%) | 0 | 25 (100%) | 0.00 (0.00–NaN**) | |
| Medical doctor/doctor of osteopathy | 523 (30.0%) | 18 (3.4%) | 505 (96.6%) | 0.63 (0.27–1.47) | |
| Nursing | 716 (41.1%) | 50 (7.0%) | 666 (93.0%) | 1.27 (0.59–2.75) | |
†Fisher’s Exact Test, otherwise, Chi Squared.
*Statistically significant when p-value < 0.05.
**NaN is Not a Number, unable to divide by 0.
Column percentages may not always add up to 100% due to missing data. Missing data ranged from 0.11–1.32%.
Administrative: Director, Supervisor, Manager, Chairs, Admission personnel.
Allied Health: Nonpatient facing: Lab personnel, Lab Technologist, Radiology.
Allied Health: Patient facing: Technologist, Medical Assistant, Speech Pathologist, Team Leader, Occupational Therapist, Dentist, Phlebotomy, Patient Education, Pharmacy, Other.
Facilities Management: Electrician, Environmental Services, Housekeeping, Support Staff.
Medical Doctor/ Doctor of Osteopathy: Attending, Resident.
Nursing: Registered Nurse, Advanced Practice Registered Nurse, Nurse Technicians.
Seroprevalence
Table 1 shows selected demographics and clinical characteristics of the study participants by antibody status. Overall, 95 of 1743 were positive for SARS-CoV-2 IgG antibody corresponding to a hospital-wide HCW seroprevalence rate of 5.5%. Seropositive participants were more likely to be female and smokers, although not statistically significant. Individuals that identified as LatinX were significantly more likely to be seropositive [RR 1.79 (95% CI 1.02–3.14)]. Obese participants had a significantly increased risk of being seropositive [RR 1.97 (95% CI 1.25–3.32)]. The relative risk of being seropositive was higher among nurses [RR 1.27 (95% CI 0.59–2.75)] and patient facing allied health [RR 1.56 (95% CI 0.67–3.62)], but these findings did not reach statistical significance. Serious and chronic illness was not significantly associated with seropositivity (p = 0.89; data not shown).
The average day between RT-PCR and serology test date was 102 days for study participants, the range was 25–145 days. The distribution of SARS-CoV-2 IgG Days between RT-PCR and serology test date are show a detectable response at 145 days (Supplemental Figure 1). Among the 441 participants with a previous RT-PCR test, 85 (19.3%) were positive by RT-PCR. Of these, 64 (75.3%) were also seropositive by antibody testing but 21 (24.7%) were seronegative. 350 of 441 participants that had undergone diagnostic testing were negative by RT-PCR. Of these, 7 (2.0%) were seropositive. A total of 1302 participants had no prior RT-PCR test and of these participants, 23 (1.8%) were positive for SARS-CoV-2 IgG (Table 2).
Table 2.
SARS-CoV-2 RT-PCR result by SARS-CoV-2 IgG status.
| Total n |
SARS-CoV-2 IgG Status | ||
|---|---|---|---|
| Positive n (row %) |
Negative n (row %) |
||
| Total | 1743 | 95 (5.5%) | 1648 (94.5%) |
| RT-PCR tested | 441 | 72 (16.3%) | 369 (83.7%) |
| Indeterminate/no result | 6 | 1 (16.7%) | 5 (83.3%) |
| Negative | 350 | 7 (2.0%) | 343 (98.0%) |
| Positive | 85 | 64 (75.3%) | 21 (24.7%) |
| RT-PCR not tested | 1302 | 23 (1.8%) | 1279 (98.2%) |
We also report seropositivity status stratified by self-reported presence of COVID-19 symptoms. Seropositivity status was significantly associated with a self-report history of fever, sore throat, cough, shortness of breath, chills, myalgia, loss of appetite, loss of smell and taste, fatigue, and neurological signs (Table 3). Runny nose, nausea/vomiting, diarrhea, headache, rash, seizures, and altered consciousness were not associated with seropositivity of SARS-CoV-2 antibody status (Table 3). Seropositive participants, on average, had approximately 2 more symptoms relative to the seronegative participants.
Table 3.
Self-Reported symptoms by SARS-CoV-2 IgG status.
| Symptoms | Total n (column %) |
SARS-CoV-2 IgG Status | p-value (all data) |
|
|---|---|---|---|---|
| Positive n (row %) |
Negative n (row %) |
|||
| Total | 1743 | 95 (5.5%) | 1648 (94.5%) | |
| Fever** | 252 (14.5%) | 43 (17.1%) | 209 (82.9%) | p < 0.001†* |
| Sore throat*** | 608 (34.9%) | 33 (5.4%) | 575 (94.6%) | p < 0.001* |
| Cough | 611 (35.1%) | 45 (7.4%) | 566 (92.6%) | 0.02* |
| Runny nose | 672 (38.6%) | 32 (4.8%) | 640 (95.2%) | 0.39 |
| Shortness of breath | 280 (16.1%) | 30 (10.7%) | 250 (89.3%) | p < 0.001* |
| Chills | 362 (20.8%) | 43 (11.9%) | 319 (88.1%) | p < 0.001†* |
| Nausea/vomiting | 318 (18.2%) | 16 (5.0%) | 302 (95.0%) | 0.83 |
| Diarrhea | 409 (23.5%) | 26 (6.4%) | 383 (93.6%) | 0.43 |
| Headache | 869 (49.9%) | 49 (5.6%) | 820 (94.4%) | 0.81 |
| Rash | 73 (4.2%) | 3 (4.1%) | 70 (95.9%) | 0.79† |
| Muscle/joint pain | 566 (32.5%) | 56 (9.9%) | 510 (90.1%) | p < 0.001* |
| Loss of appetite | 285 (16.4%) | 36 (12.6%) | 249 (87.4%) | p < 0.001* |
| Loss of Smell/Taste | 116 (6.7%) | 44 (37.9%) | 72 (62.1%) | p < 0.001* |
| Fatigue | 815 (46.8%) | 64 (7.9%) | 751 (92.1%) | p < 0.001* |
| Seizures | 5 (0.3%) | 0 | 5 (100%) | 1.00† |
| Altered consciousness | 9 (0.5%) | 2 (22.2%) | 7 (77.8%) | 0.08† |
| Other neurological signs | 21 (1.2%) | 5 (23.8%) | 16 (76.2%) | 0.0045†* |
†Fisher’s Exact Test, otherwise, Chi Squared.
*Statistically significant when p-value < 0.05.
**Missing data included in analysis due to accounting for 5.2% of responses.
***Missing data included in analysis due to accounting for 5.3% of responses.
Column percentages may not always add up to 100% due to missing data. Missing data ranged from 0–1.03%.
Seropositive participants with a negative RT-PCR (n = 7), all reported symptoms (average of four symptoms), however, thee reported symptoms were variable from person to person. Whereas seronegative participants with a positive RT-PCR (n = 21) had an average of seven symptoms. Of the 23 seropositive study participants who did not undergo diagnostic testing, 12 (52.2%) reported fever, cough, fatigue, shortness of breath or loss of taste/smell. Seven (6.7%) seropositive study participants reported no COVID-19 symptoms and 5 (4.8%) reported less common symptoms of nausea/vomiting, diarrhea, headache, rash, or runny nose.
The majority of the participants (84.1–95.8%) reported following recommended hand hygiene practices and wearing the recommended personnel protective equipment (PPE) when being exposed to patients, body fluids, and/or patient surroundings. No statistical significance was observed in seropositivity status and hand hygiene practices or PPE usage. However, these same practices were not always followed among their peers. Lack of physical distancing among health care workers in work areas and break room was significantly associated with seropositivity (p = 0.05, p = 0.003, respectively) (Table 4).
Table 4.
Infection prevention and control measures by SARS-CoV-2 IgG status.
| Total n (column %) |
SARS-CoV-2 IgG Status | p-value (all data) | ||
|---|---|---|---|---|
| Positive n (row %) |
Negative n (row %) |
|||
| Total | 1743 | 95 (5.5%) | 1648 (94.5%) | |
| Hand hygiene before and after patient contact? | 0.38† | |||
| Always | 1598 (91.7%) | 85 (5.3%) | 1513 (94.7%) | |
| Most of the time | 123 (7.1%) | 9 (7.3%) | 114 (92.7%) | |
| Occasionally | 0 | 0 | 0 | |
| Rarely | 2 (0.1%) | 0 | 2 (100%) | |
| Hand hygiene after body fluid exposure? | 0.25† | |||
| Always | 1670 (95.8%) | 89 (5.3%) | 1581 (94.7%) | |
| Most of the time | 24 (1.4%) | 3 (12.5%) | 21 (87.5%) | |
| Occasionally | 0 | 0 | 0 | |
| Rarely | 7 (0.4%) | 0 | 7 (100%) | |
| Hand hygiene after contact with patient surroundings? | 0.50† | |||
| Always | 1466 (84.1%) | 85 (5.8%) | 1381 (94.2%) | |
| Most of the time | 233 (13.4%) | 8 (3.4%) | 225 (96.6%) | |
| Occasionally | 5 (0.3%) | 0 | 5 (100%) | |
| Rarely | 4 (0.2%) | 0 | 4 (100%) | |
| PPE when interacting with patients or their specimens? | 0.95† | |||
| Always | 1549 (88.9%) | 85 (5.5%) | 1464 (94.5%) | |
| Most of the time | 141 (8.1%) | 8 (5.7%) | 133 (94.3%) | |
| Occasionally | 7 (0.4%) | 0 | 7 (100%) | |
| Rarely | 12 (0.7%) | 0 | 12 (100%) | |
| Physical distancing in work areas? | 0.05 | |||
| Always | 334 (19.2%) | 13 (3.9%) | 321 (96.1%) | |
| Most of the time | 721 (41.4%) | 33 (4.6%) | 688 (95.4%) | |
| Occasionally | 320 (18.4%) | 21 (6.6%) | 299 (93.4%) | |
| Rarely | 351 (20.1%) | 28 (8.0%) | 323 (92.0%) | |
| Physical distancing in break room? | 0.003* | |||
| Always | 447 (25.6%) | 14 (3.1%) | 433 (96.9%) | |
| Most of the time | 689 (39.5%) | 37 (5.4%) | 652 (94.6%) | |
| Occasionally | 316 (18.1%) | 15 (4.7%) | 301 (95.3%) | |
| Rarely | 222 (12.7%) | 22 (9.9%) | 200 (90.1%) | |
†Fisher’s Exact Test, otherwise, Chi Squared.
*Statistically significant when p-value < 0.05.
Column percentages may not always add up to 100% due to missing data. Missing data ranged from 0.17–2.93%.
Discussion
This is the first study, to our knowledge, that reports on the seroprevalence of HCWs in Boston, an area that was hard hit early on by the COVID-19 pandemic in the US. Serological testing allows us to assess the total proportion of asymptomatic and symptomatic individuals infected with SARS-CoV-2. Our results show a seroprevalence of 5.5% amongst HCWs at BMC. We identified 2% cases amongst HCW that were originally negative by RT-PCR testing. We also report a 1.8% prevalence amongst individuals that were not tested by RT-PCR. We report that seropositivity was associated with lack of physical distance in work areas and break rooms. Taken together, these results provide support for efficacy of hand hygiene, PPE and social distancing but also highlight the need to reinforce these practices when engaging with colleagues.
The present data suggest that racial disparities exist even among healthcare workers and support previous findings of higher odds of SARS-CoV-2 infection in LatinX individuals18. These disparities could be attributable due to inequities in social determinants of health such as, limited access to healthcare, socio-economic status, crowded housing conditions, and occupation19,20. Minority groups are disproportionately represented in essential work settings and have increased chances of being exposed to COVID-1920. There was suggestive evidence of a higher likelihood of being seropositive among patient-facing allied health care workers and nurses. This might be attributable to higher frequency and duration of exposure to COVID-19 patients. Alternatively, it could reflect increased hours working in environments where distancing is not possible or be reflective of community acquired transmission.
The seroprevalence of our institution (5.5%) was lower relative to Northwell Health Systems in New York Area and tertiary NYC hospitals (13.7 and 10%, respectively)13,21. Additionally, a recent meta-analysis estimated that prevalence of 7% (95% CI 4–11%) for SARS-CoV-2 antibodies amongst HCWs around the world22. A study among HCWs from 13 academic medical centers across the US reported a similar seroprevalence (6%) to ours3. The peak of cases in MA was slightly later than the peak in NY, and MA possibly had the advantage of being able to learn from the experience in NY. The governor of Massachusetts declared a state of emergency on March 10th, 2020, banned all public gatherings of more than 25 and closed all public schools on March 15th, 2020 and instituted a stay-at-home order on March 24th, 202023. At the hospital level, BMC offered a diagnostic RT-PCR test on March 23rd, 2020, coupled with vigilant efforts to manage suspected COVID-19 exposure and wearing PPE24. In addition, universal masking policy for all people entering the hospital went into effect on March 27th, 2020. These early measures likely contributed to lower-than-expected COVID-19 infection rates in the hospital compared to other HCWs.
Whereas twelve seropositive HCWs never received a diagnostic test but reported sentinel symptoms of COVID-19, including fever, cough, fatigue, shortness of breath, loss of smell and taste. These individuals pose a risk of virus transmission as they are thought to have a period of viral shedding. Similar to previous findings, seropositive individuals experienced a wide-range of symptoms22. The prevalence of seropositive HCWs with no symptoms (n = 7) or mild symptoms (n = 5) (i.e. fever cough fatigue, shortness of breath, loss of smell/taste) was low but not zero at our institution. These individuals experienced mild symptoms and also pose a risk of virus transmission in their community and in the hospital. Studies suggest that asymptomatic individuals are a source of transmission, however, transmission dynamics remain to be elucidated25–27. One study reported that the viral load is found to be similar in symptomatic and asymptomatic patients while Zhou et al. demonstrated a lower viral load among asymptomatic cases28,29. Our results suggest that HCWs should be screened routinely with a symptom checklist and even one reported symptom should prompt diagnostic testing to help control SARS-CoV-2 spread in the workplace.
Nosocomial transmission cannot be ruled out even if there were no cluster outbreaks in the hospital. Encouragingly, most health care workers reported almost always following recommended hand hygiene measures and wearing PPE. The CDC found that detection of SARS-CoV-2 antibodies was less common among HCW who reported using PPE1. However, a remarkable increasing seropositivity trend was observed amongst HCWs that were always (3.9%), mostly (4.6%), occasionally (6.6%) and rarely (8.8%) able to physically distance in work areas (Table 4). A similar trend was also observed in seropositivity and HCWs who weren’t able to physically distance in break rooms. It remains unclear whether the observed prevalence was due to nosocomial or community acquired infection. However, the likelihood of community acquired infection is low as Boston regulations prohibited gatherings of more than 10 people from the start of the pandemic until July 2nd, 2020. In addition, mask coverings were required in public places at all times. This data highlights the need to promote infection prevention measures amongst co-workers to help mitigate infection rates and the need for further investigation to better understand transmission dynamics of SARS-CoV-2 amongst HCWs at BMC.
The dynamics of SARS-CoV-2 immunoglobulin durability is not completely known. It is unclear as to why RT-PCR positive with an average of seven symptoms were negative by SARS-CoV-2 IgG. The average time between RT-PCR and serology test for these individuals was 101 days and perhaps their levels started to wane. Most individuals became detectable for SARS-CoV-2 IgG levels by 14 days after infection but may wane as early as 3 months30,31. Our data supports that antibody response is quite variable from person to person and antibodies are detectable up to 145 days (Supplemental Figure 1). This variation could be attributable to various factors (i.e., age, disease severity, etc.). By conducting our sero-survey within two months of peak hospital and community prevalence, it negates the impact of delays in seroconversion and provides a better seroprevalence estimate. Additionally, the present study, benefited from a sensitive [93.8% (95% CI 82.80–98.69)] and specific [99.4% (95% CI 96.41–99.98%)] serological assay32.
Certain limitations are acknowledged. First, the study is limited by cross-sectional study design, which may underestimate or overestimate the seroprevalence. Second, this study utilized a qualitative antibody assay, whose sensitivity wanes with increased time post PCR, presumably secondary to waning antibody levels33. We intend to follow a subset of the participants to assess antibody durability over time. Some of our analyses were limited by small sample sizes which limit the interpretation of our findings. In addition, self-reported questionnaire data can lead to recall error, which could have led to underreporting or overreporting. Selection bias may also be present due to individuals with a belief and/or curiosity of concerning past infection. The study also suffered from under-enrollment of support staff at the hospital, even after targeted efforts for recruitment, which could affect the generalizability of results. Lastly, this study is limited to the hospital environment and not directly generalizable to the community setting.
In conclusion, we report a seroprevalence of 5.5% among HCWs at BMC. While these data cannot be utilized to make recommendations at an individual level, they can be used at a population level to reinforce the efficacy of personal hygiene, social distancing and PPE to prevent transmission. The importance of distance in the workplace at all times, even among our colleagues, is clear. Our findings suggest that aggressive efforts to protect our employees, including an early universal mask policy, were effective in protecting healthcare workers. Our data also highlight the presence of asymptomatic prevalence and support the need for studies characterizing transmissibility among asymptomatic individuals.
Supplementary Information
Acknowledgements
We would like to acknowledge the clinical chemistry, phlebotomy, and central receiving staff in the Department of Laboratory Medicine and Pathology at Boston Medical Center for working with the research team to accomplish this study.
Author contributions
Y.K. wrote the main manuscript text. M.C. and K.C. did the analysis. M.C. prepared the tables and figures. E.D., E.M.S. and C.A heavily revised manuscript. E.D., E.M.S., T.C.B., M.M.W., C.P., E.J.R., S.E.W. K.R.J., C.A. significantly helped design, implement the study and interpret the results. All authors reviewed the manuscript.
Funding
The study was funded, in part, by BMC Development Philanthropy Funds for COVID-19 research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-89107-5.
References
- 1.Hughes, M. M. Update: Characteristics of Health Care Personnel with COVID-19—United States, February 12–July 16, 2020. MMWR Morb. Mortal. Wkly. Rep.69 (2020). [DOI] [PMC free article] [PubMed]
- 2.Nguyen LH, et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Self, W. H. et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network—13 academic medical centers, April–June 2020 (2020). [DOI] [PMC free article] [PubMed]
- 4.Massachusetts Department of Public Health. First presumptive positive case of COVID-19 identified by Massachusetts State Public Health Laboratory (2020).
- 5.Lemieux, J. et al. Phylogenetic analysis of SARS-CoV-2 in the Boston area highlights the role of recurrent importation and superspreading events. medRxiv (2020).
- 6.Havers, F. P. et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23–May 12, 2020. JAMA Intern. Med. (2020). [DOI] [PubMed]
- 7.Xu X, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat. Med. 2020 doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 8.Sood N, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10–11, 2020. JAMA. 2020;323:2425–2427. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollan, M. et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 396, 535–544 (2020). [DOI] [PMC free article] [PubMed]
- 10.Krátká Z, Luxová Š, Malíčková K, Fürst T, Šimková H. Testing for COVID-19: A few points to remember. Cas Lek Cesk. 2020;159:72–77. [PubMed] [Google Scholar]
- 11.Iversen K, et al. Risk of COVID-19 in health-care workers in Denmark: An observational cohort study. Lancet Infect. Dis. 2020 doi: 10.1016/s1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mani NS, et al. Prevalence of COVID-19 infection and outcomes among symptomatic healthcare workers in Seattle, Washington. Clin. Infect. Dis. 2020;71:2702–2707. doi: 10.1093/cid/ciaa761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeremias A, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern. Med. 2020;180:1707–1709. doi: 10.1001/jamainternmed.2020.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan F-Y, Wei C-F, Hsu Y-T, Christiani DC, Kales SN. Work-related COVID-19 transmission in six Asian countries/areas: A follow-up study. PLoS ONE. 2020;15:e0233588. doi: 10.1371/journal.pone.0233588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massachusetts Department of Public Health. “Massachusetts Department of Public Health COVID-19 Dashboard.” Dashboard of Public Health Indicators, 31 July 2020. http://www.mass.gov/doc/covid-19-dashboard-july-31-2020/download.
- 16.Harris PA, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2020). https://www.R-project.org/.
- 18.Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labor force characteristics by race and ethnicity, 2018. United States Bureau of Labor Statistics (2018).
- 20.U.S. Department of Health and Human Services. Social Determinants of Health (2020).
- 21.Moscola J, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Ochoa SA, et al. COVID-19 in healthcare workers: A living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am. J. Epidemiol. 2020;190:161–175. doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coronavirus Disease (COVID-19) Timeline. https://www.boston.gov/departments/public-health-commission/coronavirus-timeline (2020).
- 24.Vanuytsel K, et al. Rapid implementation of a SARS-CoV-2 diagnostic qRT-PCR test with emergency use authorization at a large academic safety-net hospital. Medicine. 2020;1:152–157. doi: 10.1016/j.medj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothe C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu P, Zhu J, Zhang Z, Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J. Infect. Dis. 2020;221:1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, R. et al. Viral dynamics in asymptomatic patients with COVID-19. Int. J. Infect. Dis.96, 288–290 (2020). [DOI] [PMC free article] [PubMed]
- 29.Zou L, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isho B, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020;5:5511. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibarrondo FJ, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, M. S. et al. Clinical performance of two SARS-CoV-2 serologic assays. Clin. Chem.66, 1055–1062 (2020). [DOI] [PMC free article] [PubMed]
- 33.Muecksch, F. et al. Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents. MedRxiv (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


