Abstract
Background
Currently an echocardiographic threshold for the tricuspid regurgitation gradient (TRG) of > 31 mmHg is recommended for screening for pulmonary hypertension (PH). Invasively diagnosed PH was recently redefined as mean pulmonary arterial pressure (mPAP) > 20 mmHg instead of ≥ 25 mmHg. We investigated the ability of TRG to screen for the new PH-definition.
Methods
Retrospective assessment of echocardiography and right heart catheterisation data from 1572 patients entering the Giessen PH-Registry during 2008–2018. Accuracy of different TRG thresholds and other echocardiographic parameters was evaluated using receiver operating characteristic curves.
Findings
1264 patients fulfilled the new PH-definition. Positive (PPV) and negative predictive values and accuracy of TRG > 46 mmHg were 95%, 39%, and 73%, respectively, for the new PH-definition. Lowering the TRG cut-off to 31 mmHg and below worsened PPV to ≤ 89%. The PPV of TRG for pre-capillary PH (mPAP > 20 mmHg and pulmonary vascular resistance ≥ 3 Wood Units) was ≤ 85%. In patients with TRG ≤ 46 mmHg, tricuspid annular plane systolic excursion/TRG and TRG/right ventricular outflow tract acceleration time were superior to TRG in screening for newly defined pre-capillary PH.
Interpretation
In patients with suspected PH referred to a tertiary care centre, the PPV of TRG to meet the new PH-definition depended strongly on the TRG cut-off used. Our data do not support lowering the TRG cut-off. Combining TRG with other echocardiographic parameters might improve the validity of echocardiographic screening for PH.
Keywords: Pulmonary hypertension, Screening, Echocardiography, Diagnostic algorithm
Research in context.
Evidence before this study
The diagnostic threshold of mean pulmonary arterial pressure (mPAP) for the definition of chronic pulmonary hypertension (PH) has been lowered from ≥ 25 mmHg to > 21 mmHg, as measured by gold-standard invasive right heart catheterisation (RHC). The rationale for this change was based on an extensive review of the existing literature on normal pressures in the pulmonary circulation, and an increasing body of evidence that even mild elevations of pressure are of prognostic relevance for many common diseases (e.g. heart failure and chronic lung diseases). Recommendations regarding the respective cut-off values for non-invasive echocardiographic screening have been made, but have not yet been validated.
Added value of this study
In our study, data from more than 1500 patients who underwent both RHC and echocardiography within a short time frame were investigated regarding the validity of echocardiographic screening for the new definition of PH, using different thresholds of the tricuspid regurgitation gradient (TRG, the main non-invasive parameter for the derivation of pulmonary pressures). In essence, we show that lowering the TRG threshold below 31 mmHg reduced the positive predictive value below 89% for screening for PH defined as mPAP > 20 mmHg. In patients with TRG ≤ 46 mmHg, TRG combined with concomitantly assessed tricuspid annular plane systolic excursion or right ventricular outflow tract acceleration time was superior to TRG alone for screening for the new definition of pre-capillary PH (mPAP > 20 mmHg and pulmonary vascular resistance ≥ 3 Wood Units).
Implications of all the available evidence
Using echocardiography as a meaningful screening method for the prediction of PH is still to be regarded as the most appropriate approach. However, for the new definition of PH, reducing the previously recommended threshold of TRG for echocardiographic screening results in an unacceptable reduction of the positive predictive value. We suggest combining reduced TRG cut-off values with other echocardiographic parameters to improve the validity of echocardiographic screening for PH.
Alt-text: Unlabelled box
1. Introduction
For many years, pulmonary hypertension (PH) has been defined as a mean pulmonary arterial pressure (mPAP) of ≥ 25 mmHg measured invasively by right heart catheterisation (RHC) [1]. The most important non-invasive screening parameter for PH is the transthoracic echocardiographic tricuspid regurgitation gradient (TRG), which can be estimated from the regurgitation velocity at the tricuspid valve (TRV) as 4 x TRV2 [2]. Current guidelines separate patients into low-, intermediate-, and high-risk groups for having PH based on TRG values of ≤ 31 mmHg, >31–46 mmHg, and >46 mmHg, respectively [1]. The risk groups are further defined by additional echocardiographic parameters. If TRG is > 46 mmHg, the probability of PH is high even without additional echocardiographic parameters [1]. If TRG is ≤ 31 mmHg without additional echocardiographic parameters, the probability of PH is low [1]. If TRG is ≤ 31 mmHg with other echocardiographic signs of PH (in at least two of the following three categories: the ventricles [right ventricle/left ventricle basal diameter ratio > 1•0 and/or flattening of the interventricular septum (left ventricular eccentricity index > 1•1)]; the pulmonary artery [right ventricular outflow tract Doppler acceleration time (RVOT AT) <105 ms and/or mid-systolic notching, early diastolic pulmonary regurgitation velocity >2•2 m/s, and/or pulmonary arterial diameter >25 mm]; and the inferior vena cava [IVC] and right atrium [IVC diameter >21 mm with decreased inspiratory collapse, and/or right atrial (RA) area at end-systole > 18 cm2]), the probability of PH is intermediate [1]. If TRG is between 31 mmHg and 46 mmHg, the presence of other echocardiographic signs of PH indicates a high probability of PH whereas their absence indicates an intermediate probability of PH [1]. Diagnostic invasive RHC is indicated for patients with a TRV of > 2•8 m•s−1 [1], which corresponds to an estimated TRG of >31 mmHg.
Based on data determining the upper confidence limit of mPAP in healthy controls to be 20•6 mmHg [3], the recent 6th World Symposium on PH proposed a new haemodynamic definition of PH, with a new, reduced mPAP cut-off of > 20 mmHg. The inclusion of pulmonary vascular resistance (PVR) ≥ 3 Wood Units (WU) into the definition of pre-capillary PH was also proposed [4]. However, the World Symposium did not recommend changing the diagnostic algorithm; in particular, the key echocardiographic cut-off for assessment of the risk of PH remained unchanged, although validation of this cut-off was based on the previous definition of PH as mPAP ≥ 25 mmHg [5] and lower cut-offs (e.g. TRV ≥2•5 m•s−1) have been associated with mortality [6].
Therefore, the validity of the key echocardiographic screening parameter TRG for the prediction of the new haemodynamic definition of PH is currently unknown. We evaluated whether or not the estimated TRG is still appropriate for predicting PH and if there is any benefit in lowering the estimated TRG threshold below 31 mmHg.
2. Methods
2.1. Database
Consecutive incident patients who underwent transthoracic echocardiography and RHC at our tertiary referral centre and entered the Giessen PH Registry [7] between 1 January 2008 and 31 December 2018 were included in this retrospective analysis (HG, AY, NS, FG, WS, MJR, KT, and HAG had access to the Giessen PH Registry). All patients were transferred to our expert centre either by a family physician or by a specialist physician because of unclear symptoms (such as dyspnoea, fatigue, chest pain, or oedema) leading to a suspicion of PH. All participating patients gave written informed consent to be enrolled in the Giessen PH Registry. An external validation cohort comprised of 703 from the Hannover PH centre (see below). The investigation complies with the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of the Faculty of Medicine at the University of Giessen (Approval No. 186/16, 266/11). Final diagnoses were made by the local multidisciplinary board. Information on survival status was obtained in December 2020.
2.2. Echocardiography
Two-dimensional echocardiographic and Doppler measurements were performed using GE Vivid S5 and E9 systems (GE Healthcare GmbH, Solingen, Germany) at the time of the initial visit. Several parameters including TRG, RA area, RVOT AT, tricuspid annular plane systolic excursion (TAPSE), and IVC diameter were collected from the registry. We assessed PH based on TRG (≈4 x TRV2) rather than estimated pulmonary arterial systolic pressure (PASP; calculated as TRG+RA pressure [RAP]), because echocardiographic RAP is limited by low accuracy [1]. RAP was estimated as recommended in the current PH guidelines [1], based on evaluation of IVC diameters (expiratory and inspiratory) and percent collapse during inspiration [8]. All other measurements were performed according to the available imaging guidelines [9]. If both TAPSE and TRG were measurable, TAPSE was divided by TRG to give the TAPSE/TRG ratio (mm/mmHg). Longitudinal myocardial velocity, right ventricular to left ventricular ratio, pulmonary arterial diameter, and left ventricular eccentricity index or septal bowing are not included in the registry.
2.3. Right heart catheterisation
Measurements of invasive systolic pulmonary arterial pressure (sPAP), mPAP, PVR, cardiac index, RAP, and pulmonary arterial wedge pressure (PAWP) at the initial RHC were retrieved from the registry database. All RHC parameters were obtained after a short waiting period in order to provide a standardised diagnostic procedure by addressing time-dependant variability of these measurements [10].
2.4. External validation
External validation was performed in a second cohort including patients who were referred to the tertiary expert centre of the Hannover Medical School for diagnostic work-up of suspected PH. All incident patients who entered the Hannover PH Registry between 2013 and 2020 were included (n = 703).
2.5. Statistical analyses
Statistical analyses were performed in patients with measurable TRG (patients without measurable TRG were excluded). First, the association of echocardiographic TRG with invasively measured sPAP was assessed by Pearson's correlation, with p < 0•05 considered statistically significant. In a second step, analyses were performed in the whole cohort to assess the value of echocardiographic TRG as a screening tool in a PH referral centre using the new definition of PH. We assessed the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of different TRG cut-offs for screening for PH defined as mPAP ≥ 25 mmHg or as mPAP > 20 mmHg, respectively.
Subgroup analyses were then performed in patients with echocardiographic TRG ≤ 46 mmHg (corresponding to low-/intermediate-risk groups [1]) to evaluate the impact of using additional echocardiographic parameters to screen for PH (new definition). Then, these patients were grouped according to PAWP for further subgroup analyses (PAWP < 15 mmHg and PAWP ≥ 15 mmHg).
For each of the studied echocardiographic parameters, receiver operating characteristic (ROC) analyses were performed to assess discriminatory power and the Youden Index was used to identify the optimal cut-off value for screening for PH (new definition) [11]. Areas under the ROC curves (AUROCs) were compared using the bootstrap method in each subgroup.
A priori, we defined a NPV of 80% as appropriate for a screening method in patients with echocardiographic TRG ≤ 46 mmHg in a tertiary referral centre. Sensitivity and specificity of 80% and 60%, respectively, were also considered appropriate for a screening tool.
Survival analyses were truncated at 5 years after the initial RHC. Associations of echocardiographic parameters with mortality were assessed by univariate Cox regression.
Statistical analyses were performed as appropriate using SPSS (Version 26, IBM, Armonk, USA), GraphPad Prism software (Version 6, GraphPad, La Jolla, CA, USA) and R (The R Foundation, Vienna, Austria).
2.6. Role of the funding source
The study sponsors had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
3. Results
3.1. Patient characteristics
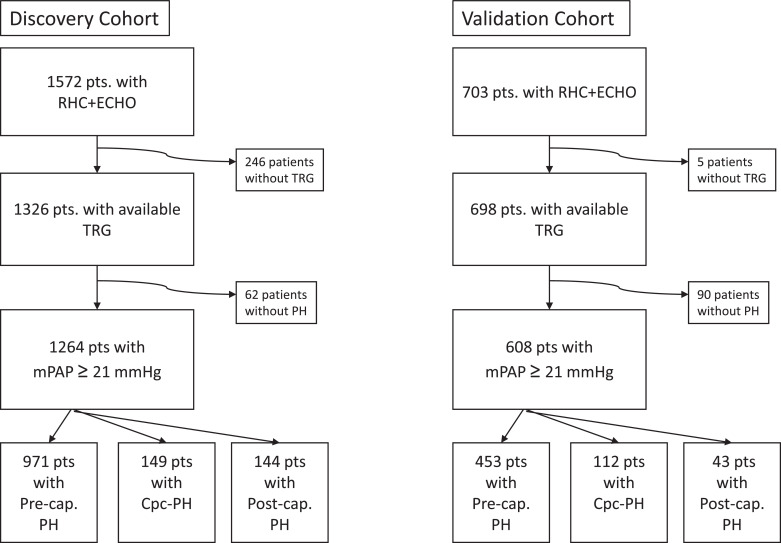
Between 2008 and 2018, 1572 patients with available echocardiographic and RHC data were included in the Giessen PH Registry because of suspected PH (Fig. 1). Of these patients, 80% had PH according to the new definition, of whom 211 patients had mPAP > 20 mmHg but < 25 mmHg. Compared with patients who had the new definition of PH overall, those without PH were slightly younger and had lower RAP, mPAP, and PVR and a higher cardiac index (Table 1). The median [quartile 1; quartile 3] time delay between echocardiography and RHC was 29 [3; 55] days. Patients with PH without post-capillary involvement (mPAP > 20 mmHg and PAWP ≤ 15 mmHg) and patients with combined pre- and post-capillary PH (mPAP > 20 mmHg, PAWP > 15 mmHg, and PVR ≥ 3 WU) showed higher mean TRG and TRG/RVOT AT ratio and lower mean TAPSE/TRG and RVOT AT than patients with post-capillary PH alone (mPAP > 20 mmHg, PAWP > 15 mmHg, and PVR < 3 WU; Table 1). The most common diagnoses in patients with PH without post-capillary involvement were PH due to lung diseases and/or hypoxia (33%) and pulmonary arterial hypertension (23%).
Fig. 1.
Flow chart of the study population and analysed subgroups .
Table 1.
Patient characteristics and right heart catheterisation data.
| All | Pre-capillary PH* | Post-capillary PH† | Combined pre- and post-capillary PH‡ | PH ruled out | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| n (%) | 1572 (100) | 971 (62) | 144 (9) | 149 (9) | 308 (20) |
| Age, years | 66 ± 12 | 66 ± 13 | 70 ± 11 | 68 ± 12 | 65 ± 11 |
| Female/male, n/n | 870/702 | 511/460 | 81/63 | 99/50 | 179/129 |
| BMI, kg•m−2 | 27•2 [24•0; 31•2] | 26•8 [23•7; 30•5] | 30•1 [26•8; 33•3] | 29•0 [24•5; 34•3] | 26•8 [24•1; 29•9] |
| Right heart catheterisation data | |||||
| mPAP, mmHg | 31•0 [22•0; 42•0] | 35•0 [27•0; 44•0] | 30•0 [27•0; 33•0] | 42•0 [38•0; 50•0] | 17•0[13•0; 19•0] |
| PVR, WU | 5 ± 4 | 7 ± 4 | 2 ± 1 | 6 ± 3 | 2 ± 1 |
| Cardiac index, L•min−1•m−2 | 2•6 ± 0•7 | 2•5 ± 0•7 | 2•7 ± 0•8 | 2•3 ± 0•5 | 2•7 ± 0•7 |
| PAWP, mmHg | 10•8 ± 6•1 | 8•8 ± 3•5 | 20•9 ± 3•8 | 21•2 ± 4•2 | 7•2 ± 3•6 |
| RAP, mmHg | 6•1 ± 5•2 | 5•7 ± 4•5 | 10•5 ± 6•3 | 11•6 ± 5•0 | 2•6 ± 2•9 |
| Echocardiographic data | |||||
| TRG, mmHg (n = 1326) | 53 ± 20 | 58 ± 19 | 44 ± 14 | 61 ± 17 | 34 ± 12 |
| PASP, mmHg (n = 1395) | 59 ± 21 | 65 ± 20 | 51 ± 14 | 68 ± 17 | 39 ± 12 |
| RVOT AT, ms (n = 1240) | 81 ± 27 | 75 ± 22 | 85 ± 29 | 72 ± 20 | 103 ± 31 |
| IVC diameter, mm (n = 510) | 21 ± 6 | 21 ± 5 | 23 ± 7 | 22 ± 5 | 19 ± 6 |
| RA area, cm2 (n = 885) | 21 ± 8 | 21 ± 8 | 23 ± 9 | 23 ± 9 | 17 ± 7 |
| TAPSE/TRG, mm•mmHg−1 (n = 1267) | 0•44 ± 0•30 | 0•37 ± 0•19 | 0•53 ± 0•54 | 0•31 ± 0•22 | 0•73 ± 0•28 |
| TRG/RVOT AT, mmHg•ms−1 (n = 1115) | 0•82 ± 1•53 | 0•94 ± 1•61 | 0•85 ± 2•77 | 0•91 ± 0•38 | 0•37 ± 0•20 |
| Left atrial area, cm2 (n = 248) | 13.4 ± 4.9 | 12.6 ± 4.8 | 16.4 ± 4.2 | 16.0 ± 4.8 | 13.1 ± 4.9 |
Data are presented as n (%), n/n, mean ± standard deviation, or median [first quartile; third quartile]. BMI=body mass index. IVC=inferior vena cava. mPAP=mean pulmonary arterial pressure. PAWP=pulmonary arterial wedge pressure. PH=pulmonary hypertension. PVR=pulmonary vascular resistance. RA=right atrial. RAP=right atrial pressure. RVOT AT=right ventricular outflow tract acceleration time. TAPSE=tricuspid annular plane systolic excursion. TRG=tricuspid regurgitation gradient. WU=Wood Units.
Defined as mPAP ≥ 21 mmHg, and PAWP ≤ 15 mmHg.
Defined as mPAP ≥ 21 mmHg, PAWP > 15 mmHg, and PVR < 3 WU.
Defined as mPAP ≥ 21 mmHg, PAWP > 15 mmHg, and PVR ≥ 3 WU.
3.2. Echocardiographic screening for PH based on the new haemodynamic definition
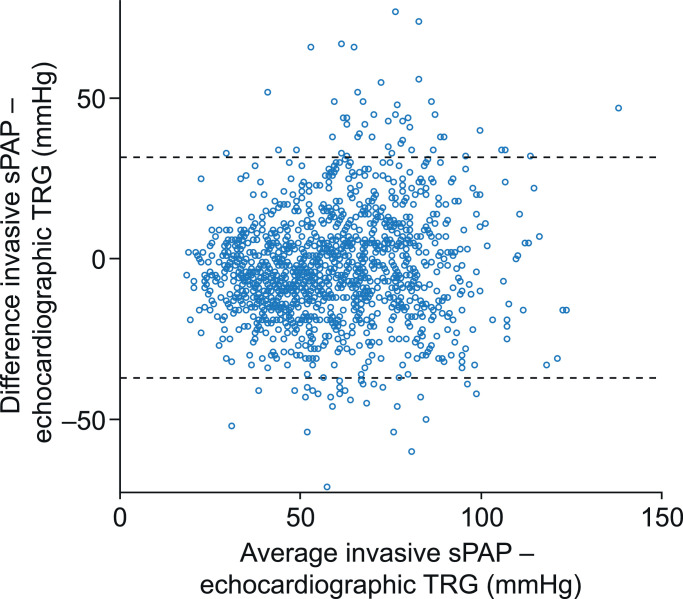
Echocardiographic TRG showed a low bias of −2•7 ± 17•6 mmHg compared with invasively measured sPAP, with wide limits of agreement (Fig. 2). Echocardiographic TRG correlated modestly with invasively measured sPAP in the whole cohort (r = 0•671, p < 0•001). In stratified analysis, invasively measured sPAP showed a modest correlation with high TRG (> 46 mmHg; r = 0•576, p < 0•001) and a low correlation with TRG between 31 mmHg and 46 mmHg (r = 0•208, p < 0•001), but was not significantly correlated with low TRG (≤ 31 mmHg; r = −0•128, p = 0•221).
Fig. 2.
Bland-Altman plot of TRG estimated by echocardiography versus sPAP measured by right heart catheterisation
Horizontal lines indicate upper (31•7 mmHg) and lower (−37•1 mmHg) limits of agreement. Echo=echocardiography. RHC=right heart catheterisation. sPAP=systolic pulmonary arterial pressure (invasively measured). TRG=tricuspid regurgitation gradient.
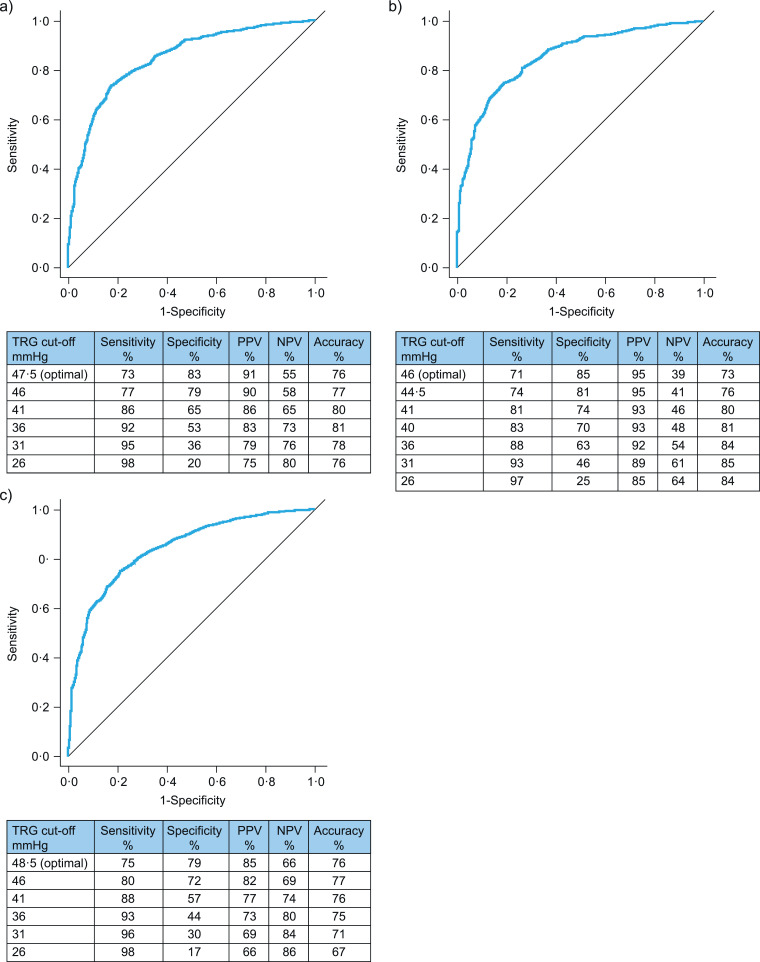
TRG had slightly lower sensitivity, higher specificity, higher PPV, and lower NPV when screening for PH defined as mPAP > 20 mmHg compared with the old definition (mPAP ≥ 25 mmHg) (Fig. 3A,B). Accuracy was good, with the highest accuracy (85%) being achieved using a TRG cut-off of 31 mmHg (Fig. 3B). We observed extremely low specificity and a slight increase in sensitivity without a substantial decrease in accuracy when the TRG cut-off for screening for newly defined PH was reduced below 31 mmHg (Fig. 3B).
Fig. 3.
Receiver operating characteristic analysis, PPV, NPV, and accuracy of different cut-offs of echocardiographic TRG for screening for pulmonary hypertension defined as (A) mPAP ≥25 mmHg (old definition), (B) mPAP >20 mmHg alone, or (C) mPAP >20 mmHg combined with pulmonary vascular resistance ≥ 3 Wood Units (pre-capillary pulmonary hypertension). Diagonal segments indicate ties. mPAP=mean pulmonary arterial pressure. NPV=negative predictive value. PPV=positive predictive value. TRG=tricuspid regurgitation gradient.
Because of the low correlation of TRG with invasively measured sPAP in patients with low TRG (≤ 46 mmHg), we performed subgroup analyses to evaluate the usefulness of other echocardiographic parameters in this population. In the subgroup of patients with an estimated TRG ≤ 46 mmHg (n = 550), 62% (n = 341) fulfilled the newly proposed diagnostic criterion of mPAP > 20 mmHg. The RVOT AT, PASP, IVC diameter, and RA area were evaluated for their ability to improve the PPV, NPV, and accuracy of TRG for screening for PH in this subgroup. The optimal cut-off for each parameter (Table 2) was tested. RVOT AT showed the highest accuracy and NPV but the lowest PPV for predicting mPAP > 20 mmHg. RA area had the highest PPV but the lowest accuracy. Echocardiographic parameters comprising both pressure and RV longitudinal shortening/RVOT AT were tested in patients with TRG ≤ 46 mmHg. The TAPSE/TRG ratio and TRG/RVOT AT ratio combined high accuracy (relative to other tested parameters) with intermediate PPV and NPV (Table 2). PASP showed slightly higher sensitivity and accuracy than TRG (Table 2).
Table 2.
Performance of different echocardiographic parameters for the detection of PH overall and pre-capillary PH (new haemodynamic definitions) in patients with TRG ≤ 46 mmHg (n = 550).
| Optimal cut-off | Sensitivity,% | Specificity,% | PPV,% | NPV,% | Accuracy,% | |
|---|---|---|---|---|---|---|
| For the detection of PH overall* | ||||||
| RVOT AT | 97•5 | 78 | 61 | 74 | 65 | 71 |
| IVC diameter | 17•5 | 76 | 48 | 81 | 40 | 69 |
| RA area | 22•5 | 37 | 88 | 84 | 44 | 55 |
| TAPSE/TRG | 0•57 | 64 | 80 | 83 | 58 | 70 |
| TRG/RVOT AT | 0•36 | 71 | 71 | 78 | 62 | 71 |
| TRG | 33•5 | 70 | 66 | 77 | 58 | 69 |
| PASP | 38•5 | 75 | 64 | 78 | 61 | 70 |
| For the detection of pre-capillary PH† in patients with PAWP ≤ 15 mmHg (n = 452) | ||||||
| RVOT AT | 97•5 | 86 | 52 | 45 | 89 | 63 |
| IVC diameter | 17•5 | 74 | 45 | 52 | 68 | 58 |
| RA area | 22•5 | 34 | 83 | 48 | 74 | 68 |
| TAPSE/TRG | 0•56 | 65 | 73 | 55 | 80 | 70 |
| TRG/RVOT AT | 0•37 | 76 | 61 | 47 | 85 | 66 |
| TRG | 35•5 | 68 | 62 | 47 | 80 | 64 |
| PASP | 39•5 | 73 | 56 | 45 | 81 | 61 |
| For the detection of pre-capillary PH† in patients with PAWP > 15 mmHg (n = 98) | ||||||
| RVOT AT | 79•5 | 71 | 61 | 44 | 83 | 64 |
| IVC diameter | 29•5 | 100 | 29 | 29 | 100 | 45 |
| RA area | 35•6 | 31 | 93 | 57 | 82 | 79 |
| TAPSE/TRG | 0•51 | 82 | 62 | 49 | 89 | 68 |
| TRG/RVOT AT | 0•56 | 58 | 79 | 54 | 81 | 73 |
| TRG | 37•5 | 67 | 62 | 43 | 81 | 63 |
| PASP | 45•5 | 69 | 55 | 40 | 80 | 59 |
IVC=inferior vena cava. mPAP=mean pulmonary arterial pressure. NPV=negative predictive value. PAWP=pulmonary arterial wedge pressure. PH=pulmonary hypertension. PPV=positive predictive value. PVR=pulmonary vascular resistance. RA=right atrial. RVOT AT=right ventricular outflow tract acceleration time. TAPSE=tricuspid annular plane systolic excursion. TRG=tricuspid regurgitation gradient.
Defined as mPAP > 21 mmHg.
Defined as mPAP > 21 mmHg and PVR ≥ 3 WU.
3.3. Echocardiographic screening for pre-capillary PH based on the new haemodynamic definition
When pre-capillary PH was defined as mPAP > 20 mmHg combined with PVR ≥ 3 WU, TRG continued to have high sensitivity as a screening parameter, but its specificity and PPV decreased to moderate levels, with wide ranges depending on the TRG cut-off (Fig. 3C). Again, lowering the TRG threshold led to a decrease of specificity and PPV while sensitivity and NPV increased slightly. Hence, we evaluated the usefulness of other echocardiographic parameters for screening for pre-capillary PH in patients with TRG ≤ 46 mmHg with or without elevated PAWP.
In patients with PAWP ≤ 15 mmHg (n = 452), 33% (n = 150) fulfilled the newly proposed combined definition of pre-capillary PH. RVOT AT, the TRG/RVOT AT ratio, TRG, and the TAPSE/TRG ratio showed the highest NPV whereas only the TAPSE/TRG ratio and IVC diameter showed a PPV above 50% (Table 2). PASP again showed slightly higher sensitivity than TRG but had lower accuracy than TRG in this subgroup. The TAPSE/TRG ratio, RA area, and TRG/RVOT AT ratio showed the highest accuracy. Concordantly, ROC analyses revealed that echocardiographic parameters combining pressure and resistance outperformed the current cornerstone TRG: the AUROC was 0•681 (95% confidence interval [CI]: 0•630, 0•732) for TRG, compared with 0.746 (95% CI: 0•694, 0•799) for the TRG/RVOT AT ratio (p < 0•001) and 0.731 (95% CI: 0•680, 0•782) for the TAPSE/TRG ratio (p = 0•014). RA area showed a significantly lower discriminatory power than TRG (AUROC: 0•597 [95% CI: 0•499, 0•659], p = 0•046). Combining the TRG/RVOT AT ratio with TAPSE slightly increased the discriminatory power (AUROC: 0•755 [95% CI: 0•703, 0•807], p = 0•795) and accuracy (68%) compared with TRG/RVOT AT alone. Amongst other key echocardiographic parameters, RVOT AT was the best discriminator between elevated (≥ 3 WU) and non-elevated PVR (< 3 WU) with an AUROC of 0•743 (95% CI: 0•692, 0•793). Consistent with this finding, RVOT AT showed an inverse correlation with sPAP (r = −0•442, p < 0•001) and PVR (r = −0•398, p < 0•001). ROC curves for TRG, TAPSE/TRG ratio and TRG/RVOT AT ratio are shown in the supplementary Fig. S1A.
In patients with PAWP > 15 mmHg (n = 98), 31% (n = 30) fulfilled the newly proposed combined definition of combined pre- and post-capillary PH. RA area, the TAPSE/TRG ratio, and TRG/RVOT AT ratio showed higher accuracy and PPV than all other tested parameters (Table 2). PASP again showed slightly higher sensitivity than TRG while accuracy was impaired (Table 2). However, PPV was impaired whereas NPV was high for all parameters (Table 2). ROC analyses revealed that the TAPSE/TRG ratio and TRG/RVOT AT ratio had slightly better discriminatory power than TRG in these patients: the AUROC was 0•652 (95% CI: 0•539, 0•765) for TRG, compared with 0•685 (95% CI: 0•560, 0•818) for the TRG/RVOT AT ratio (p = 0•470) and 0•763 (95% CI: 0•662, 0•863) for the TAPSE/TRG ratio (p = 0•143) (Table 2a).
Table 2a.
Patient characteristics and right heart catheterisation data for the validation cohort
| Validation cohort - MHH | All | Pre-capillary PH* | Post-capillary PH† | Combined pre- and post-capillary PH‡ | PH ruled out |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| n (%) | 703 (100) | 459 (65) | 42 (6) | 111 (16) | 91 (13) |
| Age, years | 66 ± 14 | 64 ± 15 | 73 ± 8 | 71 ± 12 | 63 ± 13 |
| Female/male, n/n | 407/296 | 253/206 | 24/18 | 70/41 | 60/31 |
| BMI, kg•m−2 | 26.9 [23.9; 31.2] | 26.5 [23.3; 30.5] | 29.2 [25.7; 32.6] | 29.1 [25.7; 34.7] | 25.4 [23.0; 29.7] |
| Right heart catheterisation data | |||||
| mPAP, mmHg | 36 [26; 47] | 39 [30; 48] | 29 [26; 32] | 43 [37; 50] | 17 [14; 19] |
| PVR, WU | 6 ± 5 | 8 ± 5 | 2 ± 1 | 6 ± 3 | 2 ± 1 |
| Cardiac index, L•min−1•m−2 | 2.4 ± 0.7 | 2.3 ± 0.7 | 2.7 ± 0.8 | 2.3 ± 0.6 | 2.8 ± 1.0 |
| PAWP, mmHg | 11.4 ± 5.5 | 9.4 ± 3.4 | 19.4 ± 3.0 | 19.5 ± 3.3 | 7.3 ± 2.9 |
| RAP, mmHg | 8.0 ± 4.9 | 7.6 ± 4.5 | 10.6 ± 5.3 | 11.9 ± 4.8 | 3.6 ± 2.4 |
| Echocardiographic data | |||||
| TRG, mmHg (n = 699) | 51 ± 20 | 54 ± 19 | 42 ± 13 | 57 ± 17 | 29 ± 9 |
| PASP, mmHg (n = 698) | 60 ± 21 | 63 ± 20 | 51 ± 14 | 68 ± 17 | 35 ± 10 |
| RVOT AT, ms (n = 595) | 95 ± 43 | 88 ± 42 | 122 ± 55 | 93 ± 32 | 121 ± 44 |
| IVC diameter, mm (n = 697) | 20 ± 6 | 20 ± 6 | 21 ± 5 | 22 ± 6 | 16 ± 5 |
| RA area, cm2 (n = 446) | 27 ± 17 | 28 ± 20 | 21 ± 5 | 29 ± 10 | 19 ± 6 |
| TAPSE/TRG, mm•mmHg−1 (n = 693) | 0.47 ± 0.34 | 0.40 ± 0.25 | 0.54 ± 0.18 | 0.39 ± 0.46 | 0.88 ± 0.36 |
| TRG/RVOT AT, mmHg•ms−1 (n = 594) | 0.81 ± 1.58 | 0.90 ± 1.49 | 1.04 ± 3.72 | 0.69 ± 0.33 | 0.39 ± 1.21 |
External validation was performed in 703 incident patients (Fig. 1). Baseline characteristics are shown in Table 2A. Overall, the distribution of pre-capillary, post-capillary, combined pre- and post-capillary, and invasively excluded PH was similar to that observed in the derivation cohort. However, patients in the validation cohort showed more severe impairment of pulmonary haemodynamic parameters than those in the derivation cohort.
Optimal thresholds in the validation cohort (derived by the Youden Supplementary Table S1) and associated sensitivity, specificity, PPV, NPV, and accuracy are shown in Supplementary Table S1. Of note, the TAPSE/TRG and TRG/RVOT AT ratios outperformed routinely assessed parameters such as RVOT AT, RA area, and IVC diameter in all tested subgroups with low estimated TRG (Supplementary Table S1). ROC curves of TRG, TAPSE/TRG ratio and TRG/RVOT AT ratio for detection of the new definition of PH in patients with PAWP ≤ 15 mmHg and TRG ≤ 46 mmHg are shown in the Supplementary Fig. S1A.
3.4. Echocardiographic parameters predict mortality in patients with PH
Information regarding survival status was available for 1236 of the 1264 patients with PH in the derivation cohort. Of these 1236 patients, 374 died within 5 years after the initial RHC (median [quartile 1; quartile 3] follow-up time: 60 [34; 60] months). All tested echocardiographic parameters except the TRG/RVOT AT ratio predicted mortality (Table 3).
Table 3.
Prognostic value of echocardiographic right heart parameters in patients with suspected PH and PH with mPAP > 20 mmHg and < 25 mmHg.
| Univariate Cox Regression | Univariate Cox Regression (Subgroup: mPAP > 20 mmHg and < 25 mmHg) | |||
|---|---|---|---|---|
| Parameter | HR (CI) | p-value | HR (CI) | p-value |
| RA area | 1.038 (1.022, 1.054) | < 0.001 | 1.014 (0.939, 1.095) | 0.724 |
| TAPSE | 0.917 (0.899, 0.936) | < 0.001 | 0.988 (0.903, 1.081) | 0.791 |
| S´ | 0.882 (0.839, 0.927) | < 0.001 | 0.774 (0.602, 0.997) | 0.047 |
| RIMP | 2.848 (1.922, 4.219) | < 0.001 | 3.322 (0.282, 39.124) | 0.340 |
| PASP | 1.015 (1.010, 1.020) | < 0.001 | 1.002 (0.968, 1.037) | 0.915 |
| TRG | 1.014 (1.009, 1.019) | < 0.001 | 1.005 (0.973, 1.037) | 0.781 |
| RAP | 1.056 (1.029, 1.083) | < 0.001 | 0.878 (0.722, 1.068) | 0.194 |
| AT | 0.985 (0.980, 0.989) | < 0.001 | 1.011 (0.993, 1.029) | 0.222 |
| VCI | 1.044 (1.016, 1.073) | 0.002 | 1.214 (0.951, 1.551) | 0.120 |
| TAPSE/PASP | 0.134 (0.074, 0.241) | < 0.001 | 1.099 (0.515, 2.343) | 0.808 |
| TRG/AT ratio | 1.018 (0.969, 1.070) | 0.471 | 2.114 (0.222, 20.120) | 0.515 |
3.5. Performance of echocardiography in patients with mPAP values between 21 mmHg and 25 mmHg
Of the 211 patients with mPAP > 20 mmHg and < 25 mmHg, 142 had echocardiographic TRG data available. Mean TRG in this subgroup was 41 ± 14 mmHg. Interestingly, the usefulness and thresholds of echocardiographic parameters for the prediction of pre-capillary PH in this subgroup differed substantially from those observed in patients with TRG ≤ 46 mmHg. Indeed, only TRG, PASP, and the TAPSE/TRG and TRG/RVOT AT ratios showed appropriate accuracy in patients with mPAP > 20 mmHg and < 25 mmHg (Table 4). The optimal TAPSE/TRG cut-off was meaningfully lower than in patients with TRG ≤46 mmHg whereas the optimal TRG, PASP, and TRG/RVOT AT cut-offs were meaningfully higher. The NPV of all tested parameters was ≥ 75% but PPV was severely impaired (Table 4). Moreover, only tissue Doppler-derived tricuspid lateral annular systolic velocity (S’) predicted mortality in this subgroup of patients (Table 3).
Table 4.
Performance of different echocardiographic parameters for the detection of PH defined by mPAP > 20 mmHg and < 25 mmHg.
| Parameter | Optimal cut-off | Sensitivity,% | Specificity,% | PPV,% | NPV,% | Accuracy,% |
|---|---|---|---|---|---|---|
| RVOT AT | 54.5 | 98 | 3 | 29 | 75 | 30 |
| VCI | 19.5 | 75 | 53 | 38 | 85 | 59 |
| RA Area | 15.4 | 62 | 57 | 27 | 85 | 58 |
| TAPSE/PASP ratio | 0.36 | 28 | 87 | 45 | 76 | 71 |
| TRG/RVOT AT ratio | 0.61 | 50 | 80 | 48 | 80 | 71 |
| TRG | 45.5 | 47 | 74 | 40 | 79 | 67 |
| PASP | 50.5 | 53 | 76 | 48 | 80 | 69 |
4. Discussion
We have evaluated the validity of echocardiographic TRG as a screening parameter for the new haemodynamic definitions of PH and pre-capillary PH as proposed at the 6th World Symposium on PH. In a patient population with a high pre-test probability for PH referred to a tertiary referral centre, echocardiographic TRG was somewhat imprecise as a measure of sPAP, and the specificity and PPV of TRG as a screening parameter for the new definition of PH were strongly dependant on the TRG cut-off used. The combination of pressure and resistance within the new definitions of pre-capillary PH and combined pre- and post-capillary PH resulted in an even lower PPV of echocardiography than previously described [5]. Moreover, the association of TRG with invasively measured sPAP was limited at low pressures (TRG ≤ 46 mmHg). In patients with TRG ≤ 46 mmHg, other echocardiographic parameters (the TAPSE/TRG ratio and TRG/RVOT AT ratio) were superior to TRG for screening for the new definition of pre-capillary PH. External validation was performed in a second cohort of incident patients who were referred for diagnostic work-up to the Hannover tertiary referral centre.
Our study suggests that the echocardiographic screening approach to determine the need for invasive confirmation of pH (based on the new criteria) in referral centres needs to be reconsidered. The specificity, PPV, and NPV of TRG > 31 mmHg for screening for pH defined as mPAP > 20 mmHg in our study were below the values reported previously by Greiner and co-workers using the old definition of PH (mPAP ≥ 25 mmHg) [5]. Greiner and co-workers showed that a PASP (TRG+RAP) threshold of 36 mmHg had excellent sensitivity (87%) with 79% specificity, 92% PPV, and 85% accuracy for screening for the old haemodynamic definition of PH in a large expert-centre cohort. In comparison, a TRG cut-off of 31 mmHg in our cohort showed similar sensitivity but lower specificity in screening for patients with mPAP ≥ 25 mmHg. The reason for this discrepancy cannot be directly assessed by our study, however we speculate that a general referral bias and differences between the study cohorts may have contributed. Indeed, patients included in the validation cohort had increased mPAP which may affect the validity of TRG. Compared with the results of Greiner and co-workers using the old definition of PH and a TRG cut-off of 36 mmHg, the accuracy of TRG for screening in our cohort (using a cut-off of 31 mmHg) was similar if PH was defined as mPAP > 20 mmHg. The bias of echocardiographic TRG as a measure of sPAP in our study is comparable to that reported previously by Greiner and co-workers for echocardiographic PASP (−2•0 ± 8•2 mmHg) [5]. Our data suggest that lowering the estimated TRG cut-off below 31 mmHg (without considering other echocardiographic PH signs) to screen for the new PH definition would lead to extremely low specificity and further impair PPV as well as accuracy without a substantial increase of sensitivity. This supports the approach taken by the 6th World Symposium on PH, which (in the absence of data) did not recommend reducing the TRG threshold for invasive confirmation of PH.
The accuracy and PPV of TRG for screening for pre-capillary and combined pre- and post-capillary PH (mPAP > 20 mmHg and PVR ≥ 3 WU) in our study were worse than those for screening for PH overall (mPAP > 20 mmHg), indicating that addition of PVR to the new definition of pre-capillary PH alters the usefulness of TRG as a screening parameter. Our study provides evidence that lowering the TRG threshold for intermediate risk of PH below the currently recommended level of 31 mmHg results in extremely low specificity with unacceptable PPV and moderate accuracy for screening for pre-capillary and combined pre- and post-capillary PH. The sensitivity of TRG did not meaningfully increase when lowering the TRG threshold. This is mirrored by the fact that echocardiographic TRG has been shown to correlate with invasively measured sPAP [2], but with low precision [12]. In addition, a noticeable divergence between echocardiographic PASP (TRG+RAP) and invasively measured sPAP has been described at low pressures [12,13], consistent with our data which showed a correlation between invasively measured sPAP and echocardiographic TRG overall but not at TRG values ≤ 31 mmHg. In a tertiary referral centre, a high PPV is crucial to avoid unnecessary RHC in individuals without PH. High sensitivity, however, avoids cases of manifest PH being missed. This is even more important than avoiding unnecessary RHC (which is associated with low morbidity and mortality when performed by experts [14]), because a missed or delayed diagnosis of PH leads to delayed PH-specific therapy which may result in a significant deterioration in life expectancy [1]. Therefore, we suggest maintaining the current TRG cut-off of 31 mmHg because it combines high sensitivity with acceptable PPV.
Additional echocardiographic parameters which are already recommended in the PH guidelines for assessment of the probability of PH [1] can improve the accuracy of PH screening when used in conjunction with TRG. Interestingly, in our analysis conducted in a tertiary referral centre, the TAPSE/TRG ratio and TRG/RVOT AT ratio emerged as screening parameters for the new definition of PH in patients with low estimated TRG (≤ 46 mmHg). In our sub-analysis of patients with TRG ≤ 46 mmHg, both ratios had high accuracy in screening for PH overall, and the TAPSE/TRG ratio had a considerably higher accuracy than all other tested parameters for screening for pre-capillary PH in patients with PAWP ≤ 15 mmHg. Neither ratio is mentioned in the European PH guidelines [1].
Theoretically, the TAPSE/TRG ratio mirrors the ability of the right ventricle to generate pressure and longitudinal shortening, similar to the TAPSE/PASP ratio [15]. The TAPSE/PASP ratio is a meaningful parameter for patients with pulmonary arterial hypertension and PH due to chronic lung disease [[16], [17], [18]], and was recently validated as an echocardiographic surrogate of RV-arterial coupling in PH [19].
Potential advantages of the TAPSE/TRG ratio over TRG alone might include its additional association with afterload, pulmonary arterial compliance, and maladaptive processes such as right ventricular dilatation. In particular, patients with a low estimated echocardiographic TRG often present with a slight tricuspid insufficiency leading to difficulties in obtaining an appropriate signal. This often leads to underestimation of TRG [20]. The current prioritisation of TRG is therefore to be questioned in these patients. Combining parameters, for instance by using the TAPSE/TRG ratio, reduces the error caused by each parameter. Measures of RV-arterial coupling have been proven to be more sensitive correlates of RV function during the course of PH than TAPSE, since TAPSE mostly decreases in advanced stages of RV dysfunction [19]. This could explain why echocardiographic parameters mirroring RV-arterial coupling might be superior to TRG alone at slightly elevated pulmonary arterial pressures.
The RVOT AT was the best echocardiographic screening parameter for elevated PVR (≥ 3 WU) in our cohort. RVOT AT is an established echocardiographic surrogate of pulmonary haemodynamics and RV afterload [1,[21], [22], [23], [24]] and has shown a high correlation with invasively measured pulmonary arterial pressure [25,26]. RVOT AT reflects pressure and flow analogous to the definition of pre-capillary PH [1,24], which may explain why RVOT AT is a sensitive predictor of pre-capillary PH [24]. It is noteworthy that sPAP showed a better correlation with RVOT AT than with TRG in patients with low TRG in our study. Furthermore, RVOT AT correlated with PVR. Therefore, the TRG/RVOT AT ratio outperformed TRG in patients with low TRG and showed a high discriminatory power when screening for pre-capillary PH in patients with PAWP ≤ 15 mmHg. One major drawback of TRG is the requirement for a reliable TR envelope [27]. Notably, RVOT AT alone showed the highest sensitivity for the prediction of PH overall in patients with low TRG and is often available even if the TR jet is insufficient [23]. Therefore, RVOT AT might be an alternative to TRG or the TRG/RVOT AT ratio in case of insufficient imaging of the TR jet, as known from the echocardiographic assessment of pulmonary haemodynamics in paediatric patients [24,25].
AUROCs of all tested parameters remained moderate even though they were improved compared with the TRG AUROC in patients with low TRG. Therefore, decision making for patients with low TRG remains complex and cannot be based on echocardiography alone. Clinicians need to consider clinical signs, electrocardiograms, cardiopulmonary exercise testing, and further tests to assess the necessity of RHC [1].
Our study has several limitations. Further evidence is needed from prospective studies and the results are limited by the small number of patients who have the newly proposed phenotype but not the old definition of PH. Only patients who underwent RHC were included. Hence, it is unclear how many patients with manifest PH but a low echocardiographic probability of PH have been missed because they were ruled out before RHC. Although in the literature TAPSE/PASP is an established parameter for RV-arterial coupling in PH [19,28], we used TAPSE/TRG for consistency with our target parameter TRG, without adjusting for central venous pressure. Furthermore, the transferability of these results to non-expert centres is uncertain, acknowledging that the prevalence of PH in the general population is low, impacting particularly the PPVs and NPVs (a higher pre-test probability of PH results in higher PPVs and lower NPVs) [29]. Therefore, the ability of TRG to screen for PH according to the new haemodynamic definition must be re-assessed in a large cohort without any pre-selection, as a referral bias is likely. Another limitation is the fact that we were not able to include all echocardiographic parameters mentioned in the current guidelines or left heart parameters due to missing data.
In a tertiary referral centre, echocardiographic TRG showed reduced specificity and PPV as a screening parameter for the new haemodynamic definition of pre-capillary PH. Lowering the TRG screening threshold even below 31 mmHg should not be considered for the new haemodynamic definitions of PH overall and pre-capillary PH in particular, owing to the resulting loss of specificity and PPV. Our study suggests that a combination of TRG with additional echocardiographic indices beyond those currently recommended in the European guidelines can improve predictive ability in a cohort with a high pre-test probability of PH. In patients with estimated TRG ≤ 46 mmHg, the TAPSE/TRG ratio or the TRG/RVOT AT ratio may provide an accurate assessment of the risk of PH overall and pre-capillary PH based on the new haemodynamic definitions, and should be evaluated further as a simple screening procedure for PH in tertiary referral centres.
Contributors
HG conceived the idea for the analyses detailed in this manuscript. All authors contributed to the design and data collection in the study. AY and HG undertook statistical analyses of the data in the manuscript. All authors contributed to drafting and critical review of the manuscript. All authors approved the manuscript for submission. Claire Mulligan PhD (Beacon Medical Communications Ltd, Brighton, UK) edited the final draft of the manuscript prepared by the authors.
Data sharing statement
All data presented in this work will be shared upon request.
Declaration of Competing Interest
HG reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Actelion, AstraZeneca, Bayer, BMS, GSK, Janssen-Cilag, Lilly, MSD, Novartis, OMT, Pfizer, and United Therapeutics outside the submitted work. AY reports non-financial support from the University of Giessen during the conduct of the study. JF reports no conflicts of interest. NS reports personal fees from Actelion outside the submitted work. WS reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Pfizer and Bayer Pharma AG outside the submitted work. KMO reports personal fees from GSK, United Therapeutics, Pfizer, Bayer, Actelion, personal fees from Acceleron, and MSD, outside the submitted work. MMH reports personal fees from Acceleron, Actelion, Bayer, GSK, Janssen, MSD, and Pfizer, all outside the submitted work. MJR reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and grants from United Therapeutics, grants and personal fees from Bayer, and personal fees from Actelion, Mundipharma, Roche, and OMT outside the submitted work. KT reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Actelion and Bayer outside the submitted work. HAG reports grants from German Research Foundation, non-financial support from University of Giessen, during the conduct of the study; personal fees from Bayer, Actelion, Pfizer, Merck, and GSK, grants and personal fees from Novartis, Bayer HealthCare, Encysive/Pfizer, grants from Aires, German Research Foundation, Excellence Cluster Cardiopulmonary Research, and German Ministry for Education and Research, and personal fees from Takeda. FG and JF report no conflicts of interest.
Acknowledgments
Acknowledgments
Editorial assistance was provided by Claire Mulligan, PhD (Beacon Medical Communications Ltd, Brighton, UK), funded by the University of Giessen.
Funding
This work was supported by the Excellence Cluster Cardio-Pulmonary System (ECCPS) and the Collaborative Research centre (CRC/SFB) 1213 - Pulmonary Hypertension and Cor Pulmonale, grant number SFB1213/1, project B08 (German Research Foundation, Bonn, Germany).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100822.
Appendix. Supplementary materials
References
- 1.Galie N., Humbert M., Vachiery J.L. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): endorsed by: association for european paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Berger M., Haimowitz A., Van Tosh A., Berdoff R.L., Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6(2):359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs G., Berghold A., Scheidl S., Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G., Montani D., Celermajer D.S. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greiner S., Jud A., Aurich M. Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolte D., Lakshmanan S., Jankowich M.D., Brittain E.L., Maron B.A., Choudhary G. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(18) doi: 10.1161/JAHA.118.009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gall H., Felix J.F., Schneck F.K. The Giessen pulmonary hypertension registry: survival in pulmonary hypertension subgroups. J Heart Lung Transpl. 2017;36(9):957–967. doi: 10.1016/j.healun.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Kircher B.J., Himelman R.B., Schiller N.B. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 9.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 10.Yogeswaran A., Richter M.J., Sommer N. Evaluation of pulmonary hypertension by right heart catheterisation - does timing matter? Eur Respir J. 2020;56(3) doi: 10.1183/13993003.01892-2019. [DOI] [PubMed] [Google Scholar]
- 11.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M.R., Forfia P.R., Chamera E. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habash F., Gurram P., Almomani A. Correlation between echocardiographic pulmonary artery pressure estimates and right heart catheterization measurement in liver transplant candidates. J Cardiovasc Imaging. 2018;26(2):75–84. doi: 10.4250/jcvi.2018.26.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeper M.M., Lee S.H., Voswinckel R. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48(12):2546–2552. doi: 10.1016/j.jacc.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 15.Tello K., Seeger W., Naeije R. Right heart failure in pulmonary hypertension: diagnosis and new perspectives on vascular and direct right ventricular treatment. Br J Pharmacol. 2019 doi: 10.1111/bph.14866. [DOI] [PubMed] [Google Scholar]
- 16.Tello K., Axmann J., Ghofrani H.A. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. 2018;266:229–235. doi: 10.1016/j.ijcard.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 17.Tello K., Ghofrani H.A., Heinze C. A simple echocardiographic estimate of right ventricular-arterial coupling to assess severity and outcome in pulmonary hypertension on chronic lung disease. Eur Respir J. 2019;54(3) doi: 10.1183/13993003.02435-2018. [DOI] [PubMed] [Google Scholar]
- 18.Richter M.J., Peters D., Ghofrani H.A. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2020;201(1):116–119. doi: 10.1164/rccm.201906-1195LE. [DOI] [PubMed] [Google Scholar]
- 19.Tello K., Wan J., Dalmer A. Validation of the Tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging. 2019;12(9) doi: 10.1161/CIRCIMAGING.119.009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amsallem M., Sternbach J.M., Adigopula S. Addressing the controversy of estimating pulmonary arterial pressure by echocardiography. J Am Soc Echocardiogr. 2016;29(2):93–102. doi: 10.1016/j.echo.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Patel M.D., Breatnach C.R., James A.T. Echocardiographic assessment of right ventricular afterload in preterm infants: maturational patterns of pulmonary artery acceleration time over the first year of age and implications for pulmonary hypertension. J Am Soc Echocardiogr. 2019;32(7):884–894. doi: 10.1016/j.echo.2019.03.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammad Nijres B., Bokowski J., Mubayed L., Jafri S.H., Davis A.T., Abdulla R.I. Utility of pulmonary artery acceleration time to estimate systolic pulmonary artery pressure in neonates and young infants. Pediatr Cardiol. 2020;41(2):265–271. doi: 10.1007/s00246-019-02251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yared K., Noseworthy P., Weyman A.E., McCabe E., Picard M.H., Baggish A.L. Pulmonary artery acceleration time provides an accurate estimate of systolic pulmonary arterial pressure during transthoracic echocardiography. J Am Soc Echocardiogr. 2011;24(6):687–692. doi: 10.1016/j.echo.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Koestenberger M., Grangl G., Avian A. Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ Cardiovasc Imaging. 2017;10(1) doi: 10.1161/CIRCIMAGING.116.005336. [DOI] [PubMed] [Google Scholar]
- 25.Levy P.T., Patel M.D., Groh G. Pulmonary artery acceleration time provides a reliable estimate of invasive pulmonary hemodynamics in children. J Am Soc Echocardiogr. 2016;29(11):1056–1065. doi: 10.1016/j.echo.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granstam S.O., Bjorklund E., Wikstrom G., Roos M.W. Use of echocardiographic pulmonary acceleration time and estimated vascular resistance for the evaluation of possible pulmonary hypertension. Cardiovasc Ultrasound. 2013;11:7. doi: 10.1186/1476-7120-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Riel A.C.M.J., Opotowsky A.R., Santos M. Accuracy of echocardiography to estimate pulmonary artery pressures with exercise. Circ Cardiovasc Imaging. 2017;10(4) doi: 10.1161/CIRCIMAGING.116.005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guazzi M. Use of TAPSE/PASP ratio in pulmonary arterial hypertension: an easy shortcut in a congested road. Int J Cardiol. 2018;266:242–244. doi: 10.1016/j.ijcard.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 29.Moreira E.M., Gall H., Leening M.J.G. Prevalence of pulmonary hypertension in the general population: the rotterdam study. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0130072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.