Abstract
Background
Pulmonary embolism (PE) and acute ischemic stroke (AIS) are common disorders with high morbidity and mortality, rarely presenting simultaneously. There is a paucity of data regarding the management of this uncommon presentation. The treatment of these two entities is complex in the acute phase due to the concomitant need for thrombolysis in AIS and anticoagulation for PE.
Methods
We retrospectively reviewed confirmed ischemic stroke cases to identify patients presented with simultaneous PE from June 2018 to May 2019. Additionally, a literature review was performed. Two reviewers assessed the manuscripts' quality, and relevant data regarding clinical course and management was extracted.
Results
We reviewed 439 patient charts, identifying two cases of concomitant AIS and PE. Additionally, twelve articles (n = 15 subjects) fulfilled our literature review criteria for a total of 17 cases, including ours. Intravenous anticoagulation (70.5%) was the most frequent intervention targeting both disorders. Therapies such as intravenous thrombolysis (23.53% (n = 4)) and mechanical thrombectomy (23.53% (n = 4)) were specific in AIS. Catheter-directed thrombolysis (5.88%) was used for PE. Clinical outcomes were favorable (asymptomatic or mild disable symptoms) in 47.05% (N = 8) of patients, while 41.17% had poor outcomes (severe disable symptoms or death).
Conclusions
AIS and PE stand for a challenge when they present simultaneously. The evaluation of risks and benefits of therapies such as intravenous thrombolysis, mechanical thrombectomy, and catheter-directed-thrombolysis in the clinical context is essential. According to our review, the ischemic stroke burden guides systemic anticoagulation decisions over interventional procedures when the hemodynamic status remains unaffected.
Keywords: Ischemic stroke, Pulmonary embolism
Highlights
-
•
Acute ischemic stroke incidence after a pulmonary embolism is approximately 1–10%.
-
•
Concomitant ischemic stroke and pulmonary embolism presentation remain infrequent.
-
•
PFO, prolonged immobility, and hypercoagulable states are common risk factors.
-
•
Frequent symptoms are hemiparesis/hemiplegia, aphasia, tachycardia, tachypnea and hypoxemia.
-
•
Management with systemic anticoagulation and interventional procedures is challenging.
1. Introduction
Pulmonary embolism (PE) and acute ischemic stroke (AIS) are common disorders with high morbidity and mortality, rarely presenting together. Theoretically, PEs and AIS can co-occur as a result of a paradoxical embolism. The most common scenario encompasses the lower extremities deep venous thrombus (DVT) embolizing to the lungs, with the subsequent right to left cardiac/pulmonary shunt resulting in cerebral infarction.
The treatment of these two entities is complex due to the concurrent need for intravenous thrombolysis in AIS in the first hours of symptoms onset and systemic anticoagulation for PE, which is contraindicated after pharmacological thrombolysis in stroke due to increased risk of intracranial bleeding. A review is indicated as there are no specific guidelines for the management of this unusual presentation.
AIS's management is based on the rapid application of intravenous recombinant tissue plasminogen activator (IV rt-PA) up to 4.5 h and mechanical thrombectomy up to 24 h of symptoms onset when indicated [1]. In the same way, PE treatment is based on the quick administration of anticoagulants to hinder clot progression and new clot formation. Although, in theory, both therapies have a similar underlying physiologic target and goal, the intricates of each treatment protocol adds an extra layer of challenge. Although rt-PA is relatively safe and the risk of adverse events is low, when they do occur, it can be devastating, particularly by the presence of symptomatic intracerebral hemorrhage and extracranial bleeding [2]. Therefore, suitable candidates for this therapy should be carefully selected. On the other hand, in patients with PE, the risk of pulmonary hemorrhagic necrosis is still not well established as a complication of rt-PA administration. Furthermore, patients who undergo acute rt-PA administration should avoid the simultaneous use of antithrombotic, which is contradictory to acute PE management guidelines, as patients should be started on anticoagulation therapy as soon as possible [3].
All of the above makes the treatment of this entity (AIS + PE) extremely challenging; therefore, a multidisciplinary approach involving vascular neurology, interventional radiology, cardiology, and cardiothoracic surgery should be sought to achieve better outcomes [4]. Our objective is to review the current evidence on this matter and establish/identify frequent pathways followed when approaching this complicated situation.
2. Methods
This manuscript consists of two parts: (1) a retrospective chart review and (2) a review of the current literature.
2.1. Retrospective chart study
We retrospectively reviewed the chart of adult patients whose an acute ischemic stroke with concomitant PE was diagnosed at the University of Chicago Medical Center between June 2018 to May 2019.
We included only cases in which the diagnosis of PE was simultaneous or within the AIS's prior or subsequent 72 h. The purpose was to detect instances in which an asymptomatic event was present but not identified on presentation. We excluded patients in which PE was diagnosed >72 h as we considered that it was a consequence of the AIS and not a simultaneous event. The local Institutional Review Board approved the study. Informed consent was waived.
2.2. Retrospective chart review
For the retrospective analysis at UCMC, patient charts were reviewed, and the authors extracted data. We collected the following variables: baseline demographics, vascular risk factors, antithrombotics, time from symptom onset (last-known-well), baseline functional status according to the modified Rankin Scale (mRS), stroke severity according to the National Institutes of Health Stroke Scale (NIHSS), acute interventions for recanalization including intravenous thrombolysis (rt-PA), mechanical thrombectomy and catheter-directed thrombolysis, final diagnosis and clinical outcomes (discharge mRS, discharge disposition). The vascular neurologist confirmed the definitive diagnosis for AIS, and the internist established PE. The authors reviewed the diagnosis of PE independently for the accuracy of the study.
2.3. Literature review
We performed a literature review in the databases “PubMed” and “Google Scholar” using the following Medical Subject Headings (mesh) terms and Boolean Operators: ((acute ischemic stroke) OR (ischemic stroke) AND (pulmonary embolism) OR (lung-embolism) AND simultaneous OR associated with identifying articles that included patients with the simultaneous diagnosis of AIS and PE.
2.4. Inclusion and exclusion criteria
We added articles that fulfilled the following inclusion criteria to the analysis: 1). Subjects diagnosed with an AIS and PE; 2) the diagnosis was either simultaneous or within 72 prior or subsequent hours (as to include a potential subject that arrived with a silent or asymptomatic PE/Stroke on admission); 3) case reports, case series, clinical trials, and cohort studies; 4). A clinician confirmed the diagnosis with the addition of imaging studies corroborating the findings. We excluded metanalysis, review articles, and other types of publications. We banned grey literature from our search as well. No restrictions were applied in the year of publication or language. We inspected all studies' references to decrease publication bias and avoid potential missed articles in the electronic search.
2.5. Review of the literature and data extraction
The manuscripts retrieved were screened for potential eligibility initially by title and abstract analysis, then the full text of potentially eligible studies was screened for appropriateness of inclusion (Fig. 1). After reviewing in detail the included articles, the authors independently identified and collected the following variables: demographics (age, gender, race), chief complaint, past medical history, risk factors, admitting symptom, initial general and neurological exam, follow up general and neurological exam, imaging, laboratories, admission NIHSS and mRS, management, disposition and discharge NIHSS and mRS.
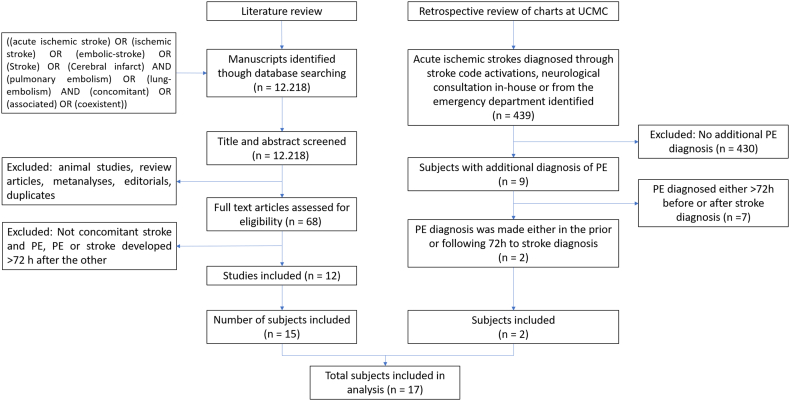
Fig. 1.
Literature review and retrospective chart review flow charts.
2.6. Quality assessment and risk of bias analysis)
To assess the studies' quality, we applied the checklist developed by Murad et al. (2018) following the standard procedure described by the authors [5]. This tool was designed to assess case reports and case series and evaluates eight questions encompassed in four main domains: selection, ascertainment, causality, and reporting. We assigned a value of zero if they did not detail and justify a question and one if they do so. Then, we calculated the total score per each study; a higher number corresponded to high quality. The quality was assessed by the authors independently.
3. Results
3.1. Retrospective chart review results
A total of 439 confirmed stroke cases were reviewed, identifying nine individuals that had an additional diagnosis of PE; among those, seven had a PE either >72 h before or > 72 h after the stroke diagnosis; therefore, they were not included in this analysis as it was considered not a concomitant presentation. We only identified two cases of simultaneous AIS and PE; both are described below.
3.2. Case 1
A 72-year-old African American woman with a history of hypertension was brought to the Emergency Department (ED) after collapsing at home. Upon arrival, she was comatose (GCS = 5), had flaccid quadriplegia, and no gag reflex requiring emergent intubation (NIHSS = 26). Vital signs were remarkable for a blood pressure of 173/101 mmHg, heart rate (HR) 98 bpm, temperature 36C, respiratory rate (RR) 26 pm, and oxygen saturation (O2Sat) of 91%. An initial non-contrast CT head was unremarkable. Rapidly after, she became hypotensive and went into a cardiac arrest requiring cardiopulmonary resuscitation until achieving the return of spontaneous circulation at 13 min.
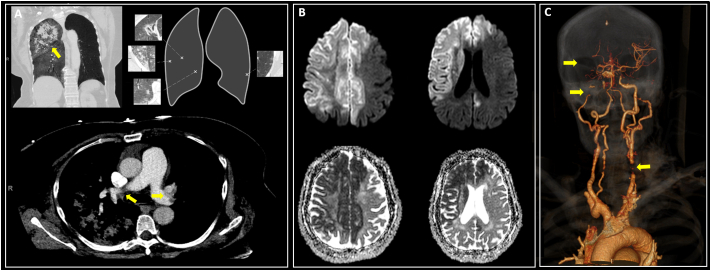
Given the persistent flaccid quadriplegia, an urgent CT angiogram (CTA) of the intracranial and extracranial vasculature was obtained, revealing a right internal carotid artery (ICA) occlusion. Subsequently, she had several bouts of hemoptysis accompanied by hypoxemia and tachycardia. A CT pulmonary angiogram (CTPA) demonstrated an acute PE involving the distal left main pulmonary artery and bilateral lobar, segmental, and subsegmental branches associated with a right pulmonary hemorrhage (Fig. 2a). In the setting of severe hemodynamic instability, pulmonary hemorrhage, and PE, IV rt-PA administration was deferred due to the presence of active bleeding. A brain-MRI revealed a large right-hemispheric cerebral infarction in the right middle cerebral artery (MCA) and bilateral anterior cerebral artery (ACA) vascular territories (Fig. 2b). We deferred the systemic anticoagulation for PE management in light of a massive ischemic stroke and the high risk of hemorrhagic transformation. A transthoracic echocardiogram (TTE) demonstrated an atrial septum aneurysm with a patent foramen ovale (PFO) through the injection of agitated saline contrast (bubble study). Also, there was no underlying malignancy detected on the chest, abdomen, and pelvis tomography, nor a DVT on the lower extremity duplex. The patient continued deteriorating despite full medical support and died five days after.
Fig. 2.
A: Bilateral PEs and right lobar hemorrhage in CT; B: DWI changes suggestive AIS; C: CTA embolic occlusion in bilateral ICAs and R-MCA.
3.3. Case 2
A 75-year-old African American woman with hypertension was brought to the ED after being found down. On arrival, she had mild dysarthria, right arm paresis (NIHSS = 2) and, complained of severe pleuritic pain and shortness of breath. Vital signs were remarkable for a blood pressure of 159/75 mmHg; HR95bpm, RR40pm, and O2Sat80%. She was emergently intubated due to hypoxia. Laboratories were unremarkable except for an elevated D-dimer. A non-contrast CT head demonstrated a left frontal lobe hypodensity. A CTA of the intracranial and extracranial vasculature showed a stenotic left-MCA.
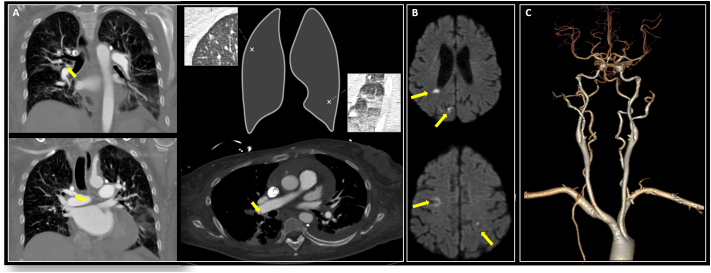
Additionally, a CTPA showed a massive bilateral PE (Fig. 3a). The last time seen well was unclear; therefore, IV rt-PA for management of AIS was not offered. However, catheter-directed thrombolysis (EKOS Ultrasound catheter) with local arterial rt-PA infusion was performed for 48 h, followed by heparin drip for PE management. A brain-MRI showed acute bilateral supra/infra-tentorial cerebral infarctions (Fig. 3b); an additional workup revealed an acute left popliteal and peroneal DVTs with a fluttering thrombus. A TTE displayed a PFO through injection of contrast (bubble study), a severely dilated right ventricle with reduced performance, and no atrial septal aneurysm.
Fig. 3.
A: Bilateral PEs CT; B: Bilateral DWI changes revealing AIS; C: Normal CTA-3D reconstruction.
Further workup for underlying malignancy was unrevealing. On day 3 of admission, a CTPA revealed a significant decrease in the clot burden and the distal lungs' robust perfusion. The patient was extubated and had a marked improvement in her pulmonary and neurological status. She was discharged on oral anticoagulation.
4. Literature review results
Sixty-eight manuscripts were derived from our initial search. After excluding and reviewing the articles, 12 fulfilled our criteria, with 15 patients having concomitant AIS and PE, in addition to our two in-house patients for a total of seventeen cases (Table 1). Overall, the mean age was 54.11 years (SD18.49); female sex was predominant (82.35%), the race was not described for most patients. Demographic, medical, and clinical characteristics are presented in Table 2.
Table 1.
Summary of eligible studies.
| Author | Age/Gender | Stroke location | PE location (Pulmonary artery) | Management | Outcomes |
|---|---|---|---|---|---|
| Lapostolle, et al. 2003 [51] | 53-yo-F | R-MCA | Bilateral | IVC filter; IV-heparin | Severe symptoms |
| 67-yo-F | L-MCA | Bilateral | IV-heparin | Severe symptoms | |
| 51-yo-F | L-MCA | Bilateral | IV-heparin | Dead | |
| 56-yo-M | L-MCA | Not reported | IV-heparin | Severe symptoms | |
| Belvís, et al. 2004 [11] | 36-yo-F | L-MCA | Bilateral segmental/subsegmental | IV-heparin | Mild symptoms |
| Bracey, et al., 2005 [52] | 29-yo-M | L-MCA | Bilateral | rt-PA for PE. | Dead |
| Iwanaga, et al., 2007 [48] | 84-yo-F | Bilat-MCA | Bilateral lobar | IV-heparin | Not reported |
| Allport & Butcher, 2008 [47] | 47-yo-F | L-MCA | Bilateral lobar | rt-PA; IV-heparin | Mild symptoms |
| Pavesi, et al., 2008 [50] | 65-yo-F | R-MCA | Bilateral main | rt-PA; IV-heparin, PFO closure | Asymptomatic |
| Pelletier, et al., 2010 [49] | 35-yo-F | L-MCA | Bilateral main | rt-PA, IVC filter, IV-heparin | Mild symptoms |
| Naidoo & Hift, 2011 [24] | 38-yo-F | L-MCA | Bilateral main | Streptokinase | Mild symptoms |
| Gunta & Kamath, 2012 [53] | 16-yo-F | L-MCA | Bilateral lobar | IV-heparin, IVC-filter, MT | Mild symptoms |
| Omar, et al., 2013 [4] | 69-yo-M | L-MCA | Unilateral segmental/subsegmental | MT, IVC-filter | Severe symptoms |
| Christiansen, et al., 2017 [22] | 59-yo-F | R-MCA | Bilateral lobar | rt-PA, MT, IVC filter, IV-heparin | Asymptomatic |
| Barros-Gomes, et al., 2017 [20] | 68-yo-F | L-ICA | Bilateral | MT | Not reported |
| Saleh Velez & Ortiz-Garcia, 2020 | 72-yo-F | R-ICA | Bilateral | Medical Therapy | Dead |
| 75-yo-F | L-MCA | Bilateral main pulmonary artery | Catheter-directed-Thrombolysis, IV-heparin | Asymptomatic |
Table 2.
Subjects demographics characteristics.
| Characteristic | Simultaneous AIS + PE (n = 17) |
|---|---|
| Mean age(SD), year | 54.11 (18.49) |
| Woman, no. (%) | 14 (82.35%) |
| Race, no. (%) | |
| Unknown/not reported | 15 (76.47%) |
| Black | 2 (11.76%) |
| White | 2 (11.76%) |
| Medical history, no. (%) | |
| Recent intercontinental flight | 7 (41.17%) |
| Hypertension | 4 (23.52%) |
| Tobacco Use | 3 (17.64%) |
| Stroke | 2 (11.76%) |
| Cancer | 2 (11.76%) |
| Recent surgery | 2 (11.76%) |
| Pulmonary embolism | 1 (5.88%) |
| Medications at presentation,no.(%) | |
| Oral contraceptives | 2 (11.76%) |
| Hormonal replacement therapy | 2 (11.76%) |
| Aspirin | 1 (5.88%) |
| Tamoxifen | 1 (5.88%) |
| Antiretrovirals | 1 (5.88%) |
4.1. Quality assessment results
We evaluated the quality of the 12 studies. We included the score ranged from 4 to 8 (out of 8 possible points). They mostly did not adequately report the causality domain as there was a lack in detail regarding the dose-response and follow up period in all manuscripts. Additionally, the ascertainment domain was also not well reported in 10 articles as they were missing data regarding the measured outcomes.
4.2. Epidemiology and risk factors
No clinical trials or observational studies are evaluating the incidence or prevalence of concomitant AIS and PE. It is estimated that AIS incidence after a PE is approximately 1–10%, and PEs are the leading cause of death in the first 2–4 weeks post-stroke [[6], [7], [8]]. However, they rarely co-occur. Few attempts have been made to account for the concomitant appearance of AIS and PE. Clergeau et al. performed MRIs in hospitalized patients with a diagnosis of acute PE to evaluate for asymptomatic AIS revealing restriction on diffusion-weighted imaging (DWI) in 6 out of 60 patients, and 25% (n = 15) had a PFO [7]. Among those patients with DWI changes, five patients had a silent stroke with an underlying PFO, and only one patient had restricted diffusion in DWI without a PFO [7]. The same authors performed a logistic regression analysis showing that PFO was an independent predictor of silent AIS in these patients [7]. These findings support the hypothesis that distal clots travel, affecting both lung and brain tissue [7].
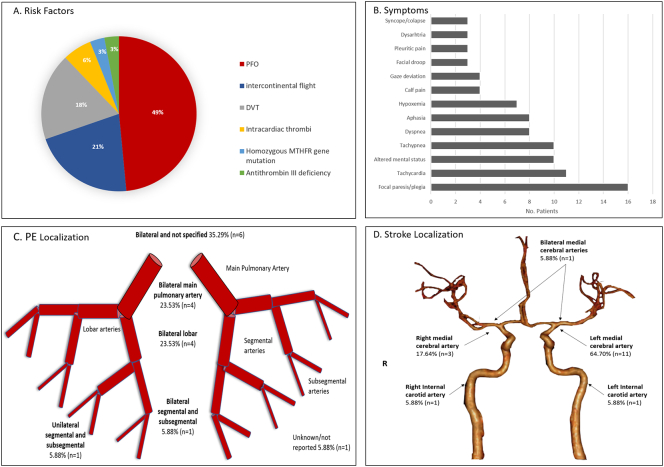
Both disorders' pathophysiological mechanism suggests that any independent factor that increases each event's risk plays an important role [9,10]. Therefore, factors such as dilated cardiomyopathy, prolonged immobility (i.e., long flight, bedbound), oral contraceptives, smoking, cancer, and hypercoagulable states are relevant [9,10]. However, most of the patients with either AIS or PE do not present with simultaneous disorders; therefore, additional elements might play a role. PFOs are common congenital cardiac anomalies, seen in approximately 25% of adults. An interatrial defect provides a feasible pathway for the passage of embolic material traveling from distant regions [7,11]. The incidence of ischemic stroke has been described in up to 16–33% among patients with PFO [7,12] and patients with cryptogenic stroke have found to have PFO in up to 40–50% of the times [11,13]. The etiology most likely is via paradoxical embolization of a systemic venous thrombus into the systemic arterial circulation [14,15]. Similar to that, our review showed that 94.11% (n = 16/17) subjects had a PFO. Among six different types of risk factors encountered in our patients, PFO (49%) and intercontinental flight (21%) were the most frequent (Fig. 4a), indicating that these two elements are crucial in simultaneous AIS and PE.
Fig. 4.
A: Risk factors; B: Clinical manifestation; C: Pulmonary vascular distribution of the PEs; D: Vascular distribution of AIS.
4.3. Clinical presentation
Thromboembolic events such as AIS and PE have unique clinical presentations [16,17]. The presence of neurological deficits and impaired consciousness is not frequent in patients with PE, while hemoptysis and dyspnea are uncommon in stroke. Therefore, the combination of respiratory and neurological symptoms should raise the concern of a simultaneous process. We found focal paresis/plegia (94.11%), altered mental status (58.82%) and aphasia (47.05%) as the most frequent neurological symptoms; whereas tachycardia (64.70%), tachypnea (58.82%), dyspnea (47.05%) and hypoxemia (41.17%) for PE (Fig. 4b).
4.4. Imaging
Imaging studies are necessary to make a definite diagnosis of both pathologies, being non-contrast brain-MRI and CTPA, respectively, the standards to identify and localize the injured vascular territory in both disorders (Fig. 4c, d). Interestingly, a transthoracic echocardiogram (TTE) with agitated saline contrast provides essential information regarding the potential etiology of the AIS, assessing for the presence of PFO (bubble study) or intra-atrial thrombi, while providing information on PE severity due to identification of right heart strain, and giving prognostic information. Furthermore, the Transesophageal echocardiogram increases the sensitivity and specificity to detect thrombi and PFO when TTE is unclear [[18], [19], [20]]. Other relevant tests include lower extremity ultrasound to identify DVTs' presence, which should always be considered in this scenario. Moreover, for elderly subjects, there should be regarded as the addition of occult malignancy workup (e.i, pan scan) and pelvic MR Venogram to assess for May-Thurner syndrome (MTS). There was little to no information about the frequency of testing for both occult malignancy and MTS in our search, which could explain that several patients had PFO and DVTs.
4.5. Management
The current guidelines are specialty-specific and do not address this unusual situation. In the acute phase, management modalities include anticoagulation, systemic intravenous thrombolysis, intra-arterial thrombolysis (when intravenous thrombolysis carries an excessive systemic bleeding risk), IVC filter placement, and endovascular pulmonary/cerebral embolectomy [21].
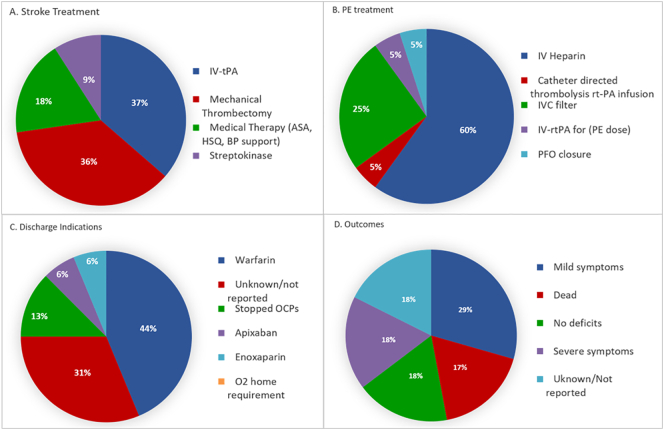
Our review revealed a dichotomized treatment, either priming the brain or the lungs as the primary target. We found that 23.52% (n = 4) received IV rt-PA while 23.52% underwent mechanical thrombectomy (MT) to manage cerebral infarction. A single patient underwent both [22], while the remainder were managed medically and with supportive measures due to the severity of both injuries and hemodynamic instability. Most of the patients that underwent IV rt-PA waited for 24 h to start infusion with unfractionated-heparin for PE management, being this the most common therapeutic approach (64.70%) in our analysis (Fig. 5a, b). One patient had an intracranial hemorrhage as a complication post-MT. Most patients were treated with IV-heparin only, despite a low NIHSS, deeming low risk of hemorrhagic conversion for rt-PA. Acute thrombolysis played a significant role in AIS therapy. From a neurological standpoint, the outcomes were favorable, showing either a complete recovery in 75% of the patients (n = 3/4) who underwent rt-PA or only minimal disabled symptoms. On the other hand, patients treated with only IV-heparin had less favorable neurological outcomes (Fig. 5c, d).
Fig. 5.
A: Stroke treatments; B: PE treatments; C: Discharge indications; D: Patient's outcomes.
Overall, this review's heterogeneous treatment strategies are likely due to the lack of treatment guidelines for simultaneous disease. For the case of AIS, current stroke guidelines advise delaying anticoagulation for two weeks post-event in patients with atrial fibrillation that will require lifelong anticoagulation [3]. This deferred initiation on anticoagulation is opposite to the treatment indicated in patients post PE as the standard of care is the immediate initiation of anticoagulation therapy. In this regard, it is well established that a delay in more than 24 h to initiate anticoagulation in PE leads to a 3-fold increase in mortality [23], hence the challenge of deciding whether or not to acutely start anticoagulation for PE patients with concomitant AIS due to the increased risk of hemorrhagic transformation. Even more challenging when a patient has been deemed a candidate for acute IV rt-PA, anticoagulation initiation should be deferred 24 h post-rt-PA [1]. Therefore, other alternative techniques should be considered for PE management, such as catheter-directed thrombolysis. In scenarios in which the risk of rt-PA exceeds its benefits, MT and endovascular therapy with stent retriever should be highly considered [24].
In massive PE, there is clear indication to reverse right ventricular pressure overload and potential fulminant cardiac failure with emergent recanalization of the pulmonary vasculature by either mechanical thrombolysis or pharmacological thrombolysis [24,25]. Therefore, in large AIS cases and high risk of hemorrhagic conversion, the former option (IV rt-PA) should be considered immediately. Guidelines for thrombolytic therapy in PE state as absolute contraindication the presence of a stroke in the previous six months [26]; however, further consensus guidelines indicate that despite being absolute contraindications, any listed contraindication should be deemed relative when there is a life-threatening situation associated with the PE [24].
The possibility of performing concomitant thrombolysis at two different and distant vascular territories is an unprecedented event. For obvious reasons, a too complicated clinical picture was only reported once in the literature [4]. The main issue relays in the significant differences in dosage, duration, and protocol administration recommendations between AIS (i.e., AIS 0.9 mg/kg, 10% in 1 min and 90% 1 h infusion) and PE (alteplase for PE in patients ≥65 kg, loading bolus 10 mg over 1–2 min, followed by 90 mg over 2 h) [27,28]. Therefore, a case-by-case approach should be applied depending on the patient's severity and hemodynamic status rather than individual guidelines.
4.6. Systemic anticoagulation
Systemic anticoagulation is managed very differently in these two pathologies. We have specific recommendations for the initiation of long term anticoagulation after a stroke, related to the etiology and further prevention of new events [29,30]. However, its initiation is usually deferred to the subacute phase due to risks of hemorrhagic conversion. Contrary to that, anticoagulation is the cornerstone for acute PE management as it is shown that delay in its initiation leads to poor prognosis and worsening outcomes [23]. Current guidelines suggest the initiation of immediate IV anticoagulation with unfractionated heparin for at least 5–10 days from the event followed by a bridge to a vitamin K antagonist with a target INR of 2–3 or a novel oral anticoagulant [23,[31], [32], [33]]. On the other hand, solely use of anticoagulation for AIS has proven to be of no benefit in the acute phase of an AIS, and it has shown no reduction in recurrences, no benefit in death rate, or no impact on disability while increasing the risk of hemorrhagic conversion [34].
Our review revealed that 70.5% of subjects were started in IV-heparin. Of those, eight were initiated immediately, and four were delayed 24 h as they received rt-PA. Among those 8, one died, and three more had severe neurological long-term sequela (aphasia, plegia). Of the remaining four, two had mild symptoms, while outcomes were not reported for the remaining two. Among the four individuals that received rt-PA, one was utterly asymptomatic, 2 had mild symptoms, and the remainder was not reported. Upon discharge, warfarin was the most used anticoagulant in 41.7% of subjects, and 29.41% were discharged on anticoagulation, but the agent was not mentioned. A single patient was discharged on apixaban. Lastly, for patients that were not candidates for systemic anticoagulation, IVC filter placement was implemented.
4.7. Mechanical thrombectomy, catheter-directed reperfusion
MT with the Penumbra System (Penumbra, Inc. USA, Alameda, CA) is utilized for clot retrieval in PE. For patients with absolute contraindications to thrombolysis, interventional options include thrombus fragmentation with pigtail or balloon catheter, thrombectomy with hydrodynamic catheters, suction thrombectomy with aspiration catheters, and rotational thrombectomy [35,36].
Catheter-directed thrombolysis (CDT) allows thrombolytic agents' administration directly into the obstructing thrombus, increasing the clot's drug concentration and decreasing the systemic circulation concentration compared with peripherally administered systemic thrombolysis. There are no studies comparing CDT and systemic thrombolysis. The ULTIMA trial and the SEATTLE-II study were performed with the CDT system named Ekosonic Endovascular System (EKOS Corporation; Bothell, Washington, US), utilizing it to compare CDT implementing ultrasound-assisted catheter-directed thrombolysis versus systemic anticoagulation. These trials favor the Ekosonic Endovascular CDT system over isolated systemic anticoagulation, as it demonstrated benefits in decreasing right ventricle dilation, reducing pulmonary hypertension, decreasing anatomic thrombus burden, and lowering the rate of intracranial hemorrhage in patients with acute massive and submassive PE [[37], [38], [39]].
4.8. PFO closure
Transfemoral device closure of PFO is frequently recommended for secondary stroke prevention following a cryptogenic stroke in selected cases, including patients younger than 60 years with cryptogenic non-lacunar stroke, large right-left interatrial shunt, or atrial septal aneurysm [[40], [41], [42]]. Based on the evidence of device safety and effectiveness, the Amplatzer Septal Occluder (Abbott-Structural, Abbott-Park, IL) and The Gore CARDIOFORM Septal Occluder (W.L.Gore & Associates, Flagstaff, Arizona) were approved for use by the Food and Drug Administration for PFO closure [14,40,41].
According to the American Academy of Neurology (AAN), patients who have a PFO after an ischemic stroke should be considered for PFO closure after appropriate evaluation by both a vascular neurologist and cardiologist and be advised about its risks and benefits. The risk of a second stroke in people with PFO and no other possible causes of stroke is very low (1% per year) while being treated with medical therapy. The AAN recommends for patients younger than 60 years old who have had a stroke thought to be caused by a PFO; may benefit from PFO closure. Patients should be aware of a 3.4% reduction in the risk of a second stroke in five years, as well as the potential risks, such as a 3.9% chance of procedural complications with atrial fibrillation. Eventual treatment to reduce the risk of recurrent stroke due to paradoxical embolism implementing transcatheter closure of the patent foramen ovale should be addressed case by case [43].
5. Discussion
Current guidelines for AIS presenting within 4.5 h of symptom onset without any contraindications include IV rt-PA [1]. The efficacy of rt-PA is inversely proportional to time to treatment, and outcomes are better when rt-PA is administered rapidly [1]. Recent trial demonstrated MT's role as an adjuvant treatment to rt-PA [44], with most recent studies showing the benefit of this intervention in selected patients up to 24 h based on advanced images with perfusion studies [45]. Whereas for PE, treatment consists of parenteral anticoagulation, followed by oral anticoagulation for a minimum of 3 months. The addition of thrombolytic therapy for PE, such as IV or IA rt-PA, increases pulmonary reperfusion speed, reduces the pulmonary artery pressure, and improves the right ventricular function faster than anticoagulation alone [46]. Thrombolysis is only recommended for PE patients with systemic shock (Class I, B) or patients with intermediate-high-risk PE and clinical signs of hemodynamic decompensation (Class IIa, B) [26]. It is not recommended in PE when not experiencing shock or hypotension [47]. Furthermore, the use of thrombolytic therapy in PE assumes anticoagulation administration immediately after.
The simultaneous disease is extremely rare, including our cases; only 17 reports are describing this entity. We treated the first patient with supportive therapy as she was not a candidate for any intervention due to hemodynamic instability after the cardiac arrest. However, the second patient received catheter-directed therapy for severe PE using an ultrasound-enhanced thrombolysis system catheter with a local rt-PA infusion followed by systemic anticoagulation with unfractionated heparin and transition LMWH. Whether combined treatment with rt-PA and MT [48], based on the interventional trials' protocols, would have been more successful is unknown. The other case reports describe multiple permutations of the available treatment combinations [22].
Assuming that rt-PA can be administered acutely, there are two critical questions. First, how safe is it to start anticoagulation immediately after IV rt-PA administration? Secondly, if it is not immediately safe to treat AIS patients after rt-PA with anticoagulant medications for 24 h, how safe is it from the pulmonary perspective to delay anticoagulation for 24 h after rt-PA administration?
In our review, a patient received IV rt-PA and IV-heparin 24 h later [49], while another was given IV rt-PA followed immediately by an IVC filter and IV-heparin 24 h later [50] a third one was started on IV-heparin immediately after IV rt-PA [11]. All three patients had good outcomes; however, the data remains anecdotal. The purpose of this review is to convey different approaches to facilitate decision-making to clinicians in this challenging scenario.
5.1. Limitations
There are several limitations to this study. The first part is a retrospective chart review on a single center in Chicago; however, our combined stroke and PE detection rate is similar to the literature. Additionally, the sample is small, and despite adding cases from the literature review, the number of patients remains small. Also, this literature review is based only on case reports and small case series. Therefore, the small and heterogeneous sample size reduces the likelihood of detecting and drawing precise conclusions on treatment effects; consequently, we could not assess statistically significant differences in treatment effects between cases. The risk of bias tools for case reports and case series are scarce; however, we believe that we applied the best available tool for this review.
6. Conclusion
Simultaneous AIS and PE remain an infrequent clinical scenario from a literature perspective. There might be additional cases, but publication biases may have limited the reporting of treatment to only those with favorable outcomes. The few reported cases are insufficient to suggest one specific approach. AIS and PE stand for a challenge when they present simultaneously. Given this clinical presentation's rarity, there likely will never be any case series large enough to define a preferred approach. The evaluation of risks and benefits of therapies such as intravenous thrombolysis, mechanical thrombectomy for AIS or PE, and catheter-directed thrombolysis in the clinical context is essential. According to our review, the ischemic stroke burden guides systemic anticoagulation decisions over interventional procedures when the hemodynamic status remains unaffected. According to the current guidelines, eventual treatment to reduce the risk of recurrent stroke due to paradoxical embolism implementing transcatheter closure of the patent foramen ovale should be addressed case by case.
Source of funding
No funding to disclose.
Conflict of interest and disclosures
No conflicts of interest reported by other authors.
References
- 1.Friedman H.S., Koroshetz W.J., Qureshi N., Marler J.R., Del Zoppo G.J. Tissue plasminogen activator for acute ischemic stroke [2] N. Engl. J. Med. 1996 doi: 10.1056/NEJM199605233342114. [DOI] [PubMed] [Google Scholar]
- 2.Miller D.J., Simpson J.R., Silver B., Silver B. Safety of thrombolysis in acute ischemic stroke: a review of complications, risk factors, and newer technologies. Neurohospitalist. 2011 doi: 10.1177/1941875211408731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers W.J., Rabinstein A.A., Ackerson T. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke A. Stroke. 2019 doi: 10.1161/STR.0000000000000211. [DOI] [Google Scholar]
- 4.Omar H.R., Mangar D., Camporesi E.M. Simultaneous thrombosis of 2 vascular territories: is thrombolytic therapy a better option? Am. J. Emerg. Med. 2013 doi: 10.1016/j.ajem.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. Evid. Based Med. 2018 doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HAGEN P.T., SCHOLZ D.G., EDWARDS W.D. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin. Proc. 1984 doi: 10.1016/S0025-6196(12)60336-X. [DOI] [PubMed] [Google Scholar]
- 7.Clergeau M.R., Hameon M., Morello R., Saloux E., Viader F., Hamon M. Silent cerebral infarcts in patients with pulmonary embolism and a patent foramen ovale: a prospective diffusion-weighted mri study. Stroke. 2009 doi: 10.1161/STROKEAHA.109.559898. [DOI] [PubMed] [Google Scholar]
- 8.Pongmoragot J., Rabinstein A.A., Nilanont Y., Swartz R.H., Zhou L., Saposnik G. Pulmonary embolism in ischemic stroke: clinical presentation, risk factors, and outcome. J. Am. Heart Assoc. 2013 doi: 10.1161/JAHA.113.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turetz M., Sideris A.T., Friedman O.A., Triphathi N., Horowitz J.M. Epidemiology, pathophysiology, and natural history of pulmonary embolism. Semin. Interv. Radiol. 2018 doi: 10.1055/s-0038-1642036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehme A.K., Esenwa C., Elkind M.S.V. Stroke risk factors, genetics, and prevention. Circ. Res. 2017 doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belvís R., Masjuan J., García-Barragán N. Stroke and pulmonary thromboembolism after a long flight. Eur. J. Neurol. 2005 doi: 10.1111/j.1468-1331.2005.01070.x. [DOI] [PubMed] [Google Scholar]
- 12.Di Tullio M.R., Jin Z., Russo C. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J. Am. Coll. Cardiol. 2013 doi: 10.1016/j.jacc.2013.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechat P., Mas J.L., Lascault G. Prevalence of patent foramen ovale in patients with stroke. N. Engl. J. Med. 1988 May 5;18(18):1148–1152. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 14.Favilla C.G., Messé S.R. Patent foramen ovale and stroke: current evidence and treatment options. Curr. Opin. Neurol. 2020 doi: 10.1097/WCO.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 15.Michel P., Villablanca P.A., Ranka S., Lemor A., Jain T., Ramakrishna H. Patent foramen ovale and risk of cryptogenic stroke – analysis of outcomes and perioperative implications. J. Cardiothorac. Vasc. Anesth. 2020 doi: 10.1053/j.jvca.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Brazis P.W., Masdeu J.C., Biller J. Sixth edition. 2012. Localization in Clinical Neurology. [DOI] [Google Scholar]
- 17.Miniati M., Cenci C., Monti S., Poli D. Clinical presentation of acute pulmonary embolism: survey of 800 cases. PLoS ONE. 2012 doi: 10.1371/journal.pone.0030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mojadidi M.K., Bogush N., Caceres J.D., Msaouel P., Tobis J.M. Diagnostic accuracy of transesophageal echocardiogram for the detection of patent foramen ovale: a meta-analysis. Echocardiography. 2014 doi: 10.1111/echo.12462. [DOI] [PubMed] [Google Scholar]
- 19.Romero J., Husain S.A., Kelesidis I., Sanz J., Medina H.M., Garcia M.J. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ. Cardiovasc. Imaging. 2013 doi: 10.1161/CIRCIMAGING.112.000153. [DOI] [PubMed] [Google Scholar]
- 20.Barros-Gomes S., El Sabbagh A., Eleid M.F., Mankad S.V. Concomitant acute stroke, pulmonary and myocardial infarction due to in-transient thrombus across a patent foramen ovale. Echo Res. Pract. 2018 doi: 10.1530/ERP-18-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omar H.R., Huang C., Miller J.H., Mangar D., Kabemba A., Camporesi E.M. Simultaneous pulmonary embolism and cerebrovascular stroke. Herz. 2013 doi: 10.1007/s00059-013-3782-6. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen M.E., Kumar G., Mahabir R.C., Helmers R.A., Bendok B.R., O’Carroll C.B. Intravenous alteplase for acute stroke and pulmonary embolism in a patient with recent abdominoplasty. Neurologist. 2017 doi: 10.1097/NRL.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 23.Smith S.B., Geske J.B., Maguire J.M., Zane N.A., Carter R.E., Morgenthaler T.I. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010 doi: 10.1378/chest.09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naidoo P., Hift R. Massive pulmonary thromboembolism and stroke. Case Rep. Med. 2011 doi: 10.1155/2011/398571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lankeit M., Konstantinides S. Mortality risk assessment and the role of thrombolysis in pulmonary embolism. Crit. Care Clin. 2011 doi: 10.1016/j.ccc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Konstantinides S.V., Torbicki A., Skoro-Sajer N. ESC guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the european society of cardiology (ESC) Russ. J. Cardiol. 2014;2015 doi: 10.15829/1560-4071-2015-08-67-110. [DOI] [Google Scholar]
- 27.Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995 doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 28.Hughes R.E., Bhimji S.S. StatPearls; 2018. TPA Therapy. [Google Scholar]
- 29.Seiffge D.J., Werring D.J., Paciaroni M. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019 doi: 10.1016/S1474-4422(18)30356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homma S., Thompson J.L.P., Pullicino P.M. Warfarin and aspirin in patients with heart failure and sinus rhythm. N. Engl. J. Med. 2012 doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michota F. Transitions of care in anticoagulated patients. J. Multidiscip. Healthc. 2013 doi: 10.2147/JMDH.S44068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel P., Pandya J., Goldberg M. 2017. NOACs vs. Warfarin for Stroke Prevention in Nonvalvular Atrial Fibrillation. Cureus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011 doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 34.Robinson A.A., Ikuta K., Soverow J. Anticoagulation for the acute management of ischemic stroke. Yale J. Biol. Med. 2014 Jun;87(2):199. [PMC free article] [PubMed] [Google Scholar]
- 35.Douketis J.D. The 2016 American College of Chest Physicians treatment guidelines for venous thromboembolism: a review and critical appraisal. Intern. Emerg. Med. 2016 doi: 10.1007/s11739-016-1553-0. [DOI] [PubMed] [Google Scholar]
- 36.Jaber W.A., Fong P.P., Weisz G. Acute pulmonary embolism: with an emphasis on an interventional approach. J. Am. Coll. Cardiol. 2016 doi: 10.1016/j.jacc.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Piazza G., Hohlfelder B., Jaff M.R. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc. Interv. 2015 doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Engelberger R.P., Kucher N. Reperfusion treatment for acute pulmonary embolism. Hamostaseologie. 2018 doi: 10.1055/s-0038-1641717. [DOI] [PubMed] [Google Scholar]
- 39.Kucher N., Boekstegers P., Müller O.J. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 40.Javois A.J., Rome J.J., Jones T.K. Results of the U.S. food and drug administration continued access clinical trial of the GORE HELEX septal occluder for secundum atrial septal defect. JACC Cardiovasc. Interv. 2014 doi: 10.1016/j.jcin.2014.01.169. [DOI] [PubMed] [Google Scholar]
- 41.Suradi H.S., Hijazi Z.M. Patent foramen ovale: stroke and device closure. Cardiol. Clin. 2016 doi: 10.1016/j.ccl.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Collado F.M.S., Poulin M.F., Murphy J.J., Jneid H., Kavinsky C.J. Patent foramen ovale closure for stroke prevention and other disorders. J. Am. Heart Assoc. 2018 doi: 10.1161/JAHA.117.007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messé S.R., Gronseth G., Kent D.M. Practice advisory: recurrent stroke with patent foramen ovale (update of practice parameter): report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2016 Aug 23;87(8):815–821. doi: 10.1212/WNL.0000000000002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkhemer O.A., Fransen P.S.S., Beumer D. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015 doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira R.G., Jadhav A.P., Haussen D.C. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018 doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 46.Wan S., Quinlan D.J., Agnelli G., Eikelboom J.W. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004 doi: 10.1161/01.CIR.0000137826.09715.9C. [DOI] [PubMed] [Google Scholar]
- 47.Allport L.E., Butcher K.S. Thrombolysis for concomitant acute stroke and pulmonary embolism. J. Clin. Neurosci. 2008 doi: 10.1016/j.jocn.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Iwanaga T., Iguchi Y., Shibazaki K., Inoue T., Kimura K. Paradoxical brain embolism in an acute stroke. J. Neurol. Sci. 2007 doi: 10.1016/j.jns.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Pelletier M., Bugeaud R., Ibrahim R., Morency G., Kouz S. Successful thrombolysis of a stroke with a pulmonary embolism in a young woman. J. Emerg. Med. 2010 doi: 10.1016/j.jemermed.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 50.Pavesi P.C., Pedone C., Crisci M., Piacentini A., Fulvi M., Di Pasquale G. Concomitant submassive pulmonary embolism and paradoxical embolic stroke after a long flight: which is the optimal treatment? J. Cardiovasc. Med. 2008 doi: 10.2459/JCM.0b013e328306f2ea. [DOI] [PubMed] [Google Scholar]
- 51.Lapostolle F., Surget V., Borron S.W. Severe pulmonary embolism associated with air travel. N. Engl. J. Med. 2001 doi: 10.1056/NEJMoa010378. [DOI] [PubMed] [Google Scholar]
- 52.Bracey T.S., Langrish C., Darby M., Soar J. Cerebral infarction following thrombolysis for massive pulmonary embolism. Resuscitation. 2006 doi: 10.1016/j.resuscitation.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 53.Gunta S., Kamath S. A case of pulmonary embolism and stroke in a 16-year-old girl. Wis. Med. J. 2012;111(2):58–60. [PubMed] [Google Scholar]