Highlights
-
•
Poor performances in neuropsychological tests were associated with cortical atrophy.
-
•
Neural substrates in Aβ (−) SVCI differed from those in ADCI.
-
•
Neural substrate of episodic memory was frontal regions in Aβ (−) SVCI.
-
•
Neural substrates of three neuropsychological tests showed laterality.
Keywords: Neural substrate, Subcortical vascular cognitive impairment, Cortical atrophy, Neuropsychological test
Abstract
Objective
Neuropsychological test-specific neural substrates in subcortical vascular cognitive impairment (SVCI) are expected to differ from those in Alzheimer’s disease-related cognitive impairment (ADCI) but the details are unclear. To determine neural substrates related to cerebral small vessel disease, we investigated the correlations between cognitive dysfunctions measured by standardized neuropsychological tests and cortical thickness in a large sample of participants with amyloid negative (Aβ (−)) SVCI.
Methods
One hundred ninety-eight participants with Aβ (−) SVCI were recruited from the memory clinic between November 2007 to August 2018. To acquire neural substrates, we performed linear regression using the scores of each neuropsychological test as a predictor, cortical thickness as an outcome, and age, sex, education years, intracranial volume and white matter hyperintensity (WMH) as confounders.
Results
Poor performances in each neuropsychological test were associated with cortical atrophy in certain brain regions regardless of WMH. Especially, not the medial temporal but the frontal and posterior cingulate regions with cortical atrophy were mainly associated with memory impairment. Poor performance in animal fluency was more likely to be associated with cortical atrophy in the left hemisphere, while poor performance in the visuospatial memory test was more likely to be associated with cortical atrophy in the right hemisphere.
Conclusions
Our findings suggested that cortical atrophy was an important factor of cognitive impairment in Aβ (−) SVCI regardless of WMH. Furthermore, our findings might give clinicians a better understanding of specific neural substrates of neuropsychological deficits in patients with SVCI.
1. Introduction
Subcortical vascular cognitive impairment (SVCI) is characterized by extensive cerebral small vessel disease (CSVD), such as white matter hyperintensities (WMH) and lacunes on subcortical regions, which in turn leads to frontal subcortical circuit damage and eventually to frontal executive dysfunction. Although SVCI is one form of subcortical dementia, it was recently shown that cortical atrophy in SVCI might occur primarily in the frontal and perisylvian regions through several mechanisms including secondary degeneration (Fein et al., 2000; C. H. Kim et al., 2012), direct cortical ischemia (Kovari et al., 2007), or concomitant Alzheimer’s disease (AD) pathology (Fein et al., 2000). SVCI is also associated with frontal executive dysfunction and other cognitive dysfunctions including memory, language, and visuospatial dysfunctions. However, brain regions related to cognitive dysfunction in SVCI patients have focused on subcortical areas, unlike in AD-related cognitive impairment (ADCI) or other dementia syndromes.
Traditionally, cognitive dysfunction is explained by cortical atrophy in many studies through an investigation of the cognitive-anatomical association (Ahn et al., 2011, Cheng et al., 2018, Teipel et al., 2006). The cognitive-anatomical association, called neural substrate, is defined as the correlation of cognitive deficits with specific anatomical regions after brain damage due to various reasons including degenerative diseases. The neural substrate is important in clinical practice, not only to understand a patient’s current cognitive impairment but also to predict the progression of cognitive dysfunction. Since ADCI is the most common neurodegenerative disease causing cognitive impairment, many studies investigated certain brain areas associated with the specific types of cognitive dysfunctions in patients with ADCI (Ahn et al., 2011, Teipel et al., 2006). In contrast, neural substrates of cognitive functions in patients with SVCI were disregarded, although SVCI also leads to multiple cognitive dysfunctions and cortical atrophy. Considering that SVCI has a completely different pathogenesis, atrophy pattern, and neuropsychological findings in comparison with ADCI (Seo et al., 2010, Seo et al., 2009), there might be distinct neural substrates of cognitive functions for SVCI which are different from those observed in ADCI. A better understanding of neural substrates specific to SVCI might enable clinicians to predict brain atrophy patterns from the results of neuropsychological tests in patients with SVCI and vice versa.
The goal of our study was, therefore, to investigate neuropsychological test-specific neural substrates in a large sample of participants with SVCI, by correlating cognitive dysfunction as measured by standardized neuropsychological tests with cortical thickness. Especially, to prevent the impact of concomitant AD pathology on cortical thinning, we excluded amyloid-positive SVCI participants in our study. Given that cortical atrophy in SVCI might be distributed primarily in the frontal and perisylvian regions, we hypothesized that neural substrates in SVCI might be related to these regions.
2. Materials and methods
2.1. Participants
We included 365 participants with SVCI from the memory clinic in the department of neurology at the Samsung Medical Center (SMC) in Seoul Korea between November 2007 and August 2018. All participants underwent comprehensive dementia evaluation including a standardized neuropsychological battery, high-resolution T1-weighted magnetic resonance imaging (MRI), and blood tests. The time interval between neuropsychological tests and MRI was less than six months. Participants with subcortical vascular mild cognitive impairment (svMCI) met Petersen's criteria (Petersen et al., 1999), with the following modifications (Seo et al., 2009): (1) a subjective complaint of cognitive impairment by the patient or caregiver; (2) an objective cognitive decline below the 16th percentile on the neuropsychological test; (3) normal activities of daily living; (4) not demented; (5) a subcortical vascular feature defined as a focal neurologic symptom or sign including corticobulbar signs (dysarthria, dysphagia, facial palsy, or pathologic laughing or crying), pyramidal signs (hemiparesis, hyperactive/asymmetric deep tendon reflexes, or Babinski sign), or parkinsonism (bradykinesia, rigidity, short-step gait, shuffling gait, festination, or postural instability); and (6) the presence of significant ischemia on MRI. Significant ischemia was defined as WMH on fluid-attenuated inversion recovery (FLAIR) images that satisfied the following criteria: (1) WMH of 10 mm or more in the periventricular white matter (caps or rim) and (2) WMH of 25 mm or more (maximum diameter) in the deep white matter, consistent with an extensive white matter lesion or diffusely confluent lesion. Participants with subcortical vascular dementia (SVaD) were diagnosed by fulfillment of the criteria described in the Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition (DSM-IV) (Association, 1994) and had severe WMH on MRI as described above. Based on our previous studies (H. J. Kim et al., 2016; H. J. Kim et al., 2014), participants with SVCI were composed of participants with svMCI and SVaD. We excluded participants who had any of the following conditions: 1) WMH due to etiologies other than vascular pathology, such as radiation injury, multiple sclerosis, leukodystrophy, or metabolic/toxic disorders, 2) participants with cerebral infarction including large territory infarction and small cortical infarction.
Four hundred eight participants with normal cognition (NC) were also enrolled. These participants were composed of spouses of patients who visited the memory clinic, volunteers who applied for comprehensive dementia evaluation advertised in the paper, and participants who had cognitive complaints. They visited the memory clinic in the department of neurology at the SMC and underwent comprehensive dementia evaluation. All participants with NC met the following criteria: (1) no medical history which is likely to affect cognitive function based on Christensen's health screening criteria (Christensen et al., 1991); (2) no objective cognitive impairment from comprehensive neuropsychological test battery on any cognitive domains (at least −1.0 SD above age-adjusted norms on any cognitive tests); (3) independent in activities of daily living; (4) neither structural lesions nor severe WMH on brain MRI; and (5) no amyloid deposition in the amyloid PET.
The institutional review boards at all participating centers approved this study. Written informed consent was obtained from participants and caregivers.
2.2. Acquisition of amyloid positron emission tomography and data analysis
All participants underwent amyloid PET: 11C-PiB PET scans were conducted in 143, 18F-florbetaben PET scans in 373, and 18F-flutemetamol PET scans in 257 participants at the SMC using a Discovery STe PET/computed tomography scanner (GE Medical Systems, Milwaukee, WI, USA). For 11C-PiB PET, a 30-minute static emission PET scan was performed 60 min after a bolus injection of a mean dose of 420 MBq. For 18F-florbetaben PET and 18F-flutemetamol PET, a 20-minute emission PET scan with dynamic mode (consisting of 4 × 5 min frames) was performed 90 min after an injection of a mean dose of 311.5 MBq 18F-florbetaben and 197.7 MBq 18F-flutemetamol. 11C-PiB PET was regarded as positive if the global PiB uptake value was >1.5 (Lee et al., 2011). 18F-florbetaben PET was classified as positive if the amyloid-plaque load on the florbetaben PET scan was visually rated as 2 or 3 on the brain amyloid-plaque load scoring system, and 18F-flutemetamol PET was considered positive when one of five brain regions (frontal, parietal, posterior cingulate, precuneus, striatum, and lateral temporal lobes) systematically reviewed for flutemetamol PET was positive in either hemisphere (S. E. Kim et al., 2018).
Overall, 35.1% (128/365) of the participants with SVCI and 19.1% (78/408) of the participants with normal cognition were excluded due to positive amyloid PET scan results.
2.3. Acquisition of three-dimensional magnetic resonance images
We acquired three-dimensional T1 Turbo Field Echo MRI scans of 237 participants with Aβ (−) SVCI and 330 participants with Aβ (−) NC using a 3.0 T MRI scanner (Philips 3.0T Achieva) with the following imaging parameters: sagittal slice thickness, 1.0 mm with 50% overlap; no gap; repetition time of 9.9 ms; echo time of 4.6 ms; flip angle of 8°; and matrix size of 240 × 240 pixels reconstructed to 480 × 480 over a field view of 240 mm.
2.4. MRI data processing for cortical thickness measurements
Images were processed using the CIVET anatomical pipeline (version 2.1.0) (Zijdenbos et al., 2002). The native MRI images were registered to the Montreal Neurological Institute −152 template by a linear transformation (Collins et al., 1994) and corrected for intensity non-uniformities using the N3 algorithm (Sled et al., 1998). The registered and corrected images were divided into white matter, gray matter, cerebrospinal fluid, and background. The Constrained Laplacian-based Automated Segmentation with Proximities algorithm (J. S. Kim et al., 2005) extracted the surfaces of the inner and the outer cortices automatically. The inner and outer surfaces had the same numbers of vertices and there were close correspondences between the counterpart vertices of the inner and outer cortical surfaces. The presence of extensive WMH in the MRI scans made it difficult to completely delineate the inner cortical surface with the correct topology due to tissue classification errors. To overcome this technical limitation, we automatically defined the WMH region using a FLAIR image and substituted it for the intensity of peripheral, normal-appearing tissue on the high-resolution T1 image after affine co-registration, as described in earlier studies (C. H. Kim et al., 2012). Cortical thickness, which was defined as the Euclidean distance between the linked vertices of the inner and outer surfaces (Lerch and Evans, 2005), was not calculated in Talairach spaces but in native brain spaces due to the limit of linear stereotaxic normalization. As expected, there was a significant positive correlation between cortical thickness and intracranial volume (ICV) in native space (Im et al., 2008). Controlling for ICV, which reflected a brain size effect, is necessary to compare the cortical thickness among participants. We proposed in a previous study (Im et al., 2008) that the measurement of native space cortical thickness, followed by analyses that include brain size as a covariate, is an efficient method and explains the relationship between cortical thickness and brain size in depth. ICV is defined as the total volume of gray matter, white matter, and cerebrospinal fluid. It is calculated by measuring the total volumes of the voxels within the brain mask, which were obtained via the Functional Magnetic Resonance Imaging of the Brain Software Library bet algorithm (Smith, 2002). As we extracted cortical surface models from MRI volumes transformed into stereotaxic space, cortical thickness was measured in the native space by applying an inverse transformation matrix to the cortical surfaces and reconstructing them in native space (Im et al., 2006).
We applied surface-based two-dimensional registration with a sphere-to-sphere warping algorithm and normalized the cortical thickness values spatially to compare the thickness of corresponding regions among subjects. We used an improved surface registration algorithm and an unbiased iterative group template showing enhanced anatomic detail (Lyttelton et al., 2007) to transform the thickness information for the vertices into an unbiased iterative group template.
Surface-based diffusion smoothing with a full-width at half-maximum of 20 mm was used to blur each map of cortical thickness, which increased the signal-to-noise ratio and statistical power (Chung et al., 2003, Im et al., 2006).
Thirty-nine participants with Aβ (−) SVCI and 22 participants with Aβ (−) NC were excluded due to an error during analysis for cortical thickness including CIVET pipeline errors (n = 46) which contained errors due to MR image problems such as motion artifacts (n = 8) and segmentation process error by WM and GM due to poor image quality (n = 38) and WMH correction errors (n = 15). As a result, a total of 198 Aβ (−) SVCI participants and 308 Aβ (−) NC were included.
2.5. Asymmetric index
As described in a previous study (S. H. Kang et al., 2019), to measure asymmetric degrees of neuroanatomical correlates for neuropsychological tests, we obtained an asymmetric index (AI), which was calculated by the following formula: (R-L/R + L), where R indicates the number of vertices with significant correlations in the right hemisphere and L indicates the number of vertices with significant correlations in the left hemisphere [4]. After obtaining the AI, we divided the extent of asymmetry into three groups according to the absolute AI value. When the absolute value of the AI was ≤ 0.1, > 0.1 and ≤ 0.5, or > 0.5, we classified the case as no hemispheric dominance, weak hemispheric dominance, or strong hemispheric dominance, respectively.
2.6. Neuropsychological tests
All participants underwent neuropsychological testing using the Seoul Neuropsychological Screening Battery 2nd edition (SNSB-II) (S. H. Kang et al., 2019; Y. Kang et al., 2012). A small number of participants could not complete all tests. We chose to use eight cognitive measures, which were representative and important neuropsychological tests to evaluate the cognitive function in five cognitive domains as follow: 1) Memory: the Seoul Verbal Learning Test (SVLT) delayed recall (verbal memory) and Rey-Osterrieth Complex Figure Test (RCFT) delayed recall (visual memory); 2) Language: Korean version of the Boston Naming Test (K-BNT); 3) Visuospatial function: RCFT copying Test; 4) Frontal executive function: animal and phonemic portion of the Controlled Oral Word Association Test (COWAT) and the Stroop Test (color reading); and 5) Attention: Digit Span Test backward. The results with numeric, continuous values were used in the analysis.
2.7. Propensity score matching
We performed a propensity score matching to balance the age and education years between Aβ (−) SVCI and Aβ (−) NC group. The propensity score of the two groups (SVCI and NC) was obtained including age and education variables for each participant. Using propensity score, 1:1 nearest neighbor matching algorithm was applied to match the participants with Aβ (−) NC to participants with Aβ (−) SVCI between the two groups within 0.2*SD of the logistic propensity score. As a result, 198 out of 308 participants with Aβ (−) NC were selected as control group.
2.8. Statistical analyses
We used analysis of variance and Chi-square tests to compare the demographic data and the results of the neuropsychological tests between the SVCI and NC groups. SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the statistical data. A two-sided p-value < 0.05 was considered significant. For cortical thickness analyses of MRI data from SVCI participants, we used a MATLAB-based toolbox (freely available online at the University of Chicago website: http://galton.uchicago.edu/faculty/InMemoriam/worsley/research/surfstat/). To identify the cortical thinning pattern in SVCI, we analyzed localized differences in cortical thickness between the NC and SVCI groups using a general linear model after controlling for age, sex, education years, and ICV. For SVCI group, to validate the correlation between each neuropsychological test score and cortical thickness, we entered the score of each neuropsychological test as a predictor and vertex-by-vertex cortical thickness as an outcome for analyzing the relationship between cortical thickness and neuropsychological performance in the surface model. Linear regression was then performed after controlling for age, sex, education years, ICV and WMH volume as covariates. Furthermore, to control for multiple comparisons with neuropsychological results, we performed false discovery rate correction as suggested by Benjamini-Hochberg(Benjamini and Hochberg, 1995). The statistical maps were thresholded using the random field theory at p < 0.05.
3. Results
3.1. Clinical characteristics of the participants
Table 1 shows the baseline demographics of participants with Aβ (−) NC and Aβ (−) SVCI. Participants with SVCI were older (75.2 ± 7.2 years) and had fewer years of education (8.8 ± 5.2 years) and a lower MMSE score (23.3 ± 4.5) than those with Aβ (−) NC (Table 1). There was no difference in the female ratio between the NC and SVCI groups (p = 0.143).
Table 1.
Demographic variables and cognitive profiles of the participants in the Aβ(−) SVCI and Aβ(−) NC groups.
| Aβ(−) SVCI |
Aβ(−) NC (n = 198) | ||||
|---|---|---|---|---|---|
| Total (n = 198) | svMCI (n = 116) | SVaD (n = 82) | p-value | ||
| Demographics | |||||
| Age, years | 75.2 ± 7.2* | 74.4 ± 7.6 | 76.3 ± 6.4* | 73.3 ± 6.7 | 0.004 |
| Gender, female | 135 (68.2%) | 79 (68.1%) | 56 (68.3%) | 127 (64.1%) | 0.697 |
| Education, years | 8.8 ± 5.2* | 9.0 ± 5.1* | 8.5 ± 5.4* | 10.5 ± 4.8 | 0.002 |
| MMSE | 23.3 ± 4.5* | 25.8 ± 2.7* | 19.7 ± 4.2* | 27.9 ± 2.0 | < 0.001 |
| Neuropsychological test | |||||
| Attention | |||||
| Digit Span Test Backward | 2.9 ± 1.1* | 3.2 ± 0.9* | 2.5 ± 1.2* | 3.7 ± 1.1 | < 0.001# |
| Language | |||||
| K-BNT | 36.1 ± 10.4* | 39.7 ± 9.6* | 31.1 ± 9.4* | 47.1 ± 6.8 | < 0.001# |
| Visuospatial function | |||||
| RCFT: copying | 23.8 ± 9.6* | 27.8 ± 6.5* | 18.1 ± 10.3* | 32.0 ± 4.0 | < 0.001# |
| Memory | |||||
| SVLT: delayed recall | 2.9 ± 2.8* | 4.1 ± 2.7* | 1.2 ± 1.8* | 6.6 ± 2.1 | < 0.001# |
| RCFT: delayed recall | 7.2 ± 6.4* | 10.0 ± 6.2* | 3.3 ± 4.1* | 13.6 ± 6.3 | < 0.001# |
| Frontal/executive function | |||||
| COWAT: animal | 9.7 ± 4.1* | 11.5 ± 3.7* | 7.2 ± 3.2* | 15.3 ± 4.5 | < 0.001# |
| COWAT: phonemic | 14.1 ± 9.6* | 17.1 ± 9.4* | 9.1 ± 7.6* | 25.1 ± 10.7 | < 0.001# |
| Stroop test: color reading | 48.6 ± 28.4* | 58.9 ± 25.0* | 31.0 ± 25.2* | 82.4 ± 20.3 | < 0.001# |
Values are presented as mean ± standard deviation. The p values were obtained by analysis of variance model and chi-square tests.
The p values were obtained by analysis of covariance after controlling for age and education.
p < 0.05 compared to Aβ(−) NC.
Aβ(−) SVCI: amyloid-negative subcortical vascular cognitive impairment, Aβ(−) NC: amyloid-negative normal cognition, MMSE: Mini Mental State Examination, K-BNT: Korean version of Boston naming test, RCFT: Rey-Osterrieth complex figure test, CDT: clock drawing test, SVLT: Seoul verbal learning test, COWAT: controlled oral word association test, TMT-B: part B of the trail making test, DSC: digit symbol coding.
3.2. Topography of cortical thinning
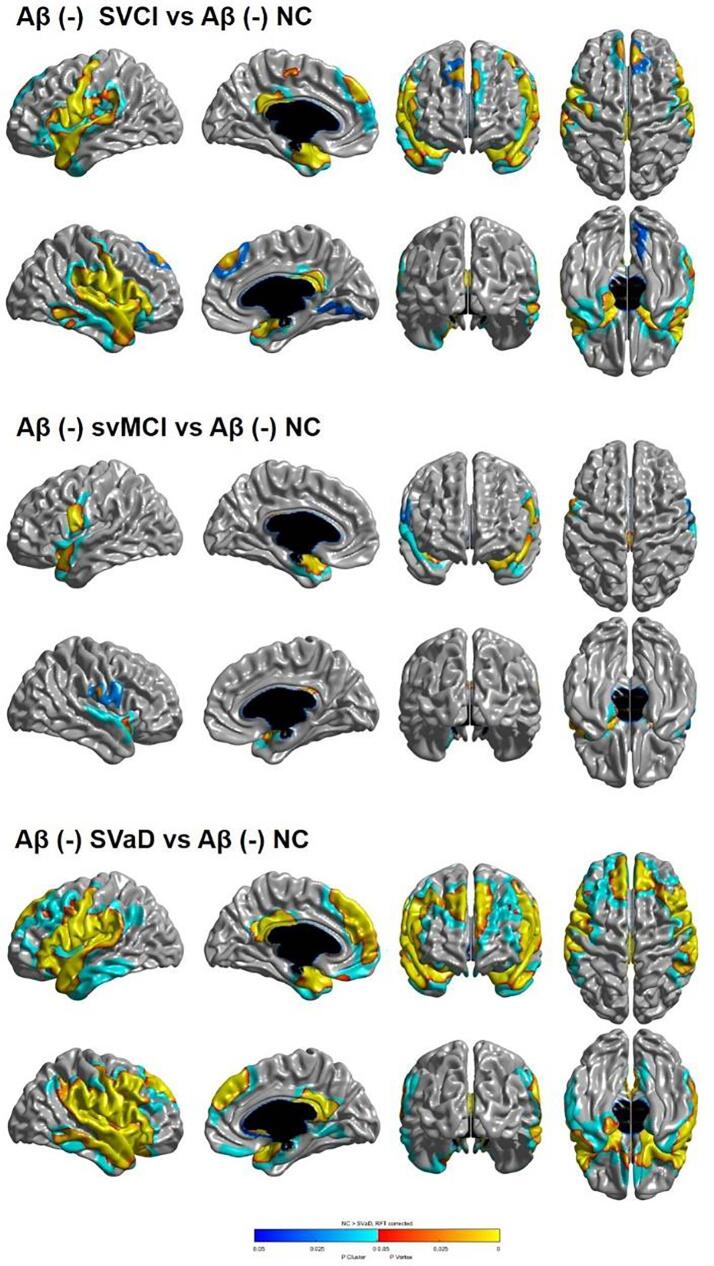
Fig. 1 shows the topography of cortical thinning in participants with Aβ (−) SVCI. Compared with Aβ (−) NC participants, Aβ (−) SVCI participants exhibited significant cortical thinning in the bilateral perisylvian, the inferior, medial frontal and posterior cingulate, the temporal pole, and superior and medial temporal regions. Specifically, compared with Aβ (−) NC participants, Aβ (−) svMCI participants exhibited significant cortical thinning in the bilateral perisylvian, inferior frontal, and superior temporal regions. Aβ (−) SVaD participants exhibited significant cortical thinning in the bilateral perisylvian, inferior, middle, superior, medial frontal, orbitofrontal, posterior cingulate, temporal pole, superior, middle, inferior, and medial temporal regions.
Fig. 1.
Statistical representation of cortical thickness in Aβ(−) NC, Aβ(−) SVCI, Aβ(−) svMCI and Aβ(−) SVaD groups. NC: amyloid-negative normal cognition, Aβ(−) svMCI: amyloid-negative subcortical vascular mild cognitive impairment, Aβ(−) SVaD: amyloid-negative subcortical vascular dementia, Aβ(−) SVCI: amyloid-negative subcortical vascular cognitive impairment.
3.3. Correlation between neuropsychological tests and cortical thickness
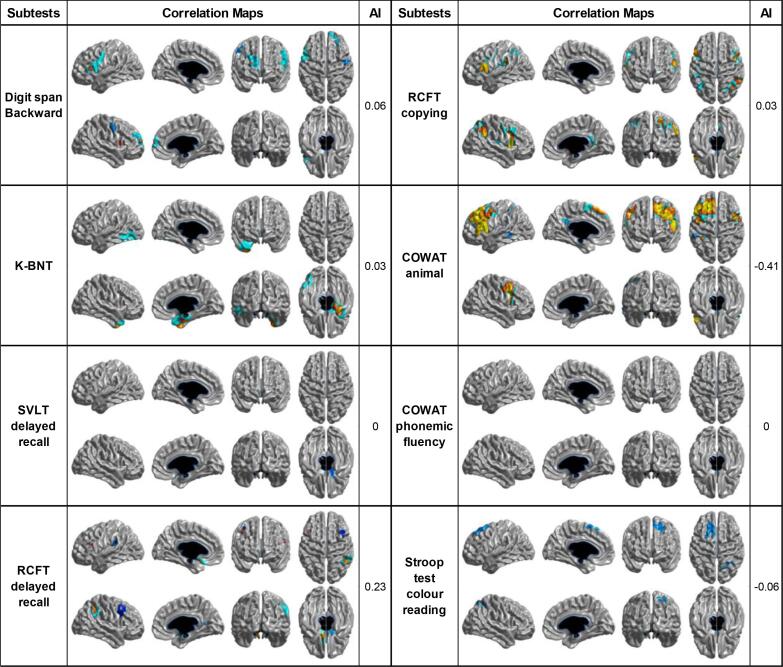
The statistical map showed that cortical thinning in specific brain regions was associated with raw scores on all neuropsychological tests except for the phonemic component of the COWAT (Fig. 2). Specifically, the score in the Digit Span Test backward, which reflects attention and working memory, was positively associated with cortical thickness in the bilateral inferior frontal, and right superior and middle frontal gyri. The score in the K-BNT reflecting confrontational naming was positively associated with cortical thickness in the left inferior temporal, fusiform gyri, and the right anterio-medial temporal region. The score in delayed recall in the SVLT was positively associated with cortical thickness in the right orbitofrontal gyrus. The score in delayed recall of the RCFT was positively correlated with cortical thickness in the bilateral orbitofrontal, left medial frontal, right middle frontal, posterior cingulate, and supramarginal gyri. The score in the copying test of the RCFT composing visuospatial domain was positively correlated with cortical thickness in the bilateral inferior and middle frontal, superior parietal, right posterior cingulate, and supramarginal gyri. The score in the semantic component of the COWAT (animal) was positively correlated with cortical thickness in the bilateral middle frontal, left superior, inferior and medial frontal gyrus, and posterior cingulate gyri. The score in the color reading portion of the Stroop test was positively associated with cortical thickness in the left frontal and right superior parietal gyri.
Fig. 2.
Correlation maps demonstrating the association between cortical thickness and neuropsychological tests in participants with Aβ(−) SVCI (AI > 0 means right-sided correlated areas > left-sided correlated areas, and vice versa for AI < 0). K-BNT: Korean version of Boston naming test, SVLT: Seoul verbal learning test, RCFT: Rey-Osterrieth complex figure test, COWAT: controlled oral word association test, AI: asymmetric index.
3.4. Hemispheric dominance of cortical thickness related to neuropsychological results
We also investigated hemispheric dominance of neural substrates by using AI. Neural substrates for only delayed recall of the RCFT, and semantic component of the COWAT (animal) showed hemispheric dominance. Among these, RCFT delayed recall was dominantly associated with the right hemisphere, while semantic component of the COWAT were dominantly related to the left hemisphere.
4. Discussion
In the present study, we investigated the neural substrates in a large cohort of carefully phenotyped participants with Aβ (−) SVCI. The major findings in this study were as follows: 1) poor performances in most of the neuropsychological tests were associated with cortical atrophy in specific cortical regions regardless of CSVD burden.; 2) poor performances of three neuropsychological tests are more likely to be associated with cortical atrophy in one hemisphere than the other, but their lateralities were modest. Taken together, our findings suggested that cortical atrophy was an important factor of cognitive impairment in CSVD population regardless of WMH. Furthermore, our findings might give clinicians a better understanding about specific neural substrates of neuropsychological deficits in participants with SVCI.
The first major finding was that poor performances in each neuropsychological test were associated with cortical atrophy in certain brain regions regardless of CSVD burden. Impairment of the backward digit span was associated with decreased cortical thickness in the bilateral inferior frontal, right superior frontal, and middle frontal gyri. The backward digit span test has been used to evaluate working memory, which consists of four components. Of these four components, the central executive system regulates the other three subordinate subsystems and is associated with the frontal cortex (Baddeley, 2000). It was reason backward digit span was mainly related to frontal cortex. Our results might be also consistent with previous neuroimaging studies showing that prefrontal regions play an important role in working memory (Cabeza et al., 2000, Cabeza et al., 1997, Majerus et al., 2010).
In the present study, poor performances in K-BNT were associated with cortical atrophy in the left inferior temporal and fusiform gyri and right anterio-medial temporal regions. K-BNT is a representative tool for evaluation of confrontational naming. It is well known that the left inferior and fusiform gyri are responsible for naming. Previous MRI studies revealed that the BNT score is positively correlated with cortical thickness in the left middle and inferior temporal gyri and the inferior parietal cortex (Baldo et al., 2013; S. H. Kang et al., 2019, Lau et al., 2015). In addition, poor performances in BNT were associated with hypometabolism in the left middle temporal and fusiform gyri in a previous FDG PET study (Teipel et al., 2006). However, there is little evidence that the right anterio-medial temporal areas may be involved in naming function (Apostolova et al., 2008). One study showed that cortical atrophy of the right anterio-medial temporal regions including the temporal pole was related to a low BNT score (Apostolova et al., 2008). Semantic variant primary progressive aphasia, a neurodegenerative disease presents with naming difficulty, shows bilateral anterior temporal atrophy despite a left-sided preference (Gorno-Tempini et al., 2004). Therefore, it seems appropriate that the right anterior temporal regions may be associated with semantic processing including naming.
Especially, the neural substrate of episodic memory was totally different from those previously known in ADCI (Ahn et al., 2011; S. H. Kang et al., 2019). Contrary to expectations, poor performances on the delayed recall task of the SVLT were related to cortical thinning not in the medial temporal areas but in the right orbitofrontal gyrus. In addition, a low score in the RCFT delayed recall was related to cortical atrophy in the bilateral orbital frontal, left medial frontal, right middle frontal, posterior cingulate, and supramarginal gyri. Previous studies revealed that cortical thinning in the bilateral medial temporal regions was closely associated with delay recall task scores of the SVLT and RCFT in patients with ADCI (Ahn et al., 2011; S. H. Kang et al., 2019). The essential role of the medial temporal area including hippocampus structures for the consolidation of new information is known for a long time (Squire and Wixted, 2011), while other networks that include the hippocampus also have an important role in episodic memory performance. Among these networks, the Papez circuit includes the hippocampus, entorhinal cortex, mammillary body, anterior thalamic nuclei, cingulate cortices, and the parahippocampal gyrus and is a core pathway of the limbic system involved in the consolidation of episodic memory (Callen et al., 2001, Jicha and Carr, 2010). Functional connectivity of the hippocampus has reflected network in Papez circuit and the connectivity with the hippocampus in the subcortical regions as well as cortical regions including frontal, insula, posterior cingulate cortices was associated with episodic memory performance (Li et al., 2014). SVCI is a disease with cognitive impairment due to CSVD, which is a representative disease of cerebral white matter tissue. In this regard, CSVD may first affect the white matter tracts associated with networks in the Papez circuit and may damage secondarily the posterior cingulate and frontal cortices. This is the reason why the posterior cingulate and frontal regions were mainly associated with episodic memory function in our study. In fact, a previous SPECT study investigating the neural substrates in a small number of participants with SVCI showed that a low score of delayed recall tests was associated with hypoperfusion in the right frontal and left occipital regions (Baker et al., 2013). A functional MRI study also revealed that worse performance in memory was related to disruption of functional connectivity between the medial frontal gyrus and the posterior cingulate cortex (Chen et al., 2019).
In contrast to the results of previous studies with participants with ADCI which showed that the neural substrate of the RCFT copying tests were predominantly temporo-parietal regions (Ahn et al., 2011; S. H. Kang et al., 2019), poor performances on the copy task of the RCFT were associated with cortical atrophy in the bilateral inferior and middle frontal, superior parietal, right posterior cingulate, and supramarginal gyri. Visuo-perceptive and visuo-constructive functions are necessary to perform the copy task of the RCFT and are linked to parietal and frontal areas, respectively. Our findings might suggest that poor performances in the RCFT copy test were triggered by visuo-constructive rather than visuo-perceptive dysfunction in SVCI participants (Lei et al., 2016, Melrose et al., 2013).
Our study showed that semantic fluency was mainly associated with the bilateral middle frontal, left superior, inferior and medial frontal, and posterior cingulate gyri. This was in contrast to results from previous studies exploring the neural substrate of semantic fluency in participants with ADCI, which showed that anterior and inferior temporal regions as well as frontal regions were associated with poor performance in the semantic fluency task (Ahn et al., 2011; S. H. Kang et al., 2019). Semantic fluency reflects language function and frontal executive function, and impairment of semantic fluency was associated predominantly with the temporal and frontal cortex in a meta-analysis (Henry and Crawford, 2004). The different results might be explained by the cortical atrophy pattern of participants with SVCI, who display profound cortical atrophy in the frontal regions. However, on phonemic fluency, no cortical neural substrates were identified after WMH volume correction. Given that, before WMH volume correction, phonemic fluency was associated with the left middle, inferior and medial frontal, and posterior cingulate gyri, poor performance in phonemic fluency might not be directly associated with cortical atrophy in the frontal cortex, but rather with primary damage in subcortical structure connected to frontal cortex. In our study, poor performances in the color reading task of the Stroop test were related to cortical atrophy in the bilateral inferior frontal gyri. Stroop color reading has been used to evaluate selective attention and cognitive flexibility. Functional neuroimaging studies showed that Stroop color reading-related activations are detected in the lateral prefrontal cortex (Cieslik et al., 2015, Cipolotti et al., 2016). Our results had a similar neural substrate pattern as those in the previous studies.
The second major finding was that poor performances in three neuropsychological tests were more likely to be associated with cortical atrophy in one hemisphere than the other, but the lateralities were not strong. As expected, neuropsychological tests to evaluate generative naming such as animal fluency and phonemic fluency related to language and frontal function were associated with cortical atrophy in the left hemisphere, while results of the visual memory function test were associated with right hemisphere atrophy. Different from expectations, however, neural substrates of some tests did not have hemispheric asymmetry. This might be explained by the fact that cerebral atrophy in patients with SVCI usually develops and progresses bilaterally.
5. Limitations and conclusion
In this study, we investigated the neural substrates of neuropsychological tests in a large sample of participants with SVCI who did not have amyloid pathology. The study has five limitations. First, the study participants underwent amyloid PET with different types of tracers. However, this limitation is mitigated to some degree by the very high correlations among amyloid PET tracers (Landau et al., 2014, Villemagne et al., 2012). Second, although we excluded amyloid positive participants, we did not consider the effects of other mixed pathologies (e.g., α-synuclein and frontotemporal lobar degeneration), which might be associated with SVCI. Third, although we revealed that certain brain regions were associated with poor performance in neuropsychological tests, it is questionable that all brain regions that we found represent test-specific brain areas. Rather, some neural substrates for each neuropsychological test may be relevant to basic cognitive processes shared by a variety of neuropsychological tests. Fourth, we used cortical thickness which reflects gray matter structure to investigate the neural substrates. Therefore, we did not cover changes in micro-white matter structure using advanced imaging such as diffusion tensor imaging and functional network using functional MRI with cognitive task. Previous studies from our group suggested that structural and functional connectivities measured by DTI and resting state fMRI were associated with cognitive dysfunction (Franzmeier et al., 2019; H. J. Kim et al., 2015). However, since the goal of our study was to investigate the pattern of cortical atrophy related to poor neuropsychological results, we did not include it. Further study should be needed to investigate further mechanisms underlying cognitive dysfunction in SVCI. Finally, since our study population was comprised of participants with SVCI, our results may be affected by the cortical thinning pattern in SVCI which was primary atrophy in the frontal and perisylvian regions, and the findings may not be generalizable to subjects with other neurodegenerative diseases such as Parkinson’s disease and frontotemporal dementia. Further studies using participants with other neurodegenerative disease are needed to address this limitation. Despite these limitations, our study is note-worthy since we reported associations between neuropsychological tests and anatomical features in participants with SVCI, which implied that cortical atrophy was also an important factor of cognitive impairment in CSVD population regardless of WMH. Our results may help clinicians diagnose SVCI and make a prognosis about disease progression by understanding the neural substrates of cognitive impairment related to subcortical vascular damage.
In conclusion, poor performances of most neuropsychological tests were associated with cortical atrophy in the specific brain areas in patients with SVCI. Thus, we were able to predict the cortical atrophy patterns in patients with SVCI by an accurate analysis of neuropsychological tests in clinical practice.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare and Ministry of science and ICT, Republic of Korea (grant number : HU20C0111); the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A5A2027340); a fund (2021-ER1006-00) by Research of Korea Centers for Disease Control and Prevention and Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL) and grant funded by Korea University Medicine (K2107411).
CRediT authorship contribution statement
Sung Hoon Kang: Writing - original draft, Writing - review & editing, Formal analysis, Data curation. Yu Hyun Park: Writing - review & editing, Formal analysis, Data curation. Jun Pyo Kim: Data curation. Ji-Sun Kim: Data curation. Chi Hun Kim: Data curation. Hyemin Jang: Data curation. Hee Jin Kim: Data curation. Seong-Beom Koh: Data curation. Duk.L. Na: Data curation. Juhee Chin: Writing - review & editing, Conceptualization, Data curation. Sang Won Seo: Writing - review & editing, Conceptualization, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Alice Hahn for her contribution to English language editing.
Contributor Information
Juhee Chin, Email: juheechin@hanmail.net.
Sang Won Seo, Email: sangwonseo@empal.com, sw72.seo@samsung.com.
References
- Ahn H.J., Seo S.W., Chin J., Suh M.K., Lee B.H., Kim S.T., Im K., Lee J.M., Lee J.H., Heilman K.M., Na D.L. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and Alzheimer's disease: a surface-based morphometric analysis. Neuropsychologia. 2011;49:3931–3945. doi: 10.1016/j.neuropsychologia.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Apostolova L.G., Lu P., Rogers S., Dutton R.A., Hayashi K.M., Toga A.W., Cummings J.L., Thompson P.M. 3D mapping of language networks in clinical and pre-clinical Alzheimer's disease. Brain Language. 2008;104:33–41. doi: 10.1016/j.bandl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A.P. American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn. Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baker J.G., Williams A.J., Wack D.S., Miletich R.S. Correlation of cognition and SPECT perfusion: easy Z score and SPM analysis of a pilot sample with cerebral small vessel disease. Dement. Geriatr. Cogn. Disord. 2013;36:290–299. doi: 10.1159/000339587. [DOI] [PubMed] [Google Scholar]
- Baldo J.V., Arevalo A., Patterson J.P., Dronkers N.F. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex J. Devoted Study Nerv. Syst. Behav. 2013;49:658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Cabeza R., Anderson N.D., Houle S., Mangels J.A., Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J. Cognit. Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Mangels J., Nyberg L., Habib R., Houle S., McIntosh A.R., Tulving E. Brain regions differentially involved in remembering what and when: a PET study. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- Callen D.J., Black S.E., Gao F., Caldwell C.B., Szalai J.P. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–1674. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- Chen X., Huang L., Ye Q., Yang D., Qin R., Luo C., Li M., Zhang B., Xu Y. Disrupted functional and structural connectivity within default mode network contribute to WMH-related cognitive impairment. NeuroImage Clin. 2019;24:102088. doi: 10.1016/j.nicl.2019.102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.P., Cheng S.T., Tam C.W., Chan W.C., Chu W.C., Lam L.C. Relationship between cortical thickness and neuropsychological performance in normal older adults and those with mild cognitive impairment. Aging Dis. 2018;9:1020–1030. doi: 10.14336/ad.2018.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K.J., Multhaup K.S., Nordstrom S., Voss K. A cognitive battery for dementia: development and measurement characteristics. Psychol. Assess. J. Consult. Clin. Psychol. 1991;3:168–174. doi: 10.1037/1040-3590.3.2.168. [DOI] [Google Scholar]
- Chung M.K., Worsley K.J., Robbins S., Paus T., Taylor J., Giedd J.N., Rapoport J.L., Evans A.C. Deformation-based surface morphometry applied to gray matter deformation. NeuroImage. 2003;18:198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- Cieslik E.C., Mueller V.I., Eickhoff C.R., Langner R., Eickhoff S.B. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti L., Spano B., Healy C., Tudor-Sfetea C., Chan E., White M., Biondo F., Duncan J., Shallice T., Bozzali M. Inhibition processes are dissociable and lateralized in human prefrontal cortex. Neuropsychologia. 2016;93:1–12. doi: 10.1016/j.neuropsychologia.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Fein G., Di Sclafani V., Tanabe J., Cardenas V., Weiner M.W., Jagust W.J., Reed B.R., Norman D., Schuff N., Kusdra L., Greenfield T., Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N., Rubinski A., Neitzel J., Kim Y., Damm A., Na D.L., Kim H.J., Lyoo C.H., Cho H., Finsterwalder S., Duering M., Seo S.W., Ewers M. Functional connectivity associated with tau levels in ageing, Alzheimer's, and small vessel disease. Brain J. Neurol. 2019;142:1093–1107. doi: 10.1093/brain/awz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Dronkers N.F., Rankin K.P., Ogar J.M., Phengrasamy L., Rosen H.J., Johnson J.K., Weiner M.W., Miller B.L. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J.D., Crawford J.R. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Im K., Lee J.M., Lee J., Shin Y.W., Kim I.Y., Kwon J.S., Kim S.I. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage. 2006;31:31–38. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Im, K., Lee, J.M., Lyttelton, O., Kim, S.H., Evans, A.C., Kim, S.I., 2008. Brain size and cortical structure in the adult human brain. Cerebral cortex (New York, N.Y.: 1991) 18, 2181–2191. https://doi.org/10.1093/cercor/bhm244. [DOI] [PubMed]
- Jicha G.A., Carr S.A. Conceptual evolution in Alzheimer's disease: implications for understanding the clinical phenotype of progressive neurodegenerative disease. J. Alzheimer's Dis. JAD. 2010;19:253–272. doi: 10.3233/jad-2010-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.H., Park Y.H., Lee D., Kim J.P., Chin J., Ahn Y., Park S.B., Kim H.J., Jang H., Jung Y.H., Kim J., Lee J., Kim J.S., Cheon B.K., Hahn A., Lee H., Na D.L., Kim Y.J., Seo S.W. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer's continuum. Dement. Neurocogn. Disord. 2019;18:77–95. doi: 10.12779/dnd.2019.18.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Jahng S., Na D.L. 2nd ed. Human Brain Research & Consulting Co.; Seoul: 2012. Seoul Neuropsychological Screening Battery. [Google Scholar]
- Kim C.H., Seo S.W., Kim G.H., Shin J.S., Cho H., Noh Y., Kim S.H., Kim M.J., Jeon S., Yoon U., Lee J.M., Oh S.J., Kim J.S., Kim S.T., Lee J.H., Na D.L. Cortical thinning in subcortical vascular dementia with negative 11C-PiB PET. J. Alzheimer's Dis. JAD. 2012;31:315–323. doi: 10.3233/jad-2012-111832. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Im K., Kwon H., Lee J.M., Kim C., Kim Y.J., Jung N.Y., Cho H., Ye B.S., Noh Y., Kim G.H., Ko E.D., Kim J.S., Choe Y.S., Lee K.H., Kim S.T., Lee J.H., Ewers M., Weiner M.W., Na D.L., Seo S.W. Clinical effect of white matter network disruption related to amyloid and small vessel disease. Neurology. 2015;85:63–70. doi: 10.1212/wnl.0000000000001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Yang J.J., Kwon H., Kim C., Lee J.M., Chun P., Kim Y.J., Jung N.Y., Chin J., Kim S., Woo S.Y., Choe Y.S., Lee K.H., Kim S.T., Kim J.S., Lee J.H., Weiner M.W., Na D.L., Seo S.W. Relative impact of amyloid-β, lacunes, and downstream imaging markers on cognitive trajectories. Brain J. Neurol. 2016;139:2516–2527. doi: 10.1093/brain/aww148. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Ye B.S., Yoon C.W., Noh Y., Kim G.H., Cho H., Jeon S., Lee J.M., Kim J.H., Seong J.K., Kim C.H., Choe Y.S., Lee K.H., Kim S.T., Kim J.S., Park S.E., Kim J.H., Chin J., Cho J., Kim C., Lee J.H., Weiner M.W., Na D.L., Seo S.W. Cortical thickness and hippocampal shape in pure vascular mild cognitive impairment and dementia of subcortical type. Eur. J. Neurol. 2014;21:744–751. doi: 10.1111/ene.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Singh V., Lee J.K., Lerch J., Ad-Dab'bagh Y., MacDonald D., Lee J.M., Kim S.I., Evans A.C. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kim S.E., Woo S., Kim S.W., Chin J., Kim H.J., Lee B.I., Park J., Park K.W., Kang D.Y., Noh Y., Ye B.S., Yoo H.S., Lee J.S., Kim Y., Kim S.J., Cho S.H., Na D.L., Lockhart S.N., Jang H., Seo S.W. A nomogram for predicting amyloid PET positivity in amnestic mild cognitive impairment. J. Alzheimer's Dis. JAD. 2018;66:681–691. doi: 10.3233/jad-180048. [DOI] [PubMed] [Google Scholar]
- Kovari E., Gold G., Herrmann F.R., Canuto A., Hof P.R., Bouras C., Giannakopoulos P. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology. 2007;68:927–931. doi: 10.1212/01.wnl.0000257094.10655.9a. [DOI] [PubMed] [Google Scholar]
- Landau S.M., Thomas B.A., Thurfjell L., Schmidt M., Margolin R., Mintun M., Pontecorvo M., Baker S.L., Jagust W.J. Amyloid PET imaging in Alzheimer's disease: a comparison of three radiotracers. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:1398–1407. doi: 10.1007/s00259-014-2753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.K., Humphreys G.W., Douis H., Balani A., Bickerton W.L., Rotshtein P. The relation of object naming and other visual speech production tasks: a large scale voxel-based morphometric study. NeuroImage Clin. 2015;7:463–475. doi: 10.1016/j.nicl.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Kim S.H., Kim G.H., Seo S.W., Park H.K., Oh S.J., Kim J.S., Cheong H.K., Na D.L. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011;77:18–25. doi: 10.1212/WNL.0b013e318221acee. [DOI] [PubMed] [Google Scholar]
- Lei Y., Su J., Guo Q., Yang H., Gu Y., Mao Y. Regional gray matter atrophy in vascular mild cognitive impairment. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2016;25:95–101. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.041. [DOI] [PubMed] [Google Scholar]
- Lerch J.P., Evans A.C. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Li W., Antuono P.G., Xie C., Chen G., Jones J.L., Ward B.D., Singh S.P., Franczak M.B., Goveas J.S., Li S.J. Aberrant functional connectivity in Papez circuit correlates with memory performance in cognitively intact middle-aged APOE4 carriers. Cortex J. Dev. Stud. Nerv. Syst. Behav. 2014;57:167–176. doi: 10.1016/j.cortex.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton O., Boucher M., Robbins S., Evans A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Majerus S., D'Argembeau A., Martinez Perez T., Belayachi S., Van der Linden M., Collette F., Salmon E., Seurinck R., Fias W., Maquet P. The commonality of neural networks for verbal and visual short-term memory. J. Cognit. Neurosci. 2010;22:2570–2593. doi: 10.1162/jocn.2009.21378. [DOI] [PubMed] [Google Scholar]
- Melrose R.J., Harwood D., Khoo T., Mandelkern M., Sultzer D.L. Association between cerebral metabolism and Rey-Osterrieth Complex Figure Test performance in Alzheimer's disease. J. Clin. Exp. Neuropsychol. 2013;35:246–258. doi: 10.1080/13803395.2012.763113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Seo S.W., Ahn J., Yoon U., Im K., Lee J.M., Tae Kim S., Ahn H.J., Chin J., Jeong Y., Na D.L. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J. Neuroimag. Off. J. Am. Soc. Neuroimag. 2010;20:37–45. doi: 10.1111/j.1552-6569.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- Seo S.W., Cho S.S., Park A., Chin J., Na D.L. Subcortical vascular versus amnestic mild cognitive impairment: comparison of cerebral glucose metabolism. J. Neuroimag. Off. J. Am. Soc. Neuroimag. 2009;19:213–219. doi: 10.1111/j.1552-6569.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L.R., Wixted J.T. The cognitive neuroscience of human memory since H.M. Annu. Rev. Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S.J., Willoch F., Ishii K., Burger K., Drzezga A., Engel R., Bartenstein P., Moller H.J., Schwaiger M., Hampel H. Resting state glucose utilization and the CERAD cognitive battery in patients with Alzheimer's disease. Neurobiol. Aging. 2006;27:681–690. doi: 10.1016/j.neurobiolaging.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Villemagne V.L., Mulligan R.S., Pejoska S., Ong K., Jones G., O'Keefe G., Chan J.G., Young K., Tochon-Danguy H., Masters C.L., Rowe C.C. Comparison of 11C-PiB and 18F-florbetaben for Abeta imaging in ageing and Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:983–989. doi: 10.1007/s00259-012-2088-x. [DOI] [PubMed] [Google Scholar]
- Zijdenbos A.P., Forghani R., Evans A.C. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med. Imaging. 2002;21:1280–1291. doi: 10.1109/tmi.2002.806283. [DOI] [PubMed] [Google Scholar]