Abstract
Diabetic Foot Infection (DFI), in its severest form the acute infected ‘diabetic foot attack’, is a limb and life threatening condition if untreated. Acute infection may lead to tissue necrosis and rapid spread through tissue planes, in the patient with poorly controlled diabetes facilitated by the host status. A combination of soft tissue infection and osteomyelitis may co-exist, in particular if chronic osteomyelitis serves as a persistent source for recurrence of soft tissue infection. This “diabetic foot attack” is characterised by acutely spreading infection and substantial soft tissue necrosis.
In the presence of ulceration, the condition is classified by the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (IDSA/IWGDF Class 3 or 4) presentation requiring an urgent surgical intervention by radical debridement of the infection. Thus, ‘time is tissue’, referring to tissue salvage and maximal limb preservation. Emergent treatment is important for limb salvage and may be life-saving. We provide a narrative current treatment practices in managing severe DFI with severe soft tissue and osseous infection. We address the role of surgery and its adjuvants, the long term outcomes, potential complications and possible future treatment strategies.
Keywords: Foot attack, Severe diabetic foot infection, Surgery, Debridement, Outcomes
Abbreviations: Diabetic Foot Infection, DFI; Diabetic Foot Ulcer, DFU; International Working Group of the Diabetic Foot, IWGDF; National Institute for Health and Care Excellence, NICE
Highlights
-
•
3 important pathologies; infection, ischemia & neuropathy, cause acute necrosis and life threatening sepsis in diabetic feet.
-
•
Careful history, examination within a MDFT – for neuropathy, ischemia and infection are essential for effective management.
-
•
Early diagnose, anatomical spread, infection load assessment in the foot will correctly prioritise the patient.
-
•
Debridement of severe or significant infection should be performed by a trained surgeon in a protocolised manor within 24 h.
-
•
Infection control is the 1st-stage of limb salvage followed by surgical reconstruction for long-term functional salvage.
1. Introduction
Diabetic foot infection (DFI) is a serious condition and is most commonly the sequel of an infected diabetic foot ulcer (DFU). Over 50% of DFUs become infected, presenting either acute or chronic, often complicated by osteomyelitis.1 Occasionally, the presentation proceeds with rapid progression, spreading cellulitis, tissue necrosis and a systemic inflammatory response. Such infections can be limb threatening without timely intervention and are described as a ‘diabetic foot attack’.2 This clinical presentation is one of three acute conditions of the diabetic foot that may be considered a “foot attack”. The other two conditions include: acute critical ischemia, usually in sensate feet, and acute Charcot neuroarthropathy, commonly known as a Charcot foot. All these presentations can precipitate loss of tissue and lead to amputation if not reversed or treated in a timely manner.
Despite necrosis and deep tissue infection, irreversible damage may be underestimated by both patient and clinician.3 Consequent, delayed presentation with minimal symptoms and subtle signs is seen. However, if not promptly treated it can lead to acute lower extremity amputation (LEA)4 and 5-year mortality of greater than 60%, with a significant psycho-socio-economic burden to the health economy.5,6 Vas et al. (2018),2 described emergent management as infection control and sequential rapid correction of ischemia. The principle, for severe infections or Infectious Diseases Society of America/International Working Group on the Diabetic Foot grade 3 or 47,8 includes rapid diagnosis, identifying pathogens, targeted intravenous antibiotic therapy, emergent and radical surgical debridement. Differentiating factors between classes relate to the systemic condition of the patient. Management is structured within a Multi-Disciplinary Foot Team (MDFT), but the exact process for the management of the acute presentation of a DFI is still not clearly defined.
We provide a narrative review of severe DFI, and where, and when surgical debridement should be considered, the surgical techniques used, and general management for best outcomes.
2. Methods
This review assessed accessible and published current literature in the management of the severe DFI to provide an update on ‘best practice approaches’ of the management of severe diabetic foot infection. A literature search up to November 2020 was conducted by interrogating the following search engines: PubMed, Embase, (CINAHL), Cochrane, and Web of Science databases. The search was conducted by two of the authors using the following search terms: “diabetic foot infection”, “IDSA class 3 foot infection or University of Texas grade 3B or Wagner classification grade 5” requiring an urgent surgical debridement of the infection “diabetic foot osteomyelitis” and “surgical approach” and “foot attack”.
3. Results
We identified a number of guidelines reflecting the multitude of disciplines involved in treatment, but no specific guidelines on the surgical management of severe DFI or the ‘diabetic foot attack’. The most inclusive guidance has been published by the International Working Group on the Diabetic Foot (IWGDF) but only provide 6 recommendations (see Table 1)8,9; but no specific information of surgical process including details of operative management and consequent clinical outcomes in this specific cohort of patients is given. Organisations such as NICE (NG19.9), emphasise the importance of acute debridement and drainage of pus as an emergency in any hospital setting.10,11 The joint colleges released an expertise for the document “Operational Delivery of the Multi-Disciplinary Care Pathway for Diabetic Foot” concluding acute infection control was an emergency.11
Table 1.
IWGDF evidence in the management of severe diabetic foot infection. The quality of evidence was graded ‘high’, ‘moderate’ or ‘low’ based on the risk of bias, and the strength of recommendation rated as ‘weak’ or ‘strong’.
| Guidance from IWGDF for management of severe foot infection | IWGDF evidence grade & Strength | |
|---|---|---|
| 1 | Diagnose a soft tissue DFI clinically, based on the presence of local or systemic signs and symptoms of inflammation | (Strong; low) |
| 2 | Consider hospitalization of all patients with diabetes and a severe foot infection and those with a moderate infection that is complex or associated with key relevant morbidities. | (Strong; low) |
| 3 | Non-surgeons should urgently consult with a surgical specialist in cases of severe infection or of moderate infection complicated by extensive gangrene, necrotizing infection, signs suggesting deep (below the fascia) abscess or compartment syndrome, or severe lower limb ischemia. | (Strong; low) |
| 4 | In a patient with probable diabetic foot osteomyelitis with concomitant soft tissue infection, urgently evaluate for the need for surgery as well as intensive post-operative medical and surgical follow-up. (Strong; moderate) but in a patient with uncomplicated forefoot osteomyelitis, you may consider treating with antibiotic therapy without surgical resection of bone | (Strong; moderate) |
| 5 | During surgery to resect bone for diabetic foot osteomyelitis, consider obtaining a specimen of bone for culture (and, if possible, histopathology) at the stump of the resected bone to identify if there is residual bone infection. (Weak; moderate) and if an aseptic collected culture specimen obtained during surgery grows pathogens, or if the histology demonstrates osteomyelitis, administer appropriate antibiotic therapy for up to six weeks | (Strong; moderate) |
| 6 | Diabetic foot osteomyelitis with antibiotic therapy for no longer than 6 weeks. If the infection does not clinically improve within the first 2–4 weeks, reconsider the need for collecting a bone specimen for culture, undertaking (further) surgical resection, or selecting an alternative antibiotic regimen | (Strong; moderate) |
The current literature is heterogeneous and not conductive to a formal analysis; management is based predominantly on surgical/medical principles. We present the clinically relevant assessment, management and surgical treatment and, outcomes based on our review of guidelines and supporting literature.
3.1. Identification of infection requiring surgical management
Identification can commonly divided, into clinical, radiological and microbiological. The key is not to underestimate the extent of damage caused by the speed of deterioration. The infection may appear local but might have already advanced along tendon sheaths and dermal plains. Both conduits of spread will need to be considered to achieve adequate debridement.13 Patients, and non-specialists often cannot judge the severity of their infections and lack concordance.14,15
3.2. History and clinical examination
An adequate history to identify all risk factors is important including previous events and hospital admissions. The clinical examination can be divided into local, limb and systematic review as described below:
-
a.
Local Foot Assessment:
A detailed general and foot examination is vital in a diabetes patient presenting with foot problem. Identification of a pre-existing ulcer can give a possible indication of the aetiology of the ulcer. The location of the ulcer, whether it is plantar, dorsal, medial, lateral or interdigital, must be defined and examined in detail as it may point to an associated tendinous infectious. Physical examination is crucial to identify the point of bacterial entry which is frequently attributed to the presence of an ulcer can lead to a osteomyelitis and spreading infection.16 The probe to bone test has a high degree of sensitivity for diabetic foot osteomyelitis.17
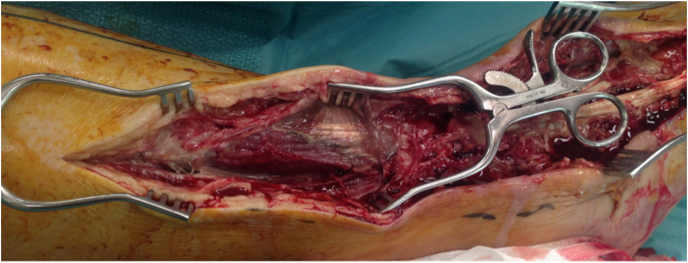
Clinical photographs (with permission) aid monitoring. Clinicians should have a high index of suspicion of spreading tendon sheath infection especially when an ulcer is located close approximation to the tendon (see Fig. 1a). Infections spread through the foot along the tendons and their sheaths as they are poorly vascularized structures.13 Equally diabetic foot infections spread from the plantar to the dorsal aspect of the foot through the interosseous compartment.13 The same group describe deeper infection producing a neutrophilic vasculitis of the digital arteries, subsequent thrombosis and necrosis of the involved toes. In cases complicated by osteomyelitis of the involved metatarsal heads, the joint capsule is violated, and purulence may commonly drain to the dorsum of the foot.
-
b.
Local Limb Assessment
Fig. 1a.
Infection from a proceeding ulcer spreading through the tendon sheaths and during surgical exploration, was found to have purulent material extending from the long extensor of the 5th metatarsal into the common digital sheath of the Flexor Digitorum Longus. Further extension revealed all muscle of the anterior compartment were affected, and further debridement was undertaken, a 5th ray amputation was undertaken at the time of debridement.
The surgeon must be vigilant of signs akin to necrotising fasciitis including erythema, purpuric rash, swelling and tracking pus associated in our experience with streptococcus infection. A focused assessment should include assessment of neuropathy, ischemia and deformity. One should also consider assessing mechanical deformities, e.g., claw toes, prominent metatarsal heads leading to pressure areas that may predispose to ulceration and infection. These may be considered as a long-term problem that would require elective surgical offloading. However, this must not take away from the immediate primary goal of effective infection control.
3.2.1. Systemic review
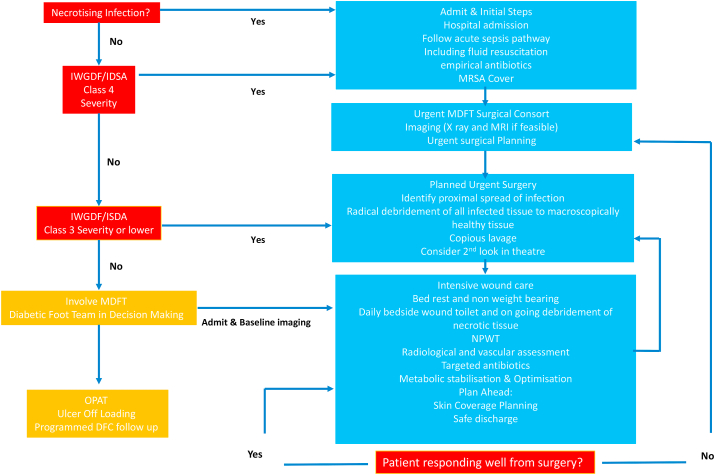
Systemic symptoms (e.g., feverishness and chills), marked leucocytosis or major metabolic disturbances, are uncommon in patients with a DFI, but their presence denotes a more severe, potentially limb-threatening (or even life-threatening) infection.10,17 Systematic signs include Temperature >38 °C or <36 °C; a Heart rate >90 beats/min, Respiratory rate >20 breaths/min or PaCO2 < 4.3 kPa (32 mmHg) and a White blood cell count >12 000/mm3 or <4000/mm3 or >10% immature (band) forms10.17 However, this is not always the case and the surgeon must be careful not to miss a subtle presentation. Many patients with diabetes present with retinopathy, nephropathy and cardiac related complications and these require adequate assessment on presentation and management of these complications.18,19 Fig. 2 outlines the management pathway of diabetic foot infection.
Fig. 2.
Flow chart for the management of diabetic foot attack infections, we have divided the clinical presentation, into obvious necrosis, class 4 and class 3. The differentiating factors between the classes represents the differences between the systemic condition of the patient. In the acute severe foot attack attention is paid to resuscitation and emergent debridement and infection control and in less severe cases planned urgent surgical debridement with imaging can take place and MDFT review.
3.3. Use of radiological investigation
The perceived wisdom is that emergent systematic debridement is required before imaging, however in certain situations surgical debridement is withheld or not freely available, then in these instances additional imaging is required.
Advanced imaging e.g., Computer Tomography (CT) and Magnetic Resonance Imaging (MRI), provide different information useful for the surgeon. The combination of clinical and necessary radiological assessment aims to mark out three zones: necrotic/infected tissue followed by the area of damaged tissue that is likely to be colonized with infecting bacteria, and lastly, the remaining healthy tissue.20 Resection can be guided by radiographic and, time permitting, MRI, that demarcate the extent of tissue destruction. Radiographs will show gas spreading within soft tissue and T1/2 weighted MRI images showing abnormal fluid and cortical bone destruction. Thus, appropriate imaging aids planning of minimum resection and limits of additional and possibly unnecessary exploration prior to entering the operating room (see Fig. 1a, Fig. 1b, Fig. 1ca–c). The differential diagnosis to acute infection, may be a rapidly progressing active Charcot foot or an acutely ischemic foot. The former can be diagnosed with the help of radiographic assessment.
Fig. 1b.
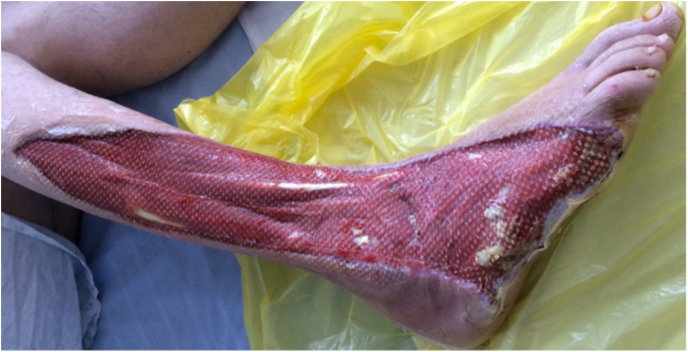
3 weeks after the first debridement, a split skin graft was second debridement was undertaken, in between a second debridement and wound monitoring with bedside podiatric debridement was continued.
Fig. 1c.
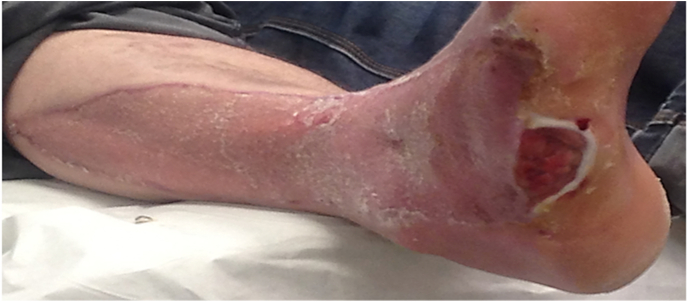
at 1 year the wounds have healed, but a recurrent ulcer had developed as a result of muscle – tendon imbalance. The patient was counselled for definitive reconstructive fusion surgery with a hind foot nail due to hind foot inversion and lateral plantar surface overload.
3.4. Microbiology: are there benefits to wound swabs and is early bone biopsy essential?
IWGDF guidelines suggest that the culture-specific antibiotic therapy is of crucial importance in all diabetic foot infections and IWGDF guidelines strongly recommend appropriate, reliable sampling techniques to determine the causative pathogen.16 In urgent clinical cases, empirical antibiotics must be started. Culture specific antibiotics should be instituted once bacterial isolation and identification has been achieved and are continued until there is clinical and serological evidence of infection eradication. Manas et al. (2020) compared deep wound swap taken from admission through the actively infected tissue against subsequent surgical bone sample taken during surgical debridement.21 Concluding only a fair concordance between the 2 specimens. Unlike, radical tumour surgery, intra-operative bone and tissue sampling during the primary debridement aim to identify the relevant bacteriology.
Modern molecular techniques including PCR may have a role in difficult cases of microbiological diagnostics.22 However, in the acute clinical situation and infection load, expedient surgery resulting in removal of all necrotic bone and infected soft tissue along with quality microbiological analysis of bone and deep tissue should be prioritized.2,23
Studies exploring microbiological characteristics in diabetic foot infection consistently highlight S. aureus as the most prevalent pathogen, as it has a bone cell penetration time of less than 30 min upon exposure22,23,.24 Other important gram-positive organisms include Enterococcus spp. and Streptococcus spp. We have also seen synergistic activity of S. aureus with Group B haemolytic streptococcus in severe infections, demonstrating classical stigmata of vascular purpura and these patients should be treated aggressively.
3.5. Arterial assessment
For practical purposes, vascular assessment is important, but it is not essential to stop emergent debridement. There is also a growing body of evidence suggesting that deformity caused by Charcot neuropathy is associated with peripheral arterial disease (PAD). Sohn et al. (2009)25 reported that PAD was present in 26.9% of US military veterans with Charcot foot. Peripheral arterial disease (PAD) is a independent risk factor for developing ulceration, osteomyelitis and subsequent amputation and a risk factor for mortality in the presence of ulceration.25,26 In chronic cases associated with long standing deformity, Bem et al. (2015)27 estimated the incidence of clinically significant PAD to be as high as 48%. Adequate vascularity will be required to provide an optimal situation for soft tissue and bone healing after debridement.
3.6. MDFT management & time to theatre
There are no specific studies that address the time to theatre in the severe diabetic foot attack, however the IWGDF identified two single-centre studies that investigated the effect of treatment with “early” surgery (variously defined, but usually within 72 h of presentation) versus delayed surgery, 3–6 days after admission.12,28 Both studies, found a significant reduction in LEA with early surgery. Inherent bias, i.e., a lack of randomization of the subjects and lack of standardized protocols for surgical treatment. Therefore, the IWGDF rated the evidence as low.29
Ensuring early surgical debridement of all infected tissue and obtaining bone specimens should be considered a clinical priority, within 24hrs if the CRP is over 100. A reported case series by Ahluwalia et al. (2015) indicated a reduced rate of return to theatre and length of stay with early infection control.30 Further, studies are required to define and understand the implications of blood marker levels, in the acute phase, and a prognostic role in the healing time of DFUs.31,32
3.7. The art of successful debridement
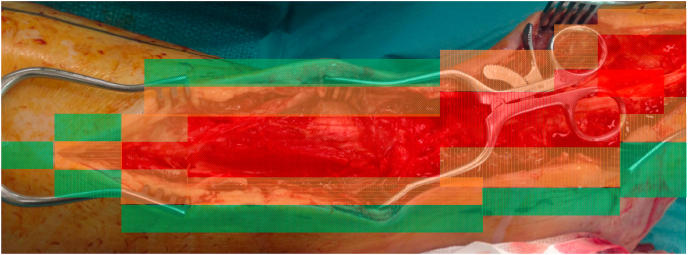
Severe foot infection requires resection of the infected and necrotic tissue, informed by radiology, to achieve eradication of infection and achieve predictable ulcer healing. In 2019, Ahluwalia et al. described the RAG (Red-Amber-Green) model providing a structure for debridement of diabetic foot infection.20 The central part of the infected ulcer with necrotic portion is recognised as the ‘red zone’ and included in routine surgical debridement. The red zone is surrounded by relatively avascular and fibrous tissue that often harbours infection. This is considered as the ‘amber zone’. The pathological area often forms a continuum with the normal healthy tissue - the ‘green zone’. It is essential that all tissue in the red and amber zone is excised completely, to the adjacent green zone of unaffected tissue (Fig. 1c, Fig. 1d shows a clinical example of this process).
Fig. 1d.
Illustration of the red amber and green technique. Each area has been colour-coded to include the different areas for debridement. Exploration must warrant the assessment of all tissues that are related to infection, including the spread along tendon sheaths to muscle bellies and into fascial planes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
This system is applicable to bone debridement. Deep tissue samples obtained from the periphery of the necrotic area, after resection of the red zone, are used for microbiological analysis. Elliptical incisions are preferred to facilitate apposition and accommodate negative pressure wound therapy once the debulking and debridement of dead tissue has been completed. Following radical debridement of tissues, exploration of all involved tendon sheaths up to the muscle belly, lavage with copious amounts of warmed Normal Saline is required. Re-inspection of all wounds is undertaken with a repeat of the above steps as indicated prior to leaving the operating room. Continued bedside wound management and supplementary soft tissue debridement when necessary.30
Protocolisation can reduce length of stay and further theatre debridement. If instability exists in the foot and ankle due to the extent of bone or soft tissue resection temporarily stabilisation either in a windowed/bivalve total contact cast, using a K-wire or an external fixator especially if acute infected Charcot foot is considered. Stability whilst not proven to directly support wound healing is thought to provide a stable environment to allow defects to heal. The authors advocate planning ahead; consider 2nd look at 48 h if clinical improvement is not seen. Further careful re-assessment of the vascularity is always recommended.
3.8. Local antibiotic loading
Local antibiotic eluding calcium sulphate preparations or similar products may be used to fill the bone voids or tissue defects and may be considered for dead-space management. A recent systematic review concluded there is little evidence to support routine local antibiotic delivery devices in the treatment of diabetic foot infections.33
However, local antibiotic delivery yield very high local concentrations of antibiotics in targeted areas, especially in the presence of osteomyelitis: this approach maybe especially useful in poorly perfused tissues and ‘hard-to-reach’ locations. For example, the Silo technique, describes debridement delineated by MRI, drilling of bone tunnels and injection of antibiotic-loaded bio-ceramic, allowing infection control.34 There is a current focus on biodegradable vehicles. However, the only RCT conducted utilizes non-absorbable polymethylmethacrylate impregnated cement in diabetic foot infection. Lipsky et al. (2012) reported a higher proportion of successful treatment in patients randomized to a gentamicin-collagen sponge compared to standard treatment of moderately infected foot ulcers in 38 patients.35 Novel local delivery systems combined with off-loading may aid early healing; however, this should not take away from appropriate debridement.20
3.9. Appropriate post-surgical wound care
Once rapid infection control has been achieved, a standardised evaluation of the clinical condition including assessment of the vascular status, empirical broad spectrum antibiotic treatment, with subsequent targeted antibiotic therapy guided by the intraoperative tissues and bones specimens is performed.16 The principles of strict off-loading, debridement, wound agitation (on going freshening and scrapping of wound edges) and biofilm disruption usually allows a chronic ulcer to heal.
The type of off-loading is dependent on the location of the wound and is achieved commonly by the application of a total contact cast. However, off-loading can also be achieved with a removable knee-high off-loading device, as an aircast boot. A removable ankle-high off-loading device such as heel-weightbearing shoes or plaster booties would offer a third choice.36 Regular wound care and therewith biofilm disruption by agitation is essential for antibiotic therapy to be effective.37 The rationale for serial debridement is to activate senescent cells, stimulate the release of growth factors, remove inflammatory factors and reduce bioburden.38
In conjunction with appropriate wound care as outlined above (including debridement) studies have suggested that NPWT promotes local perfusion and angiogenesis in wounds.39,40 Some authors report the use of NPWT will reduce the frequency of debridement required in diabetic foot wounds, and its application is unlikely to reduce the efficacy of topical antibiotics in a preclinical animal model.41 Assessment and input by the plastic surgeons is sought early. Where required, lower limb revascularization is prioritized to treat the peripheral arterial disease.42 The authors would advocate close monitoring and if infection does not clinically improve, reassessment (incl. vascular tree) and reconsider the need for collecting a bone specimen for culture, undertaking surgical resection and biopsy to ensure antibiotic regimen.
Thereafter, skin grafting, or a similar form of plastic surgical procedure, may be performed to achieve soft tissue coverage of the wound, and follow the reconstructive ladder.41 Residual bony instability or deformity can be managed with a brace or surgical stabilisation. A second stage of definitive fixation may be performed at this time point.
3.10. Dressings and topical treatments and on-going wound care
Little evidence exists to advocate one dressing or wound healing method over another.44 Sucrose-octasulfate dressing for the management of patients with vascular indices above the threshold denoting critical limb ischemia has been demonstrated in a multicentre randomised study.42 Various molecular growth factors43 and supplementary therapies44 are used to stimulate wound healing but there is limited evidence to support their use in routine clinical practice. Indeed, the IWGDF is yet to endorse any of these methods in their wound healing guidance.10 Recurring ulcers or poor healing signify the need for a thorough assessment to exclude an underlying significant vascular compromise or chronic osteomyelitis and/or systematic issues e.g., hypoxia.
3.11. Monitoring and on-going assessment and need for definitive surgical treatment
The aim of surgical debridement is to clear infection and ensure adequate infection control. In order to meet this goal, the surgeon may have to sacrifice functional structures such as tendons. Residual muscular imbalance after debridement, (e.g., excision of the peroneal tendons) can result in a consequent loss of foot shape (Fig. 1a, Fig. 1b, Fig. 1ca and b,c) and this should be monitored. In these cases, one should consider prophylactic surgery e.g. exostectomy and staged surgical reconstruction especially if there is osseous instability and recurrent ulceration.
3.12. Outcomes and limb salvage
There is a paucity of literature, therefore meaningful comparison is not possible in regard to the outcomes after an “acute foot attack”. Most studies reporting clinical outcomes in diabetic foot ulceration invariably contain a composite patient group, with varying pathologies and differing outcome criteria. Registries such as the United Kingdom National Diabetes Foot Audit only report early outcomes to 24 weeks or specifically a cohort of patients with an acute infective foot attack (e.g., IDSA class 3+) who required an urgent surgical debridement. Recent, evidence suggests age>60, the presence of PAD and a higher CRP were key negative predictive factors for a poorer outcome.46,47
4. Summary
Emergent surgical resection of all devitalised tissue is mandatory in the acute diabetic infective foot attack. The key to success is an appreciation of infection load/extent based on current imaging and knowledge of anatomical pathways for infection to spread, and surgical debridement of the infected load. Formal protocol-driven treatment can only be achieved in a joined-up collaboration between surgical, medical and podiatric teams (see Fig. 2). Prospective research is required to collect robust data to support treatment algorithms.
5. Conclusion
This manifestation of a DFI, with significant soft tissue loss is often combined with osseous destruction. We have highlighted key components of surgical care. The role of urgent MDFT assessment, systematic resuscitation, protocolled surgical debridement, stabilisation and long term follow up are required.
References
- 1.Zhang P., Lu J., Jing Y., Tang S., Zhu D., Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49(2):106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 2.Vas P.R.J., Edmonds M. The diabetic foot attack: “Tis too late to retreat! Int J Low Extrem Wounds. 2018;17:7–13. doi: 10.1177/1534734618755582. [DOI] [PubMed] [Google Scholar]
- 3.Edmonds M.E., Foster A.V. Diabetic foot ulcers. BMJ. 2006;332(7538):407–410. doi: 10.1136/bmj.332.7538.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes U.K. Diabetes UK; London, England: 2013. Putting Feet First. Fast Track for a Foot Attack: Reducing Amputations. [Google Scholar]
- 5.Brownrigg J.R., Hinchliffe R.J., Apelqvist J. International Working Group on the Diabetic Foot. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(suppl 1):128–135. doi: 10.1002/dmrr.2704. [DOI] [PubMed] [Google Scholar]
- 6.NHS Digital National diabetes foot care audit—2014-2016. https://digital.nhs.uk/catalogue/PUB23525 Published March 8, 2017.
- 7.Lavery L.A., Armstrong D.G., Murdoch D.P., Peters E.J., Lipsky B.A. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis. 2007;44:562–565. doi: 10.1086/511036. [DOI] [PubMed] [Google Scholar]
- 8.Lipsky B.A., Senneville E. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36(S1) doi: 10.1002/dmrr.3280. [DOI] [PubMed] [Google Scholar]
- 9.Senneville E., Lipsky B.A. Diagnosis of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36(S1) doi: 10.1002/dmrr.3281. [DOI] [PubMed] [Google Scholar]
- 10.NG19 N.I.C.E. Diabetic Foot Problems: Prevention and Management. https://www.nice.org.uk/guidance/ng19/ifp/chapter/Diabetic-foot-infection
- 11.Operational Delivery of the Multi-Disciplinary Care Pathway for Diabetic Foot https://www.bofas.org.uk/Portals/0/newsfiles/DiabeticFoot%20FINAL.pdf?ver=2016-05-23-204311-560.
- 12.Tan J.S., Friedman N.M., Hazelton-Miller C., Flanagan J.P., File T.M., Jr. Can aggressive treatment of diabetic foot infections reduce the need for above-ankle amputation? Clin Infect Dis. 1996;23:286–291. doi: 10.1093/clinids/23.2.286. [DOI] [PubMed] [Google Scholar]
- 13.Aragón-Sánchez J., Lázaro-Martínez J.L., Pulido-Duque J., Maynar M. From the diabetic foot ulcer and beyond: how do foot infections spread in patients with diabetes? Diabet Foot Ankle. 2012;3 doi: 10.3402/dfa.v3i0.18693. Epub 2012 Oct 1. PMID: 23050067; PMCID: PMC3464072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manu C., Lacopi E., Bouillet B. Delayed referral of patients with diabetic foot ulcers across Europe: patterns between primary care and specialised units. J Wound Care. 2018;27(3) doi: 10.12968/jowc.2018.27.3.186. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Ríos J.P., García-Klepzig J.L., Manu C., Ahluwalia R. Referral of patients with diabetic foot ulcers in four European countries: patient follow-up after first GP visit. J Wound Care. 2019;28(No) doi: 10.12968/jowc.2019.28.Sup8.S4. Sup8 Practice. [DOI] [PubMed] [Google Scholar]
- 16.Lavery L.A., Crisologo P.A., La Fontaine J., Bhavan K., Oz O.K., Davis K.E. Are we misdiagnosing diabetic foot osteomyelitis? Is the gold standard gold? J Foot Ankle Surg. 2019;58:713–716. doi: 10.1053/j.jfas.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsky B.A., Aragon-Sanchez J., Diggle M. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):45–74. doi: 10.1002/dmrr.2699. [DOI] [PubMed] [Google Scholar]
- 18.Al-Maskari F., El-Sadig M. Prevalence of risk factors for diabetic foot complications. BMC Fam Pract. 2007;8:59. doi: 10.1186/1471-2296-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pradeepa R., Anjana R.M., Unnikrishnan R., Ganesan A., Mohan V., Rema M. Risk factors for microvascular complications of diabetes among South Indian subjects with type 2 diabetes: the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-5. Diabetes Technol Therapeut. 2010;12:755–761. doi: 10.1089/dia.2010.0069. [DOI] [PubMed] [Google Scholar]
- 20.Ahluwalia R., Vainieri E., Tam J. Surgical diabetic foot debridement: improving training and practice utilizing the traffic light principle. Int J Low Extrem Wounds. 2019;18:279–286. doi: 10.1177/1534734619853657. [DOI] [PubMed] [Google Scholar]
- 21.Manas A.B., Taori S., Ahluwalia R. The International Journal of Lower Extremity Wounds; 2020. Admission Time Deep Swab Specimens Compared with Surgical Bone Sampling in Hospitalized Individuals with Diabetic Foot Osteomyelitis and Soft Tissue Infection. [DOI] [PubMed] [Google Scholar]
- 22.Nelson A., Wright-Hughes A., Backhouse M.R. CODIFI (Concordance in Diabetic Foot Ulcer Infection): a cross-sectional study of wound swab versus tissue sampling in infected diabetic foot ulcers in England. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-019437. e019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aragon-Sanchez J., Lipsky B.A., Lazaro-Martinez J.L. Diagnosing diabetic foot osteomyelitis: is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet Med. 2011;28:191–194. doi: 10.1111/j.1464-5491.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu K., Song L., Kang H.P., Kwon H.-K., Back J., Lee F.Y. Recalcitrant methicillin-resistant Staphylococcus aureus infection of bone cells: intracellular penetration and control strategies. Bone Joint Res. 2020;16(9):49–59. doi: 10.1302/2046-3758.92.BJR-2019-0131.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohn M.W., Lee T.A., Stuck R.M., Frykberg R.G., Budiman-Mak E. Mortality risk of Charcot arthropathy compared with that of diabetic foot ulcer and diabetes alone. Diabetes Care. 2009;32:816–821. doi: 10.2337/dc08-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorley P.J., Sheard K.L., Sivananthan U.M. Limb blood flow measurement in technically successful balloon angioplasty. Br J Radiol. 1994;67:764–769. doi: 10.1259/0007-1285-67-800-764. [DOI] [PubMed] [Google Scholar]
- 27.Bem K., Jirkovska A., Dubsky M., Woskova V., Fejfarova V. Presented at the 25th World Congress of the International Union of Angiology. September 2015. Charcot neuropathic osteoarthropathy and peripheral arterial disease; pp. 89–90. [Google Scholar]
- 28.Faglia E., Clerici G., Caminiti M., Quarantiello A., Gino M., Morabito A. The role of early surgical debridement and revascularization in patients with diabetes and deep foot space abscess: retrospective review of 106 patients with diabetes. J Foot Ankle Surg. 2006;45:220–226. doi: 10.1053/j.jfas.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Peters E.J.G., Lipsky B.A., Senneville E. Interventions in the management of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36(S1) doi: 10.1002/dmrr.3282. [DOI] [PubMed] [Google Scholar]
- 30.Ahluwalia R., Francis S., Tremlett J. ISDF; 2015. Surgical Management of the Neuropathic Acute Foot-Attack – the Value of C-Reactive Protein in Prioritizing the ’door to Table’ Time.https://isdf.nl/wp-content/uploads/2016/06/Poster-session-16-Surgery.pdf Poster. [Google Scholar]
- 31.Tardáguila-García A., García-Álvarez Y., Sanz-Corbalán I., Álvaro-Afonso F.J., Molines-Barroso R.J., Lázaro-Martínez J.L. Role of inflammatory markers in the healing time of diabetic foot osteomyelitis treated by surgery or antibiotics. J Wound Care. 2020 Jan 2;29(1):5–10. doi: 10.12968/jowc.2020.29.1.5.PMID:31930948. [DOI] [PubMed] [Google Scholar]
- 32.Tardáguila-García A., García Álvarez Y., García-Morales E., Álvaro-Afonso F.J., Sanz-Corbalán I., Lázaro-Martínez J.L. Utility of blood parameters to detect complications during long-term follow-up in patients with diabetic foot osteomyelitis. J Clin Med. 2020 Nov 22;9(11):3768. doi: 10.3390/jcm9113768.PMID:33266483. PMCID: PMC7700132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marson B.A., Deshmukh D. J. C. Grindlay. A systematic review of local antibiotic devices used to improve wound healing following the surgical management of foot infections in diabetics. Bone Joint Lett J. 2018;100-B doi: 10.1302/0301-620X.100B11.BJJ-2018-0720. 1409–15. [DOI] [PubMed] [Google Scholar]
- 34.Drampalos E., Mohammad H.R., Kosmidis C. Single stage treatment of diabetic calcaneal osteomyelitis with an absorbable gentamicin-loaded calcium sulphate/hydroxyapatite bio-composite: the Silo technique. Foot (Edinb) 2018 Mar;34:40–44. doi: 10.1016/j.foot.2017.11.011. Epub-2017 Nov 23. [DOI] [PubMed] [Google Scholar]
- 35.Lipsky B.A., Kuss M., Edmonds M.E., Reyzelman A., Sigal F. Topical application of a gentamicin-collagen sponge combined with systemic antibiotic therapy for the treatment of diabetic foot infections of moderate severity: a randomized, controlled, multicenter clinical trial. J Am Podiatr Med Assoc. 2012;102:223–232. doi: 10.7547/1020223. [DOI] [PubMed] [Google Scholar]
- 36.Bus S.B., Armstrong D.G., Gooday C. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36(S1) doi: 10.1002/dmrr.3274. [DOI] [PubMed] [Google Scholar]
- 37.Senneville E., Robineau O. Treatment options for diabetic foot osteomyelitis. Expet Opin Pharmacother. 2017;18(8):759–765. doi: 10.1080/14656566.2017.1316375. [DOI] [PubMed] [Google Scholar]
- 38.a Kim P.J., Attinger C.E., Bigham T. Clinic-based debridement of chronic ulcers has minimal impact on bacteria. Wounds. 2018;30(5):114–119. [PubMed] [Google Scholar]; b Armstrong D.G., Lavery L.A. Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 39.a Nain P.S., Uppal S.K., Garg R., Bajaj K., Garg S. Role of negative pressure wound therapy in healing of diabetic foot ulcers. J Surg Tech Case Rep. 2011;3:17–22. doi: 10.4103/2006-8808.78466. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shiels S.M., Sgromolo N.M., W, enke J.C. Negative pressure wound therapy does not diminish efficacy of topical antibiotic powder in a preclinical contaminated wound model. Bone Joint Res. 2021;10:149–155. doi: 10.1302/2046-3758.102.BJR-2020-0171.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.a Blume P.A., Walters J., Payne W., Ayala J., Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2007;31(4):631–636. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]; b Bateman A.H., Bradford S., Hester T.W. Modern orthopedic inpatient care of the orthopedic patient with diabetic foot disease. Int J Low Extrem Wounds. 2015 Dec;14(4):384–392. doi: 10.1177/1534734615596114. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald O’Connor E.J., Vesely M., Holt P.J., Jones K.G., Thompson M.M., Hinchliffe R.J. A systematic review of free tissue transfer in the management of non-traumatic lower extremity wounds in patients with diabetes. Eur J Vasc Endovasc Surg. 2011 Mar;41(3):391–399. doi: 10.1016/j.ejvs.2010.11.013. Epub 2010 Dec 16. PMID: 21163675. [DOI] [PubMed] [Google Scholar]
- 42.Edmonds M.E., Lazaro-Martinez J.L., Alfayate-Garcia J.M. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):186–196. doi: 10.1016/S2213-8587(17)30438-2. [DOI] [PubMed] [Google Scholar]
- 43.Edmonds M.E., Bodansky H.J., Boulton A.J. Multicenter, randomized controlled, observer-blinded study of a nitric oxide generating treatment in foot ulcers of patients with diabetes - ProNOx1 study. Wound Repair Regen. 2018;26:228–237. doi: 10.1111/wrr.12630. [DOI] [PubMed] [Google Scholar]
- 44.Sridharan K., Sivaramakrishnan G. Growth factors for diabetic foot ulcers: mixed treatment comparison analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(3):434–444. doi: 10.1111/bcp.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vainieri E., Ahluwalia R., Slim H. Outcomes after emergency admission with a diabetic foot attack indicate a high rate of healing and limb salvage but increased mortality: 18-month follow-up study. Exp Clin Endocrinol Diabetes. 2020 Dec 22 doi: 10.1055/a-1322-4811. Epub ahead of print. PMID: 33352595. [DOI] [PubMed] [Google Scholar]
- 47.Ahn J., Raspovic K.M., Liu Gt. Lower extremity necrotizing fasciitis in diabetic and nondiabetic patients: mortality and amputation. Int J L Extrem Wound Jour. 2019 Jun;18(2):114–121. doi: 10.1177/1534734619836464. Epub-2019 Apr-1. PMID: 30929530. [DOI] [PubMed] [Google Scholar]