Highlights
-
•
No significant reduction in brain glial activation measured by PBR28-PET SUVR.
-
•
No significant reduction in serum neurofilament light levels over 36–40 weeks.
-
•
Adverse event related dose reductions/discontinuations common.
Keywords: Biomarker endpoint, Phase 1b, Clinical trial, Ibudilast, PBR28, Neurofilament, MIF, TNF-alpha
Abstract
Ibudilast (MN-166) is an inhibitor of macrophage migration inhibitory factor (MIF) and phosphodiesterases 3,4,10 and 11 (Gibson et al., 2006; Cho et al., 2010). Ibudilast attenuates CNS microglial activation and secretion of pro-inflammatory cytokines (Fujimoto et al., 1999; Cho et al., 2010). In vitro evidence suggests that ibudilast is neuroprotective by suppressing neuronal cell death induced by microglial activation. People with ALS have increased microglial activation measured by [11C]PBR28-PET in the motor cortices. The primary objective is to determine the impact of ibudilast on reducing glial activation and neuroaxonal loss in ALS, measured by PBR28-PET and serum Neurofilament light (NfL). The secondary objectives included determining safety and tolerability of ibudilast high dosage (up to 100 mg/day) over 36 weeks.
In this open label trial, 35 eligible ALS participants underwent ibudilast treatment up to 100 mg/day for 36 weeks. Of these, 30 participants were enrolled in the main study cohort and were included in biomarker, safety and tolerability analyses. Five additional participants were enrolled in the expanded access arm, who did not meet imaging eligibility criteria and were included in the safety and tolerability analyses. The primary endpoints were median change from baseline in (a) PBR28-PET uptake in primary motor cortices, measured by standard uptake value ratio (SUVR) over 12–24 weeks and (b) serum NfL over 36–40 weeks. The secondary safety and tolerability endpoints were collected through Week 40.
The baseline median (range) of PBR28-PET SUVR was 1.033 (0.847, 1.170) and NfL was 60.3 (33.1, 219.3) pg/ml. Participants who completed both pre and post-treatment scans had PBR28-PET SUVR median(range) change from baseline of 0.002 (−0.184, 0.156) , P = 0.5 (n = 22). The median(range) NfL change from baseline was 0.4 pg/ml (−1.8, 17.5), P = 0.2 (n = 10 participants). 30(86%) participants experienced at least one, possibly study drug related adverse event. 13(37%) participants could not tolerate 100 mg/day and underwent dose reduction to 60–80 mg/day and 11(31%) participants discontinued study drug early due to drug related adverse events.
The study concludes that following treatment with ibudilast up to 100 mg/day in ALS participants, there were no significant reductions in (a) motor cortical glial activation measured by PBR28-PET SUVR over 12–24 weeks or (b) CNS neuroaxonal loss, measured by serum NfL over 36–40 weeks. Dose reductions and discontinuations due to treatment emergent adverse events were common at this dosage in ALS participants. Future pharmacokinetic and dose-finding studies of ibudilast would help better understand tolerability and target engagement in ALS.
1. Introduction
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disorder with no treatments that halt progression. There is a large and growing pipeline of disease modifying investigational products in late preclinical or early clinical stages of development for ALS. These experimental therapies need to be tested efficiently to decide the best to bring forward for phase 3 testing. There is an urgent need to improve efficiency of early phase clinical trials to accelerate therapeutic discovery. Robust, reliable and sensitive disease biomarkers can provide proof of mechanism in relevant patient populations and can also facilitate dose-selection in relatively small and short clinical trials (Parmar et al., 2020).
Neuroinflammation and glial activation have been implicated in ALS progression (Brettschneider et al., 2012, Bakkar et al., 2015). Microglial activation has been observed consistently and in association with degenerating upper and lower motor neurons, where neuronal and corticospinal atrophy ensue subsequently (Brettschneider et al., 2012). Several biomarkers have emerged to investigate neuroinflammation and neuronal loss in people with ALS.
[11C]PBR28 radioligand binds to 18 kDa translocator protein (TSPO), which is highly expressed in the mitochondria of activated microglia and reactive astrocytes (Downer et al., 2020). Regional [11C]PBR28-positron emission tomography (PBR28-PET) uptake is greater in the motor cortices in ALS participants relative to healthy controls and it correlates with clinical measures, including upper motor neuron burden and the fine motor subscale of revised ALS functional rating scale (ALSFRS-R) (Alshikho et al., 2016, Alshikho et al., 2018). Furthermore, a sample size of 30 is adequate to show a statistically significant treatment effect of 0.02 change in PBR28-PET uptake SUVR in the motor cortices (Alshikho et al., 2018).
Neurofilament-light (NfL) is a marker of CNS neuroaxonal loss. Neurofilaments are highly expressed in axons. NfL levels are increased in serum and cerebrospinal fluid in several neurodegenerative disorders, including ALS. (Lu et al., 2015, Bridel et al., 2019). NfL levels in serum and cerebrospinal fluid samples from ALS participants are highly correlated to one another and remain stable over time (Lu et al., 2015, Kuhle et al., 2016, Novakova et al., 2017, Canto et al., 2019, Huang et al., 2020). Reduction in serum-NfL levels were observed in conjunction with slowing of disease progression in SOD1 ALS patients after treatment with antisense oligonucleotides (Miller et al., 2020).
Ibudilast (MN-166) is an orally administered small molecule with well-established safety and tolerability profiles in other CNS disorders at 60–100 mg/day dosages. Ibudilast inhibits multiple phosphodiesterases (PDE 3, 4, 10, 11) and macrophage migratory inhibitory factor (MIF), which suppress neuroinflammation and microglial activation in vitro (Gibson et al., 2006, Cho et al., 2010, Cox et al., 2013, Ha et al., 2019). Ibudilast also reduces the production of tumor necrosis factor-alpha (TNF-α) in a dose-dependent manner in in vitro studies (Suzumura et al., 1999) and suppresses neuronal cell death induced by microglial activation in vitro (Mizuno et al., 2004). Ibudilast is shown to lower proinflammatory cytokines secreted by activated glia in plate-based chemotaxis assays and in experimental autoimmune encephalitis models (Fujimoto et al., 1999, Cho et al., 2010). Ibudilast (up to 100 mg/day) has been shown to slow the rate of cortical atrophy in primary progressive multiple sclerosis (Fox et al., 2018). However, it is unknown if ibudilast crosses an intact blood–brain-barrier.
We hypothesized that ibudilast can slow ALS progression by reducing neuroinflammation/glial activation. This Phase 1b trial employs PBR28-PET and serum NfL to determine whether ibudilast 100 mg/day can reduce these markers in people with ALS. We selected the highest dosage (100 mg/day) with known favorable safety profile from prior clinical trials in other disorders for this trial (DeYoung et al., 2016, Ray et al., 2017).
2. Materials and methods
2.1. Trial design and outcomes
This is an investigator-initiated, open-label, phase 1b trial of oral ibudilast in ALS. This trial was conducted at Massachusetts General Hospital(MGH), Boston and South Shore Neurological Associates, New York. The trial was approved by the institutional review boards at both sites and conducted in accordance with the Good Clinical Practice guidelines of the International Conference on Harmonization. All participants provided written informed consent prior to onset of any study procedures. The study was registered on clinicaltrials.gov NCT02714036.
The primary objective of this trial was to measure biological impact of oral ibudilast up to 100 mg/day measured by changes of brain PBR28-PET and serum NfL levels. This trial included two arms: (a) the main cohort, included participants who met all the eligibility criteria and (b) expanded access arm, included five participants who did not meet the imaging specific eligibility criteria and hence did not complete imaging outcomes, but were otherwise eligible for the trial. All eligible participants in both arms, received 100 mg/day of ibudilast per protocol. Participants were allowed dose reduction to 60 or 80 mg/day in two or three divided doses in the first 12 weeks of treatment if experiencing drug related adverse events (AEs). The treatment period included an initial 2-week dose-escalation, 36-week stabilization phase, and a 4-week follow up telephone visit after 36-week completion. During every in-person visit, standard ALS clinical outcomes including revised ALS functional rating scale (ALSFRS-R) (Cedarbaum et al., 1999), slow vital capacity (SVC %predicted) (Knudson et al., 1983) and quantitative limb muscle strength using handheld dynamometry (HHD) (Shefner et al., 2016). Safety labs were collected pre-treatment and at Week 4, 8, 12, 24 and 36 visits and adverse events were recorded at each visit.
2.2. Power calculations
A sample size of ten participants would be required to show a true pre- and post- treatment, minimal detectable group difference of 0.096 point reduction in PBR28-PET SUVR in the motor cortices with 90% probability, at a one-sided 0.1 significance level. This is based on prior data showing a mean difference in PBR28-PET SUVR of 0.116, with standard deviation of 0.08 (Zurcher et al., 2015, Alshikho et al., 2018).
2.3. Participants and eligibility criteria
All enrolled participants met trial eligibility criteria including ALS diagnosis based on the revised El Escorial criteria for definite, probable, probable with laboratory supported or possible ALS (Brooks et al., 2000), and ability to swallow study medication in the opinion of the investigator at study entry and throughout the study. All main cohort participants had SVC ≥ 50% predicted and were either not taking or on stable dose of riluzole, and not on any other investigational agents, immunosuppressive or immunomodulatory treatments for ≥ 30 days of baseline. When edaravone became US FDA approved for ALS during the trial, participants were allowed to start edaravone without trial restrictions. The exclusion criteria included serum aminotransferases > 3 times the upper limit of normal or serum creatinine > 1.5 times the upper limit of normal, known study drug allergies, concomitant medications that interacted with ibudilast such as cimetidine, cyclosporine, dronedarone, lopinavir, probenecid, quinidine (with the exception of Nuedexta, which was allowed with frequent clinical EKG monitoring), ranolazine, rifampin, ritonavir, tipranavir, retigabine, and mexiletine. Other exclusion criteria included history of human immunodeficiency virus, clinically significant chronic hepatitis or other active infections, inflammatory or autoimmune conditions, presence of unstable psychiatric illnesses or dementia that impaired ability to provide informed consent and women who were pregnant, lactating or not on birth control.
The imaging specific inclusion criteria for the main cohort were: (a) presence of clinical upper motor neuron burden (UMNB) score as measured by the MGH-UMNB scale of ≥ 25 (range 0–45) at screening (Zurcher et al., 2015, Alshikho et al., 2016) (b) absence of low binding rs6971 polymorphism for PBR28-PET (Owen et al., 2012). Five to 30% of people are low-binders for this PET ligand and do not provide a useful imaging signal, based on a “low binding” polymorphism in rs6971 (Owen et al., 2012, Mizrahi et al., 2012), (c) ability to lie flat for scan duration, (d) absence of any contraindications for PET and MRI as per institutional clinical guidelines and (e) no concomitant use of nicotine containing products, benzodiazepines or anti-inflammatory medications one week prior to PET scan.
2.4. Data monitoring and oversight
The site PIs reviewed all data for clinical adverse events (AEs) and safety laboratory tests throughout the study. All treatment emergent AEs (TEAEs) and serious adverse events (SAEs) were followed for resolution or up until the final study contact. Participants were re-educated as necessary to ensure treatment compliance. The imaging and serological data were quality checked by independent imaging evaluators who were blinded to clinical data (BH, CE and PW).
2.5. Primary, secondary and exploratory endpoints
The primary endpoints of this study were imaging and biofluid biomarker changes from baseline including (a) brain PBR28-PET uptake as a marker of glial activation over 12–24 weeks, and (b) serum NfL levels as a marker of neurodegeneration over 36 weeks.
Baseline PBR28-PET uptake in the primary motor cortices region of interest was compared to post-treatment (week 12–24) uptake levels and was carried out on all participants in the main cohort, who received at least one dose of study drug and completed both pre- and post- treatment scans. The post-treatment PBR28-PET scan visit was chosen to have a large visit window between 12 and 24 weeks post treatment to allow for more participants, especially faster progressors, to complete the scan safely. Similarly, levels of serum NfL was compared to post-treatment (weeks 36–40) levels in the main cohort participants who received at least one dose of study drug. Participants with incomplete pairs of pre- or post- treatment (Weeks 36–40) serum samples were excluded from analyses.
Safety and tolerability were secondary endpoints. Safety outcomes included all serious adverse events (SAEs), and treatment emergent adverse events (TEAEs) that were assessed as possibly, probably or definitely related to study drug and occurred in >5% of participants or led to early drug discontinuation. Tolerability of ibudilast was reported as percentage of participants who completed all 36 weeks of study treatment on study drug and remained free from any possibly, probably or definitely drug related AEs leading to permanent study drug discontinuation to week 36. All safety and tolerability analyses were done on the safety sample which included all participants in both expanded access and main cohorts, who received at least one dose of study drug. All observed AEs were reported and grouped by system organ class and preferred term.
Exploratory endpoints included analyses of rates of decline of ALSFRS-R, SVC and HHD from baseline to 36 weeks. Secondary endpoints were analyzed using all available data from enrolled participants who received at least the first ibudilast dose. Exploratory endpoint analyses were known to lack statistical power to detect change based on trial sample size and were analyzed to provide information about directionality and trends.
2.6. Imaging acquisition and analyses
PBR28-PET scans were completed at AA Martinos Center at MGH, for participants enrolled at both sites. [11C]PBR28 radiotracer was synthesized in-house at MGH in accordance with the US federal USP823 standards as previously reported (Fujita et al., 2008). PET scans were obtained in a simultaneous MR/PET scanner (Siemens 3T Magnetom Tim Trio scanner, Germany with BrainPET insert). An eight-channel MR head receiver coil was used and a high-resolution multi-echo magnetization-prepared rapid acquisition gradient echo (MEMPRAGE) sequence was acquired. All MR sequences and PET data acquired over a 90-minute scan session were analyzed using automated processing pipelines as previously reported (Zurcher et al., 2015, Alshikho et al., 2016, Alshikho et al., 2018) and are described in Supplement 1. The mean(SD) administered dose of the [11C]PBR28 was 13.62(1.79) mCi. The occipital lobe was selected apriori as the pseudoreference region, since it is biologically unaffected in ALS and scan data acquired within the same field of view and hence subjected to similar technical quality conditions as the region of interest (Albrecht et al., 2018). The region of interest comprised of the bilateral primary motor cortical gray and white matter parcellates.
The PBR28-PET uptake at 60–90 min post radiotracer injection was reported as standard uptake value (SUV) averaged across the whole brain spanning the region of interest in 2 mm3 voxels. At the individual level, the median SUV in the region of interest was then normalized by the median SUV in the occipital lobe to obtain the primary imaging outcome PBR28-PET SUVRocc for each participant (Albrecht et al., 2018).
Median PBR28-PET SUVRocc = Median SUVPrimary Motor Cortices/Median SUVoccipital lobe
PET scan image quality was assessed by an independent imaging expert rater (BH), who remained blinded to the clinical data except TSPO genotyping. All poor-quality scans, defined by incomplete scans (<20 min of PET data collected or incomplete anatomical field of view affecting reliable PET attenuation correction) and excessive motion degradation, were excluded from the study.
2.7. Biofluid biomarker analyses
Serum NfL was analyzed using the Quanterix Simoa SR-X platform. Samples were analyzed in duplicate and the CoV for each pair calculated as (standard deviation of duplicate values) divided by (mean of duplicate values) and expressed as a percentage. Mean(SD) CoV for NfL was 5.0 ± 4.6% (range 0.01–19%). Frozen serum aliquots were stored onsite at −80 °C and batch analyzed at end of study. Several serum aliquots from 11 participants were thawed and then refrozen during storage due to a freezer malfunction. These samples were included in NfL analysis because it is known to be robust to freeze–thaw cycles (Keshavan et al., 2018).
An attempt was made to analyze serum MIF and serum TNF-α changes, as peripheral markers for neuroinflammation. However, the results of serum MIF or TNF-α analyses were not interpretable due to preanalytical issues as follows and hence not presented in this paper. There was (a) false 3x elevation of serum MIF in visibly hemolyzed samples, presumably due to release of MIF from erythrocytes and without relation to treatment (data not shown) and (b) serum TNF-α levels were near lower limits of quantification (ELISA; electrochemoluminescence) and highly variable (coefficient of variance (CoV) 25–40%).
2.8. Statistical analyses
All primary and secondary analyses were performed as pre-specified in statistical analyses plan. Wilcoxon signed rank tests were used to assess if changes from baseline to post-treatment were significantly different from zero and the pre-treatment and change values are expressed as median(range). Exploratory analyses describing the 36-week trajectory of clinical outcomes (ALSFRS-R, SVC, and HHD-mega Z-scores) and serum biomarkers were performed using mixed effects models with a fixed effect for continuous time (visits at Weeks 0, 4, 12, 24, 36/40) and a random slope, and intercept for each participant with an unstructured covariance on the intent to treat sample. Analyses were performed for all participants in the main cohort who had any data available for the given measure. Analyses were performed using R (Core team 2019). Alpha of 0.05 was split between PET and serum biomarker outcomes. Non-primary analyses were not corrected for multiple testing.
2.9. Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
3. Results
3.1. Participants
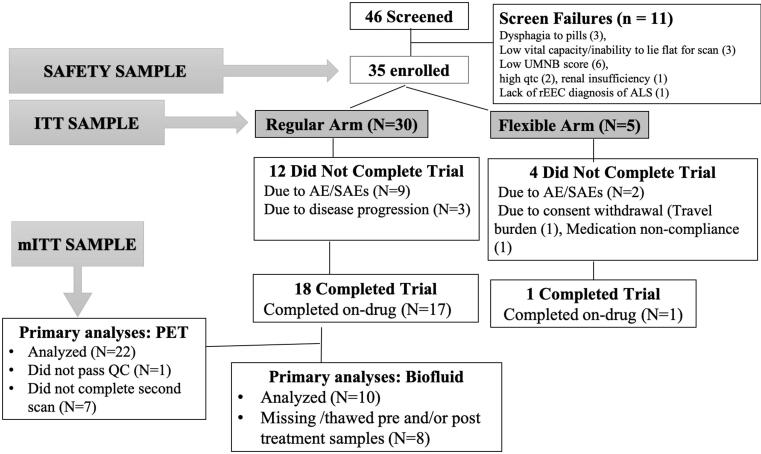
Forty-six participants were screened and 35 enrolled between May 2016 and June 2018 (Fig. 1; Consort Diagram). Of enrolled participants, 30 were in the main cohort and five in the expanded access arm. All 35 participants received at least one dose of the study drug and were included in the safety and tolerability analyses. The intent-to-treat sample used for clinical marker analyses included all 30 main cohort participants. The imaging sample (modified intent-to-treat) included 22 of the regular participants. Nineteen (54%) out of the 35 enrolled participants completed the study.
Fig. 1.
Consort diagram Some participants had more than one reason for screen failure.
The baseline characteristics are shown in Table 1. Compared to the main cohort of the study, the expanded access arm, included a higher proportion of males (80% versus 53.3% in main cohort), lower mean ALSFRS-R (31.6 vs. 37.2 points in main cohort) and a slightly faster estimated rate of progression (0.70 versus 0.58 points/month drop in ALSFRS-R in the main cohort) (Labra et al., 2016). Among the main cohort participants included in the imaging and biofluid biomarker analyses, 23% (n = 7 out of 30) started edaravone before entering the study.
Table 1.
Baseline characteristics.
| Baseline Characteristics | Main cohort (n = 30) | Expanded access arm (n = 5) |
|---|---|---|
| % (n)/Mean (SD) | % (n)/Mean (SD) | |
| Age at Screening (years) | 57.1 (10.3) | 53.8 (13.9) |
| Family History of ALS | 10.0% (3) | 0.0% (0) |
| Male | 53.3% (16) | 80.0% (4) |
| Caucasian | 93.3% (28) | 100.0% (5) |
| Symptom onset to Diagnosis (in months) | 13.2 (12.6) | 8.9 (6.4) |
| Symptom onset to Screening (in months) | 24.4 (15.7) | 22.8 (9.0) |
| Limb Onset | 66.7% (20) | 60.0% (3) |
| Baseline SVC (% predicted) | 77.0 (23.2) | 81.0 (17.6) |
| ALSFRS-R at Baseline | 37.2 (4.7) | 31.6 (11.6) |
| Estimated ALSFRS-R Slope Pre-Baseline (48-Baseline ALSFRS-R/disease duration) (points/month) | 0.58 (0.37) | 0.70 (0.33) |
| UMNB Total at Screening | 29.4 (3.5) | 27.0 (10.5) |
| Exposure to 30-day stable dose of Riluzole | 83% (25) | 100% (5) |
| Exposure to edaravone | 37% (11) | 20% (1) |
3.2. Pharmacodynamic marker outcomes
3.2.1. [11C]PBR28-PET uptake in the motor cortices
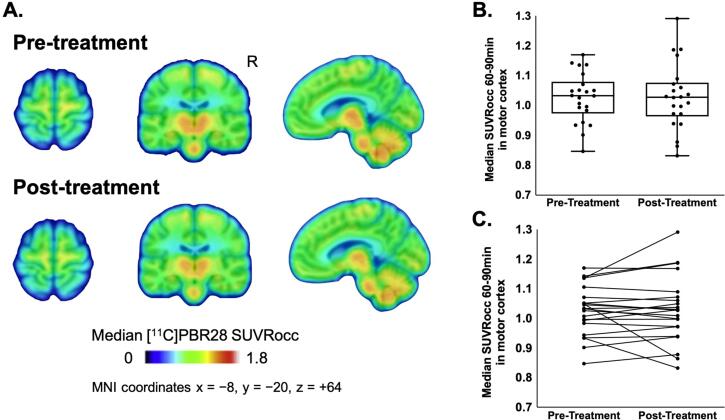
All 30 main cohort participants completed the pre-treatment baseline scan and 23 participants (77%) completed the post–treatment scan. One scan pair was excluded in the final analyses due to failing scan quality check by an independent imaging analysts (BH), due to excessive motion artifacts. This participant was noted to have advanced bulbar ALS and sialorrhea interfering with scan quality. There was no significant change from baseline in SUVRocc (Wilcoxon Signed Rank; NS) (Fig. 2). The percent change in SUVRocc in motor cortices remained within the previously reported average test–retest variability (Alshikho et al., 2018) of 7% in all but three participants. SUVRocc decreased by 11% in one participant and 18% in another and increased by 14% in one participant (Fig. 2C). The pre-treatment SUVR images for all 22 participants included in the final analyses are shown in Fig. S4.
Fig. 2.
[11C]PBR28-PET uptake in bilateral motor cortices for Ibudilast pre-treatment and post-treatment groups (n = 22 participants). [A] Group median [11C]PBR28 SUVRocc for pre-treatment (top) and 12–24 weeks from baseline (bottom) groups. The median pre-treatment SUVRocc in the motor cortices was 1.033 (range 0.847, 1.170). All images are projected onto standard MNI space at coordinates (x = −8, y = − 20, z = +64). The color bar represents the group median [11C]PBR28 SUVRocc values in the motor cortices. [B] The box plots show that there were no significant changes from baseline observed in median [11C]PBR28 SUVRocc in the motor cortices at 12–24 weeks of ibudilast treatment [n = 22, median 0.002 (range −0.184, 0.156), Wilcoxon-Signed-Rank-Test V = 149, p = 0.5] [C] The spaghetti plot represents individual changes from baseline in median [11C]PBR28 SUVRocc in the motor cortices following 12–24 weeks of ibudilast treatment.
3.2.2. Serum biomarkers (NfL)
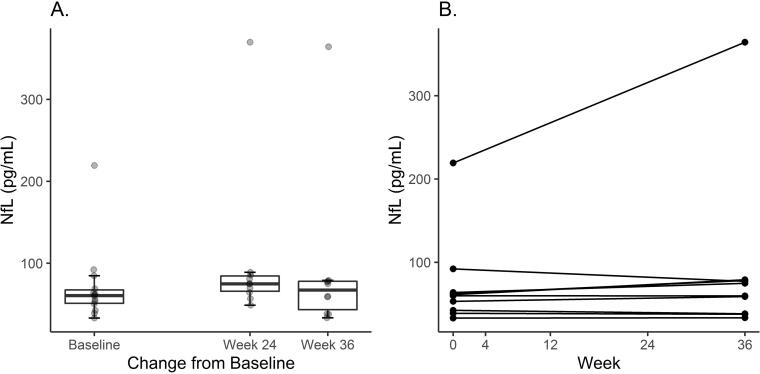
Out of the 18 main cohort trial completers, ten participants had complete sets of pre-treatment and post-treatment (weeks 36–40) serum samples. There were no significant changes from baseline in serum NfL (Wilcoxon Signed Rank; NS) (Fig. 3A, 3B).
Fig. 3.
[Fig. 3A]: Baseline serum NfL was 60.6 (range 33.1, 219.3) pg/ml. The box plots show median serum NfL levels at (a) baseline (b) 24 weeks of ibudilast treatment [n = 10 participants, median(range) change 1.7 pg/ml (−2.7, 27.3), Wilcoxon-Signed-Rank-Test V = 41, p = 0.19] and (c) 36–40 weeks of ibudilast treatment [n = 10 participants, median (range) change 0.4 pg/ml (−1.8, 17.5), Wilcoxon-Signed-Rank-Test V = 40, p = 0.23] and. [Fig. 3B] The spaghetti plot represents individual changes from baseline in median serum NfL following 36–40 weeks of ibudilast treatment.
3.3. Safety and tolerability
The adverse events (AEs) assessed as at least possibly related to study drug or leading to permanent study drug discontinuation reported by ≥ 5% of study participants, and serious adverse events are listed in Table 2. Ten serious adverse events occurred in six participants between treatment initiation and within four weeks after drug discontinuation. None of the serious adverse events were assessed as related to study drug and were thought to be due to ALS disease progression. These serious adverse events included pulmonary embolism (3), deep vein thrombosis in leg (1), respiratory failure (1), brief unresponsive episode of unclear etiology (1), dysphagia (1), dehydration (1), IV catheter related infection (used for concomitant edaravone infusion) (1) and severe abdominal pain (1). Two serious adverse events occurred while on study drug and resulted in death (1 pulmonary embolism and 1 respiratory failure); both were assessed as unrelated to study drug and were deemed by site investigator as related to ALS disease progression.
Table 2.
Only AEs occurring 5% overall are included in this table.
| Adverse Events (AEs) | Adverse Events % (N) participants | ||
|---|---|---|---|
| Regular (n = 30) | Flexible (n = 5) | Total (n = 35) | |
| [A] ALL ADVERSE EVENTS | 100% (30) | 100%(5) | 100%(35) |
| [B] SERIOUS ADVERSE EVENTS (SAE) | |||
| Serious Adverse Events- Total | 20%(6) | 0%(0) | 17%(6) |
| SAEs at least “Possibly Related” to study drug | 0% (0) | 0% (0) | 0%(0) |
| [C] AEs leading to permanent study drug discontinuation | 37%(11) | 20%(1) | 34%(12) |
| GI disorders- other | 13% (4) | 0% (0) | 11%(4) |
| Nausea | 10%(3) | 0%(0) | 9%(3) |
| Diarrhea | 3% (1) | 0% (0) | 6% (2) |
| Dysphagia | 10% (3) | 0% (0) | 9% (3) |
| Dehydration | 7% (2) | 0% (0) | 6% (2) |
| [E]AEs AT LEAST “POSSIBLY RELATED” TO STUDY DRUG | 83%(25) | 100%(5) | 86%(30) |
| Nausea | 37% (11) | 60% (3) | 40%(14) |
| Fatigue | 23% (7) | 40% (2) | 26%(9) |
| Diarrhea | 17% (5) | 40%(2) | 20%(7) |
| Other GI | 20% (6) | 20%(1) | 20%(7) |
| Insomnia | 17% (5) | 40% (2) | 20%(7) |
| Hot flashes | 20% (6) | 0% (0) | 17%(6) |
| Headache | 13% (4) | 20% (1) | 14%(5) |
| Abdominal/Stomach/GI pain | 13% (4) | 20% (1) | 14%(5) |
| Dizziness | 13% (4) | 0% (0) | 11%(4) |
| Other skin and subcutaneous tissue disorders | 10%(3) | 20%(1) | 11%(4) |
| Vomiting | 7%(2) | 20%(1) | 9%(3) |
| Flatulence | 10%(3) | 0%(0) | 9%(3) |
| Abdominal distension | 7%(2) | 0%(0) | 6%(2) |
| Weight loss | 3% (1) | 20%(1) | 6% (2) |
| Anorexia | 3% (1) | 20% (1) | 6% (2) |
All participants experienced at least one treatment emergent AE (TEAE). The most common AEs at least possibly related to study drug were nausea (40%), fatigue (26%), diarrhea (20%), other GI symptoms (bloating, abdominal discomfort/pain, 20%), insomnia (20%) and hot flashes (17%). Thirty-three percent (101/304) of AEs were assessed by the investigator as at least possibly related to study drug. AEs that led to early study drug discontinuation included nausea (17%), diarrhea (9%), other GI symptoms (9%) and weight loss (6%).
A total of sixteen participants (45%) did not complete the trial. A total of 13 of the 35 participants (37%) underwent permanent dose reduction to 60 mg/day (n = 11) and 80 mg/day (n = 2), due to at least one TEAE that was possibly, probably or definitely drug related AEs. Subsequently, 11 participants (31%) discontinued drug early and were deemed intolerant. Three participants (9%) discontinued drug early due to disease progression and travel burden and two withdrew consent (6%). The cumulative intolerance rates at week 12, 24 and 36 were 11%, 20% and 31%, respectively (Supplementary Fig. 3). The mean on-drug duration after permanent dose reduction to 60 mg/day was 17(SD 13) weeks and 80 mg/day was 33 (SD 5) weeks.
3.4. Exploratory clinical outcome and biomarker analyses
The estimated change mean(SD) in ALSFRS-R was −0.88 (0.17) pts per month, SVC was −1.38 (0.35) %-points per month. HHD arm muscle MegaZ-score was −0.28(0.24) per month and HHD leg muscle MegaZ-score was +0.01 (0.17) per month (Supplementary Fig. 1). The estimated mean (SE) change in NfL was +1.04 pg/ml (0.90) (Supplementary Fig. 2).
4. Discussion
This multi-site, Phase 1b, open label ALS clinical trial of high dosage (up to 100 mg/day) of MN-166 (Ibudilast) showed no detectable changes on (i) imaging biomarker of neuroinflammation (PBR28-PET uptake in motor cortices) over 12–24 weeks or (ii) serum biomarker of CNS neuroaxonal loss (NfL levels) over 36–40 weeks of study treatment respectively. Overall ibudilast was observed to be safe and showed no drug related SAEs. However, the tolerability was limited due to GI side effects, fatigue and insomnia.
Preclinical evidence suggests that ibudilast reduces microglial activation and offer neuroprotection by promoting release of neurotropic factors. (Cho et al., 2010, Cox et al., 2013, Suzumura et al., 1999, Mizuno et al., 2004). In ALS, among other neurodegenerative diseases, abnormal protein aggregation is implicated in its pathophysiology, to which neuroinflammation is a response. In a recently published, in vitro study, ibudilast-treated HEK293 and NSC-34 cells were observed to contain fewer TDP-43 and SOD1 aggregates, which are pathological hallmarks of ALS. This suggests that ibudilast may have a neuroprotective effect in ALS by protecting against TPD-43-induced toxicity in motor neuron-like NSC-34 cells (Chen et al., 2020). A randomized controlled trial of ibudilast 60 mg/day in relapsing remitting multiple sclerosis slowed the progression of cortical atrophy but no effect on MRI gadolinium enhancing lesions, suggesting that ibudilast may be neuroprotective but without substantial impact on neuroinflammation (Barkhof et al., 2010). Ibudilast slowed brain atrophy in patients with primary progressive multiple sclerosis in the SPRINT-MS trial. However, the effects of ibudilast on neurofilament levels and other biofluid biomarkers remain unknown (Fox et al., 2018). Brooks et al conducted an early phase adaptive design clinical trial of ibudilast 60 mg/day in ALS, peer-reviewed results of which have not been published to date (Brooks et al, MND symposium abstract 2018).
PBR28-PET is increasingly being used as an outcome measure in ALS trials to evaluate experimental treatments with anti-inflammatory properties [(RNS60 (Paganoni et al., 2019), BLZ945 (NCT04066244), AMX0035 (NCT03127514)]. There is an ongoing debate of whether a pseudoreference ratio metric (SUVR) versus the “gold standard” volume of distribution (VT) would be best implemented in treatment trials. The SUVR measure has high specificity and high test–retest reliability but may reduce sensitivity and responsiveness to change, especially for experimental treatments with global brain reduction of glial activation (Albrecht et al., 2018). On the other hand, VT measure using radial arterial sampling for kinetic modeling has high sensitivity and responsiveness of the biomarker to uptake change in interventional drug trials or to upper motor neuron disease progression (Jucaite et al., 2015). Jucaite et al study showed that PBR28-PET VT reduced significantly and as early as six weeks following an experimental myeloperoxidase inhibitor treatment in Parkinson’s disease population, indicating the promise of PBR28-PET uptake as a proof of mechanism biomarker in future trials. However, one must balance the potential improved analytical rigor with the burden of using arterial lines and multiple blood sampling in ALS patients.
Both neurofilament light and heavy subunits are reported in several papers over the past decade, to have excellent test–retest reliability and accuracy for diagnostic usefulness in ALS. Recent observations from dimethylfumarate (Phase IV) and Toferson (Phase I) trials in MS and ALS respectively reveal that blood and CSF neurofilaments have excellent sensitivity to change with neuroprotective treatments (Sejbaek et al., 2019, Miller et al., 2020). These studies also showed that both CSF and blood NfL levels were highly correlated. Serum NfL levels have been shown to be stable in up to four freeze–thaw cycles (Keshavan et al., 2018). There is currently some variability in the choice of light or heavy neurofilament subunit use as a pharmacodynamic marker in ALS trials (Poesen and Van Damme, 2019). Although promising, it is still early days for using neurofilaments in interventional drug trials in ALS. They are increasingly being used as primary and secondary outcomes in early phase clinical trials in ALS (Ravulizumab NCT04248465, TUDCA trial NCT03800524).
We observed a high early drug discontinuation rate due to TEAEs (31%). Early drug discontinuations due to TEAEs in other ALS trials of similar trial durations (~40 weeks) have been variable and dependent on tolerability to study drug rather than duration of study treatment. For example, the early drug discontinuation rate was 0% in the 40-week CoQ10 trial (Kaufmman et al., 2009), 25% in the 9-month Talampanel trial (Pascuzzi et al., 2010). The 42-week creatine -tamoxifen selection design ALS trial showed that participants in the creatine arm who had higher TEAEs also had higher early drug discontinuation rates (50% versus 24% Tamoxifen arm) (Babu et al., 2020).
The goal of this study was to answer the key question about the biological activity of an experimental treatment in an adequately powered study, while conserving sample size and eliminating need for placebo to improve access to study drug to ALS participants. Both PBR28-PET SUVR and NfL markers are known from prior studies to remain elevated at stable levels in ALS participants over disease course without experimental intervention (Huang et al., 2020, Alshikho et al., 2018, Lu et al., 2015). PBR28-PET uptake and NfL are observed to decrease significantly in 6 weeks and 12 weeks respectively following other experimental treatments in clinical drug trials indicating their responsiveness to change with intervention (Miller, 2020; Jucaite, 2015). The authors acknowledge that the small sample size, lack of placebo comparison arm and attrition issues due to AEs are limitations for this trial for interpreting clinical efficacy. Review of peer-reviewed literature and the large 8000 + ALS database PRO-ACT show that the trial sample is representative of ALS trial populations in the USA (Zach et al., 2015, Proudfoot et al., 2016) including mean estimated ALSFRS-R slope at baseline (this trial: 0.58 ± 0.37 points/month versus PRO-ACT: 0.59 ± 0.49, Proudfoot et al., 2016).
There is paucity of in vivo evidence of CNS penetration properties of ibudilast in human ALS and therapeutic potential of ibudilast in ALS. To date, there are no published postmortem or in vivo human studies describing CSF concentrations or brain and spinal cord tissue concentrations of oral administered ibudilast at 50–100 mg/day dosages. Evidence about the brain permeability of ibudilast comes from a small rat study, which showed that 6 hours after dosing with oral ibudilast at 50 mg/kg once daily for 7 days, the brain and spinal cord tissue sampling of three euthanized rats showed higher brain tissue than plasma concentrations of ibudilast (Sanftner et al., 2009). This preclinical study also showed substantial inter-species variation in oral bioavailability of ibudilast related to variation in first-pass metabolism enzymes expression and gut-wall transporters of study drug across different animal species. Based on these findings, some future directions include conducting rigorous pharmacokinetic and dose-finding studies of ibudilast to better understand tolerability, pharmacodynamic markers of biological efficacy. This study was not designed to answer whether ibudilast is clinically effective in ALS. These questions are being addressed in COMBAT-ALS, a Phase 2b/3 randomized, placebo-controlled, double-blind, parallel, multi-center study being conducted by Medicinova in the US and Canada (NCT04057898). Participants living with ALS are treated with MN-166 (30–50 mg BID) for 12 months followed by an open-label extension for six months. Along with efficacy, safety, and tolerability, the pharmacokinetic properties of ibudilast will be evaluated.
There is undoubtedly an urgent need for more disease modifying therapies that prolong survival and functional ability in ALS. Until today, edaravone and riluzole are the only US Food and Drug Administration approved medications for ALS, which have modest benefit of slowing disease progression. There is a great need to design efficient early phase, proof-of-mechanism trials to demonstrate the biological efficacy of experimental treatments using reliable molecular endpoints.
Lessons learned from this trial prompt us to suggest for a future biomarker-driven, early phase, ALS clinical trial, to include arterial sampling to measure the more sensitive PBR28-PET uptake VT metric in addition to SUVR metric, and to include a control group to provide a more conclusive biological efficacy results interpretation. Rather than using historical control data, the presence of a placebo group would provide important information about the biomarker endpoint variance and distribution for the study drug versus placebo under the trial specific eligibility conditions, neuroimaging analyses pipelines and biomarker assays. The optimal treatment duration in such a trial would be dependent on the pharmacokinetics of the experimental drug being tested. Based on the Jucaite et al study, it is reasonable to consider that a 12-week duration trial may be sufficient to measure PBR28-PET signal changes for drugs with rapid time-to-peak effect and good brain permeability properties (Jucaite et al, 2015). An adaptive trial design which is adequately powered to include additional Pk/PD and dose finding objectives, in addition to safety and target engagement would answer all these questions systematically. The design of such an early phase, biomarker-driven clinical trial could follow a platform approach, where multiple novel drugs with anti-neuroinflammatory properties could be tested simultaneously using a shared trial infrastructure comprising of pooled placebo groups, central IRB and shared trial operations and regulatory oversight teams. The platform approach has been successful in accelerating treatment discoveries in oncological trials (Platform Trials Coalition, 2019, Park et al., 2020) and is currently being launched for late phase drug development in the Healey ALS platform trial (NCT04297683). Innovative and high impact study designs and thoughtful use of reliable biomarkers, either singly or in combination, will play an important role in shaping the future of ALS clinical drug trials.
5. Authorship statement
All persons designated as authors qualify for authorship based on International Committee of Medical Journal Editors (ICMJE) criteria for authorship. All authors have been involved in drafting/revision of the article and have read and approved the final version of the manuscript.
CRediT authorship contribution statement
Suma Babu: Investigation, Writing - original draft, Visualization, Supervision. Baileigh G. Hightower: Formal analysis, Writing - original draft, Visualization. James Chan: Formal analysis, Data curation, Visualization. Nicole R. Zürcher: Formal analysis, Validation, Visualization. Pia Kivisäkk: Formal analysis, Validation. Chieh-En J. Tseng: Formal analysis, Writing - original draft, Visualization. Danica L. Sanders: Investigation. Ashley Robichaud: Investigation. Haruhiko Banno: Investigation. Armineuza Evora: Project administration. Akshata Ashokkumar: Project administration. Lindsay Pothier: Project administration. Sabrina Paganoni: Investigation. Sheena Chew: Investigation. Joanna Dojillo: Project administration, Resources. Kazuko Matsuda: Resources. Mark Gudesblatt: Investigation. James D. Berry: Resources. Merit E. Cudkowicz: Conceptualization, Funding acquisition. Jacob M Hooker: Supervision, Visualization. Nazem Atassi: Conceptualization, Methodology, Investigation, Resources, Supervision, Funding acquisition.
Declaration of Competing Interest
Nazem Atassi is an employee of Sanofi.
Baileigh Hightower, Pia Kivisäkk, Nicole R Zürcher, Chieh-En J Tseng, Danica L Sanders, Ashley Robichaud, Armineuza Evora, Akshata Ashokkumar and Lindsay Pothier report no COI related to this trial.
Suma Babu reports research support from American Academy of Neurology, AANEM Foundation, The ALS Association., Muscular Dystrophy Association, Biogen Inc, Orion Corporation, Voyager Therapeutics and Novartis pharmaceuticals.
James D. Berry has attended advisory boards at Alexion, Biogen and Clene Nanomedicine, has received research support from Alexion, Amylyx Therapeutics, Biogen, MT Pharma of America, Anelixis Therapeutics, Brainstorm Cell Therapeutics, Genentech, nQ Medical, ALS Finding a Cure, NINDS, Muscular Dystrophy Association, and ALS One.
Sabrina Paganoni reports research grants from Amylyx Therapeutics, Revalesio Corporation, Ra Pharma/UCB, Biohaven Pharmaceuticals, Clene Nanomedicine, Prilenia Therapeutics, The ALS Association, the American Academy of Neurology, ALS Finding a Cure, the Salah Foundation, the Spastic Paraplegia Foundation and reports personal consulting fees for advisory panels from Orion Corporation.
Sheena Chew is a full-time employee of Biogen and reports research support from the American Academy of Neurology and Biogen Inc.
Haruhiko Banno reports research grant from Time Therapeutics and scientific advising fee from Sumitomo Dainippon Pharma.
Jacob Hooker holds equity or stock options and/or has received income from Eikonizo Therapetics, Psy Therapeutics, Fuzionaire Diagnostics, and Delix Therapeutics.
Merit Cudkowicz reports consulting fees from Biogen, Takeda, Sunovion, Cytokinetics, Immunitypharm, and Wave.
Kazuko Matsuda is an employee of MediciNova.
Joanna Dojillo is an employee of MediciNova.
Acknowledgements
This project was funded by an anonymous donor to the Mass General ALS research program and Ride for Life. Medicinova provided study drug, electronic database support, monitoring and partial funding support. We express our thanks and deep appreciation to all our ALS study participants and their families for their participation in the study. We extend acknowledgements to everyone who supported the study including (A) Catherine Cebulla, Olivia Pijanowski, Reagan Church, Jianing Liu, Beverly Reynolds and Lori Fafard for clinical research coordinator support, (B) Grae Arabasz, Regan Butterfield and Shirley Hsu for nuclear medicine imaging technical support and (C) Steven Arnold, MD, Bianca Trombetta, Dario Gelevsky, Cassandra Leiberman and Alanna Farrar for biofluid technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102672.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Albrecht D.S., Normandin M.D., Shcherbinin S., Wooten D.W., Schwarz A.J., Zurcher N.R. Pseudoreference regions for glial imaging with (11)C-PBR28: investigation in 2 clinical cohorts. J. Nucl. Med. 2018;59(1):107–114. doi: 10.2967/jnumed.116.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshikho M.J., Zurcher N.R., Loggia M.L., Cernasov P., Chonde D.B., Izquierdo Garcia D. Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology. 2016;87(24):2554–2561. doi: 10.1212/WNL.0000000000003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshikho M.J., Zurcher N.R., Loggia M.L., Cernasov P., Reynolds B., Pijanowski O. Integrated magnetic resonance imaging and [(11) C]-PBR28 positron emission tomographic imaging in amyotrophic lateral sclerosis. Ann. Neurol. 2018;83(6):1186–1197. doi: 10.1002/ana.25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu S., Macklin E.A., Jackson K.E., Simpson E., Mahoney K., Yu H. Selection design phase II trial of high dosages of tamoxifen and creatine in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. Frontotemporal Degener. 2020;21(1–2):15–23. doi: 10.1080/21678421.2019.1672750. [DOI] [PubMed] [Google Scholar]
- Bakkar N., Boehringer A., Bowser R. Use of biomarkers in ALS drug development and clinical trials. Brain Res. 2015;14(1607):94–107. doi: 10.1016/j.brainres.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof F., Hulst H.E., Drulovic J., Uitdehaag B.M., Matsuda K., Landin R. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74(13):1033–1040. doi: 10.1212/WNL.0b013e3181d7d651. [DOI] [PubMed] [Google Scholar]
- Brettschneider J., Toledo J.B., Van Deerlin V.M., Elman L., McCluskey L., Lee V.M. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFL Group, et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol 2019 Jun 17. [DOI] [PMC free article] [PubMed]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. World federation of neurology research group on motor neuron diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Bravver E.K., Sanjak M.S., Bockenek W.L., Lindblom S.S., Lary C.L., Ranzinger L.H., Sturdivant A.N., Langford V.L., Holsten S.E., Ward A.L., Hillberry R., Wright A., Williamson T.A., Linville A.N., Lucas N.M., Brandon N.B., Dojillo J., Matsuda K., Iwaki Y., Graves D.C. Novel composite endpoint extended analysis during extension of Ibudilast Phase 1a/2b clinical trial better predicts post-wash-out survival. Platform Commun. Amyotroph. Lateral Scler. Frontotemporal Degener. 2018;19(sup1):1–84. doi: 10.1080/21678421.2018.1510202. [DOI] [Google Scholar]
- Canto E, Barro C, Zhao C, Caillier SJ, Michalak Z, Bove R, et al. Association Between Serum Neurofilament Light Chain Levels and Long-term Disease Course Among Patients With Multiple Sclerosis Followed up for 12 Years. JAMA Neurol 2019 Aug 12. [DOI] [PMC free article] [PubMed]
- Cedarbaum J.M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J. Neurol. Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang H., Ying Z., Gao Q. Ibudilast enhances the clearance of SOD1 and TDP-43 aggregates through TFEB-mediated autophagy and lysosomal biogenesis: The new molecular mechanism of ibudilast and its implication for neuroprotective therapy. Biochem. Biophys. Res. Commun. 2020;526(1):231–238. doi: 10.1016/j.bbrc.2020.03.051. [DOI] [PubMed] [Google Scholar]
- Cho Y., Crichlow G.V., Vermeire J.J., Leng L., Du X., Hodsdon M.E. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc. Natl. Acad. Sci. USA. 2010;107(25):11313–11318. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coalition Adaptive Platform Trials. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- Cox G.M., Kithcart A.P., Pitt D., Guan Z., Alexander J., Williams J.L. Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation. J. Immunol. 2013;191(3):1043–1054. doi: 10.4049/jimmunol.1200485. [DOI] [PubMed] [Google Scholar]
- DeYoung D.Z., Heinzerling K.G., Swanson A.N., Tsuang J., Furst B.A., Yi Y. Safety of intravenous methamphetamine administration during ibudilast treatment. J. Clin. Psychopharmacol. 2016;36(4):347–354. doi: 10.1097/JCP.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer OM, Marcus REG, Zurcher NR, Hooker JM. Tracing the History of the Human Translocator Protein to Recent Neurodegenerative and Psychiatric Imaging. ACS Chem Neurosci 2020 Jul 23. [DOI] [PubMed]
- Fox R.J., Coffey C.S., Conwit R., Cudkowicz M.E., Gleason T., Goodman A. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N. Engl. J. Med. 2018;379(9):846–855. doi: 10.1056/NEJMoa1803583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Sakoda S., Fujimura H., Yanagihara T. Ibudilast, a phosphodiesterase inhibitor, ameliorates experimental autoimmune encephalomyelitis in Dark August rats. J. Neuroimmunol. 1999;95(1–2):35–42. doi: 10.1016/s0165-5728(98)00251-3. [DOI] [PubMed] [Google Scholar]
- Fujita M., Imaizumi M., Zoghbi S.S., Fujimura Y., Farris A.G., Suhara T. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40(1):43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson L.C., Hastings S.F., McPhee I., Clayton R.A., Darroch C.E., Mackenzie A. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur. J. Pharmacol. 2006;538(1–3):39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Ha W, Sevim-Nalkiran H, Zaman AM, Matsuda K, Khasraw M, Nowak AK, et al. Ibudilast sensitizes glioblastoma to temozolomide by targeting Macrophage Migration Inhibitory Factor (MIF). Sci Rep 2019 Feb 27;9(1):2905-019-39427-4. [DOI] [PMC free article] [PubMed]
- Huang F, Zhu Y, Hsiao-Nakamoto J, Tang X, Dugas JC, Moscovitch-Lopatin M, et al. Longitudinal biomarkers in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 2020 Jun 9. [DOI] [PMC free article] [PubMed]
- Jucaite A., Svenningsson P., Rinne J.O., Cselenyi Z., Varnas K., Johnstrom P. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson's disease. Brain. 2015;138(Pt 9):2687–2700. doi: 10.1093/brain/awv184. [DOI] [PubMed] [Google Scholar]
- Kaufmann P., Thompson J.L., Levy G., Buchsbaum R., Shefner J., Krivickas L.S. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 2009;66(2):235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan A., Heslegrave A., Zetterberg H., Schott J.M. Stability of blood-based biomarkers of Alzheimer's disease over multiple freeze-thaw cycles. Alzheimers Dement (Amst) 2018;2(10):448–451. doi: 10.1016/j.dadm.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson R.J., Lebowitz M.D., Holberg C.J., Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am. Rev. Respir. Dis. 1983;127(6):725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- Kuhle J., Barro C., Andreasson U., Derfuss T., Lindberg R., Sandelius A. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016;54(10):1655–1661. doi: 10.1515/cclm-2015-1195. [DOI] [PubMed] [Google Scholar]
- Labra J., Menon P., Byth K., Morrison S., Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry. 2016;87(6):628–632. doi: 10.1136/jnnp-2015-310998. [DOI] [PubMed] [Google Scholar]
- Lu C.H., Macdonald-Wallis C., Gray E., Pearce N., Petzold A., Norgren N. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84(22):2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T., Cudkowicz M., Shaw P.J., Andersen P.M., Atassi N., Bucelli R.C. Phase 1–2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2020;383(2):109–119. doi: 10.1056/NEJMoa2003715. [DOI] [PubMed] [Google Scholar]
- Mizrahi R., Rusjan P.M., Kennedy J., Pollock B., Mulsant B., Suridjan I. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J. Cereb. Blood Flow Metab. 2012;32(6):968–972. doi: 10.1038/jcbfm.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kurotani T., Komatsu Y., Kawanokuchi J., Kato H., Mitsuma N. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46(3):404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Novakova L., Zetterberg H., Sundstrom P., Axelsson M., Khademi M., Gunnarsson M. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230–2237. doi: 10.1212/WNL.0000000000004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D.R., Yeo A.J., Gunn R.N., Song K., Wadsworth G., Lewis A. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 2012;32(1):1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganoni S., Alshikho M.J., Luppino S., Chan J., Pothier L., Schoenfeld D. A pilot trial of RNS60 in amyotrophic lateral sclerosis. Muscle Nerve. 2019;59(3):303–308. doi: 10.1002/mus.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.J.H., Harari O., Dron L., Lester R.T., Thorlund K., Mills E.J. An overview of platform trials with a checklist for clinical readers. J. Clin. Epidemiol. 2020;13(125):1–8. doi: 10.1016/j.jclinepi.2020.04.025. [DOI] [PubMed] [Google Scholar]
- Parmar A., Zurcher N.R., Hooker J.M. Time Will Tell the Utility of Biomarkers. ACS Chem. Neurosci. 2020;11(12):1692–1695. doi: 10.1021/acschemneuro.0c00238. [DOI] [PubMed] [Google Scholar]
- Pascuzzi R.M., Shefner J., Chappell A.S., Bjerke J.S., Tamura R., Chaudhry V. A phase II trial of talampanel in subjects with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11(3):266–271. doi: 10.3109/17482960903307805. [DOI] [PubMed] [Google Scholar]
- Poesen K., Van Damme P. Diagnostic and prognostic performance of neurofilaments in ALS. Front. Neurol. 2019;18(9):1167. doi: 10.3389/fneur.2018.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener 2016 Jul-Aug;17(5-6):414-425. [DOI] [PMC free article] [PubMed]
- Ray L.A., Bujarski S., Shoptaw S., Roche D.J., Heinzerling K., Miotto K. Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: a randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology. 2017;42(9):1776–1788. doi: 10.1038/npp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanftner L.M., Gibbons J.A., Gross M.I., Suzuki B.M., Gaeta F.C., Johnson K.W. Cross-species comparisons of the pharmacokinetics of ibudilast. Xenobiotica. 2009;39(12):964–977. doi: 10.3109/00498250903254340. [DOI] [PubMed] [Google Scholar]
- Sejbaek T., Nielsen H.H., Penner N., Plavina T., Mendoza J.P., Martin N.A. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naive relapsing MS patients. J. Neurol. Neurosurg. Psychiatry. 2019;90(12):1324–1330. doi: 10.1136/jnnp-2019-321321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefner J.M., Liu D., Leitner M.L., Schoenfeld D., Johns D.R., Ferguson T. Quantitative strength testing in ALS clinical trials. Neurology. 2016;87(6):617–624. doi: 10.1212/WNL.0000000000002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A., Ito A., Yoshikawa M., Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999;837(1–2):203–212. doi: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Zach N., Ennist D.L., Taylor A.A., Alon H., Sherman A., Kueffner R. Being PRO-ACTive: what can a clinical trial database reveal about ALS? Neurotherapeutics. 2015;12(2):417–423. doi: 10.1007/s13311-015-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurcher N.R., Loggia M.L., Lawson R., Chonde D.B., Izquierdo-Garcia D., Yasek J.E. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. Neuroimage Clin. 2015;19(7):409–414. doi: 10.1016/j.nicl.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.